Introduction
Gestational diabetes mellitus (GDM) is a type of
diabetes characterized by abnormal insulin deficiency and
pancreatic β cell dysfunction that occurs in pregnant females with
a prevalence of 6-20% worldwide (1,2). It is
one of the most common diseases that occurs during pregnancy,
normally leading to serious complications, such as cardiovascular
illness, type 2 diabetes and obesity, as well as an increase in
fetal morbidity and mortality (3,4).
During the process of GDM, insulin secretion is defective and
pancreatic β cells are unable to compensate for insulin resistance,
inevitably leading to hyperglycemia (5). Recently, lifestyle habits, including
diet and physical activity, have been suggested as key factors that
can prevent the symptoms and development of GDM in females
(6). However, in patients with GDM
where lifestyle management cannot result in the recommended
glycemia, additional therapeutic treatments, including exercise and
insulin therapy, are required to control GDM (7,8).
Therefore, it is crucial to identify additional therapeutic
strategies for GDM.
Dysfunction of pancreatic β-cells have been reported
to be a central component in the pathogenesis and development of
GDM (9,10). Reduction of pancreatic β cell
function is characterized by increased rates of cell apoptosis
(11) and defects in insulin
generation and secretion (12).
During pregnancy, insulin resistance results in increments of the
insulin requirement (13), while
insulin secretion by β cells is reduced under the stimulation of
prolactin and placental lactogens, and fetuin generation and
secretion (12). During pregnancy,
insulin resistance results in increments of the insulin requirement
(13), while insulin secretion by β
cells is reduced under the stimulation of prolactin, placental
lactogens and fetal growth (14-16).
It was shown that hyperglycemia participated in the induction of
pancreatic β cell apoptosis (17)
and insulin secretion (18).
Therefore, investigating preventive mechanisms that can maintain
glucose homeostasis by protecting and improving the functions of
pancreatic β cells is a significant research topic in the field of
maternal medicine. However, to the best of our knowledge, the
cellular and molecular mechanisms of pancreatic β cell dysfunction
remain to be elucidated.
TP53-regulated inhibitor of apoptosis 1 (TRIAP1) is
a small conserved protein containing 76 amino acids that was
initially identified as a p53-induced cell survival factor
(19,20). It was previously reported that
TRIAP1 results in cell death resistance via a
mitochondrial-dependent pathway (21,22).
It was also shown that TRIAP1 can modulate apoptotic pathways by
interacting with Hsp70-binding protein 1, prevent the formation of
apoptotic protease-activating factor 1 (APAF1), release of
cytochrome c and induction of caspase-9, which reduced the
formation of the apoptosome complex (23). These molecular events lead to
insulin resistance by suppressing apoptosis and allowing DNA damage
repair (23). Due to the
significant function of TRIAP1 in apoptosis and the interaction
between pancreatic β cell apoptosis and GDM, the current study
explored the involvement of TRIAP1 in the regulation of GDM.
Materials and methods
Clinical sample collection
Peripheral blood samples were collected from 30
female patients with GDM (age range, 24-37 years) and 30 female
subjects that were undergoing healthy pregnancies (age range, 23-36
years) at the Obstetrics and Gynecology Hospital of Fudan
University Hospital (Shanghai, China) between December 2017 and
December 2018. The blood samples were separated by centrifugation
(1,000 x g for 10 min at 4˚C) and stored in liquid nitrogen
instantly for subsequent experiments. There were no statistically
significant differences in age, gestational week and weight between
patients with GDM and subjects with healthy pregnancies. The
present study was approved by the Ethics Committee of the
Obstetrics and Gynecology Hospital of Fudan University, and written
informed consent was acquired from all participants prior to their
enrollment.
Females with a fasting plasma glucose (FPG) level of
≥4.4 mmol/l but ≤5.1 mmol/l underwent a 75 g oral glucose tolerance
test (OGTT). In such cases, a diagnosis of GDM was made when at
least one glucose value was elevated (FPG ≥5.1 mmol/l, 1-h OGTT
≥10.0 mmol/l or 2-h OGTT ≥8.5 mmol/l). Pregnant females with the
following conditions were excluded from the present study: i)
αbnormal blood lipid (low-density lipoprotein cholesterol,
triglyceride and high-density lipoprotein cholesterol) levels,
hypertension and chronic kidney and liver diseases; ii) endocrine
diseases, including obesity, diabetes, thyroid disease,
osteoporosis, adrenal cortical disease and hyperthyroidism prior to
pregnancy; iii) pregnant females currently undergoing long-term
drug treatments (such as sodiumlevothyroxine) and iv) other
pregnancy complications (pre-eclampsia, pregnancy-induced
hypertension or pregnancy with chronic nephritis).
Cell culture and transfection
INS-1 cells were obtained from American Type Culture
Collection. The cells were cultured and maintained in RPMI-1640
medium (HyClone; Cytiva) supplemented with 10% fetal bovine serum
(HyClone; Cytiva), 11.1 mmol/l glucose, 50 mM b-mercaptoethanol and
1% penicillin/streptomycin. The cells were maintained in 5%
CO2 at 37˚C.
TRIAP1-small interfering RNA (siRNA; 0.2 µM; 5'-AGG
CAUGCACGGACAUGAATT-3') or control-siRNA (0.2 µM;
5'-GCACCACGTGACGGAGCGT-3'), 1 µg TRIAP1 CRISPR activation plasmid
(cat. no. sc-427287-ACT) or control-plasmid (cat no. sc-437275)
were purchased from Santa Cruz Biotechnology, Inc.
Lipofectamine® 2000 transfection reagent (Invitrogen;
Thermo Fisher Scientific, Inc.) was used for transfection according
to the manufacturer's instructions. Following transfection, the
cells were cultured for 48 h and collected for further experiments.
Cells without any treatment were used as the control.
