Introduction
Gastric cancer (GC) is a challenging disease for
general surgeons to manage and is one of the most common types of
cancer worldwide, and GC has the third leading mortality rate
worldwide, causing 723,000 deaths every year (1,2). In
developing countries, 70% of deaths result from GC compared with
40% in China (3). Changes in
lifestyle and eating habits, for example unhealthy diet, may
increase the possibility of the incidence of GC (4). GC is more prevalent in East Asia than
other geographic areas (5).
Surgery, including open surgery and minimally invasive surgery, is
the main treatment option for GC; however, as <10% patients with
GC in developing countries are diagnosed early, there is a poor
survival rate (6,7). Thus, the mechanisms underlying GC
require further investigation.
Previous studies have demonstrated that NF-κB served
a crucial role during the progression of GC (8,9). The
NF-κB complex is activated in the cytoplasm following the
degradation of inhibitor (I)κB, and is then involved in the nuclear
physiologic response (10). Tumor
necrosis factor receptor-associated factor 6 (TRAF6), which belongs
to the TRAF family (11), is an
adaptor protein that has important roles in innate immune responses
and is a participator in the activatory process of the NF-κB
signaling pathway (12). In
addition, Han et al (11)
reported that TRAF6 promoted the invasion and metastasis of GC and
was an index for GC prognosis; Sun et al (13) concluded that the expression levels
of TRAF6 in the skeletal muscle of patients with GC were
significantly upregulated; and Maeda et al (14) suggested that Helicobacter
pylori (H. pylori) may lead to NF-κB activation through
a TRAF6 intracellular signaling pathway, which to the authors' best
knowledge, is the only report of a relationship between NF-κB and
TRAF6 in GC.
The NOD-like receptor (NLR) family serves a crucial
role in immune defense and inflammation (15,16). A
new member of the NLR family member, NLRX1, has been identified as
a protein localized to the membrane of the mitochondria (16,17).
Previous studies have revealed that NLRX1 suppressed tumorigenesis
by inhibiting NF-κB signaling (18,19);
however, the role of NLRX1, the correlation between NLRX1, TRAF6
and NF-κB expression levels, and the relationship between NLRX1 and
the clinicopathological characteristics of GC have not been
established.
In the present study, the expression levels of
NLRX1, TRAF6 and NF-κB in GC tissues were determined and the
association between these three proteins and the
clinicopathological characteristics of gastric adenocarcinoma (GA)
were examined. The present research may offer insight into novel
molecular mechanisms underlying GC, specifically GA.
Materials and methods
Patients studies
The present study included 60 patients (age range,
34-83 years; 51 males and 9 females), who were diagnosed with GA
based on post-operative pathologic evaluations at The Third
People's Hospital of Dalian (Dalian, China) between October 2017
and April 2019. All patient demographic and clinicopathological
data were recorded (Table I). The
patients were diagnosed with GA by gastroscopy. Patients that
received pre-operative chemotherapy or radiotherapy were excluded
from the present study. The tissues were obtained at the time of
surgery and immediately stored at -80˚C. GA and adjacent normal
gastric tissues (>5 cm from the tumor) were collected. Written
informed consent regarding information and tissue samples were
acquired from the patients. The present study was approved by the
Ethics Committee of The Third People's Hospital of Dalian (approval
no. 2017-KY-004).
 | Table IDemographic and clinicopathological
characteristics of the patients. |
Table I
Demographic and clinicopathological
characteristics of the patients.
| Clinicopathological
features |
|---|
| Age, years (mean ±
SD) | 66.5±11.4 |
| Male, number
(%) | 51(85) |
| Female, number
(%) | 9(15) |
| Gross type,
number | |
|
Early
GC | 8 |
|
Advanced
GC | 52 |
| Tumor size,
number | |
|
<5
cm | 29 |
|
≥5 cm | 31 |
| Differentiation,
number | |
|
Moderately-to-well | 33 |
|
Poorly
differentiated | 27 |
| Lymph node
metastasis, number | |
|
Positive | 31 |
|
Negative | 29 |
| Vascular invasion,
number | |
|
Yes | 35 |
|
No | 25 |
| Nerve invasion,
number | |
|
Yes | 26 |
|
No | 34 |
| Depth of
infiltration, number | |
|
T1 or
T2 | 18 |
|
T3 or
T4 | 42 |
| Clinical stage,
number | |
|
I/II | 34 |
|
III/IV | 26 |
The histologic grade was evaluated using the World
Health Organization tumor classification: i) grade well if gland
tissue is present, possibly including metaplasia; ii) grade poorly
if highly irregular glands are indistinguishable; and iii) grade
moderately if the condition is between the grade well and grade
poorly classifications (20).
Immunohistochemistry (IHC)
IHC staining was performed to analyze the expression
levels of NLRX1, TRAF6 and NF-κB in GC and normal gastric tissues.
The tissues were fixed in 10% formalin for 24 h at room
temperature, Subsequently, the tissues were embedded in paraffin
and cut into 4-µm thick sections. Paraffin sections were placed in
an incubator at 60˚C for 120 min. Xylene was used for
deparaffinization and ethanol was used for rehydration at room
temperature. Then the sections were subsequently incubated at 37˚C
with 3% H2O2 for 10 min to block endogenous
peroxidase activity. Antigen retrieval was performed by boiling the
sections with 0.01 M citric acid buffer (pH 6.0) at 95˚C for 20 min
and the sections were then blocked using goat serum (cat. no.
