Introduction
Lemurs tyrosine kinase 2 (LMTK2) belongs to the
transmembrane serine/threonine protein kinase family anchoring
membrane with unique structure (1,2).
LMTK2, widely expressed in brain, is involved in regulating key
cellular events, apoptosis and cell differentiation (3-7).
According to a review, LMTK2 affects the sensitivities of cells to
cytotoxicity depending on apoptotic and survival pathways (3). The latest research shows that LMTK2
can activate the NRF/ARE signaling pathway to reduce neurons injury
induced by ischemia reperfusion (8). LMTK2 could be phosphorylated through
CDK5/p35 in neurons, however, there is only a small amount of the
phosphorylated forms of LMTK2 in non-neuron cells with inactive
CDK5 (9-12).
Furthermore, LMTK2 can exert vital roles through phosphorylating
downstream targets in non-neuron cells (3). It has been proved that LMTK2 regulates
NF-κB signals through the PP1/GSK3β/p65 pathway or PP1/IKK pathway
in colon cancer cells (13).
Neuroinflammation is associated with the progression
of multiple neurological diseases. Microglia, as the main immune
effector cells of the central nervous system (CNS), play a
substantial role in CNS diseases (14). Although microglia have essential
neuroprotection functions, including sensing changes of the
environment, maintaining normal neuronal function and defensing
these changes, they can damage neurons in response to a particular
stimulus or with neuroinflammation (15). Activated microglia are involved in
the pathologic processes of CNS diseases, such as neurodegenerative
disease, pain, infection and brain trauma (16-19).
The activation of microglia could damage neurons in the brain by
releasing inflammatory cytokines and generating oxidative stress,
which further triggers neurological diseases (20,21).
Therefore, the present study aims to explore the role of LMTK2 in
lipopolysaccharide (LPS)-induced microglia inflammation and to
explore whether it can activate Nrf2 signaling. LPS was used to
activate mouse microglia (BV2) cells to construct a cell model of
neuroinflammation.
Materials and methods
Cells
Mouse microglial cells, BV2, were purchased (The
Institute of Cell Biology, Chinese Academy of Sciences, China) and
cultured with DMEM, a high-glucose medium containing 10% fetal
bovine serum (Gibco; Thermo Fisher Scientific, Inc.), at a constant
temperature of 37˚C in 5% CO2. The microglia were used
in the experiment when the cells reached the logarithmic growth
stage.
Western blotting (WB)
BV2 cell suspension was placed in a 6-well plate
(2x105 cells/ml). The cells were stimulated with LPS at
different concentrations (100, 200 and 500 ng/ml; Sigma-Aldrich;
Merck KGaA) for 24 h. For LMTK2 overexpression, the cells were
transfected with plasmids overexpressing LMTK2. After 24 h, the
cells were stimulated with LPS (500 ng/ml) for 24 h. Subsequently,
cells were collected to extract total protein using RIPA lysis
solution (cat. no. R0278; Sigma-Aldrich; Merck KGaA). The protein
concentrations were detected by BCA method. Then, 10% SDS-PAGE
electrophoresis was performed to separate the proteins (40 µg
protein in each well). Skim milk powder (5%) was utilized to block
the PVDF membrane for 40 min at room temperature. Primary
antibodies (LMTK2 (1:500; cat. no. DF3344; Affinity Biosciences),
inducible nitric oxide synthase (1:1,000; iNOS; cat. no. ab178945;
Abcam), cyclooxygenase 2 (1:1,000; COX2; cat. no. ab179800),
nuclear factor erythroid 2-related factor 2 (1:1,000; NRF2;
ab137550; Abcam), heme oxygenase-1 (1:2,000; HO-1; cat. no.
ab189491; Abcam), NAD(P)H dehydrogenase quinone 1 (1:20,000; NQO1;
cat. no. ab28947; Abcam), Histone H3 (1:2,000; cat. no. ab1791;
Abcam), GAPDH (1:5,000; cat. no. ab8245; Abcam) were incubated with
the membrane at 4˚C overnight. This was followed by incubation with
the secondary antibodies goat anti-rabbit IgG (1:10,000; cat. no.
ab6721; Abcam) and rabbit anti-mouse IgG (1:10,000; cat. no.
ab6728; Abcam) at room temperature for 1 h. ECL was used to
visualize the protein bands which then was quantified with ImageJ
1.52v software (National Institutes of Health).
Plasmids transfection
The plasmids overexpressing LMTK2 were constructed
by Shanghai GenePharma Co., Ltd. BV2 cells were seeded into 6-well
plates (2x105 cells/ml). LMTK2 overexpression plasmids
(Oe-LM; 4 µg) and empty plasmids (Oe-NC; 4 µg) were respectively
transfected into BV2 cells using Lipofectamine™3000 (Invitrogen;
Thermo Fisher Scientific, Inc.) according to manufacturer's
protocol. After transfection of 24 h at 37˚C, cells were activated
by LPS (500 ng/ml).
