Introduction
Osteoarthritis (OA) is an aging-associated
progressive joint disease characterized by cartilage loss and
damage (1,2). However, to date, there is no effective
and safe medication available against OA. Most patients with
advanced OA require total joint replacement (3). Recently, it was hypothesized that
oxidative stress is closely associated with the progression of
cartilage degeneration during OA, and promotion of endogenous
antioxidant activity was demonstrated to confer protection against
OA (4-8).
Nuclear factor erythroid 2-related factor 2 (Nrf2) is a
transcription factor, which binds to antioxidant response elements
(AREs) to regulate numerous phase II antioxidant enzymes, including
heme oxygenase 1 (HO-1) (9). Our
previous study showed that Nrf2 deficiency aggravated the damage of
cartilage in an inflammatory model and a post-traumatic model of OA
(10). Therefore, the present study
aimed to investigate potential drugs targeting Nrf2 activation.
Ergosterol (ER), which is extracted from the fungus Agaricus
campestris, exhibits a wide range of pharmacological
properties, including anti-inflammatory and anti-oxidative effects
(11,12). Xu et al (13) reported that ER increased the
expression of Nrf2 and HO-1 in rat hearts and exerted a
cardioprotective effect in a lipopolysaccharide-induced sepsis
model. However, to the best of our knowledge, the role of ER in OA
remains unclear. The aim of the present study was to investigate
the chondroprotective effects of ER in a destabilization of the
medial meniscus (DMM) surgery-induced OA model and elucidate the
underlying mechanisms.
Materials and methods
Chemicals and reagents
ER (cat. no. 45480; purity, 95%) was supplied by
Sigma-Aldrich; Merck KGaA. Anti-Nrf2 (cat. no. 12721; 1:1,000),
anti-lamin B (cat. no. 13435; 1:1,000) and anti-GAPDH (cat. no.
5174; 1:5,000) antibodies were purchased from Cell Signaling
Technology, Inc.. Anti-HO-1 (cat. no. BS6626; 1:1,000), anti-MMP1
(cat. no. BS62563; 1:1,000), anti-MMP3 (cat. no. BS90872; 1:1,000),
anti-MMP9 (cat. no. BS6893; 1:1,000), anti-MMP13 (cat. no. BS6668;
1:1,000), anti-ADAMTS5 (cat. no. BS74041; 1:2,000) and secondary
HRP-conjugated goat anti-rabbit (cat. no. BS13278; 1:5,000)
antibodies were purchased from Bioworld Technology, Inc.. A nuclear
and cytoplasmic protein extraction kit (cat. no. P0028) was
obtained from Beyotime Institute of Biotechnology.
Cell culture
Primary mouse chondrocytes were collected from the
costal cartilage of 14 male neonatal C57BL/6 mice (age, 6 days;
weight, 3.08±0.85 g) obtained from the Comparative Medical Center
of Yangzhou University (Yangzhou, China) and housed in
specific-pathogen-free conditions under a 12 h light-dark cycle at
25±2˚C with 45-50% humidity with food and water available ad
libitum. Murine cartilage was collected and collagen II
immunofluorescence staining was used for identification of
chondrocytes, as previously described (14). Following digestion with collagenase
D overnight at 37˚C, harvested chondrocytes were seeded on a 10-cm
dish and incubated in DMEM/F12 (Gibco; Thermo Fisher Scientific,
Inc.) with 10% FBS (Gibco; Thermo Fisher Scientific, Inc.), 100
U/ml penicillin and 100 µg/ml streptomycin with 5% CO2
at 37˚C. To avoid loss of the chondrocyte phenotype with successive
passages, cells at 80% confluence were detached and plated in
six-well plates at a density of 3x105 cells/well (2.4 cm
in diameter) for further assays. Primary mouse chondrocytes
pre-treated with IL-1β (10 ng/ml; as previously described)
(15,16) for 12 h were cultured in various
concentrations (0, 5, 10 or 20 µM) of ER for 24 h at 37˚C followed
by protein or RNA extraction.
Cell viability assays
A Cell Counting Kit-8 (CCK-8) assay was used to
determine cell viability following ER treatment, as detailed in our
previous study (14). Briefly,
primary chondrocytes were seeded in a 96-well plate at a density of
5x103 cells/well. Following incubation with different
concentrations (0, 2, 5, 10, 20 or 40 µM) of ER at 37˚C for 24 h,
cells were incubated with CCK-8 solution (Bioworld Technology, Inc;
10 µl/well) for 1 h in the dark at room temperature. The absorbance
of each well was subsequently measured using a microplate reader
(Bio-Rad Laboratories, Inc.) at a wavelength of 450 nm according to
the manufacturer's protocols.
Protein expression analysis
Male mice were kept at 24˚C with a 12-h light/dark
cycle and food and water access ad libitum. Mice were
monitored for health and weight every 2 or 3 days. In total 68
knees from 34 mice were used for protein expression analysis. OA
was induced by sectioning of the medial meniscotibial ligament,
also known DMM surgery. The pre-surgery grouping was separate from
the surgery grouping. For pre-surgery, two groups of mice (n=8 per
group) that had undergone no sham or DMM surgery were administered
saline (vehicle) or ER (25 mg/kg/day) dissolved in saline (0.1
ml/10 g) every day by oral gavage for 2 weeks, and 16 knee joints
were harvested for WB analysis to confirm whether ER could increase
expression of Nrf2/HO-1 in cartilage. For surgery groups, two
groups of mice (n=9 per group) that had undergone DMM surgery on
the right knee and sham surgery on the left knee were administered
the aforementioned doses of vehicle or ER for 2 weeks. At the
eighth week after surgery, atotal of 36 knees were harvested and
formed the four groups (sham surgery + vehicle, sham surgery + ER,
DMM surgery + vehicle and DMM surgery + ER). The knees were
prepared as described previously (17). Briefly, cartilage tissue obtained
from knee joints was harvested using a scalpel blade with a
surgical microscope and stored in liquid nitrogen. To obtain a
suitable amount of protein, sample pooling was performed. The
cartilage collected from each individual knee (including a femur
and tibia) was treated as one compartment. Each experimental unit
of mouse samples was pooled from three compartments from different
mice. When pooling was performed, the experimental unit was
regarded as one sample. For animal tissues, the cartilage tissue
was extracted in PBS containing 1% Triton X-100, 0.1% SDS, 20 nM
sodium orthovanadate, 1 µg/ml aprotinin, 1 mM phenylmethylsulfonyl
fluoride and 5 mM ethylenediaminetetraacetic acid. The homogenates
were centrifuged at 12,000 x g for 30 min at 4˚C and the protein in
the supernatant was used for further study. When analyzing
fractionated protein from cultured cells the cytoplasmic and
nuclear fractions were separated using the aforementioned nuclear
and cytoplasmic protein extraction kit according to the
manufacturer's instruction. Briefly, the cultured chondrocytes were
washed twice with pre-cooled PBS and harvested by centrifugation at
1,000 x g for 5 min at 4˚C, and the cell pellet was lysed in a
cytoplasmic extraction reagent. After incubation for 30 min on ice,
the homogenate was centrifuged at 10,000 x g for 10 min at 4˚C and
the supernatant was removed. The nuclear extraction reagent was
subsequently added to the precipitate. After incubation for 30 min
on ice, nuclear protein fraction was harvested in the supernatant
after centrifugation at 10,000 x g for 10 min at 4˚C. In a separate
total protein extraction, cultured chondrocytes were lysed with
pre-cooled RIPA lysis buffer (Sigma-Aldrich; Merck KGaA cat. no.