Reverse transcription-quantitative PCR
(RT-qPCR)
Total RNA was isolated from peripheral blood and
INS-1 cells using TRIzol® reagent (Invitrogen; Thermo
Fisher Scientific, Inc.). RNA concentration was detected using a
NanoDrop ND-1000 spectrophotometer (Thermo Fisher Scientific, Inc.)
at wavelengths of 260 and 180 nm. Subsequently, 800 ng RNA was
reversed transcribed into cDNA using HiScriptTM Q RT SuperMix
(Vazyme Biotech Co., Ltd.). The following temperature conditions
for reverse transcription were as follows: 70˚C for 5 min, 37˚C for
5 min and 42˚C for 60 min. qPCR was performed using an ABI Prism
7500 Real-Time PCR System (Applied Biosystems; Thermo Fisher
Scientific, Inc.) with the SYBR-Green Real-Time PCR kit (Toyobo
Life Science) for the detection of RNA expression levels. The
following thermocycling conditions were used for the qPCR: Initial
denaturation for 5 min at 95˚C, followed by 35 cycles of
denaturation at 94˚C for 1 min, annealing at 60˚C for 1 min and
extension at 72˚C for 1 min, followed by a final extension step at
72˚C for 10 min. GAPDH was used as the internal control and the
relative expression levels of the transcripts were calculated using
the 2-ΔΔCq method (24).
Primer sequences were listed as following: TRIAP1 forward,
5'-GCACCGACCTCTTCA AGC-3' and reverse, 5'-CCATGAACTCCAGTCCTTCA3';
GAPDH forward, 5'-CTTTGGTATCGTGGAAGGACTC-3' and reverse,
5'-GTAGAGGCAGGGATGATGTTCT-3'; APAF1 forward,
5'-AACCAGGATGGGTCACCATA-3' and reverse, 5'-ACTGAAACCCAATGCACTCC-3';
caspase-3 forward, 5'-AGAACTGGACTGTGGCATTG-3' and reverse,
5'-CACAAAGCGACTGGATGAAC-3'; caspase-7 forward,
5'-CTACCGCCGTGGGAACGATGGCAGA-3' and reverse,
5'-CGAAGGCCCATACCTGTCACTTTATC-3' and caspase-9 forward,
5'-TTCCCAGGTTTTGTTTCCTG-3' and reverse,
5'-CCTTTCACCGAAACAGCATT-3'.
Western blot analysis
To obtain total protein, INS-1 cells and peripheral
blood samples were lysed with RIPA buffer containing proteinase
inhibitors (Beyotime Institute of Biotechnology) for 30 min and
subsequently centrifuged at 12,000 x g for 3 min at 4˚C. Protein
concentration was determined using a bicinchoninic acid protein
assay kit (Beyotime Institute of Biotechnology). Subsequently, the
proteins were mixed with 4X loading buffer, boiled at 95˚C for 10
min and centrifuged at 10,000 x g at 4˚C for 1 min. Equal amounts
of protein (20 µg) were loaded on 10% SDS gels and analyzed by
PAGE. The gels were transferred to PVDF membranes, and the
membranes were blocked with 5% non-fat milk for 1 h at room
temperature. The membranes were washed with PBS-0.1% Tween-20
(PBST) three times and subsequently incubated overnight at 4˚C with
the following primary antibodies: TRIAP1 (cat. no. 515801; 1:1,000;
Santa Cruz Biotechnology, Inc.), APAF1 (cat. no. 8723; 1:1,000;
Cell Signaling Technology, Inc.), caspase-3 (cat. no. 14220;
1:1000; Cell Signaling Technology, Inc.), caspase-7 (cat. no.
12827; 1:1,000; Cell Signaling Technology, Inc.), caspase-9 (cat.
no. 9508; 1:1,000; Cell Signaling Technology, Inc.) and GAPDH (cat.
no. 5174; 1:1,000; Cell Signaling Technology, Inc.). The following
day, the membranes were washed with PBST four times and
subsequently incubated with horseradish peroxidase-conjugated
anti-rabbit immunoglobulin G secondary antibody (cat. no. 7074;
1:2,000; Cell Signaling Technology, Inc.) for 1 h at room
temperature. Finally, protein bands were visualized using the ECL
Western blot substrate (Pierce; Thermo Fisher Scientific, Inc.) and
the relative band intensities were analyzed using ImageJ software
version 1.48u (National Institutes of Health).
Cell viability assay
INS-1 cells were transfected with TRIAP1-siRNA,
control-siRNA, TRIAP1-plasmid and control-plasmid, and then seeded
into 96-well plates at a density of 1x104 cells/well. Following
incubation for 48 h at 37˚C, 10 µl MTT solution (5 mg/ml;
Sigma-Aldrich; Merck KGaA) was added to each well and the cells
were cultured for 4 h at 37˚C. Following incubation, DMSO (200 µl;
Sigma-Aldrich; Merck KGaA) was added to each well and the plate was
shaken for 10 min. Finally, the absorbance was measured using a Neo
Multi-Mode Reader (BioTek Instruments, Inc.) at a wavelength of 570
nm.
Flow cytometry analysis
To detect cell apoptosis, INS-1 cells were
transfected with TRIAP1-siRNA, control-siRNA, TRIAP1-plasmid and
control-plasmid, and then cultured for 48 h. Following
transfection, the Annexin V/PI Cell Apoptosis Detection kit
(Nanjing KeyGen Biotech Co., Ltd) was used to determine the
percentage of apoptotic cells. A total of 1x106 INS-1
cells were harvested and resuspended in 500 μl binding buffer
containing 5 µl Annexin V and 5 µl propidium iodide. The resulting
solution was incubated for 15 min in the dark and the BD
FACSCalibur™ flow cytometer (BD Biosciences) was used to evaluate
the fluorescence intensity. Data were analyzed using the CellQuest™
version 5.1 software (BD Biosciences).
Insulin secretion detection
INS-1 cells were transfected with TRIAP1-siRNA,
control-siRNA, TRIAP1-plasmid and control-plasmid, and then
cultured for 48 h. Following transfection, the cells were cultured
with normal glucose (3.3 mM) or high glucose (16.7 mM) for 1 h.
Subsequently, the total insulin content was extracted following
sonication of the cells in acid ethanol (2%
H2SO4 in 75% ethanol), accompanied with three
cycles of freezing and thawing. The cells were centrifuged at 500 x
g at 4˚C for 5 min and the supernatant was used to determine
insulin release levels using ELISA kit (cat. no. PI606; Beyotime
Institute of Biotechnology) according to the manufacturer's
instructions.
Statistical analysis
Statistical analyses were performed with SPSS 21.0
software (IBM Corp.). The derived values were expressed as the mean
± SD. One-way ANOVA with Tukey's post hoc test was used for
comparisons between multiple groups. Unpaired Student's t-test was
used to analyze the difference between two groups. P<0.05 was
considered to indicate a statistically significant difference.
