Introduction
Osteoporosis (OP) and vascular calcification (VC)
are known worldwide as major risk factors of mortality and
morbidity (1,2). VC causes alterations to the blood
pressure profile and an increased pulsatile pressure and flow load,
which can damage target organs, such as the heart, brain and
kidneys, and therefore increases the risk of cardiovascular
morbidity and mortality (3). OP has
been indicated to result in increased susceptibility to fracture
and painful morbidity (4). Although
both factors were considered independently for a long time, studies
have suggested a close relationship between OP and VC (1,2). Both
OP and VC share a number of common risk factors, pathophysiologic
mechanisms and etiology (5,6), which is well summarized in the
expression ‘bone-vascular axis’ that was coined to describe the
cross-talk between bone and cardiovascular metabolism (7). Several emerging findings have
suggested that bone loss may promote VC, and that VC also has an
impact on bone metabolism (8,9);
therefore, it is of crucial importance to establish a treatment
that targets both disorders.
Although associations between VC and bone loss have
been demonstrated (3), it remains
unclear which exact mechanisms are core in the bone-vascular axis.
VC and osteoporosis share a number of risk factors, including
aging, chronic sterile inflammation and metabolic disorders
(3). However, considerable research
is required to identify the mechanisms that link VC to bone
deterioration. Several molecules have been reported to serve a
crucial role in the bone-vascular axis (9), among which, sclerostin might serve a
major role (10-13).
Sclerostin, a 22-kDa glycoprotein, is a well-known inhibitor of
bone formation (14). However,
previous studies have also reported the potential role of
sclerostin in the development of VC (14-16).
Sclerostin positively correlates with the presence of
atherosclerosis, and is present in atherosclerotic plaques, mainly
expressed by vascular smooth muscle cells (VSMCs) in patients
(14,15). Furthermore, the expression of
sclerostin in calcifying VSMC has been observed, and it has been
demonstrated to have a protective role in the vasculature (16). These results suggest that sclerostin
may be a target for the simultaneous regulation of VC and
osteoporosis.
Injection of Shuxuetong (SXT), a Chinese Materia
Medica standardized product extracted from Hirudo and
Pheretima, has been widely used in Traditional Chinese
Medicine, particularly in patients with stroke and myocardial
infarction (17-19).
Each ampoule of SXT (2 ml) is derived from ~0.5 g leech and ~0.5 g
earthworm (18), and the main
active ingredients are considered to be peptides, glycopeptides and
oligosaccharides (20). In addition
to its antithrombotic, anticoagulant and fibrinolytic activities
(21), injection of SXT injection
has also been shown to confer vascular protection (18,20).
In addition, SXT has been reported to decrease the levels of
pro-inflammatory cytokines and inhibit the inflammation (22-24)
that contributes to progression of VC and osteoporosis (5). Therefore, it was hypothesized that the
injection of SXT could be used for the simultaneous treatment of VC
and osteoporosis by regulating the vascular-bone axis.
Epidemiologic research demonstrated that
glucocorticoids (GCs) have long been used globally for
inflammatory, immunologic and allergic disorders (25). However, long-term exposure to GCs
simultaneously induces osteoporosis and VC (26-29).
The synthetic long-acting GC dexamethasone (DEX) may cause more
severe adverse effects compared with short-acting medication such
as methylprednisolone (26). DEX
may induce VC by downregulating calcification-inhibiting molecules
and accelerating osteoblastic differentiation of VSMCs (27,28),
while osteonecrosis of the femoral head could also be caused by DEX
via activation of endoplasmic reticulum stress (ERS) (29). Thus, a rat model of simultaneous VC
and OP induced by DEX was used in the present study to investigate
the ameliorative effect of SXT injections.
Materials and methods
Experimental protocols
All animal care and experimental protocols were in
compliance with the P.R. China Animal Management Rule (30) and the National Institutes of Health
Guide for the Care and Use of Laboratory Animals (31), and were approved by the Animal Care
Committee of the Hebei Provincial Hospital of Chinese Medicine
(Shijiazhuang, China; approval no. 2019-KY-012-01). Male
Sprague-Dawley (SD) rats (8 weeks old; 200-220 g) were supplied by
the Animal Center of the Hebei Medical University (Hebei, China).
All animals were housed at 22±2˚C with a relative humidity of
50±10% and a 12-h light/dark cycle. The animals had free access to
water and food.
A total of 32 rats were randomly divided into four
groups: i) Control group (Con), only treated with vehicle; ii) SXT
group, intraperitoneally injected with 0.6 ml/kg/day (19,20)
SXT (Mudanjiang Youbo Pharmaceutical Co. Ltd.) for 4 weeks; iii) VC
and osteoporosis group (DEX), intramuscularly injected with 1
mg/kg/day DEX (Guangzhou Baiyunshan Tianxin Pharmaceutical Co.,
Ltd.) for 4 weeks; iv) DEX plus SXT injection group (DEX + SXT).
The recommended dose of SXT injection in the manufacturer's package
insert is 0.1 ml/kg/day. The coefficient of conversion between
human (60 kg) and rats (200 g) is ~6. Therefore, the dose of 0.6
ml/kg/day was used in the present study. After 4 weeks of
treatment, the rats were anesthetized with 50 mg/kg sodium
pentobarbital, and then sacrificed for further assessment.
Histological staining
Thoracic aortas were fixed in 10% neutral buffered
formaldehyde for 24 h at 4˚C, embedded in paraffin, cut into
6-µm-thick sections, and stained with Alizarin Red S at room
temperature to measure the amount of calcium deposition in the
vessels. The two femoral heads were harvested through dissection
and placed in 10% neutral buffered formaldehyde for 72 h at 4˚C for
fixation. After fixation, the femoral heads were decalcified and
stained with hematoxylin and eosin (H&E) staining. The sections
were stained with hematoxylin for 10 min at room temperature, and
after washing with distilled water, they were stained with eosin
for 3 min at room temperature. The slides were visualized using a
digital light microscope (magnification, x200; Olympus
Corporation).
Immunohistochemistry
For immunohistochemistry, the left femoral heads
were harvested through dissection and placed in 10% neutral
buffered formaldehyde for 72 h at 4˚C for fixation. The tissues
were embedded in paraffin and cut into 6-µm thick sections. The
sections were then incubated with primary antibodies (Table I) at 4˚C overnight. Bovine serum
albumin (1%; Sigma-Aldrich; Merck KGaA) was used as the blocking
agent at room temperature for 1 h. The primary antibodies were
detected after incubation with secondary antibody (Table I) conjugated to horseradish peroxide
for 30 min at 37˚C, and visualized with 3,3'-diaminobenzidine
tetrahydrochloride. The slides were visualized using a digital
light microscope (Olympus Corporation), and images were analyzed
using ImageJ software (v1.48; National Institutes of Health). The
positive rate of sclerostin was defined using the following
formula: (Positive area/specimen area) x100.
 | Table IAntibody information. |
Table I
Antibody information.
| Antibody | Cat. no. | Supplier | Dilution |
|---|
| GRP78 | ab21685 | Abcam | 1:2,000 |
| CHOP | ab10444 | Abcam | 1:500 |
| Phosphorylated
Akt | 4534 | Cell Signaling
Technology, Inc. | 1:2,000 |
| Akt | 4691 | Cell Signaling
Technology, Inc. | 1:1,000 |
| Sclerostin | ab85799 | Abcam | 1:1,000 |
| Calponin | ab203047 | Abcam | 1:1,000 |
| SM22α | ab14106 | Abcam | 1:2,000 |
| RUNX2 | ab114133 | Abcam | 1:1,000 |
| BMP | ab14933 | Abcam | 1:1,000 |
| β-actin | GTX109639 | GeneTex, Inc. | 1:2,000 |
| Goat anti-rabbit
secondary antibody (HRP conjugate) | GTX213110 | GeneTex, Inc. | 1:5,000-10,000 |
Terminal deoxynucleotidyl
transferase-mediated dUTP nick-end labeling (TUNEL) staining
TUNEL staining was performed for in situ
detection of late apoptosis. The bilateral femora were harvested
through dissection and placed in 10% neutral buffered formaldehyde
for 72 h at 4˚C for fixation. The femora were embedded in paraffin
and cut into 6-µm thick sections. The sections underwent TUNEL
staining using commercial kits according to the manufacturer's
instructions (cat. no. C1098; Beyotime Institute of
Biotechnology).
