1. Introduction
Regenerative medicine and tissue engineering aim to
restore both the structure and functions of damaged tissues
(1). In recent years, novel and
more efficient regenerative strategies have been devised. These
emerging approaches employ specific biocompatible and bioactive
composite or natural scaffolds, into which cells or bioactive
molecules are incorporated to construct a dynamic environment for
wound healing in damaged tissues (2). However, the development and
optimization of low-cost and effective therapeutic methods remains
the primary goal (3).
Several studies have focused on autologous platelet
concentrate derivatives, which may delay complications and boost
tissue regeneration. It has been >40 years since the first
generation of autologous platelet concentrates, termed
platelet-rich plasma (PRP), was produced (4). Since then, other platelet concentrates
have been established, including platelet-rich fibrin (PRF) and
concentrated growth factor (CGF). Platelet concentrates have three
standard characteristics: i) They act as scaffolds, ii) they serve
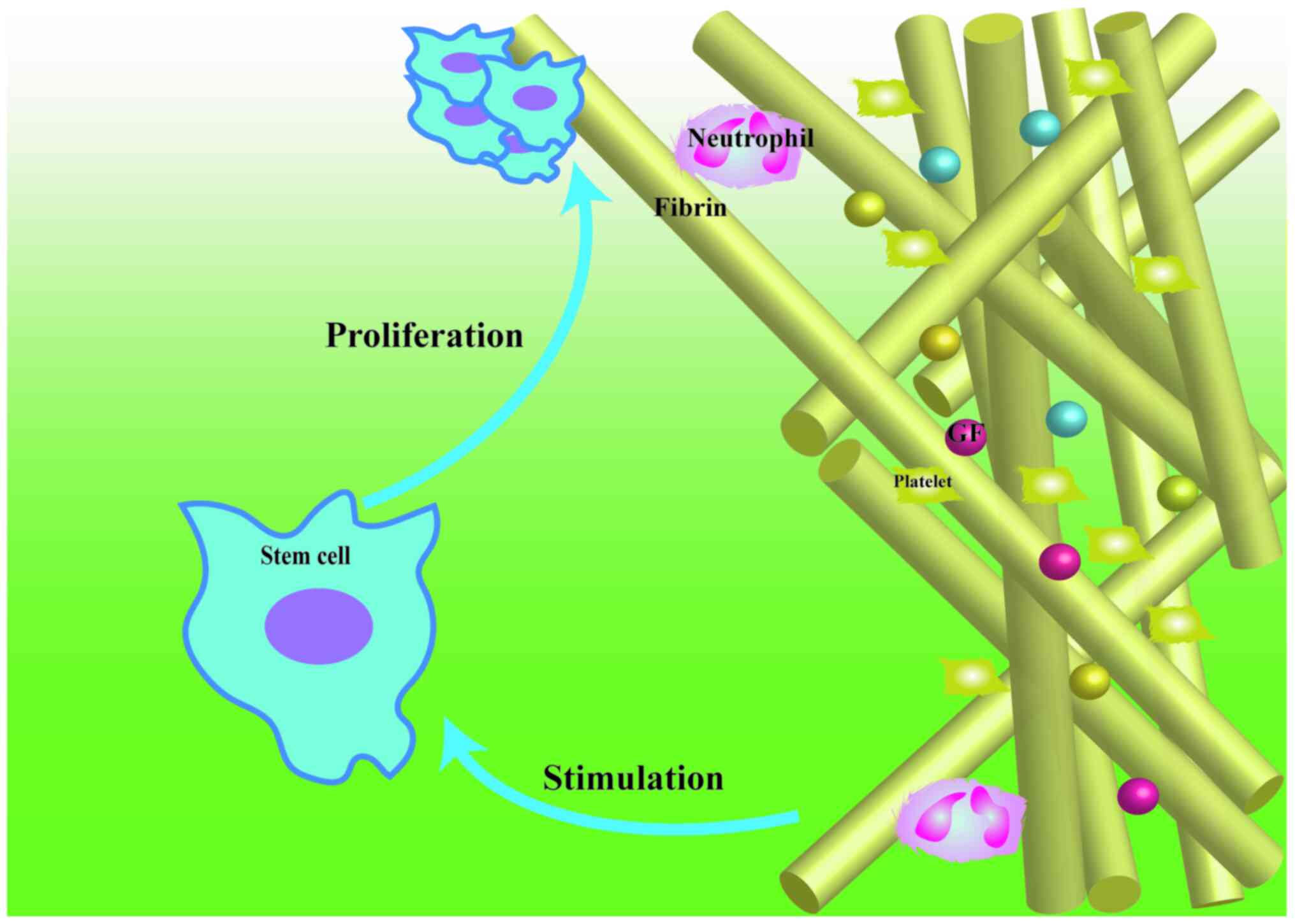
as a source of GF and iii) they contain live cells (Fig. 1 describes the rationale of using
platelet concentrates in tissue regeneration) (5). These characteristics make platelet
concentrates suitable candidates for tissue regeneration in
clinical practice. The clinical applications of these types of
derivatives of platelet concentrates in sports, spine and
musculoskeletal medicine, ophthalmology and oral surgery have
increased over the past few decades, resulting in positive clinical
effects due to their ability to rapidly regenerate tendon, muscle
and bone cartilage and speed up wound healing (6). Platelet concentrate injections in the
knee for curing early osteoarthritis have achieved satisfying
clinical results (7,8). Platelet concentrates may serve as
filling material for post-extraction alveolar sockets, resulting in
increased bone quality and soft-tissue healing (9,10). In
addition, dormant corneal ulcers associated with surgery may be
healed by platelet concentrates prospectively (11). Chronic ulcers, characterized by a
prolonged, self-perpetuating inflammatory phase, slow and defective
formation of extracellular matrix and a decreased rate or a failure
of re-epithelialization are general complications associated with
diabetes or bedsores (12-17).
Platelet concentrates provide a novel standard of chronic wound
care and achieve satisfying healing effects in the clinic. These
positive results suggest that platelet concentrates may be used to
effectively manage simple clinical issues.
However, the single use of platelet concentrates may
not always provide satisfactory clinical results, particularly as
complex clinical issues are concerned, such as substantial wounds
accompanied with infection, where the high demands of wound healing
result in a high degree of tissue tension. Occasionally, the goal
is to restore tissues rapidly with improved healing results, such
as in skin and cartilage, with the restored tissue being similar to
the normal tissue, thus making it functionally better or compared
with the simple use of platelet concentrates. In addition,
prolonged degradation of platelet concentrates is required to keep
pace with the slow regeneration of bones. Thus, it is necessary to
develop platelet concentrates further, such that they are suitable
for use in complex clinical applications and meet regenerative
expectations. The present review highlights novel strategies
utilizing platelet concentrates for tissue engineering and
discusses several aspects of these strategies, such as lyophilized
platelet concentrates, as well as the combination of platelet
concentrates with biomaterials, stem cells or medications. The aim
of the present review is to discuss the current progress in the
field and highlight potential avenues for development strategies
utilizing platelet concentrates with improved quality and
performance for different therapeutic applications. Therefore, a
comprehensive and detailed review on modifying strategies of
platelet concentrates in various types of tissue regeneration is
provided. An overview is presented in Fig. 2.
