Introduction
Genus Erigeron and Clematis belong to
the Asteraceae (Compositae) and Ranunculaceae family respectively,
and have been used in traditional remedies (1,2)
because some species possess anti-apoptotic activity and
anti-inflammatory properties (3,4). Many
studies have reported the synergistic effects of a mixture of
extracts from two or more natural resources in neuroprotection
against ischemic brain insults (5-7).
In this regard, we recently published a paper that showed that
pretreatment with YES-10®, a combination of extracts
from Erigeron annuus (L.) PERS (EALP) and Clematis
mandshurica RUPR. (CMR), protected cornu ammonis 1 (CA1)
pyramidal neurons and attenuated gliosis (astrogliosis and
microgliosis) in the hippocampus of a gerbil model of 5-min
transient forebrain ischemia (5).
The hippocampus consists of several subfields. Among
them, the CA1 field is vulnerable to 5-min transient ischemia
(8). Namely, pyramidal cells in the
CA1 field, which are called CA1 pyramidal cells or neurons, die
several days (4-5 days) after 5-min transient ischemia. Hence, the
death phenomenon is called delayed neuronal death (DND) (8,9). Until
now, the mechanisms of DND following 5-min transient ischemia have
included glutamate-induced excitotoxicity, oxidative stress due to
the immoderate production of reactive oxygen species (ROS) and
inflammatory response by pro-inflammatory cytokines (10-13).
Among the mechanisms, oxidative stress is one of the major
mechanisms of DND (10,14). The overproduction of ROS decreases
or stops the functions of molecules such as DNA, lipids and
proteins and eventually leads to cell death (15,16).
In this regard, many studies on the mechanisms of neuroprotection
against ischemic insults have focused on enhancing endogenous
antioxidant enzymes, including SOD1 and SOD2 to scavenge
overproduced ROS (10,16-19).
The neuroprotective mechanisms of YES-10®
against brain ischemic injury have been poorly investigated,
although we reported neuroprotective effects in a gerbil model of
5-min transient forebrain ischemia (5). In addition, it has been demonstrated
that plants belonging to the genus Clematis, specifically
Clematis chinensis, contains scutellarin, a flavonoid
glycoside compound (20,21). The scutellarin is also commonly
found in the genus Erigeron and, in particular, Erigeron
breviscapus (2,22,23).
In addition, Erigeron annuus contains chlorogenic acid, a
polyphenolic compound, which consists of ester bonds with caffeine
acid and quinic acid (24-26).
Interestingly, it has been reported that scutellarin and/or
chlorogenic acid display neuroprotective effects against brain
ischemic insults in rodents (27,28).
Therefore, the objective of this research was to analyze
scutellarin and chlorogenic acid in YES-10® and examine
the antioxidant efficacy of YES-10® in neuroprotection
against ischemic injury induced by 5-min transient forebrain
ischemia in gerbils, which have been used as a model of transient
ischemia (TI) in the forebrain (19,29).
Materials and methods
Experimental animals
Male gerbils at 6.5 months old (body weight, 77-82
g) obtained from the Experimental Animal Center at Kangwon National
University (Chuncheon, Republic of Korea) were used in this study.
The animals were housed in conventional environment with suitable
room temperature (23±0.5˚C) and humidity (approximately 60%). Their
room was controlled under constant dark and light cycle every 12 h.
The gerbils were provided accessible water and feed. The protocol
of this research was approved at Feb. 18, 2020 by the Institutional
Animal Care and Use Committee (AICUC) at Kangwon National
University (approval no. KW-200113-1). The animal caring and
handling complies with the guidelines with the current
international laws and policies from the NIH Guide for the Care and
Use of Laboratory Animals (The National Academies Press, 8th Ed,
2011).
Preparation of YES-10®
YES-10® was prepared as previously
described in our published paper (5,30).
Briefly, CMR and EALP were cultivated in Metro-Mix potting soil
with a slow releasing fertilizer (Osmocote Plus). They had been
grown for 6 weeks, and their leaves were harvested. The collected
leaves were washed, dried at 50˚C and ground into powder (<1
mm). In turn, 150 g of CMR was extracted with 7-fold volume of 50%
EtOH for 60 min and refluxed 2 times (2 h/reflux). The resulting
suspension (extract) was filtered, concentrated to be powder by
using a rotary evaporator and stored at 4˚C. EALP was extracted
with 50% EtOH, filtered and dried to be powder by using the same
procedure for CMR extract. These extracts were mixed 1:1 ratio to
be YES-10® which was provided from Famenity Co.,
Ltd.
Qualitative analysis of
YES-10®
The test sample (YES-10®) and standard
samples (chlorogenic acid and scutellarin) were precisely weighed
and dissolved into 50% methanol. And, thereafter, the test sample
(0.5 µl) and standard samples (0.5 µl respectively) were subjected
to HPLC (Agilent 1260 infinity II; Agilent Technologies, Inc.)
using a Supelco Dicovery C18 column (diameter, 4.6 mm; length, 250
mm) filled with octadecylsilyl silica gel (diameter, 5 µm) at 1.0
ml/min of flow rate. Optimum HPLC separation was achieved at 30˚C.
UV wave length was 335 nm. The mobile phases were set as A (aqueous
phosphoric acid) and B (methanol) under following conditions: 0-5
min (5% B), 5-27 min (5-30% B), 27-47 min (30-55% B), 47-47.1 min
(55-100% B), 47.1-52 min (100% B), 52-52.1 min (100-5%) and 52.1-55
min (5% B).
Experimental groups and treatment with
YES-10®
Forty two gerbils were assigned to four groups: 1)
vehicle/sham group (n=14) treated with vehicle (0.85%
saline) and sham operation; 2) vehicle/TI group (n=28)
treated with vehicle (0.85% saline) and TI; 3) YES/sham group
(n=14) treated with 200 mg/kg YES-10® and sham
operation; 4) YES/TI group (n=28) treated with 200 mg/kg
YES-10® and TI.
The administration of the vehicle or
YES-10® was orally given with a feeding needle once/day
for 7 days because extracts from natural products have orally been
ingested in traditional medicine. The dose of YES-10®
(200 mg/kg) was chosen on the basis of our published paper, in
which pretreatment with 200 mg/kg of YES-10® strongly
protected the pyramidal neurons located in the hippocampal CA1
field after TI (5). We applied 7
days for YES-10® administration according to previous
studies with plant extracts, and since data regarding to ADME
properties of YES-10® had been rarely reported (18,31).
TI induction
Surgical procedure for TI was performed as
previously described (29,32). In short, the gerbils were
anesthetized with 2.5% isoflurane (Hana Pharmaceutical Co., Ltd.)
in mixture of oxygen and nitrous oxide (33%:67%). Under anesthesia,
a midline incision was made on ventral surface of the neck to find
right and left common carotid arteries. Both arteries were exposed
and occluded for 5 min by using non-traumatic aneurysm clips. Five
minutes after TI, the clips were removed for blood circulation.
Complete stop and circulation of blood flow was confirmed in the
central arteries located at retinae with ophthalmoscope (HEINE
K180®) (Heine Optotechnik, Herrsching, Germany). Body
temperature was regulated at normal temperature (37.5±0.5˚C) with a
rectal temperature probe (TR-100; Fine Science Tools) by using
thermometric blanket during and after TI surgery. Sham operated
gerbils were subjected to the same TI surgery process without
occlusion of both common carotid arteries.
Expression levels of antioxidant
enzymes
To investigate changes in antioxidant enzymes
following treatment with YES-10®, Western blot analysis
was carried out according to previous study (33,34).
Five gerbils per group were deeply anesthetized by intraperitoneal
injection of 90 mg/kg pentobarbital sodium (JW Pharm Co Ltd.)
(32) and their hippocampal tissues
were harvested and homogenized. We used a buffer for homogenization
as 50 mM phosphate-buffered saline (PBS, pH 7.4) solution
containing 0.1 mM EGTA (pH 8.0), 0.2% Nonidet P-40, 10 mM EDTA (pH
8.0), sodium pyrophosphate, 100 mM β-glycerophosphate, 50 mM NaF,
150 mM NaCl, 2 mM sodium orthovanadate, 1 mM PMSF and 1 mM DTT.
After homogenization, the tissue were centrifuged and the
supernatants were taken to determine protein levels via using a
Micro BCA assay kit with bovine serum albumin as the standard
(Pierce Chemical). The aliquots including total protein (20 µg)
were boiled in loading buffer containing 150 mM Tris (pH 6.8), 3 mM
DTT, 6% SDS, 0.3% bromophenol blue and 30% glycerol. The samples
were separated by SDS-PAGE (10%) and subsequently the gels were
transferred to nitrocellulose membranes (Pall Co.) at 350 mA and
4˚C for 90 min. The membranes were incubated with 5% defatted milk
at room temperature for 60 min to block non-specific staining. The
membrane, thereafter, immunoreacted with each primary antibody at
4˚C during overnight: Rabbit anti-Cu, Mn-superoxide dismutase
(SOD1; 1:2,000; Abcam), rabbit anti-Mn-superoxide dismutase (SOD2;
1:2,000; Abcam) and rabbit anti-β-actin (1:2,000; Sigma-Aldrich;
Merck KGaA). The membrane, subsequently, incubated with each
peroxidase-conjugated secondary antibody at room temperature for 60
min: Donkey anti-rabbit IgG (1:5,000, Santa Cruz Biotechnology,
Inc.). Using a luminol-based chemiluminescence kit (Pierce; Thermo
Fisher Scientific, Inc.) was used for enhancement of
visualization.