Quantification of calcium and alkaline
phosphatase (ALP) activity
For the determination of calcium content, aortic
tissues were dried at 55˚C, and dissolved in 0.6 N HCl at 4˚C for
24 h. The supernatant fluid was used to measure calcium content by
colorimetry in a reaction with o-Cresolphtalein complexone (cat.
no. C004-2; Nanjing Jiancheng Bioengineering Institute Co., Ltd.).
ALP activity in the aortic vessels was measured using an ALP
colorimetric assay kit according to the manufacturer's instructions
(cat. no. A059-2-2; Nanjing Jiancheng Bioengineering
Institute).
Western blotting
Aortic tissues were homogenized in a lysis buffer
[1% NP-40, 20 mmol/l Tris-HCl (pH 8.0), 137.5 mmol/l NaCl, l mmol/l
Na3VO4, l mmol/l PMSF and 10 µg/ml
aprotinin]. The lysate's protein concentration was determined using
the Bradford method. An equal volume of 2X SDS sample buffer [0.125
mol/l Tris-HCl (pH 7.4), 4% SDS and 20% glycerol] was added, then
the samples were boiled for 5 min. The samples comprising 50 µg of
protein underwent 10% SDS-PAGE for 3 h at 60 mA. The proteins were
then electrophoretically transferred to a nitrocellulose membrane
and incubated for 1 h in TBS containing 5% non-fat powdered milk at
room temperature. The membranes were then incubated with the
primary antibodies (Table I) at 4˚C
overnight. After washing each membrane with TBS-0.1% Tween-20
(TBST) three times for 10 min, the membranes were incubated with
the secondary antibody (Table I)
for 1 h at room temperature. The membranes were then washed with
TBST three times for 10 min each and detected using enhanced
chemiluminescence (cat. no. P1050-500; Applygen Technologies,
Inc.). Autoradiographs were scanned, and the relative densities
were semi-quantified by ImageJ software (v1.48; National Institutes
of Health).
Statistical analysis
Continuous data are presented as the mean ± SD
(n=8/group). Unpaired Student's t-test was performed to compare the
results of the two groups. One-way analysis of variance followed by
Tukey's post hoc test was performed to compare the results of >2
groups. P<0.05 was considered to indicate a statistically
significant difference.
Results
Ameliorative effect of SXT on
DEX-induced VC
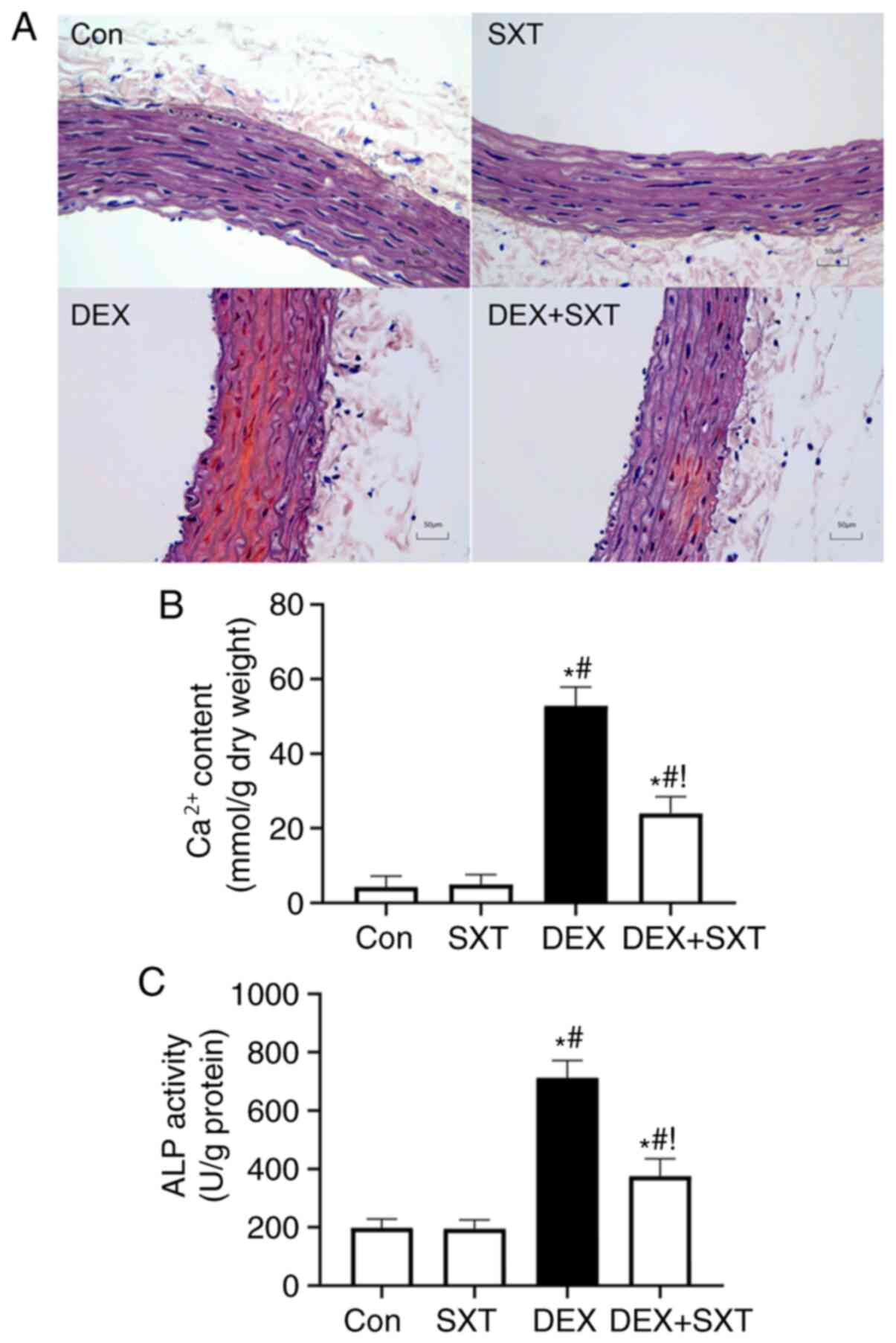
Alizarin red staining revealed increased calcium
deposition, and destruction of elastic fibers and VSMCs in the
aortic tunica media of DEX rats, which was notably ameliorated by
treatment with SXT (Fig. 1A).
Additionally, the DEX-induced increase in calcium and ALP activity
in the aorta was also attenuated by injection of SXT (Fig. 1B and C). SXT injection alone did not
significantly influence calcium content and ALP activity.
