Introduction
Polycystic ovary syndrome (PCOS), also known as
Stein-Leventhal syndrome, is a series of symptoms caused by
increased levels of male hormones in women, which include irregular
menstruation, excessive menstruation, hirsutism, acne, pelvic pain,
difficulty in conception, acanthosis nigricans and other symptoms
(1-3).
There are several conditions related to PCOS, such as obesity,
obstructive sleep apnea, cardiovascular diseases, affective
disorders and endometrial cancer (4). PCOS is affected by genetic and
environmental factors; the risk factors include obesity,
insufficient exercise or family history (5). Currently, there is no specific cure
for PCOS (6,7). Therefore, the development of novel and
effective treatment strategies for PCOS are required.
The ovary is an important organ for follicle
development and ovulation, and the development of the follicle is
accompanied by the growth, development and differentiation of
granulosa cells (8,9). The proliferation and apoptosis of
ovarian granulosa cells was discovered to be closely associated
with ovarian development (10,11),
thus, ovarian granulosa cells are usually used to study the
associated mechanisms of PCOS (12-14).
For this reason, the present study also studied PCOS using ovarian
granulosa cells.
MicroRNAs (miRNAs/miRs) are a set of small
non-coding RNAs of 20-22 nucleotides in length, which are involved
in the growth of several types of cell and regulate the expression
levels of target genes (15). An
increasing number of studies have suggested that miRNAs may be
associated with numerous types of human disease, including cancer,
cardiovascular diseases, gynecological diseases and inflammatory
diseases (16-19).
In addition, previous studies have reported the roles of miRNAs in
PCOS, such as miR-27a-3p and miR-518f-3p (20,21).
The mechanisms by which miRNAs are involved in the pathogenesis of
PCOS are multifaceted and include their ability to affect
follicular development or promote ovarian dysfunction, and to
regulate ovarian hormones, and granulosa cell proliferation and
apoptosis (22,23). In previous studies, the expression
levels of miR-206, which is located on human chromosome 6, were
discovered to be downregulated in patients with PCOS; however, its
specific role remains unclear (24).
The present study aimed to determine whether miR-206
served a significant role in patients with PCOS and further
investigated its molecular mechanisms to provide a deeper
theoretical basis and potential novel strategies for the treatment
of PCOS.
Materials and methods
Cell culture
Normal ovarian surface epithelial IOSE80 cells were
used as the control cells in the present study (25). IOSE80 cells and human ovarian
granulosa cell-like KGN cells were obtained from the American Type
Culture Collection. The cells were cultured in DMEM (Gibco; Thermo
Fisher Scientifc, Inc.) supplemented with 10% FBS (Gibco; Thermo
Fisher Scientific, Inc.), and maintained at 37˚C in a humidified
atmosphere of 5% CO2.
RNA extraction and reverse
transcription-quantitative PCR (RT-qPCR)
Total RNA was extracted from the cells using an
PicoPure™ RNA Isolation kit (Invitrogen; Thermo Fisher Scientific,
Inc.) and reverse transcribed into cDNA using a High Capacity cDNA
Reverse Transcription Kit (Invitrogen; Thermo Fisher Scientific,
Inc.). The following temperature conditions for RT were as follows:
70˚C for 5 min, 37˚C for 5 min and 42˚C for 60 min. qPCR was
subsequently performed to quantify the expression levels of miRNA
and mRNA on a Prism 7000 Real-Time PCR system (Applied Biosystems;
Thermo Fisher Scientific, Inc.) using the SYBR qPCR Master Mix
(Vazyme Biotech Co., Ltd.) according to the manufacturer's
protocol. The primers were supplied by Sangon Biotech Co., Ltd. The
following thermocycling conditions were used for the qPCR: Initial
denaturation for 5 min at 95˚C; followed by 40 cycles of
denaturation at 95˚C for 10 sec, annealing at 60˚C for 30 sec and
extension at 72˚C for 34 sec. GAPDH was used as the internal
loading control for mRNA expression levels, while U6 was used as
the internal loading control for miR-206 expression levels. The
primer sequences used for the PCR were listed as follows: GAPDH
forward, 5'-CTTTGGTATCGTGGAAGGACTC-3' and reverse,
5'-GTAGAGGCAGGGATGATGTTCT-3'; U6 forward,
5'-GCTTCGGCAGCACATATACTAAAAT-3' and reverse,
5'-CGCTTCACGAATTTGCGTGTCAT-3'; miR-206 forward,
5'-GCGTCTGGAATGTAAGGAAGTG-3' and reverse, 5'-GTGCAGGGTCCGAGGT-3';
and CCND2 forward, 5'-TCCAAACTCAAAGAGACCAGC-3'; and reverse,
5'-TTCCACTTCAACTTCCCCAG-3'.
The relative mRNA expression levels of miR-206 and
cyclin D2 (CCND2) were quantified using the 2-ΔΔCq
method (26). All experiments were
performed ≥3 times.
