Introduction
As the leading cause of mortality and disability
worldwide, stroke refers to cerebral vascular injury, local or
whole brain tissue damage caused by various reasons, resulting in
clinical symptoms for >24 h or sudden death (1,2).
Stroke is commonly classified into two categories, ischemic stroke
(cerebral infarction) and hemorrhagic stroke (parenchymal
hemorrhage, intraventricular hemorrhage and subarachnoid
hemorrhage), of which ischemic stroke accounts for ~80% (3,4). To
date, few drugs have been approved for the treatment of ischemic
stroke (5), and there is still
considerable capacity and demand for the development of effective
drugs to cure ischemic stroke. An improved understanding of the
pathophysiology of ischemic stroke could provide basis for clinical
research.
Cerebral ischemia-reperfusion (I/R) injury is
generally considered as a complex pathophysiological process of
ischemic stroke (6). Cerebral
ischemia initiates a series of cascades, including inflammation,
followed by the production of reactive oxygen species (ROS) and
apoptosis, which aggravate the damage of brain cells, destroy the
extracellular matrix and blood-brain barrier (7).
FABPs are lipid-binding chaperones that are
expressed in various tissue types. FABPs may regulate the transport
of fat, promote the formation of blood vessels and cell
differentiation, and participate in the occurrence of various
metabolic diseases (8-10).
FABP4 participates in ERS and inflammation, contributing toward the
release of inflammatory cytokines (11,12).
FABP4 protects the kidney from I/R-induced acute kidney injury
(13). A recent study has
demonstrated that FABP4 may be a novel therapeutic target for
hepatic I/R injury (14). In
addition, high levels of FABP4 in plasma were associated with poor
prognosis in patients with acute ischemic stroke (15). However, the role of FABP4 in
cerebral I/R injury has rarely been investigated. Therefore, the
aim of the present study was to investigate the function of FABP4
in the pathogenesis of ischemic stroke. The PC12 cell line is a
differentiated cell line of rat adrenal medulla pheochromocytoma.
It is widely used in neurophysiological and neuropharmacological
research because it possesses the general characteristics of
neuroendocrine cells and can be passaged. In the present study,
PC12 cells were selected to establish an OGD/R model in
vitro.
Materials and methods
Cell culture
PC12 cell line (Type Culture Collection of the
Chinese Academy of Sciences) were cultured in Dulbecco's modified
Eagle's medium (DMEM; Gibco; Thermo Fisher Scientific, Inc.)
supplemented with 10% fetal bovine serum (FBS) at 37˚C in an
atmosphere of 5% CO2. Cells were transfected with 10 µM
FABP4 small interfering RNA (siRNA; Santa Cruz Biotechnology, Inc.)
or negative control siRNA using Lipofectamine 2000 (Thermo Fisher
Scientific, Inc.), according to the manufacturer's protocol. A
total of 6 h post-transfection, fresh DMEM, supplemented with 10%
fetal bovine serum, was replaced, followed by incubation for 48 h.
GW9662 (MedChemExpress Co., Ltd.), a selective PPARγ antagonist was
used to treat cells 2 h prior to OGD/R. ERS agonist tunicamycin
(MedChemExpress Co., Ltd.) was obtained to incubate cells 2 h prior
to OGD/R to induce ERS.
OGD/R model
PC12 cells that have been exposed to OGD/R have been
widely used to simulate cerebral I/R injury in vitro
(16,17). Cells were maintained in a
glucose-deprivation buffer (Gibco; Thermo Fisher Scientific, Inc.)
and incubated in an anaerobic chamber in an atmosphere of 95%
N2/5% CO2 for 2, 4, 6, 8 or 10 h, followed by
fresh DMEM (containing 10% fetal bovine serum) under normoxic
conditions with 95% air/5% CO2 for 24 h.
CCK-8 assay
PC12 cells were resuspended in culture medium and
then planted onto a 96-well plate (5x103 cells per
well). Following reoxygenation for 2, 4, 6, 8 or 12 h, 10 µl CCK-8
reagent (MedChemExpress Co., Ltd.) was added to each well and
incubated for 2 h. Subsequently, the absorbance value of each well
was recorded using a microplate reader (BioTek Instruments, Inc.)
at 450 nm wavelength.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
TRIzol reagent (Invitrogen; Thermo Fisher
Scientific, Inc.) was used to extract total RNA from PC12 cells.
The PrimeScript RT reagent kit (Takara Biotechnology, Inc.) was
used for reverse transcription of RNA (42˚C for 30 min; 99˚C for 5
min). Amplification reaction was performed using SYBR-Green PCR kit
(Qiagen, Inc.) and subjected to Applied Biosystems 7500 system
(Applied Biosystems; Thermo Fisher Scientific, Inc.). The primer
sequences were listed as follows: FABP4 sense,
5'-GCCAGGAATTTGACGAAGTCAC-3' and antisense,
5'-TTCTGCACATGTACCAGGACAC-3'; IL-6 sense,
5'-ATTGTATGAACAGCGATGATGCAC-3' and antisense,
5'-CCAGGTAGAAACGGAACTCCAGA-3'; IL-1β sense,
5'-CCCTGAACTCAACTGTGAAATAGCA-3' and antisense,
5'-CCCAAGTCAAGGGCTTGGAA-3'; TNF-α sense,
5'-TCAGCCTCTTCTCATTCCTGC-3' and antisense,
5'-TTGGTGGTTTGCTACGACGTG-3'; β-actin sense,
5'-GGAGATTACTGCCCTGGCTCCTA-3' and antisense,
5'-GACTCATCGTACTCCTGCTTGCTG-3'. β-actin served as an endogenous
control. PCR reaction conditions were as follows: 95˚C for 2 min,
followed by 40 cycles of 95˚C for 20 sec and 65˚C for 40 sec.
