Introduction
IgA nephropathy (IgAN) is the most common type of
primary glomerulonephritis worldwide. In China, it accounts for
30-40% of primary glomerulonephritis cases (1). At 10-20 years after diagnosis, 20-40%
of IgAN patients progress to end-stage renal disease (1). IgAN requires to be diagnosed by renal
biopsy. However, as renal biopsy is traumatic and patients
frequently refuse to undergo the procedure, it cannot be used as a
routine means to detect the disease. In the clinic, urinary
microalbumin, 24-h urinary protein, serum creatinine and glomerular
filtration rate (GFR) are commonly used to evaluate the condition
and prognosis of IgAN. However, these indicators have numerous
influencing factors, and their sensitivity and specificity are
poor.
It has been indicated that certain microRNAs
(miRNAs/miRs) have important roles in the pathogenesis,
inflammatory response, renal fibrosis and prognosis of IgAN
(2,3). miRNAs are expressed not only in
tissues and cells but also in plasma and urine (3). Studies have identified that miRNAs are
stable in peracid or alkali environments, and may persist after
long storage at room temperature, multiple thaws and exposure to
certain active RNAses (2,4). In addition, the collection of plasma
and urine samples is easy and non-invasive, and is more acceptable
as opposed to biopsy for patients (5). Therefore, plasma and urine miRNAs
associated with the pathophysiological changes of IgAN may be a
novel non-invasive marker for IgAN (6). However, research on miRNAs in IgAN is
still in its infancy, and the correlation between the expression of
certain miRNAs and the pathological changes and clinical
manifestations of IgAN patients requires further study (7,8). It
has been indicated that miRNAs in urinary sediment are easy to
obtain and may be potential non-invasive biomarkers for IgAN
(9). For instance, urinary
miR-3613-3p was reported to be downregulated in IgAN patients and
correlated with the severity of the disease (8). Hence, exploration of the association
between miRNAs and IgAN may provide approaches for the early
diagnosis of IgAN.
A recent study has indicated a reduction of
miR-33-5p levels in the urine of db/db mice and type 2 diabetes
mellitus patients, and miR-33-5p levels were negatively correlated
with albuminuria (10). In the
pathogenesis of HIV-associated nephropathy (HIVAN), miR-33-5p was
reported to be decreased in subjects with HIV-1 infections.
However, whether miR-33-5p is involved in the progression of IgAN
has remained elusive.
The present study aimed to detect the expression of
miR-33-5p in the renal tissue, serum and urine of patients with
primary IgAN, thereby preliminarily exploring the association
between the expression of miR-33-5p and the condition of primary
IgAN to test the possibility of utilizing the expression of
miR-33-5p in the serum and urine of IgAN patients as biomarkers.
The present study provides a reference and a novel idea for the
utilization of these non-invasive diagnostic markers in the
diagnosis of IgAN.
Materials and methods
Patients and samples
A total of 100 patients diagnosed with IgAN by renal
biopsy and clinical and laboratory examinations at the Department
of Nephrology, the Second Hospital of Jilin University (Changchun,
China) between December 2016 and June 2017 were enrolled in the
study as the IgAN group. This group comprised 59 males and 41
females, with an average age of 35.55±9.66 years. On the morning of
renal biopsy, morning urine and fasting venous blood samples were
simultaneously collected, of which 20 patients were enrolled in
renal biopsy. The kidney tissue of these patients was collected as
control specimens for patients with IgAN. The patients with IgAN
were divided into five subgroups according to the Lee
classification (11): 24 patients
with grade I, 24 patients with grade II, 18 patients with grade
III, 17 patients with grade IV and 17 patients with grade V. In the
same time window, kidney tissue samples were collected from 20
patients receiving nephrectomy due to upper-tract urothelial
carcinoma, including 12 males and 8 females, with an average age of
43.2±7.5 years, at the Second Hospital of Jilin University
(Changchun, China) (12). Kidney
tissues that were far away (minimum distance, 2 cm) from the tumor
tissues and were of normal pathology were selected. Informed
consent was obtained from all participants.