R0278) containing protease and phosphatase inhibitors. The protein
concentration of the lysates was measured with a bicinchoninic acid
protein quantitation kit (Pierce; Thermo Fisher Scientific, Inc.).
Equal amounts of protein (5 µg of protein per lane for tissues and
10 µg of protein per lane for cells) were loaded on SDS-PAGE (10%
gel) and transferred onto polyvinylidene fluoride membranes (EMD
Millipore). The membranes were blocked with 5% BSA (Sigma-Aldrich;
Merck KGaA) at room temperature for 1 h and then incubated with the
aforementioned primary antibodies for 18 h at 4˚C. Following three
washes and probing with the aforementioned secondary HRP-conjugated
goat anti-rabbit antibodies for 2 h at 4˚C, membranes were
visualized using an Pierce™ ECL Western Blotting
Substrate (Thermo Fisher Scientific, Inc. cat. no. 32209) and
quantified using ImageJ software (version 1.51; National Institutes
of Health).
Gene transcript analysis
In total 24 knees were harvested from 12 mice for
gene transcript analysis. Pooling was performed to obtain a
suitable amount of cartilage and each experimental unit was a pool
of two compartments. The cartilage collected from each individual
knee (including a femur and tibia) was treated as one compartment.
Total RNA from cartilage in knee joints of mice was isolated with
TRIzol® reagent (Invitrogen; Thermo Fisher Scientific,
Inc.). First strand cDNA was synthesized from total RNA using the
PrimeScript RT Reagent kit (Promega Corporation) according to the
manufacturer's protocols. mRNA expression of MMP-9 and MMP-13 was
measured on a 7500 Real-Rime PCR system with SYBR Green PCR Master
Mix (Thermo Fisher Scientific, Inc.). ACTB was used as a reference
gene (18). Gene-specific primer
sequences used in the present study are listed in Table I. The expression levels of genes
were calculated using the 2-ΔΔCq method (19).
 | Table IGene-specific primer sequences used
for the quantitative PCR. |
Table I
Gene-specific primer sequences used
for the quantitative PCR.
| Target gene | Primer sequence
(5'-3') |
|---|
| MMP-9 | Forward:
TGGCTTTTGTGACAGGCACTTC |
| | Reverse:
CGGTGGTGTTCTCCAATGTAAGAG |
| MMP-13 | Forward:
ATGCATTCAGCTATCCTGGCCA |
| | Reverse:
AAGATTGCATTTCTCGGAGCCTG |
| ACTB | Forward:
TGACGGGGTCACCCACACTGTGCCCATCTA |
| | Reverse:
CTAGAAGCATTTGCGGTGGACGATGGAGGG |
Luciferase assays
The HO-1 promoter was amplified by PCR from RAW cell
genome DNA and the product was then inserted into a pGL3 vector
(Promega Corporation) at the HindIII and Bg1II sites
(20). DNA from RAW cells was
extracted using the TIANamp Genomic DNA Kit (cat. no. DP304;
Tiangen Biotech Co., Ltd.) and amplified with AmpliTaq
Gold™ DNA Polymerase (Thermo Fisher Scientific, Inc.)
following the manufacturer's instructions. The thermocycling
conditions were as follows: 2 min at 94˚C; 30 cycles of 30 sec at
94˚C, 30 sec at 56˚C and 30 sec at 72˚C, and a final 5 min at 72˚C.
All the constructs were subjected to 4% agarose gel electrophoresis
in an ABI Prism 377 DNA sequencer (Applied Biosystems; Thermo
Fisher Scientific, Inc.). A nonspecific oligonucleotide was used to
construct a control plasmid. The primer sequences for HO-1 were as
follows: Forward, 5'-GGAAGATCTCTGCAGAGCCCCACTGGA G-3' and reverse,
5'-CCCAAGCTTGGAACAGCAACGCTGT-3'. All the constructs were confirmed
by sequencing. 293 cells (The Cell Bank of Type Culture Collection
of the Chinese Academy of Sciences) were transfected with the
HO-1-ARE-promoter-driven luciferase plasmids using
Lipofectamine® 2000 (Invitrogen; Thermo Fisher
Scientific, Inc.) according to the manufacturer's instructions.
Following transfection for 24 h, the cells (5x103
cells/well in a 96 well plate) were treated with different
concentrations of ER (0, 10 or 20 µM) for 24 h at 37˚C. The
Dual-luciferase reporter assay system (Promega Corporation) was
used to measure luciferase activity by normalizing firefly
luciferase activity to Renilla luciferase activity.