Results
TRIAP1 expression is downregulated in
peripheral blood samples from patients with GDM
To examine the pathological relevance of TRIAP1 in
GDM, the expression levels of TRIAP1 was detected in peripheral
blood samples obtained from 30 patients with GDM and subjects with
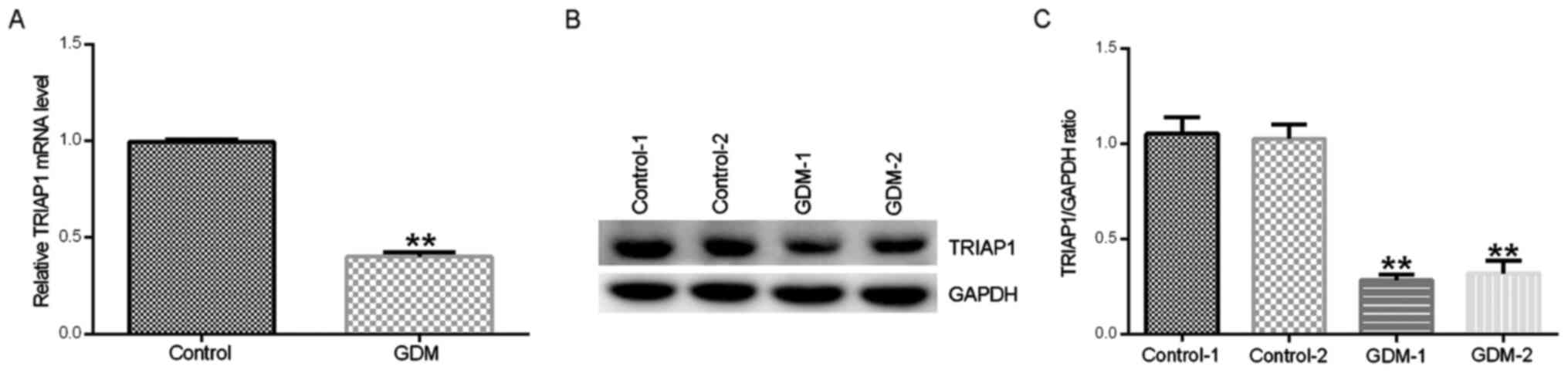
healthy pregnancies by RT-qPCR and western blot assays. As shown in
Fig. 1A-C, the mRNA and protein
expression levels of TRIAP1 in peripheral blood samples from
patients with GDM were significantly lower compared with samples
from healthy pregnancies.
Downregulation of TRIAP1 suppresses
insulin secretion and total insulin content
To determine whether TRIAP1 is involved in
regulating pancreatic β cell function, control-siRNA and
TRIAP1-siRNA were transfected into INS-1 cells and the transfection
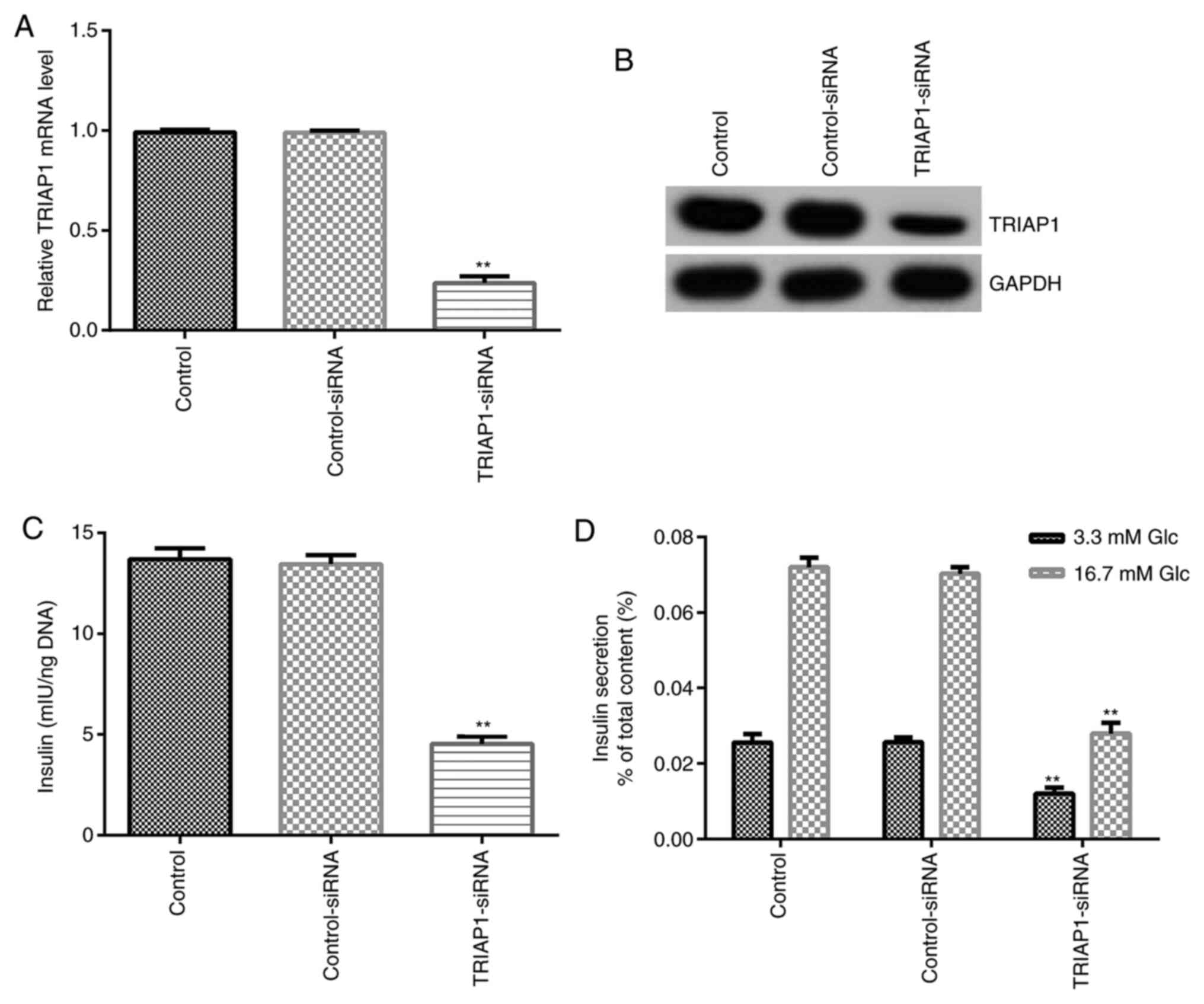
efficiency was confirmed by RT-qPCR and western blot assays. As
shown in Fig. 2A and B, transfection of cells with TRIAP1-siRNA
significantly downregulated the mRNA and protein expression levels
of TRIAP1 compared with the control-siRNA group. In addition, the
results presented in Fig. 2C
demonstrated that downregulation of TRIAP1 could inhibit the total
insulin content in INS-1 cells compared with control-siRNA group.