Cell transfection
Following the overnight culture, KGN cells
(5x104 cells per well) were transfected with the 100 nM
mimic control (5'-UUUGUACUACACAAAAGUACUG-3'; Shanghai GenePharma
Co., Ltd.), 100 nM miR-206 mimic (5'-TGGAATGTAAGGAAGTGTGTGG-3';
Shanghai GenePharma Co., Ltd.), 1 µg cyclin D2 CRISPR Activation
Plasmid (CCND2-plasmid; cat. no. sc-401236-ACT; Santa Cruz
Biotechnology, Inc.), 1 µg Control CRISPR Activation Plasmid
(control-plasmid; cat. no. sc-437275; Santa Cruz Biotechnology,
Inc.), 100 nM miR-206 mimic + 1 µg control-plasmid or 100 nM
miR-206 mimic + 1 µg CCND2-plasmid using Lipofectamine®
2000 reagent (Invitrogen; Thermo Fisher Scientific, Inc.).
Following transfection at 37˚C for 48 h, the transfection
efficiency of the cells was analyzed using RT-qPCR. Cells without
any treatment were used as the control.
miRNA target analysis and
Dual-Luciferase reporter assay
The relationship between miR-206 and CCND2 was
determined using TargetScan release 7.1 software (www.targetscan.org/vert_71). The 3'-untranslated
region (UTR) of CCND2 containing the target sequence of miR-206 was
obtained using RT-qPCR and cloned into a pmirGLO vector (Promega
Corporation) to construct the wild-type (WT) reporter vector,
CCND2-WT. Similarly, a mutant (MUT) vector, CCND2-MUT, was created.
A QuikChange Site-Directed Mutagenesis kit (Stratagene; Agilent
Technologies, Inc.) was applied according to the manufacturer's
instructions to point-mutate the miR-206 binding domain in the
3'UTR of CCND2. Subsequently, 293 cells (5x104 cells per
well) were cultured at 37˚C for 24 h and then co-transfected with 1
ng CCND2-WT or 1 ng CCND2-MUT and the 100 nM miR-206 mimic or 100
nM mimic control using Lipofectamine 2000 reagent at 37˚C for 48 h.
The relative luciferase activity was measured using a
Dual-Luciferase reporter assay system (Promega Corporation),
according to the manufacturer's protocol. The relative luciferase
activity was normalized to Renilla luciferase activity. All
the experiments were performed ≥3 times.
Western blotting
Total protein was extracted from IOSE80 and KGN
cells using RIPA Lysis Buffer (Beyotime Institute of
Biotechnology). Total protein was quantified using a BCA protein
assay kit (Pierce; Thermo Fisher Scientific, Inc.) and equal
amounts (40 µg per lane) of protein were separated via 10%
SDS-PAGE. The separated proteins were subsequently transferred onto
PVDF membranes and blocked with PBS-(0.1%) Tween-20 (PBST) solution
containing 5% non-fat milk at room temperature for 1 h. The
membranes were then incubated with the following primary antibodies
at 4˚C overnight: anti-CCND2 (cat. no. sc-376676; 1:1,000; Santa
Cruz Biotechnology, Inc.), anti-cleaved-caspase-3 (cat. no. 9664;
1:1,000; Cell Signaling Technology), anti-pro-caspase-3 (cat. no.
sc-373730; 1:1,000; Santa Cruz Biotechnology, Inc.) or anti-GAPDH
(cat. no. sc-166574; 1:1,000; Santa Cruz Biotechnology, Inc.).
Following the primary antibody incubation, the membranes were
washed 3 times with PBST and incubated with the horseradish
peroxidase-conjugated secondary antibodies (cat. nos. bs-0296G-HRP
and bs-0295G-HRP; 1:500; BIOSS) for 40 min at room temperature. The
protein bands were visualized using an ECL reagent (Pierce; Thermo
Fisher Scientifc, Inc.), according to the manufacturer's
instructions. All experiments were performed 3 times. ImageJ
software (version 2.0; National Institutes of Health) was used to
quantify the band intensity.
MTT assay
KGN cells (5x104 cells per well) were
cultured in 96-well plates at 37˚C for 24 h, then the cells were
transfected with 100 nM mimic control, 100 nM miR-206 mimic, 100 nM
miR-206 mimic + 1 µg control-plasmid or 100 nM miR-206 mimic + 1 µg
CCND2-plasmid for 48 h. Subsequently, 10 µl MTT solution (Beyotime
Institute of Biotechnology) was added/well and incubated at 37˚C
for a further 4 h. Subsequently, 100 µl DMSO was added into each
well at 37˚C for 3 h to solubilize the formazan product after the
culture medium was removed. The absorbance was measured at 570 nm
using a microplate reader (Bio-Rad Laboratories, Inc.). The cell
viability was calculated by normalizing each group with the control
group using the optical density values.