SP-9000; OriGene Technologies, Inc.) at 37˚C for 15 min. Subsequent
incubation was performed with primary antibodies against NLRX1
(1:100; cat. no. ABP57527; Abbkine Scientific Co., Ltd.), TRAF6
(1:100; cat. no. ABP52637; Abbkine Scientific Co., Ltd.) and NF-κB
(1:100; cat. no. ABP51957; Abbkine Scientific Co., Ltd.) overnight
in a humidified chamber at 4˚C. Negative controls were performed
using PBS. Following the primary antibody incubation, the samples
were then incubated with HRP-conjugated anti-rabbit secondary
antibody (cat. no. SP-9000; OriGene Technologies, Inc.) at 37˚C for
15 min. Finally, DAB (cat. no. ZLI-9018; OriGene Technologies,
Inc.) was added for 5 min and the tissue sections were
counterstained with hematoxylin for 20 sec (both at room
temperature).
IHC-stained slides were accessed by two pathologists
who were blinded to the nature of the research using a light
microscope (Nikon Eclipse Ni-E; Nikon Corporation; magnification,
x400). The stained slides were evaluated semi-quantitatively and
the intensity of staining was categorized into four levels as
follows: No staining=0; weak staining=1; moderate staining=2; and
strong staining=3. Positive stained cells were scored as follows:
1, 0-25; 2, 26-50; 3, 51-75; and 4, 76-100%. The final score was
based on the above scores (score of positive stained cells
multiplied by the intensity of staining) (21). There were four categories based on
the final staining scores as follows: 0, -; 1-4, +; 5-8, ++; and
9-12, +++. The results were evaluated as negative and moderate for-
and + (grouped as negative) and positive for ++ and +++ (21).
Western blotting
Total protein was extracted using RIPA lysis buffer,
proteinase inhibitor, phosphatase inhibitors and PMSF (all from
Beyotime Institute of Biotechnology). Proteins (30 µg; quantified
using the BCA method) and the molecular weight marker were
separated by 10% SDS-PAGE (cat. no. P0015A; Beyotime Institute of
Biotechnology). The separated proteins were subsequently
transferred onto PVDF membranes, which were blocked with 5% non-fat
milk in 0.1% Tween-20 in TBS (TBST) for 1 h at room temperature,
and incubated overnight at 4˚C with rabbit polyclonal anti-NLRX1
(1:1,000), anti-TRAF6 (1:1,000), anti-NF-κB (1:500) and
anti-β-actin (1:1,000; cat. no. ABP57456; Abbkine Scientific Co.,
Ltd.) diluted in TBST. Following the primary antibody incubation,
the membranes were washed three times with TBS-T for 10 min and
incubated for 1 h at room temperature with an anti-rabbit IgG
HRP-conjugated secondary antibody (1:5,000; cat. no. A21020;
Abbkine Scientific Co., Ltd.). BeyoECL Plus (Beyotime Institute of
Biotechnology) was used to visualize the protein bands. All results
were analyzed using Gel-Pro Analyzer (version 4.0; Media
Cybernetics, Inc.).
Statistical analysis
Data are presented as the mean ± SD of three
experimental repeats. Statistical analyses were performed using
SPSS 20.0 software (IBM Corp.) and GraphPad Prism 7.0 software
(GraphPad Software, Inc.). A χ2 and Fisher's exact test
were used to determine the associations between NLRX1, TRAF6 and
NF-κB expression levels and the clinicopathological parameters. A
paired Student's t-test was used for the western blotting analysis.
Spearman's rank correlation was used for correlation analyses.
P<0.05 was considered to indicate a statistically significant
difference.
Results
Demographic and clinicopathological
characteristics of the enrolled patients
Demographic information was collected; the average
age of the patients was 66.5±11.4 years, and there were 51 males
and 9 females in the present study. Clinicopathological
characteristics were also collected, including age, sex, tumor
size, histologic grade, lymph node metastasis, vascular invasion,
nerve invasion, depth of infiltration (22) and clinical stage (Table I).
