Introduction
Carotid stenosis is caused by atherosclerosis,
characterized by the accumulation of plaques accumulating in the
artery wall, thus occluding the flow of blood (1). In Western countries, carotid artery
stenosis accounts for approximately 20-30% of cerebral infarction
cases (2). It is one of the main
risk factors for the appearance of cerebral infarction (3). More than 60% of cerebral infarctions
are caused by carotid stenosis and severe cerebral infarction may
lead to disability or even death (4). It is also a major risk factor for
stroke that leads to brain damage (5). Therefore, carotid stenosis poses a
serious health threat. At present, carotid angioplasty and stenting
(CAS) is used as an efficient treatment for patients with
symptomatic moderate- and high-grade stenosis (6). However, the frequent occurrence of
restenosis after surgery seriously affects the treatment of CAS.
Thus, restenosis remains an unsolved concern following CAS
treatment (7). In-stent restenosis
(ISR) is the gradual renarrowing of a stented coronary artery
lesion due to arterial damage with subsequent neointimal tissue
proliferation (8). Increasing
evidence has revealed that intimal hyperplastic lesions are
correlated with ISR and involve the participation of various cells,
including endothelial cells and vascular smooth muscle cells
(VSMCs) (9,10). VSMCs exhibit increased
proliferation, thereby leading to neointimal hyperplasia and
ultimately initiating the progression of various cardiovascular
diseases, including ISR (11).
Proliferation of VSMCs and the consequent intimal thickening caused
by their migration to the intima are the major pathological
mechanisms leading to ISR. Therefore, the present study aimed to
explore the regulatory mechanisms of VSMC proliferation and
migration and to provide novel ideas and targets for the prevention
of ISR.
Previous studies have indicated that circulating
microRNA (miRNA/miR)-143 levels are associated with the occurrence
of ISR and may serve as novel noninvasive biomarkers for ISR
(12,13). Furthermore, long non-coding (lnc)RNA
cyclin-dependent kinase inhibitor 2B antisense RNA 1 (CDKN2B-AS1)
is able to target and regulate miR-143-3p (14). Of note, CDKN2B-AS1 participates in
the pathological process of atherosclerosis and promotes the
formation of atherosclerotic plaques (15,16).
Atherosclerosis is one of the important causes of carotid stenosis,
but whether CDKN2B-AS1 participates in restenosis after stenting by
targeting miR-149-3p has remained elusive. In previous studies,
CDKN2B-AS1 was indicated to regulate the biological activities of
numerous types of cell, including cell proliferation, migration,
invasion and apoptosis (17,18).
Given the importance of the biological function of VSMCs in the
process of restenosis, the present study aimed to further analyze
whether CDKN2B-AS1 is also able to regulate the biological activity
of VSMCs, thus further revealing the function of CDKN2B-AS1 and
providing a novel biomarker and therapeutic target for restenosis
after stenting.
Materials and methods
Patient inclusion and sample
collection
A total of 52 patients who underwent CAS for carotid
stenosis at Cangzhou Central Hospital (Cangzhou, China) between
February 2014 and October 2016 were analyzed, including 20 patients
with ISR (ISR group) and 32 patients without restenosis (non-ISR
group). The degree of stenosis was determined using the criteria of
the North American Symptomatic Carotid Endarterectomy trial and
restenosis was based on stenosis of ≥50% of the stented segment
(19). None of the patients had any
acute infection, chronic inflammation, severe liver or kidney
diseases or malignant tumors. Blood samples were collected from
subjects after CAS and serum was centrifuged and stored at -80˚C
for future use. The demographic and clinicopathological
characteristics of the patients were recorded, including age, sex,
body mass index (BMI), smoking history, drinking history, diabetes,
hypertension, hyperlipidemia, total cholesterol (TC), triglyceride
(TG), high-density lipoprotein cholesterol (HDL-C) and low-density
lipoprotein cholesterol (LDL-C). Written informed consent was
obtained from each patient and the experimental procedures were
approved by the Ethics Committee of Cangzhou Central Hospital
(Cangzhou, China; no. CZCH14h0283).