Reverse transcription-quantitative
(RT-q)PCR
BV2 cell suspension was placed in a 6-well plate
(2x105 cells/ml). After experimental treatment, total
RNA was extracted using TRIzol method and reverse transcribed into
cDNA 42˚C for 1 h (RevertaidTM First Strand cDNA Synthesis kit,
Fermentas; Thermo Fisher Scientific, Inc.). Relative determination
of LMTK2 and GADPH mRNA was performed by SYBR-Green dye method
(Clontech Laboratories, Inc.) and calculated using the
2-ΔΔCq method (22). The
primers of LMTK2 mRNA were as follows: Forward,
5'-TTGCCCGCCACAGTCTAAAC-3' and reverse,
5'-GATGACTCTTGCTACGCTAGT-3'; The primers of GADPH mRNA were as
follows: Forward 5'-GCCTTCCGTGTTCCTACCC -3' and reverse 5'-TGCCTG
CTTCACCACCTTC-3'. The target mRNA was amplified in the following
thermocycling conditions: Predenaturation at 95˚C for 3 min; 30
cycles of 95˚C 30 sec, 58˚C for 30 sec and 72˚C for 1 min. The
total extension at 72˚C for 10 min.
MTT assay
Cells viability was detected as per the
manufacturer's protocol. BV2 cells were seeded into 96-well plates
(1x105 cells/ml). After cells were treated, MTT solution
of 5 g/l (APExBIO Technology LLC) was added and placed in an
incubator at 37˚C and 5% CO2 for 4 h. The dimethyl
sulfoxide (DMSO) solution of 100 µl was added to each well. After
shaking the mixture, the absorbance (A) value at the wavelength of
490 nm was detected by a microplate reader and the cell survival
rate was calculated.
Griess assays
NO2 was formed from NO in aqueous and reacted with
Griess reagent (Beyotime Institute of Biotechnology). Therefore, NO
levels in the supernatant were indirectly detected. Cells were
seeded into 24-well plates (2x105 cells/ml). After LPS
treatment (500 ng/ml) for 24 h, the supernatant in the medium was
collected and then 50 µl of Griess reagent was supplemented into
the supernatant. After 10 min, the absorbance at 540 nm was
detected.
Enzyme-linked immunosorbent assay
(ELISA)
BV2 cells were cultured in 6-well plates
(2x105 cells/ml). After treatment, the supernatant in
medium was collected. Interleukin (IL)-1β, IL-6 and IL-10 levels
were analyzed through ELISA kits (Mouse IL-1β ELISA kit; cat. no.
PI301; IL-6, Mouse IL-6 ELISA kit; cat. no. PI326; and IL-10; Mouse
IL-10 ELISA kit, cat. no. PI522; all from Beyotime Institute of
Biotechnology), along with the detection of tumor necrosis factor
(Mouse TNF-α ELISA Standard Recombinant Protein; cat. no.
29-8321-65; Invitrogen; Thermo Fisher Scientific, Inc.) and
prostaglandin E2 (PGE2) levels (Prostaglandin E2 ELISA; ab133021;
Abcam).
Statistical analysis
GraphPad Prism 8.0 software (GraphPad Software,
Inc.) was used for statistical analysis of data, and one-way ANOVA
was used to perform the comparison among groups, followed by
Turkey's test. P<0.05 was considered to indicate a statistically
significant difference.