The inclusion criteria were as follows: i) Patients
agreed to provide renal puncture tissue, plasma and urine samples
to be analyzed in the present study and provided written informed
consent; ii) age ≥18 years; iii) no previous treatment with
glucocorticoids, immunosuppressive agents or kidney
transplantation; and iv) kidney pathology indicated that the
deposition of IgA-based IgGs or complements in the mesangial region
was strongly positive for IgA under light microscopy (XDS-500D;
Shanghai Caikon Optical Instrument Co., Ltd.).
The exclusion criteria were as follows: i) Secondary
IgAN caused by allergic purpura, systemic lupus erythematosus,
hepatitis B virus infection, cirrhosis, tumors, or inflammatory
bowel disease; ii) other kidney diseases, including membranous
nephropathy, hypertensive nephropathy and diabetic nephropathy,
minimal-change disease diagnosed under electronic microscopy
(JEM-1011; JEOL); iii) systemic diseases including diabetes
mellitus and connective tissue disease; iv) glomerulus number in
renal biopsy tissues <10; v) urinary tract infections; and vi)
cases of lactation and pregnancy.
The clinical data of the cohort are listed in
Table I. The above-mentioned
clinical indicators were determined prior to the renal biopsy in
patients with IgAN after admission. The estimated GFR (eGFR) was
calculated according to the Chronic Kidney Disease Epidemiology
group equation for Chinese individuals (13).
 | Table IClinical data of patients with IgA
nephropathy. |
Table I
Clinical data of patients with IgA
nephropathy.
| Clinical
indicator | Value |
|---|
| Sex (male/female,
%) | 59/41 (59%/41%) |
| Age (years) | 35.55±9.66 |
| Course of disease
(months) | 8.12±3.95 |
| Family history | 26 |
| Scr (µM) | 79.00
(57.75-108.25) |
| eGFR (ml/min/1.73
m2) | 90.67±42.52 |
| BUN (mM) | 5.05 (4.06-9.43) |
| UA (µM) | 339.24±109.71 |
| CYS (mg/l) | 0.98 (0.76-1.69) |
| TP (g/l) | 55.45
(43.65-63.48) |
| ALB (g/l) | 32.59±9.61 |
| GLOB (g/l) | 20.95
(18.75-26.43) |
| T-CHO (mM) | 6.23±3.17 |
| TG (mM) | 1.94±1.29 |
| HDL (mM) | 1.36±0.49 |
| LDL (mM) | 4.27±2.67 |
| HB (g/l) | 131.26±23.28 |
| U-Prot (g/24 h) | 2.53 (0.94-4.25) |
| Urine RBC count
(/µl) | 38.79
(12.00-393.50) |
| C3 (g/l) | 1.08±0.21 |
| C4 (g/l) | 0.22 (0.18-0.26) |
| Systolic blood
pressure (mmHg) | 126.50
(120.00-144.00) |
| Diastolic blood
pressure (mmHg) | 82.00
(73.00-100.00) |
Age- and gender-matched healthy controls were
selected at the Second Hospital of Jilin University during the same
time window, and their blood and urine were collected. This healthy
control group (n=50) included 27 males and 23 females with an
average age of 35.07±6.13 years.
RNA extraction
Total RNA was isolated from the serum samples (5 ml,
collected in tubes containing EDTA) or urine or tissues using
RNAVzol LS or RNAVzol (Vigorous Biotechnology Beijing Co., Ltd.)
according to the manufacturer's protocol. The concentration and
purity of the RNA samples were determined by measuring the optical
density ratio at 260/280 nm.
Reverse transcription-quantitative
(RT-q)PCR
A total of 1 µg of RNA was reverse transcribed using
Moloney Murine Leukemia Virus RT enzyme (Applied Biosystems; Thermo
Fisher Scientific, Inc.) with specific primers. The protocol used
for RT as follows: 72˚C for 10 min, 42˚C for 60 min, 72˚C for 5 min
and 95˚C for 2 min. To quantify the relative mRNA levels, qPCR was
performed using SYBR Green SuperMix (Bio-Rad Laboratories, Inc.) in
an iCycleriQ real-time PCR detection system. The PCR amplifications
were performed in a 10-µl reaction system containing 5 µl SYBR
Green SuperMix, 0.4 µl forward primer, 0.4 µl reverse primer, 2.2
µl double-distilled H2O and 2 µl template complementary
DNA. Thermocycling conditions were as follows: 95˚C for 10 min,
followed by 40 cycles of 95˚C for 15 sec and 60˚C for 1 min.