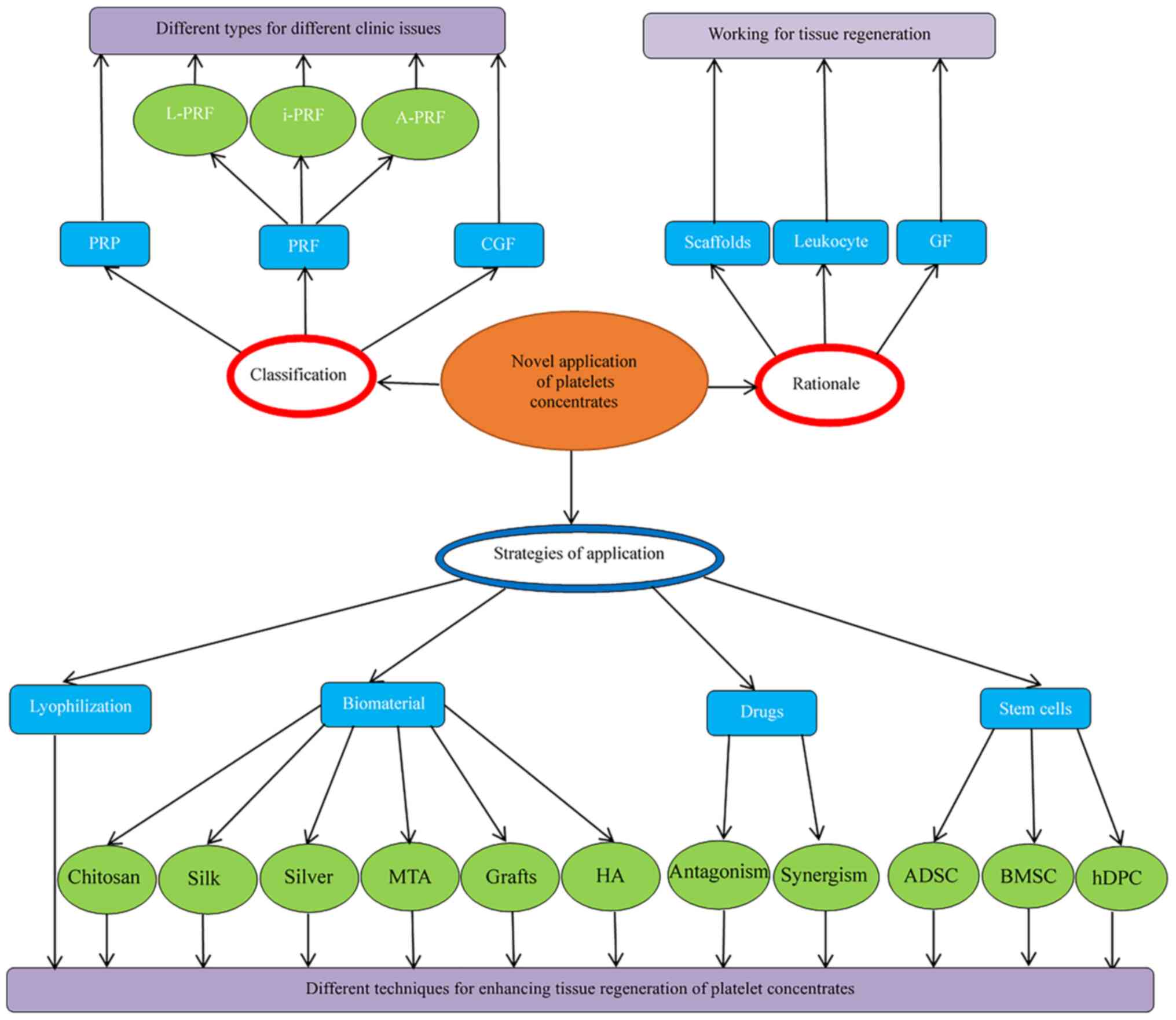 | Figure 2Platelet concentrates and application
strategies. PRP, platelet-rich plasma; i-PRF, injectable
platelet-rich fibrin; GF, growth factor; BMSC, bone marrow stromal
cells; A-PRF, advanced PRF; HA, hydroxyapatite; L-PRF, leukocyte
PRF; CGF, concentrated GFs; ADSC, adipose-derived stem cell; h-DPC,
human dental pulp cells; MTA, mineral trioxide aggregate. |
2. Methods
This detailed review focuses on research employing
platelet concentrates for novel tissue engineering applications. A
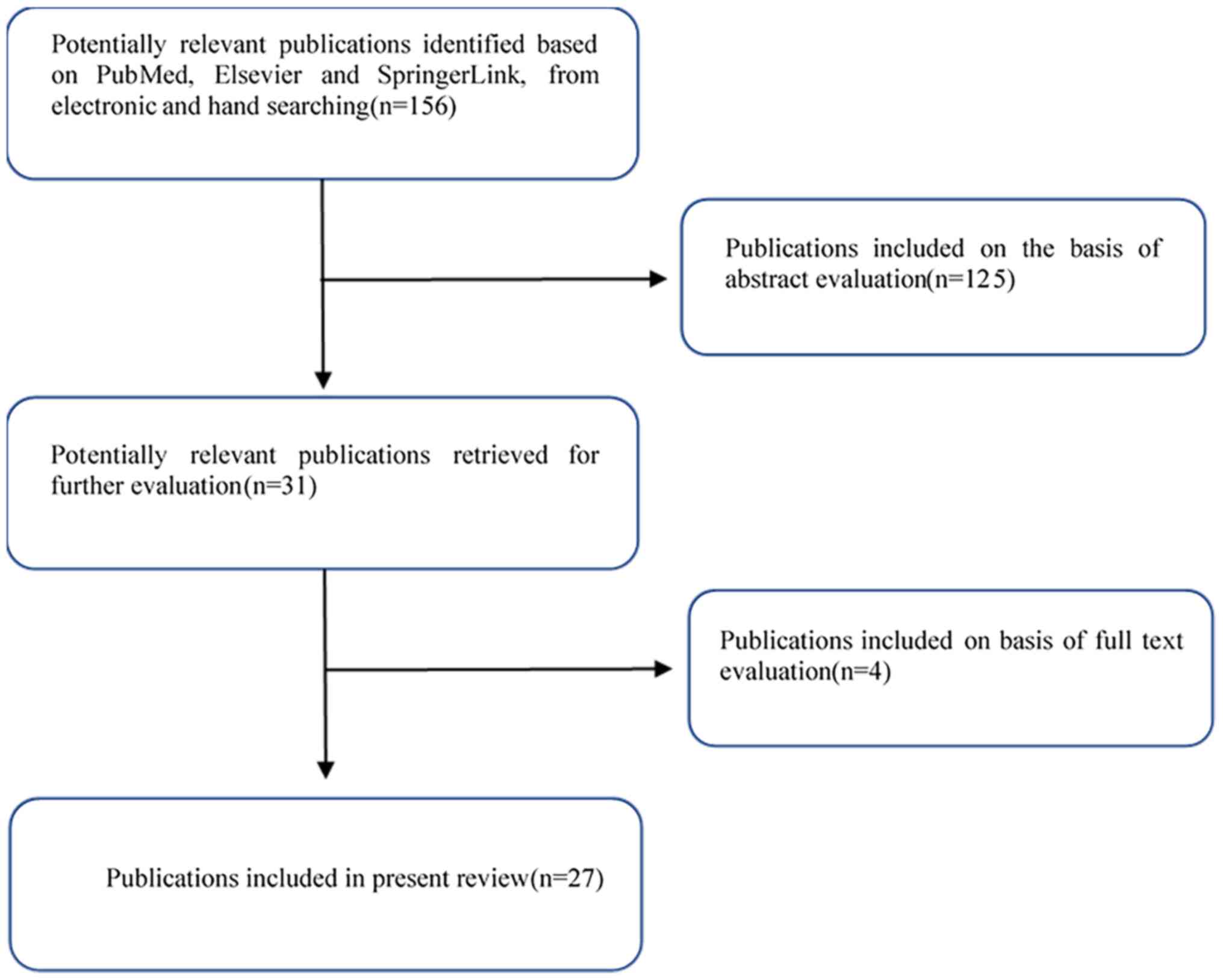
comprehensive literature search was performed to identify
appropriate studies for inclusion, incorporating several databases
(Fig. 3) and using permutations of
multiple search terms as follows: Platelet Rich Plasma; PRP;
Platelet Rich fibrin; Platelet Rich Fibrin; PRF; Platelet-Rich
Fibrin; Leukocyte Platelet Rich Fibrin; Leukocyte Platelet-Rich
Fibrin; LPRF; L-PRF; Advanced Platelet Rich Fibrin; Advanced PRF;
A-PRF; APRF; Injectable Platelet Rich Fibrin; IPRF; Concentrated
Growth Factors; CGF and Soft Tissue Regeneration; Soft Tissue Wound
Regeneration; Soft Tissue Wound-Healing; Wound Healing; Bone
Regeneration; Bone Formation; Tissue Engineering. The inclusion
criteria comprised studies published in the English language and
both in vitro and in vivo evaluations of novel tissue
regeneration applications of platelet concentrates. Methods,
objectives, conclusions, potential future directions and potential
shortcomings of included studies were reviewed.
3. Classification and preparation of
platelet concentrates
Platelet concentrates may be defined as autologous
biological products obtained following centrifugation of peripheral
blood. Different centrifugal speeds result in various types of
platelet concentrates and the characteristics are compared and
summarized in Table I. PRP was
introduced in the 1970s and is recognized as the first generation
of platelet concentrates (4). PRP
is produced by a two-step procedure: Blood is collected and
centrifuged at high speeds after the addition of an anticoagulant.
The plasma, erythrocytes and buffy coat separate at the top,
bottom, and in the middle layer, respectively. The buffy coat
contains platelets and leukocytes. Blood cells are removed and the
remnants are centrifuged at a higher speed. Finally, PRP is
collected from the bottom layer. To obtain insoluble PRP, bovine
serum or 2% of calcium chloride are added. This process converts
plasmatic fibrinogen into insoluble fibrin scaffolds and activates
the platelets (18-20).
However, the primary concern with this procedure is the presence of
chemical or biological additives that may have adverse effects on
tissue regeneration and delay wound healing.
 | Table IComparison of characteristics of
platelet concentrates. |
Table I
Comparison of characteristics of
platelet concentrates.
| | PRF | |
|---|
|
Classification/author (year) | Platelet-rich
plasma | L-PRF | i-PRF | A-PRF | Concentrated growth
factors | Ref. |
|---|
| Anticoagulants | | | | | | |
|
Miron et
al (2017) | Yes | No | No | No | No | (26) |
|
Isobe
(2017) | | | | | | (86) |
| Preparation (RCF/g)
centrifuge tube | | | | | | |
|
Hatakeyama
et al (2014) | 700/8+1600/8
(plastic tubes) | 708/12 (glass
tubes) | 60/3 (plastic
tubes) | 208/8 (glass
tubes) | Altered speed
(glass tubes) | (19) |
|
Miron et
al (2017) | (26) |
|
Pitzurra
et al (2020) | | | | | | (25) |
| Contents | | | | | | |
|
Miron et
al (2017) | Leukocytes, GF and
fibrinogen | Leukocytes, GF and
fibrin | Leukocytes, GF and
fibrinogen | Leukocytes, GF and
fibrin | Leukocytes, GF and
fibrin | (5) |
|
Masuki
(2016) | (87) |
| Morphological
characteristics | | | | | | |
|
Miron et
al (2018) | Liquid | Solid | Liquid | Solid | Solid | (3) |
|
Isobe
(2017) | | | | | | (86) |
| Fibrin
formation | | | | | | |
|
Miron and
Zhang (2018) | Requirement to be
induced | Natural | Delayed
formation | Natural | Natural | (3) |
|
Hatakeyama
et al (2014) | (19) |
|
Anitua et
al (2019) | (20) |
|
Ghanaati
et al (2014) | (23) |
|
Malli et
al (2019) | (27) |
| Fibrin density | | | | | | |
|
Masuki
(2016) | - | + | - | ++ | ++ | (87) |
|
Isobe
(2017) | (86) |
| Degradable
rates | | | | | | |
|
Isobe
(2017) | Fast | Moderate | Fast | Moderate | Moderate | (86) |
Consequently, in 2001, the optimization of the
preparation protocol was introduced by Choukroun, a second
generation of platelet concentrates called PRF, which was
subsequently renamed as leukocyte PRF (L-PRF), an enhanced version
of PRP (21,22). PRF has three primary advantages: i)
Chemical or biological additives are absent, which avoids their
related adverse reactions; ii) fibrin clots are naturally formed,
so there is no requirement to add platelet activators, such as
bovine serum or calcium chloride; iii) a higher concentration of
host immune cells is collected in the fibrin matrix (3,23).