Expression levels of target genes were analyzed using the
2-ΔΔCq method (18).
Cell apoptosis assay
A total of 1x106/ml PC12 cells were
collected and apoptosis was detected by double staining with
fluorescein isothiocyanate (FITC)-Annexin V and propidium iodide
(PI) for 10 min in the dark at room temperature. Subsequently,
cells were subjected to a FACSCalibur flow cytometer (BD
Biosciences) to detect the apoptotic rate. Data were analyzed using
flow cytometry software (iSort™ Automated Cell Sorter; version A.0;
Thermo Fisher Scientific, Inc.).
Western blotting
Total protein was extracted from PC12 cells using
RIPA lysis buffer (Beyotime Institute of Biotechnology). Following
centrifugation for 10 min at 4˚C (12,000 x g), cell supernatant was
collected and the protein concentration was estimated using a BCA
assay kit (Beyotime Institute of Biotechnology). A total of 20 µg
protein per lane was separated by 10% SDS-PAGE gel and transferred
onto PVDF membranes. Membranes were blocked with 5% skimmed milk at
room temperature for 2 h followed by incubation with primary
antibodies at 4˚C overnight. FABP4 (1:5,000; cat. no.
ab92501), cleaved-caspase-3 (1:500; cat. no. ab49822) and caspase-3
(1:2,000; cat. no. ab184787) antibodies were obtained from Abcam,
GRP78 (1:1,000; cat. no. sc-13539) and CHOP (1:1,000; cat. no.
sc-7351) antibodies were purchased from Santa Cruz Biotechnology,
Inc. β-actin (1:5,000; A1978) antibody was obtained from
Sigma-Aldrich (Merck KGaA). Horseradish peroxidase-conjugated goat
anti-rabbit IgG (1:5,000; cat. no. ab6721; Abcam) and goat
anti-mouse IgG secondary antibodies (1:5,000; cat. no. ab6789;
Abcam) were used for detection (room temperature; 2 h). The protein
bands were visualized with an Enhanced Chemiluminescence Detection
kit (Thermo Fisher Scientific, Inc.). Protein expression levels
were semi-quantified using Image-Pro Plus software version 6.0
(Roper Technologies, Inc.).
Determination of ROS
The production of ROS was estimated using a ROS
assay kit (Beyotime Institute of Biotechnology). PC12 cells
(5x106 cells/ml) were collected and suspended in the
diluted DCFH-DA (probe) at a final concentration of 10 µM. Next,
cells were incubated at 37˚C for 20 min and mixed every 5 min to
enable the probe to be in full contact with the cells. The cells
were washed three times with DMEM, and ROS was determined at
wavelengths of 488 and 525 nm using a fluorescence microplate
reader (Omega Bio-Tek, Inc.).
The measurement of SOD activity
SOD activity was determined using an SOD assay kit
(Beyotime Institute of Biotechnology). In brief, SOD sample
preparation solution was used to lyse PC12 cells, and the solution
was centrifugated for 5 min at 4˚C (12,000 x g) to collect cell
supernatant. Corresponding solution was added for 30 min at 37˚C
and the absorbance value was recorded using a microplate reader
(Omega Bio-Tek, Inc.) at 450 nm wavelength.
Statistical analysis
All the experiments were performed in triplicate and
results are expressed as the mean ± standard deviation of data from
triplicate experiments. A t-test or one-way analysis of variance
followed by Tukey's test was used for comparisons between two
groups or multiple groups, respectively. P<0.05 was considered
to indicate a statistically significant difference.
Results
Downregulation of FABP4 in PC12 cells
under OGD/R
PC12 cells subjected to 2, 4, 6, 8 or 10 h OGD,
respectively, followed by 24 h reoxygenation. As shown in Fig. 1A, prolonged duration of OGD led to a
decrease in cell activity. The expression of FABP4 in PC12 cells
exposed to OGD/R was gradually increased, which matched the
decrease in cell viability. However, FABP4 was downregulated when
cells were exposed to OGD for 10 h, compared with OGD for 8 h,
which may involve several underlying mechanisms (Fig. 1B). Therefore, OGD for 8 h and
reoxygenation for 24 h served as an invariant OGD/R condition for
subsequent experiments. PC12 cells under normal conditions were
transfected with FABP4 siRNA, and the transcription and translation
of FABP4 was significantly decreased (Fig. 1C and D). Additionally, FABP4 siRNA decreased the
protein expression of FABP4 under OGD/R (Fig. 1E). Furthermore, FABP4 was
downregulated in PC12 cells exposed to OGD/R.