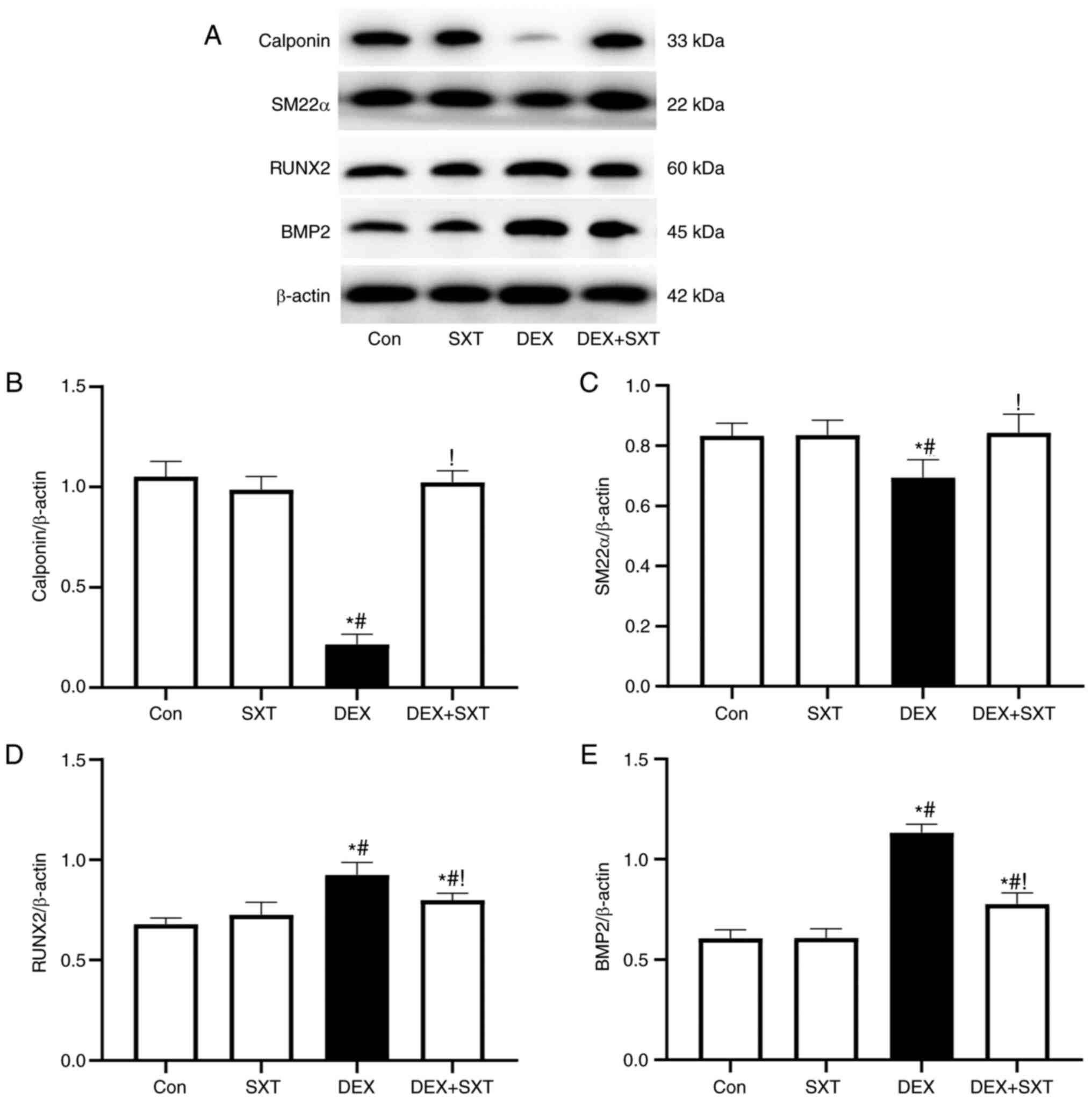
The protein expression levels of contractile
phenotype markers of VSMCs, including calponin and smooth muscle
22α (SM22α), were significantly reduced in the DEX-treated group
compared with in the control group, but were restored following
injection of SXT (Fig. 2).
Injection of SXT also attenuated the increased protein expression
levels of the osteoblastic phenotype markers runt-related
transcription factor 2 (RUNX2) and bone morphogenetic protein-2
(BMP2) in rats with DEX, whereas the protein expression levels of
these markers in the aorta were not significantly influenced in the
SXT alone group (Fig. 2).
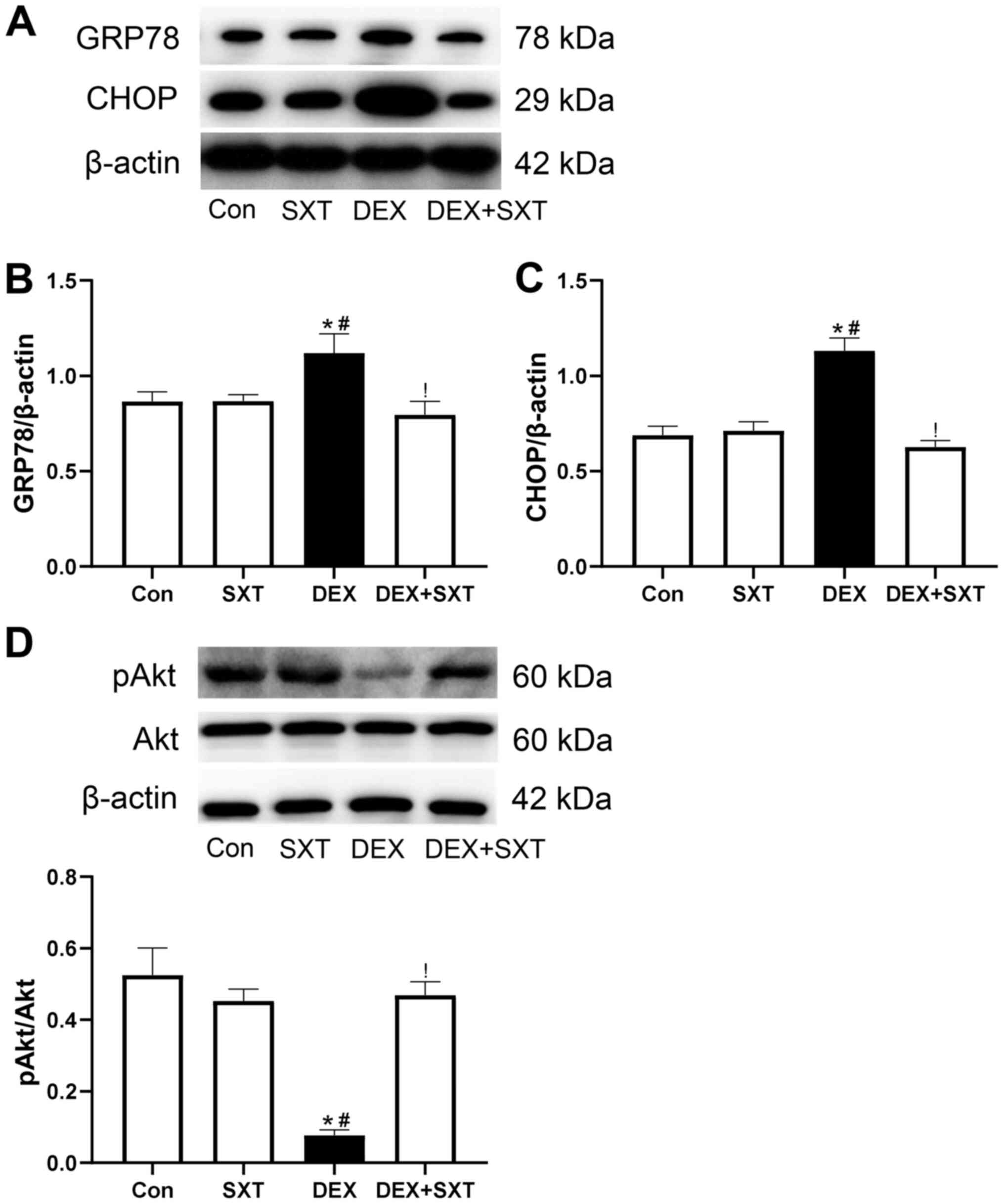
SXT inhibits DEX-induced activation of
ERS and increased levels of sclerostin in the aorta
The protein expression levels of ERS markers,
including glucose-regulated protein 78 (GRP78) and C/EBP-homologous
protein (CHOP), were determined by western blotting. Compared with
the Con and SXT groups, the protein expression levels of GRP78 and
CHOP in aortic tissue were significantly increased in the DEX
group, and were attenuated in the DEX + SXT group (Fig. 3A-C). Conversely, SXT injection
rescued the decrease in relative levels of phosphorylated (p)Akt
induced by DEX treatment (Fig. 3D).