Results
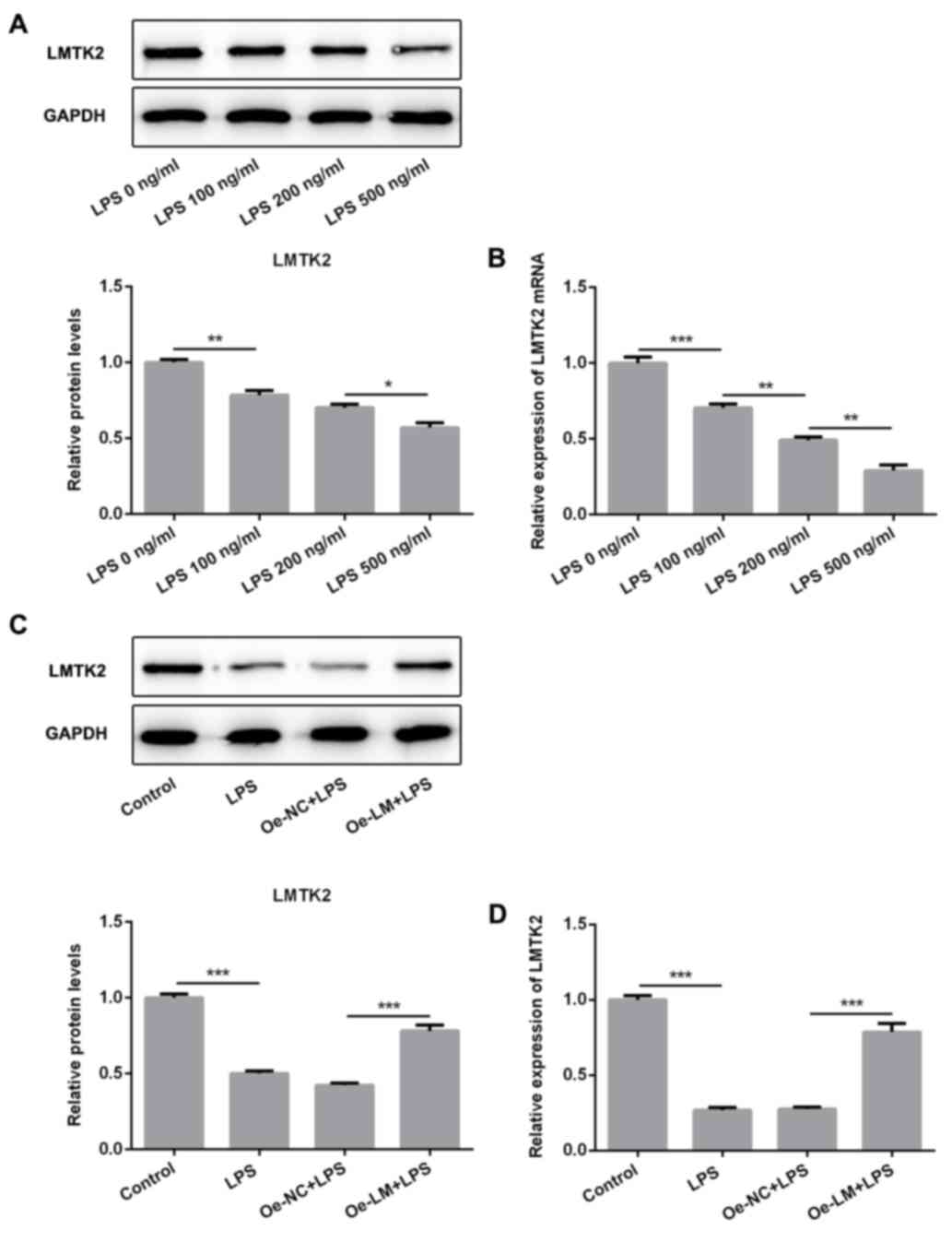
LMTK2 was significantly decreased in
LPS-induced BV2 cells
LPS of different concentrations (100, 200 and 500
ng/ml) was used to stimulate BV2 cells. The LMTK2 protein and mRNA
levels presented a gradual decrease with the increasing dose of LPS
(Fig. 1A and B). Therefore, 500 ng/ml LPS was utilized
to perform the follow-up experiments. Subsequently, the plasmids
Oe-LM and Oe-NC were utilized to pre-treat BV2 cells. A significant
upregulation of LMTK2 mRNA levels was observed in cells transfected
with Oe-LM. (Fig. S1). Following
plasmid transfection for 24 h, LPS was used to activate BV2 cells.
A marked upregulation of LMTK2 protein and mRNA levels was observed
in BV2 cells transfected with Oe-LM compared with Oe-NC (Fig. 1C and D).
LMTK2 overexpression notably decreased
the levels of pro-inflammatory mediators in LPS-stimulated BV2
cells
In subsequent experiments, cell viability was
evaluated through MTT assay in BV2 cells stimulated with LPS.
Compared with the control group, LPS stimulation increased the cell
viability of BV2 cells (Fig. 2A).
As shown in a previous study (23),
the levels of cytoskeletal protein α-tubulin and Iba1 were
significantly increased in BV2 cells following LPS induction.
Moreover, succinic acid dehydrogenase decreased exogenous MTT into
water-insoluble blue-purple crystal formazan, contributing to the
increase in OD value. In the present study, LMTK2 overexpression
significantly decreased cell viability compared with cells treated
with LPS alone (Fig. 2A).
Subsequently, it was observed that the levels of proinflammatory
mediators, consisting of NO generated by iNOS, PGE2 generated by
COX-2), iNOS and COX-2, showed significant decreases in the
presence of LMTK2 overexpression in LPS-activated BV2 cells
(Fig. 2B-E).
