Introduction
Breast cancer is the most frequently diagnosed
malignancy and ranks as the leading cause for cancer-related deaths
among females worldwide, for which surgery has become the primary
treatment strategy (1). Patients
with malignant tumors often also display immune dysfunction
(2). A number of factors may
aggravate immune dysfunction during the perioperative period
(3-5).
Recently, there has been increasing interest in the influence of
perioperative immune function secondary to the surgical stress
response, anesthesia and postoperative acute pain (6). Perioperative immune dysfunction may
predispose patients to infections, homeostasis disturbances, organ
dysfunction and other related complications, including an increased
mortality rate (7,8). Therefore, it is crucial to take
measures to protect the immune function of patients during the
perioperative period.
Postoperative pain may trigger a strong stress
response, stimulate the release of inflammatory factors and inhibit
immune function (9). Therefore, an
ideal postoperative analgesic is conducive to the recovery of
patients and shortening the length of hospital stay. Currently,
opioids providing high analgesic efficacy are commonly used as
effective postoperative analgesics (10). However, opioid-induced
immunosuppressive effects and adverse reactions including nausea,
vomiting, sedation, dizziness and decreased gut motility may delay
patient recovery, which limits the application of opioids (11,12).
A recently published meta-analysis suggested that
acupuncture is effective in alleviating cancer-related pain,
particularly malignancy- and surgery-induced pain (13). Transcutaneous electrical acupoint
stimulation (TEAS), as a novel non-invasive acupuncture alternative
therapy, combines acupoint stimulation and transcutaneous
electrical nerve stimulation (TENS), and displays similarities with
traditional acupuncture and moxibustion (14). Due to the advantages of the
non-invasive, convenient application and reduced number of side
effects of TEAS, it has been increasingly applied in conventional
medical settings (15). To date,
there have been a few prospective studies comparing the effects of
TEAS on postoperative analgesia control and postoperative recovery
(16,17).
The concentration of immune cytokines and immunocyte
in tumor tissues and plasma is closely related to the cellular
immune response to malignant tumors, which may implicate the
prognosis of malignant tumors (18,19).
Immune cytokines are primarily secreted by T helper (Th)1 and Th2
cells, which are associated with the cellular immune responses to
malignant tumors. Th1 cells release IFN-γ, IL-2 and TNF, which are
essential for anti-inflammatory and antineoplastic processes
(20,21), whereas Th2 cells secrete
proinflammatory cytokines, primarily including IL-4, IL-6 and IL-10
(19,22). Therefore, the release and balance of
cytokines during the perioperative period have substantial
implications for alterations to immune function (23). The present study investigated the
serum levels of IL-2, IFN-γ and IL-4, and estimated the balance of
Th1/Th2 by calculating the IL-2/IL-4 ratio to explore whether TEAS
could improve immune dysfunction. The present study aimed to
identify the effects of TEAS on immune function and postoperative
analgesia for patients undergoing radical mastectomy.
Materials and methods
Participants
The present study was based on the Declaration of
Helsinki and the Guidelines on Good Clinical Practice (24). The present study was approved by the
Ethics Committee of Human Research of Tangshan People's Hospital
(approval no. RMYY-YWLL-2017-1110; Tangshan, China). The trial was
also registered prior to participant enrollment at www.chictr.org.cn (clinical trial no.
ChiCTR1800017768). Written informed consent was obtained from each
patient. Adult female patients (age, 20-65 years) with American
Society of Anesthesiologists physical status I or II (25), who were scheduled to undergo
elective radical mastectomy of breast cancer in the Department of
Anesthesiology, Tangshan People's Hospital and Tangshan Cancer
Hospital, North China University of Science and Technology
(Tangshan, China) between August 2018 and December 2019, were
enrolled in the present study. The exclusion criteria were as
follows: i) Body mass index >30 kg/m2; ii) severe
cardiac or respiratory diseases, significant renal or hepatic
impairment or immune disorders; iii) history of immunosuppressive
therapy (chemotherapy or radiation) or previous acupuncture/TAES
therapies; iv) history of chronic pain, steroid or opioid
administration, or alcohol or illegal substance abuse; v) high
levels of C-reactive protein or leukocytosis (>10,000/ml) prior
to surgery; and vi) puncture site infection or systemic
infection.
Randomization and blinding
A total of 70 patients were enrolled and randomly
allocated to the TEAS group or the sham TEAS group using
sequentially-numbered sealed envelopes and a random number
generator. The allocation ratio between the two groups was 1:1. The
envelopes were prepared and distributed by an assistant who was not
involved in the present study. An experienced acupuncturist, who
was independent of the present study, performed the corresponding
interventions. All surgeries were performed by the same group of
surgeons. Similarly, a blinded anesthesiologist provided anesthesia
implementation and perioperative care. A second anesthesiologist,
who was also blinded to the treatment regimen and was not involved
in the data analysis, conducted the anesthesia follow-ups.
Study protocol
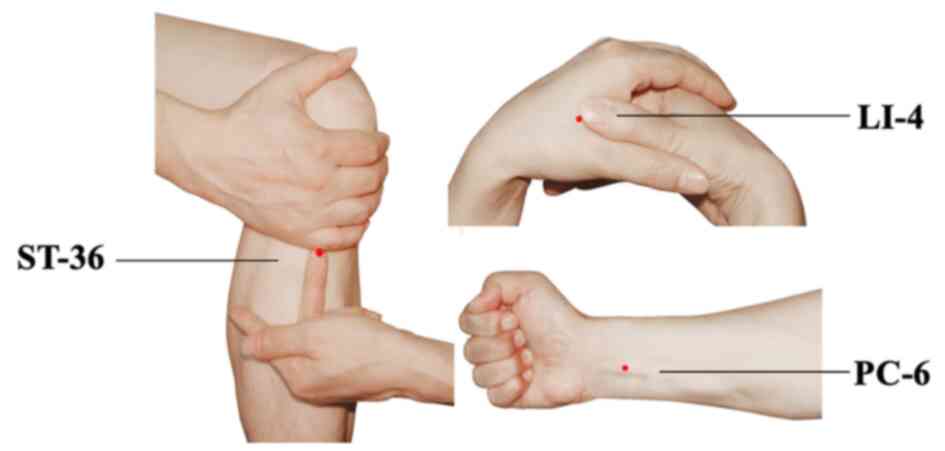
For patients in both groups, the application of TEAS
was implemented at bilateral Hegu (LI-4), Neiguan (PC-6) and
Zusanli (ST-36) acupoints simultaneously. The acupoints were
identified according to the traditional anatomical location
(Fig. 1). LI-4 Hegu is located at
the radial side of the dorsum surface of the hand, between the
first and second metacarpal bones. ST-36 Zusanli is located at 3
cun below the patella, outside of the anterior crest of the tibia.