Relative mRNA expression was normalized to U6 using the
2-∆∆Cq method (14).
Primer sequences were as follows: miR-33-5p-RT,
5'-GTCGTATCCAGTGCAGGGTCCGAGGTATTCGCACTGGATACGACTGCAAT-3'; U6-RT,
5'-GTCGTATCCAGTGCAGGGTCCGAGGTATTCGCACTGGATACGACAAAATG-3';
miR-33-5p, forward 5'-GCGCGUGCAUUGUAGUUGC-3'; U6, forward
5'-GCGCGTCGTGAAGCGTTC-3'; and universal reverse primer,
5'-GTGCAGGGTCCGAGGT-3'.
Statistical analysis
Values are expressed as the mean ± standard
deviation. Two-tailed unpaired Student's t-tests were used for
comparisons between two groups using SPSS version 13.0 (SPSS,
Inc.). Receiver operating characteristic (ROC) curves were used to
assess the diagnostic accuracy of miR-33-5p as a biomarker, and the
area under the ROC curve (AUC) was determined with SPSS version
20.0 (IBM Corp). P<0.05 was considered to indicate a
statistically significant difference.
Results
Expression of miR-33a-5p in blood,
urine and kidney tissues
RT-qPCR indicated that the relative levels of
miR-33a-5p in the serum and urine of the IgAN group (n=100) were
significantly lower than those in the healthy control group (n=50)
(0.28±0.25 vs. 1.00±0.45, P<0.05; 0.34±0.28 vs. 1.00±0.53,