Each immune blot was analyzed as described
previously (35). In brief, the
bands were scanned and in order to quantify the bands,
densitometric analysis was performed via using the Scion Image
software (Scion Crop.). Each level of the target protein was
normalized via the corresponding level of β-actin.
Tissue preparation of
histopathology
In order to examine histopathological and
immunohistochemical changes in the gerbil hippocampus following TI,
brain sections containing the hippocampus were prepared according
to published method (36). Briefly,
the gerbils were anesthetized with intraperitoneal injection of
pentobarbital sodium (90 mg/kg) (JW Pharm Co Ltd., Republic of
Korea) (32) at 2 and 5 days after
TI. Under deep anesthesia, the gerbils were transcardially perfused
with 0.1 M PBS (pH 7.4) to wash their brains and followed by
solution of 4% paraformaldehyde (in 0.1 M PB, pH 7.4) to fix the
brains. The fixed brains were removed and post-fixed with the same
fixative for 4 h. These brains were cryoprotected with solution of
30% sucrose (in 0.1 M PB, pH 7.4) for 10 h. Finally, serial 30-µm
coronal sections were made in cryostat (Leisa).
Immunohistochemistry
To study antioxidant and neuroprotective effects of
YES-10® against TI, immunohistochemistry was done
according to our previously published method (37). The sections were treated with 0.3%
hydrogen peroxide (H2O2) solution (in 0.1 M
PBS, pH 7.4) and immersed in 10% normal goat, horse or chicken
serum (in 0.05 M PBS, pH 7.4) for 40 min at room temperature. They
were then reacted in solution of each primary antibody for 24 h at
4˚C. Primary antibodies were rabbit anti-SOD1 (1:1,500; EMD
Millipore) and sheep anti-SOD2 (1:1,500; EMD Millipore) for
examining antioxidant enzymes, mouse anti-neuronal nuclei-specific
protein (NeuN; a marker for neurons; 1:1,000; Chemicon) for
detecting neurons and rabbit anti-4-hydroxy-2-nonenal (4-HNE;
1:1,000; Alexis Biochemicals) for investigating oxidative stress.
Subsequently, these sections were exposed to solution of
biotinylated goat anti-rabbit IgG (1:250; Vector), chicken
anti-sheep IgG (1:250; Vector) or horse anti-mouse IgG (1:250;
Vector) as secondary antibody. Finally, these tissues were reacted
with solution of avidin-biotin complex (1:300; Vector) and
visualized by reacting them with solution of 3, 3'-diaminobenzidine
tetrahydrochloride (Sigma-Aldrich; Merck KGaA) (in 0.1 M PBS, pH
7.4).
Quantitative analyses of SOD1, SOD2 and 4-HNE
immunoreactivity were done according to our published protocol
(19). In brief, digital image of
each immunoreactive structure was captures in the regions of
interest with light microscope (BX53) (Olympus Corporation). Each
image was calibrated into an array of 512x512 pixels, and each
immunoreactivity was measured by 0-255 gray scale system. After the
background density was subtracted, change in each immunoreactivity
was calibrated ratio of relative immunoreactivity (RI) by using
Adobe Photoshop (version 8.0) and analyzed by using NIH image 1.59
software. The ratio of RI was presented as percent compared with
the vehicle/sham group (100%).
Counts of NeuN immunoreactive neurons were done
according to our published method (19). Briefly, NeuN immunoreactive neurons
were examined with light microscope (BX53) (Olympus Corporation).
Digital images of NeuN immunoreactive neurons were captured in the
regions of interest (a 250x250 µm square), and background density
was subtracted as described above. Finally, Cell numbers were
analyzed by using an image analyzing software (Optimas 6.5;
CyberMetrics).
Fluoro-Jade B (FJB) histofluorescence
staining
To examine neuronal loss (death) following TI, F-JB
(a fluorescent marker for neuronal degeneration) histofluorescence
staining was performed as previously described (38). Briefly, the prepared sections were
mounted onto microscopy slides which were coated with gelatin. The
tissues on the slides were immersed in solution of 0.06% potassium
permanganate and incubated in solution of 0.0004% F-JB (Histochem).
These tissues were washed with distilled water and placed on slide
warmer (approximately 50˚C) to be reacted. These stained tissues
were dehydrated and mounted by cover glasses with dibutylphthalate
polystyrene xylene (D.P.X.; Sigma-Aldrich; Merck KGaA).
As described previously (19), F-JB positive cells were counted. In
short, F-JB positive cells were examined with epifluorescent
microscope (Carl Zeiss) equipped with a digital camera (DP72)
(Olympus Corporation). Digital images of F-JB positive cells were
capture in the regions of interest (250x250 µm2), and
the back ground density was subtracted as described above
(immunohistochemistry section). Finally, F-JB positive cells were
counted and analyzed by using image analyzing software (Optimas
6.5; CyberMetrics).
Dihydroethidium (DHE) fluorescence
staining
In this study, oxidative stress was observed by DHE
(Sigma-Aldrich; Merck KGaA) fluorescence staining, which is a
method to examine in situ production of superoxide anion. As
we previously described (36),
briefly, the sections were incubated in Krebs-HEPES buffer
containing 130 Mm NaCl, 5.6 Mm KCL, 2 Mm CaCl2, 0.24 mM
MgCl2, 8.3 mM HEPES, 11 mM glucose (pH 7.4) for 30 min
at 37˚C. Ten µM DHE solution was topically applied on the sections
for 2 h at 37˚C. At this stage, DHE was oxidized at reaction with
superoxide to ethidium, which could bind nuclear DNA and fluoresced
red.
Fluorescent intensity of DHE was analyzed as
previously described (38).
Shortly, the image of DHE histofluorescence was captured in regions
of interest with epifluorescence microscope like the method for
F-JB staining section). DHE fluorescent intensity was analyzed by
using Image-pro Plus 6.0 software. Ratio of DHE fluorescent
intensity was calibrated as percentage compared with the
vehicle/sham group (100%).
Statistical analysis
Data shown in this study represent the means ±
standard error of the mean (SEM) among the groups were
statistically analyzed by two-way analysis of variance (ANOVA) with
a post hoc Bonferroni's multiple comparison test was done to
determine differences among groups. They were statistically
analyzed using SPSS 18.0 (SPSS, Inc.). P<0.05 was considered to
indicate a statistically significant difference.