It has been frequently reported that high glucose can promote
insulin secretion (25). In the
present study, the data showed that downregulation of TRIAP1
significantly inhibited insulin release under high and low glucose
conditions in INS-1 cells compared with control-siRNA groups
(Fig. 2D). The aforementioned
results indicated that downregulation of TRIAP1 expression
suppressed total insulin content and reduced insulin release in
INS-1 cells under glucose stimulation.
Downregulation of TRIAP1 effects INS-1
cell viability and apoptosis
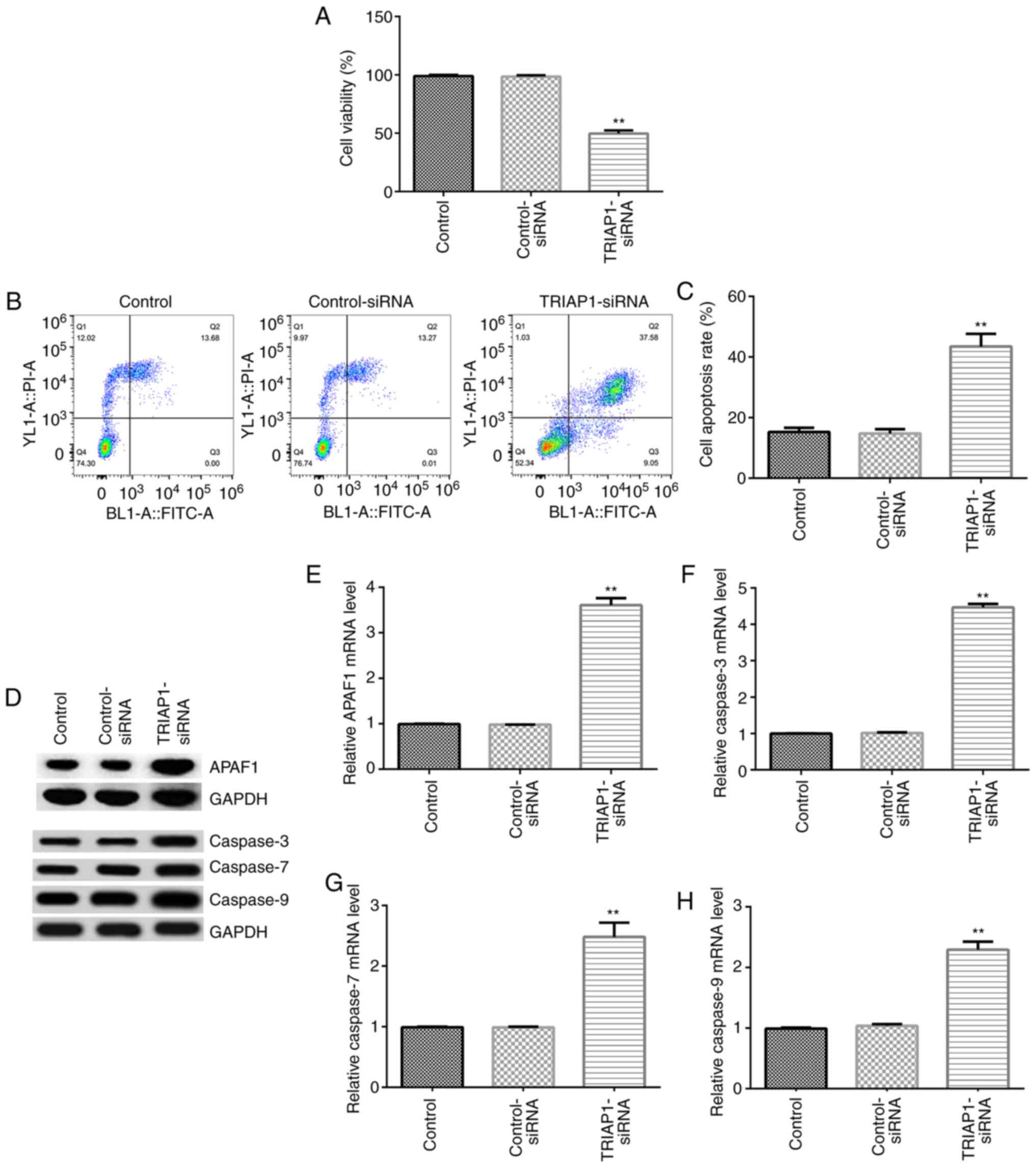
Subsequently, the effects of TRIAP1 on the viability
and apoptosis of INS-1 cells were investigated. Control-siRNA and
TRIAP1-siRNA were transfected into INS-1 cells for 48 h, and MTT
and flow cytometry analyses were performed to detect the viability
and apoptosis of INS-1 cells, respectively. As shown in Fig. 3A-C, downregulation of TRIAP1
significantly decreased cell viability and significantly increased
the cell apoptotic population compared with their respective
control-siRNA groups. Furthermore, the expression levels of cell
apoptosis-associated genes, including APAF1, caspase-3, caspase-7
and caspase-9, were determined by RT-qPCR and western blotting. The
findings demonstrated that the protein and mRNA expression levels
of APAF1, caspase-3, caspase-7 and caspase-9 were significantly
increased in the TRIAP1-siRNA-transfected group compared with the
control-siRNA group (Fig. 3D-H).
The aforementioned results indicated that TRIAP1-siRNA suppressed
insulin secretion by inhibiting cell viability, promoting cell
apoptosis and regulating the expression of apoptosis-associated
genes in INS-1 cells.