NLRX1, TRAF6 and NF-κB protein
expression levels in GC and normal gastric tissues
To investigate the roles of NLRX1, TRAF6 and NF-κB
in the progression of GC, IHC staining was performed to evaluate
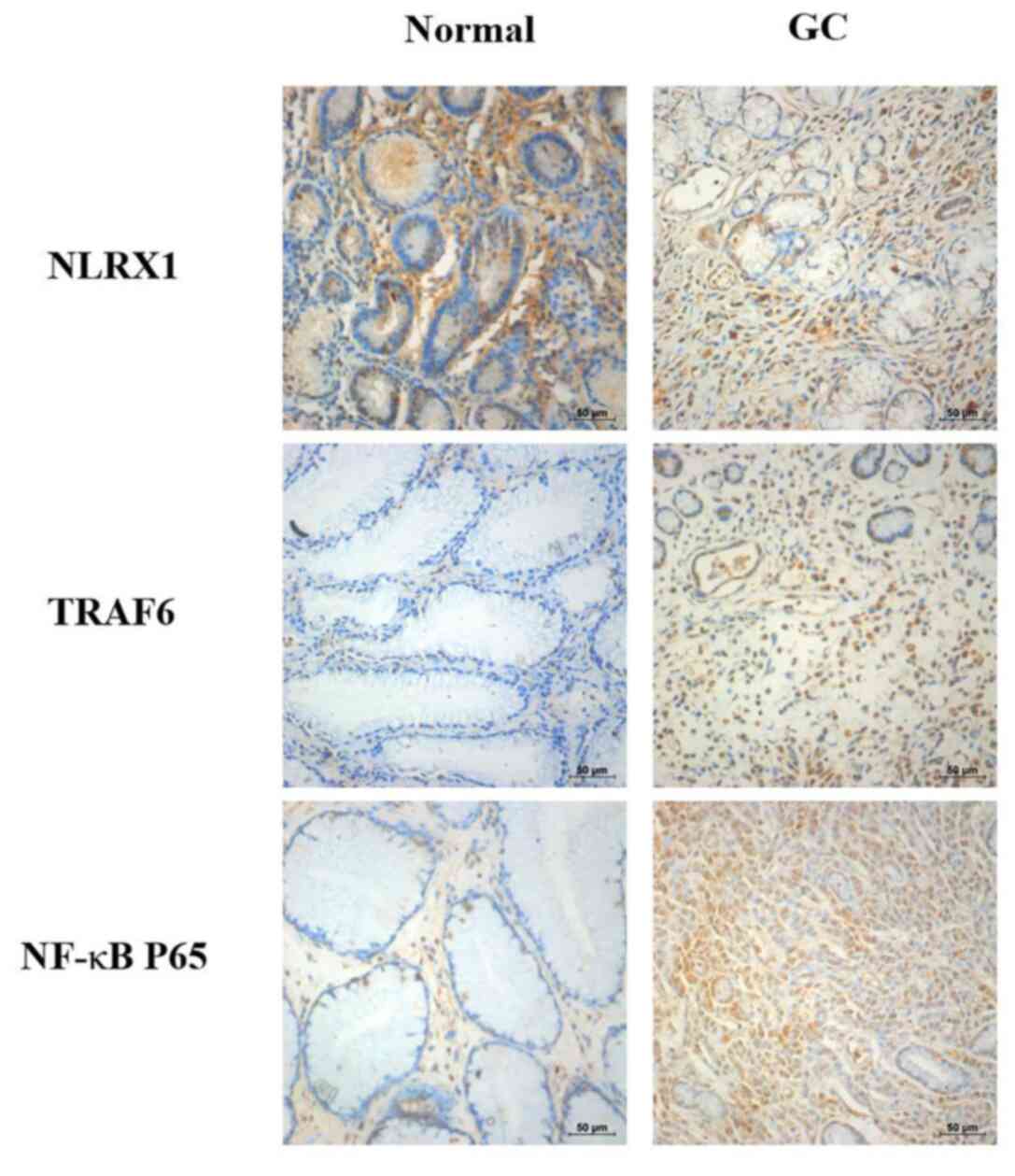
the changes in the protein expression levels. IHC staining of NLRX1
was markedly reduced in the GC tissues compared with the normal
tissues (Fig. 1). In contrast, IHC
staining of TRAF6 and NF-κB were markedly increased in the GC
tissues compared with the normal tissues.
Association between NLRX1, TRAF6 and
NF-κB expression levels and clinicopathological
characteristics
NLRX1, TRAF6 and NF-κB IHC staining of GC samples
and clinicopathological characteristics are presented in Table II. There were no significant
differences identified between positive or negative expression
levels of NLRX1, TRAF6 or NF-κB for either patient sex or age (≤65
or >65 years). However, significant differences were identified
between positive or negative NLRX1, TRAF6 or NF-κB expression
levels for tumor size (<5 cm or ≥5 cm), vascular invasion (yes
or no), neural invasion (yes or no), lymph node metastasis (yes or
no), differentiation (poorly or moderately-to-well differentiated),
gross stage (early or advanced) and clinical stage (I/II or III/IV)
(23).
 | Table IIAssociation between NLRX1, TRAF6 and
NF-κB expression levels and the clinicopathologic features of
patients with gastric cancer. |
Table II
Association between NLRX1, TRAF6 and
NF-κB expression levels and the clinicopathologic features of
patients with gastric cancer.