Flow cytometric analysis of
apoptosis
To analyze cell apoptosis, Annexin V/PI Apoptosis
Detection kit (Beyotime Institute of Biotechnology) according to
the manufacturer's protocol. KGN cells were collected by
trypsinization through centrifugation (1,000 x g, 5 min, 4˚C),
washed with PBS and then resuspended in 1X binding buffer at a
density of 1x106 cells/ml. Subsequently, 100 µl cell
suspension was added into a 5 ml tube, which was incubated with 5
µl Annexin V-FITC and PI at room temperature for 20 min, according
to the manufacturers' protocols. The stained cells were analyzed
using a BD FACSCalibur flow cytometer (BD Biosciences) and
apoptotic rate (the percentage of early + late apoptotic cells) was
calculated. Data were analyzed using CellQuest™ software
(version 5.1; BD Biosciences). All the experiments were performed
≥3 times.
Detection of caspase-3 activity
KGN cells (5x104 cells) were plated into
96-well plates and incubated at 37˚C overnight. Following the
incubation, the cells were transfected for 48 h, as described
above, and then collected by trypsinization through centrifugation
(600 x g; 4˚C; 5 min). Subsequently, the caspase-3 activity was
immediately detected using a caspase-3 activity assay kit (Beyotime
Institute of Biotechnology), according to the manufacturer's
protocol. The absorbance was recorded at 405 nm using a microplate
reader (Bio-Rad Laboratories, Inc.); caspase-3 activity was
measured using optical density values.
Statistical analysis
Statistical analysis was performed using GraphPad
Prism 6 software (GraphPad Software, Inc.) and data are presented
as the mean ± standard deviation of ≥3 independent experiments.
Statistical differences between groups were determined using an
unpaired Student's t-test or one-way ANOVA followed by Tukey's post
hoc test. P<0.05 was considered to indicate a statistically
significant difference.
Results
miR-206 expression levels are
downregulated in KGN cells
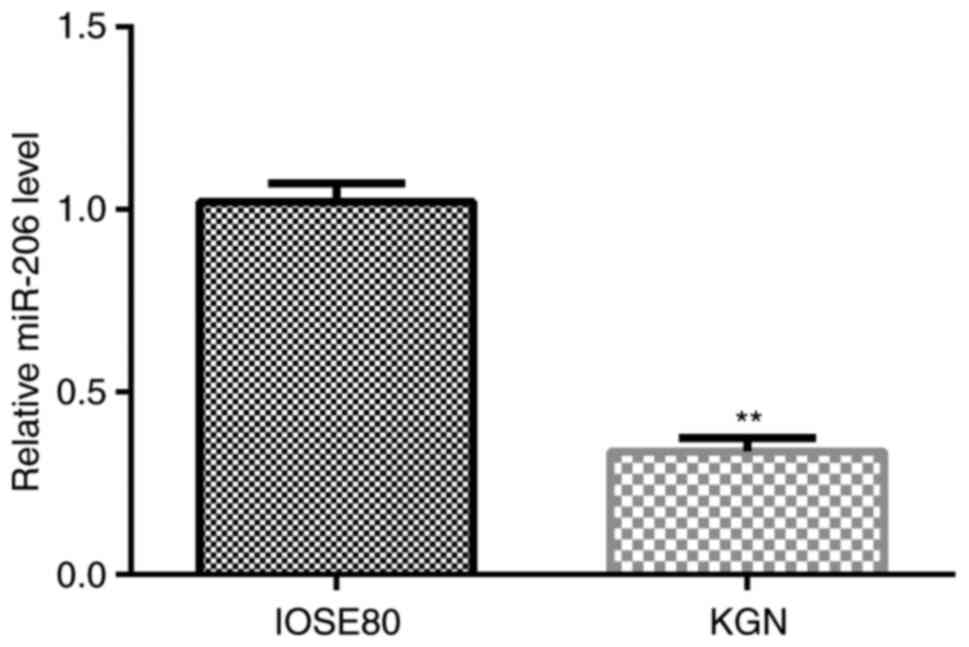
The relative expression levels of miR-206 in normal
ovarian surface epithelial IOSE80 cells and human ovarian granulosa
KGN cells were analyzed using RT-qPCR. As shown in Fig. 1, the expression levels of miR-206
were significantly downregulated in KGN cells compared with in
IOSE80 cells.
CCND2 is a direct target gene of
miR-206 and demonstrates upregulated expression levels in KGN
cells
To investigate the molecular mechanisms of miR-206
in ovarian granulosa cells, the direct target gene of miR-206 was
identified through the bioinformatics tool TargetScan. The
predicted results indicated that CCND2 was a potential target of
miR-206 (Fig. 2A). In addition, a
Dual-Luciferase reporter assay was performed in 293 cells
co-transfected with a luciferase vector plasmid carrying
CCDND2-WT/MUT and the miR-206 mimic or mimic control to validate
the predicted binding site of miR-206 on the CCND2 3'-UTR; the
miR-206 mimic significantly decreased the relative luciferase
activity of the CCND2-WT 3'-UTR compared with the mimic control and
CCDN2-WT-transfected cells, whereas no significant differences were
observed in the relative luciferase activity between the cells
co-transfected with the CCND2-MUT 3'-UTR and the miR-206 mimic or
mimic control (Fig. 2B). These
results indicated that miR-206 may directly target CCND2. In
addition, the mRNA and protein expression levels of CCND2 in IOSE80
cells and KGN cells were investigated. As shown in Fig. 2C-E, the mRNA and protein expression
levels of CCND2 were significantly upregulated in KGN cells
compared with IOSE80 cells.