PC-6 Neiguan is located at 2 cun above the transverse crease of the
wrist, between the palmaris longus tendons and the flexor carpi
radialis. After acupoint selection and skin disinfection, the
electrodes were attached to the target acupoints. A constant
electrical stimulation was applied for 30 min each time with a
dense-and-disperse frequency of 2/100 Hz (26) via a HANS LH-202 electrical
stimulator (Nanjing Jisheng Medical Technology Co., Ltd.). The
optimal intensity was set to mild twitching of the surrounding
muscle and individual maximum tolerance (5-10 mA for upper limbs;
10-30 mA for lower limbs) (27,28).
The TEAS effect was confirmed by de qi sensation (27,29).
Participants in the sham TEAS group underwent electrode attachment
on the target acupoints without electronic stimulation.
TEAS was performed for 30 min prior to the induction
of anesthesia. Postoperative TEAS was performed for 30 min each
time at 4 and 12 h post-surgery on the day of surgery, and
administrated three times (8 a.m., 2 p.m. and 8 p.m.) daily at
postoperative days 1 and 2. Participants in each group were also
provided with a patient-controlled analgesia (PCA) pump for
postoperative pain control, which was maintained for 48 h
post-surgery. The PCA consisted of 1.5 µg/kg sufentanil (cat. no.
81A09131; Yichang Humanwell Pharmaceutical Co., Ltd.), diluted to
150 ml with normal saline. The basal infusion rate was set to 2
ml/h, with a bolus dose of 0.5 ml and a lockout interval of 15
min.
Standardized anesthesia
Upon arrival in the operating room, all patients
were continuously monitored with electrocardiography, and the
following parameters: Blood pressure, pulse oximetry, pressure of
end-tidal carbon dioxide (PETCO2) and
bispectral index (BIS) (cat. no. MG8001; Sichuan Kehong Medical
Equipment Co., Ltd.) were monitored (30). Before anesthesia induction, all
patients received midazolam (1-2 mg; cat. no. 20190108; Jiangsu
Nhwa Pharmaceutical Co., Ltd.) intravenously. General anesthesia
was induced using intravenous sufentanil (0.3-0.5 µg/kg; cat. no.
81A09131; Yichang Humanwell Pharmaceutical Co., Ltd.), propofol
(2-3 mg/kg; cat. no. 1809208; Fresenius Kabi) and cisatracurium
(0.2 mg/kg; cat. no. 18082021; Jiangsu Hengrui Medicine Co., Ltd.).
After intubation, ventilation was adopted with an 8 ml/kg tidal
volume and PETCO2 was maintained at 35-40
mmHg. Propofol and remifentanil (cat. no. 80A06221; Yichang
Humanwell Pharmaceutical Co., Ltd.) were titrated to maintain
hemodynamic stability intraoperatively by using a target-controlled
infusion. During the surgery, the BIS value was maintained at
40-60. Muscle relaxation was facilitated by the intermittent
administration of cisatracurium according to surgical requirements.
After surgery, muscle relaxation was antagonized with 1 mg
neostigmine (cat. no. 1810605; Shanghai Xinyi Pharmaceutical Co.,
Ltd.) and 0.5 mg atropine (cat. no. 1809131; Tianjin Jinyao
Pharmaceutical Co., Ltd.). The patients were sent to the
postanesthetic care unit for further monitoring after extubation.
The anesthesia time, operation time, volume of blood loss and urine
were recorded.
Observation indexes
Visual analogue scale (VAS) scores (31) were recorded quantitatively to assess
the postoperative pain intensity at the following time points
post-surgery: 4 h (T1), 12 h (T2), 24 h
(T3) and 48 h (T4). VAS scores ranged from
0-10, 0 indicated no pain and 10 indicated the worst pain. The use
of the VAS score was detailed to each patient to ensure the
accuracy of the assessment. Subjects were asked to make a mark on
the VAS line to indicate the instant pain intensity.
The PCA pump bolus and the total consumption of
opioids within 48 h post-surgery were recorded. In addition, the
occurrence of postoperative adverse complications, including
postoperative nausea and vomiting (PONV), pruritus, dizziness and
headache, were observed up to 48 h after operation.
Blood sample collection
Peripheral venous blood (5 ml) was drawn from each
participant at 30 min before the first treatment of TEAS
(T0), at T1, T2, T3 and
T4. The blood samples were collected using a heparinized
anticoagulation tube for cytokine concentration analysis.
Cytokine assays
For cytokine measurements, the blood samples were
centrifuged at 2,200 x g for 10 min at 4˚C. The supernatant was
stored at -20˚C until subsequent use. Plasma concentrations of IL-2
(cat. no. D2050), IL-4 (cat. no. D4050) and IFN-γ (cat. no. DIF50C)
were detected using ELISA kits (all from R&D Systems, Inc.) The
absorbance was measured at a wavelength of 450 nm using a Spectra
Max 190 microplate reader (Molecular Devices, LLC). To determine
the balance of Th1/Th2, the IL-2/IL-4 ratio was also
calculated.
Statistical analysis
Statistical analyses were performed using the SPSS
software, (version 19.0; IBM Corp.). Data were tested for normality
using the Kolmogorov-Smirnov test. Continuous variables are
presented as the mean ± SD. Continuous variables were analyzed
using the independent Student's t-test or Mann-Whitney U test. A
mixed two-way ANOVA followed by Bonferroni correction were used to
detect comparisons of cytokine concentrations between groups at the
same time and within groups at the different time points.
Categorical variables are presented as numbers or frequencies.
Categorical variables were analyzed using the χ2 test or
Fisher's exact test, where appropriate. P<0.05 was considered to
indicate a statistically significant difference.