| A, NLRX1 expression
levels |
|---|
| Clinicopathological
variables | Number of
patients | Positive
expression | Negative
expression | P-value |
|---|
| Age, years | | | | 0.955 |
|
≤65 | 27 | 8 | 19 | |
|
>65 | 33 | 10 | 23 | |
| Sex | | | | 0.431 |
|
Male | 51 | 14 | 37 | |
|
Female | 9 | 4 | 5 | |
| Tumor size, cm | | | | 0.012 |
|
<5 | 28 | 13 | 15 | |
|
≥5 | 32 | 5 | 27 | |
| Vascular
invasion | | | | 0.010 |
|
Yes | 35 | 6 | 29 | |
|
No | 25 | 12 | 13 | |
| Neural
invasion | | | | <0.001 |
|
Yes | 26 | 1 | 25 | |
|
No | 34 | 17 | 17 | |
| Lymph node
metastasis | | | | 0.011 |
|
Yes | 29 | 4 | 25 | |
|
No | 31 | 14 | 17 | |
|
Differentiation | | | | 0.001 |
|
Poorly | 27 | 2 | 25 | |
|
Moderately-to-well | 33 | 16 | 17 | |
| Gross stage | | | | 0.005 |
|
Early | 8 | 6 | 2 | |
|
Advanced | 52 | 11 | 41 | |
| Clinical stage | | | | <0.001 |
|
I/II | 34 | 17 | 17 | |
|
III/IV | 26 | 1 | 25 | |
| B, TRAF6 expression
levels |
| Clinicopathological
variables | Number of
patients | Positive
expression | Negative
expression | P-value |
| Age, years | | | | 0.180 |
|
≤65 | 27 | 17 | 10 | |
|
>65 | 33 | 20 | 13 | |
| Sex | | | | 0.284 |
|
Male | 51 | 33 | 18 | |
|
Female | 9 | 4 | 5 | |
| Tumor size, cm | | | | <0.001 |
|
<5 | 28 | 10 | 18 | |
|
≥5 | 32 | 27 | 5 | |
| Vascular
invasion | | | | <0.001 |
|
Yes | 35 | 29 | 6 | |
|
No | 25 | 8 | 17 | |
| Neural
invasion | | | | 0.002 |
|
Yes | 26 | 22 | 4 | |
|
No | 34 | 15 | 19 | |
| Lymph node
metastasis | | | | <0.001 |
|
Yes | 29 | 25 | 4 | |
|
No | 31 | 12 | 19 | |
|
Differentiation | | | | 0.001 |
|
Poorly | 27 | 23 | 4 | |
|
Moderately-to-well | 33 | 14 | 19 | |
| Gross stage | | | | <0.001 |
|
Early | 8 | 0 | 8 | |
|
Advanced | 52 | 37 | 15 | |
| Clinical stage | | | | 0.002 |
|
I/II | 34 | 15 | 19 | |
|
III/IV | 26 | 22 | 4 | |
| C, NF-κB expression
levels |
| Clinicopathological
variables | Number of
patients | Positive
expression | Negative
expression | P-value |
| Age, years | | | | 0.957 |
|
≤65 | 27 | 17 | 10 | |
|
>65 | 33 | 21 | 12 | |
| Sex | | | | 0.111 |
|
Male | 51 | 38 | 13 | |
|
Female | 9 | 4 | 5 | |
| Tumor size, cm | | | | 0.020 |
|
<5 | 28 | 12 | 16 | |
|
≥5 | 32 | 26 | 6 | |
| Vascular
invasion | | | | 0.002 |
|
Yes | 35 | 28 | 7 | |
|
No | 25 | 10 | 15 | |
| Neural
invasion | | | | 0.017 |
|
Yes | 26 | 21 | 5 | |
|
No | 34 | 17 | 17 | |
| Lymph node
metastasis | | | | 0.007 |
|
Yes | 29 | 24 | 5 | |
|
No | 31 | 15 | 16 | |
|
Differentiation | | | | 0.015 |
|
Poorly | 27 | 22 | 5 | |
|
Moderately-to-well | 33 | 16 | 17 | |
| Gross stage | | | | <0.001 |
|
Early | 8 | 0 | 8 | |
|
Advanced | 52 | 39 | 13 | |
| Clinical stage | | | | 0.017 |
|
I/II | 34 | 17 | 17 | |
|
III/IV | 26 | 21 | 5 | |
NLRX1, TRAF6 and NF-κB expression
levels based on western blotting
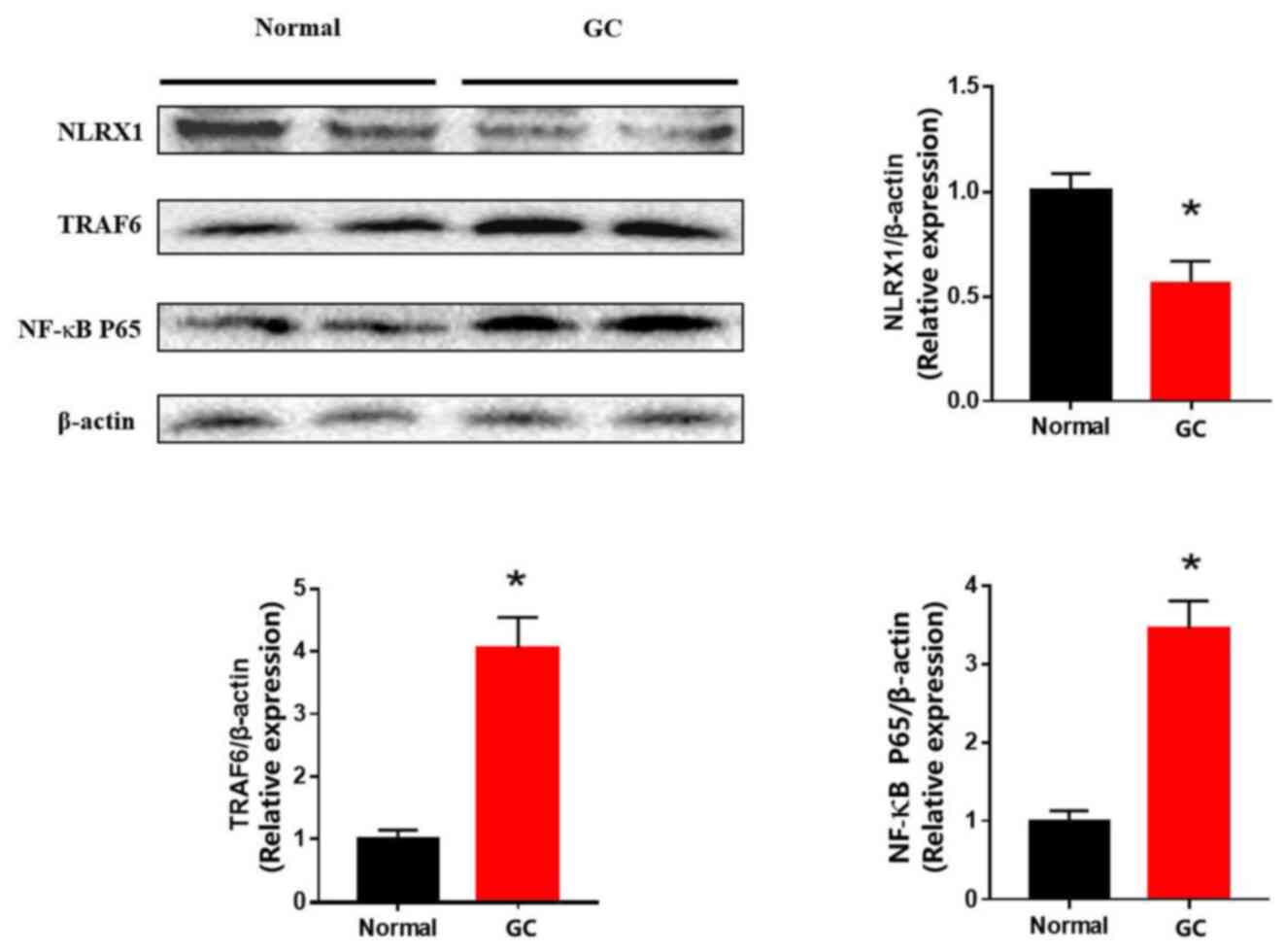
The results from the western blotting experiments
revealed that NLRX1 expression levels were significantly
downregulated in the GC tissues compared with the normal gastric
tissues (Fig. 2). Conversely, TRAF6
and NF-κB expression levels were discovered to be significantly
upregulated in the GC tissues compared with the normal tissues.