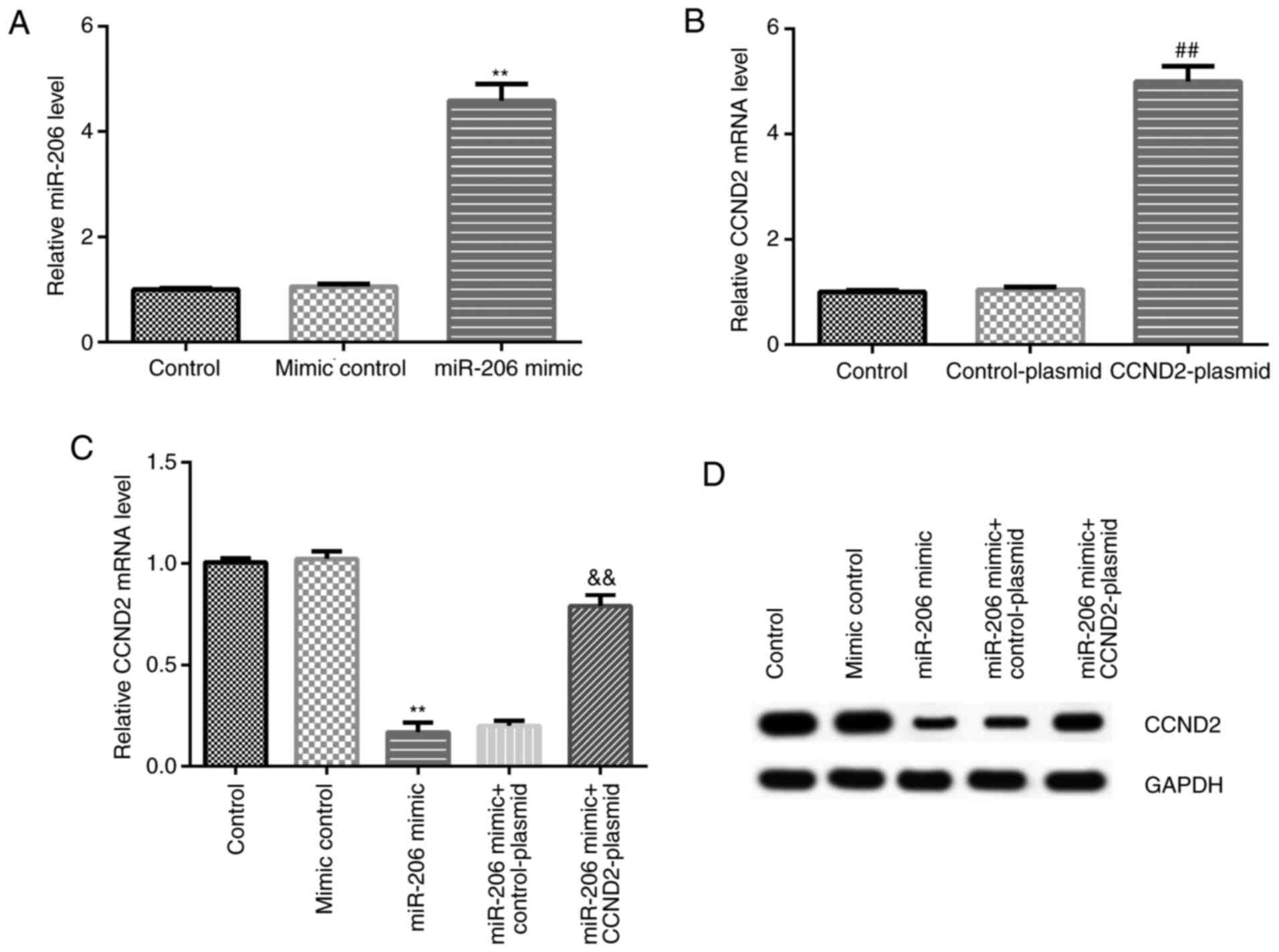
miR-206 downregulates the expression
levels of CCND2 in KGN cells
KGN cells were transfected with the mimic control,
miR-206 mimic, CCND2-plasmid, or control-plasmid, and RT-qPCR was
used to analyze the transfection efficiency. Compared with the
mimic control group, the miR-206 mimic significantly upregulated
the expression levels of miR-206 in KGN cells, while compared with
the control-plasmid group, the CCND2-plasmid significantly
upregulated CCND2 mRNA expression levels in KGN cells (Fig. 3A and B). In addition, the results of the
co-transfection in KGN cells revealed that compared with the mimic
control group, the miR-206 mimic downregulated the mRNA and protein
expression levels of CCND2 in KGN cells; however, this reduction
could be reversed by the co-transfection with the CCND2-plasmid
(Fig. 3C and D).