OA model and histological
analysis
A total of 86 C57BL/6 male mice (age, 10 weeks;
weight, 24.78±4.05 g) purchased from the Comparative Medical Center
of Yangzhou University (Yangzhou, China) were kept at 24˚C in
standard mouse cages (5 animals per cage) with a 12-h light/dark
cycle and food and water access ad libitum. Mice were
monitored for health and weight every 2 or 3 days. When pain or
distress were observed, the animals were treated with buprenex
(0.1-2.0 mg/kg; Reckitt & Colman Pharmaceuticals, Inc.), which
was added to crushed or wet food. If pain or distress continued,
the mice were sacrificed regardless of the scheduled endpoints. The
criteria that determined discomfort/distress/pain were any three of
the following signs: Abnormal posture, slow, careful or abnormal
(waddling) gait, low activity levels, slow eating, cowering or
vocalizing on handling, change in eye or coat appearance and weight
loss. Animal death following sacrifice was confirmed by one of the
following criteria: No response to tail or toe pinch, no
respiration or heartbeat following continuous monitoring for 30 sec
or rigor mortis. Mice were euthanized using 100% CO2
anesthesia using an air displacement rate of 20% of the chamber
volume/min. Experiment duration was 8 weeks. The animal research
was performed in accordance with Nanjing Medical University
Institutional Animal Care and Use Committee guidelines (approval
no. IACUC 1903044). The mice were anesthetized with intraperitoneal
ketamine hydrochloride (120 mg/kg) and xylazine hydrochloride (5
mg/kg). OA was induced by sectioning of the medial meniscotibial
ligament, also known as the coronary ligament, which anchors the
medial meniscus (MM) to the tibial plateau (21). Forty mice were divided into four
groups with 10 mice per group (sham surgery + vehicle, sham surgery
+ ER, DMM surgery + vehicle and DMM surgery + ER). The sham surgery
(ligament was exposed but not transected) was performed on the left
knee of the same mice that underwent DMM on the right knee. Mice
were administered with saline as vehicle or ER (25 mg/kg/day)
dissolved in saline by oral gavage for 2 weeks immediately after
surgery. Mice were sacrificed at 8 weeks post-DMM surgery. Knee
joints were dissected free of skin or excess muscle and fixed with
10% buffered formalin for 24 h at 22˚C. Obtained sections (5 µm)
were placed in 70% ethyl alcohol for 15 min and then stained with
0.04% safranin O/sodium acetate buffer (pH 4.0) for 10 min at 22˚C.
Sectioned murine joint tissues were observed under an Olympus BX51
light microscope and photographed by a computer-operated Olympus
DP72 digital camera (Olympus Corporation). Sections of knee joints
(10 slides per joint) were evaluated by an assessor experienced in
this technique and blinded to the origin of the sample using the
Osteoarthritis Research Society International scoring system (0-6
subjective scoring system) where the higher the score, the more
severe the joint degeneration (22).
Statistical analysis
All data are presented as the mean ± SEM. All assays
were repeated at least three times independently. Statistical
analysis was performed using Mann-Whitney U test or one-way ANOVA
followed by Tukey's test using GraphPad Prism software (version
6.01; GraphPad Software, Inc.). Datasets containing a mixture of
paired and unpaired samples were analyzed using mixed ANOVA
followed by Bonferroni correction using SPSS 22.0 (SPSS, IBM Inc.).
P<0.05 was considered to indicate a statistically significant
difference.
Results
ER activates the Nrf2 pathway in
chondrocytes and cartilage
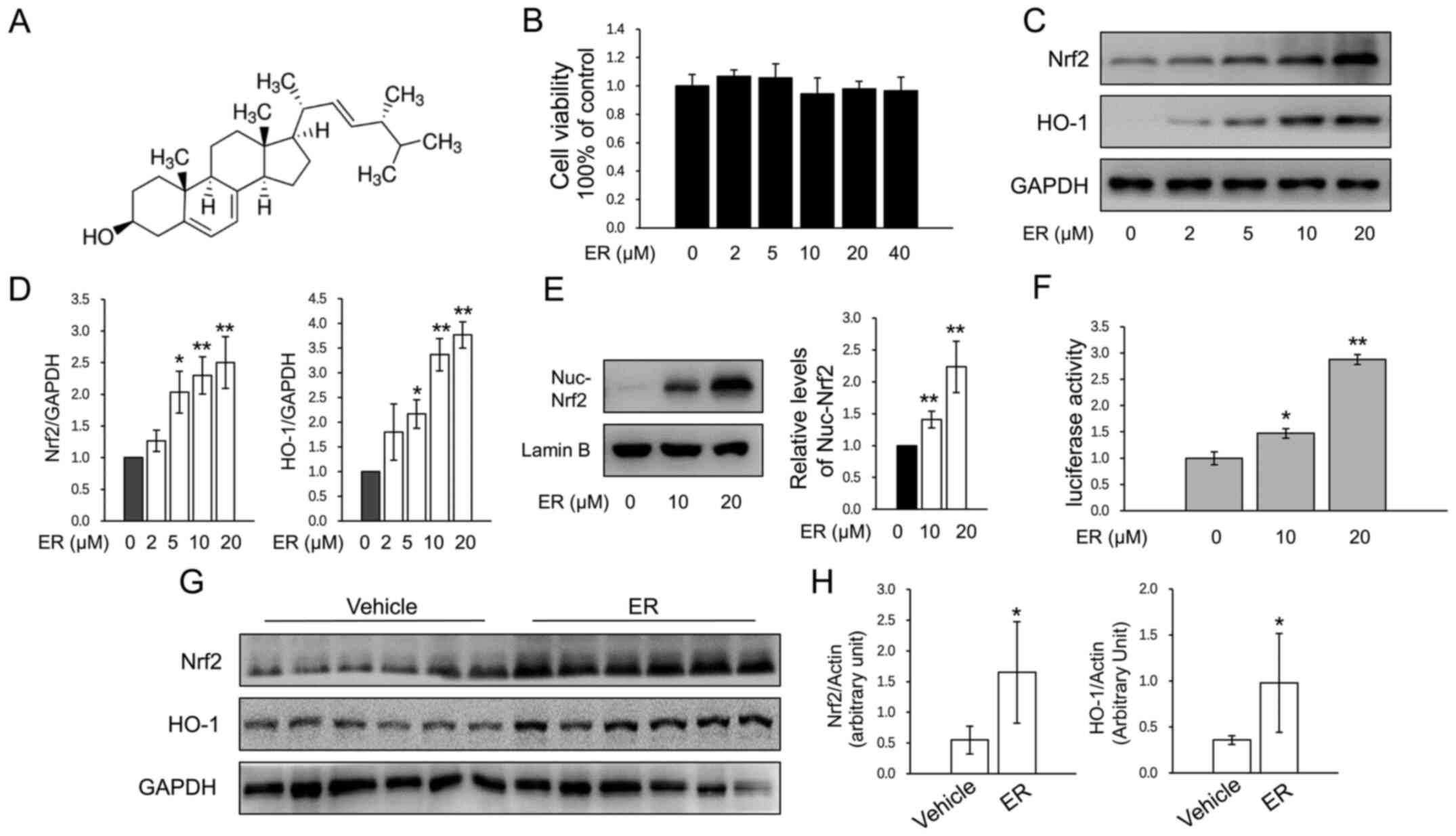
The chemical structure of ER is presented in
Fig. 1A. The cytotoxicity of ER was
measured by CCK-8 assay, and concentrations <40 µM were used in
primary murine chondrocytes (Fig.