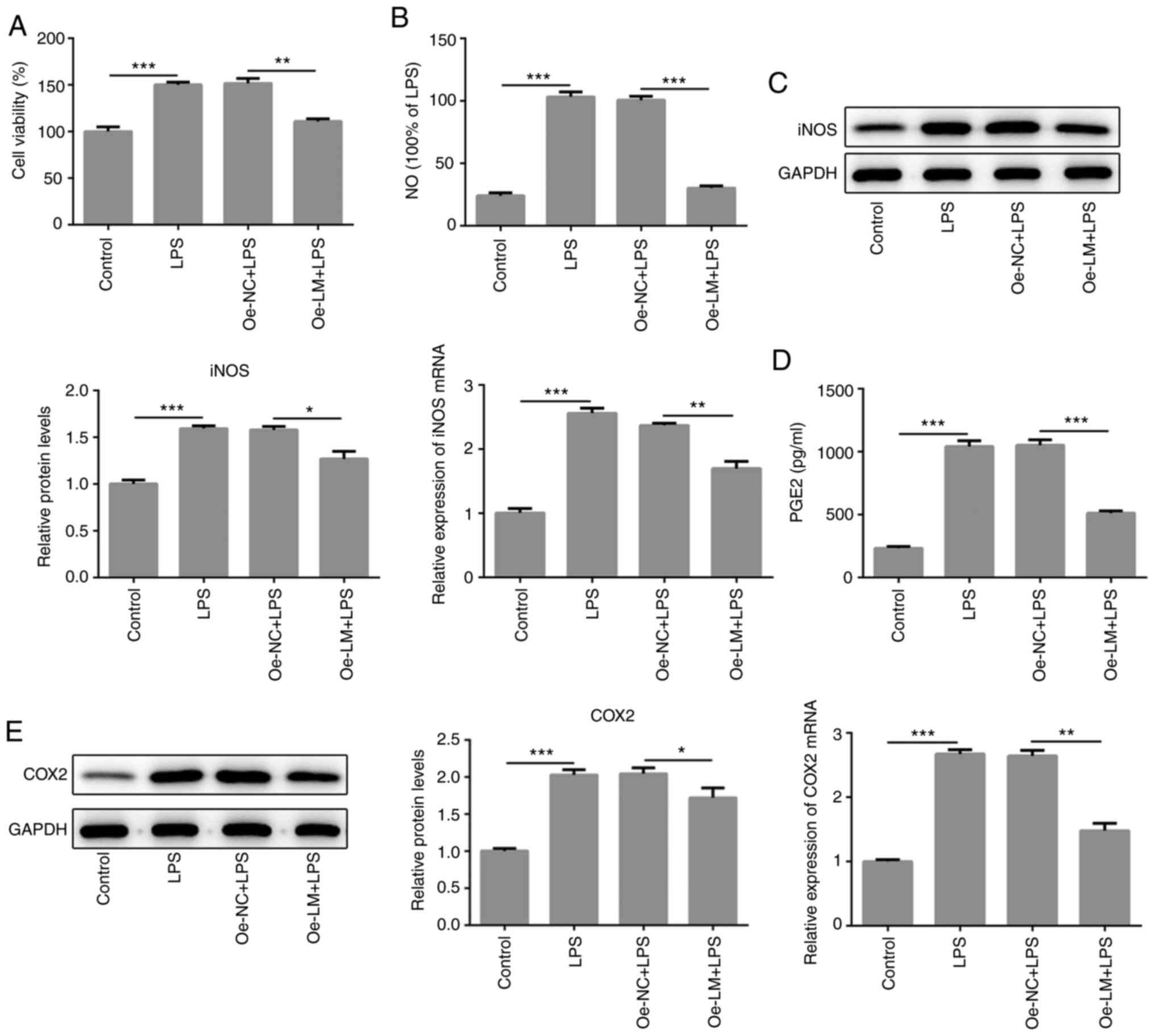 | Figure 2LMTK2 overexpression contributed to a
reduction in the expression levels of pro-inflammatory mediators.
(A) LMTK2 overexpression significantly decreased cell viability, as
detected by MTT assay in LPS-induced BV2 cells. (B) Griess assays
analyzed NO release in supernatant following LMTK2 overexpression
in LPS-stimulated BV2 cells. (C) Reverse transcription-quantitative
PCR and WB was used to evaluate the expression of iNOS in
LPS-induced BV2 cells following LMTK2 overexpression. (D) PGE2
levels were markedly reduced, by WB analysis, in LPS-induced BV2
cells following LMTK2 overexpression. (E) LMTK2 overexpression
significantly decreased COX2 expression in LPS-induced BV2 cells
overexpressing LMTK2, by WB analysis. Data are shown as mean ± SD.