Results
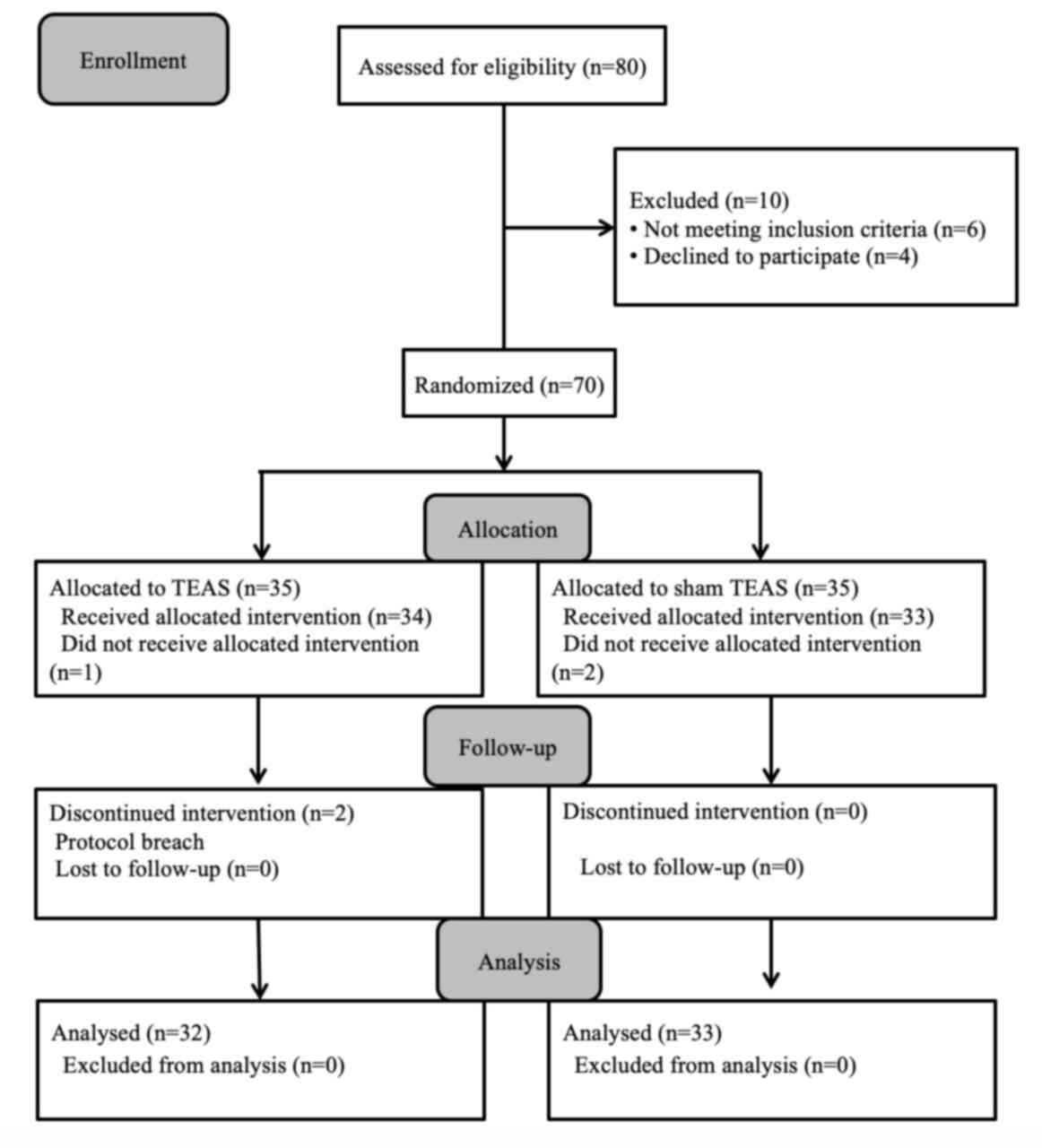
Participant enrollment
Initially, 80 participants were recruited. Among
them, 10 participants (12.50%) were excluded due to meeting the
exclusion criteria and 5 participants (6.25%) were excluded for
other reasons (Fig. 2). A total of
one patient in the TEAS group and two patients in the sham TEAS
group were excluded as they refused to receive TEAS after surgery.
Similarly, two patients in the TEAS group had not completed all
time point stimulations. Therefore, available data from 65
participants (81.25%) were included in the analysis.
Demographics and operation
details
The demographic characteristics were comparable
between the two groups. There were no significant differences
between the two groups regarding the details of anesthesia and
operation, in terms of duration, operation site, blood loss,
infusion volume and urine volume (Table
I).
 | Table IClinical characteristics of patients
between two groups. |
Table I
Clinical characteristics of patients
between two groups.
| Variable | TEAS | Sham TEAS | P-value |
|---|
| Average age
(years) | 45.6±9.8 | 46.9±8.6 | 0.572 |
| BMI
(kg/m2) | 23.4±4.2 | 22.6±3.3 | 0.396 |
| ASA physical
status | | | 0.426 |
|
I | 26 | 24 | |
|
II | 6 | 9 | |
| Operation site | | | 0.444 |
|
Left | 18 | 16 | |
|
Right | 14 | 17 | |
| Anesthesia duration
(min) | 131.6±21.2 | 128.6±25.6 | 0.609 |
| Operation duration
(min) | 98.8±16.8 | 96.1±20.2 | 0.561 |
| Blood loss volume
(ml) | 109.5±28.6 | 105.6±25.7 | 0565 |
| Infusion volume
(ml) | 895.2±120.5 | 910.7±143.6 | 0.636 |
| Urine volume
(ml) | 170.3±25.4 | 158.6±30.1 | 0.095 |
Postoperative analgesia indexes
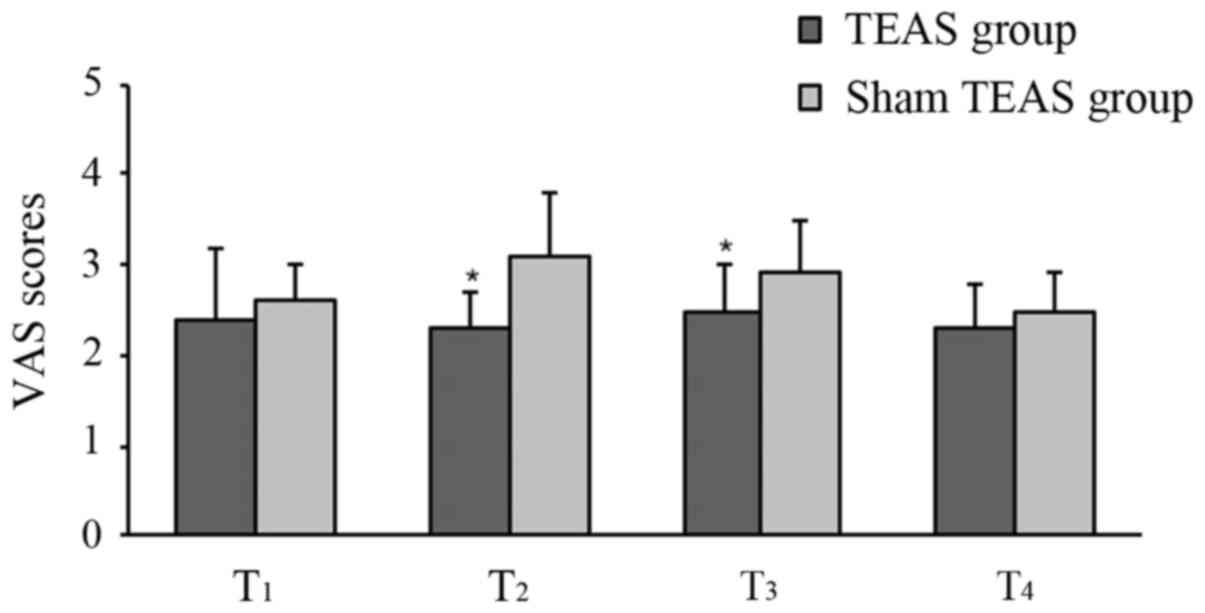
The postoperative VAS scores at T2 and
T3 in the TEAS group were significantly lower compared
with the sham TEAS group. There were no statistical differences at
T1 and T4 between the groups (Fig. 3). Moreover, the total consumption of
opioids in PCA pump and cumulative times of rescue analgesia during
the 48-h postoperative period were significantly lower in the TEAS
group compared with the sham TEAS group (Table II).