Correlation analysis between NLRX1 and
TRAF6, NLRX1 and NF-κB and TRAF6 and NF-κB expression levels
Spearman's rank correlation was used to determine
the correlation between NLRX1 and TRAF6, NLRX1 and NF-κB and TRAF6
and NF-κB expression levels. A negative correlation was identified
between NLRX1 and TRAF6 expression levels (Table III), NLRX1 and NF-κB protein
expression levels (Table IV) and a
positive correlation between TRAF6 and NF-κB protein expression
levels in GC tissues (Table V).
 | Table IIICorrelation between NLRX1 and TRAF6
expression levels. |
Table III
Correlation between NLRX1 and TRAF6
expression levels.
| | NLRX1
expression | |
|---|
| | Staining score,
(n) | - (23) | + (19) | ++ (14) | +++ (4) | Correlation
coefficient | P-value |
|---|
| TRAF6
expression | - (3) | - | - | 2 | 1 |
rs=-0.635 | P<0.001 |
| | + (20) | 2 | 7 | 8 | 3 | | |
| | ++ (18) | 8 | 6 | 4 | - | | |
| | +++ (19) | 13 | 6 | - | - | | |
 | Table IVCorrelation between NLRX1 and NF-κB
expression levels. |
Table IV
Correlation between NLRX1 and NF-κB
expression levels.
| | NLRX1
expression | |
|---|
| | Staining score,
(n) | - (23) | + (19) | ++ (14) | +++ (4) | Correlation
coefficient | P-value |
|---|
| NF-κB P65
expression | - (4) | - | 1 | 1 | 2 |
rs=-0.530 | P<0.001 |
| | + (18) | 3 | 6 | 8 | 1 | | |
| | ++ (17) | 6 | 7 | 3 | 1 | | |
| | +++ (21) | 14 | 5 | 2 | - | | |
 | Table VCorrelation between TRAF6 and NF-κB
expression levels. |
Table V
Correlation between TRAF6 and NF-κB
expression levels.
| | TRAF6
expression | |
|---|
| | Staining score,
(n) | - (3) | + (20) | ++ (17) | +++ (20) | Correlation
coefficient | P-value |
|---|
| NF-κB P65
expression | - (4) | 1 | 3 | - | - |
rs=0.781 | P<0.001 |
| | + (18) | 2 | 14 | 2 | - | | |
| | ++ (17) | - | 3 | 7 | 7 | | |
| | +++ (21) | - | - | 8 | 13 | | |
Discussion
The worldwide incidence of GC has decreased markedly
in recent decades (24). Data from
the 2014 Chinese National Cancer Center (NCCRC) have also revealed
a decreased incidence of GA (25).
Nevertheless, the poor prognosis among patients with GC remains a
serious threat to global health. The incidence of GC varies by
country and is 2-3-fold higher in males compared with females
(26). In the present study, the
male-to-female GC ratio was 51:9 (5.7-fold higher in males). The
2014 NCCRC reported a high incidence of GC in the 60-79-year group
(27), which coincides with the age
data (66.5±11.4 years) obtained in the present research.
NLRX1 is a negative regulator of antiviral immunity
during the early stage of virus infection (16). With additional research involving
NLRX1, other functions of NLRX1 have been discovered, such as its
ability to regulate inflammation (28), metabolism (29) and development of histiocytic sarcoma
(18). Previous studies have also
reported that NLRX1 served as a tumor suppressor in colorectal
cancer (30,31). For example, the deletion of NLRX1 in
intestinal epithelial cells did not alter the architecture of the
intestines, but there was an increased susceptibility among
NLRX1-/- mice for developing colitis-associated
colorectal cancer (30,31). Wang et al (32) also concluded that NLRX1 expression
levels were downregulated in hepatocellular carcinoma tissues and
that NLRX1 expression levels may be used as a prognostic marker in
HCC hepatectomy. Castano-Rodriguez et al (33) reported that NLRX1 expression levels
were downregulated in H. pylori-infected gastric tissues.