Results
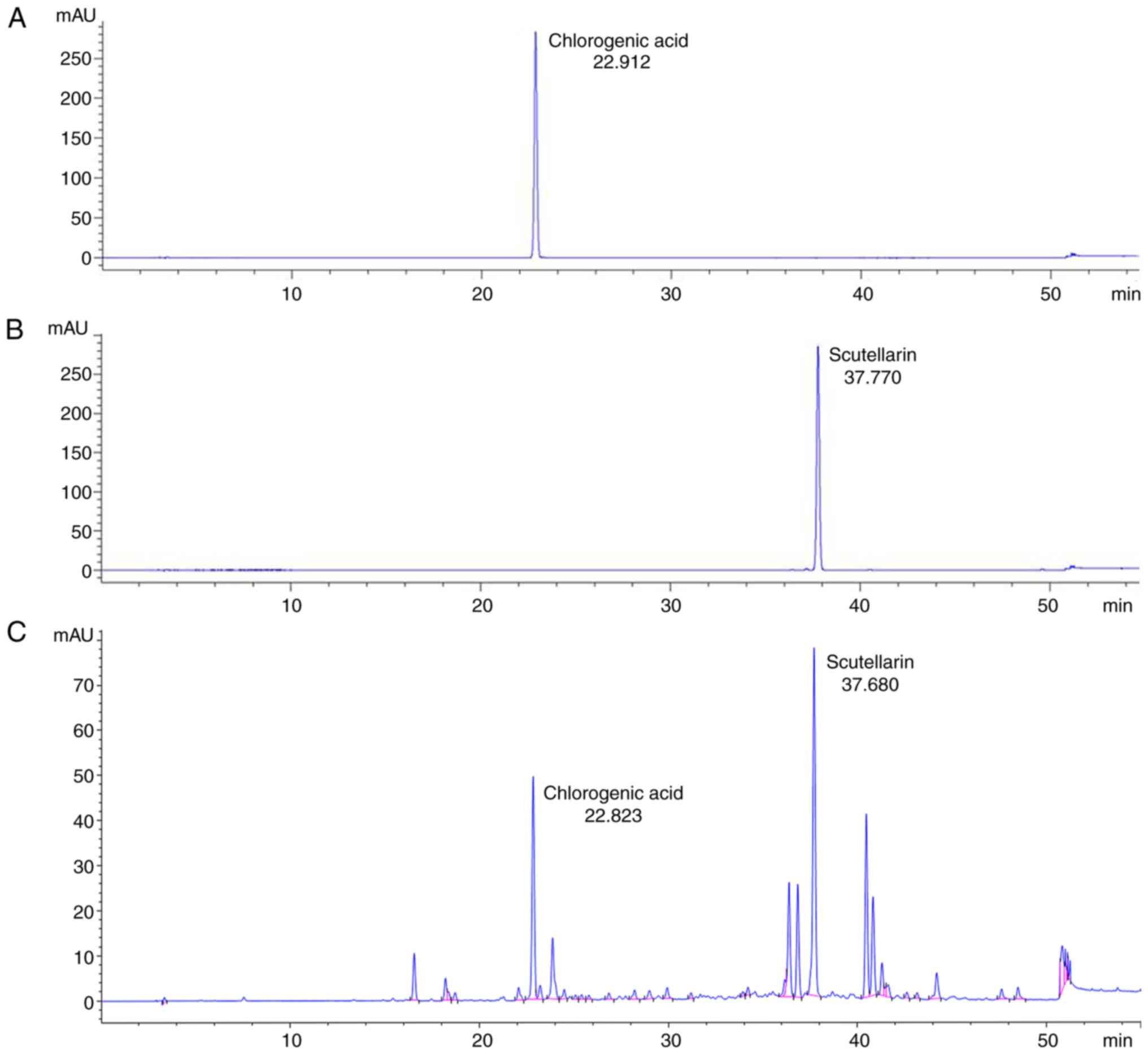
Major ingredients of
YES-10®
The result from the HPLC, the retention time of the
standard samples were respectively 22.912 min (chlorogenic acid;
Fig. 1A) and 37.770 min
(scutellarin; Fig. 1B). The major
ingredients of the YES-10® were revealed as chlorogenic
acid (retention time, 22.823 min) and scutellarin (retention time,
22.823 min) (Fig. 1C).
Expression levels of SODs
Western blot analysis for SOD1 and SOD2 was carried
out to examine alterations in expression levels of endogenous
antioxidant enzymes in the hippocampal CA1 field of the sham and TI
groups following YES-10® pretreatment.
Expression level of SOD1 of the vehicle/sham group
was fundamentally observed (Fig.
2A). In the vehicle/TI group, expression levels of SOD1 and
SOD2 were decreased at 2 days after TI and significantly reduced at
5 days after TI compared to that in the vehicle/sham group
(Fig. 2A and Ba). However, in the YES/sham group
expression level of SOD1 was significantly increased compared to
that in the vehicle/sham group. In the YES/TI group, expression
level of SOD1 was not significantly changed compared with that in
the YES/sham group (Fig. 2A and
Ba).
 | Figure 2Western blot analysis of changes in
antioxidant enzymes in the hippocampus following TI. (A)
Representative western blot images of SOD1 and SOD2 in the
hippocampal CA1 field of the vehicle/sham, vehicle/TI, YES/sham and
YES/TI groups (A), and densitometric analyses of the bands of (Ba)
SOD1 and (Bb) SOD2. In the vehicle/TI group, levels of SOD1 and
SOD2 are reduced at 2 days post-TI and more significantly decreased
at 5 days post-TI. However, levels of SOD1 and SOD2 are elevated in
the YES/sham group. In the YES/TI group, the increased levels of
SOD1 and SOD2 are maintained at 2 and 5 days post-TI (n=7 at each
point in time). *P<0.05 vs. vehicle/sham group,
#P<0.05 vs. corresponding vehicle/sham or TI group.
Data are presented as the mean ± SEM. CA1, cornu ammonis 1; SOD,
superoxide dismutase; TI, transient ischemia. |
A basic expression level of SOD2 was detected in the
vehicle/sham group (Fig. 2A). In
the vehicle/TI group, expression level of SOD2 was significantly
decreased at 2 days after TI and more significantly reduced at 5
days after TI compared to that in the vehicle/sham group (Fig. 2A and Bb). On the other hand, in the YES/sham
group, expression level of SOD2 was significantly increased
compared to that in the vehicle/sham group (Fig. 2A and Bb). In the YES/TI group, expression level
of SOD2 was not markedly altered compared with that in the YES/sham
group (Fig. 2A and Bb).
Increases of SODs immunoreactivities
by YES-10®
Immunohistochemistry for SOD1 and SOD2 was conducted
in order to investigate changes in endogenous antioxidant enzymes
in the hippocampal CA1 field of the sham and TI groups following
YES-10® pretreatment.
SOD1 immunoreactivity in the CA1 field of the
hippocampus of the vehicle/sham group was found in pyramidal cells
of the stratum pyramidale, which are called CA1 pyramidal cells (or
neurons), and in non-pyramidal cells which are distributed in
strata oriens and radiatum (Fig.
3Aa). In the vehicle/TI group, SOD1 immunoreactivity was
significantly reduced in the CA1 pyramidal cells (approximately 60%
of the vehicle/sham group) at 2 days after TI and more
significantly decreased (approximately 33% of the vehicle/sham
group) at 5 days after TI compared to that in the vehicle/sham
group (Fig. 3Ab, Ac and B).
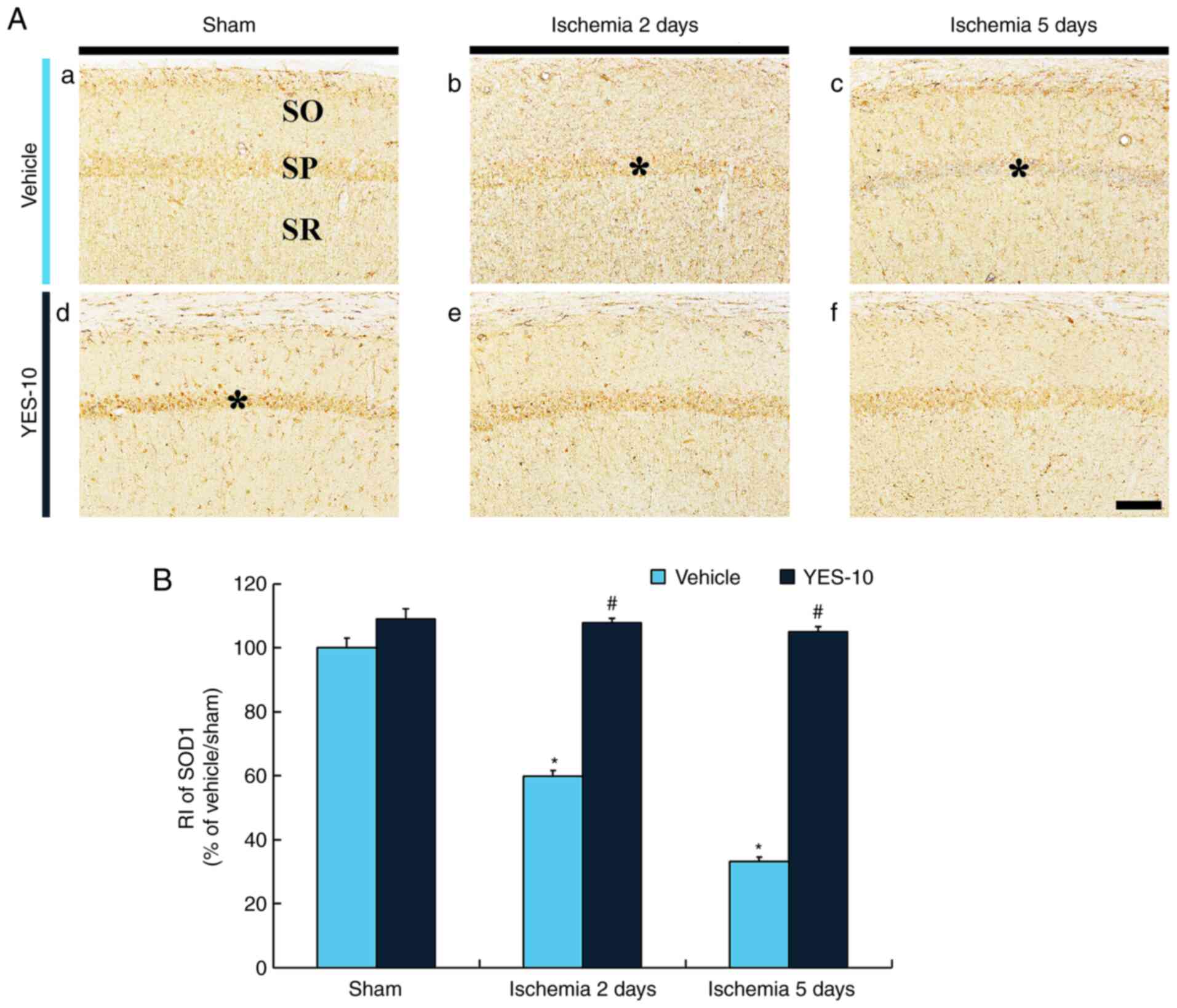 | Figure 3Immunohistochemistry for SOD1 in the
hippocampal CA1 field following TI. (Aa) SOD1 immunohistochemistry
in the hippocampal CA1 field of the vehicle/sham, (Ab and Ac)
vehicle/TI, (Ad) YES/sham and (Ae and Af) YES/TI groups. In the
vehicle/TI group, SOD1 immunoreactivity is gradually reduced in the
SP (asterisks), and SOD1 immunoreactivity in the SP (asterisk) at 5
days post-TI is hardly shown. SOD1 immunoreactivity in the SP
(asterisk) of the YES/sham group is significantly increased
compared with that in the vehicle/sham group. SOD1 immunoreactivity
in the SP of the YES/TI group is significantly higher than that in
the vehicle/TI group. Scale bar, 40 µm. (B) RI as % of SOD1
immunoreactivity in the SP (n=7 at each point in time).