The increased protein expression levels of sclerostin induced by
DEX in the aorta were also significantly attenuated by treatment
with SXT injection (Fig. 4).
SXT ameliorates DEX-induced
osteoporosis and apoptosis of the femoral head
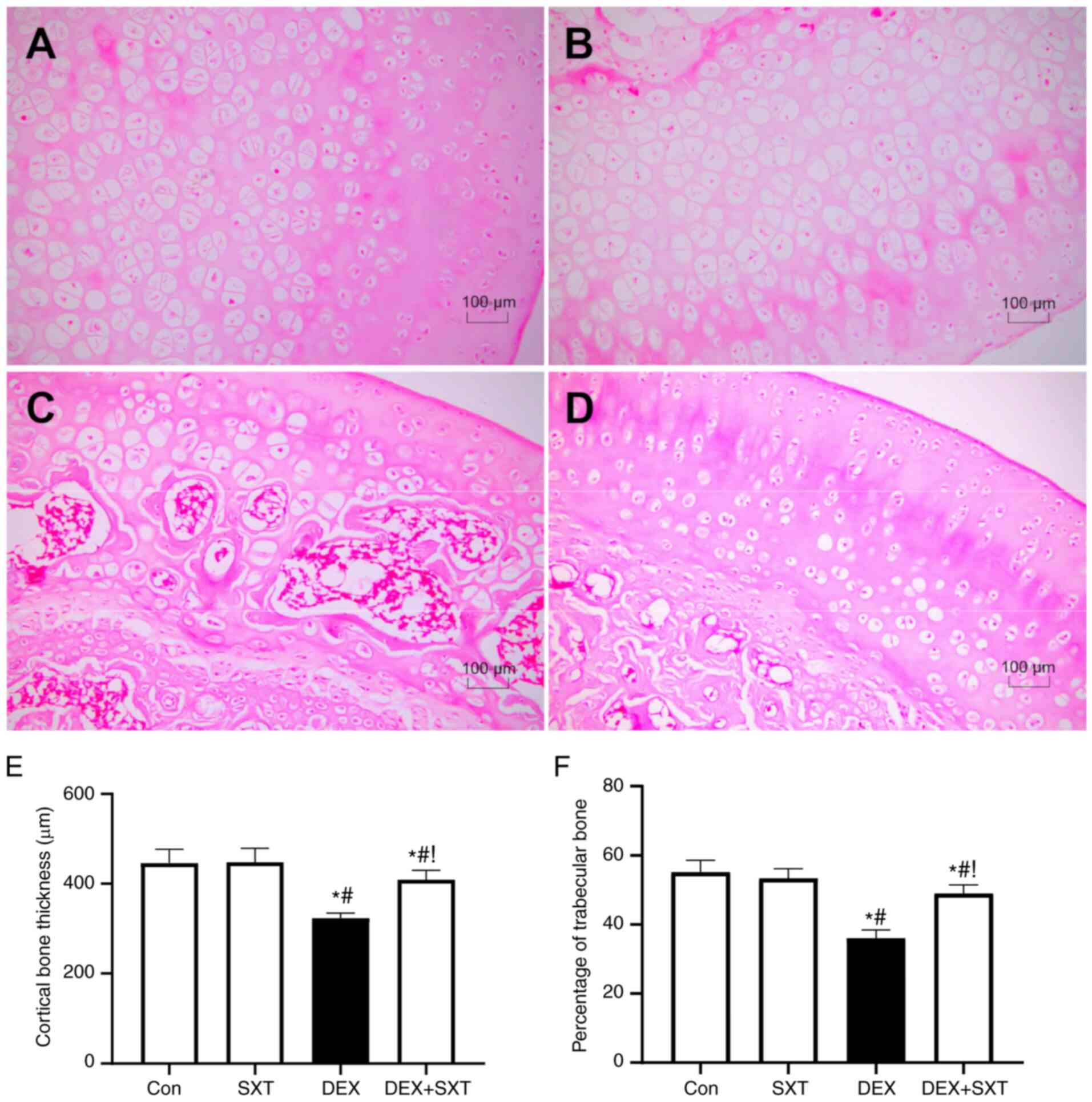
H&E staining demonstrated the destroyed
structure of the femoral head in rats treated with DEX, which was
markedly ameliorated by injection with SXT (Fig. 5A-D). In line with these structural
changes, decreases in cortical bone thickness and trabecular bone
area percentage were also significantly ameliorated by SXT
treatment (Fig. 5E and F). There was no clear effect of SXT
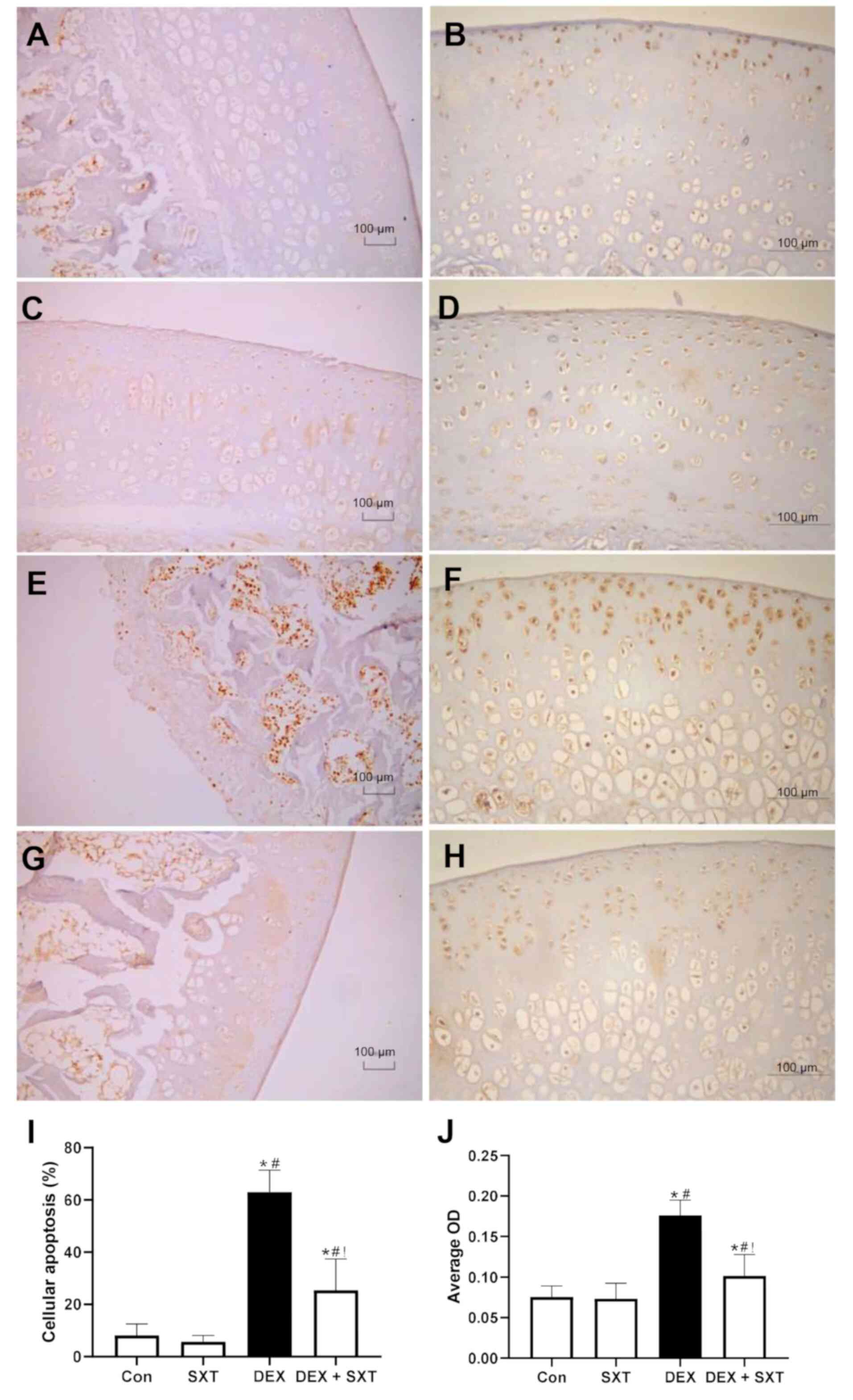
treatment alone on the structure of the femoral head (Fig. 5A-D). Similarly, the apoptotic cell
counts determined by TUNEL staining were significantly increased in
the DEX group, and SXT injection attenuated this increase in
cellular apoptosis (Fig. 6A-E).