Each experiment was repeated in triplicate. *P<0.05;
**P<0.01; ***P<0.001. LMTK2, Lemurs
tyrosine kinase 2; LPS, lipopolysaccharide 2; Oe-NC, negative
control plasmid; Oe-LM, LMTK2-overexpression plasmid; WB, western
blotting; NO, nitric oxide; iNOS, inducible nitric oxide synthase;
PGE2, prostaglandin E2; COX2, cyclooxygenase 2. |
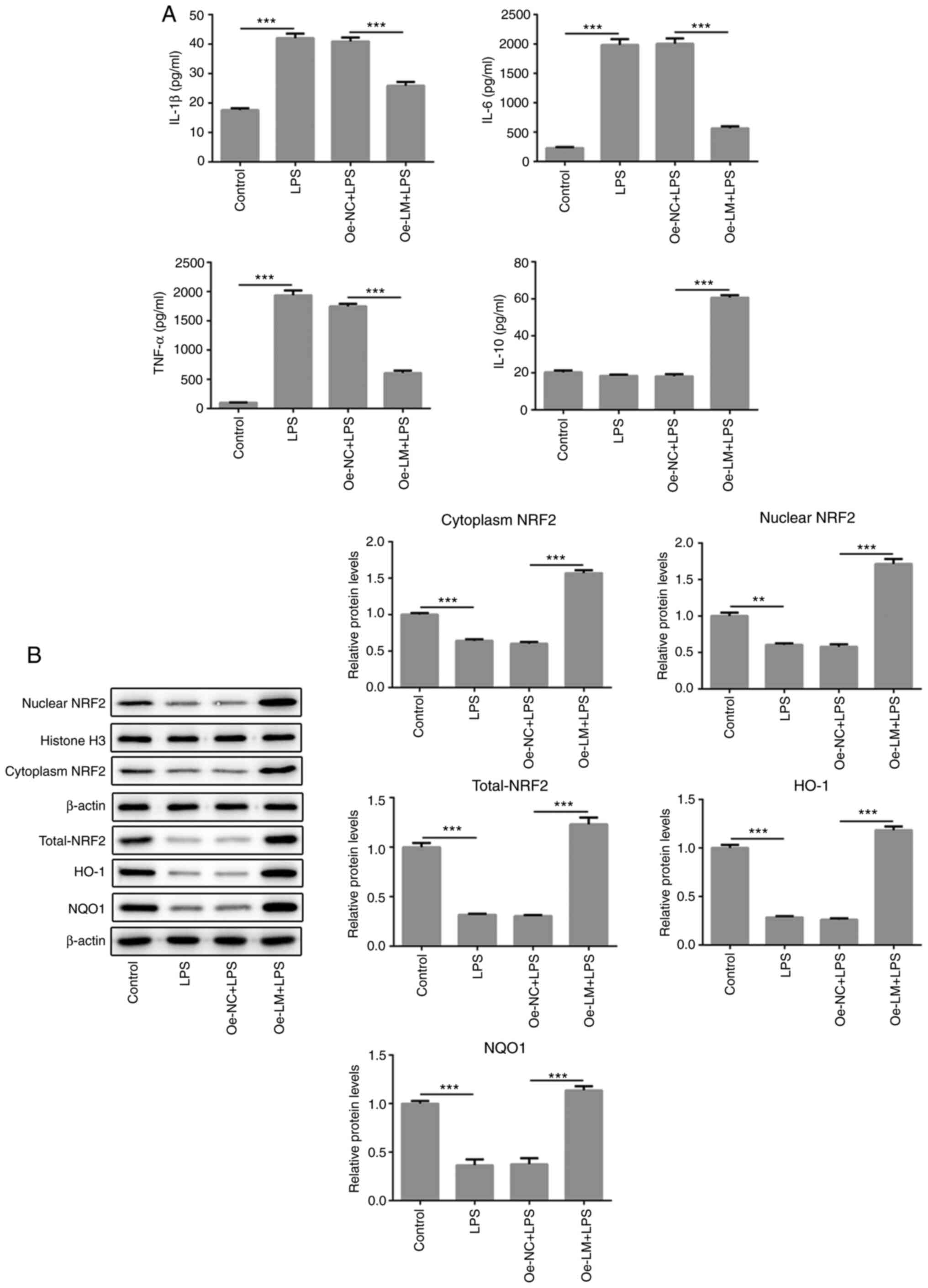
The overexpression of LMTK2 regulated
the release of inflammatory factors in LPS-induced BV2 cells
The levels of proinflammatory and anti-inflammatory
factors were analyzed in LPS-treated BV2 cells with or without
transfection of Oe-LM plasmids. As the result displayed, the
proinflammatory mediators, TNF-α, IL-1β and IL-6, were markedly
decreased in response to LMTK2 overexpression compared with LPS
treatment alone (Fig. 3A).