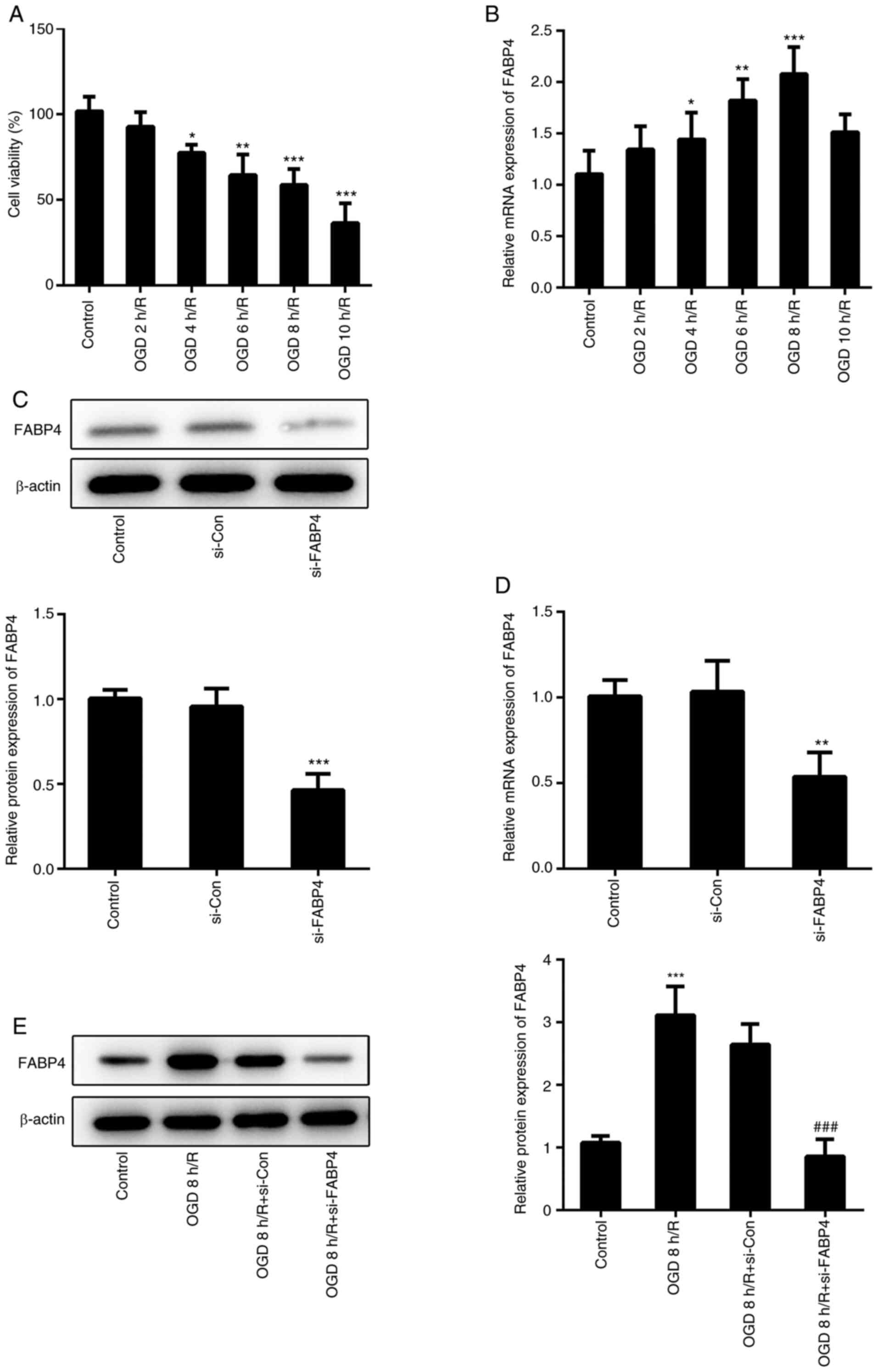 | Figure 1Downregulation of FABP4 in PC12 cells
under OGD/R conditions. (A) PC12 cells were maintained under OGD
for 2, 4, 6, 8 or 10 h followed by reoxygenation for 24 h. Cell
viability was estimated using a Cell Counting Kit-8 assay. (B) mRNA
expression of FABP4 in PC12 cells exposed to OGD/R were determined
by RT-qPCR. *P<0.05, **P<0.01,
***P<0.001 vs. control. The (C) protein and (D) mRNA
expression of FABP4 in PC12 cells were determined by RT-qPCR and
western blotting following transfection with FABP4 siRNA or control
siRNA. **P<0.01, ***P<0.001 vs. si-Con.
(E) The protein expression of FABP4 in PC12 cells exposed to OGD 8
h/R (OGD for 8 h and reoxygenation for 24 h) was determined by
western blotting. ***P<0.001 vs. control;
###P<0.001 vs. OGD 8 h/R+si-Con. FABP4, fatty acid
binding protein 4; siFABP4, FABP4 small interfering RNA; siCon,
control small interfering RNA; OGD/R, oxygen glucose
deprivation/reoxygenation; RT-qPCR, reverse
transcription-quantitative polymerase chain reaction. |
FABP4 knockdown inhibits oxidative
stress and apoptosis
Oxidative stress is the main event in the
pathogenesis of I/R injury (19).
The present study demonstrated that OGD/R inhibited cell
proliferation and induced a potent increment of ROS production.