Furthermore, the results suggested that there was no effect of SXT
treatment alone on apoptosis (Fig.
6A-E). Accordingly, the protein expression levels of CHOP, a
common marker of ERS that mediates ERS-associated apoptosis
(32), were significantly increased
in the DEX group and rescued by SXT injection (Fig. 6F-J).
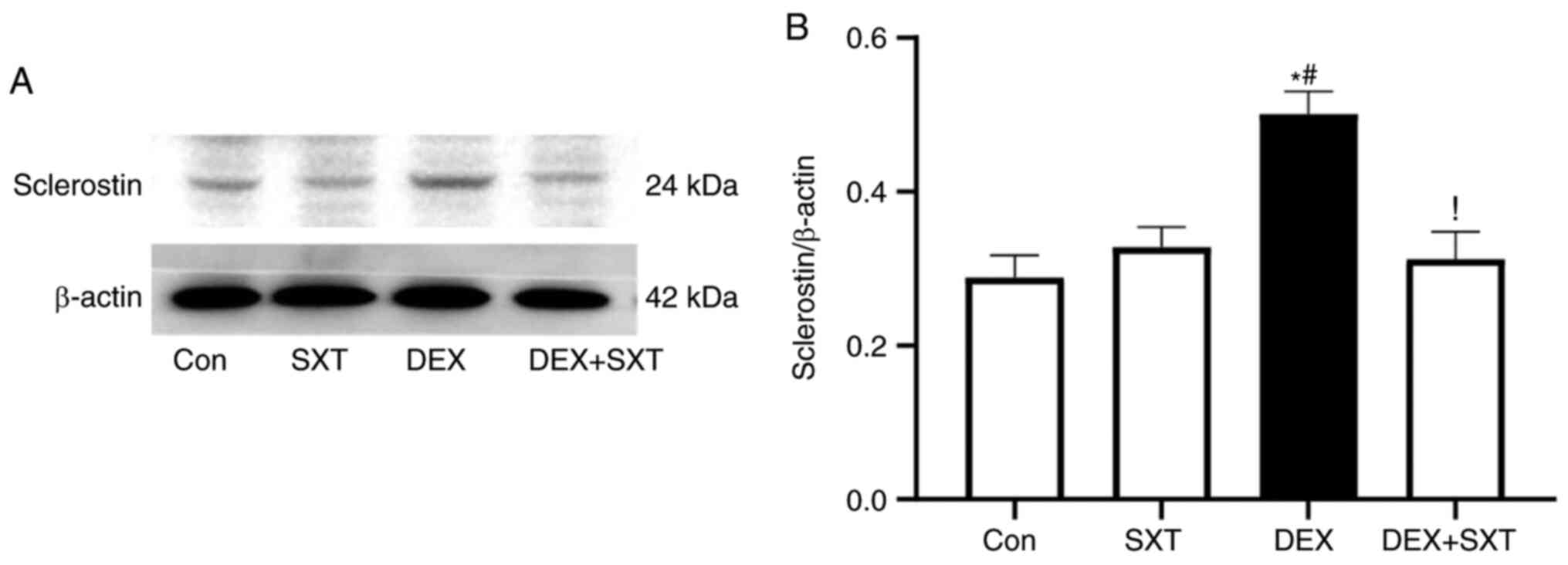
Increases in DEX-induced expression
levels of sclerostin are attenuated by SXT
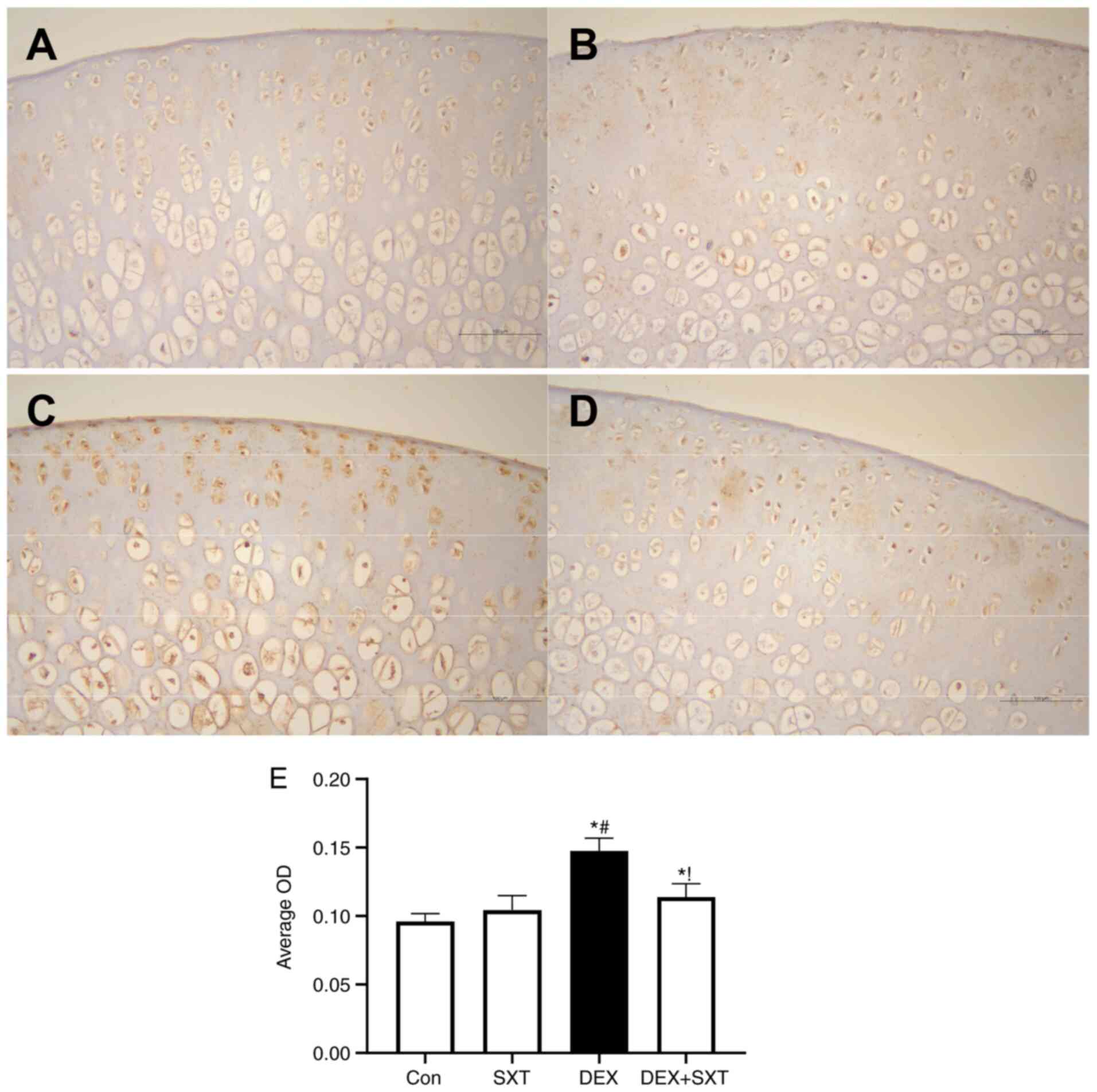
The sclerostin protein expression levels in the
femoral head were detected by immunohistochemical staining
(Fig. 7). The sclerostin protein
expression levels in the femoral head of rats with DEX were
significantly increased compared with those in the control rats,
and SXT treatment attenuated the DEX-induced increase in sclerostin
levels (Fig. 7). Additionally, SXT
injection alone did not exhibit any observable effect on sclerostin
protein levels in the femoral head compared with the control group
(Fig. 7).