Since low-speed centrifugation preserves more immune cells, GF and
cytokines (24), this procedure has
gained popularity, resulting in the production of advanced PRF
(A-PRF) and injectable PRF (i-PRF). In a standard protocol, PRF is
centrifuged at 708 x g for 12 min, whereas A-PRF is processed at
208 x g for 8 min (25) and i-PRF
is centrifuged at 60 x g for 3 min (26). Another advantage of i-PRF is that it
produces a solid fibrin clot within 15 min, which gives operators
adequate time to handle it and combine with other materials.
CGF is considered the third generation of autologous
plasma and is prepared by a different centrifugation method. These
platelet concentrates are produced by centrifuging blood samples at
dynamic speeds, which increases the density of the fibrin matrix
and the concentration of GF (27).
However, it is not easy to differentiate A-PRF and CGF either
macroscopically or microscopically due to their similar principles
of preparation and they are thus classified as the same type of
platelet concentrate (Table I)
(28).
4. Rationale of using platelet concentrates
in tissue restoration
The wound-healing process consists of five stages:
Hemostasis, inflammation, cell proliferation, migration and tissue
remodeling/maturation (29,30). Platelets, GF, leukocytes and fibrous
proteins serve essential roles in these phases. The aim of the
present review is not to describe their functions in detail. The
respective functions of the primary ingredients of platelet
concentrates in the wound-healing process may be generally
summarized as stated below.
GF serve critical roles in wound healing. These
cytokines originate from cells, including platelets and leukocytes,
or plasma. In general, GF stimulates the proliferation of stem
cells (Fig. 1). These factors
include platelet-derived growth factor (PDGF), vascular endothelial
growth factor (VEGF), basic fibroblast growth factor (bFGF) and
transforming growth factor-β (TGF-β) (31,32).
They serve different roles in different phases of the wound-healing
process. The function of PDGF may be summarized as follows: First,
it initiates and drives the wound-healing process by guiding
neutrophils, macrophages and fibroblasts into the wound site from
the early stage; subsequently, it activates macrophages and other
cells possessing the potential for differentiation, such as
fibroblasts, thereby resulting in provisional extracellular matrix
synthesis, stem cell proliferation and collagen synthesis; finally,
it triggers remodeling of the wound by activating and cross-linking
collagen (33). Angiogenesis is a
part of the tissue remodeling/maturation phase in wound healing. In
this phase, both bFGF and VEGF promote endothelial cells to migrate
and proliferate and differentiate into new blood vessels.
Furthermore, TGF-β may promote angiogenesis, collagen synthesis and
collagenase secretion and modulate osteoblastic cell division
(31).
During the synthesis of autologous fibrin scaffolds,
the 3-dimensional fibrin nano-scaffold incorporates the platelets
in a non-diffusible mode and binds platelet- and plasma-derived GF
before they attach to their corresponding cell-surface receptors
(20). As the fibrin matrix is
gradually degraded, GF and platelets are slowly released into the
injured site at a rate consistent with that required for tissue
growth (34,35). Furthermore, the fibrin matrix
provides mechanical support, plastic-elastic stiffness and space
for cellular migration and proliferation (Fig. 1) (36,37).
Thus, platelet concentrates fulfill different requirements for
tissue restoration by linking molecular and cellular events.
5. Strategies to enhance the properties of
platelet concentrates for clinical applications
General
The tissue regeneration process involves cells,
signaling biomolecules and scaffolding. The reparative protocols
introduced in the present review focus on the application of
platelet concentrates in several forms, either lyophilized or
blended with (bio)materials (chitosan, silk fibrin, metal
nanoparticles, mineral trioxide aggregate graft materials and
hydroxyapatite) and/or stem cells [adipose-derived stem cells, bone
marrow stromal cells (BMSC) and human dental pulp cells] or those
modulated with drugs (antagonism and synergism) to enhance tissue
regeneration. An overview is provided in Table II.
 | Table IIStudies evaluating the effects of
strategies/advanced techniques of platelet concentrates in tissue
regeneration. |
Table II
Studies evaluating the effects of
strategies/advanced techniques of platelet concentrates in tissue
regeneration.
| A,
Lyophilization |
|---|
| Author (year) | Setting | Research
direction | Conclusion | Ref. |
|---|
| Wang et al
(2019) | In vivo and
in vitro | Bone and
osteoblasts | LPRF promotes BMSC
proliferation and osteogenic differentiation; bone regeneration
with LPRF and BMSC in mice. | (41) |
| Li (2014) | In vivo and
in vitro | Bone and MSC | Lyophilization
favors PRF promotes Runx2-mediated osteogenic lineage commitment in
alveolar bone cells in alveolar bone cells mainly; bone
regeneration and mineralization. | (42) |
| Liu et al
(2019) | In vivo and
in vitro | Bone and BMSC | Combination of
fresh and lyophilized PRF favors BMSC osteogenic differentiation
in vitro and bone formation in vivo. | (44) |
| Zhang et al
(2017) | In vivo | Bone | Lyophilization
preserves the clinical effects. of PRF with tissue healing | (43) |
| B, Combination with
(bio)materials |
| Method/author
(year) | Setting | Research
direction | Conclusion | Ref. |
| Chitosan | | | | |
|
Mohammadi
et al (2016) | In vivo | Full-thickness
wound healing | Chitosan and PRP
combinations promote wound healing by promoting collagen
synthesis. | (47) |
|
Shimojo
et al (2016) | In
vitro | Human ADMSCs | Composite scaffolds
control the release of and enhance the proliferation of
ADMSCs. | (48) |
|
Wang et
al (2019) | In
vitro | Murine-derived
cell | CGF loaded on
chitosan-alginate stably releases GF and presents superior
osteogenic effects. | (49) |
|
Ansarizadeh
et al (2019) | In
vitro | Bone marrow
MSCs | Composite scaffolds
present higher Young's modulus and MSC viability and lower
degradation rate. | (50) |
| Silk fibrin | | | | |
|
Lee et
al (2010) | In vivo | Bone | A combination of
PRF and silk fibroin presented faster bone formation than the
unfilled group. | (54) |
| SNPs | | | | |
|
Khorshidi
et al (2018) | In
vitro | Antimicrobial
properties | Modification of
L-PRF by SNPs exhibits antimicrobial properties, mainly for
viridans streptococci and higher mechanical strength. | (57) |
| MTA | | | | |
|
Woo et
al (2018) | In
vitro | h-DPCs | The synergic
effects of MTA and PRF promote the differentiation of h-DPCs into
odontoblast-like cells through the activation of BMP/Smad signaling
pathway. | (58) |
| Graft
materials | | | | |
|
Abdullah
(2016) | In vivo | Bone | A combination of
PRF and β-TCP accelerates bone regeneration compared to PRF
alone. | (59) |
|
Karayurek
et al (2019) | In vivo | Bone | A combination of
PRF and autograft presents superior bone regeneration than other
graft materials. | (60) |
| HA | | | | |
|
Ohba et
al (2012) | In vivo | Bone | Electrically
polarized HA/PRP gel activated osteogenic cells to enhance bone
regeneration. | (61) |
|
Sadeghinia
et al (2019) | In
vitro | h-DPSCs | PRP-fibrin
glue/chitosan-gelatin/nano HA provides rich GF to promote
differentiation and proliferation of h-DPSCs. | (62) |
| C, Combination with
stem cells |
| Method/author
(year) | Setting | Research
direction | Conclusion | Ref. |
| ADSC | | | | |
|
Stessuk
et al (2016) | In
vitro | Fibroblasts and
keratinocytes | A combination of
ADSC and PRP stimulates fibroblasts and keratinocytes to
proliferate. | (67) |
|
Chen et
al (2014) | In
vitro | Maxillofacial soft
tissue | A mixture of PRF
and ADSC cures injury of maxillofacial soft tissue by irradiation
better than PRF or ADSC alone. | (70) |
|
Sun et
al (2014) | In vivo | Myocardial
tissue | PRF-embedded
autologous ADMSC protects left ventricle. | (71) |
| Bone mesenchymal
cells | | | | |
|
Park et
al (2017) | In vivo | Bone | Increased bone
formation. | (68) |
|
Wang et
al (2017) | In vivo | Bone | The combined
application of an MSC sheet with nano-HA and granular PRF promotes
bone regeneration in large bone defects. | (72) |
|
Wang et
al (2016) | In vivo and
in vitro | Periodontal
ligament and jaw bone MSC | Periodontal
ligament stem cells/PRF/jaw bone MSCs induce periodontal ligament-
and bone-like tissue. | (73) |
|
Wu et
al (2017) | In vivo | Bone | MSC + PRF releasate
induce hyaline-like cartilage in defects and present better results
than PRF or MSC alone. | (74) |
| h-DPC | | | | |
|
He et
al (2016) | In
vitro | h-DPC | When h-DPC is added
before blood centrifugation during the preparation of PRF, the
PRF-h-DPC complex is successfully developed. | (75) |
| D, Combination with
drugs |
| Method/author
(year) | Setting | Research
direction | Conclusion | Ref. |
| Antagonism | | | | |
|
Steller
et al (2019) | In
vitro | Osteoblasts | PRF and PRP enhance
bone healing in patients with osteonecrosis of the jaw and PRF is
better than PRP. | (81) |
|
Borsani
et al (2018) | In
vitro | Osteoblasts | Cotreatment with
resveratrol and CGF protect osteoblasts treated with
bisphosphonates. | (83) |
| Synergism | | | | |
|
Xu et
al (2016) | In vivo and
in vitro | Human breast
ADSC | A combination of
G-Rg1 and PRF augments the effect of neovascularization and
adipogenesis compared to G-Rg1 or PRF alone. | (84) |
|
Raafat et
al (2018) | In vivo | Bone formation | Statins loaded on
PRF promote better bone healing and bone maturation than each
material alone. | (85) |
Lyophilized platelet concentrates
Lyophilization, a process comprising sublimation and
desorption of water from the frozen sample, may improve protein
stability, extend shelf-life and preserve the biological activity
of samples (38-41).