P<0.05; Fig. 1A and B, respectively). Furthermore, the relative
expression level of miR-33a-5p was decreased in kidney tissues of
the IgAN group compared with those of the patients who received
biopsy due to renal cancer (n=20; 0.47±0.27 vs. 1.00±0.38,
P<0.05; Fig. 1C).
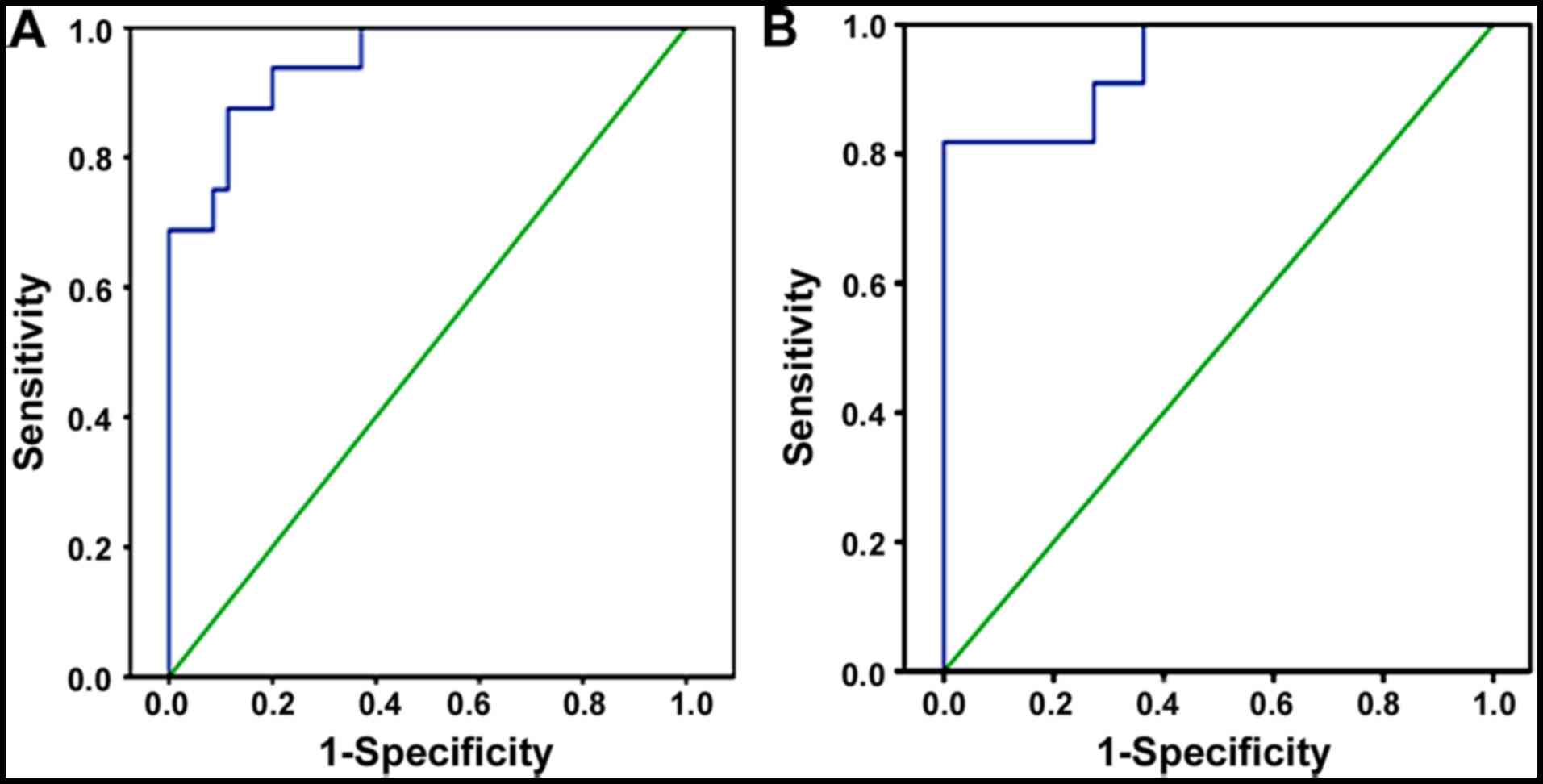
Blood and urine miR-33a-5p levels may
differentiate IgAN patients from healthy controls
When the cutoff value was 0.13, the AUC of serum
miR-33a-5p in IgAN patients was 0.912 (95% CI=0.819-1.000,
P<0.001), with a sensitivity of 88.6% and specificity of 97.4%.
The results indicated that serum miR-33a-5p could distinguish IgAN
patients from normal controls (Fig.
2A). Furthermore, when the cutoff value was 0.18, the AUC of
urine miR-33a-5p in IgAN patients (n=100) was 0.942 (95%
CI=0.858-1.000, P<0.001), with a sensitivity of 87.8% and
specificity of 98.7% (Fig. 2B).
These results indicated that serum as well as urine miR-33a-5p may
be used as diagnostic markers for IgAN patients. The aforementioned
results indicated that serum and tissue miR-33a-5p could
distinguish patients with IgAN from control individuals.