Discussion
The present study demonstrated that DEX treatment
induced calcium deposition, ALP activation, calponin and SM22α
downregulation, and RUNX2, BMP2, GRP78 and CHOP upregulation in the
aortic tissue of rats; all these effects were ameliorated by SXT
injection. In the femoral head, SXT treatment rescued the observed
structural disturbance, osteoporosis and apoptosis. Notably,
sclerostin protein expression levels in the aorta and femoral head
significantly increased in the DEX group compared with in the
control group, but this was attenuated in the DEX + SXT group.
Although SXT injection is mainly used clinically to
stimulate blood circulation (17),
several studies have reported its effects on blood vessels,
including angiogenesis facilitation (18), amelioration of lower extremity
arteriosclerosis in patients with diabetes (33), protection of cerebral microvascular
endothelial cells (20) and
inhibition of inflammation (22-24).
The present study demonstrated the ameliorative effect of SXT
injection on VC, supported by decreased deposition of calcium in
the aortic media, and the inhibition of ALP activity. Thus, the
results suggested that SXT may have potential for treating VC in
the future.
Increasing evidence has indicated an important role
for ERS in VC progression; studies have demonstrated that ERS
activation can exaggerate VC by contributing to the phenotypic VSMC
differentiation from contractile-like to osteoblastic-like
structures (32,34). The results of the present study
demonstrated that the protein expression levels of two universal
ERS markers, GRP78 and CHOP (32,34),
were increased in DEX-treated rats, alongside decreases in calponin
and SM22α levels, both which are markers of contractile VSMCs, and
increased RUNX2 and BMP2 levels, both of which are markers of
osteoblastic VSMCs (35-37).
These results indicated the contribution of ERS-induced VSMC
differentiation in DEX-induced VC progression, as well as
suggesting that ERS inhibition can attenuate VSMC differentiation
and VC (35-37).
Moreover, the present study also observed ERS inhibition, and VSMC
differentiation and VC amelioration following SXT injection in
DEX-treated rats. These findings suggested that SXT injection may
ameliorate VC by preventing ERS-induced VSMC differentiation.
Akt may represent a link among SXT, DEX and ERS
(38-40).
Previous studies have demonstrated the negative effect of Akt on
ERS (38-40).
Activation of Akt signaling can significantly rescue ERS
stimulation (38-40).
Notably, Akt signaling was activated by SXT injection (19), whereas DEX prevented the activation
of Akt (29). To investigate
whether SXT injection can activate Akt signaling, the protein
expression level of pAkt was detected via western blotting. The
results demonstrated that the decreased level of pAkt in
DEX-treated animals was significantly rescued by SXT injection.
Collectively, these findings suggested that SXT may attenuate
DEX-induced ERS via activating the Akt signaling pathway.
In addition to vascular calcification, DEX can also
cause osteoporosis (41-43).
DEX treatment led to structural disturbance, reduction of cortical
bone thickness, reduction in the percentage of trabecular bone and
increased cellular apoptosis in the femoral head. Notably,
injection of SXT significantly ameliorated the DEX-induced femoral
head impairment. Thus, the results of the present study suggested
that SXT could be used to prevent and rescue the side-effects of
GCs, including VC and osteoporosis.
To further investigate the mechanisms via which SXT
ameliorated VC and osteoporosis, sclerostin protein expression
levels in the aorta and femoral head were examined. Sclerostin was
involved in both VC and osteoporosis progression (12,44).
Consistent with previous studies (12,13),
increased sclerostin protein expression levels were observed in the
aortic tissue and femoral head of DEX-treated rats, and SXT
injection successfully attenuated the increased sclerostin levels
in both the aorta and bone. These results suggested that sclerostin
inhibition may be involved in the suppressive effects of SXT on VC
and osteoporosis. Unfortunately, in the present study, whether the
injection of SXT ameliorated the symptoms induced by DEX by
inhibiting sclerostin was not studied; the causal role of
sclerostin should be fully investigated in the future.
There are two sclerostin inhibitors, romosozumab and
baicalin; romosozumab is a human monoclonal antibody against
sclerostin (45), and baicalin, a
herb-derived flavonoid compound, binds to sclerostin via
hydrophobic interactions with the amino acid residues on loop2
region, but outside the Pro-Asn-Ala-Ile-Gly motif, particularly the
Arg-Gly-Lys-Trp-Trp-Arg motif (46). The beneficial effects of these two
agents against osteoporosis have been reported in a number of
studies (47-50).
Although the effect of romosozumab on cardiovascular events
including VC is controversial (45,51),
baicalin has been reported to relax VSMCs (52), ameliorate atherosclerosis (53), prevent trans-differentiation of
VSMCs (54) and inhibit vascular
remodeling (55), via which
baicalin may ameliorate VC. These results suggested that inhibition
of sclerostin may exert beneficial effects on both VC and
osteoporosis.
In conclusion, the results of the present study
indicated that the injection of SXT ameliorated both VC and
osteoporosis, which may be mediated via regulation of sclerosis,
the crosslink in the bone-vascular axis. Thus, injection of SXT may
provide a novel strategy for the treatment of GC-induced VC and
osteoporosis.
Acknowledgements
Not applicable.
Funding
This study was supported by the project for talents
of Finance Department of Hebei Province (grant no. 2018133214), and
the National Natural Science Foundation of China (grant no.
81770499).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
All authors contributed to experimental design. ZX,
XL, YL, HG, TH and CZ performed the experiments. ZX, XL and YL
collected and analyzed the data. ZX, XL, WH and XT interpreted the
data. WH and XT wrote and revised the manuscript. WH and XT confirm
the authenticity of all the raw data. All authors read and approved
the final manuscript.
Ethics approval and consent to
participate
The present study was approved by the Animal Care
Committee of Hebei Provincial Hospital of Chinese Medicine
(Shijiazhuang, China; approval no. 2019-KY-012-01).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Towler DA: Arteriosclerosis, bone biology,
and calciotropic hormone signaling: Learning the ABCs of disease in
the bone-vascular axis. J Am Soc Nephrol. 26:243–245.