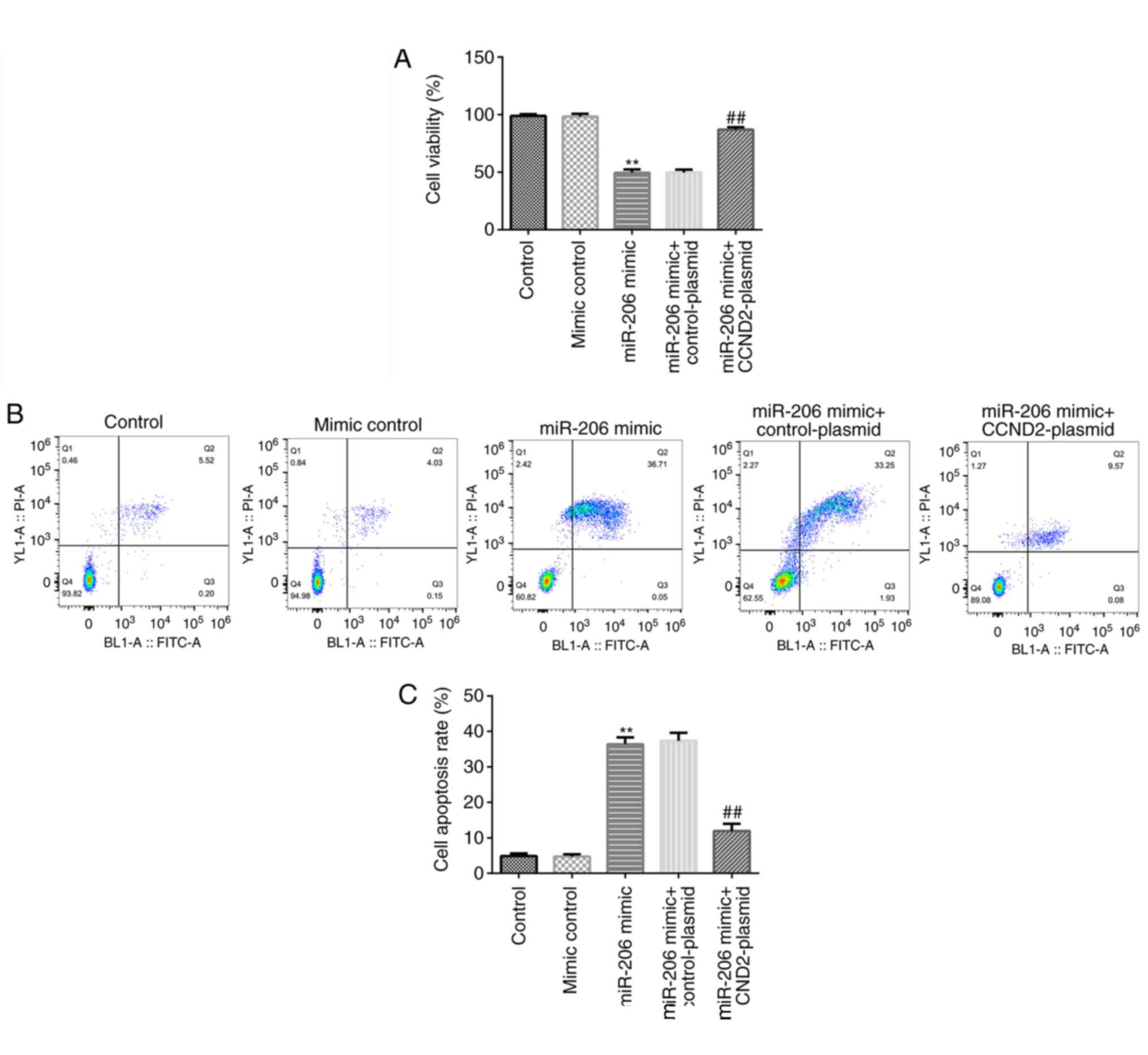
miR-206 inhibits cell viability and
induces cell apoptosis in KGN cells by targeting CCND2
An MTT assay and flow cytometric analysis were
performed to determine the cell viability and levels of apoptosis.
As shown in Fig. 4A-C, compared
with the mimic control, the miR-206 mimic significantly reduced the
cell viability and induced apoptosis in KGN cells, while these
effects were significantly reversed following the co-transfection
with the CCND2-plasmid.