FABP4 siRNA caused a significant increase in cell proliferation and
decrease in the ROS level, compared with control siRNA under OGD/R
(Fig. 2A and B). On the other hand, the decrease in SOD
activity under OGD/R, compared with the control group, demonstrated
that oxidative stress occurred under OGD/R conditions (Fig. 2C). Apoptotic rates of PC12 cells
under different conditions were determined by flow cytometry. OGD/R
induced marked cell apoptosis, while FABP4 siRNA potently decreased
the apoptotic rate (Fig. 2D).
Furthermore, increased cleaved caspase-3 under an OGD/R and FABP4
knockdown-mediated decrease in cleaved caspase-3 under OGD/R
further suggested an anti-apoptotic effect of FABP4-knockdown
(Fig. 2E). Therefore, FABP4 siRNA
protected PC12 cells against OGD/R-induced oxidative stress and
apoptosis.
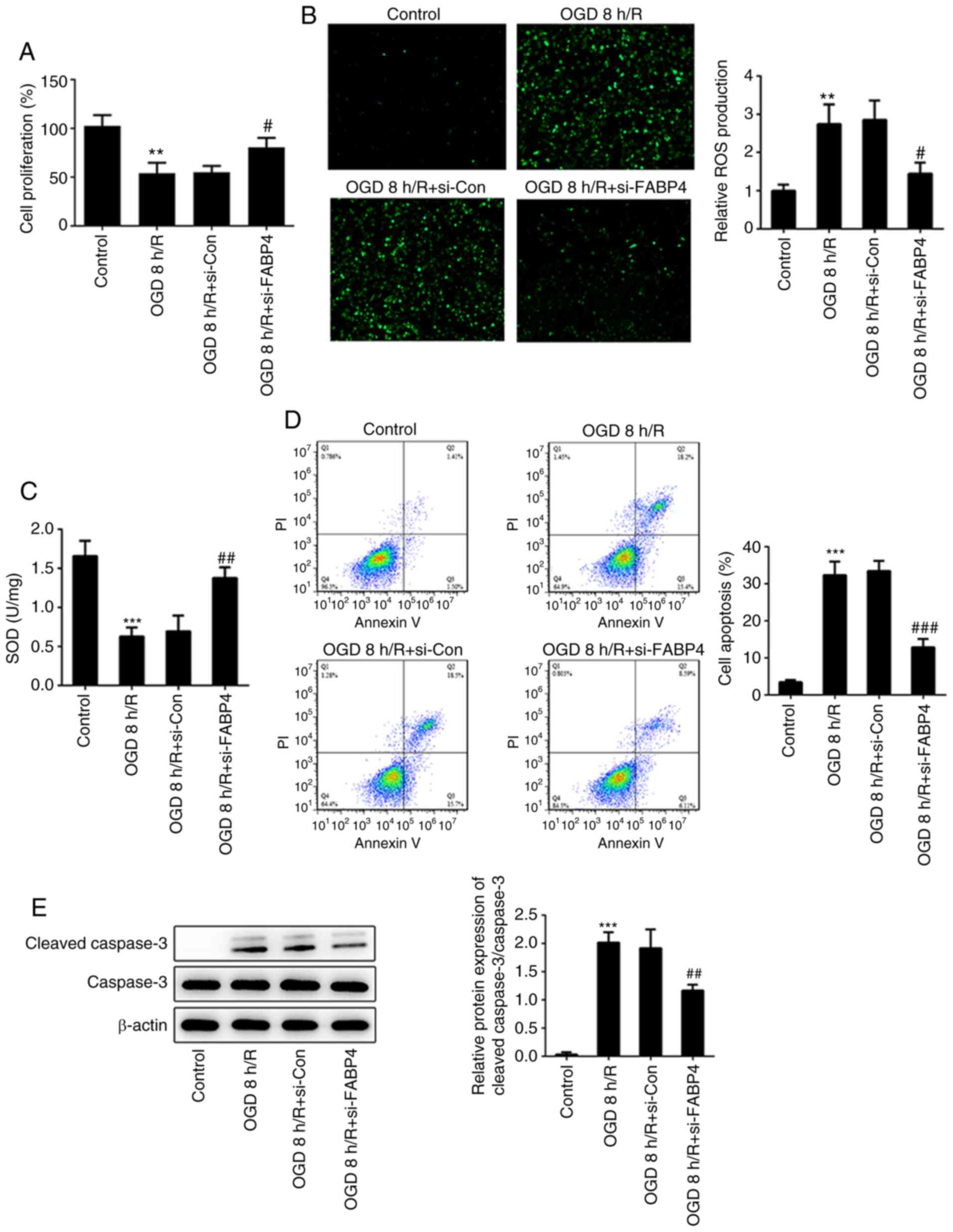 | Figure 2FABP4-knockdown inhibits oxidative
stress and apoptosis. (A) Cell proliferation was measured by Cell
Counting Kit-8 assay. (B) ROS production and (C) SOD activity were
assessed using a ROS assay kit (magnification, x100) or SOD assay
kit, respectively. (D) Cell apoptosis was detected by flow
cytometry. (E) Cleaved caspase-3, caspase-3 and β-actin were
estimated by western blotting. **P<0.01,
***0.001 vs. control; #P<0.05,
##P<0.01, ###P<0.001 vs. OGD 8
h/R+si-Con. FABP4, fatty acid binding protein 4; ROS, reactive
oxygen species; SOD, superoxide dismutase; siCon, control small
interfering RNA; OGD/R, oxygen glucose
deprivation/reoxygenation. |
Interference of FABP4 suppresses
inflammation, ERS and activated PPARγ
A recent study illustrated that FABP4 regulated the
apoptosis of tubular epithelial cells via inactivating ERS
(20). ERS is generally considered
to contribute toward neuronal apoptosis following cerebral IR
(21). ERS-related proteins, GRP78
and CHOP, were enhanced under OGD/R (Fig. 3A), accompanied by upregulation of
inflammatory factors TNF-α, IL-1β and IL-6 (Fig. 3B). Notably, FABP4 siRNA alleviated
OGD/R-induced ERS and inflammation. Activation of PPARγ may protect
the brain from cerebral I/R injury via inhibiting ERS (22). As shown in Fig. 3C, the protein expression of PPARγ
was markedly decreased under OGD/R; however, it was reinforced in
PC12 cells transfected with FABP4 siRNA. Taken together, these
finding suggested that FABP4 siRNA repressed inflammation, ERS and
activated PPARγ under OGD/R.