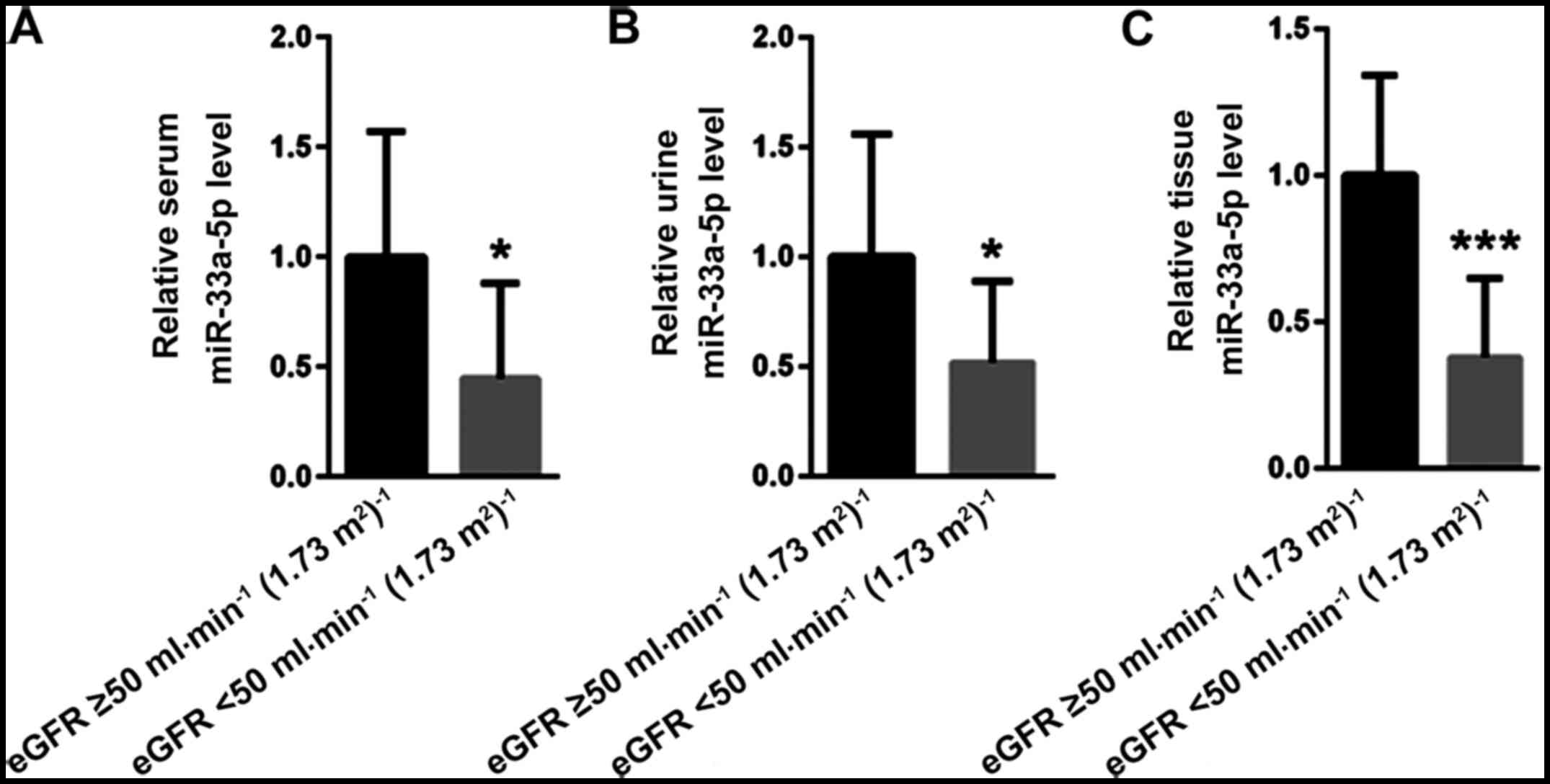
miR-33a-5p is decreased along with
impaired renal function
The IgAN patients were divided into two groups
according to the eGFR, namely <50 ml/min/1.73 m2 and
≥50 ml/min/1.73 m2 (52 vs. 48 cases). The relative
levels of miR-33a-5p were decreased in serum, urine and kidney
tissues in the eGFR <50 ml/min/1.73 m2 group compared
with those in the eGFR ≥50 ml/min/1.73 m2 group
(0.45±0.43 vs. 1.00±0.57, P<0.05; 0.52±0.37 vs. 1.00±0.56,
P<0.05; and 0.38±0.27 vs. 1.00±0.34, P<0.001, respectively;
Fig. 3).
Serum miR-33a-5p is reduced along with
enhanced urinary protein level
The IgAN group was divided into a ≤1.5 g/24 h group
and a >1.5 g/24 h group according to the amount of urinary
protein (46 vs. 54 cases). The levels of miR-33a-5p in serum, urine
and kidney tissues were decreased in the group with a urine protein
content of >1.5 g/24 h compared with those in the group with a
urine protein content of ≤1.5 g/24 h (0.45±0.39 vs. 1.00±0.53,
P<0.05; 0.55±0.42 vs. 1.00±0.61, P>0.05; 0.58±0.49 vs.