1B). ER significantly increased the protein expression of Nrf2
and HO-1 in chondrocytes in a dose-dependent manner (Fig. 1C and D). Nuclear protein was extracted for
assays and the results showed that ER upregulated nuclear Nrf2
expression (Fig. 1E). ER treatment
caused a significant increase in luciferase activity (Fig. 1F), which indicated that ER activated
the HO-1 promoter transactivation activity. To investigate the
effects of ER on Nrf2 and HO-1 expression in the cartilage of knee
joints, mice that had undergone no treatment were administered ER
(25 mg/kg/day) for 2 weeks and cartilage samples were harvested for
western blot analysis. The results revealed that the expression
levels of both of Nrf2 and HO-1 were significantly higher in the
cartilage of the ER-treated group compared with the expression
levels in the saline-treated group (Fig. 1G and H).
ER inhibits the expression of MMPs in
chondrocytes
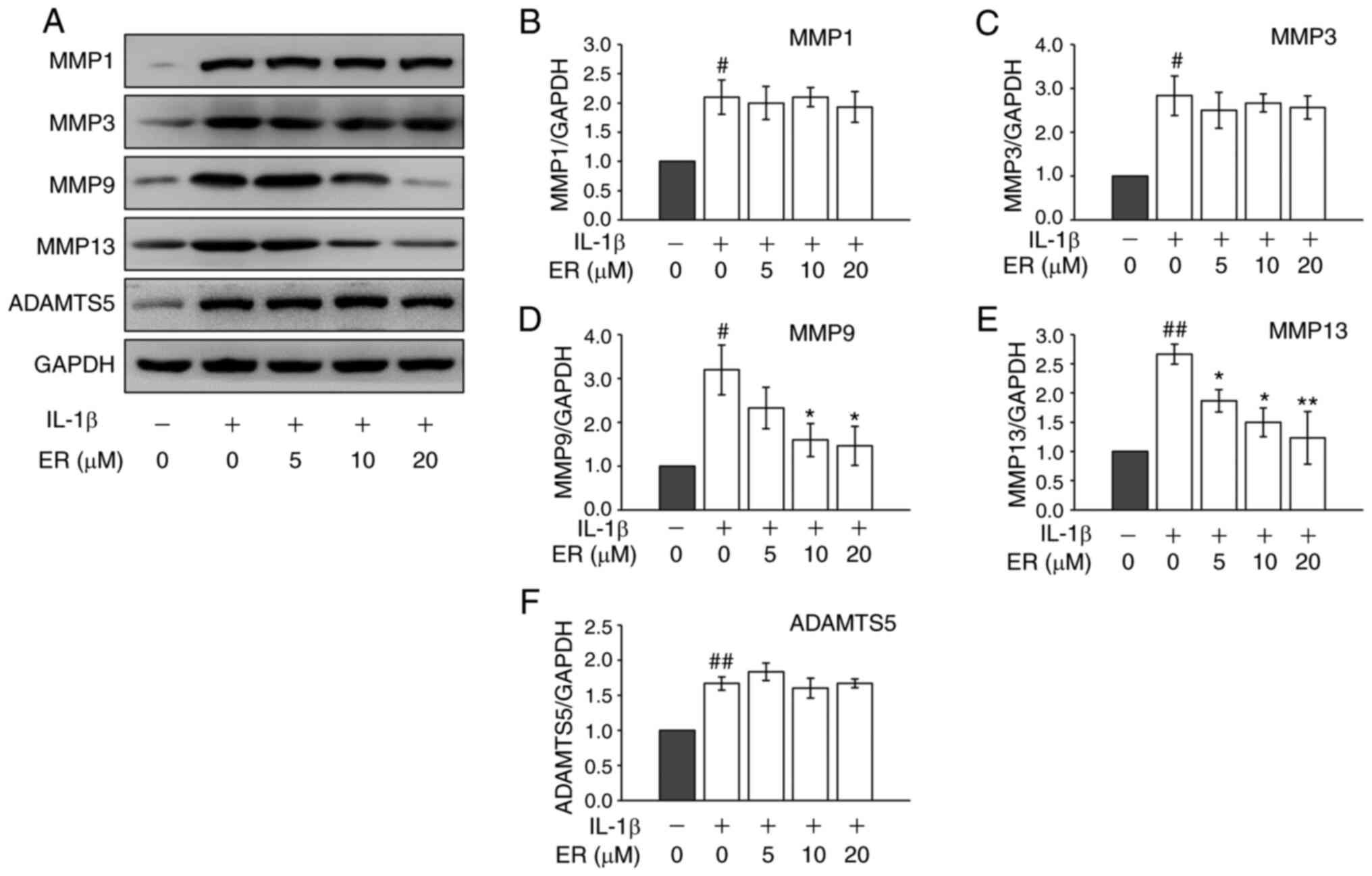
MMPs and a disintegrin and metalloproteinase with
thrombospondin motifs (ADAMTS) play a role in cartilage destruction
during OA. Both MMP-9 and MMP-13 are important proteolytic enzymes
in ECM breakdown, and abnormal up-regulation of these enzymes can
induce excess catabolism in cartilage, gradually leading to
cartilage breaking down (23,24).
To investigate the potential therapeutic effects of ER on OA, the
protein expression of matrix-degrading enzymes were further
examined. As predicted, IL-1β increased the protein expression
levels of MMP-1, MMP-3, MMP-9, MMP-13 and ADAMTS-5 in the cells
(Fig. 2). Although IL-1β induced
expression of MMP-1 and MMP-3 were not regulated by ER (Fig. 2A-C), significant inhibition of
matrix-degrading enzymes MMP-9 and MMP-13 was observed at the
protein level (Fig. 2D and E). ADAMTS-5 expression also did not appear
to be regulated by ER (Fig. 2F).