2015.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Vassalle C and Mazzone A: Bone loss and
vascular calcification: A bi-directional interplay? Vascul
Pharmacol. 86:77–86. 2016.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Tap L, Kirkham FA, Mattace-Raso F, Joly L,
Rajkumar C and Benetos A: Unraveling the links underlying arterial
stiffness, bone demineralization, and muscle loss. Hypertension.
76:629–639. 2020.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Stafford RS, Drieling RL and Hersh AL:
National trends in osteoporosis visits and osteoporosis treatment,
1988-2003. Arch Intern Med. 164:1525–1530. 2004.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Viaene L, Behets GJ, Heye S, Claes K,
Monbaliu D, Pirenne J, D'Haese PC and Evenepoel P: Inflammation and
the bone-vascular axis in end-stage renal disease. Osteoporos Int.
27:489–497. 2016.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Zoppellaro G, Faggin E, Puato M, Pauletto
P and Rattazzi M: Fibroblast growth factor 23 and the bone-vascular
axis: Lessons learned from animal studies. Am J Kidney Dis.
59:135–144. 2012.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Kim JM, Lee WS and Kim J: Therapeutic
strategy for atherosclerosis based on bone-vascular axis
hypothesis. Pharmacol Ther. 206(107436)2020.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Brandenburg VM, D'Haese P, Deck A, Mekahli
D, Meijers B, Neven E and Evenepoel P: From skeletal to
cardiovascular disease in 12 steps-the evolution of sclerostin as a
major player in CKD-MBD. Pediatr Nephrol. 31:195–206.
2016.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Fadini GP, Rattazzi M, Matsumoto T,
Asahara T and Khosla S: Emerging role of circulating calcifying
cells in the bone-vascular axis. Circulation. 125:2772–2781.
2012.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Pieralice S, Vigevano F, Del Toro R,
Napoli N and Maddaloni E: Lifestyle management of diabetes:
Implications for the bone-vascular axis. Curr Diab Rep.
18(84)2018.PubMed/NCBI View Article : Google Scholar
|
|
11
|
D'Onofrio L, Maddaloni E and Buzzetti R:
Osteocalcin and sclerostin: Background characters or main actors in
cardiovascular disease? Diabetes Metab Res Rev.
36(e3217)2020.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Figurek A, Rroji M and Spasovski G:
Sclerostin: A new biomarker of CKD-MBD. Int Urol Nephrol.
52:107–113. 2020.PubMed/NCBI View Article : Google Scholar
|
|
13
|
De Maré A, Maudsley S, Azmi A, Hendrickx
JO, Opdebeeck B, Neven E, D'Haese PC and Verhulst A: Sclerostin as
regulatory molecule in vascular media calcification and the
Bone-Vascular Axis. Toxins (Basel). 11(428)2019.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Leto G, D'Onofrio L, Lucantoni F, Zampetti
S, Campagna G, Foffi C, Moretti C, Carlone A, Palermo A, Leopizzi
M, et al: Sclerostin is expressed in the atherosclerotic plaques of
patients who undergoing carotid endarterectomy. Diabetes Metab Res
Rev. 35(e3069)2019.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Morales-Santana S, García-Fontana B,
García-Martín A, Rozas-Moreno P, García-Salcedo JA, Reyes-García R
and Muñoz-Torres M: Atherosclerotic disease in type 2 diabetes is
associated with an increase in sclerostin levels. Diabetes Care.
36:1667–1674. 2013.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Saag KG, Petersen J, Brandi ML, Karaplis
AC, Lorentzon M, Thomas T, Maddox J, Fan M, Meisner PD and Grauer
A: Romosozumab or alendronate for fracture prevention in women with
osteoporosis. N Engl J Med. 377:1417–1427. 2017.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Cai L, Huang W and Lin D: Effects of
traditional Chinese medicine Shuxuetong injection on random skin
flap survival in rats. ScientificWorldJournal.
2014(816545)2014.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Jin X, Shen G, Gao F, Zheng X, Xu X, Shen
F, Li G, Gong J, Wen L, Yang X and Bie X: Traditional Chinese drug
ShuXueTong facilitates angiogenesis during wound healing following
traumatic brain injury. J Ethnopharmacol. 117:473–477.
2008.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Liu X, Qing Wang, Cui Y, Li X and Yang H:
In-depth transcriptomic and proteomic analyses of the hippocampus
and cortex in a rat model after cerebral ischemic injury and repair
by Shuxuetong (SXT) injection. J Ethnopharmacol.
249(112362)2020.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Sun ZY, Wang FJ, Guo H, Chen L, Chai LJ,
Li RL, Hu LM, Wang H and Wang SX: Shuxuetong injection protects
cerebral microvascular endothelial cells against oxygen-glucose
deprivation reperfusion. Neural Regen Res. 14:783–793.
2019.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Zhang X, Xiao B and Hu CL: Study in the
mechanism of Shuxuetong injection on antithrombosis and
thrombolysis. Zhongguo Zhong Yao Za Zhi. 30:1950–1952.
2005.PubMed/NCBI
|
|
22
|
Chen X, Ding X, Liu L, Zhao L, Jiang Z and
Wang X: Effect of Shuxuetong injection on reducing expression of
vascular adhesion molecules in cerebral ischemia and reperfusion
rats. J New Chin Med. 45:128–130. 2013.
|
|
23
|
Wei J, Chen B, Tian Z, Luo K and Deng W:
Effect of Shuxuetong injection on VEGF expression of hind-limb
ischemic vascular disorder in diabetic rats. Zhong Xi Yi Jie He Xin
Nao Xue Guan Bing Za Zhi. 7:1048–1049. 2009.(In Chinese).
|
|
24
|
Zhang Y, Zhou S and Wang B: Effect of
Shuxuetong injection on ET-1, sICAM-1, TNF-α and P-selection in
diabetes mellitus with coronary artery disease. Zhong Xi Yi Jie He
Xin Nao Xue Guan Bing Za Zhi. 6:1271–1272. 2008.(In Chinese).
|
|
25
|
Xi L, Song Y, Wu W, Qu Z, Wen J, Liao B,
Tao R, Ge J and Fang D: Investigation of bone matrix composition,
architecture and mechanical properties reflect structure-function
relationship of cortical bone in glucocorticoid induced
osteoporosis. Bone. 136(115334)2020.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Ren H, Liang D, Jiang X, Tang J, Cui J,
Wei Q, Zhang S, Yao Z, Shen G and Lin S: Variance of spinal
osteoporosis induced by dexamethasone and methylprednisolone and
its associated mechanism. Steroids. 102:65–75. 2015.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Mori K, Shioi A, Jono S, Nishizawa Y and
Morii H: Dexamethasone enhances In vitro vascular calcification by
promoting osteoblastic differentiation of vascular smooth muscle
cells. Arterioscler Thromb Vasc Biol. 19:2112–2118. 1999.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Kirton JP, Wilkinson FL, Canfield AE and
Alexander MY: Dexamethasone downregulates calcification-inhibitor
molecules and accelerates osteogenic differentiation of vascular
pericytes: Implications for vascular calcification. Circ Res.
98:1264–1272. 2006.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Tao SC, Yuan T, Rui BY, Zhu ZZ, Guo SC and
Zhang CQ: Exosomes derived from human platelet-rich plasma prevent
apoptosis induced by glucocorticoid-associated endoplasmic
reticulum stress in rat osteonecrosis of the femoral head via the
Akt/Bad/Bcl-2 signal pathway. Theranostics. 7:733–750.
2017.PubMed/NCBI View Article : Google Scholar
|
|
30
|
China Animal Management Rule,
documentation No. 55,. 2001, the Ministry of Health of P.R.