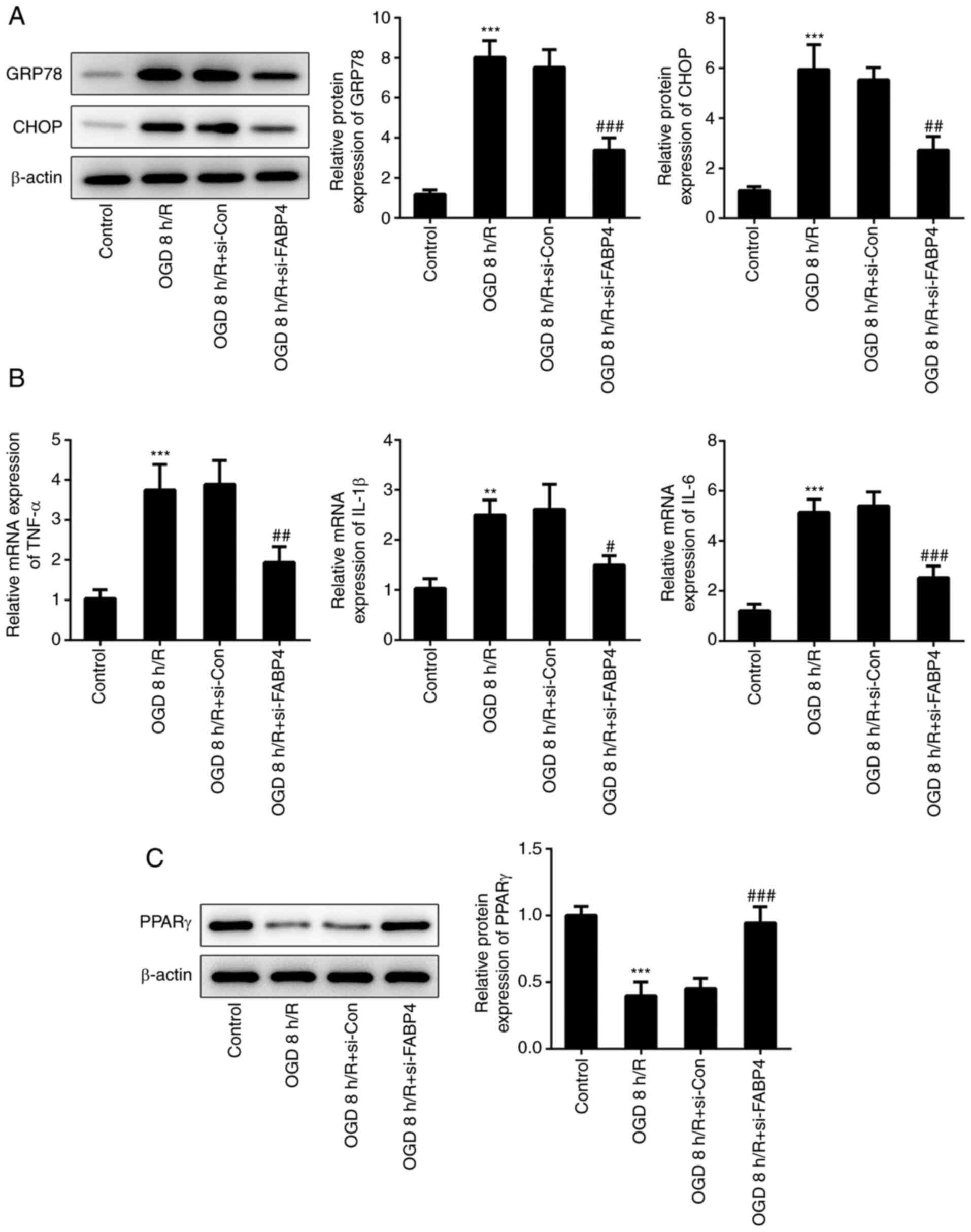 | Figure 3Interference of FABP4 suppresses
inflammation and ERS and activates PPARγ. (A) GRP78, CHOP and
β-actin expression was estimated by western blotting. (B) mRNA
expression of TNF-α, IL-1β and IL-6 were determined by reverse
transcription-quantitative polymerase chain reaction. (C) Protein
expression of PPARγ was detected by western blotting.