Therefore, although it is not necessary to use platelet
concentrates after lyophilization immediately, there may be
concerns regarding whether lyophilization affects the restorative
abilities of platelet concentrates. Based on the current body of
knowledge, it is not easy to differentiate between the number of GF
between lyophilized and fresh PRF. Furthermore, during the process
of lyophilization, the structure of the fibrin matrix is altered
substantially. For instance, the pore size of the fibrin matrix is
enlarged, which accelerates its degradation rate. The process of
lyophilization may also damage the structure of leukocytes and this
may reduce immunological rejection. Since the amount of GF in
lyophilized and fresh platelet concentrates retains consistency,
the in vitro osteogenic capacity of BMSC sheets treated with
fresh or lyophilized platelet concentrates is unaffected (41). Owing to the changes in the structure
of lyophilized platelet concentrates, adhesion and migration of
stem cells in vivo may in turn be affected, which may
improve bone regeneration in bone defects compared with the fresh
preparations (42). However,
results regarding lyophilized PRF are inconsistent. For instance,
one study reported that lyophilized PRF appears to retain an
architecture similar to that of fresh PRF (both lyophilized and
fresh PRF exhibit an abundance of leukocytes with nuclei
distributed within the fibrin matrix) (43). However, the sponge structure of
lyophilized PRF has a larger internal space than that of fresh PRF,
while the structure of fibrin is similar in both types of PRF. The
conclusion derived from this contrasting scenario is that
lyophilization of PRF may preserve its bioactivity in tissue
regeneration. On the other hand, another concern is whether mixing
lyophilized and fresh PRF at different ratios is better than the
use of lyophilized or fresh PRF alone. Studies have indicated that
mixing lyophilized and fresh PRF at a 1:1 ratio may exert improved
healing effects in vivo, as TGF-β was released more
steadily, which confirmed the hypothesis that fresh/lyophilized PRF
not only prolongs the release of GF but also delays the peak of
release (44).
We hypothesized that lyophilization may not
influence cell sheets in vitro due to the constant levels of
GF in lyophilized and fresh PRF preparations. However, structural
changes occur due to lyophilization, such as enlarged pore size,
which serve pivotal roles in wound healing and influence
proliferation, adhesion and migration of stem cells in vivo.
Consequently, further investigations are required to verify whether
lyophilized PRF exhibits increased tissue-restorative capacities
compared with fresh PRF.
Platelet concentrates combined with
biomaterials
Biomaterials are widely used in tissue engineering
for their unique biological characteristics, which may be used to
cover the shortcomings of platelet concentrates. Potential
shortcomings of platelet concentrates include the possibility that
released cytokines may not sustain tissue regeneration for a
prolonged period and that platelet membranes may be vulnerable to
mechanical stress (4,30,45),
limiting their surgical applicability. Therefore, the following
section addresses several approaches that have been proposed to
overcome this problem.
Chitosan. Chitosan contributes to several
phases of wound repair via the promotion of cellular organization
(46). This biomaterial serves a
role during proliferation and matrix generation, which may reduce
the formation of disoriented connective tissue. Biocompatible
membranes adsorbed with PRP and chitosan have excellent
wound-healing effects (47). The
composite scaffolds control the release of cytokines and enhance
the proliferation of stem cells compared with platelet concentrates
alone.
Furthermore, chitosan may be made porous and
stabilized by different treatments, yielding improved properties
that may then be combined with platelet concentrates (48). Lyophilized platelet concentrates are
more rapidly combined with biomaterials when compared with fresh
ones. By loading the lyophilized platelet concentrates on the
chitosan membrane, the physical properties may be tuned; two
primary advantages have been described (49,50).
First, the degradation rate is slow, which ensures a steady and
prolonged release of GF. Furthermore, their enhanced mechanical
strength makes them more suitable for surgery. Combination with
absorbable biomaterials, such as chitosan, provides a feasible
approach to prepare wound dressings. The synthetic membrane may be
designed to not only provide adequate mechanical strength for
surgery but also delay the release of GF in platelet concentrates,
matching the speed of tissue regeneration.
Silk fibroin. Silk fibroin offers good
biocompatibility, slow degradation and excellent mechanical
properties (51-53).
When tissue defects are too large, platelet concentrates filling
the defects may not be sufficient and in such cases, the
combination of silk fibroin and PRF is a potentially suitable
option (54). Owing to the
excellent biocompatibility of silk fibrin, tissue regeneration may
be accelerated by such synthetic biomaterials. However, slow
degradation may induce immunoreaction and has an adverse impact on
bone formation, as the scaffolds would occupy the space for bone
formation. Thus, it is necessary to further explore the
relationship between biodegradability and osteo-induction and
calibrate the degradation rates of scaffolds for optimal bone
formation.
Metal nanoparticles. Metal-derived
nanoparticles are biocompatible, multifunctional and versatile
materials, which are widely used as diagnostic and drug delivery
tools in anticancer and antibacterial therapy (55). In the majority of cases, platelet
concentrates are exposed to the oral cavity, where a large number
of microorganisms may interfere with the treatment (56). Silver nanoparticles (SNP) have both
high biocompatibility and antimicrobial properties. Preparation of
platelet concentrates with the addition of SNP improved both the
antibacterial abilities, primarily via the inhibition of viridans
streptococci, and enhanced mechanical strength (57). A range of metal particles are being
applied in medical research for different purposes and these
nanoscale particles may endow platelet concentrates with novel
biological properties (57).
Appropriate nanoalloy particles not only improve the properties of
platelet concentrates but also broaden the scope of clinical
application.
Mineral trioxide aggregate (MTA). MTA is
widely used in endodontic applications, including pulp capping,
root perforation repair and root-end filling, to induce pulp
regeneration and facilitate dentin bridge formation without causing
inflammation. In 2016, Woo et al (58) aimed to evaluate the effect of
combined MTA and PRF on odontoblastic differentiation in human
dental pulp cells (h-DPC). Indeed, the combination exhibited a
synergistic effect by accelerating differentiation via activation
of the bone morphogenetic protein (BMP)/Smad signaling pathway.
Since both materials promote pulp and dentin regeneration, their
combination may represent a novel therapeutic strategy in the
context of pulpitis.
Graft materials. Graft materials are
frequently employed to improve bone volumes for dental surgery,
such as autografts, allografts, xenografts and alloplastic grafts.
However, the effects of grafts on osteogenesis may be limited
without the assistance of autologous bioactive ingredients. Of
note, platelet concentrates may help overcome these issues. The
bone regeneration capacity of PRF mixed with β-tricalcium phosphate
is higher than that of PRF alone, although the difference is only
significant in the initial two weeks post-surgery (59). Furthermore, there are discrepancies
regarding the effects of PRF combined with different grafts on bone
formation and maturation (60).
Autograft-PRF exhibits outstanding results in the formation of new
bone when compared with any other combinations and the
xenograft-PRF combination is also promising for such applications
(60). This may be ascribed to the
abundance of bioactive BMSC in autografts, which may cooperate with
platelet concentrates to actively regenerate bone tissue. In
addition, autografts may reduce immunological rejection compared to
other types of grafts. In general, platelet concentrates provide a
plethora of bioactive molecules, which may promote bone
regeneration and deposition in graft materials (59,60).
Thus, the use of platelet concentrates for improving the effects of
graft materials should be further studied.
Hydroxyapatite (HA). HA is a calcium
phosphate compound that serves as a bone stuffer due to its mineral
composition being similar to that of bone tissue. However, its use
in surgery is limited owing to the challenge of handling HA at
operation sites. Consequently, it is necessary to deliver HA
granules using biomaterials. A liquid platelet concentrate may be
appropriate for carrying HA granules (61). After thoroughly mixing the HA with
platelet concentrates, the blend may be solidified prior to being
implanted into tissue defects. On the other hand, fibrin glue and
activated PRP loaded on composite scaffolds based on
chitosan-gelatin/nanohydroxyapatite may improve osteogenic
differentiation and proliferation of dental pulp stem cells
(62). However, synthetic scaffolds
offer certain advantages. First, platelets are typically scattered
on the surface of the membrane, suggesting that the release of GF
is smooth. In addition, the fibrin glue may guide the stem cells to
adhere to the network structure and stimulate cells without further
migration, which limits mesenchymal cell mobility. Finally, the
scaffold provides a microenvironment rich in GF to promote
differentiation and proliferation of stem cells. Both fibrin glue
and PRP may promote the synthesis of the extracellular matrix.