Subsequently, Nrf2 signals were analyzed by detecting the
expression of Nrf2 and its downstream genes (HO-1 and NQO1). Nrf2
was upregulated in the cytoplasm and nucleus, as well as HO-1 and
NQO1, following overexpression of LMTK2 in BV cells (Fig. 3B), implying that the activation of
Nrf2 is dependent on LMTK2.
Discussion
The present study showed that exogenous LMTK2
significantly upregulated the expression of Nrf2 in the nucleus and
the levels of HO-1 and NQO1 proteins in BV2 cells stimulated with
LPS; which implied that LMTK2 promoted the transcription of
Nrf2-mediated downstream genes. A recent study demonstrated that
LMTK2 regulates GSK-3β/Nrf2/ARE signaling to ameliorate neuronal
injury induced by oxygen-glucose deprivation/reoxygenation
(8).
There is a crosstalk between the Nrf and NF-κB
pathways (24). Lack of Nrf2 is
associated with enhanced production of cytokines (25), which could lead to the
neurodegenerative changes in Nrf2 knockdown animals (26,27).
Previously, Nrf2 was reported to show anti-inflammatory abilities
through the downregulation of COX-2, TNFα and iNOS in LPS-induced
peritoneal macrophages (28). HO-1,
as a Nrf2-mediated downstream protein, has been demonstrated to
inhibit Nrf2-modulated NF-κB (29).
Collectively, the upregulation of HO-1 expression could suppress
NF-κB activation and cause the decrease in pro-inflammatory factors
in the present study. LPS treatment frequently induces the
activation of NF-κB, along with the enhancement of iNOS, COX-2,
PGE2 and pro-inflammatory factors in microglia cells (30,31).
PGE2 produced by microglial cells are the main source for
neuroinflammation, showing marked increase upon LPS stimulation in
microglia (32,33).
The aforementioned studies imply that the Nrf2
pathway could negatively regulate the NF-κB pathway. Taken
together, LMTK2 overexpression reduced the levels of iNOS, COX-2
and pro-inflammatory factors, TNF-α, IL-1β and IL-6, but increased
IL-10 level; possibly due to the dependence of LPS-induced
microglia on Nrf2 pathway. However, no significant changes were
observed in IL-10 levels following LPS stimulation, which was
consistent with a previous report (34). IL-10 is an important inflammatory
suppressor in vivo and can inhibit the release of
pro-inflammatory cytokines in microglia cells in the central
nervous system (35). Besides, LPS
could induce the increase of NO, the production of iNOS, in
microglia (36). In addition, NF-κB
is considered upstream of NO and could initiate the synthesis of NO
(37). A study has also shown that
in lipoteichoic acid-induced microglia, matrix metalloprotease
(MMP)-8 inhibitor regulates NF-κB and Nrf2 signals (38). Thus, LPS-mediated increase in
pro-inflammatory factors were markedly reduced by inducement of
exogenous LMTK2 expression, which implied the involvement of LMTK2
in regulating MMP-8 levels; however, this requires further study.
In conclusion, the present study implies that LMTK2 regulates
inflammation potentially by activating Nrf2 pathway.
Supplementary Material
Detection of LMTK2 mRNA through
reverse transcription quantitative PCR in BV2 cells following
transfection with LMTK2 overexpressing plasmids or empty plasmids.
Data were shown as mean ± SD. ***P<0.001. LMTK2,
Lemurs tyrosine kinase 2; NC, negative control.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
QYR, and QX made substantial contributions to the
conception and design of the study, acquired, analyzed and
interpreted the data, and drafted and revised the manuscript for
important intellectual content; QY, SGC, XZW, XYD, XI, WLD, QF and
XGZ performed the experiments and interpreted the data. All authors
read and approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Tomomura M, Morita N, Yoshikawa F, Konishi
A, Akiyama H, Furuichi T and Kamiguchi H: Structural and functional
analysis of the apoptosis-associated tyrosine kinase (AATYK)
family. Neuroscience. 148:510–521. 2007.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Wendler F: The LMTK-family of kinases:
Emerging important players in cell physiology and disease
pathogenesis. Biochim Biophys Acta Mol Basis Dis
S0925-4439(18)30515-5, 2018.