However, the role of NLRX1 in GC has not been elucidated.
By examining IHC staining and western blotting of
NLRX1 in GC and normal gastric tissues, the present study revealed
that NLRX1 expression levels were downregulated in GC tissues,
indicating that NLRX1 may be a tumor suppressor. The changes in
NLRX1 expression levels were significantly associated with tumor
size, vascular invasion, neural invasion, lymph node metastasis,
differentiation, gross stage and clinical stage, which indicated
that NLRX1 may serve as an index in assessing GC. However, no
statistical differences were observed between NLRX1 expression
levels and the age or sex of the patient. A previous study
concluded that NLRX1 was a tumor suppressor in primary solid tumors
of the breast (34). Hu et
al (19) also reported that
downregulated expression levels of NLRX1 were associated with liver
cancer prognosis.
NF-κB is a pivotal mammalian transcription factor
that was discovered to exert protumorigenic effects in liver
(35), lung (36), breast (37) and prostate (38) cancer, in addition to in GC (39). In normal cells, NF-κB is located in
the cytosol in the form of an inactive complex bound to IκBα. Once
stimulated, IκBα is phosphorylated and separated from NF-κB for
degradation (40). NF-κB has been
demonstrated to remain in an active status in pancreatic cancer
cells (41). The results of the
present study revealed that NF-κB expression levels were
upregulated in GC tissues through IHC staining and western
blotting, which is consistent with the aforementioned studies.
microRNA-146a was discovered to upregulate NF-κB by
targeting TRAF6 in human cervical cancer (42). In multiple myeloma, TRAF6 was
discovered to mediate NF-κB activation and was suggested as a
potential therapeutic target (43).
Both of these studies illustrated that TRAF6 could regulate NF-κB
as an upstream gene. However, to the best of our knowledge, the
relationship between TRAF6 and NF-κB in GC remains to be clarified.
It has also been reported that in response to lipopolysaccharide,
NLRX1 interacted with TRAF6 and negatively regulated NF-κB
activation in 293T cells (16,44).
Allen et al (16)
demonstrated that in response to viral infection, NLRX1 decreased
the inflammatory responses by interacting with TRAF6.
TRAF6, as a TRAF protein family member, activates
IκB kinase, thus resulting in the degradation of IκB and the
activation of NF-κB (45,46). Therefore, a positive correlation is
suggested to exist between TRAF6 and NF-κB. In the present study, a
positive correlation was also discovered and TRAF6 and NF-κB
expression levels, as tumor promotors, were increased and
correlated with tumor size, vascular and neural invasion, lymph
node metastasis, differentiation, gross stage and clinical
stage.
In the present study, a negative correlation between
NLRX1 and TRAF6 was discovered using Spearman's rank correlation.
Therefore, it was speculated that NLRX1 may also interact with
TRAF6 to exert an antitumor effect in GC.
In conclusion, NLRX1 expression levels were
discovered to be downregulated in GC tissues and the expression
levels of NLRX1 were associated with the clinicopathological
characteristics of GC. A negative correlation was identified
between NLRX1 and TRAF6/NF-κB expression levels, while a positive
correlation was observed between TRAF6 and NF-κB expression levels.
Thus, NLRX1 may be a potential biomarker for the diagnosis of GC
and warrants further investigation.
Acknowledgements
Not applicable.
Funding
The present study was funded by the Dalian Medical
Science Research Project (grant no. 1711038), the National Natural
Science Foundation of China (grant nos. 81701965 and 81872255), the
Key Medical Talents Fund of Jiangsu Province (grant no.
2016KJQWZDRC-03) and the Natural Science Foundation of Liaoning
Province (grant nos. 20180550116 and 2019-MS-069).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
ZF and JP designed the study. JP contributed to the
collection and storage of the tissues. HW analyzed the pathology.
ZF, JP, HW and YZ performed the remaining experiments. YZ revised
the manuscript. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
The present study was approved by the Ethics
Committee of The Third People's Hospital of Dalian (approval no.
2017-KY-004). Written informed consent regarding information and
tissue samples were acquired from the patients.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Cancer Genome Atlas Research Network.
Comprehensive molecular characterization of gastric adenocarcinoma.
Nature. 513:202–209. 2014.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Gong Z, Mu Y, Chen J, Chu H, Lian P, Wang
C, Wang J and Jiang L: Expression and significance of cyclophilin J
in primary gastric adenocarcinoma. Anticancer Res. 37:4475–4481.
2017.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Wadhwa R, Song S, Lee JS, Yao Y, Wei Q and
Ajani JA: Gastric cancer-molecular and clinical dimensions. Nat Rev
Clin Oncol. 10:643–655. 2013.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2016. CA Cancer J Clin. 66:7–30. 2016.PubMed/NCBI View Article : Google Scholar
|
|
5
|
McGuire S: World Cancer Report 2014
Geneva, Switzerland: World Health Organization, International
Agency for Research on Cancer, WHO Press, 2015. Adv Nutr.
7:418–419. 2016.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Graziosi L, Marino E and Donini A:
Minimally invasive surgery for advanced gastric cancer: Are we
sure? Gastric Cancer. 20:1013–1014. 2017.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Peddanna N, Holt S and Verma RS: Genetics
of gastric cancer. Anticancer Res. 15:2055–2064. 1995.PubMed/NCBI
|
|
8
|
Lv Y, Zhao Y, Wang X, Chen N, Mao F, Teng
Y, Wang T, Peng L, Zhang J, Cheng P, et al: Increased intratumoral
mast cells foster immune suppression and gastric cancer progression
through TNF-α-PD-L1 pathway. J Immunother Cancer.