**P<0.01, ***0.001 vs. control;
#P<0.05, ##P<0.01,
###P<0.001 vs. OGD 8 h/R+si-Con. FABP4, fatty acid
binding protein 4; ERS, endoplasmic reticulum stress; PPARγ,
peroxisome proliferator-activated receptor γ; GRP78, glucose
regulating protein 78; CHOP, C/EBP homologus protein; TNF-α, tumor
necrosis factor α; IL, interleukin; OGD/R, oxygen glucose
deprivation/reoxygenation. |
FABP4 modulates ERS and apoptosis via
regulation of PPARγ
PPARγ antagonist GW9662 was employed in subsequent
experiments to further investigate the role of PPARγ in
FABP4-mediated ERS and apoptosis. The levels of GRP78 and CHOP were
decreased following transfection with FABP4 siRNA under OGD/R.
GW9662 reversed the function of FABP4 siRNA, showing a similar
result to the ERS agonist, tunicamycin (Fig. 4A). Cleaved caspase-3 was decreased
in PC12 cells transfected with FABP4 siRNA, compared with that of
the OGD/R group, and cleaved caspase-3 in cells treated with GW9662
or tunicamycin on the basis of FABP4 siRNA transfection was
increased, compared with FABP4 siRNA transfection only (Fig. 4B). Furthermore, apoptotic rates of
PC12 cells indicated that the anti-apoptotic effects of FABP4 siRNA
were abolished by GW9662 or tunicamycin (Fig. 4C). These results implied that PPARγ
involved FABP4-regulated ERS and apoptosis.
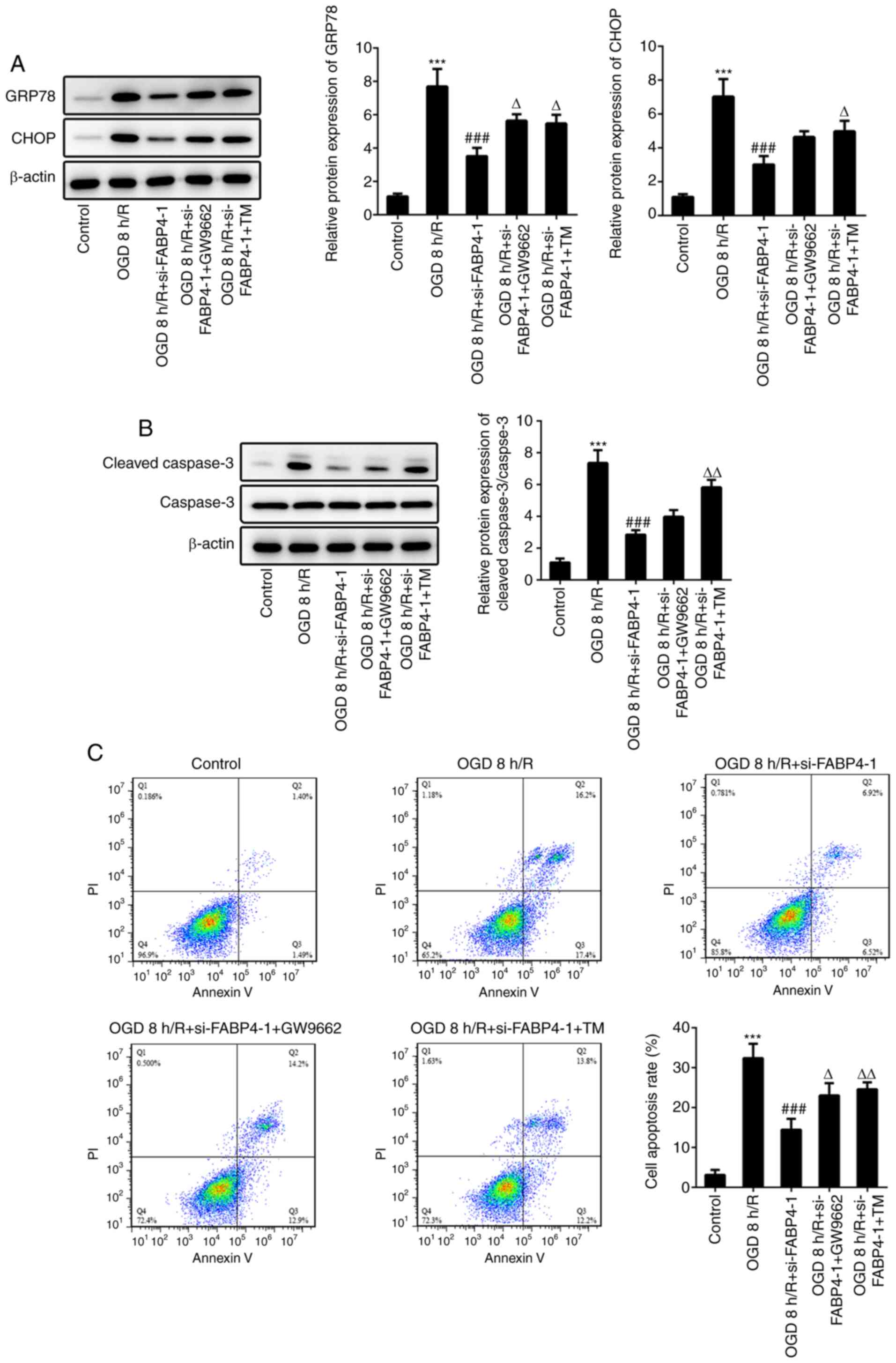 | Figure 4FABP4 modulates ERS and apoptosis via
regulation of PPARγ. (A) PC12 cells exposed to OGD 8 h/R. Cells
were pretreated with 1 µΜ GW9662 or 1 µg/ml TM for 2 h before OGD 8
h/R. GRP78, CHOP and β-actin expression was estimated by western
blotting. (B) Cleaved caspase-3, caspase-3 and β-actin expression
was estimated by western blotting. (C) Cell apoptosis was detected
by flow cytometry. ***P<0.001 vs. control;
###P<0.001 vs. OGD 8 h/R; ΔP<0.05,
ΔΔP<0.01 vs. OGD 8 h/R+si-FABP4-1. FABP4, fatty acid
binding protein 4; ERS, endoplasmic reticulum stress; PPARγ,
peroxis(^#me proliferator-activated receptor γ; TM, tunicamycin;
OGD/R, oxygen glucose deprivation/reoxygenation; GRP78, glucose
regulating protein 78; CHOP, C/EBP homologous protein. |
Discussion
Cerebral I/R injury is a core pathophysiological
process of ischemic stroke (6).