 | Table IIEffective press, consumption of
opioids and incidence of adverse events. |
Table II
Effective press, consumption of
opioids and incidence of adverse events.
| Variable | TEAS | Sham TEAS | P-value |
|---|
| Number of effective
press |
6.2±3.7a | 12.3±4.6 | <0.001 |
| Consumption of
opioids (ml) |
102.8±7.4a | 120.6±9.2 | <0.001 |
| Dizziness | 7 | 9 | 0.614 |
| Pruritis | 2 | 4 | 0.672 |
| Headache | 2a | 9 | 0.044 |
| PONV | 4a | 12 | 0.042 |
Compared with the sham TEAS group, the incidences of
PONV and headache were significantly lower in the TEAS group. No
significant differences between the two groups were detected
regarding the incidence of pruritis and dizziness (Table II).
Immunological indexes
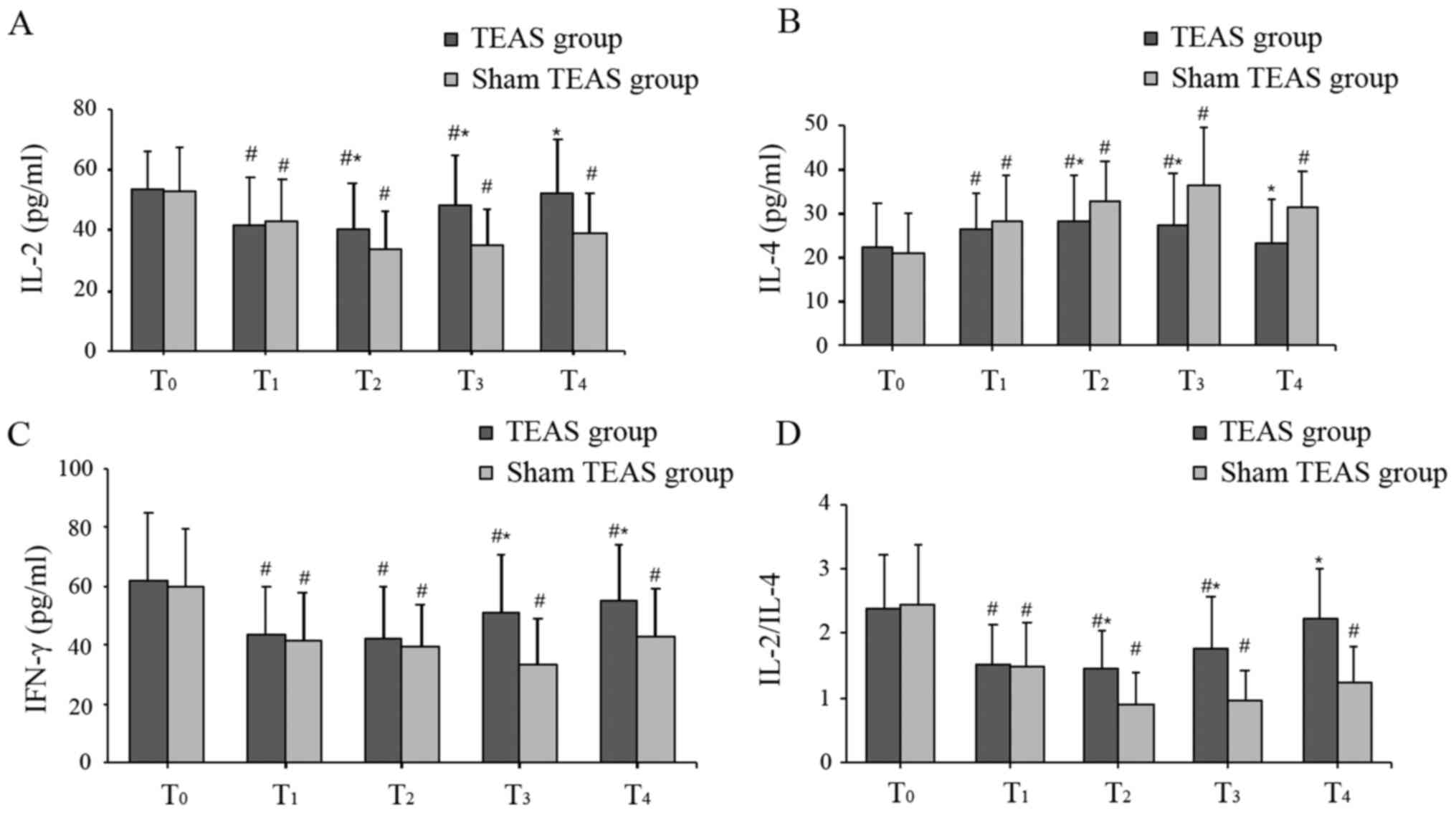
The baseline levels of IL-2, IL-4 and IFN-γ at
T0 were similar between the two groups. Compared with
baseline levels at T0, serum levels of IL-2, IFN-γ and
the ratio of IL-2/IL-4 were significantly decreased at
T1-T4 in the sham TEAS group, whereas the
serum levels of IL-4 were significantly increased at all
postoperative time points. Similarly, in the TEAS group,
significantly lower serum levels of IL-2 and IFN-γ, a decreased
IL-2/IL-4 ratio, and higher levels of IL-4 were observed at
T1, T2 and T3 compared with
T0. However, postoperative serum levels of IL-2 and
IL-4, and the IL-2/IL-4 ratio at T4 were similar to
those at T0 in the TEAS group, and the serum levels of
IFN-γ were still decreased at T4.
In the TEAS group, the serum levels of IL-2 at
T2-T4 and IFN-γ at T3 and
T4 were higher compared with the sham TEAS group. By
contrast, the serum levels of IL-4 were lower at
T2-T4. Moreover, the IL-2/IL-4 ratio in the
TEAS group was significantly higher compared with the sham TEAS
group at T2-T4 (Fig. 4).