7(54)2019.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Xie Y, Li F, Li Z and Shi Z: miR-135a
suppresses migration of gastric cancer cells by targeting
TRAF5-mediated NF-κB activation. Onco Targets Ther. 12:975–984.
2019.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Deptala A, Bedner E, Gorczyca W and
Darzynkiewicz Z: Activation of nuclear factor kappa B (NF-kappaB)
assayed by laser scanning cytometry (LSC). Cytometry. 33:376–382.
1998.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Han F, Zhang L, Qiu W and Yi X: TRAF6
promotes the invasion and metastasis and predicts a poor prognosis
in gastric cancer. Pathol Res Pract. 212:31–37. 2016.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Inoue J, Gohda J and Akiyama T:
Characteristics and biological functions of TRAF6. Adv Exp Med
Biol. 597:72–79. 2007.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Sun YS, Ye ZY, Qian ZY, Xu XD and Hu JF:
Expression of TRAF6 and ubiquitin mRNA in skeletal muscle of
gastric cancer patients. J Exp Clin Cancer Res.
31(81)2012.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Maeda S, Yoshida H, Ogura K, Mitsuno Y,
Hirata Y, Yamaji Y, Akanuma M, Shiratori Y and Omata M: H.
pylori activates NF-kappaB through a signaling pathway
involving IkappaB kinases, NF-kappaB-inducing kinase, TRAF2, and
TRAF6 in gastric cancer cells. Gastroenterology. 119:97–108.
2000.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Takeuchi O and Akira S: Innate immunity to
virus infection. Immun Rev. 227:75–86. 2009.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Allen IC, Moore CB, Schneider M, Lei Y,
Davis BK, Scull MA, Gris D, Roney KE, Zimmermann AG, Bowzard JB, et
al: NLRX1 protein attenuates inflammatory responses to infection by
interfering with the RIG-I-MAVS and TRAF6-NF-κB signaling pathways.
Immunity. 34:854–865. 2011.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Moore CB, Bergstralh DT, Duncan JA, Lei Y,
Morrison TE, Zimmermann AG, Accavitti-Loper MA, Madden VJ, Sun L,
Ye Z, et al: NLRX1 is a regulator of mitochondrial antiviral
immunity. Nature. 451:573–577. 2008.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Coutermarsh-Ott S, Simmons A, Capria V,
LeRoith T, Wilson JE, Heid B, Philipson CW, Qin Q,
Hontecillas-Magarzo R, Bassaganya-Riera J, et al: NLRX1 suppresses
tumorigenesis and attenuates histiocytic sarcoma through the
negative regulation of NF-κB signaling. Oncotarget. 7:33096–33110.
2016.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Hu B, Ding GY, Fu PY, Zhu XD, Ji Y, Shi
GM, Shen YH, Cai JB, Yang Z, Zhou J, et al: NOD-like receptor X1
functions as a tumor suppressor by inhibiting
epithelial-mesenchymal transition and inducing aging in
hepatocellular carcinoma cells. J Hematol Oncol.
11(28)2018.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Fléjou JF: WHO Classification of digestive
tumors: The fourth edition. Ann Pathol. 31 (5 Suppl):S27–S31.
2011.PubMed/NCBI View Article : Google Scholar : (In French).
|
|
21
|
Zheng M, Zang S, Xie L, Fang X, Zhang YU,
Ma X, Liu J, Lin D and Huang A: Rheb phosphorylation is involved in
p38-regulated/activated protein kinase-mediated tumor suppression
in liver cancer. Oncol Lett. 10:1655–1661. 2015.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Lyros O, Thomaidis T, Muller M, Sivanathan
V, Grimminger P, Lang H, Gockel I, Hartmann JT and Moehler M:
External Validation of the Proposed Kiel Staging System and
Comparison with the Old (6th edition) and the Currently Used (7th
edition) TNM Classification in Gastric Cancer. Oncol Res Treat.
41:122–128. 2018.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Harino Y, Imura S, Kanemura H, Morine Y,
Fujii M, Ikegami T, Uehara H and Shimada M: Role of tumor
angiogenesis in gallbladder carcinoma: With special reference to
thymidine phosphorylase. Int J Clin Oncol. 13:452–457.
2008.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Bertuccio P, Chatenoud L, Levi F, Praud D,
Ferlay J, Negri E, Malvezzi M and La Vecchia C: Recent patterns in
gastric cancer: A global overview. Int J Cancer. 125:666–673.
2009.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Wang FH, Shen L, Li J, Zhou ZW, Liang H,
Zhang XT, Tang L, Xin Y, Jin J, Zhang YJ, et al: The Chinese
society of clinical oncology (CSCO): Clinical guidelines for the
diagnosis and treatment of gastric cancer. Cancer Commun (Lond).
39(10)2019.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Makino Y, Nishimura Y, Oshita S, Mizosoe T
and Akihiro T: Storage in high-barrier pouches increases the
sulforaphane concentration in broccoli florets. PLoS One.