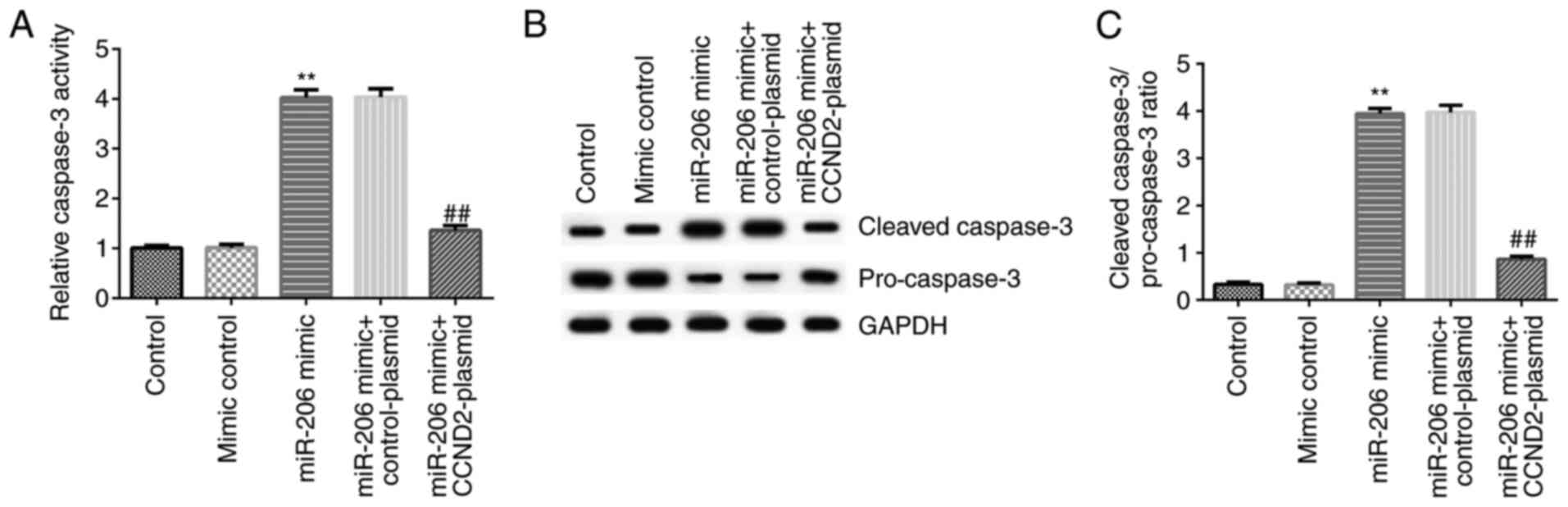
miR-206 induces the apoptosis of KGN
cells by regulating caspase-3 activity
To investigate the potential mechanism of miR-206 in
the regulation of apoptosis in KGN cells, the activity and related
protein expression levels of caspase-3 were analyzed in KGN cells
following cell transfection. The results revealed that compared
with the mimic control, the miR-206 mimic significantly increased
the activity of caspase-3, and upregulated the protein expression
levels of cleaved-caspase-3 and the cleaved-caspase-3/pro-caspase-3
ratio, while downregulating the protein expression levels of
pro-caspase-3, in KGN cells. All of these effects were
significantly reversed following the co-transfection with the
CCND2-plasmid (Fig. 5A-C).
Discussion
PCOS is one of the most common types of endocrine
disease among women aged 18-44 years (4). In fact, it has been suggested that the
incidence of PCOS accounts for 6-10% of women's reproductive years
(27,28). PCOS is a primary cause of
infertility and the growth of ovarian granulosa cells has been
associated with the development of PCOS (29). In the present study, IOSE80 cells
and human ovarian granulosa cell-like KGN cells were used and it
was discovered that the expression levels of miR-206 were
downregulated in KGN cells, which is consistent with a previous
report (24). The results also
suggested that the downregulated miR-206 expression levels may be
associated with the occurrence and development of PCOS.
The present study further investigated the
mechanisms of miR-206 in PCOS. It was previously reported that
CCND2 was a target gene of miR-206(30), and the present study validated this
finding. In addition, miR-206 mimic significantly reduced CCND2
expression in KGN cells, indicating a negative regulatory mechanism
was identified between miR-206 and its target gene CCND2. To
determine whether miR-206 affected the viability of KGN cells by
directly targeting CCND2, several experiments were performed in KGN
cells following the overexpression of miR-206 or CCND2. The results
of the MTT and flow cytometric analysis indicated that the
overexpression of miR-206 reduced the cell viability and induced
cell apoptosis in KGN cells, while these effects were reversed
following the upregulation of CCND2. However, in the present study,
the cell viability and apoptosis of cells was detected using an MTT
assay and flow cytometric analysis, respectively; it is more
reliable and convincing to detect cell viability and the levels of
apoptosis by various experimental methods (such as TUNEL and BrdU
assay), thus this is a limitation of the present study.
The caspase family serve an important role in the
process of mediating cell apoptosis (31). Caspase-3, which belongs to the cell
death protein 3 subfamily, is the crucial executor molecule that
participates in numerous pathways of apoptosis signal transduction
(32,33). The present study analyzed caspase-3
activity and expression levels, and the results showed that miR-206
mimic significantly enhanced caspase-3 activity, increased
cleaved-caspase-3 protein level, reduced pro-caspase-3 protein
level and enhanced the ratio of cleaved-caspase-3/pro-caspase-3,
and all these changes were reversed by CCND2-plasmid
co-transfection. The findings indicated that miR-206 and CCND2 may
affect the viability of KGN cells via regulating caspase-3.