Discussion
Increasing evidence suggested that surgical
trauma-induced stress or general anesthesia could lead to
immunosuppression (3-5,32).
A recent prospective and randomized pilot analysis investigated the
effect of anesthetic technique on immunocyte infiltration in breast
cancer (33). The analysis
indicated that balancing general anesthesia with opioid analgesia
could attenuate perioperative immunity. A previous study also
indicated that regional anesthesia and avoidance of opioids could
reduce perioperative residual disease (34), which was consistent with another
study (35).
Postoperative pain could lead to the release of
inflammatory factors and result in immune disorder (9). Therefore, minimizing the
immunosuppressive regimen has been beneficial for patients with
cancer (34-36).
Opioids are recommended to relieve postoperative or cancer-related
pain (10,37). However, intractable opioid-induced
adverse reactions, especially immunosuppression, could be easily
overlooked (38). Although weak
opioids (for example, tramadol) and non-steroidal anti-inflammatory
drugs cannot inhibit immune function (39,40),
they are rarely used alone for postoperative pain control, given
their weak analgesic effects and side effects. In the present
study, TEAS in combination with low-dose opioids was selected for
analgesia. The results suggested that patients in the TEAS group
displayed significantly lower VAS scores at 12 and 24 h
post-operation, as well as reduced consumption of PCA within 48 h
post-surgery, indicating that TEAS displayed potent analgesic
effects, which was consistent with a previous study (27). Therefore, TEAS might be associated
with effective postoperative pain relief and lower analgesic
consumption. In addition, the incidences of nausea, vomiting and
headache were lower in the TEAS group, which suggested that TEAS
could attenuate the incidence of adverse effects. TEAS displayed an
analgesic role via certain intensity stimulation on specific
acupoints, which was characterized by low stimulation, strong
operability and few side effects. In a previous trial, the
therapeutic advantage of pretreatment with TEAS at Jia ji EX-B2 was
assessed, and the results indicated reduced post-procedural
discomfort and abdominal pain following colonoscopy (41). A meta-analysis including a total of
1,350 participants demonstrated that the use of TENS could
significantly reduce the consumption of postoperative analgesics
(42), whereas TEAS combined the
effect of TENS and acupuncture. In addition, Liang et al
(43) reported that TEAS was an
effective adjunct to opioid therapy for moderate and severe
cancer-related pain, and could reduce side effects and improve
immune function. Therefore, the utilization of TEAS may have a
direct clinical application value, especially for patients with
cancer. In a previous study, the PC6 acupoint stimulation
effectively prevented and relieved nausea and vomiting that was
associated with surgery (44). In
addition, two previously published randomized controlled trials
reported that acupuncture at PC6 was associated with the relief of
postoperative nausea and vomiting (45,46).
ST36 and LI-4 are the most common acupoints for analgesia, and
stimulation at LI-4 is especially effective for head and neck pain
(47). A previous study indicated
that acupuncture at LI-4 was as effective as analgesics in
relieving headaches with fewer side effects, which was suggested as
an alternative to the non-pharmacological analgesia method
(48). In an animal experiment,
electroacupuncture (EA) at LI-4 and ST-36 significantly enhanced
the immune function of rats with gastric carcinoma after operation
(49). Moreover, EA at acupoint
ST36 alone in mice may ameliorate inflammation and modulate immune
function in collagen-induced arthritis (50). EA at acupoint ST36 has been revealed
to be beneficial for preserving immune function and improving
postoperative recovery (17,29).
Consequently, PC-6, LI-4 and ST-36 acupoints were investigated in
the present study.
Although TEAS is widely used in daily clinical
practice, the specific analgesic mechanism is not fully understood.
TEAS serves as an alternative to acupuncture, which provides
stimulation by needles on the surface of target acupoints. TEAS can
also generate an action potential to activate nerve fibers
(51,52) as signals are transmitted to the
central nervous system to induce corresponding analgesic effects by
modulating the release of neurotransmitters, such as endorphins and
enkephalins, and blocking pain signaling pathways (53). EA has also been reported to slowly
increase pain thresholds, producing analgesia via
counter-regulation of glial activation (54,55).
Furthermore, a neuromodulator with antinociceptive properties,
adenosine, has been implicated in mediating the analgesia effect of
EA, which could be prolonged by manipulation of adenosine
metabolism (56).
The present study demonstrated that immune function
in both groups was suppressed after surgery, which was associated
with downregulated serum levels of IL-2 and IFN-γ, and increased
serum levels of IL-4. In the TEAS group, the levels of IL-2 and
IL-4 were restored to the preoperative level at 48 h post-surgery.
However, the levels of IL-2 and IL-4 in the sham TEAS group did not
restore to the baseline level until 48 h post-surgery. Moreover,
the serum levels of IL-2 at T2, T3 and
T4, and IFN-γ at T3 and T4 in the
sham TEAS group were lower compared with the TEAS group. By
contrast, the serum levels of IL-4 were higher in the sham TEAS
group at T2, T3 and T4. TEAS
returned the aforementioned cellular immune factors to the
preoperative control value at a faster rate, exerting a modulatory
effect on the immune system. A previous animal study indicated that
EA at the ST36 acupoint significantly enhanced the levels of IFN-γ
and IL-2(57). Similarly, surgical
trauma has contributed to postoperative immunosuppression, which is
associated with decreased expression levels of IL-2 and IFN-γ, and
increased expression of IL-4 in a surgical traumatized rat model
(58). In the present study, TAES
treatment increased the serum levels of IL-2, IFN-γ, and decreased
IL-4 secretion, suggesting that TAES could attenuate the
postoperative immune dysfunction of patients with breast cancer via
altering the expression of Th1/Th2 cell-associated cytokines. The
results were consistent with a previous study (59). Moreover, Li et al (60) demonstrated that EA combined with
anesthesia was able to reduce perioperative immunosuppression
compared with general anesthesia alone. Under normal conditions,
Th1 and Th2 cells are in a relatively balanced state. Since the
shift from Th1 to Th2 is associated with immunosuppression and
cancer development, the balance between Th1 and Th2 cells is of
importance in patients with cancer (61). The ratio of IL-2/IL-4 is typically
used to represent the Th1/Th2 cell ratio (32,62).
In the present study, the balance between Th1/Th2 was measured to
investigate the impact of EA on immune function. The results
suggested that the IL-2/IL-4 ratio was significantly altered
secondary to mastectomy, whereas TEAS partially restored the
imbalance of Th1/Th2.
Although the exact mechanisms underlying EA
stimulation-mediated promotion of the functions of the immune
system are not completely understood, previous studies have
indicated that certain signaling pathways could be associated with
the signaling mechanisms (57,58,63).