Platelet concentrates combined with
stem cells Adipose-derived stem cells (ADSC)
ADSC have a high potential to differentiate into
several types of cells. In addition to their multipotent
differentiation ability, the paracrine functions of ADSC also
influence tissue regeneration (63). Certain studies suggested that the
ability of ADSC to repair tissues originates from stimulating
collagen synthesis, cellular matrix protein synthesis and dermis
revascularization (64-66).
The scaffolds and GF in platelet concentrates favor the
proliferation of ADSC. It may thus be assumed that the combination
of platelet concentrates and ADSC enhances tissue regeneration.
PRP functions co-operatively with conditioned medium
obtained from adipose-derived MSC (ADMSC) to promote the
proliferation and migration of fibroblasts and keratinocytes in
vitro (66). It is assumed that
PRP is able to interact with the conditioned medium from ADMSC to
exert beneficial effects. However, although conditioned medium
stimulates proliferation in a concentration-dependent manner,
excessive PRP concentrations may have adverse effects on
proliferation. This may be attributed to GF-inhibiting proteins
within the complex mixture produced by platelets (such as platelet
concentrates containing high levels of proteolytic enzymes
inhibiting cell growth) (67-69).
Furthermore, a deeper association between the activities of
different cells was determined; a low concentration of PRP may
promote the proliferation and migration of ADMSC and fibroblasts.
In addition, the paracrine activity of ADMSC stimulates
keratinocyte proliferation. Thus, there is a promising prospect for
healing of cutaneous ulcers by exploiting the reparative synergy
between ADMSC and PRP in the clinic.
It is particularly challenging to re-establish a
sufficient blood supply after maxillofacial soft-tissue defects
caused by irradiation. This leads to difficulties in recovering the
damaged tissue to its pre-damaged condition both in terms of
function and aesthetics. The abundance of GF in platelet
concentrates and the multipotent differentiation potential and
paracrine ability of ADSC provide an opportunity for healing this
kind of tissue damage. A combination of platelet concentrates and
stem cells induces adequate angiogenesis and reduces apoptotic
activities (70). Acute myocardial
infarction is another vital issue. Embedding ADMSC in the PRF
scaffold was reported to improve acute myocardial infarction. An
ADMSC-embedded PRF scaffold preserved left ventricle thickness and
function, ameliorated LV remodeling and inhibited inflammatory
reactions and oxidative stress in a rat model (71). This improvement may be due to three
reasons: First, PRF scaffolds provide a suitable environment for
ADMSC to survive for a longer period; furthermore, the
ADMSC-embedded PRF scaffold may induce the formation of more
abundant vessels than direct ADMSC implantation alone; finally,
implanting a PRF scaffold on the infarcted LV surface provides
mechanical support and limits LV dilatation, thereby preserving the
cardiac function to a certain extent.
BMSC. BMSC serve important roles in
osteogenesis. It is possible to combine BMSC with platelet
concentrates to produce bone tissue. The combined autologous BMSC
and PRP may promote structural allogenic bone graft healing in a
rabbit bone defect model. Abundant bridging trabeculae and
increased expression of relevant osteoblastic proteins were
determined in BMSC and PRP, suggesting the effectiveness of this
combination (68). In 2017, Wang
et al (72) also
demonstrated that therapeutic application of MSC in isolation
induces a lesser degree of central bone regeneration in bone
defects when compared to combination treatment with both MSC and
PRF. In the future, this cotreatment may improve clinical outcomes
in patients receiving structural allografts. Periodontitis, a
widespread chronic disease, destroys both soft and hard tissues.
With traditional treatment, it is hard to regenerate the complete
periodontium, including periodontal ligament (PDL), cementum and
alveolar bone. Loading the PDL and jaw bone MSC sheets on PRF to
restore periodontal defects is a novel approach (73). The two above-mentioned stem-cell
types were used to form cell sheets that were able to differentiate
into PDL- and bone-like tissues, respectively. Furthermore, PRF was
used as a 3-dimensional fibrin network to support, sustain and
nourish the cell sheets in the space between the simulated root
surface and alveolar bone. After the implantation, PDL and
bone-like structures were successfully induced in corresponding
sites. It is not easy for articular cartilage to regenerate after
injuries, even though progenitor cells are present in the
superficial zone. Though autologous chondrocyte implantation is an
established strategy for repairing cartilage injuries, there is a
chance that fibrocartilage may form. In addition, pure BMSC
injection leads to a small proportion of stem cells attaching to
the defect sites to repair the injured cartilage, which may lead to
unsatisfactory cartilage restoration. However, BMSC injection
combined with PRF releasates offers improved results (74). PRF releasates may increase the
thickness of the regenerated cartilage and improve the histological
morphology, which may be ascribed to the roles of GF. Thus, the
combination of PRF releasates and BMSC highlights an alternative
strategy for autologous chondrocyte implantation to cure cartilage
defects.
Stem-cell treatment has gained popularity in recent
years. It is a feasible means of combining stem-cell treatment with
platelet concentrates, as the platelet concentrates provide
scaffolds for cells to adhere to and migrate from and the GF may
promote stem-cell proliferation (67,68,70-74).
In addition, the activities of different types of cells may
synergistically interact to produce beneficial outcomes.
h-DPC. Dental pulp regeneration is an
important aspect of recovery from pulpitis and periapical disease.
Therapeutic assistance, aimed at facilitating such regeneration,
currently involves the use of differentiated cells, biological
scaffolds and GF. There is an urgent requirement to develop more
appropriate biocompatible scaffolds. Addition of an h-DPC
suspension to blood prior to centrifugation during PRF preparation
may be a feasible approach (75).
This method distributes h-DPC to the buffy coat along with
leukocytes, preserving h-DPC viability. This is because h-DPC are
naturally occurring human cells, such as leukocytes. The reviewed
study provides a novel method for preparing a combination cell-PRF
complex, which may be generalizable to the incorporation of other
stem cell types (such as BMSC) (75).
Combination of platelet concentrates
with drugs Antagonism
Certain medications used during the treatment
process may cause adverse effects. For instance, bisphosphonates
are used to manage osteoclast-mediated osseous resorption. However,
long-term administration of bisphosphonates may result in a rare
but adverse complication, namely osteonecrosis of the maxillary and
mandibular bones (76-78).
This side-effect may be ascribed to cytotoxic effects on
osteoblasts, which may prevent cells from proliferating, adhering
and migrating (79,80). Owing to the impact of GF in platelet
concentrates in terms of promoting the settlement, adhesion,
proliferation and migration of osteoblasts, Steller et al
(81) combined bisphosphonates with
platelet concentrates to preserve the activity of osteoblasts.
Different types of platelet concentrates may be able to inhibit
bisphosphonates on different levels. However, PRF may have
advantages over PRP in promoting stem-cell proliferation. This
discrepancy may be due to two reasons. First, the release of GF
from PRP occurs more rapidly than that of PRF due to the natural
scaffold in PRF. Furthermore, a higher concentration of leukocytes
in PRF may positively influence cell proliferation and migration,
although the function of leukocytes is disputed (81). The plant-derived stilbenoid
resveratrol has anti-inflammatory, antioxidant and bone-protective
properties (82). In addition, it
may enhance the therapeutic outcomes of CGF treatment in
bisphosphonate-related osteonecrosis of the jaw (83). In particular, resveratrol may
modulate the effects of CGF to protect osteoblasts. However, the
influence of resveratrol on CGF against bisphosphonate-induced
osteonecrosis is not always synergistic, as the outcome is
dependent on experimental conditions.
Synergism. As mentioned above, engineered
adipose tissues have recently gained popularity for use in
reconstruction of soft-tissue defects. However, adipocytes are
fragile and it is difficult to preserve them after grafting due to
an inadequate blood supply. Ginsenoside Rg1 (G-Rg1) and PRF have
been used to pretreat human breast adipose-derived stem cells
(HBASC) prior to being seeded in a 3-dimensional porous sponge
scaffold of collagen type I (84).
G-Rg1 and PRF may induce the expression of angiogenic factors and
G-Rg1 may increase the paracrine activity of HBASCs. Therefore, it
is reasonable that the combination of G-Rg1 and PRF may result in
the production of more adipose tissue than that produced by any
material alone. Statins may increase BMP-2 expression directly to
promote bone formation and may modulate the activities of
osteoclasts and osteoblasts. PRF, an autologous fibrin scaffold,
may not only serve as a carrier for statins, but also enhances bone
formation due to its biological properties (85). The combination of PRF and statins
provides superior osteosis, upregulation of both BMP-2 and VEGF
protein and elevated bone anabolic serum markers as compared with
those in samples treated with simvastatin or PRF separately.
Reasonable combinations of platelet concentrates
with drugs may reduce side effects and enhance the effects of drugs
due to the mutual effects between drugs and cytokines in platelet
concentrates (81,83-85).
Furthermore, the fibrin matrix scaffold may serve as carriers for
drugs in the target location. Thus, it is recommended to implement
a combination of platelet concentrates and drugs to solve clinical
issues.