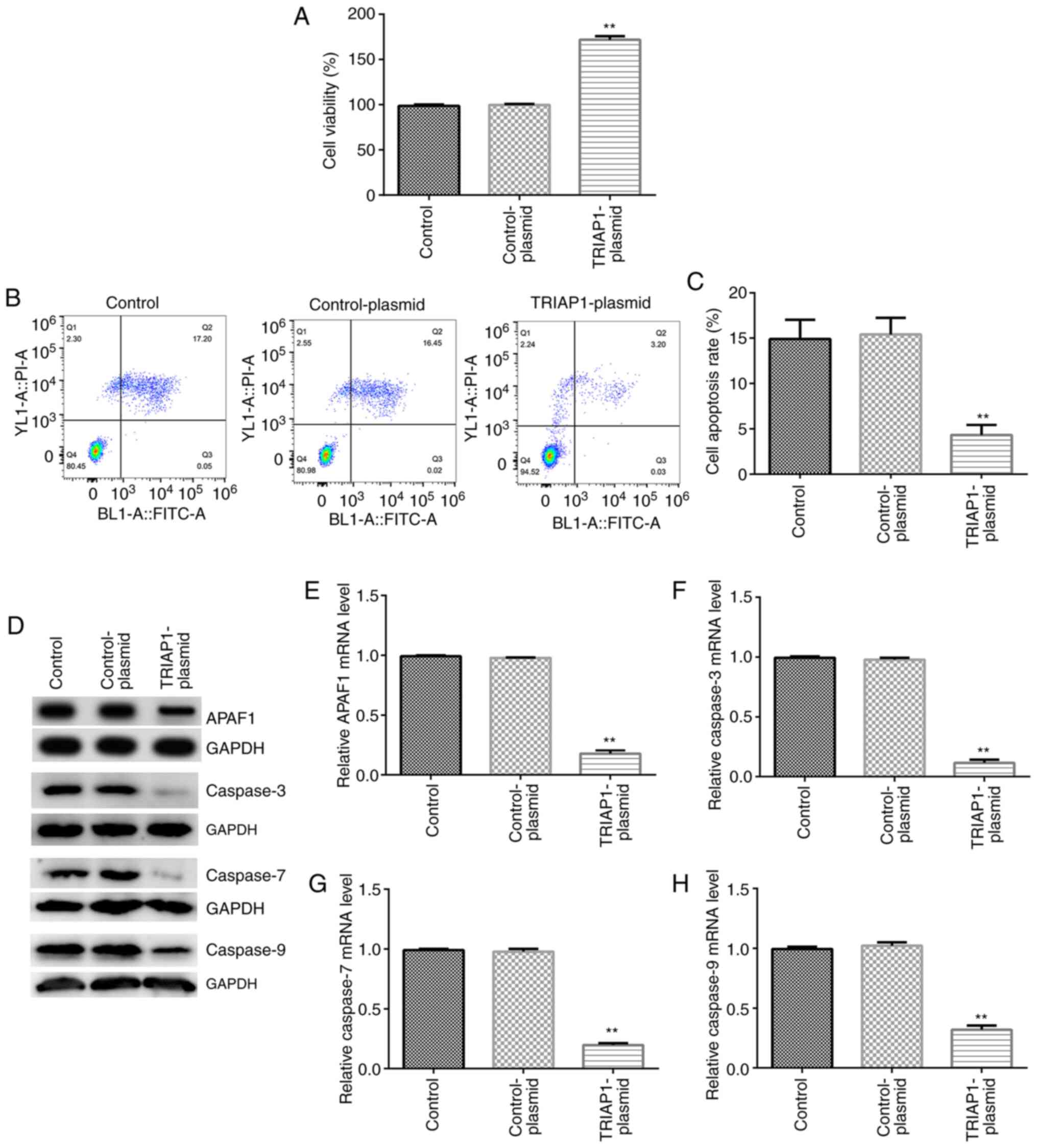
Upregulation of TRIAP1 promotes
insulin secretion and total insulin content
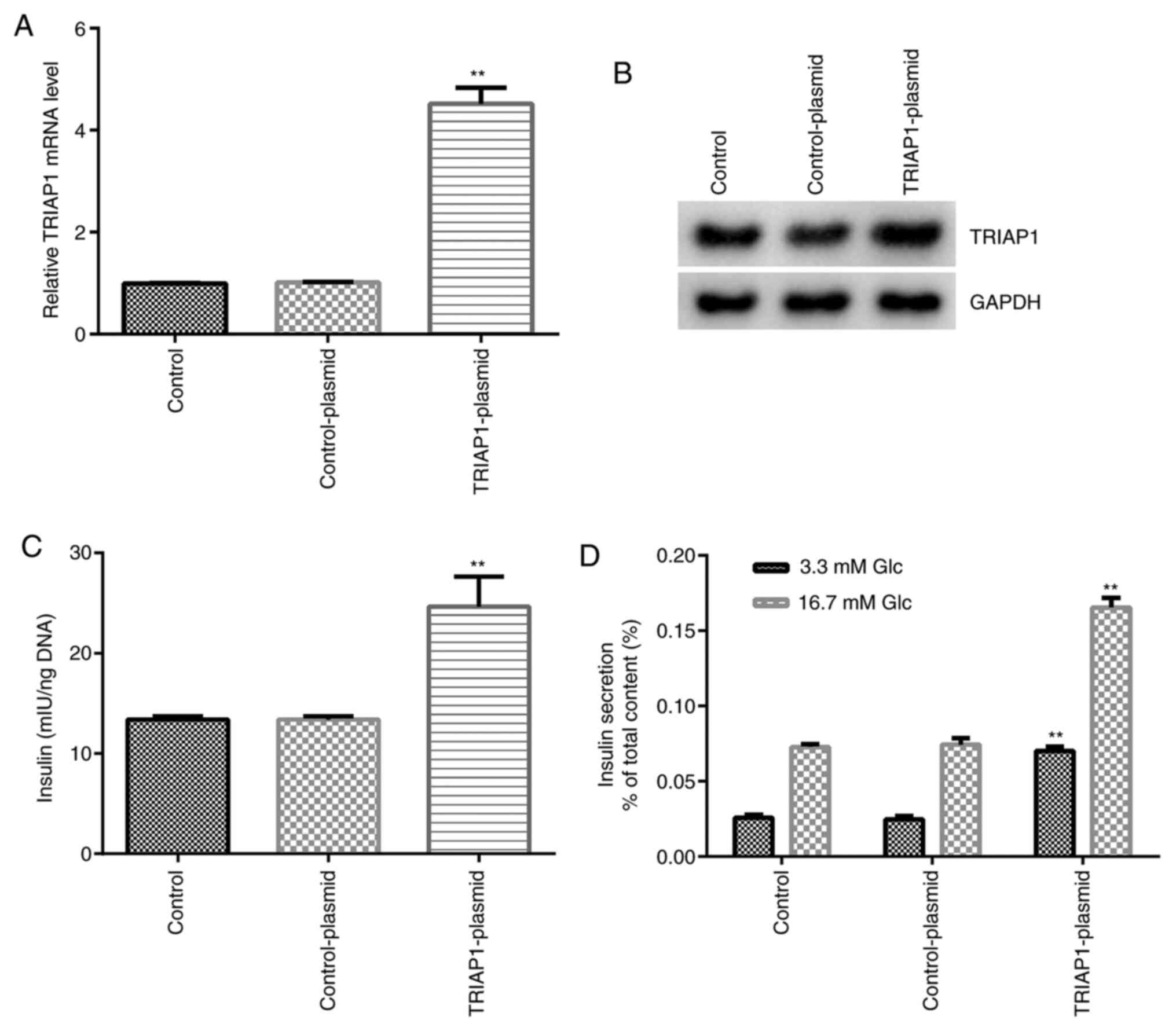
Subsequently, the regulation of TRIAP1 expression
was investigated with regard to the metabolic function of
pancreatic β cells. INS-1 cells were transfected with
TRIAP1-plasmid and control-plasmid and the transfection efficiency
was evaluated by RT-qPCR and western blot assays. As shown in
Fig. 4A and B, the mRNA and protein expression levels
of TRIAP1 were significantly increased in the TRIAP1-plasmid group
compared with the control-plasmid group. The results also
demonstrated that upregulation of TRIAP1 expression could
significantly increase the total insulin content in INS-1 cells
compared with control-plasmid group (Fig. 4C). Upregulation of TRIAP1 expression
further significantly promoted insulin secretion under high and low
glucose conditions in INS-1 cells compared with the control-plasmid
group (Fig. 4D). These data suggest
that TRIAP1 serves an essential role in regulating insulin
secretion of pancreatic β cells.
Upregulation of TRIAP1 influences of
INS-1 cell viability and apoptosis
To further investigate the role of TRIAP1 in the
regulation of proliferation and apoptosis of INS-1 cells,
control-plasmid and TRIAP1-plasmids were transfected into the cells
for 48 h. MTT assay and flow cytometry analysis indicated that
upregulation of TRIAP1 significantly increased cell viability and
significantly decreased apoptosis of INS-1 cells (Fig. 5A-C) compared with the
control-plasmid groups. Western blotting and RT-qPCR results
demonstrated that the protein and mRNA expression levels of APAF1,
caspase-3, caspase-7 and caspase-9 were significantly decreased in
the TRIAP1-plasmid-transfected group compared with the
control-plasmid group (Fig. 5D-H).
Taken together, these findings demonstrated that TRIAP1 could
influence insulin secretion by regulating cell viability, cell
apoptosis and the expression levels of apoptosis-associated genes
in INS-1 cells.
Discussion
GDM is a common condition in which hyperglycemia
occurs in females during pregnancy (26), which usually leads to an enhanced
risk of adverse outcomes for mothers and newborns (27). It was reported that during
pregnancy, strict control of blood glucose can ameliorate potential
poor health outcomes of the mothers and newborns during pregnancy
(28). Individualized management,
including exercise, dietary guidance and encouragement for physical
activity, was suggested to improve glycemic control in patients