Considering their roles in the cartilage degradation network
(25,26), it was hypothesized that ER may
suppress MMP-9 and MMP-13 expression in chondrocytes and reduce
cartilage breakdown.
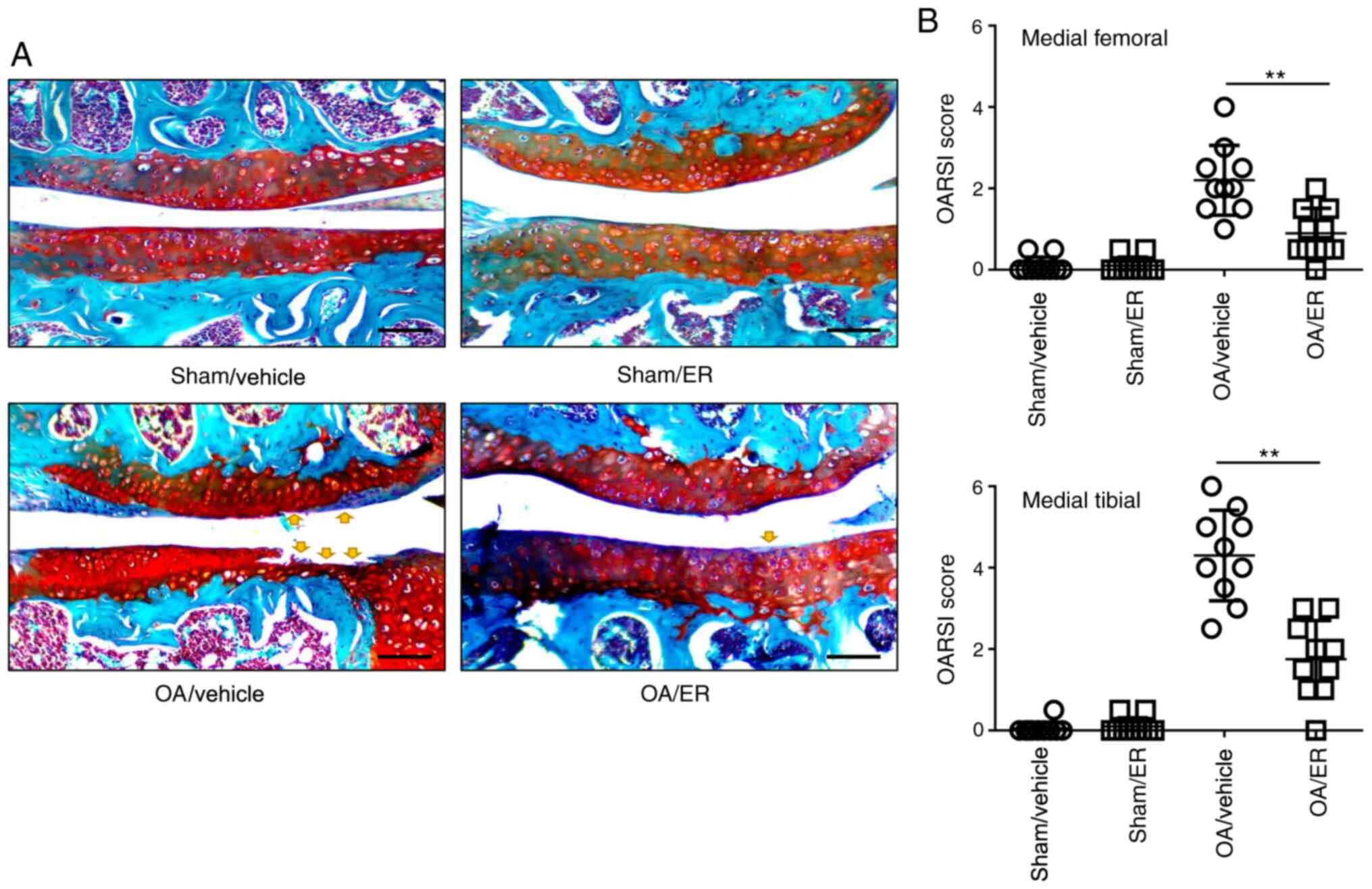
ER alleviates cartilage erosion in
experimental OA
To determine the protective effects of ER against
OA, a murine model of OA was induced by transecting the medial
meniscotibial ligament. ER (25 mg/kg/day) or vehicle was
administered for 2 weeks post-operation. Mice were sacrificed at 8
weeks post-DMM surgery. The knee joint tissues were collected for
Safranin O/Fast Green staining. Histological sections were assessed
using OARSI scores in a blinded manner. The data showed that the
ER-treated group significantly improved femur and tibia
proteoglycan loss or cartilage damage compared with the
vehicle-treated group at 8 weeks post-DMM surgery. Knee cartilage
harvested from mice administrated with ER or vehicle that underwent
sham surgery showed no damage, indicating the ER administration had
no effect on an undamaged knee. These results suggested that that
oral administration of ER in mice effectively delayed the
progression of OA (Fig. 3A and
B).