6. Conclusions
Autologous platelet-derived concentrates are
biocompatible products containing cytokines, platelets, leukocytes
and fibrin, and may be regarded as a slow-release system, which
ensures sustained delivery of active principles and GF for ~2
weeks. Of note, the release kinetics may influence the entire
process of tissue regeneration. However, certain problems remain
that must be addressed to optimize the formulation intended for
tissue regeneration. The present review provided numerous novel
applications of platelet concentrates for a wide range of tissue
regeneration applications. Such applications aim to meet the
demands for treatments and overcome the shortcomings of platelet
concentrates. The freeze-drying method is an effective method for
preserving the viability of platelet concentrates for comparatively
longer periods, without any concerns regarding the loss of their
effects in tissue regeneration. Combination with biomaterials, such
as chitosan and silk fibrin, may enhance physical strength, making
it suitable for suture in surgery. The synergistic effects of
platelet concentrates and bone regenerative materials, including HA
and graft materials, may induce rapid bone regeneration in diseases
associated with bone loss, such as periodontitis and bone defects
caused by tumors, amongst others. The addition of stem cells
provides a novel approach for the rehabilitation of tissue
morphology. Particularly in the skin and cartilage, it is necessary
to restore the defective tissue to normal levels, which is
important for aesthetics and normal function. Due to the complexity
of clinical issues, tissue defects associated with infection and
tissue damage caused by side effects of drugs are common in the
clinic. Nanometal particles, such as nano-silver, have significant
anti-bacterial properties. Combination of nano-silver and platelet
concentrates provides integrated methods to inhibit bacteria and
promote tissue regeneration for patients with burn damage. The
antagonism of platelet concentrates to osteonecrosis induced by
long-term administration of bisphosphonates provides a suitable
method for curing patients with osteonecrosis. The efficacy and
suitability of these creative approaches require to be further
verified in clinical trials. Certain limitations remain in the
field of tissue regeneration using platelet concentrations as
follows: i) No studies are currently available on the application
of modified methods using platelet concentrates, to the best of our
knowledge, and thus, modified methods that may be used in the
clinic were not reviewed; ii) particularly the underlying
mechanisms remain elusive. In the future, studies on the detailed
mechanisms of modification should be performed and evaluation of
these experimental methods in the clinic should be performed. The
use of platelet concentrates has demonstrated positive clinical
effects in several fields of regenerative medicine. Simple
administration of platelet concentrates cannot always meet the
complex demands of clinical issues. Furthermore, different types of
tissue have different healing periods. Future studies focusing on
the methods of establishment of platelet concentrates as a
multifunctional medical procedure, meeting the complex clinical
issues, are thus required. In addition, the properties of platelet
concentrates are important for clinical use, such as the ability to
tightly bind with the tissue-surrounding defects and strong
resilience, avoiding disruption. Thus, additional studies on
modified strategies using platelet concentrates and prospects for
clinical use are required.
Acknowledgements
Not applicable.
Funding
The present review was supported by a grant from the
Natural Science Foundation of Hunan Province (project no.
2018JJ2546).
Availability of data and materials
Not applicable.
Authors' contributions
ZYD performed the primary literature searches and
prepared the manuscript. QP, NL, JZ and YT read, edited and
reviewed the manuscript. NL and JZ confirmed the authenticity of
the raw data. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Bishop CJ, Kim J and Green JJ: Biomolecule
delivery to engineer the cellular microenvironment for regenerative
medicine. Ann Biomed Eng. 42:1557–1572. 2014.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Abou Neel EA, Chrzanowski W, Salih VM, Kim
HW and Knowles JC: Tissue engineering in dentistry. J Dent.
42:915–928. 2014.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Miron RJ and Zhang Y: Autologous liquid
platelet rich fibrin: A novel drug delivery system. Acta Biomater.
75:35–51. 2018.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Kobayashi E, Flückiger L,
Fujioka-Kobayashi M, Sawada K, Sculean A, Schaller B and Miron RJ:
Comparative release of growth factors from PRP, PRF, and
advanced-PRF. Clin Oral Investig. 20:2353–2360. 2016.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Miron RJ, Fujioka-Kobayashi M, Bishara M,
Zhang Y, Hernandez M and Choukroun J: Platelet-rich fibrin and soft
tissue wound healing: A systematic review. Tissue Eng Part B Rev.
23:83–99. 2017.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Alsousou J, Ali A, Willett K and Harrison
P: The role of platelet-rich plasma in tissue regeneration.
Platelets. 24:173–182. 2013.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Görmeli G, Görmeli CA, Ataoglu B, Çolak C,
Aslantürk O and Ertem K: Multiple PRP injections are more effective
than single injections and hyaluronic acid in knees with early
osteoarthritis: A randomized, double-blind, placebo-controlled
trial. Knee Surg Sports Traumatol Arthrosc. 25:958–965.
2017.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Huang Y, Liu X, Xu X and Liu J:
Intra-articular injections of platelet-rich plasma, hyaluronic acid
or corticosteroids for knee osteoarthritis: A prospective
randomized controlled study. Orthopade. 48:239–247. 2019.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Bhujbal R, A Malik N, Kumar N, Kv S, I
Parkar M and Mb J: Comparative evaluation of platelet rich plasma
in socket healing and bone regeneration after surgical removal of
impacted mandibular third molars. J Dent Res Dent Clin Dent
Prospect. 12:153–158. 2018.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Del Fabbro M, Bucchi C, Lolato A, Corbella
S, Testori T and Taschieri S: Healing of postextraction sockets
preserved with autologous platelet concentrates. A systematic
review and meta-analysis. J Oral Maxillofac Surg. 75:1601–1615.
2017.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Alio JL, Rodriguez AE, De Arriba P,
Gisbert S and Abdelghany AA: Treatment with platelet-rich plasma of
surgically related dormant corneal ulcers. Eur J Ophthalmol.
28:515–520. 2018.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Tambella AM, Attili AR, Dini F, Palumbo
Piccionello A, Vullo C, Serri E, Scrollavezza P and Dupré G:
Autologous platelet gel to treat chronic decubital ulcers: A
randomized, blind controlled clinical trial in dogs. Vet Surg.
43:726–733. 2014.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Tambella AM, Attili AR, Dupré G,
Cantalamessa A, Martin S, Cuteri V, Marcazzan S and Del Fabbro M:
Platelet-rich plasma to treat experimentally-induced skin wounds in
animals: A systematic review and meta-analysis. PLoS One.
13(e0191093)2018.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Burgos-Alonso N, Lobato I, Hernández I,
San Sebastian K, Rodríguez B, March AG, Perez-Salvador A, Arce V,
Garcia-Alvarez A, Gomez-Fernandez MC, et al: Autologous
platelet-rich plasma in the treatment of venous leg ulcers in
primary care: A randomised controlled, pilot study. J Wound Care.
27 (Suppl 6):S20–S24. 2018.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Martinez-Zapata MJ, Martí-Carvajal AJ,
Solà I, Expósito JA, Bolíbar I, Rodríguez L, Garcia J and Zaror C:
Autologous platelet-rich plasma for treating chronic wounds.
Cochrane Database Syst Rev: May 25, 2016. doi:
10.1002/14651858.CD006899.pub3.
|
|
16
|
Moneib HA, Youssef SS, Aly DG, Rizk MA and
Abdelhakeem YI: Autologous platelet-rich plasma versus conventional
therapy for the treatment of chronic venous leg ulcers: A
comparative study. J Cosmet Dermatol. 17:495–501. 2018.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Picard F, Hersant B, Bosc R and Meningaud
JP: The growing evidence for the use of platelet-rich plasma on
diabetic chronic wounds: A review and a proposal for a new standard
care. Wound Repair Regen. 23:638–643. 2015.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Masoudi E, Ribas J, Kaushik G, Leijten J
and Khademhosseini A: Platelet-rich blood derivatives for stem
cell-based tissue engineering and regeneration. Curr Stem Cell Rep.
2:33–42. 2016.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Hatakeyama I, Marukawa E, Takahashi Y and
Omura K: Effects of platelet-poor plasma, platelet-rich plasma, and
platelet-rich fibrin on healing of extraction sockets with buccal
dehiscence in dogs. Tissue Eng Part A. 20:874–882. 2014.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Anitua E, Nurden P, Prado R, Nurden AT and
Padilla S: Autologous fibrin scaffolds: When platelet- and
plasma-derived biomolecules meet fibrin. Biomaterials. 192:440–460.
2019.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Dohan DM, Choukroun J, Diss A, Dohan SL,
Dohan AJ, Mouhyi J and Gogly B: Platelet-rich fibrin (PRF): A
second-generation platelet concentrate. Part II: platelet-related
biologic features. Oral Surg Oral Med Oral Pathol Oral Radiol
Endod. 101:e45–e50. 2006.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Shah R, M G T, Thomas R and Mehta DS: An
Update on the protocols and biologic actions of platelet rich
fibrin in dentistry. Eur J Prosthodont Restor Dent. 25:64–72.
2017.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Ghanaati S, Booms P, Orlowska A, Kubesch
A, Lorenz J, Rutkowski J, Landes C, Sader R, Kirkpatrick C and
Choukroun J: Advanced platelet-rich fibrin: A new concept for
cell-based tissue engineering by means of inflammatory cells. J
Oral Implantol. 40:679–689. 2014.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Wend S, Kubesch A, Orlowska A, Al-Maawi S,
Zender N, Dias A, Miron RJ, Sader R, Booms P, Kirkpatrick CJ, et
al: Reduction of the relative centrifugal force influences cell
number and growth factor release within injectable PRF-based
matrices. J Mater Sci Mater Med. 28(188)2017.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Pitzurra L, Jansen IDC, de Vries TJ,
Hoogenkamp MA and Loos BG: Effects of L-PRF and A-PRF+
on periodontal fibroblasts in in vitro wound healing experiments. J
Periodontal Res. 55:287–295. 2020.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Miron RJ, Fujioka-Kobayashi M, Hernandez
M, Kandalam U, Zhang Y, Ghanaati S and Choukroun J: Injectable
platelet rich fibrin (i-PRF): Opportunities in regenerative
dentistry? Clin Oral Investig. 21:2619–2627. 2017.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Malli Sureshbabu N, Selvarasu K, v JK,
Nandakumar M and Selvam D: Concentrated growth factors as an
ingenious biomaterial in regeneration of bony defects after
periapical surgery: A report of two cases. Case Rep Dent.