FABP4 has been investigated in I/R injury of the kidney, liver and
myocardium (13,14,23).
The roles of FABP4 in cerebral I/R injury are largely unknown. In
the present study, cerebral I/R injury was simulated in
vitro to investigate the effects of FABP4 in PC12 cells under
OGD/R conditions.
During 8 h of OGD, cell viability was gradually
downregulated and FABP4 was gradually increased, suggesting that
FABP4 had a harmful effect on PC12 cells under OGD/R conditions,
which was consistent with the results reported in myocardial IR
injury (23). Oxidative stress and
inflammation serve as the main pathogenesis of I/R injury, and
effective prevention of oxidative stress and inflammation is
conducive to ameliorate I/R injury (24). In the present study, FABP4-silencing
decreased the expression of inflammatory factors, IL-1β, IL-6 and
TNF-α, and the decrease in ROS and increase in SOD demonstrated
that FABP4 siRNA relieved oxidative stress.
Bosquet et al (25) reported that exogenous FABP4 promoted
ERS in HepG2 cells. In addition, another study reported by Bosquet
et al (26) described that
the FABP4 inhibitor prevented lipid-induced ERS-associated
inflammation in skeletal muscle. The present study demonstrated
that FABP4-silencing ameliorated ERS and ERS-associated apoptosis.
ERS contributes toward the development of cerebral I/R injury
(25).
PPARγ accelerated the elimination of additional
superoxide by promoting the transcription of SOD. Inhibition of
PPARγ promoted neuron apoptosis in ischemia by upregulating ROS
scavenging genes (26). FABP4 siRNA
repressed inflammation and enhanced PPARγ signaling in proximal
tubular epithelial cells (27). In
PC12 cells, FABP4 siRNA contributed toward the increase in PPARγ
expression. The PPARγ antagonist, GW9662, counteracted the effects
of FABP4 siRNA on ERS and apoptosis.
In summary, the present study demonstrated that
FABP4 was overexpressed in PC12 cells under OGD/R, and
FABP4-silencing inhibited ERS-associated inflammation, oxidative
stress and apoptosis via upregulating PPARγ signaling.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
XLY performed the experiments and drafted the
manuscript, JHM analyzed and interpreted the data and QD designed
the study. All authors read and approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Khoshnam SE, Winlow W, Farzaneh M, Farbood
Y and Moghaddam HF: Pathogenic mechanisms following ischemic
stroke. Neurol Sci. 38:1167–1186. 2017.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Siniscalchi A, Gallelli L, Malferrari G,
Pirritano D, Serra R, Santangelo E and De Sarro G: Cerebral stroke
injury: The role of cytokines and brain inflammation. J Basic Clin
Physiol Pharmacol. 25:131–137. 2014.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Tobin MK, Bonds JA, Minshall RD,
Pelligrino DA, Testai FD and Lazarov O: Neurogenesis and
inflammation after ischemic stroke: What is known and where we go
from here. J Cereb Blood Flow Metab. 34:1573–1584. 2014.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Kim JY, Kawabori M and Yenari MA: Innate
inflammatory responses in stroke: Mechanisms and potential
therapeutic targets. Curr Medb Chem. 21:2076–2097. 2014.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Blakeley JO and Llinas RH: Thrombolytic
therapy for acute ischemic stroke. J Neurol Sci. 261:55–62.
2007.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Fu Y, Liu Q, Anrather J and Shi FD: Immune
interventions in stroke. Nat Rev Neurol. 11:524–535.
2015.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Danton GH and Dietrich WD: Inflammatory
mechanisms after ischemia and stroke. J Neuropathol Exp Neurol.
62:127–136. 2003.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Bogdan D, Falcone J, Kanjiya MP, Park SH,
Carbonetti G, Studholme K, Gomez M, Lu Y, Elmes MW, Smietalo N, et
al: Fatty acid-binding protein 5 controls microsomal prostaglandin
E synthase 1 (mPGES-1) induction during inflammation. J Biol Chem.
293:5295–5306. 2018.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Yu CW, Liang X, Lipsky S, Karaaslan C,
Kozakewich H, Hotamisligil GS, Bischoff J and Cataltepe S: Dual
role of fatty acid-binding protein 5 on endothelial cell fate: A
potential link between lipid metabolism and angiogenic responses.