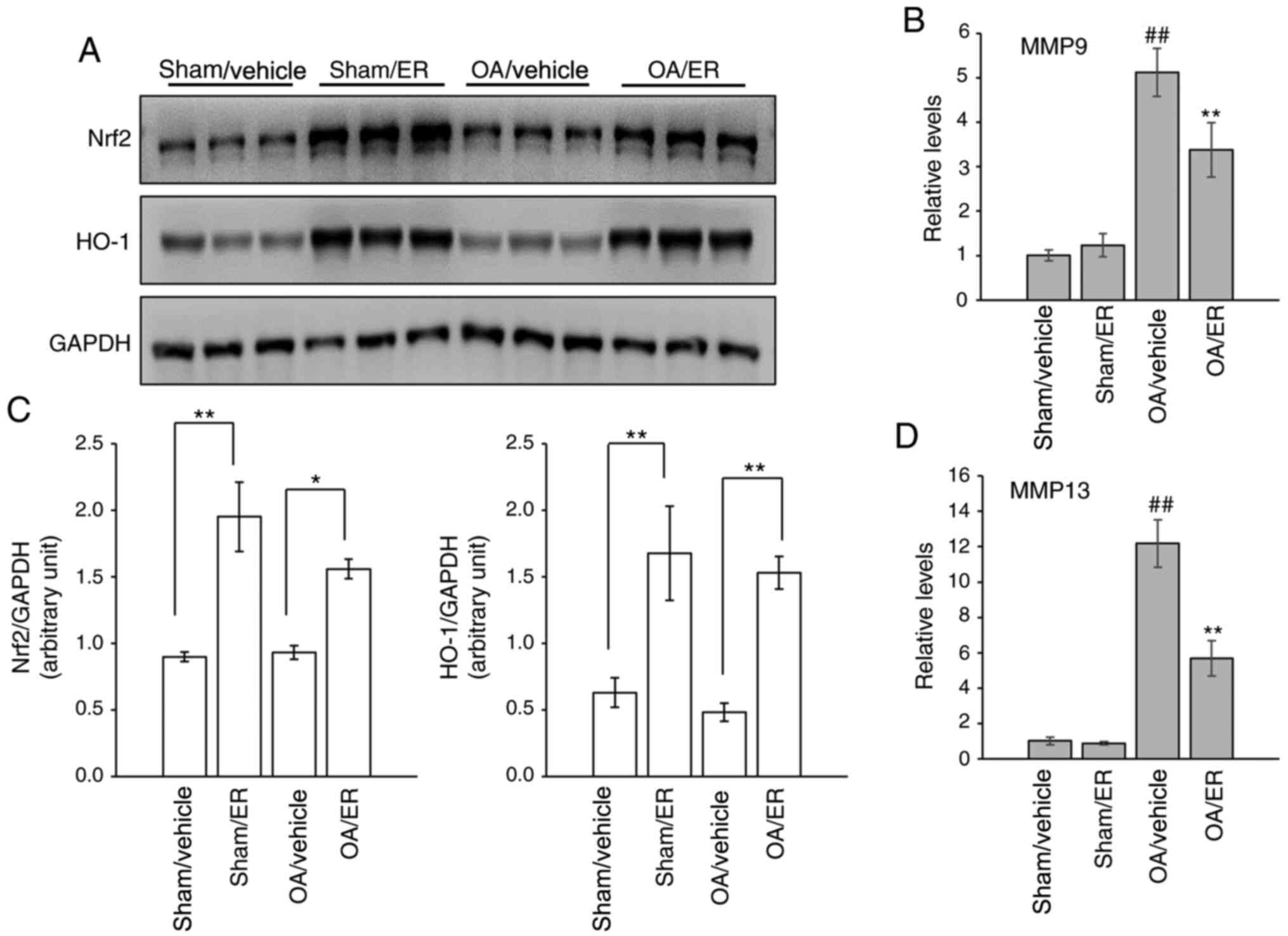
ER promotes expression of Nrf2/HO-1
and suppresses gene expression of MMPs in experimental OA
The expression levels of Nrf2 and HO-1 in the
cartilage of knee joints were measured by western blot analysis to
determine whether activation of the Nrf2 signaling pathway in
response to ER was similar to that observed in vitro. The
results showed that protein expression levels of Nrf2 and HO-1 were
increased in the cartilage of mice administrated orally with ER
compared with the respective vehicle-treated groups (Fig. 4A and C). The effect of ER on the expression of
MMP-9 and MMP-13 in articular cartilage was further assessed. Gene
transcript analysis results showed that the mRNA expression levels
of MMP-9 and MMP-13 were increased in the knee cartilage from the
OA/vehicle group compared with the sham/vehicle group, and this
upregulation was suppressed by ER (Fig.
4B and D), similar to the
aforementioned in vitro assay results.
Discussion
Excessive oxidative stress is associated with OA and
triggers chondrocyte senescence and apoptosis, extracellular matrix
(ECM) degradation, dysfunction of the subchondral bone and synovial
inflammation during OA (27). The
transcription factor Nrf2 regulates the expression of a set of
genes that counteract oxidative stress. Under physiological
conditions, Nrf2 is tethered in the cytoplasm by its inhibitor,
Kelch-ECH associated protein 1 (KEAP1), which controls its
proteasomal degradation. During increased oxidative or
electrophilic stress, Nrf2 is released from KEAP1 and translocates
to the nucleus, binding to AREs located in the promoter regions to
activate its target genes, including HO-1, which is an important
defense against reactive oxygen species-mediated damage in various
tissue injuries (28). A previous
study revealed that HO-1 expression markedly decreased in the
articular cartilage of wild-type mice with age, and that
maintenance of HO-1 expression had the potential to protect against
OA development (29). The present
study confirmed that ER enhanced the nuclear translocation of Nrf2,
promoted HO-1 promoter transactivation and upregulated the
expression of genes downstream of Nrf2, indicating that ER
activated the Nrf2 signaling pathway, which contributed to
preventing cartilage degeneration.
Common medication for OA aims to relieve patient's
joint pain of to improve quality of life (30,31).
Trichostatin A, a histone deacetylase inhibitor, was used to
activate Nrf2 signaling pathways, subsequently leading to a
significantly decreased severity of cartilage damage in DMM
surgery-induced OA mice in our previous study (10). While disease-modifying OA drugs
(DMOAD) require long-term use, the search for potential drugs
targeting Nrf2 activation continues, considering the side effects
caused by trichostatin A (32). In
the present study, ergosterol significantly activated the Nrf2
pathway in primary chondrocytes. The present study further
demonstrated that oral administration of ER in mice significantly
increased the expression of Nrf2 and HO-1 in murine knee cartilage,
exerting protective effects on the cartilage during OA.
Additionally, the oral administration of ER may make it more
suitable for clinical use as a potential DMOAD in comparison with
injectable therapeutic agents.
When measuring the expression of ECM-degrading
proteases, the present study found that ER significantly inhibited
the expression levels of MMP9 and MMP13 in chondrocytes and
cartilage, which were enhanced during OA and play an important role
in articular cartilage damage. Previous studies reported that
upregulation of Nrf2 downstream proteins such as HO-1 can reduce
the expression of MMPs and inhibit the production of
proinflammatory cytokines (29,33-35).
Hence, the inhibition of MMP9 and MMP13 may be partially attributed
to ER-induced Nrf2 signaling activation.