1.00±0.74, P>0.05; respectively; Fig. 4). The level of miR-33a-5p in serum
was significantly decreased (P<0.05), but there was no
significant difference in the urine and kidney tissue levels of
miR-33a-5p between the two groups.
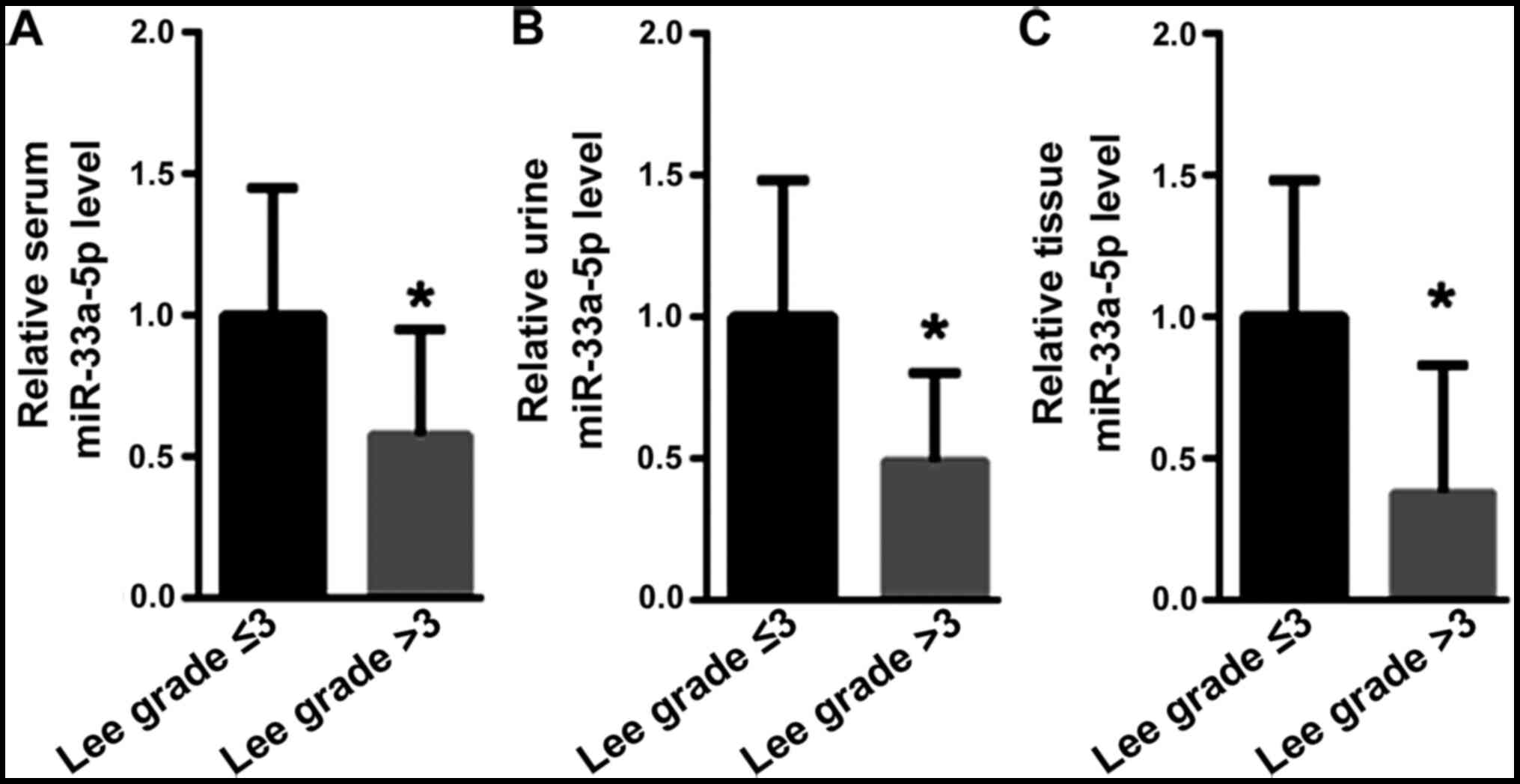
miR-33a-5p is decreased with the
severity of nephropathy
IgAN patients were divided according to Lee's
classification of nephropathy into grade ≤3 and >3 groups (38
vs. 62 cases). Compared with those in the Lee grade ≤3 group, the
miR-33a-5p levels in the serum (1.00±0.45 vs. 0.58±0.37,
P<0.05), urine (1.00±0.48 vs. 0.49±0.31, P<0.05) and renal
tissue (1.00±0.48 vs. 0.38±0.25, P<0.05) of IgAN patients with
Lee grade >3 were significantly decreased (Fig. 5).