EA administration could regulate the production of Th1 and Th2
cytokines, and the expression of mRNA splenic T cells (58). Additionally, the immunomodulatory
effects of EA are likely connected with the MAPK signaling pathway,
which serves an important role in the regulation of T cell
activation and cytokine production (58). EA applied to the ST36 acupoint
enhanced the level of immune cytokines and cluster of
differentiation 4 in spleen cells via transient receptor potential
vanilloid (TRPV) channels (57).
The aforementioned study also suggested that the activation of TRPV
channels was related to Ca2+ influx in spleen cells.
Moreover, by acupuncture application, an increase in
neurotransmitter levels, such as β-endorphin, serotonin,
met-encephalin and leu-encephalin have been detected in the central
nervous system and plasma, which have been revealed to exhibit
immunomodulator effects on the immune system (63,64).
However, pain relief could also have the potential to benefit
immune function by reducing postoperative stress and inhibiting
excessive release of inflammatory cytokines (9,36).
The present study had a number of limitations.
Firstly, the present study was a single center study with a
strictly defined participant population; therefore, the findings
might not be applicable to other centers despite high homogeneity
of both groups. Secondly, although the sham group was treated in
the same manner as the TEAS group, other than the stimulation,
blinding of TEAS treatment was not possible as patients eventually
knew whether they were receiving electrical stimulation. Thirdly,
it was not possible for the operators to be blinded to the grouping
because the effectiveness of TEAS was determined via the sensation
of de qi. In addition, the present study did not detect the
influences on long-term sequelae, such as tumor metastasis,
recurrence and mortality, which could be associated with
perioperative immunosuppression. Moreover, the relatively small
sample size of the present study may have partially affected the
outcomes. Therefore, further studies with larger sample sizes and
multi-indicators are required to evaluate the potential advantages
of TEAS.
To conclude, the present study indicated that TEAS
maintained cellular immune function, alleviated postoperative pain
and reduced the occurrence of opioid-related side effects,
providing a novel insight for selection of postoperative analgesia.
The results of the present study may have implications for
postoperative pain management in patients with cancer regarding
immune function and postoperative recovery.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
LA and JS drafted the manuscript. JS and JG were in
charge of patient recruitment. LA collected individual data. SZ and
YB performed statistical analyses. JG and YB contributed to study
conception. JG reviewed the manuscript and the approved final
submission. All authors read and approved the final manuscript.
Ethical approval and consent to
participate
The present study was approved by the Ethics
Committee of Human Research of Tangshan People's Hospital (approval
no. RMYY-YWLL-2017-1110; Tangshan, China). The trial was also
registered prior to participant enrollment at www.chictr.org.cn (clinical trial no.
ChiCTR1800017768). Written informed consent was obtained from each
patient.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Torre LA, Siegel RL, Ward EM and Jemal A:
Global cancer incidence and mortality rates and trends-an update.
Cancer Epidemiol Biomarkers Prev. 25:16–27. 2016.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Kurosawa S: Anesthesia in patients with
cancer disorders. Curr Opin Anaesthesiol. 25:376–384.
2012.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Neeman E and Ben-Eliyahu S: The
perioperative period and promotion of cancer metastasis: New
outlooks on mediating mechanisms and immune involvement. Brain
Behav Immun. 30 (Suppl):S32–S40. 2013.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Green JS and Tsui BC: Impact of anesthesia
for cancer surgery: Continuing professional development. Can J
Anaesth. 60:1248–1269. 2013.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Boland JW, McWilliams K, Ahmedzai SH and
Pockley AG: Effects of opioids on immunologic parameters that are
relevant to anti-tumour immune potential in patients with cancer: A
systematic literature review. Br J Cancer. 111:866–873.
2014.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Cho JS, Lee MH, Kim SI, Park S, Park HS,
Oh E, Lee JH and Koo BN: The Effects of perioperative anesthesia
and analgesia on immune function in patients undergoing breast
cancer resection: A prospective randomized study. Int J Med Sci.
14:970–976. 2017.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Bobocea AC, Trandafir B, Bolca C and
Cordoş I: Minimally invasive surgery in cancer. Immunological
response. Chirurgia (Bucur). 107:154–157. 2012.PubMed/NCBI
|
|
8
|
Leaver HA, Craig SR, Yap PL and Walker WS:
Lymphocyte responses following open and minimally invasive thoracic
surgery. Eur J Clin Invest. 30:230–238. 2000.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Page GG, Blakely WP and Ben-Eliyahu S:
Evidence that postoperative pain is a mediator of the
tumor-promoting effects of surgery in rats. Pain. 90:191–199.
2001.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Rawal N: Current issues in postoperative
pain management. Eur J Anaesthesiol. 33:160–171. 2016.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Wei G, Moss J and Yuan CS: Opioid-induced
immunosuppression: Is it centrally mediated or peripherally
mediated? Biochem Pharmacol. 65:1761–1766. 2003.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Alonzo NC and Bayer BM: Opioids,
immunology, and host defenses of intravenous drug abusers. Infect
Dis Clin North Am. 16:553–569. 2002.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Chiu HY, Hsieh YJ and Tsai PS: Systematic
review and meta-analysis of acupuncture to reduce cancer-related
pain. Eur J Cancer Care (Engl): Feb 7, 2017 (Epub ahead of print).
doi: 10.1111/ecc.12457.
|
|
14
|
Gavronsky S, Koeniger-Donohue R, Steller J
and Hawkins JW: Postoperative pain: Acupuncture versus percutaneous
electrical nerve stimulation. Pain Manag Nurs. 13:150–156.
2012.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Chernyak GV and Sessler DI: Perioperative
acupuncture and related techniques. Anesthesiology. 102:1031–1049.
2005.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Yao Y, Zhao Q, Gong C, Wu Y and Chen Y,
Qiu L, Wu X and Chen Y: Transcutaneous electrical Acupoint
stimulation improves the postoperative quality of recovery and
analgesia after gynecological laparoscopic surgery: A randomized
controlled trial. Evid Based Complement Alternat Med.
2015(324360)2015.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Grech D, Li Z, Morcillo P, Kalyoussef E,
Kim DD, Bekker A and Ulloa L: Intraoperative low-frequency
electroacupuncture under general anesthesia improves postoperative
recovery in a randomized trial. J Acupunct Meridian Stud.