Several limitations of the present study should be
noted. First, the mice used in the experiments were relatively
young (~10 weeks old) with immature skeletons. Therefore, the
possibility that other late developmental events might affect the
effectiveness of ER in the OA model could not be excluded. Second,
since ER was only administered at the same time as OA onset in the
experiment, it could not be determined whether ER was protective in
pre-arthritic knees. A middle-stage OA model should be used in
future investigations to fully elucidate the preventive effect of
ER. Although to the best of our knowledge there are no direct links
between ER and Nrf2 expression in the existing literature, with
further research into the physiological effect of ER, more
cross-pathways may be found to help understand the potential
regulation of Nrf2 protein expression by ER. The present study made
an assumption that ER may alter the expression of Nrf2 gene through
epigenetics, such as microRNA (miRNA/miR). Previous studies have
shown that miR-144, miR-28, miR-93 and other miRNAs can regulate
Nrf2 gene expression (36-38),
while miR-125a, miR-378 and other miRNAs have been shown to be
regulated by ER (39-41).
A possibility that ER mediated the expression of Nrf2 through
certain miRNA cannot be ruled out in the present study.
In conclusion, the present study found that ER
served a regulatory role in anti-oxidative damage and reduction of
catabolism in cartilage tissues, suggesting that ER could be
considered a promising effective option for the treatment of
OA.
Acknowledgements
Not applicable.
Funding
The present study was supported by grants from the
Natural Science Foundation of the Jiangsu Higher Education
Institutions of China (grant no. 18KJB320009), Scientific Research
Project of Hunan Education Department (grant no. 13C836) and
Technological Innovation Guidance Plan of Hunan Province (grant no.
2017SK50214).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
DC, XW and JQ conceived and designed the study. DC,
HY, JL, SC and LJ performed the experiments. DC and HY wrote the
paper. JQ and XW reviewed and edited the manuscript. All authors
read and approved the final manuscript.
Ethics approval and consent to
participate
All experiments performed with the use of animals
were approved by the Animal Ethical and Welfare Committee of
Nanjing Medical University Institutional Animal Care and Use
Committee (approval no. IACUC 1903044).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Li T, Ma J, Zhao T, Gao F and Sun W:
Application and efficacy of extracorporeal shockwave treatment for
knee osteoarthritis: A systematic review and meta-analysis. Exp
Ther Med. 18:2843–2850. 2019.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Fragkiadaki P, Nikitovic D, Kalliantasi K,
Sarandi E, Thanasoula M, Stivaktakis PD, Nepka C, Spandidos DA,
Tosounidis T and Tsatsakis A: Telomere length and telomerase
activity in osteoporosis and osteoarthritis. Exp Ther Med.
19:1626–1632. 2020.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Joly DA, Ludwig T, Mahdavi S, Khong H,
Piroozfar SG and Sharma R: Does age influence patient-reported
outcomes in unilateral primary total hip and knee arthroplasty? J
Arthroplasty. 35:1800–1805. 2020.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Park C, Hong SH, Shin SS, Lee DS, Han MH,
Cha HJ, Kim S, Kim HS, Kim GY, Park EK, et al: Activation of the
Nrf2/HO-1 signaling pathway contributes to the protective effects
of sargassum serratifolium extract against oxidative stress-induced
DNA damage and apoptosis in SW1353 human chondrocytes. Int J
Environ Res Public Health. 15(1173)2018.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Vaamonde-Garcia C, Courties A, Pigenet A,
Laiguillon MC, Sautet A, Houard X, Kerdine-Römer S, Meijide R,
Berenbaum F and Sellam J: The nuclear factor-erythroid 2-related
factor/heme oxygenase-1 axis is critical for the inflammatory
features of type 2 diabetes-associated osteoarthritis. J Biol Chem.
292:14505–14515. 2017.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Alcaraz MJ and Ferrandiz ML: Relevance of
Nrf2 and heme oxygenase-1 in articular diseases. Free Radic Biol
Med. 157:83–93. 2020.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Li X, Lin J, Ding X, Xuan J, Hu Z, Wu D,
Zhu X, Feng Z, Ni W and Wu A: The protective effect of sinapic acid
in osteoarthritis: In vitro and in vivo studies. J Cell Mol Med.
23:1940–1950. 2019.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Qiao YQ, Jiang PF and Gao YZ: Lutein
prevents osteoarthritis through Nrf2 activation and downregulation
of inflammation. Arch Med Sci. 14:617–624. 2018.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Robledinos-Antón N, Fernández-Ginés R,
Manda G and Cuadrado A: Activators and inhibitors of NRF2: A review
of their potential for clinical development. Oxid Med Cell Longev.
2019(9372182)2019.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Cai D, Yin S, Yang J, Jiang Q and Cao W:
Histone deacetylase inhibition activates Nrf2 and protects against
osteoarthritis. Arthritis Res Ther. 17(269)2015.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Landolfo S, Zara G, Zara S, Budroni M,
Ciani M and Mannazzu I: Oleic acid and ergosterol supplementation
mitigates oxidative stress in wine strains of Saccharomyces
cerevisiae. Int J Food Microbiol. 141:229–235. 2010.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Yasukawa K, Aoki T, Takido M, Ikekawa T,
Saito H and Matsuzawa T: Inhibitory effects of ergosterol isolated
from the edible mushroom Hypsizigus marmoreus on TPA-induced
inflammatory ear oedema and tumour promotion in mice. Phytother
Res. 8:10–13. 1994.
|
|
13
|
Xu J, Lin C, Wang T, Zhang P, Liu Z and Lu
C: Ergosterol attenuates LPS-induced myocardial injury by
modulating oxidative stress and apoptosis in rats. Cell Physiol
Biochem. 48:583–592. 2018.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Cai D, Feng W, Liu J, Jiang L, Chen S,
Yuan T, Yu C, Xie H, Geng D and Qin J: 7,8-Dihydroxyflavone
activates Nrf2/HO-1 signaling pathways and protects against
osteoarthritis. Exp Ther Med. 18:1677–1684. 2019.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Shakibaei M, John T, Seifarth C and
Mobasheri A: Resveratrol inhibits IL-1 beta-induced stimulation of
caspase-3 and cleavage of PARP in human articular chondrocytes in
vitro. Ann N Y Acad Sci. 1095:554–563. 2007.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Zhou PH, Liu SQ and Peng H: The effect of
hyaluronic acid on IL-1beta-induced chondrocyte apoptosis in a rat
model of osteoarthritis. J Orthop Res. 26:1643–1648.