with GDM (29). When lifestyle
management fails to retain adequate glycemic levels, alternative
therapeutic strategies, such as exercise and insulin therapy, may
be used for GDM (7,8). Recently, a study demonstrated that
pancreatic β cell dysfunction plays an important role in the
pathogenesis of GDM (30). TRIAP1
is a small conserved protein containing 76 amino acids that is
considered a regulator of cell death (19,20).
However, the role of TRIAP1 in pancreatic β cells remains to be
investigated. Therefore, the aim of the present study was to assess
the regulatory effects of TRIAP1 in GDM and its functions in
pancreatic β cells. It was hypothesized that TRIAP1 played critical
roles in regulating pancreatic β cell function.
In the present study, RT-qPCR and western blot
assays were used to determine the levels of TRIAP1 in GDM, and the
data revealed that TRIAP1 expression was downregulated in
peripheral blood samples from patients with GDM compared with those
obtained from control subjects. Since the present study focused on
the investigation of TRIAP1 in GDM, a control group for patients
with diabetes was not included in the current study (31). However, the number of samples of the
present study is too small, and large sample needs to be included.
Additionally, the association between clinicopathological
characteristics of patients with GDM and TRIAP1 expression was not
analyzed in the present study. These were the limitations of the
present study and will be further studied in the future. To further
investigate the effects of TRIAP1 in GDM, TRIAP1-siRNA and
control-siRNA were transfected into INS-1 cells. The transfection
efficiency was confirmed by RT-qPCR and western blot assays, and the
results indicated that TRIAP1-siRNA significantly downregulated the
mRNA and protein expression levels of TRIAP1. Pancreatic β cells
are a distinct population of cells that produce insulin in order to
fulfill metabolic demands (32).
The lack of adaptation of pancreatic β cells to peripheral insulin
resistance may be the main cause of GDM (33,34).
Therefore, ELISA was performed to evaluate insulin secretion and
total insulin content in INS-1 cells under high and low glucose
conditions. ELISA results revealed that TRIAP1-siRNA significantly
reduced the total insulin content and insulin release in INS-1
cells. However, further confirm the results, observation of changes
in cell and insulin secretion by setting different time gradients
and concentration gradients in cell experiments is required, which
will be investigated this in the future.