|
|
3
|
Bencze J, Mórotz GM, Seo W, Bencs V,
Kálmán J, Miller CCJ and Hortobágyi T: Biological function of Lemur
tyrosine kinase 2 (LMTK2): Implications in neurodegeneration. Mol
Brain. 11(20)2018.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Wang H and Brautigan DL: A novel
transmembrane Ser/Thr kinase complexes with protein phosphatase-1
and inhibitor-2. J Biol Chem. 277:49605–49612. 2002.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Luz S, Cihil KM, Brautigan DL, Amaral MD,
Farinha CM and Swiatecka-Urban A: LMTK2-mediated phosphorylation
regulates CFTR endocytosis in human airway epithelial cells. J Biol
Chem. 289:15080–15093. 2014.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Kawa S, Ito C, Toyama Y, Maekawa M, Tezuka
T, Nakamura T, Nakazawa T, Yokoyama K, Yoshida N, Toshimori K, et
al: Azoospermia in mice with targeted disruption of the Brek/Lmtk2
(brain-enriched kinase/lemur tyrosine kinase 2) gene. Proc Natl
Acad Sci USA. 103:19344–19349. 2006.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Cruz DF, Farinha CM and Swiatecka-Urban A:
Unraveling the Function of Lemur Tyrosine Kinase 2 Network. Front
Pharmacol. 10(24)2019.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Bao H and Gao M: Overexpression of lemur
tyrosine kinase-2 protects neurons from oxygen-glucose
deprivation/reoxygenation-induced injury through reinforcement of
Nrf2 signaling by modulating GSK-3β phosphorylation. Biochem
Biophys Res Commun. 521:964–970. 2020.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Manser C, Vagnoni A, Guillot F, Davies J
and Miller CCJ: Cdk5/p35 phosphorylates lemur tyrosine kinase-2 to
regulate protein phosphatase-1C phosphorylation and activity. J
Neurochem. 121:343–348. 2012.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Tsai LH, Delalle I, Caviness VS Jr, Chae T
and Harlow E: p35 is a neural-specific regulatory subunit of
cyclin-dependent kinase 5. Nature. 371:419–423. 1994.PubMed/NCBI View
Article : Google Scholar
|
|
11
|
Guidato S, Tsai LH, Woodgett J and Miller
CC: Differential cellular phosphorylation of neurofilament heavy
side-arms by glycogen synthase kinase-3 and cyclin-dependent
kinase-5. J Neurochem. 66:1698–1706. 1996.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Li BS, Zhang L, Gu J, Amin ND and Pant HC:
Integrin alpha(1) beta(1)-mediated activation of cyclin-dependent
kinase 5 activity is involved in neurite outgrowth and human
neurofilament protein H Lys-Ser-Pro tail domain phosphorylation. J
Neurosci. 20:6055–6062. 2000.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Zhang R, Li X, Wei L, Qin Y and Fang J:
Lemur tyrosine kinase 2 acts as a positive regulator of NF-κB
activation and colon cancer cell proliferation. Cancer Lett.
454:70–77. 2019.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Goldmann T and Prinz M: Role of microglia
in CNS autoimmunity. Clin Dev Immunol. 2013(208093)2013.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Hickman S, Izzy S, Sen P, Morsett L and El
Khoury J: Microglia in neurodegeneration. Nat Neurosci.
21:1359–1369. 2018.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Lehnardt S: Innate immunity and
neuroinflammation in the CNS: The role of microglia in Toll like
receptor mediated neuronal injury. Glia. 58:253–623.
2010.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Tsuda M, Tozaki Saitoh H and Inoue K:
Purinergic system, microglia and neuropathic pain. Curr Opin
Pharmacol. 74–79. 2012.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Mao SS, Hua R, Zhao XP, Qin X, Sun ZQ,
Zhang Y, Wu YQ, Jia MX, Cao JL and Zhang YM: Exogenous
administration of PACAP alleviates traumatic brain injury in rats
through a mechanism involving the TLR4/MyD88/NF-κB pathway. J
Neurotrauma. 29:1941–1959. 2012.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Aravalli RN, Peterson PK and Lokensgard
JR: Toll-like receptors in defense and damage of the central
nervous system. J Neuroimmune Pharmacol. 2:297–312. 2007.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Hirsch EC and Hunot S: Neuroinflammation
in Parkinson's disease: A target for neuroprotection? Lancet
Neurol. 8:382–397. 2009.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Frank-Cannon TC, Alto LT, McAlpine FE and
Tansey MG: Does neuroinflammation fan the flame in
neurodegenerative diseases? Mol Neurodegener. 4(47)2009.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) Method. Methods. 25:402–408.
2001.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Gupta M and Kaur G: Aqueous extract from
the Withania somnifera leaves as a potential anti-neuroinflammatory
agent: A mechanistic study. J Neuroinflammation.