2008.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Cai D, Huff TW, Liu J, Yuan T, Wei Z and
Qin J: Alleviation of cartilage destruction by sinapic acid in
experimental osteoarthritis. Biomed Res Int.
2019(5689613)2019.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Lorenz H, Wenz W, Ivancic M, Steck E and
Richter W: Early and stable upregulation of collagen type II,
collagen type I and YKL40 expression levels in cartilage during
early experimental osteoarthritis occurs independent of joint
location and histological grading. Arthritis Res Ther. 7:R156–R165.
2005.PubMed/NCBI View
Article : Google Scholar
|
|
19
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408.
2001.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Sherf BA, Navarro SL, Hannah RR and Wood
KV: Dual-luciferase reporter assay: an advanced co-reporter
technology integrating firefly and Renilla luciferase assays.
Promega Notes. 57:2–9. 1996.
|
|
21
|
Glasson SS, Blanchet TJ and Morris EA: The
surgical destabilization of the medial meniscus (DMM) model of
osteoarthritis in the 129/SvEv mouse. Osteoarthritis Cartilage.
15:1061–1069. 2007.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Glasson SS, Chambers MG, Van Den Berg WB
and Little CB: The OARSI histopathology initiative-recommendations
for histological assessments of osteoarthritis in the mouse.
Osteoarthritis Cartilage. 18 (Suppl 3):S17–S23. 2010.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Zeng GQ, Chen AB, Li W, Song JH and Gao
CY: High MMP-1, MMP-2, and MMP-9 protein levels in osteoarthritis.
Genet Mol Res. 14:14811–14822. 2015.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Zhou X, Cao H, Yuan Y and Wu W:
Biochemical signals mediate the crosstalk between cartilage and
bone in osteoarthritis. Biomed Res Int.
2020(5720360)2020.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Li H, Wang D, Yuan Y and Min J: New
insights on the MMP-13 regulatory network in the pathogenesis of
early osteoarthritis. Arthritis Res Ther. 19(248)2017.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Chen J, Wang C, Huang K, Chen S and Ma Y:
Acacetin suppresses IL-1β-induced expression of matrix
metalloproteinases in chondrocytes and protects against
osteoarthritis in a mouse model by inhibiting NF-κB signaling
pathways. Biomed Res Int. 2020(2328401)2020.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Lepetsos P and Papavassiliou AG:
ROS/oxidative stress signaling in osteoarthritis. Biochim Biophys
Acta. 1862:576–591. 2016.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Ahmed SM, Luo L, Namani A, Wang XJ and
Tang X: Nrf2 signaling pathway: Pivotal roles in inflammation.
Biochim Biophys Acta Mol Basis Dis. 1863:585–597. 2017.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Takada T, Miyaki S, Ishitobi H, Hirai Y,
Nakasa T, Igarashi K, Lotz MK and Ochi M: Bach1 deficiency reduces
severity of osteoarthritis through upregulation of heme
oxygenase-1. Arthritis Res Ther. 17(285)2015.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Wang L, Chen H, Lu H, Wang Y, Liu C, Dong
X, Chen J, Liu N, Yu F, Wan Q and Shang S: The effect of
transtheoretical model-lead intervention for knee osteoarthritis in
older adults: A cluster randomized trial. Arthritis Res Ther.
22(134)2020.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Zhong HM, Zhao GF, Lin T, Zhang XX, Li XY,
Lin JF, Zhao SQ and Pan ZJ: Intra-articular steroid injection for
patients with hip osteoarthritis: A systematic review and
meta-analysis. Biomed Res Int. 2020(6320154)2020.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Im GI and Choi YJ: Epigenetics in
osteoarthritis and its implication for future therapeutics. Expert
Opin Biol Ther. 13:713–721. 2013.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Park SY, Jin ML, Kim YH, Lee SJ and Park
G: Sanguinarine inhibits invasiveness and the MMP-9 and COX-2
expression in TPA-induced breast cancer cells by inducing HO-1
expression. Oncol Rep. 31:497–504. 2014.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Rousset F, Nguyen MV, Grange L, Morel F
and Lardy B: Heme oxygenase-1 regulates matrix metalloproteinase
MMP-1 secretion and chondrocyte cell death via Nox4 NADPH oxidase
activity in chondrocytes. PLoS One. 8(e66478)2013.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Lee IT, Luo SF, Lee CW, Wang SW, Lin CC,
Chang CC, Chen YL, Chau LY and Yang CM: Overexpression of HO-1
protects against TNF-alpha-mediated airway inflammation by
down-regulation of TNFR1-dependent oxidative stress. Am J Pathol.
175:519–532. 2009.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Li B, Zhu X, Ward CM, Starlard-Davenport
A, Takezaki M, Berry A, Ward A, Wilder C, Neunert C, Kutlar A and
Pace BS: MIR-144-mediated NRF2 gene silencing inhibits fetal
hemoglobin expression in sickle cell disease. Exp Hematol.
70:85–96.e5. 2019.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Yang M, Yao Y, Eades G, Zhang Y and Zhou
Q: MiR-28 regulates Nrf2 expression through a Keap1-independent
mechanism. Breast Cancer Res Treat. 129:983–991. 2011.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Singh B, Ronghe AM, Chatterjee A, Bhat NK
and Bhat HK: MicroRNA-93 regulates NRF2 expression and is
associated with breast carcinogenesis. Carcinogenesis.
34:1165–1172. 2013.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Wang L, Yang Y and Hong B: Advances in the
role of microRNAs in lipid metabolism-related anti-atherosclerotic
drug discovery. Expert Opin Drug Discov. 8:977–990. 2013.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Croston TL, Lemons AR, Beezhold DH and
Green BJ: MicroRNA regulation of host immune responses following
fungal exposure. Front Immunol. 9(170)2018.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Wu QP, Xie YZ, Deng Z, Li XM, Yang W, Jiao
CW, Fang L, Li SZ, Pan HH, Yee AJ, et al: Ergosterol peroxide
isolated from Ganoderma lucidum abolishes microRNA miR-378-mediated
tumor cells on chemoresistance. PLoS One. 7(e44579)2012.PubMed/NCBI View Article : Google Scholar
|