Discussion
In the present study, the expression of miR-33a-5p
in the renal tissue, plasma and urine of patients with IgAN was
compared with those with renal cancer (non-cancerous tissues) and
it was explored whether the miRNA expression levels were associated
with the degree of pathological damage and clinical manifestations
of IgAN patients, thereby evaluating the diagnostic value of
miR-33a-5p to distinguish IgAN patients from non-IgAN
individuals.
The results indicated that the serum, urine and
kidney tissue levels of miR-33a-5p in IgAN patients were lower than
those in patients with renal cancer (non-cancerous tissues). ROC
analysis indicated that the level of miR-33a-5p in blood and urine
may be used as a marker to differentiate IgAN patients from healthy
controls. At the same time, according to the eGFR and Lee
classification of nephropathy, the level of miR-33a-5p in kidney
tissue decreased with the progression of renal failure and the
increase of the pathological grade of kidney tissue. This result
suggested that the levels of miR-33a-5p in blood, urine and kidney
tissues were decreased with the severity of renal injury and the
progression of renal failure in patients with IgAN. Hence,
detection of miR-33a-5p in blood and urine may be used as a
non-invasive biomarker to reflect the progression of renal injury
and renal failure in IgAN patients. However, in the future study,
this should be further confirmed via ROC analysis.
However, the reason for the change in serum
miR-33a-5p levels and the underlying mechanisms remain elusive. The
change may be caused by organ secretion or disease (15,16).
It is necessary to further study the expression level of miR-33a-5p
in specific renal cell types. Similar to serum, urinary miR-33a-5p
originates from extracellular bodies or microbubbles and exists in
apoptotic bodies, which are highly stable (3,17).
miR-33a-5p in urine may be derived from glomerular ultrafiltration
or secreted by renal tubules. However, due to technical
limitations, there is currently no way to detect the specific
source of these specific miRNAs in urine, which may come from
exfoliated renal tubular epithelial cells or urinary epithelial
cells (7). In addition, a future
study with a large sample size is necessary to further validate the
diagnostic value of miR-33a-5p. It is also important to evaluate
whether miR-33a-5p acts as a marker or a mediator in the
progression of IgAN. In order to determine whether miR-33a-5p is a
specific diagnostic biomarker for IgAN, further studies involving
other kidney diseases for comparison, including diabetic
nephropathy, HIVAN, membranous nephropathy and minimal change
disease, are required.
In conclusion, in spite of the above-mentioned
limitations, the present study provided novel information regarding
the early diagnosis of IgAN. These results suggest that the
expression of miR-33a-5p in renal tissue, plasma and urine may be
associated with the pathological changes and clinical
manifestations of IgAN, providing a reference for the utilization
of urine and serum miR-33a-5p as a non-invasive biomarker of
IgAN.
Acknowledgements
Not applicable.
Funding
The present study was supported by a grant from the
Jilin Province supporting fund of the Second Hospital of Jilin
University (grant no. JLSH-20170125).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
LL performed the experiments and analyzed the data.
AD, QG and GS collected the patient samples, and analyzed and
interpreted the data. WC, XL and HY performed part of the RT-qPCR
experiments. PL designed the experiments, analyzed the data and
gave final approval of the manuscript to be published. All authors
read and approved the final manuscript.