9:234–241. 2016.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Enwere EK, Kornaga EN, Dean M, Koulis TA,
Phan T, Kalantarian M, Köbel M, Ghatage P, Magliocco AM,
Lees-Miller SP and Doll CM: Expression of PD-L1 and presence of
CD8-positive T cells in pre-treatment specimens of locally advanced
cervical cancer. Mod Pathol. 30:577–586. 2017.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Schreiber RD, Old LJ and Smyth MJ: Cancer
immunoediting: Integrating immunity's roles in cancer suppression
and promotion. Science. 331:1565–1570. 2011.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Bradley LM, Dalton DK and Croft M: A
direct role for IFN-gamma in regulation of Th1 cell development. J
Immunol. 157:1350–1358. 1996.PubMed/NCBI
|
|
21
|
Liao W, Lin JX and Leonard WJ: IL-2 family
cytokines: New insights into the complex roles of IL-2 as a broad
regulator of T helper cell differentiation. Curr Opin Immunol.
23:598–604. 2011.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Ellyard JI, Simson L and Parish CR:
Th2-mediated anti-tumour immunity: Friend or foe? Tissue Antigens.
70:1–11. 2007.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Han YF, Zhao J, Ma LY, Yin JH, Chang WJ,
Zhang HW and Cao GW: Factors predicting occurrence and prognosis of
hepatitis-B-virus-related hepatocellular carcinoma. World J
Gastroenterol. 17:4258–4270. 2011.PubMed/NCBI View Article : Google Scholar
|
|
24
|
International Conference on Harmonization
of Technical Requirements for Registration of Pharmaceuticals for
Human Use. ICH Harmonized Tripartite Guideline. [Accessed May 12,
2015]; Guideline for Good Clinical Practice E6(R1) 1996 Available
from: http://www.ich.org/fileadmin/Public_Web_Site/ICH_Products/Guidelines/Efficacy/E6/E6_R1_Guideline.pdf.
|
|
25
|
American Society of Anesthesiologists: ASA
physical status classification system. [Accessed 23 Nov 2018]
Available from: https://www.asahq.org/resources/ American Society of
Anesthesiologists.
|
|
26
|
Huang S, Peng W, Tian X, Liang H, Jia Z,
Lo T, He M and Feng Y: Effects of transcutaneous electrical
acupoint stimulation at different frequencies on perioperative
anesthetic dosage, recovery, complications, and prognosis in
video-assisted thoracic surgical lobectomy: A randomized,
double-blinded, placebo-controlled trial. J Anesth. 31:58–65.
2017.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Tu Q, Gan J, Shi J, Yu H, He S and Zhang
J: Effect of transcutaneous electrical acupoint stimulation on
postoperative analgesia after ureteroscopic lithotripsy: A
randomized controlled trial. Urolithiasis. 47:279–287.
2019.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Qu F, Li R, Sun W, Lin G, Zhang R, Yang J,
Tian L, Xing GG, Jiang H, Gong F, et al: Use of electroacupuncture
and transcutaneous electrical acupoint stimulation in reproductive
medicine: A group consensus. J Zhejiang Univ Sci B. 18:186–193.
2017.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Tu Q, Yang Z, Gan J, Zhang J, Que B, Song
Q and Wang Y: Transcutaneous electrical acupoint stimulation
improves immunological function during the perioperative period in
patients with non-small cell lung cancer undergoing video-assisted
thoracic surgical lobectomy. Technol Cancer Res Treat: Jan 1, 2018
(Epub ahead of print). doi: 10.1177/1533033818806477.
|
|
30
|
Oliveira CR, Bernardo WM and Nunes VM:
Benefit of general anesthesia monitored by bispectral index
compared with monitoring guided only by clinical parameters.
Systematic review and meta-analysis. Braz J Anesthesiol. 67:72–84.
2017.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Huskisson E: Measurement of pain. Lancet.
2:1127–1131. 1974.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Cata JP, Bauer M, Sokari T, Ramirez MF,
Mason D, Plautz G and Kurz A: Effects of surgery, general
anesthesia, and perioperative epidural analgesia on the immune
function of patients with non-small cell lung cancer. J Clin
Anesth. 25:255–262. 2013.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Fiona Desmond J, McCormack N, Mulligan M,
Stokes and Donal JB: Effect of anesthetic technique on
immune cell infiltrationin breast cancer: A follow-up pilot
analysis of a prospective, randomised, investigator-masked study.
Anticancer Res. 35:1311–1319. 2015.PubMed/NCBI
|
|
34
|
Buckley A, McQuaid S, Johnson P and Buggy
DJ: Effect of anaesthetic technique on the natural killer cell
anti-tumour activity of serum from women undergoing breast cancer
surgery: A pilot study. Br J Anaesth. 113 (Suppl 1):i56–i62.
2014.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Pérez-González O, Cuéllar-Guzmán LF, Soliz
J and Cata JP: Impact of regional anesthesia on recurrence,
metastasis, and immune response in breast cancer surgery: A
systematic review of the literature. Reg Anesth Pain Med.
42:751–756. 2017.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Kim SY, Kim NK, Baik SH, Min BS, Hur H,
Lee J, Noh HY, Lee JH and Koo BN: Effects of postoperative pain
management on immune function after laparoscopic resection of
colorectal cancer: A Randomized Study. Medicine (Baltimore).
95(e3602)2016.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Fallon M, Giusti R, Aielli F, Hoskin P,
Rolke R, Sharma M and Ripamonti CI: ESMO Guidelines Committee:
Electronic address: Clinicalguidelines@esmo.org. Management of
cancer pain in adult patients: ESMO Clinical Practice Guidelines.
Ann Oncol. 29 (Suppl 4):iv166–iv191. 2018.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Suzuki M, Sakurada T, Gotoh K, Watanabe S
and Satoh N: Correlation between the administration of morphine or
oxycodone and the development of infections in patients with cancer
pain. Am J Hosp Palliat Care. 30:712–716. 2013.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Sacerdote P, Bianchi M, Gaspani L,
Manfredi B, Maucione A, Terno G, Ammatuna M and Panerai AE: The
effects of tramadol and morphine on immune responses and pain after
surgery in cancer patients. Anesth Analg. 90:1411–1414.
2000.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Narahara H, Kadoi Y, Hinohara H, Kunimoto
F and Saito S: Comparative effects of flurbiprofen and fentanyl on
natural killer cell cytotoxicity, lymphocyte subsets and cytokine
concentrations in post-surgical intensive care unit patients:
Prospective, randomized study. J Anesth. 27:676–683.
2013.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Chen Y, Wu W, Yao Y, Yang Y, Zhao Q and
Qiu L: Transcutaneous electric acupoint stimulation at Jiaji points
reduce abdominal pain after colonoscopy: A randomized controlled
trial. Int J Clin Exp Med. 8:5972–5977. 2015.PubMed/NCBI
|
|
42
|
Bjordal JM, Johnson MI and Ljunggreen AE:
Transcutaneous electrical nerve stimulation (TENS) can reduce
postoperative analgesic consumption. A meta-analysis with
assessment of optimal treatment parameters for postoperative pain.