13(e0192342)2018.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Chen W, Sun K, Zheng R, Zeng H, Zhang S,
Xia C, Yang Z, Li H, Zou X and He J: Cancer incidence and mortality
in China, 2014. Chin J Cancer Res. 30:1–12. 2018.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Ma D, Zhao Y, She J, Zhu Y, Zhao Y, Liu L
and Zhang Y: NLRX1 alleviates lipopolysaccharide-induced apoptosis
and inflammation in chondrocytes by suppressing the activation of
NF-κB signaling. Int Immunopharmacol. 71:7–13. 2019.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Scantlebery AML, Uil M, Butter LM, Poelman
R, Claessen N, Girardin SE, Florquin S, Roelofs JJTH and Leemans
JC: NLRX1 does not play a role in diabetes nor the development of
diabetic nephropathy induced by multiple low doses of
streptozotocin. PLoS One. 14(e0214437)2019.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Soares F, Tattoli I, Rahman MA, Robertson
SJ, Belcheva A, Liu D, Streutker C, Winer S, Winer DA, Martin A, et
al: The mitochondrial protein NLRX1 controls the balance between
extrinsic and intrinsic apoptosis. J Biol Chem. 289:19317–19330.
2014.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Tattoli I, Killackey SA, Foerster EG,
Molinaro R, Maisonneuve C, Rahman MA, Winer S, Winer DA, Streutker
CJ, Philpott DJ and Girardin SE: NLRX1 acts as an
epithelial-intrinsic tumor suppressor through the modulation of
TNF-mediated proliferation. Cell Rep. 14:2576–2586. 2016.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Wang X, Yang C, Liao X, Han C, Yu T, Huang
K, Yu L, Qin W, Zhu G, Su H, et al: NLRC and NLRX gene family mRNA
expression and prognostic value in hepatocellular carcinoma. Cancer
Med. 6:2660–2672. 2017.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Castaño-Rodríguez N, Kaakoush NO, Goh KL,
Fock KM and Mitchell HM: The NOD-like receptor signalling pathway
in Helicobacter pylori infection and related gastric cancer:
A case-control study and gene expression analyses. PLoS One.
9(e98899)2014.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Singh K, Poteryakhina A, Zheltukhin A,
Bhatelia K, Prajapati P, Sripada L, Tomar D and Singh R, Singh AK,
Chumakov PM and Singh R: NLRX1 acts as tumor suppressor by
regulating TNF-α induced apoptosis and metabolism in cancer cells.
Biochim Biophys Acta. 1853:1073–1086. 2015.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Zhang M, Pan L, Xu D, Cao C, Shi R, Han S,
Liu J, Li X and Li M: The NFκB signaling pathway serves an
important regulatory role in Klebsiella pneumoniae liver abscesses.
Exp Ther Med. 15:5443–5449. 2018.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Ahmmed B, Khan MN, Nisar MA, Kampo S,
Zheng Q, Li Y and Yan Q: Tunicamycin enhances the suppressive
effects of cisplatin on lung cancer growth through PTX3
glycosylation via AKT/NF-κB signaling pathway. Int J Oncol.
54:431–442. 2019.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Bishop RT, Marino S, de Ridder D, Allen
RJ, Lefley DV, Sims AH, Wang N, Ottewell PD and Idris AI:
Pharmacological inhibition of the IKKε/TBK-1 axis potentiates the
anti-tumour and anti-metastatic effects of Docetaxel in mouse
models of breast cancer. Cancer Lett. 450:76–87. 2019.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Marino S, Bishop RT, Carrasco G, Logan JG,
Li B and Idris AI: Pharmacological inhibition of NFκB reduces
prostate cancer related osteoclastogenesis in vitro and osteolysis
ex vivo. Calcif Tissue Int. 105:193–204. 2019.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Fu J, Yu L, Luo J, Huo R and Zhu B:
Paeonol induces the apoptosis of the SGC-7901 gastric cancer cell
line by downregulating ERBB2 and inhibiting the NF-κB signaling
pathway. Int J Mol Med. 42:1473–1483. 2018.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Novack DV: Role of NF-κB in the skeleton.
Cell Res. 21:169–182. 2011.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Jeong Y, Lim JW and Kim H: Lycopene
inhibits reactive oxygen species-mediated NF-κB signaling and
induces apoptosis in pancreatic cancer cells. Nutrients.
11(762)2019.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Li T, Li M, Xu C, Xu X, Ding J, Cheng L
and Ou R: miR-146a regulates the function of Th17 cell
differentiation to modulate cervical cancer cell growth and
apoptosis through NF-κB signaling by targeting TRAF6. Oncol Rep.
41:2897–2908. 2019.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Morgan JJ, McAvera RM and Crawford LJ:
TRAF6 silencing attenuates multiple myeloma cell adhesion to bone
marrow stromal cells. Int J Mol Sci. 20(702)2019.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Xia X, Cui J, Wang HY, Zhu L, Matsueda S,
Wang Q, Yang X, Hong J, Songyang Z, Chen ZJ and Wang RF: NLRX1
negatively regulates TLR-induced NF-κB signaling by targeting TRAF6
and IKK. Immunity. 34:843–853. 2011.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Paik JH, Jang JY, Jeon YK, Kim WY, Kim TM,
Heo DS and Kim CW: MicroRNA-146a downregulates NFκB activity via
targeting TRAF6 and functions as a tumor suppressor having strong
prognostic implications in NK/T cell lymphoma. Clin Cancer Res.
17:4761–4771. 2011.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Yang WL, Wang J, Chan CH, Lee SW, Campos
AD, Lamothe B, Hur L, Grabiner BC, Lin X, Darnay BG and Lin HK: The
E3 ligase TRAF6 regulates Akt ubiquitination and activation.
Science. 325:1134–1138. 2009.PubMed/NCBI View Article : Google Scholar
|