*P<0.05 vs. vehicle/sham group, #P<0.05
vs. corresponding vehicle/sham or TI group. Data are presenred as
the mean ± SEM. SOD, superoxide dismutase; SP, stratum pyramidale;
SO, stratum oriens; SR, stratum radiatum; TI, transient ischemia;
RI, immunoreactivity; CA1, cornu ammonis 1. |
In the YES/sham group, SOD1 immunoreactivity was
apparently increased in the CA1 pyramidal cells (approximately 109%
of the vehicle/sham group) when compared to that in the
vehicle/sham group (Fig. 3Ad and
B). In the YES/TI group, SOD1
immunoreactivity in the CA1 field was not altered at 2 days post-TI
compared with that in the YES/sham group (Fig. 3Ae and B), and SOD1 immunoreactivity at 5 days
post-TI was maintained (Fig. 3Af
and B).
SOD2 immunoreactivity was detected in the CA1
pyramidal cells of the vehicle/sham group (Fig. 4Aa). In the vehicle/TI group, SOD2
immunoreactivity in the CA1 pyramidal neurons was significantly
decreased (approximately 59% of the vehicle/sham group) at 2 days
after TI and more significantly reduced (approximately 9% of the
vehicle/sham group) at 5 days after TI compared to that in the
vehicle/sham group (Fig. 4Ab,
Ac and B).
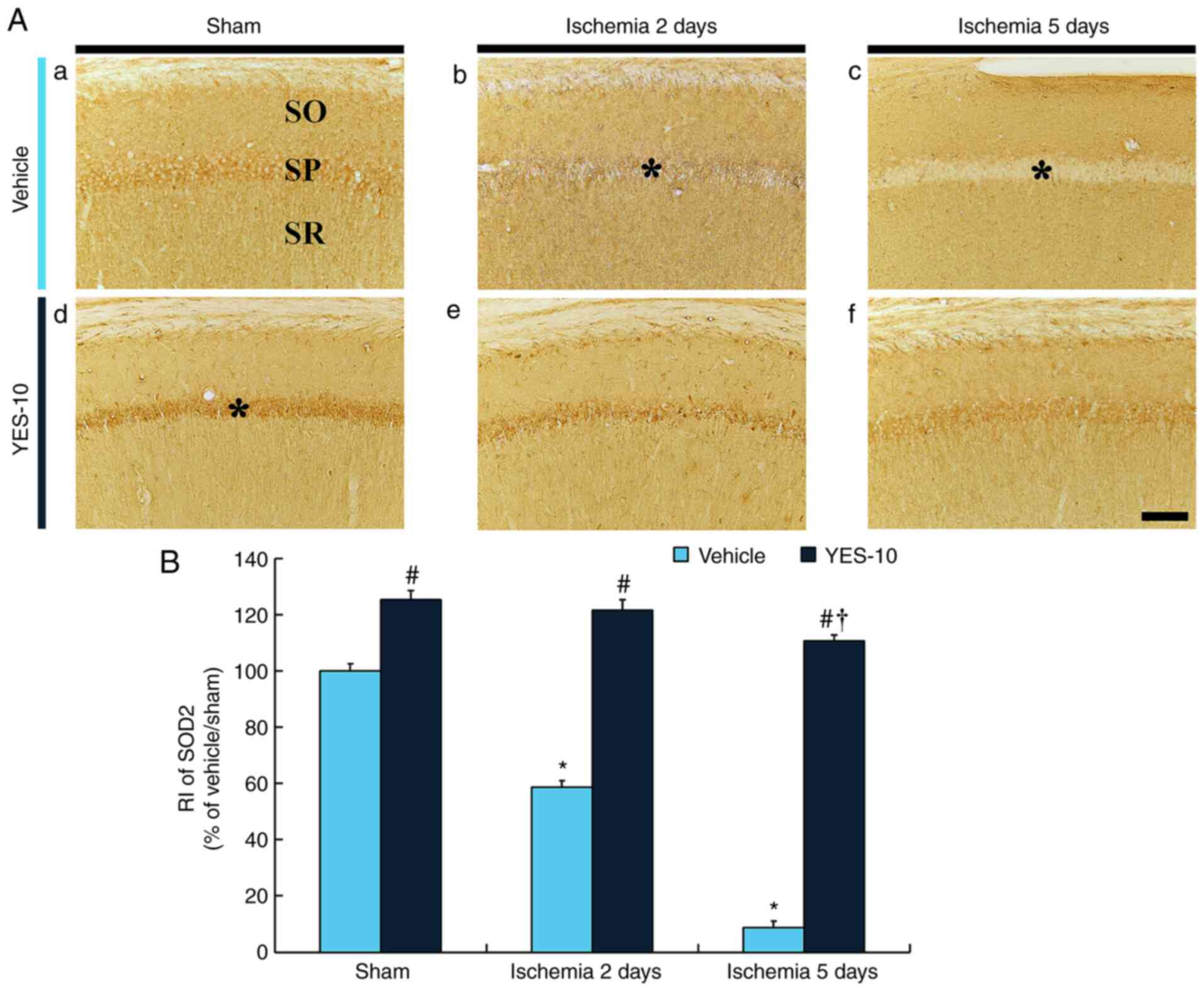 | Figure 4Immunohistochemistry for SOD2 in the
hippocampal CA1 field following TI. (Aa) SOD2 immunohistochemistry
in the hippocampal CA1 field of the vehicle/sham, (Ab and Ac)
vehicle/TI, (Ad) YES/sham and (Ae and Af) YES/TI groups. SOD2
immunoreactivity in the SP (asterisks) of the vehicle/TI group is
markedly decreased at 2 days after TI and hardly detected (astrisk)
at 5 days after TI. In the YES/sham group, SOD2 immunoreactivity in
the SP (asterisk) is significantly higher compared with that in the
vehicle/sham group. SOD2 immunoreactivity in the SP of the YES/TI
group is significantly high compared to that in the vehicle/TI
group. Scale bar, 40 µm. (B) RI as % of SOD2 immunoreactivity in
the SP (n=7 at each point in time). *P<0.05 vs.
vehicle/sham group; #P<0.05 vs. corresponding
vehicle/sham or TI group; †P<0.05 vs. YES/sham group.
The bars indicate the mean ± SEM. SOD, superoxide dismutase; SP,
stratum pyramidale; SO, stratum oriens; SR, stratum radiatum; TI,
transient ischemia; CA1, cornu ammonis 1. |
SOD2 immunoreactivity in the CA1 pyramidal cells of
the YES/sham group was significantly high (approximately 125% of
the vehicle/sham group) compared to that in the vehicle/sham group
(Fig. 4Ad and B). In the YES/TI group, SOD2
immunoreactivity in the CA1 pyramidal neurons were slightly
decreased after TI compared to that in the YES/sham group (Fig. 4Ae, Af and B).
Neuroprotective effects by
YES-10®
To investigate neuroprotective effects of
YES-10® in the ischemic hippocampus,
immunohistochemistry for NeuN and FJB histofluorescence were
performed.
NeuN immunoreactive (NeuN+) neurons in
the vehicle/sham group were found in all layers in all subfields
(CA1-3 fields) of the hippocampus (Fig.
5Aa). In this group, NeuN immunoreactivity was well shown in
cells located the stratum pyramidale, which are called pyramidal
cells or neurons (Fig. 5Ab), but
most of pyramidal cells in the CA1 field, not in the CA2/3 field of
the vehicle/TI group were not stained with NeuN (Fig. 5Ba and Bb). This finding means CA1 pyramidal
neurons were damaged or died. In the YES/sham group,
NeuN+ neurons in the hippocampus were not different from
those in the vehicle/sham group (Fig.