2019(7046203)2019.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Dohan Ehrenfest DM, Rasmusson L and
Albrektsson T: Classification of platelet concentrates: From pure
platelet-rich plasma (P-PRP) to leucocyte- and platelet-rich fibrin
(L-PRF). Trends Biotechnol. 27:158–167. 2009.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Boateng JS, Matthews KH, Stevens HN and
Eccleston GM: Wound healing dressings and drug delivery systems: A
review. J Pharm Sci. 97:2892–2923. 2008.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Broughton G II, Janis JE and Attinger CE:
The basic science of wound healing. Plast Reconstr Surg. 117 (Suppl
7):12S–34S. 2006.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Etulain J: Platelets in wound healing and
regenerative medicine. Platelets. 29:556–568. 2018.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Walsh TG, Metharom P and Berndt MC: The
functional role of platelets in the regulation of angiogenesis.
Platelets. 26:199–211. 2015.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Pierce GF, Mustoe TA, Altrock BW, Deuel TF
and Thomason A: Role of platelet-derived growth factor in wound
healing. J Cell Biochem. 45:319–326. 1991.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Briquez PS, Hubbell JA and Martino MM:
Extracellular matrix-inspired growth factor delivery systems for
skin wound healing. Adv Wound Care (New Rochelle). 4:479–489.
2015.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Martino MM, Brkic S, Bovo E, Burger M,
Schaefer DJ, Wolff T, Gürke L, Briquez PS, Larsson HM,
Gianni-Barrera R, et al: Extracellular matrix and growth factor
engineering for controlled angiogenesis in regenerative medicine.
Front Bioeng Biotechnol. 3(45)2015.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Janmey PA, Winer JP and Weisel JW: Fibrin
gels and their clinical and bioengineering applications. J R Soc
Interface. 6:1–10. 2009.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Sánchez M, Anitua E, Delgado D, Prado R,
Sánchez P, Fiz N, Guadilla J, Azofra J, Pompei O, Orive G, et al:
Ultrasound-guided plasma rich in growth factors injections and
scaffolds hasten motor nerve functional recovery in an ovine model
of nerve crush injury. J Tissue Eng Regen Med. 11:1619–1629.
2017.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Haugh MG, Murphy CM and O'Brien FJ: Novel
freeze-drying methods to produce a range of
collagen-glycosaminoglycan scaffolds with tailored mean pore sizes.
Tissue Eng Part C Methods. 16:887–894. 2010.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Roy I and Gupta MN: Freeze-drying of
proteins: Some emerging concerns. Biotechnol Appl Biochem.
39:165–177. 2004.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Shi L, Li R, Wei S, Zhou M, Li L, Lin F,
Li Y, Guo Z, Zhang W, Chen M, et al: Effects of a protective agent
on freeze-dried platelet-rich plasma. Blood Coagul Fibrinolysis.
30:58–65. 2019.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Wang Z, Han L, Sun T, Wang W, Li X and Wu
B: Preparation and effect of lyophilized platelet-rich fibrin on
the osteogenic potential of bone marrow mesenchymal stem cells in
vitro and in vivo. Heliyon. 5(e02739)2019.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Li Q, Reed DA, Min L, Gopinathan G, Li S,
Dangaria SJ, Li L, Geng Y, Galang MT, Gajendrareddy P, et al:
Lyophilized platelet-rich fibrin (PRF) promotes craniofacial bone
regeneration through Runx2. Int J Mol Sci. 15:8509–8525.
2014.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Zhang J, Qi X, Luo X, Li D, Wang H and Li
T: Clinical and immunohistochemical performance of lyophilized
platelet-rich fibrin (Ly-PRF) on tissue regeneration. Clin Implant
Dent Relat Res. 19:466–477. 2017.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Liu Z, Jin H, Xie Q, Jiang Z, Guo S, Li Y
and Zhang B: Controlled release strategies for the combination of
fresh and lyophilized platelet-rich fibrin on bone tissue
regeneration. BioMed Res Int. 2019(4923767)2019.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Sam G, Vadakkekuttical RJ and Amol NV: In
vitro evaluation of mechanical properties of platelet-rich fibrin
membrane and scanning electron microscopic examination of its
surface characteristics. J Indian Soc Periodontol. 19:32–36.
2015.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Behera SS, Das U, Kumar A, Bissoyi A and
Singh AK: Chitosan/TiO2 composite membrane improves proliferation
and survival of L929 fibroblast cells: Application in wound
dressing and skin regeneration. Int J Biol Macromol. 98:329–340.
2017.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Mohammadi R, Mehrtash M, Mehrtash M,
Hassani N and Hassanpour A: Effect of platelet rich plasma combined
with chitosan biodegradable film on full-thickness wound healing in
rat model. Bull Emerg Trauma. 4:29–37. 2016.PubMed/NCBI
|
|
48
|
Shimojo AA, Perez AG, Galdames SE, Brissac
IC and Santana MH: Stabilization of porous chitosan improves the
performance of its association with platelet-rich plasma as a
composite scaffold. Mater Sci Eng C. 60:538–546. 2016.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Wang L, Wan M, Li Z, Zhong N, Liang D and
Ge L: A comparative study of the effects of concentrated growth
factors in two different forms on osteogenesis in vitro. Mol Med
Rep. 20:1039–1048. 2019.PubMed/NCBI View Article : Google Scholar
|
|
50
|
Ansarizadeh M, Mashayekhan S and
Saadatmand M: Fabrication, modeling and optimization of lyophilized
advanced platelet rich fibrin in combination with collagen-chitosan
as a guided bone regeneration membrane. Int J Biol Macromol.
125:383–391. 2019.PubMed/NCBI View Article : Google Scholar
|
|
51
|
Dal Pra I, Freddi G, Minic J, Chiarini A
and Armato U: De novo engineering of reticular connective tissue in
vivo by silk fibroin nonwoven materials. Biomaterials.
26:1987–1999. 2005.PubMed/NCBI View Article : Google Scholar
|
|
52
|
Horan RL, Antle K, Collette AL, Wang Y,
Huang J, Moreau JE, Volloch V, Kaplan DL and Altman GH: In vitro
degradation of silk fibroin. Biomaterials. 26:3385–3393.
2005.PubMed/NCBI View Article : Google Scholar
|
|
53
|
Franco AR, Fernandes EM, Rodrigues MT,
Rodrigues FJ, Gomes ME, Leonor IB, Kaplan DL and Reis RL:
Antimicrobial coating of spider silk to prevent bacterial
attachment on silk surgical sutures. Acta Biomater. 99:236–246.
2019.PubMed/NCBI View Article : Google Scholar
|
|
54
|
Lee EH, Kim JY, Kweon HY, Jo YY, Min SK,
Park YW, Choi JY and Kim SG: A combination graft of
low-molecular-weight silk fibroin with Choukroun platelet-rich
fibrin for rabbit calvarial defect. Oral Surg Oral Med Oral Pathol
Oral Radiol Endod. 109:e33–e38. 2010.PubMed/NCBI View Article : Google Scholar
|
|
55
|
Kuchur OA, Tsymbal SA, Shestovskaya MV,
Serov NS, Dukhinova MS and Shtil AA: Metal-derived nanoparticles in
tumor theranostics: Potential and limitations. J Inorg Biochem.
209(111117)2020.PubMed/NCBI View Article : Google Scholar
|
|
56
|
Zhang J, Xu Q, Huang C, Mo A, Li J and Zuo
Y: Biological properties of an anti-bacterial membrane for guided
bone regeneration: An experimental study in rats. Clin Oral
Implants Res. 21:321–327. 2010.PubMed/NCBI View Article : Google Scholar
|
|
57
|
Khorshidi H, Haddadi P, Raoofi S, Badiee P
and Dehghani Nazhvani A: Does adding silver nanoparticles to
leukocyte- and platelet-rich fibrin improve its properties? BioMed
Res Int. 2018(8515829)2018.PubMed/NCBI View Article : Google Scholar
|
|
58
|
Woo SM, Kim WJ, Lim HS, Choi NK, Kim SH,
Kim SM and Jung JY: Combination of mineral trioxide aggregate and
platelet-rich fibrin promotes the odontoblastic differentiation and
mineralization of human dental pulp cells via BMP/Smad signaling
pathway. J Endod. 42:82–88. 2016.PubMed/NCBI View Article : Google Scholar
|
|
59
|
Abdullah WA: Evaluation of bone
regenerative capacity in rats claverial bone defect using platelet
rich fibrin with and without beta tri calcium phosphate bone graft
material. Saudi Dent J. 28:109–117. 2016.PubMed/NCBI View Article : Google Scholar
|
|
60
|
Karayürek F, Kadiroğlu ET, Nergiz Y,
Coşkun Akçay N, Tunik S, Ersöz Kanay B and Uysal E: Combining
platelet rich fibrin with different bone graft materials: An
experimental study on the histopathological and immunohistochemical
aspects of bone healing. J Craniomaxillofac Surg. 47:815–825.