13(193)2016.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Wardyn JD, Ponsford AH and Sanderson CM:
Dissecting molecular cross-talk between Nrf2 and NF-κB response
pathways. Biochem Soc Trans. 43:621–626. 2015.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Pan H, Wang H, Wang X, Zhu L and Mao L:
The absence of Nrf2 enhances NF-κB-dependent inflammation following
scratch injury in mouse primary cultured astrocytes. Mediators
Inflamm. 2012(217580)2012.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Neymotin A, Calingasan NY, Wille E, Naseri
N, Petri S, Damiano M, Liby KT, Risingsong R, Sporn M, Beal MF, et
al: Neuroprotective effect of Nrf2/ARE activators, CDDO ethylamide
and CDDO trifluoroethylamide, in a mouse model of amyotrophic
lateral sclerosis. Free Radic Biol Med. 51:88–96. 2011.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Frakes AE, Ferraiuolo L, Haidet-Phillips
AM, Schmelzer L, Braun L, Miranda CJ, Ladner KJ, Bevan AK, Foust
KD, Godbout JP, et al: Microglia induce motor neuron death via the
classical NF-κB pathway in amyotrophic lateral sclerosis. Neuron.
81:1009–1023. 2014.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Lin W, Wu RT, Wu T, Khor TO, Wang H and
Kong AN: Sulforaphane suppressed LPS-induced inflammation in mouse
peritoneal macrophages through Nrf2 dependent pathway. Biochem
Pharmacol. 76:967–973. 2008.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Soares MP, Seldon MP, Gregoire IP,
Vassilevskaia T, Berberat PO, Yu J, Tsui TY and Bach FH: Heme
oxygenase-1 modulates the expression of adhesion molecules
associated with endothelial cell activation. J Immunol.
172:3553–3563. 2004.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Xie Q, Wu GZ, Yang N, Shen YH, Tang J and
Zhang WD: Delavatine A, an unusual isoquinoline alkaloid exerts
anti-inflammation on LPS-induced proinflammatory cytokines
production by suppressing NF-κB activation in BV-2 microglia.
Biochem Biophys Res Commun. 502:202–208. 2018.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Zhao J, Bi W, Xiao S, Lan X, Cheng X,
Zhang J, Lu D, Wei W, Wang Y, Li H, et al: Neuroinflammation
induced by lipopolysaccharide causes cognitive impairment in mice.
Sci Rep. 9(5790)2019.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Jung YS, Park JH, Kim H, Kim SY, Hwang JY,
Hong KW, Bae SS, Choi BT, Lee SW and Shin HK: Probucol inhibits
LPS-induced microglia activation and ameliorates brain ischemic
injury in normal and hyperlipidemic mice. Acta Pharmacol Sin.
37:1031–1044. 2016.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Saliba SW, Jauch H, Gargouri B, Keil A,
Hurrle T, Volz N, Mohr F, van der Stelt M, Bräse S and Fiebich BL:
Anti-neuroinflammatory effects of GPR55 antagonists in
LPS-activated primary microglial cells. J Neuroinflammation.
15(322)2018.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Bao Y, Zhu Y, He G, Ni H, Liu C, Ma L,
Zhang L and Shi D: Dexmedetomidine attenuates neuroinflammation in
LPS-stimulated BV2 microglia cells through upregulation of miR-340.
Drug Des Devel Ther. 13:3465–3475. 2019.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Cianciulli A, Dragone T, Calvello R, Porro
C, Trotta T, Lofrumento DD and Panaro MA: IL-10 plays a pivotal
role in anti-inflammatory effects of resveratrol in activated
microglia cells. Int Immunopharmacol. 24:369–376. 2015.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Posadas I, Terencio MC, Guillén I,
Ferrándiz ML, Coloma J, Payá M and Alcaraz MJ: Co-regulation
between cyclo-oxygenase-2 and inducible nitric oxide synthase
expression in the time-course of murine inflammation. Naunyn
Schmiedebergs Arch Pharmacol. 361:98–106. 2000.PubMed/NCBI View Article : Google Scholar
|
|
37
|
El Moussawi L, Chakkour M and Kreydiyyeh
S: The epinephrine-induced PGE2 reduces
Na+/K+ ATPase activity in Caco-2 cells via
PKC, NF-κB and NO. PLoS One. 14(e0220987)2019.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Lee EJ, Park JS, Lee YY, Kim DY, Kang JL
and Kim HS: Anti-inflammatory and anti-oxidant mechanisms of an
MMP-8 inhibitor in lipoteichoic acid-stimulated rat primary
astrocytes: Involvement of NF-κB, Nrf2, and PPAR-γ signaling
pathways. J Neuroinflammation. 15(326)2018.PubMed/NCBI View Article : Google Scholar
|