China.
|
|
31
|
The National Institutes of Health Guide
for the Care and Use of Laboratory Animals, NIH Publications no.
8023, revised 1978.
|
|
32
|
Duan X, Zhou Y, Teng X, Tang C and Qi Y:
Endoplasmic reticulum stress-mediated apoptosis is activated in
vascular calcification. Biochem Biophys Res Commun. 387:694–699.
2009.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Feng K, Tan J and Chen Y: Treatment of
lower extremity diabetic atherosclerotic obliterans with Shuxuetong
injection. Zhongguo Zhong Xi Yi Jie He Za Zhi. 29:255–257.
2009.PubMed/NCBI(In Chinese).
|
|
34
|
Duan XH, Chang JR, Zhang J, Zhang BH, Li
YL, Teng X, Zhu Y, Du J, Tang CS and Qi YF: Activating
transcription factor 4 is involved in endoplasmic reticulum
stress-mediated apoptosis contributing to vascular calcification.
Apoptosis. 18:1132–1144. 2013.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Chang JR, Duan XH, Zhang BH, Teng X, Zhou
YB, Liu Y, Yu YR, Zhu Y, Tang CS and Qi YF: Intermedin1-53
attenuates vascular smooth muscle cell calcification by inhibiting
endoplasmic reticulum stress via cyclic adenosine
monophosphate/protein kinase A pathway. Exp Biol Med (Maywood).
238:1136–1146. 2013.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Yang R, Teng X, Li H, Xue HM, Guo Q, Xiao
L and Wu YM: Hydrogen sulfide improves vascular calcification in
rats by inhibiting endoplasmic reticulum stress. Oxid Med Cell
Longev. 2016(9095242)2016.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Hao W, Yang R, Yang Y, Jin S, Li Y, Yuan
F, Guo Q, Xiao L, Wang X, Wang F, et al: Stellate ganglion block
ameliorates vascular calcification by inhibiting endoplasmic
reticulum stress. Life Sci. 193:1–8. 2018.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Teng X, Song J, Zhang G, Cai Y, Yuan F, Du
J, Tang C and Qi Y: Inhibition of endoplasmic reticulum stress by
intermedin(1-53) protects against myocardial injury through a PI3
kinase-Akt signaling pathway. J Mol Med (Berl). 89:1195–1205.
2011.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Tian T, Zhao Y, Nakajima S, Huang T, Yao
J, Paton AW, Paton JC and Kitamura M: Cytoprotective roles of ERK
and Akt in endoplasmic reticulum stress triggered by subtilase
cytotoxin. Biochem Biophys Res Commun. 410:852–858. 2011.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Liu H, Li X, Qin F and Huang K: Selenium
suppresses oxidative-stress-enhanced vascular smooth muscle cell
calcification by inhibiting the activation of the PI3K/AKT and ERK
signaling pathways and endoplasmic reticulum stress. J Biol Inorg
Chem. 19:375–388. 2014.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Rathi A, Ishaq M, Najmi AK and Akhtar M:
Trigonelline demonstrated ameliorative effects in dexamethasone
induced osteoporotic rats. Drug Res (Stuttg). 70:257–264.
2020.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Qin Z, Li S, Zhang X, Liu G, Gu M, Zhang
N, Liu J, Ji Z, Li K, Han Y and Zhai H: Combination therapy of
wuweizi (Schisandrae Chinensis Fructus) and dexamethasone
alleviated dexamethasone-induced glucocorticoid osteoporosis in
rats with idiopathic pulmonary fibrosis. Biomed Res Int.
2020(6301697)2020.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Fu L, Wu W, Sun X and Zhang P:
Glucocorticoids enhanced osteoclast autophagy through the
PI3K/Akt/mTOR signaling pathway. Calcif Tissue Int. 107:60–71.
2020.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Pietrzyk B, Smertka M and Chudek J:
Sclerostin: Intracellular mechanisms of action and its role in the
pathogenesis of skeletal and vascular disorders. Adv Clin Exp Med.
26:1283–1291. 2017.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Asadipooya K and Weinstock A:
Cardiovascular outcomes of romosozumab and protective role of
alendronate. Arterioscler Thromb Vasc Biol. 39:1343–1350.
2019.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Alazmi M and Motwalli O: In silico virtual
screening, characterization, docking and molecular dynamics studies
of crucial SARS-CoV-2 proteins. J Biomol Struct Dyn: Aug 7, 2020
(Epub ahead of print). doi: 10.1080/07391102.2020.1803965.
|
|
47
|
Khosla S: Bone diseases: Romosozumab-on
track or derailed? Nat Rev Endocrinol. 13:697–698. 2017.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Fontalis A, Kenanidis E, Kotronias RA,
Papachristou A, Anagnostis P, Potoupnis M and Tsiridis E: Current
and emerging osteoporosis pharmacotherapy for women: State of the
art therapies for preventing bone loss. Expert Opin Pharmacother.
20:1123–1134. 2019.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Chen Z, Pan X, Sheng Z, Yan G, Chen L and
Ma G: Baicalin suppresses the proliferation and migration of
Ox-LDL-VSMCs in atherosclerosis through upregulating miR-126-5p.
Biol Pharm Bull. 42:1517–1523. 2019.PubMed/NCBI View Article : Google Scholar
|
|
50
|
Lu X, He W, Yang W, Li J, Han W, Liu Q,
Zhang T, Jiang J, Qin A and Qian Y: Dual effects of baicalin on
osteoclast differentiation and bone resorption. J Cell Mol Med.
22:5029–5039. 2018.PubMed/NCBI View Article : Google Scholar
|
|
51
|
Turk JR, Deaton AM, Yin J, Stolina M, Felx
M, Boyd G, Bienvenu JG, Varela A, Guillot M, Holdsworth G, et al:
Nonclinical cardiovascular safety evaluation of romosozumab, an
inhibitor of sclerostin for the treatment of osteoporosis in
postmenopausal women at high risk of fracture. Regul Toxicol
Pharmacol. 115(104697)2020.PubMed/NCBI View Article : Google Scholar
|
|
52
|
Ding L, Jia C, Zhang Y, Wang W, Zhu W,
Chen Y and Zhang T: Baicalin relaxes vascular smooth muscle and
lowers blood pressure in spontaneously hypertensive rats. Biomed
Pharmacother. 111:325–330. 2019.PubMed/NCBI View Article : Google Scholar
|
|
53
|
Liu Y, Jia L, Min D, Xu Y, Zhu J and Sun
Z: Baicalin inhibits proliferation and promotes apoptosis of
vascular smooth muscle cells by regulating the MEG3/p53 pathway
following treatment with ox-LDL. Int J Mol Med. 43:901–913.
2019.PubMed/NCBI View Article : Google Scholar
|
|
54
|
Lv P, Zhang F, Yin YJ, Wang YC, Gao M, Xie
XL, Zhao LL, Dong LH, Lin YL, Shu YN, et al: SM22α inhibits
lamellipodium formation and migration via Ras-Arp2/3 signaling in
synthetic VSMCs. Am J Physiol Cell Physiol. 311:C758–C767.
2016.PubMed/NCBI View Article : Google Scholar
|
|
55
|
Dong LH, Wen JK, Miao SB, Jia Z, Hu HJ,
Sun RH, Wu Y and Han M: Baicalin inhibits PDGF-BB-stimulated
vascular smooth muscle cell proliferation through suppressing
PDGFRβ-ERK signaling and increase in p27 accumulation and prevents
injury-induced neointimal hyperplasia. Cell Res. 20:1252–1262.
2010.PubMed/NCBI View Article : Google Scholar
|