Ethics approval and consent to
participate
The present study was approved by the Research
Ethics Committee of the Second Hospital of Jilin University
(Changchun, China). All patients and healthy controls provided
written informed consent for participating in this study.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Li LS and Liu ZH: Epidemiologic data of
renal diseases from a single unit in China: Analysis based on
13,519 renal biopsies. Kidney Int. 66:920–923. 2004.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Min QH, Chen XM, Zou YQ, Zhang J, Li J,
Wang Y, Li SQ, Gao QF, Sun F, Liu J, et al: Differential expression
of urinary exosomal microRNAs in IgA nephropathy. J Clin Lab Anal.
32:2018.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Szeto CC and Li PK: MicroRNAs in IgA
nephropathy. Nat Rev Nephrol. 10:249–256. 2014.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Bockmeyer CL, Sauberlich K, Wittig J, Eßer
M, Roeder SS, Vester U, Hoyer PF, Agustian PA, Zeuschner P, Amann
K, et al: Comparison of different normalization strategies for the
analysis of glomerular microRNAs in IgA nephropathy. Sci Rep.
6(31992)2016.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Tan K, Chen J, Li W, Chen Y, Sui W, Zhang
Y and Dai Y: Genome-wide analysis of microRNAs expression profiling
in patients with primary IgA nephropathy. Genome. 56:161–169.
2013.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Wang G, Kwan BC, Lai FM, Choi PC, Chow KM,
Li PK and Szeto CC: Intrarenal expression of microRNAs in patients
with IgA nephropathy. Lab Invest. 90:98–103. 2010.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Wang G, Kwan BC, Lai FM, Chow KM, Kam-Tao
Li P and Szeto CC: Expression of microRNAs in the urinary sediment
of patients with IgA nephropathy. Dis Markers. 28:79–86.
2010.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Wang N, Bu R, Duan Z, Zhang X, Chen P, Li
Z, Wu J, Cai G and Chen X: Profiling and initial validation of
urinary microRNAs as biomarkers in IgA nephropathy. PeerJ.
3(e990)2015.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Duan ZY, Cai GY, Bu R, Lu Y, Hou K and
Chen XM: Selection of urinary sediment miRNAs as specific
biomarkers of IgA nephropathy. Sci Rep. 6(23498)2016.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Tsai YC, Kuo PL, Hung WW, Wu LY, Wu PH,
Chang WA, Kuo MC and Hsu YL: Angpt2 induces mesangial cell
apoptosis through the MicroRNA-33-5p-SOCS5 Loop in Diabetic
Nephropathy. Mol Ther Nucleic Acids. 13:543–555. 2018.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Lee HS, Choi Y, Lee JS, Yu BH and Koh HI:
Ultrastructural changes in IgA nephropathy in relation to
histologic and clinical data. Kidney Int. 35:880–886.
1989.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Cheng K, Rai P, Plagov A, Lan X, Subrati
A, Husain M, Malhotra A and Singhal PC: MicroRNAs in HIV-associated
nephropathy (HIVAN). Exp Mol Pathol. 94:65–72. 2013.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Xie P, Huang JM, Lin HY, Wu WJ and Pan LP:
CDK-EPI equation may be the most proper formula based on creatinine
in determining glomerular filtration rate in Chinese patients with
chronic kidney disease. Int Urol Nephrol. 45:1057–1064.
2013.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408.
2001.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Prabu P, Rome S, Sathishkumar C, Gastebois
C, Meugnier E, Mohan V and Balasubramanyam M: MicroRNAs from
urinary extracellular vesicles are non-invasive early biomarkers of
diabetic nephropathy in type 2 diabetes patients with the 'Asian
Indian phenotype'. Diabetes Metab. 45:276–285. 2019.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Selvaskandan H, Pawluczyk I and Barratt J:
MicroRNAs: A new avenue to understand, investigate and treat
immunoglobulin A nephropathy? Clin Kidney J. 11:29–37.
2018.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Srivastava SP, Koya D and Kanasaki K:
MicroRNAs in kidney fibrosis and diabetic nephropathy: Roles on EMT
and EndMT. Biomed Res Int. 2013(125469)2013.PubMed/NCBI View Article : Google Scholar
|