In conclusion, the findings of the present study
suggested that miR-206 may serve a crucial role in PCOS through
modulating the cell viability and apoptosis of ovarian granulosa
cells. These findings indicated that miR-206 may be an effective
target for the treatment of PCOS, which provides a novel
opportunity for the development of clinical therapies for PCOS.
However, the present study was only a preliminary study of the role
of miR-206 in PCOS. In order to clarify the role and function of
miR-206 in PCOS, further in-depth research is required. For
example, the expression levels of miR-206 and its target gene CCND2
in ovarian tissue or granulosa cells of patients with PCOS needs to
be clarified. Furthermore, the role of CCND2 in regulating the cell
viability and apoptosis of ovarian granulosa cells should be
determined. In addition, the effects of miR-206 and CCND2 in PCOS
should be investigated in vivo; for instance, the
association between the expression levels of miR-206 and CCND2 and
the clinicopathological variables of patients with PCOS requires
further analysis. Future research will aim to explore these topics
in depth.
Acknowledgements
Not applicable.
Funding
The present study was supported by the Project of
Study on Meiosis and Signal Transduction Pathway of Mouse Oocytes
(grant no. LY20H040001).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
JZ and XJ contributed to the conception and design
of the study, data acquisition, analysis and interpretation, and
drafted and critically revised the manuscript. ZS collected the
data and performed statistical analysis. ZZ contributed to data
collection and prepared the manuscript. All authors read and
approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Macut D, Bjekić-Macut J, Rahelić D and
Doknić M: Insulin and the polycystic ovary syndrome. Diabetes Res
Clin Pract. 130:163–170. 2017.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Franik S, Eltrop SM, Kremer JA, Kiesel L
and Farquhar C: Aromatase inhibitors (letrozole) for subfertile
women with polycystic ovary syndrome. Cochrane Database Syst Rev.
5(CD010287)2018.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Öztürk A, Kucur SK, Seven A, Deveci E,
Şencan H, Yilmaz O and Kiliç A: Temperament and character
differences of patients with polycystic ovary syndrome. J Gynecol
Obstet Hum Reprod. 48:255–259. 2019.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Cooney LG and Dokras A: Beyond fertility:
Polycystic ovary syndrome and long-term health. Fertil Steril.
110:794–809. 2018.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Kshetrimayum C, Sharma A, Mishra VV and
Kumar S: Polycystic ovarian syndrome: Environmental/occupational,
lifestyle factors; an overview. J Turk Ger Gynecol Assoc.
20:255–263. 2019.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Sathyapalan T, Shepherd J, Arnett C, Coady
AM, Kilpatrick ES and Atkin SL: Atorvastatin increases 25-hydroxy
vitamin D concentrations in patients with polycystic ovary
syndrome. Clin Chem. 56:1696–1700. 2020.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Li L, Zhang R, Zeng J, Ke H, Peng X, Huang
L, Zhang H, Chen Z, Li TT, Tan Q, et al: Effectiveness and safety
assessment of drospirenone/ethinyl estradiol tablet in treatment of
PCOS patients: A single center, prospective, observational study.
BMC Womens Health. 20(39)2020.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Xu J, Bao X, Peng Z, Wang L, Du L, Niu W
and Sun Y: Comprehensive analysis of genome-wide DNA methylation
across human polycystic ovary syndrome ovary granulosa cell.
Oncotarget. 7:27899–27909. 2016.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Lai Q, Xiang W, Li Q, Zhang H, Li Y, Zhu
G, Xiong C and Jin L: Oxidative stress in granulosa cells
contributes to poor oocyte quality and IVF-ET outcomes in women
with polycystic ovary syndrome. Front Med. 12:518–524.
2018.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Lv X, He C, Huang C, Wang H, Hua G, Wang
Z, Zhou J, Chen X, Ma B, Timm BK, et al: Timely expression and
activation of YAP1 in granulosa cells is essential for ovarian
follicle development. FASEB J. 33:10049–10064. 2019.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Fan Y, Chang Y, Wei L, Chen J, Li J,
Goldsmith S, Silber S and Liang X: Apoptosis of mural granulosa
cells is increased in women with diminished ovarian reserve.
Version 2. J Assist Reprod Genet. 36:1225–1235. 2019.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Fu X, He Y, Wang X, Peng D, Chen X, Li X
and Wan Q: MicroRNA-16 promotes ovarian granulosa cell
proliferation and suppresses apoptosis through targeting PDCD4 in
polycystic ovarian syndrome. Cell Physiol Biochem. 48:670–682.