5Bd and Be). Also,
NeuN-immunoreactive CA1 pyramidal neurons of the YES/TI group were
similar to those in the vehicle/sham groups (Fig. 5Bd, Be and C).
This result means that YES-10® pretreatment protected
CA1 pyramidal cells from TI injury.
 | Figure 5Immunohistochemistry for NeuN and
histofluorescence for F-JB in the hippocampus and hippocampal CA1
field following TI. (Aa, Ad, Ba and Bd) NeuN immunohistochemistry
in the hippocampus and (Ab, Ae, Bb and Be) CA1 field. (Ac, Af, Bc
and Bf) F-JB histofluorescence staining in the CA1 field of the
(Aa-Ac) vehicle/sham, (Ad-Af) YES/sham, (Ba-Bc) vehicle/TI and
(Bd-Bf) YES/TI groups at 5 days after TI. In all of the sham
groups, numerous NeuN+ and no FJB+ cells are present in the SP. In
the vehicle/TI group, a few NeuN+ and many FJB+ cells were found in
the SP (asterisks). In the YES/TI group, the distribution pattern
of NeuN+ and FJB+ cells in the SP is similar to that in the
vehicle/sham. Scale bar, 400 µm (Aa, Ad, Ba and Bd) and 40 µm (Ab,
Ac, Ae, Af, Bb, Bc, Be and Bf). (C) Mean numbers of NeuN+ and (D)
FJB+ cells in the CA1 SP (n=7 in each group). *P<0.05
vs. vehicle/TI group, #P<0.05 vs. vehicle/TI group.
The bars indicate the mean ± SEM. DG, dentate gyrus; CA1, cornu
ammonis 1; SP, stratum pyramidale; SO, stratum oriens; SR, stratum
radiatum; TI, transient ischemia; NeuN, neuronal nuclei-specific
protein; F-JB, fluoro-Jade B. |
FJB positive (FJB+) cells mean that they
are dead. In the vehicle/sham group, FJB+ cells were not
found in any layers of the CA1 field (Fig. 5Ac). However, numerous
FJB+ cells were shown in the stratum pyramidale of the
CA1 field of the vehicle/TI group at 5 days after TI (Fig. 5Bc and D). This result means that most of CA1
pyramidal cells were dead at 5 days after 5-min TI. In the YES/sham
group, FJB+ cells were not found in the CA1 field
(Fig. 5Af). In the YES/TI group,
only a few FJB+ CA1 pyramidal neurons were shown at 5
days after TI (Fig. 5Bf and
D). This finding means that most of
CA1 pyramidal cells were protected from TI injury by
YES-10® pretreatment.
Decrease of oxidative stress by
YES-10®
Immunohistochemistry for 4-HNE (an end-product by
lipid peroxidation) and DHE (a marker for production of in
situ superoxide anion) were carried out to investigate
alterations in oxidative stress in the ischemic CA1 filed following
YES-10® pretreatment.
4-HNE immunoreactivity was shown in CA1 pyramidal
neurons in the vehicle/sham group (Fig.
6Aa). In the vehicle/TI group, 4-HNE immunoreactivity in the
CA1 pyramidal neurons at 2 days after TI was significantly
increased (approximately 121%) compared to that in the vehicle/sham
group (Fig. 6Ab and B), and 4-HNE immunoreactivity at 5 days
after TI was very weak due to damage of the CA1 pyramidal cells
(Fig. 6Ac and B). In the YES/sham group, 4-HNE
immunoreactivity in the CA1 pyramidal cells was not different from
that in the vehicle/sham group (Fig.
6Ad and B). In the YES/TI
group, 4-HNE immunoreactivity in the CA1 pyramidal cells was not
altered at 2 days post-TI (Fig. 6Ae
and B), and the immunoreactivity at
5 days post-TI was slightly reduced (approximately 83% of the
YES/sham group) compared to that in the YES/sham group (Fig. 6Af and B). This finding means that lipid
peroxidation in ischemic cells following TI is attenuated by
YES-10® pretreatment.
 | Figure 6Immunohistochemistry for 4-HNE in the
hippocampal CA1 field following TI. (Aa) Immunohistochemistry for
4-HNE in the CA1 field of the vehicle/sham, (Ab and Ac) vehicle/TI,
(Ad) YES/sham and (Ae and Af) YES/TI groups. 4-HNE immunoreactivity
in the vehicle/sham group is shown in the SP. In the vehicle/TI
group, 4-HNE immunoreactivity in the SP (asterisk) is significantly
increased at 2 days post-TI, and, at 5 days after TI, 4-HNE
immunoreactivity in the SP (asterisk) is more reduced. In the
YES/sham group, 4-HNE immunoreactivity in the SP is similar to that
of the vehicle/sham group. Also, 4-HNE immunoreactivity in the SP
of the YES/TI group is not significantly altered after TI. Scale
bar, 40 µm. (B) RI as % of 4-HNE in the SP (n=7 at each point in
time). *P<0.05 vs. vehicle/sham group,
#P<0.05 vs. corresponding vehicle/sham or TI group,
†P<0.05 vs. YES/sham group. The bars indicate the
means ± SEM. CA1, cornu ammonis 1; TI, transient ischemia; RI,
immunoreactivity. |
Weak DHE fluorescence of the vehicle/sham group was
easily detected in CA1 pyramidal cells (Fig. 7Aa). However, in the vehicle/TI
group, DHE fluorescence in the CA1 pyramidal cells was
significantly enhanced (approximately 168%) at 2 days after TI
compared with the vehicle/sham group (Fig. 7Ab and B), and, at 5 day after TI, DHE
fluorescence in the CA1 pyramidal cells was decreased, but the
fluorescence was higher (approximately 143%) than that in the
vehicle/sham group (Fig. 7Ac and
B). In this group, particularly,
non-pyramidal cells distributed in the other layers (strata oriens
and radiatum) expressed strong DHE fluorescence (Fig. 7Ab and Ac). In the YES/sham group, DHE
fluorescence in the CA1 pyramidal cells was similar to that in the
vehicle/sham group (Fig. 7Ad and
B). In the YES/TI group, DHE
fluorescence in the CA1 pyramidal cells was significantly low
(approximately 71% at 2 days and 73% at 5 days after TI) compared
with that in the corresponding time point of vehicle/TI group
(Fig. 7Ae, Af and B).
This finding means that the production of superoxide anion in
ischemic cells following TI is reduced by YES-10®
pretreatment.
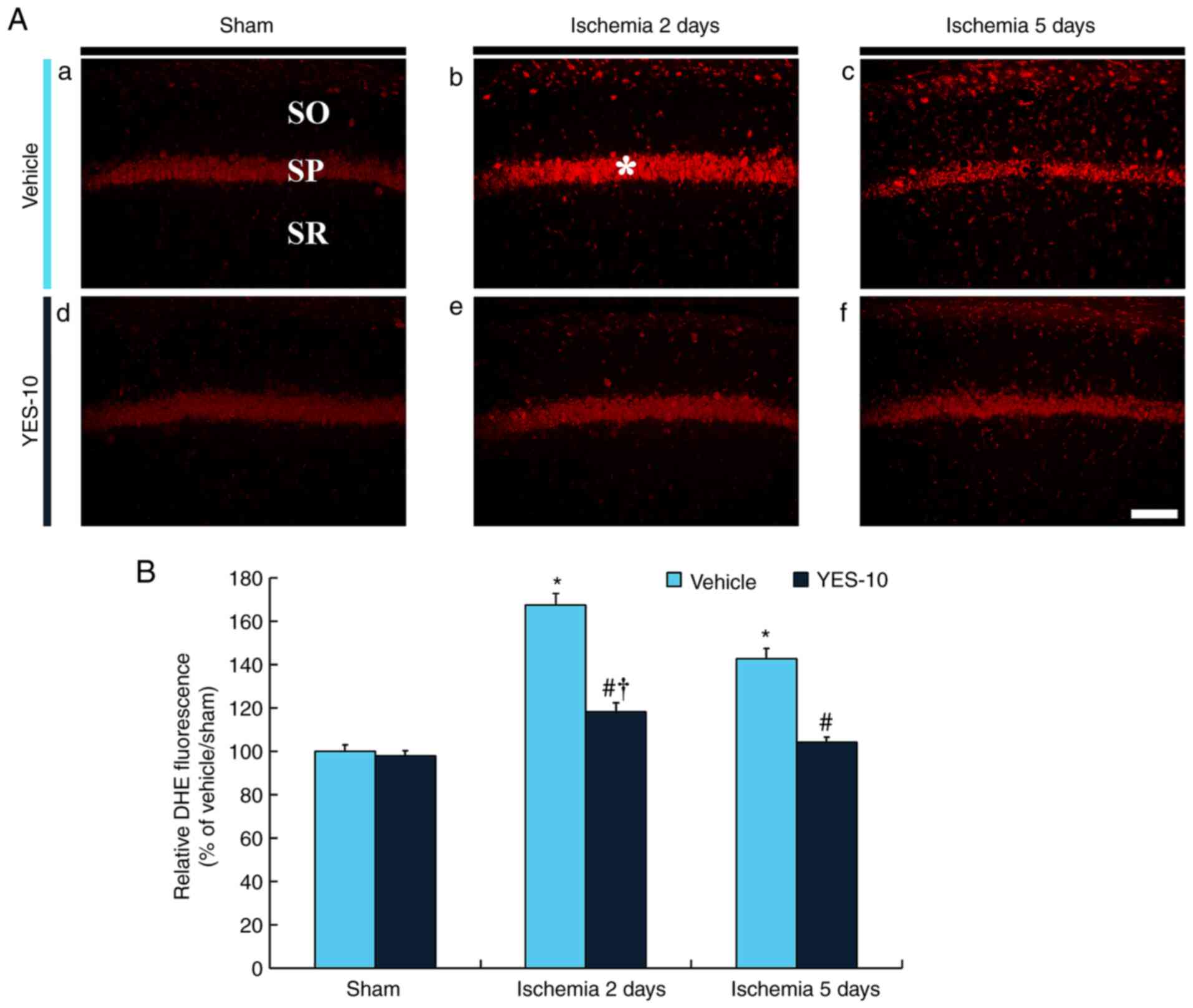 | Figure 7Histofluorescence for DHE in the
hippocampal CA1 field following TI. (Aa) DHE histofluorescence
staining in the CA1 field of the vehicle/sham, (Ab and Ac)
vehicle/TI, (Ad) YES/sham and (Ae and Af) YES/TI groups. In the
vehicle/sham group, DHE fluorescence is shown in the SP, but DHE
fluorescence is significantly increased (asterisk) at 2 days after
TI and decreased at 5 days after TI. In the YES/TI group, DHE
fluorescence in the SP is similar to that in the vehicle/sham
group. DHE fluorescence of the YES/TI group is slightly increased
after TI. Scale bar, 40 µm. (B) RI as % of DHE fluorescence in the
SP (n=7 at each point in time). *P<0.05 vs.