Angiogenesis. 19:95–106. 2016.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Rodriguez Sawicki L, Bottasso Arias NM,
Scaglia N, Falomir Lockhart LJ, Franchini GR, Storch J and Córsico
B: FABP1 knockdown in human enterocytes impairs proliferation and
alters lipid metabolism. Biochim Biophys Acta Mol Cell Biol Lipids.
1862:1587–1594. 2017.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Hotamisligil GS and Bernlohr DA: Metabolic
functions of FABPs-mechanisms and therapeutic implications. Nat Rev
Endocrinol. 11:592–605. 2015.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Chen Y, Gao H, Yin Q, Chen L, Dong P,
Zhang X and Kang J: ER stress activating ATF4/CHOP-TNF-α signaling
pathway contributes to alcohol-induced disruption of osteogenic
lineage of multipotential mesenchymal stem cell. Cell Physiol
Biochem. 32:743–754. 2013.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Shi M, Huang R, Guo F, Li L, Feng Y, Wei
Z, Zhou L, Ma L and Fu P: Pharmacological inhibition of fatty
acid-binding protein 4 (FABP4) protects against renal
ischemia-reperfusion injury. Rsc Advances. 8:15207–15214.
2018.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Hu B, Guo Y, Garbacz WG, Jiang M, Xu M,
Huang H, Tsung A, Billiar TR, Ramakrishnan SK, Shah YM, et al:
Fatty acid binding protein-4 (FABP4) is a hypoxia inducible gene
that sensitizes mice to liver ischemia/reperfusion injury. J
Hepatol. 63:855–862. 2015.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Holm S, Ueland T, Dahl TB, Michelsen AE,
Skjelland M, Russell D, Nymo SH, Krohg-Sørensen K, Clausen OP, Atar
D, et al: Fatty acid binding protein 4 is associated with carotid
atherosclerosis and outcome in patients with acute ischemic stroke.
PLoS One. 6(e28785)2011.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Li TF, Ma J, Han XW, Jia YX, Yuan HF, Shui
SF, Guo D and Yan L: Chrysin ameliorates cerebral
ischemia/reperfusion (I/R) injury in rats by regulating the
PI3K/Akt/mTOR pathway. Neurochem Int. 129(104496)2019.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Jiang D, Sun X, Wang S and Man H:
Upregulation of miR-874-3p decreases cerebral ischemia/reperfusion
injury by directly targeting BMF and BCL2L13. Biomed Pharmacother.
117(108941)2019.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Whiley H and Taylor M: Legionella
detection by culture and qPCR: Comparing apples and oranges. Crit
Rev Microbiol. 42:65–74. 2016.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Zhang HF, Li TB, Liu B, Lou Z, Zhang JJ,
Peng JJ, Zhang XJ, Ma QL, Peng J and Luo XJ: Inhibition of myosin
light chain kinase reduces NADPH oxidase-mediated oxidative injury
in rat brain following cerebral ischemia/reperfusion. Naunyn
Schmiedebergs Arch Pharmacol. 388:953–963. 2015.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Tan Z, Guo F, Huang Z, Xia Z, Liu J, Tao
S, Li L, Feng Y, Du X, Ma L and Fu P: Pharmacological and genetic
inhibition of fatty acid-binding protein 4 alleviated
cisplatin-induced acute kidney injury. J Cell Mol Med.
23:6260–6270. 2019.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Yang W and Paschen W: Unfolded protein
response in brain ischemia: A timely update. J Cereb Blood Flow
Metab. 36:2044–2050. 2016.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Chen Y, Liu S and Chen G: Aggravation of
cerebral ischemia/reperfusion injury by peroxisome
proliferator-activated receptor-gamma deficiency via endoplasmic
reticulum stress. Med Sci Monit. 25:7518–7526. 2019.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Deng T, Wang Y, Wang C and Yan H: FABP4
silencing ameliorates hypoxia reoxygenation injury through the
attenuation of endoplasmic reticulum stress-mediated apoptosis by
activating PI3K/Akt pathway. Life Sci. 224:149–156. 2019.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Wu L, Xiong X, Wu X, Ye Y, Jian Z, Zhi Z
and Gu L: Targeting oxidative stress and inflammation to prevent
ischemia-reperfusion injury. Front Mol Neurosci.
13(28)2020.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Bosquet A, Guaita-Esteruelas S, Saavedra
P, et al: Exogenous FABP4 induces endoplasmic reticulum stress in
HepG2 liver cells. Atherosclerosis. 249:191–199. 2016.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Bosquet A, Girona J, Guaita-Esteruelas S,
et al: FABP4 inhibitor BMS309403 decreases
saturated-fatty-acid-induced endoplasmic reticulum
stress-associated inflammation in skeletal muscle by reducing p38
MAPK activation. Biochimica et biophysica acta. Molecular and cell
biology of lipids. 1863:604–613. 2018.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Qiao Y, Liu L, Yin L, Xu L, Tang Z, Qi Y,
Mao Z, Zhao Y, Ma X and Peng J: FABP4 contributes to renal
interstitial fibrosis via mediating inflammation and lipid
metabolism. Cell Death Dis. 10(382)2019.PubMed/NCBI View Article : Google Scholar
|


