Eur J Pain. 7:181–188. 2003.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Liang Y, Bao G, Gong L, Zhou J, Kong X,
Ran R, Shao X, Jiang Y, Zhang W, Liu B, et al: Evaluating the
analgesic effect and advantage of transcutaneous electrical
acupoint stimulation combined with opioid drugs for moderate to
severe cancer-related pain: A study protocol for a randomized
controlled trial. Trials. 20(40)2019.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Lee A and Fan LT: Stimulation of the wrist
acupuncture point P6 for preventing postoperative nausea and
vomiting. Cochrane Database Syst Rev: April 15, 2009 (Epub ahead of
print). doi: 10.1002/14651858.CD003281.pub.
|
|
45
|
Cooke M, Rickard C, Rapchuk I, Shekar K,
Marshall AP, Comans T, Doi S, McDonald J and Spooner A: PC6
acupoint stimulation for the prevention of postcardiac surgery
nausea and vomiting: A protocol for a two-group, parallel,
superiority randomised clinical trial. BMJ Open.
4(e006179)2014.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Lee S, Lee MS, Choi DH and Lee SK:
Electroacupuncture on PC6 prevents opioid-induced nausea and
vomiting after laparoscopic surgery. Chin J Integr Med. 19:277–281.
2013.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Shen YF and Goddard G: The short-term
effects of acupuncture on myofascial pain patients after clenching.
Pain Pract. 7:256–264. 2007.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Witt CM, Reinhold T, Jena S, Brinkhaus B
and Willich SN: Cost-effectiveness of acupuncture treatment in
patients with headache. Cephalalgia. 28:334–345. 2008.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Lai M, Wang SM, Wang Y, Tang CL, Kong LW
and Xu XY: Effects of electroacupuncture of ‘Zusanli’ (ST 36),
‘Hegu’ (LI 4) and/or ‘Sanyinjiao’ (SP 9) on immunofunction in
gastric carcinectomy rats. Zhen Ci Yan Jiu. 33:245–249.
2008.PubMed/NCBI(In Chinese).
|
|
50
|
Yim YK, Lee H, Hong KE, Kim YI, Lee BR,
Son CG and Kim JE: Electro-acupuncture at acupoint ST36 reduces
inflammation and regulates immune activity in Collagen-Induced
Arthritic Mice. Evid Based Complement Alternat Med. 4:51–57.
2007.PubMed/NCBI View Article : Google Scholar
|
|
51
|
Kagitani F, Uchida S, Hotta H and Aikawa
Y: Manual acupuncture needle stimulation of the rat hindlimb
activates groups I, II, III and IV single afferent nerve fibers in
the dorsal spinal roots. Jpn J Physiol. 55:149–155. 2005.PubMed/NCBI View Article : Google Scholar
|
|
52
|
Han JS: Acupuncture: Neuropeptide release
produced by electrical stimulation of different frequencies. Trends
Neurosci. 26:17–22. 2003.PubMed/NCBI View Article : Google Scholar
|
|
53
|
Acar HV: Acupuncture and related
techniques during perioperative period: A literature review.
Complement Ther Med. 29:48–55. 2016.PubMed/NCBI View Article : Google Scholar
|
|
54
|
Zhao ZQ: Neural mechanism underlying
acupuncture analgesia. Prog. Neurobiol. 85:355–375. 2008.PubMed/NCBI View Article : Google Scholar
|
|
55
|
Lin L, Skakavac N, Lin X, Lin D, Borlongan
MC, Borlongan CV and Cao C: Acupuncture-induced analgesia: The role
of microglial inhibition. Cell Transplant. 25:621–628.
2016.PubMed/NCBI View Article : Google Scholar
|
|
56
|
Goldman N, Chen M, Fujita T, Xu Q, Peng W,
Liu W, Jensen TK, Pei Y, Wang F, Han X, et al: Adenosine A1
receptors mediate local anti-nociceptive effects of acupuncture.
Nat Neurosci. 13:883–888. 2010.PubMed/NCBI View Article : Google Scholar
|
|
57
|
Chen L, Xu A, Yin N, Zhao M, Wang Z, Chen
T, Gao Y and Chen Z: Enhancement of immune cytokines and splenic
CD4+ T cells by electroacupuncture at ST36 acupoint of
SD rats. PLoS One. 12(e0175568)2017.PubMed/NCBI View Article : Google Scholar
|
|
58
|
Wang K, Wu H, Wang G, Li M, Zhang Z and Gu
G: The effects of electroacupuncture on TH1/TH2 cytokine mRNA
expression and mitogen-activated protein kinase signaling pathways
in the splenic T cells of traumatized rats. Anesth Analg.
109:1666–1673. 2009.PubMed/NCBI View Article : Google Scholar
|
|
59
|
Wu H, Wang K, Li G, Meng D, Han J, Wang G
and Li YU: Effects of transcutaneous acupoint electrical
stimulation on the imbalance of Th1, Th2,
Th17 and Treg cells following thoracotomy of
patients with lung cancer. Exp Ther Med. 11:495–502.
2016.PubMed/NCBI View Article : Google Scholar
|
|
60
|
Li G, Li S, Wang B and An L: The effect of
electroacupuncture on postoperative immunoinflammatory response in
patients undergoing supratentorial craniotomy. Exp Ther Med.
6:699–702. 2013.PubMed/NCBI View Article : Google Scholar
|
|
61
|
Hao CJ, Li J, Liu P, Li XL, Hu YQ, Sun JC
and Wei Y: Effects of the balance between type 1 and type 2 T
helper cells on ovarian cancer. Genet Mol Res: Jun 3, 2016 (Epub
ahead of print). doi: 10.4238/gmr.15027936.
|
|
62
|
Woo JH, Baik HJ, Kim CH, Chung RK, Kim DY,
Lee GY and Chun EH: Effect of propofol and desflurane on immune
cell populations in breast cancer patients: A randomized trial. J
Korean Med Sci. 30:1503–1508. 2015.PubMed/NCBI View Article : Google Scholar
|
|
63
|
Su TF, Zhang LH, Peng M, Wu CH, Pan W,
Tian B, Shi J, Pan HL and Li M: Cannabinoid CB2 receptors
contribute to upregulation of β-endorphin in inflamed skin tissues
by electroacupuncture. Mol Pain. 7(98)2011.PubMed/NCBI View Article : Google Scholar
|
|
64
|
Cabioğlu MT and Cetin BE: Acupuncture and
immunomodulation. Am J Chin Med. 36:25–36. 2008.PubMed/NCBI View Article : Google Scholar
|