2019.PubMed/NCBI View Article : Google Scholar
|
|
61
|
Ohba S, Wang W, Itoh S, Takagi Y, Nagai A
and Yamashita K: Acceleration of new bone formation by an
electrically polarized hydroxyapatite microgranule/platelet-rich
plasma composite. Acta Biomater. 8:2778–2787. 2012.PubMed/NCBI View Article : Google Scholar
|
|
62
|
Sadeghinia A, Davaran S, Salehi R and
Jamalpoor Z: Nano-hydroxy apatite/chitosan/gelatin scaffolds
enriched by a combination of platelet-rich plasma and fibrin glue
enhance proliferation and differentiation of seeded human dental
pulp stem cells. Biomed Pharmacother. 109:1924–1931.
2019.PubMed/NCBI View Article : Google Scholar
|
|
63
|
Suga H, Glotzbach JP, Sorkin M, Longaker
MT and Gurtner GC: Paracrine mechanism of angiogenesis in
adipose-derived stem cell transplantation. Ann Plast Surg.
72:234–241. 2014.PubMed/NCBI View Article : Google Scholar
|
|
64
|
Klar AS, Güven S, Zimoch J, Zapiórkowska
NA, Biedermann T, Böttcher-Haberzeth S, Meuli-Simmen C, Martin I,
Scherberich A, Reichmann E, et al: Characterization of vasculogenic
potential of human adipose-derived endothelial cells in a
three-dimensional vascularized skin substitute. Pediatr Surg Int.
32:17–27. 2016.PubMed/NCBI View Article : Google Scholar
|
|
65
|
Lee SH, Jin SY, Song JS, Seo KK and Cho
KH: Paracrine effects of adipose-derived stem cells on
keratinocytes and dermal fibroblasts. Ann Dermatol. 24:136–143.
2012.PubMed/NCBI View Article : Google Scholar
|
|
66
|
Moon KM, Park YH, Lee JS, Chae YB, Kim MM,
Kim DS, Kim BW, Nam SW and Lee JH: The effect of secretory factors
of adipose-derived stem cells on human keratinocytes. Int J Mol
Sci. 13:1239–1257. 2012.PubMed/NCBI View Article : Google Scholar
|
|
67
|
Stessuk T, Puzzi MB, Chaim EA, Alves PC,
de Paula EV, Forte A, Izumizawa JM, Oliveira CC, Frei F and
Ribeiro-Paes JT: Platelet-rich plasma (PRP) and adipose-derived
mesenchymal stem cells: Stimulatory effects on proliferation and
migration of fibroblasts and keratinocytes in vitro. Arch Dermatol
Res. 308:511–520. 2016.PubMed/NCBI View Article : Google Scholar
|
|
68
|
Park CG, Joo MW, Jeong J, Kang YK and Lee
DR: Evaluation of the effects of the combination of autologous
mesenchymal stem cells and platelet-rich plasma on structural bone
allograft healing. Cell Tissue Bank. 18:229–238. 2017.PubMed/NCBI View Article : Google Scholar
|
|
69
|
Mills DC, Robb IA and Roberts GC: The
release of nucleotides, 5-hydroxytryptamine and enzymes from human
blood platelets during aggregation. J Physiol. 195:715–729.
1968.PubMed/NCBI View Article : Google Scholar
|
|
70
|
Chen Y, Niu Z, Xue Y, Yuan F, Fu Y and Bai
N: Improvement in the repair of defects in maxillofacial soft
tissue in irradiated minipigs by a mixture of adipose-derived stem
cells and platelet-rich fibrin. Br J Oral Maxillofac Surg.
52:740–745. 2014.PubMed/NCBI View Article : Google Scholar
|
|
71
|
Sun CK, Zhen YY, Leu S, Tsai TH, Chang LT,
Sheu JJ, Chen YL, Chua S, Chai HT, Lu HI, et al: Direct
implantation versus platelet-rich fibrin-embedded adipose-derived
mesenchymal stem cells in treating rat acute myocardial infarction.
Int J Cardiol. 173:410–423. 2014.PubMed/NCBI View Article : Google Scholar
|
|
72
|
Wang X, Li G, Guo J, Yang L, Liu Y, Sun Q,
Li R and Yu W: Hybrid composites of mesenchymal stem cell sheets,
hydroxyapatite, and platelet-rich fibrin granules for bone
regeneration in a rabbit calvarial critical-size defect model. Exp
Ther Med. 13:1891–1899. 2017.PubMed/NCBI View Article : Google Scholar
|
|
73
|
Wang ZS, Feng ZH, Wu GF, Bai SZ, Dong Y,
Chen FM and Zhao YM: The use of platelet-rich fibrin combined with
periodontal ligament and jaw bone mesenchymal stem cell sheets for
periodontal tissue engineering. Sci Rep. 6(28126)2016.PubMed/NCBI View Article : Google Scholar
|
|
74
|
Wu CC, Sheu SY, Hsu LH, Yang KC, Tseng CC
and Kuo TF: Intra-articular Injection of platelet-rich fibrin
releasates in combination with bone marrow-derived mesenchymal stem
cells in the treatment of articular cartilage defects: An in vivo
study in rabbits. J Biomed Mater Res B Appl Biomater.
105:1536–1543. 2017.PubMed/NCBI View Article : Google Scholar
|
|
75
|
He X, Chen WX, Ban G, Wei W, Zhou J, Chen
WJ and Li XY: A new method to develop human dental pulp cells and
platelet-rich fibrin complex. J Endod. 42:1633–1640.
2016.PubMed/NCBI View Article : Google Scholar
|
|
76
|
Graves LL, Bukata SV, Aghazadehsanai N,
Chang TI, Garrett NR and Friedlander AH: Patients receiving
parenteral bisphosphonates for malignant disease and having
developed an atypical femoral fracture are at risk of concomitant
osteonecrosis of the jaw: an evidence-based review. J Oral
Maxillofac Surg. 74:2403–2408. 2016.PubMed/NCBI View Article : Google Scholar
|
|
77
|
Lundberg AP, Roady PJ, Somrak AJ, Howes ME
and Fan TM: Zoledronate-associated osteonecrosis of the jaw in a
dog with appendicular osteosarcoma. J Vet Intern Med. 30:1235–1240.
2016.PubMed/NCBI View Article : Google Scholar
|
|
78
|
Kharazmi M, Hallberg P, Warfvinge G and
Michaëlsson K: Risk of atypical femoral fractures and osteonecrosis
of the jaw associated with alendronate use compared with other oral
bisphosphonates. Rheumatology (Oxford). 53:1911–1913.
2014.PubMed/NCBI View Article : Google Scholar
|
|
79
|
Street J, Bao M, deGuzman L, Bunting S,
Peale FV Jr, Ferrara N, Steinmetz H, Hoeffel J, Cleland JL,
Daugherty A, et al: Vascular endothelial growth factor stimulates
bone repair by promoting angiogenesis and bone turnover. Proc Natl
Acad Sci USA. 99:9656–9661. 2002.PubMed/NCBI View Article : Google Scholar
|
|
80
|
Krüger TB, Herlofson BB, Landin MA and
Reseland JE: Alendronate alters osteoblast activities. Acta Odontol
Scand. 74:550–557. 2016.PubMed/NCBI View Article : Google Scholar
|
|
81
|
Steller D, Herbst N, Pries R, Juhl D and
Hakim SG: Positive impact of Platelet-rich plasma and Platelet-rich
fibrin on viability, migration and proliferation of osteoblasts and
fibroblasts treated with zoledronic acid. Sci Rep.
9(8310)2019.PubMed/NCBI View Article : Google Scholar
|
|
82
|
Ginés C, Cuesta S, Kireev R, García C,
Rancan L, Paredes SD, Vara E and Tresguerres JAF: Protective effect
of resveratrol against inflammation, oxidative stress and apoptosis
in pancreas of aged SAMP8 mice. Exp Gerontol. 90:61–70.
2017.PubMed/NCBI View Article : Google Scholar
|
|
83
|
Borsani E, Bonazza V, Buffoli B, Nocini
PF, Albanese M, Zotti F, Inchingolo F, Rezzani R and Rodella LF:
Beneficial effects of concentrated growth factors and resveratrol
on human osteoblasts in vitro treated with bisphosphonates. BioMed
Res Int. 2018(4597321)2018.PubMed/NCBI View Article : Google Scholar
|
|
84
|
Xu FT, Liang ZJ, Li HM, Peng QL, Huang MH,
Li Q, Liang YD, Chi GY, Li H, Yu BC, et al: Ginsenoside Rg1 and
platelet-rich fibrin enhance human breast adipose-derived stem cell
function for soft tissue regeneration. Oncotarget. 7:35390–35403.
2016.PubMed/NCBI View Article : Google Scholar
|
|
85
|
Raafat SN, Amin RM, Elmazar MM, Khattab MM
and El-Khatib AS: The sole and combined effect of simvastatin and
platelet rich fibrin as a filling material in induced bone defect
in tibia of albino rats. Bone. 117:60–69. 2018.PubMed/NCBI View Article : Google Scholar
|
|
86
|
Isobe K, Watanebe T, Kawabata H, et al:
Mechanical and degradation properties of advanced platelet-rich
fibrin (A-PRF), concentrated growth factors (CGF), and
platelet-poor plasma-derived fibrin (PPTF). Int J Implant Dent.
3(17)2017.PubMed/NCBI View Article : Google Scholar
|
|
87
|
Masuki H, Okudera T, Watanebe T, et al:
Growth factor and pro-inflammatory cytokine contents in
platelet-rich plasma (PRP), plasma rich in growth factors (PRGF),
advanced platelet-rich fibrin (A-PRF), and concentrated growth
factors (CGF). Int J Implant Dent. 2:2016.PubMed/NCBI View Article : Google Scholar
|