vehicle/sham group, #P<0.05 vs. corresponding
vehicle/TI group, †P<0.05 vs. YES/sham group. The
bars indicate the mean ± SEM. CA1, cornu ammonis 1; SP, stratum
pyramidale; SO, stratum oriens; SR, stratum radiatum; TI, transient
ischemia; DHE, dihydroethidium; F-JB, fluoro-Jade B. |
Discussion
Many studies have reported that natural products
display protective effects against brain ischemic injury. For
example, Chrysanthemum indicum Linné belonging to the
Asteraceae family exerts neuroprotective effects in the hippocampal
CA1 field after TI in gerbils (13,19).
In addition, some studies have shown the synergistic effects of two
extracts from natural products in animal models of ischemic
insults. For instance, a combination of Ligusticum
chuanxiong (from the Apiaceae family) and Radix
Paeoniae, (from the Paeoniaceae family) ameliorated ischemic
brain damage following transient focal brain ischemia induced by
middle cerebral artery occlusion in rats (6,7).
Additionally, we recently published a paper reporting that
pretreatment with 200 mg/kg YES-10® strongly protected
hippocampal CA1 pyramidal neurons and alleviated reactive gliosis
in a gerbil model of TI (5).
Gerbils have a unique cerebrovascular system. Namely, they lack
posterior communicating arteries which compose Willis' circle
(cerebral arterial circle) to supply blood to the brain (39). Therefore, the ligation of only both
common carotid arteries is bale to evoke TI in the forebrain, not
the hindbrain which supports vital processes (40,41).
In this regard, the gerbils with TI show significant
reproducibility of TI induction (42). In addition, this model has the
advantage of long survival after TI due to intact hindbrain
(43,44).
Our current analysis of YES-10®
identified scutellarin and chlorogenic acid as the major
ingredients of YES-10®. It has been demonstrated that
these compounds confer neuroprotective effects against ischemic
insults. In detail, a precedent study reported that pretreatment
with scutellarin ameliorated infarct lesions following focal
cerebral ischemia induced by occlusion of the middle cerebral
artery (MCA) in rats (27,45). In particular, Wang et al
(27) demonstrated that the
neuroprotective effects resulted from the down regulation of
angiotensin-converting enzyme and angiotensin II type 1 receptor
and, ultimately, inhibited the production of pro-inflammatory
cytokines including tumor necrosis factor α, interleukin (IL)-1β,
and IL-6(27). Additionally, Liu
et al (28) reported the
precautionary administration of chlorogenic acid reduced the
infarcts induced by transient global cerebral ischemia from two
repeated occlusions of the bilateral common carotid artery for 10
min in ten-minute intervals in rats (28), suggesting that the neuroprotective
effect of chlorogenic acid treatment to attenuate oxidative stress,
which is regulated by the nuclear factor erythroid 2-related factor
2 (Nrf2) pathway contributed to the neuroprotective effects of
chlorogenic acid. We previously demonstrated neuroprotective effect
of chlorogenic acid in a gerbil model of 5-min transient forebrain
ischemia (35). In that study, the
neuroprotective effect was obtained by treatment of chlorogenic
acid as a single compound (35). In
our current study, contrastively, we investigated the
neuroprotective effect of YES-10® as a mixture of two
extracts derived from natural resources, which contained
scutellarin and chlorogenic acid as major compounds. Additionally,
we found that the synergistic effect in the neuroprotection might
be done via excellent antioxidant efficacy.
In this study, we studied whether pretreatment with
YES-10® exerted antioxidant effects and whether the
antioxidant effects protected hippocampal CA1 pyramidal neurons
from ischemic injury induced by 5-min TI in gerbils. For this, we
performed Western blot analysis and immunohistochemical analysis
for SODs and found that these endogenous antioxidant enzymes were
markedly increased in the CA1 pyramidal neurons of the YES/sham
group and the increases were maintained in the CA1 pyramidal
neurons after TI. A number of investigations have been concluded to
develop that exert neuroprotective effects against brain ischemia
by attenuating oxidative stress. The antioxidant efficacy and
neuroprotective effects against cerebral ischemia have been
investigated in single compounds (17,46,47),
and also natural resource-derived extracts (19,38,48).
These materials have a common characteristic that enhances
endogenous antioxidant enzymes including SOD1, SOD2, catalase and
glutathione peroxidase. It is well known that antioxidant enzymes
are defensive factors against oxidative stress (10,49).
Therefore, the enhancement of antioxidant enzymes is a driving
force in the attenuation of oxidative stress, which eventually can
protect neurons or tissues from brain ischemic insults.
Based on the above-mentioned findings, we examined
neuroprotection of YES-10® in the hippocampal CA1 field
in gerbils following TI. In this study, we found death (loss) of
the CA1 pyramidal neurons 5 days after 5-min TI. It is well known
that, in gerbils, the CA1 pyramidal neurons die 4-5 days after
5-min TI. Thus, this phenomenon is called delayed neuronal death
(8,9). When we treated YES-10®,
neuroprotection was shown in the hippocampal CA1 field of the
YES/TI group. However, the neuroprotective effect of
YES-10® after TI was not examined in other areas (i.e.,
cerebral cortex and striatum) of the brain induced by 5-min TI.
Therefore, further studies on the neuroprotective effect in
important areas of the brain should be accomplished in the
future.
Finally, we examined whether YES-10®
pretreatment decreased the production of ROS in the CA1 pyramidal
neurons after TI by immunohistochemical analysis of 4-HNE and
histofluorescence for DHE. Various approaches to evaluate ROS
production have been established and widely used. Among them, two
methodologies are commonly used: 1) detecting 4-HNE, an end-product
of excessive ROS-induced lipid peroxidation chain reaction
(38,50,51)
and 2) quantifying in situ superoxide anion by the DHE
fluorescence assay (36,52). In the YES/TI group, the production
of ROS in the CA1 pyramidal neurons was significantly reduced. This
finding indicates that the increase in antioxidant enzymes by
YES-10® ameliorated ROS overproduction, which
contributed to neuroprotection against TI injury. Adequate ROS is
needed to maintain homeostasis by participating in cellular
metabolism (53,54). However, the excessive production of
ROS can lead to oxidative stress, which can be the main cause of
neuronal damage or death following ischemic insults (10,15).
Namely, imbalanced production and neutralization of antioxidant
enzymes following ischemic insults increases ROS production and
augments cellular vulnerability through lipid peroxidation, protein
oxidation and DNA damage (55).
Among cells in the central nervous system, neurons and astrocytes
are more susceptible to ROS toxicity than other CNS cells (i.e.,
microglia), as they have high oxidative metabolism and low
antioxidant enzymes as well as high membrane fatty acid content
(15).