A previous study demonstrated that inhibition of
TRIAP1 in RPMI8226 cells increased the proportion of apoptotic
cells, increased the expression levels of APAF1 and caspase-9, and
induced caspase-9 and caspase-3/7 activities (35). Therefore, the effects of TRIAP1
knockdown on the proliferation and apoptosis of INS-1 cells was
investigated in the present study. In line with previous studies
(19,35,36),
the results indicated that TRIAP1-siRNA may lead to the induction
of apoptosis and to the inhibition of INS-1 cell proliferation. The
data revealed that TRIAP1-siRNA promoted apoptosis of pancreatic β
cells. Furthermore, the results from the RT-qPCR and western blot
assays demonstrated that TRIAP1-siRNA significantly increased the
expression levels of the apoptosis-associated genes APAF1,
caspase-3, caspase-7 and caspase9 in INS-1 cells.
Accordingly, TRIAP1 overexpression enhanced insulin
secretion and total insulin content in INS-1 cells, as demonstrated
by transfection of the cells with TRIAP1-plasmid. In addition,
TRIAP1 overexpression significantly inhibited the induction of
INS-1 cell apoptosis and promoted the viability of INS-1 cells.
Moreover, the results demonstrated the repressive effects of TRIAP1
overexpression on the expression levels of APAF1, caspase-3,
caspase-7 and caspase-9 in INS-1 cells. The aforementioned findings
demonstrated that TRIAP1 expression was downregulated in patients
with GDM and that it may play an important role in pancreatic β
cell function by regulating insulin secretion, as well as
proliferation and apoptosis of pancreatic β cells.
Taken together, the present study revealed a novel
role of TRIAP1 in GDM. This protein may serve as a potential target
for the therapy of GDM. The present study revealed that TRIAP1
could regulate insulin secretion and the proliferation and
apoptosis of pancreatic β cells. This indicated a protective role
of TRIAP1 in GDM and suggested novel possibilities for the use of
targeted therapy against this disease. Although previous studies
have analyzed the predictive and diagnostic biomarkers for GDM
(37,38), however, whether TRIAP1 plays a
prognostic or diagnostic role in GDM needs further research, which
will be performed in the future.
In conclusion, TRIAP1 increased the growth of
pancreatic β cells and their ability to secrete insulin, which in
turn resulted in a protective effect in GDM. Therefore, TRIAP1 may
be considered a promising therapeutic target for GDM treatment.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
LXL contributed to the conception and design of the
study, data acquisition, analysis and interpretation. KHY, FY, YX
and LLC contributed to data acquisition and performed statistical
analysis. JS contributed to data acquisition, data analysis and
prepared the manuscript. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
The present study was approved by the Ethics
Committee of the Obstetrics and Gynecology Hospital of Fudan
University (Shanghai, China) and written informed consent was
acquired from all participants prior to their enrollment.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Zhen XM, Li X and Chen C: Longer-term
outcomes in offspring of GDM mothers treated with metformin versus
insulin. Diabetes Res Clin Pract. 144:82–92. 2018.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Shan Z, Xu C, Wang W and Li W: Enhanced
PDGF signaling in gestational diabetes mellitus is involved in
pancreatic β-cell dysfunction. Biochem Biophys Res Commun.
516:402–407. 2019.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Goueslard K, Cottenet J, Mariet AS, Giroud
M, Cottin Y, Petit JM and Quantin C: Early cardiovascular events in
women with a history of gestational diabetes mellitus. Cardiovasc
Diabetol. 15(15)2016.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Gunderson EP, Hurston SR, Dewey KG, Faith
MS, Charvat-Aguilar N, Khoury VC, Nguyen VT and Quesenberry CP Jr:
The study of women, infant feeding and type 2 diabetes after GDM
pregnancy and growth of their offspring (SWIFT Offspring study):
prospective design, methodology and baseline characteristics. BMC
Pregnancy Childbirth. 15(150)2015.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Wolpin BM, Bao Y, Qian ZR, Wu C, Kraft P,
Ogino S, Stampfer MJ, Sato K, Ma J, Buring JE, et al:
Hyperglycemia, insulin resistance, impaired pancreatic β-cell
function, and risk of pancreatic cancer. J Natl Cancer Inst.
105:1027–1035. 2013.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Malek R and Davis SN: Pharmacokinetics,
efficacy and safety of glyburide for treatment of gestational
diabetes mellitus. Expert Opin Drug Metab Toxicol. 12:691–699.
2016.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Aberg AEB, Jönsson EK, Eskilsson I,
Landin-Olsson M and Frid AH: Predictive factors of developing
diabetes mellitus in women with gestational diabetes. Acta Obstet
Gynecol Scand. 81:11–16. 2002.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Alfadhli EM: Gestational diabetes
mellitus. Saudi Med J. 36:399–406. 2015.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Plows JF, Stanley JL, Baker PN, Reynolds
CM and Vickers MH: The Pathophysiology of Gestational Diabetes
Mellitus. Int J Mol Sci. 19(3342)2018.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Li L, Wang S, Li H, Wan J, Zhou Q, Zhou Y
and Zhang C: microRNA-96 protects pancreatic β-cell function by
targeting PAK1 in gestational diabetes mellitus. Biofactors.
44:539–547. 2018.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Chen C, Luo Y, Su Y and Teng L: The
vitamin D receptor (VDR) protects pancreatic beta cells against
Forkhead box class O1 (FOXO1)-induced mitochondrial dysfunction and
cell apoptosis. Biomed Pharmacother. 117(109170)2019.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Mandelbaum AD, Kredo-Russo S, Aronowitz D,
Myers N, Yanowski E, Klochendler A, Swisa A, Dor Y and Hornstein E:
miR-17-92 and miR-106b-25 clusters regulate beta cell mitotic
checkpoint and insulin secretion in mice. Diabetologia.
62:1653–1666. 2019.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Vejrazkova D, Vcelak J, Vankova M,
Lukasova P, Bradnova O, Halkova T, Kancheva R and Bendlova B:
Steroids and insulin resistance in pregnancy. J Steroid Biochem Mol
Biol. 139:122–129. 2014.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Hill DJ: Placental control of metabolic
adaptations in the mother for an optimal pregnancy outcome What
goes wrong in gestational diabetes? Placenta. 69:162–168.
2018.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Brelje TC, Bhagroo NV, Stout LE and
Sorenson RL: Beneficial effects of lipids and prolactin on insulin
secretion and beta-cell proliferation: A role for lipids in the
adaptation of islets to pregnancy. J Endocrinol. 197:265–276.