2018.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Zheng Q, Li Y, Zhang D, Cui X, Dai K, Yang
Y, Liu S, Tan J and Yan Q: ANP promotes proliferation and inhibits
apoptosis of ovarian granulosa cells by NPRA/PGRMC1/EGFR complex
and improves ovary functions of PCOS rats. Cell Death Dis.
8(e3145)2017.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Li M, Zhao H, Zhao SG, Wei DM, Zhao YR,
Huang T, Muhammad T, Yan L, Gao F, Li L, et al: The HMGA2-IMP2
pathway promotes granulosa cell proliferation in polycystic ovary
syndrome. J Clin Endocrinol Metab. 104:1049–1059. 2019.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Wang J, Chen J and Sen S: MicroRNA as
biomarkers and diagnostics. J Cell Physiol. 231:25–30.
2016.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Yanaihara N and Harris CC: MicroRNA
involvement in human cancers. Clin Chem. 59:1811–1812.
2013.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Liao XB, Perez VA, Król M, Yeh CH and Yuan
LQ: MicroRNA and cardiovascular disease. Biomed Res Int.
2015(734380)2015.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Gong CL, Wang Y, Lyu Y, Sun JJ and Li GP:
The effect and mechanism of microRNA199 in gynecological diseases.
J Fam Plam Reprod H. 33:60–63. 2014.
|
|
19
|
Guo J, Sun M, Teng X and Xu L:
MicroRNA-7-5p regulates the expression of TFF3 in inflammatory
bowel disease. Mol Med Rep. 16:1200–1206. 2017.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Wang M, Sun J, Xu B, Chrusciel M, Gao J,
Bazert M, Stelmaszewska J, Xu Y, Zhang H, Pawelczyk L, et al:
Functional characterization of microRNA-27a-3p expression in human
polycystic ovary syndrome. Endocrinology. 159:297–309.
2018.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Sørensen AE, Wissing ML, Englund AL and
Dalgaard LT: MicroRNA species in follicular fluid associating with
polycystic ovary syndrome and related intermediary phenotypes. J
Clin Endocrinol Metab. 101:1579–1589. 2016.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Roth LW, McCallie B, Alvero R, Schoolcraft
WB, Minjarez D and Katz-Jaffe MG: Altered microRNA and gene
expression in the follicular fluid of women with polycystic ovary
syndrome. J Assist Reprod Genet. 31:355–362. 2014.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Ilie IR and Georgescu CE: Polycystic ovary
syndrome-epigenetic mechanisms and aberrant microRNA. Adv Clin
Chem. 71:25–45. 2015.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Díaz M, Bassols J, López-Bermejo A, de
Zegher F and Ibáñez L: Low circulating levels of miR-451a in girls
with polycystic ovary syndrome: Different effects of randomized
treatments. J Clin Endocrinol Metab. 105:1–9. 2020.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Sun X, Su S, Zhang G, Zhang H and Yu X:
MiR-204 suppresses cell proliferation and promotes apoptosis in
ovarian granulosa cells via targeting TPT1 in polycystic ovary
syndrome. Biochem Cell Biol. 97:554–562. 2019.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408.
2001.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Bahri Khomami M, Boyle JA, Tay CT, Vanky
E, Teede HJ, Joham AE and Moran LJ: Polycystic ovary syndrome and
adverse pregnancy outcomes: Current state of knowledge, challenges
and potential implications for practice. Clin Endocrinol (Oxf).
88:761–769. 2018.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Cui Y, Shi Y, Cui L, Han T, Gao X and Chen
ZJ: Age-specific serum antimullerian hormone levels in women with
and without polycystic ovary syndrome. Fertil Steril. 102:230–236.
2014.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Wei D, Xie J, Yin B, Hao H, Song X, Liu Q,
Zhang C and Sun Y: Significantly lengthened telomere in granulosa
cells from women with polycystic ovarian syndrome (PCOS). J Assist
Reprod Genet. 34:861–866. 2017.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Chang L, Guo R, Yuan Z, Shi H and Zhang D:
LncRNA HOTAIR regulates CCND1 and CCND2 expression by sponging
miR-206 in ovarian cancer. Cell Physiol Biochem. 49:1289–1303.
2018.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Van Opdenbosch N and Lamkanfi M: Caspases
in cell death, inflammation, and disease. Immunity. 50:1352–1364.
2019.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Pang X, Li K, Wei L, Huang Y, Su M, Wang
L, Cao H and Chen T: IL-8 inhibits the apoptosis of MCF-7 human
breast cancer cells by up-regulating Bcl-2 and down-regulating
caspase-3. Xi Bao Yu Fen Zi Mian Yi Xue Za Zhi. 31:307–311.
2015.PubMed/NCBI(In Chinese).
|
|
33
|
Zhang Y, Jiang Q, Wang N, Dai B, Chen Y
and He L: Effects of taspine on proliferation and apoptosis by
regulating caspase-3 expression and the ratio of Bax/Bcl-2 in A431
cells. Phytother Res. 25:357–364. 2011.PubMed/NCBI View
Article : Google Scholar
|