In conclusion, our current results showed that
pretreatment with YES-10® increased SODs in the CA1
pyramidal neurons and protected the CA1 pyramidal neurons from TI
injury. The TI-induced over-production of ROS in the CA1 pyramidal
neurons was significantly reduced after TI. Therefore, we suggest
that the strong antioxidant efficacy of YES-10® makes it
a candidate for developing a therapeutic agent to protect the
brains from ischemic damage.
Acknowledgements
Not applicable.
Funding
The present research was supported by the
following: i) Korea Institute of Planning and Evaluation for
Technology in Food, Agriculture, Forestry and Fisheries, through
the High Value-added Food Technology Development Program, funded by
the Ministry of Agriculture, Food and Rural Affairs (grant no.
117055-3); ii) Cooperative Research Program for Agriculture Science
and Technology Development Rural Development Administration
(Project no. PJ01329401; and iii) the Bio-Synergy Research Project
(NRF-2018M3A9C4076478) of the Ministry of Science and ICT through
the National Research Foundation.
Availability of data and materials
The datasets used and/or analyzed during the
current study are available from the corresponding author on
reasonable request.
Authors' contributions
YP, TL, BK, CP and DK performed the experiments and
measurements. YN, JA, JL, JP, JK and YK analyzed and interpreted
data. YP and MW confirm the authenticity of all the raw data. IK,
JL, SK and MW made substantial contributions to conception and
design, and were involved in drafting, revising the manuscript and
interpreting all data. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
The protocol of this research was approved on
February 18, 2020 by the Institutional Animal Care and Use
Committee (AICUC) at Kangwon National University (approval no.
KW-200113-1).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Hao D, Gu X, Xiao P and Peng Y: Chemical
and biological research of Clematis medicinal resources. Chin Sci
Bull. 58:1120–1129. 2013.
|
|
2
|
Chledzik S, Strawa J, Matuszek K and
Nazaruk J: Pharmacological effects of scutellarin, an active
component of genus scutellaria and erigeron: A systematic review.
Am J Chin Med. 46:319–337. 2018.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Lee SW, Chung WT, Choi SM, Kim KT, Yoo KS
and Yoo YH: Clematis mandshurica protected to apoptosis of
rat chondrocytes. J Ethnopharmacol. 101:294–298. 2005.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Jo MJ, Lee JR, Cho IJ, Kim YW and Kim SC:
Roots of Erigeron annuus attenuate acute inflammation as
mediated with the inhibition of NF-κ B-associated nitric oxide and
prostaglandin E2 production. Evid Based Complement Alternat Med.
2013(297427)2013.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Lee TK, Park JH, Kim B, Park YE, Lee JC,
Ahn JH, Park CW, Noh Y, Lee JW, Kim SS, et al: YES-10, a
combination of extracts from Clematis mandshurica RUPR. and
Erigeron annuus (L.) PERS., prevents ischemic brain injury
in a gerbil model of transient forebrain ischemia. Plants (Basel).
9(154)2020.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Gu J, Su S, Guo J, Zhu Y, Zhao M and Duan
JA: Anti-inflammatory and anti-apoptotic effects of the combination
of Ligusticum chuanxiong and Radix Paeoniae against focal cerebral
ischaemia via TLR4/MyD88/MAPK/NF-kappaB signalling pathway in MCAO
rats. J Pharm Pharmacol. 70:268–277. 2018.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Gu J, Chen J, Yang N, Hou X, Wang J, Tan
X, Feng L and Jia X: Combination of Ligusticum chuanxiong and Radix
Paeoniae ameliorate focal cerebral ischemic in MCAO rats via
endoplasmic reticulum stress-dependent apoptotic signaling pathway.
J Ethnopharmacol. 187:313–324. 2016.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Kirino T and Sano K: Selective
vulnerability in the gerbil hippocampus following transient
ischemia. Acta Neuropathol. 62:201–208. 1984.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Kirino T: Delayed neuronal death in the
gerbil hippocampus following ischemia. Brain Res. 239:57–69.
1982.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Lee JC and Won MH: Neuroprotection of
antioxidant enzymes against transient global cerebral ischemia in
gerbils. Anat Cell Biol. 47:149–156. 2014.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Mdzinarishvili A, Sumbria R, Lang D and
Klein J: Ginkgo extract EGb761 confers neuroprotection by reduction
of glutamate release in ischemic brain. J Pharm Pharm Sci.
15:94–102. 2012.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Shirley R, Ord EN and Work LM: Oxidative
stress and the use of antioxidants in stroke. Antioxidants (Basel).
3:472–501. 2014.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Yoo KY, Kim IH, Cho JH, Ahn JH, Park JH,
Lee JC, Tae HJ, Kim DW, Kim JD, Hong S, et al: Neuroprotection of
Chrysanthemum indicum Linne against cerebral ischemia/reperfusion
injury by anti-inflammatory effect in gerbils. Neural Regen Res.
11:270–277. 2016.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Li P, Stetler RA, Leak RK, Shi Y, Li Y, Yu
W, Bennett MVL and Chen J: Oxidative stress and DNA damage after
cerebral ischemia: Potential therapeutic targets to repair the
genome and improve stroke recovery. Neuropharmacology. 134:208–217.
2018.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Chan PH: Reactive oxygen radicals in
signaling and damage in the ischemic brain. J Cereb Blood Flow
Metab. 21:2–14. 2001.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Lee JC, Kim IH, Park JH, Ahn JH, Cho JH,
Cho GS, Tae HJ, Chen BH, Yan BC, Yoo KY, et al: Ischemic
preconditioning protects hippocampal pyramidal neurons from
transient ischemic injury via the attenuation of oxidative damage
through upregulating heme oxygenase-1. Free Radic Biol Med.
79:78–90. 2015.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Ya BL, Li HF, Wang HY, Wu F, Xin Q, Cheng
HJ, Li WJ, Lin N, Ba ZH, Zhang RJ, et al: 5-HMF attenuates striatum
oxidative damage via Nrf2/ARE signaling pathway following transient
global cerebral ischemia. Cell Stress Chaperones. 22:55–65.
2017.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Park JH, Lee TK, Yan BC, Shin BN, Ahn JH,
Kim IH, Cho JH, Lee JC, Hwang IK, Kim JD, et al: Pretreated
Glehnia littoralis extract prevents neuronal death following
transient global cerebral ischemia through increases of superoxide
dismutase 1 and brain-derived neurotrophic factor expressions in
the Gerbil Hippocampal cornu ammonis 1 area. Chin Med J (Engl).
130:1796–1803. 2017.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Kim IH, Lee TK, Cho JH, Lee JC, Park JH,
Ahn JH, Shin BN, Chen BH, Tae HJ, Kim YH, et al: Pretreatment with
Chrysanthemum indicum Linne extract protects pyramidal neurons from
transient cerebral ischemia via increasing antioxidants in the
gerbil hippocampal CA1 region. Mol Med Rep. 16:133–142.
2017.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Li L, Li L, Chen C, Yang J, Li J, Hu N, Li
Y, Zhang D, Guo T, Liu X and Yang W: Scutellarin's cardiovascular
endothelium protective mechanism: Important role of PKG-Iα. PLoS
One. 10(e0139570)2015.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Yang B, Zhang Z, Yang Z, Ruan J, Luo L,
Long F and Tang D: Chanling Gao attenuates bone cancer pain in rats
by the IKKβ/NF-κB signaling pathway. Front Pharmacol.
11(525)2020.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Jiang NH, Zhang GH, Zhang JJ, Shu LP,
Zhang W, Long GQ, Liu T, Meng ZG, Chen JW and Yang SC: Analysis of
the transcriptome of Erigeron breviscapus uncovers putative
scutellarin and chlorogenic acids biosynthetic genes and genetic
markers. PLoS One. 9(e100357)2014.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Chen S, Li M, Li Y, Hu H, Li Y, Huang Y,
Zheng L, Lu Y, Hu J, Lan Y, et al: A UPLC-ESI-MS/MS method for
simultaneous quantitation of chlorogenic acid, scutellarin, and
scutellarein in rat plasma: Application to a comparative
pharmacokinetic study in sham-operated and MCAO rats after oral
administration of Erigeron breviscapus extract. Molecules.
23(1808)2018.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Lee JY, Park JY, Kim DH, Kim HD, Ji YJ and
Seo KH: Erigeron annuus protects PC12 neuronal cells from
oxidative stress induced by ROS-mediated apoptosis. Evid Based
Complement Alternat Med. 2020(3945194)2020.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Sato Y, Itagaki S, Kurokawa T, Ogura J,
Kobayashi M, Hirano T, Sugawara M and Iseki K: In vitro and in vivo
antioxidant properties of chlorogenic acid and caffeic acid. Int J
Pharm. 403:136–138. 2011.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Szwajgier D, Borowiec K and Pustelniak K:
The neuroprotective effects of phenolic acids: Molecular mechanism
of action. Nutrients. 9(477)2017.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Wang W, Ma X, Han J, Zhou M, Ren H, Pan Q,
Zheng C and Zheng Q: Neuroprotective effect of scutellarin on
ischemic cerebral injury by down-regulating the expression of
angiotensin-converting enzyme and AT1 receptor. PLoS One.