2008.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Boehmer BH, Brown LD, Wesolowski SR, Hay
WW Jr and Rozance PJ: Pulsatile hyperglycemia increases insulin
secretion but not pancreatic β-cell mass in intrauterine
growth-restricted fetal sheep. J Dev Orig Health Dis. 9:492–499.
2018.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Weng Q, Zhao M, Zheng J, Yang L, Xu Z,
Zhang Z, Wang J, Wang J, Yang B, Richard Lu Q, et al: STAT3
dictates β-cell apoptosis by modulating PTEN in
streptozocin-induced hyperglycemia. Cell Death Differ. 27:130–145.
2020.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Li T, Ni L, Zhao Z, Liu X, Lai Z, Di X,
Xie Z, Song X, Wang X, Zhang R, et al: Melatonin attenuates
smoking-induced hyperglycemia via preserving insulin secretion and
hepatic glycogen synthesis in rats. J Pineal Res.
64(e12475)2018.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Adams C, Cazzanelli G, Rasul S, Hitchinson
B, Hu Y, Coombes RC, Raguz S and Yagüe E: Apoptosis inhibitor
TRIAP1 is a novel effector of drug resistance. Oncol Rep.
34:415–422. 2015.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Ketteler J, Panic A, Reis H, Wittka A,
Maier P, Herskind C, Yagüe E, Jendrossek V and Klein D:
Progression-related loss of stromal caveolin 1 levels mediates
radiation resistance in prostate carcinoma via the apoptosis
inhibitor TRIAP1. J Clin Med. 8(8)2019.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Li Y, Tang X, He Q, Yang X, Ren X, Wen X,
Zhang J, Wang Y, Liu N and Ma J: Overexpression of mitochondria
mediator gene TRIAP1 by miR-320b loss is associated with
progression in nasopharyngeal carcinoma. PLoS Genet.
12(e1006183)2016.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Miliara X, Garnett JA, Tatsuta T, Abid Ali
F, Baldie H, Pérez-Dorado I, Simpson P, Yague E, Langer T and
Matthews S: Structural insight into the TRIAP1/PRELI-like domain
family of mitochondrial phospholipid transfer complexes. EMBO Rep.
16:824–835. 2015.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Alyes VLF, Zanatta DB, de Oliveira MB,
Eugenio AIP, Fernando RC, Strauss BE and Colleoni GWB: Silencing of
apoptosome regulating genes, HSP70 and TRIAP1, induces apoptosis in
MM cell lines In: Proceedings of the 106th Annual Meeting of the
American Association for Cancer Research, Philadelphia, PA, 2015.
Cancer Res. 75 (Suppl 15)(Abstract nr 1017)2015.
|
|
24
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408.
2001.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Zhong B, Ma S and Wang DH: TRPV1 mediates
glucose-induced insulin secretion through releasing neuropeptides.
In Vivo. 33:1431–1437. 2019.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Seshiah V, Kalra S, Gupte S, Divakar H,
Murugananthan A, Banerjee S, Gupta S, Balaji V, Zargar A, Das A, et
al: Classification of hyperglycemia in pregnancy. Indian J
Endocrinol Metab. 18:445–448. 2014.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Dessì A, Marincola FC and Fanos V:
Metabolomics and the great obstetrical syndromes - GDM, PET, and
IUGR. Best Pract Res Clin Obstet Gynaecol. 29:156–164.
2015.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Naseh A, Ashrafzadeh S and Rassi S:
Prevalence of vitamin D deficiency in pregnant mothers in Tehran
and investigating its association with serum glucose and insulin. J
Matern Fetal Neonatal Med. 31:2312–2318. 2018.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Wang C, Zhu WW, Wei YM, Feng H, Su R and
Yang HX: Exercise intervention during pregnancy can be used to
manage weight gain and improve pregnancy outcomes in women with
gestational diabetes mellitus. BMC Pregnancy Childbirth.
15(255)2015.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Xu K, Bian D, Hao L, Huang F, Xu M, Qin J
and Liu Y: microRNA-503 contribute to pancreatic beta cell
dysfunction by targeting the mTOR pathway in gestational diabetes
mellitus. EXCLI J. 16:1177–1187. 2017.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Zhang YL and Chen XQ: Dysregulation of
microRNA-770-5p influences pancreatic-β-cell function by targeting
TP53 regulated inhibitor of apoptosis 1 in gestational diabetes
mellitus. Eur Rev Med Pharmacol Sci. 24:793–801. 2020.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Boland BB, Rhodes CJ and Grimsby JS: The
dynamic plasticity of insulin production in β-cells. Mol Metab.
6:958–973. 2017.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Jian X and Felsenfeld G: Insulin promoter
in human pancreatic β cells contacts diabetes susceptibility loci
and regulates genes affecting insulin metabolism. Proc Natl Acad
Sci USA. 115:E4633–E4641. 2018.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Miyakoshi K, Tanaka M, Saisho Y, Shimada
A, Minegishi K, Kim SH, Asai S, Itoh H and Yoshimura Y: Pancreatic
beta-cell function and fetal growth in gestational impaired glucose
tolerance. Acta Obstet Gynecol Scand. 89:769–775. 2010.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Fook-Alves VL, de Oliveira MB, Zanatta DB,
Strauss BE and Colleoni GWB: TP53 regulated inhibitor of apoptosis
1 (TRIAP1) stable silencing increases late apoptosis by
upregulation of caspase 9 and APAF1 in RPMI8226 multiple myeloma
cell line. Biochim Biophys Acta. 1862:1105–1110. 2016.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Potting C, Tatsuta T, König T, Haag M, Wai
T, Aaltonen MJ and Langer T: TRIAP1/PRELI complexes prevent
apoptosis by mediating intramitochondrial transport of phosphatidic
acid. Cell Metab. 18:287–295. 2013.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Lorenzo-Almorós A, Hang T, Peiró C,
Soriano-Guillén L, Egido J, Tuñón J and Lorenzo Ó: Predictive and
diagnostic biomarkers for gestational diabetes and its associated
metabolic and cardiovascular diseases. Cardiovasc Diabetol.
18(140)2019.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Wójcik M, Mac-Marcjanek K, Woźniak LA,
Nadel I, Lewiński A and Cypryk K: The association of leukocyte
phosphatidylinositol 3-kinase delta overexpression with gestational
diabetes mellitus (GDM). Endokrynol Pol. 65:17–24. 2014.PubMed/NCBI View Article : Google Scholar
|