11(e0146197)2016.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Liu D, Wang H, Zhang Y and Zhang Z:
Protective effects of chlorogenic acid on Cerebral
ischemia/reperfusion injury rats by regulating oxidative
stress-related Nrf2 pathway. Drug Des Devel Ther. 14:51–60.
2020.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Lee TK, Kim H, Song M, Lee JC, Park JH,
Ahn JH, Yang GE, Kim H, Ohk TG, Shin MC, et al: Time-course pattern
of neuronal loss and gliosis in gerbil hippocampi following mild,
severe, or lethal transient global cerebral ischemia. Neural Regen
Res. 14:1394–1403. 2019.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Halloran ST, Mauck KE, Fleischer SJ and
Tumlinson JH: Volatiles from intact and Lygus-damaged Erigeron
annuus (L.) Pers. are highly attractive to ovipositing Lygus
and its parasitoid Peristenus relictus Ruthe. J Chem Ecol.
39:1115–1128. 2013.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Park JH, Lee TK, Ahn JH, Shin BN, Cho JH,
Kim IH, Lee JC, Kim JD, Lee YJ, Kang IJ, et al: Pre-treated
Populus tomentiglandulosa extract inhibits neuronal loss and
alleviates gliosis in the gerbil hippocampal CA1 area induced by
transient global cerebral ischemia. Anat Cell Biol. 50:284–292.
2017.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Carpenter JW: Exotic Animal
Formulary-eBook, 4th edition. Elsevier Health Sciences, 2012.
|
|
33
|
Engel T, Schindler CK, Sanz-Rodriguez A,
Conroy RM, Meller R, Simon RP and Henshall DC: Expression of
neurogenesis genes in human temporal lobe epilepsy with hippocampal
sclerosis. Int J Physiol Pathophysiol Pharmacol. 3:38–47.
2011.PubMed/NCBI
|
|
34
|
Zhao H, Li Z, Wang Y and Zhang Q:
Hippocampal expression of synaptic structural proteins and
phosphorylated cAMP response element-binding protein in a rat model
of vascular dementia induced by chronic cerebral hypoperfusion.
Neural Regen Res. 7:821–826. 2012.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Lee TK, Park Y, Kim B, Lee JC, Shin MC,
Ohk TG, Park CW, Cho JH, Park JH, Lee CH, et al: Long-term
alternating fasting increases interleukin-13 in the Gerbil
Hippocampus, but does not protect BBB and Pyramidal Neurons from
ischemia-reperfusion injury. Neurochem Res. 45:2352–2363.
2020.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Kim H, Ahn JH, Song M, Kim DW, Lee TK, Lee
JC, Kim YM, Kim JD, Cho JH, Hwang IK, et al: Pretreated fucoidan
confers neuroprotection against transient global cerebral ischemic
injury in the gerbil hippocampal CA1 area via reducing of glial
cell activation and oxidative stress. Biomed Pharmacother.
109:1718–1727. 2019.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Ahn JH, Noh Y, Shin BN, Kim SS, Park JH,
Lee TK, Song M, Kim H, Lee JC, Yong J, et al: Intermittent fasting
increases SOD2 and catalase immunoreactivities in the hippocampus
but does not protect from neuronal death following transient
ischemia in gerbils. Mol Med Rep. 18:4802–4812. 2018.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Ahn JH, Shin MC, Kim DW, Kim H, Song M,
Lee TK, Lee JC, Kim H, Cho JH, Kim YM, et al: Antioxidant
properties of fucoidan alleviate acceleration and exacerbation of
hippocampal neuronal death following transient global cerebral
ischemia in high-fat diet-induced obese gerbils. Int J Mol Sci.
20(554)2019.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Kuchinka J, Nowak E, Szczurkowski A and
Kuder T: Arteries supplying the base of the brain in the Mongolian
gerbil (Meriones unguiculatus). Pol J Vet Sci. 11:295–299.
2008.PubMed/NCBI
|
|
40
|
Martínez NS, Machado JM, Pérez-Saad H,
Coro-Antich RM, Berlanga-Acosta JA, Salgueiro SR, Illera GG, Alba
JS and del Barco DG: Global brain ischemia in Mongolian gerbils:
Assessing the level of anastomosis in the cerebral circle of
Willis. Acta Neurobiol Exp (Wars). 72:377–384. 2012.PubMed/NCBI
|
|
41
|
Ahn JH, Song M, Kim H, Lee TK, Park CW,
Park YE, Lee JC, Cho JH, Kim YM, Hwang IK, et al: Differential
regional infarction, neuronal loss and gliosis in the gerbil
cerebral hemisphere following 30 min of unilateral common carotid
artery occlusion. Metab Brain Dis. 34:223–233. 2019.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Traystman RJ: Animal models of focal and
global cerebral ischemia. ILAR J. 44:85–95. 2003.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Lee JC, Park JH, Ahn JH, Kim IH, Cho JH,
Choi JH, Yoo KY, Lee CH, Hwang IK, Cho JH, et al: New GABAergic
neurogenesis in the hippocampal CA1 region of a gerbil model of
long-term survival after transient cerebral ischemic injury. Brain
Pathol. 26:581–592. 2016.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Kim H, Park JH, Shin MC, Cho JH, Lee TK,
Kim H, Song M, Park CW, Park YE, Lee JC, et al: Fate of astrocytes
in the gerbil hippocampus after transient global cerebral ischemia.
Int J Mol Sci. 20(845)2019.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Qian L, Shen M, Tang H, Tang Y, Zhang L,
Fu Y, Shi Q and Li NG: Synthesis and protective effect of
scutellarein on focal cerebral ischemia/reperfusion in rats.
Molecules. 17:10667–10674. 2012.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Wu J, Chen Y, Yu S, Li L, Zhao X, Li Q,
Zhao J and Zhao Y: Neuroprotective effects of sulfiredoxin-1 during
cerebral ischemia/reperfusion oxidative stress injury in rats.
Brain Res Bull. 132:99–108. 2017.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Xue F, Huang JW, Ding PY, Zang HG, Kou ZJ,
Li T, Fan J, Peng ZW and Yan WJ: Nrf2/antioxidant defense pathway
is involved in the neuroprotective effects of Sirt1 against focal
cerebral ischemia in rats after hyperbaric oxygen preconditioning.
Behav Brain Res. 309:1–8. 2016.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Bazmandegan G, Boroushaki MT, Shamsizadeh
A, Ayoobi F, Hakimizadeh E and Allahtavakoli M: Brown propolis
attenuates cerebral ischemia-induced oxidative damage via affecting
antioxidant enzyme system in mice. Biomed Pharmacother. 85:503–510.
2017.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Fukai T and Ushio-Fukai M: Superoxide
dismutases: Role in redox signaling, vascular function, and
diseases. Antioxid Redox Signal. 15:1583–1606. 2011.PubMed/NCBI View Article : Google Scholar
|
|
50
|
Csala M, Kardon T, Legeza B, Lizák B,
Mandl J, Margittai É, Puskás F, Száraz P, Szelényi P and Bánhegyi
G: On the role of 4-hydroxynonenal in health and disease. Biochim
Biophys Acta. 1852:826–838. 2015.PubMed/NCBI View Article : Google Scholar
|
|
51
|
Yan BC, Park JH, Ahn JH, Kim IH, Lee JC,
Yoo KY, Choi JH, Hwang IK, Cho JH, Kwon YG, et al: Effects of
high-fat diet on neuronal damage, gliosis, inflammatory process and
oxidative stress in the hippocampus induced by transient cerebral
ischemia. Neurochem Res. 39:2465–2478. 2014.PubMed/NCBI View Article : Google Scholar
|
|
52
|
Peshavariya HM, Dusting GJ and Selemidis
S: Analysis of dihydroethidium fluorescence for the detection of
intracellular and extracellular superoxide produced by NADPH
oxidase. Free Radic Res. 41:699–712. 2007.PubMed/NCBI View Article : Google Scholar
|
|
53
|
Dan Dunn J, Alvarez LA, Zhang X and
Soldati T: Reactive oxygen species and mitochondria: A nexus of
cellular homeostasis. Redox Biol. 6:472–485. 2015.PubMed/NCBI View Article : Google Scholar
|
|
54
|
Vara D and Pula G: Reactive oxygen
species: Physiological roles in the regulation of vascular cells.
Curr Mol Med. 14:1103–1125. 2014.PubMed/NCBI View Article : Google Scholar
|
|
55
|
Röhnert P, Schröder UH, Ziabreva I, Täger
M, Reymann KG and Striggow F: Insufficient endogenous redox buffer
capacity may underlie neuronal vulnerability to cerebral ischemia
and reperfusion. J Neurosci Res. 90:193–202. 2012.PubMed/NCBI View Article : Google Scholar
|