Cell culture and transfection
Human carotid artery smooth muscle cells (cat. no.
3514-05a; hHCtASMCs; Cell Applications, Inc.) were cultured with
Medium 231 with Smooth Muscle Growth Supplement (Thermo Fisher
Scientific, Inc.) and placed in an incubator containing 5%
CO2 at 37˚C.
Cell transfection was applied in the present study
to achieve in vitro regulation of CDKN2B-AS1 and miR-143-3p.
Small interfering (siRNA) for CDKN2B-AS1 (si-CDKN2B-AS1), si
negative control (si-NC), mimics-NC, miR-143-3p mimics, inhibitor
NC and miR143-3p inhibitor were synthesized by GenePharma and
Transfection was performed when hHCtASMCs cells were grown to 80%
confluence using Lipofectamine 2000 (Invitrogen; Thermo Fisher
Scientific, Inc.) according to the manufacturer's protocol. Cells
transfected with only transfection reagent were set as a control
group. After 48 h of transfection, the cells were used for further
analyses. The transfection sequence was as follows (from 5' to 3'):
miR-143-3p mimics, UGAGAUGAAGCACUGUAGCUC; miR-143-3p inhibitor,
GAGCUACAGUGCUUCAUCUCA; mimics NC, UUCUCCGAACGUGUCACGU; inhibitor
NC, CAGUACUUUUGUGUAGUACAA; si-CDKN2B-AS1, UCUGUUUAAAUUAUGAAUGUG;
si-NC, UCUUCCGAACGUGUCACGUTT.
RNA extraction and reverse
transcription-quantitative (RT-q)PCR
Total RNA was extracted from fresh serum samples and
cells using TRIzol reagent (Invitrogen; Thermo Fisher Scientific,
Inc.). Subsequently, the RNA was reversely transcribed into
single-stranded complementary DNA with the PrimeScript reverse
transcriptase reagent kit (Takara Biotechnology, Inc.) following
the manufacturer's protocol. The expression levels of CDKN2B-AS1
and miR-143-3p were examined by qPCR, which was performed with a
SYBR-Green I Master Mix kit (Invitrogen; Thermo Fisher Scientific,
Inc.) in a 7500 Real-Time PCR System (Applied Biosystems; Thermo
Fisher Scientific, Inc.). U6 was used as an endogenous control for
miR-143-3p and GAPDH was used as an endogenous control for
CDKN2B-AS1. The following thermal cycling conditions were used for
qPCR: Initial denaturation at 95˚C for 10 min, followed by 40
cycles of 95˚C for 20 sec, 60˚C for 15 sec and 72˚C for 20 sec. The
primers for qPCR were as follows: U6 forward,
5'-CTCGCTTCGGCAGCACA-3' and reverse, 5'-AACGCTTCACGAATTTGCGT-3';
miR-143-3p forward, 5'-GCCGAGTGAGATGAAGCACT-3' and reverse,
5'-CTCAACTGGTGTCGTGGA-3'; GAPDH forward, 5'-TGCACCACCAACTGCTTAGC-3'
and reverse, 5'-GGCATGCACTGTGGTCATGAG-3'; CDKN2B-AS1 forward,
5'-ACAGAAGCCTACGAAGAACTC-3' and reverse,
5'-TGCATGGTGGTGCATCTGTA-3'. The final expression value was
calculated using the 2-ΔΔCq method (20).
Cell proliferation analysis
The Cell Counting Kit-8 (CCK-8) assay was used to
analyze the cell proliferation rate. Cells were seeded into a
96-well plate (5x103 cells/well) and incubated for 0,
24, 48 or 72 h. Subsequently, 10 µl of CCK-8 reagent (Beyotime
Institute of Biotechnology, Inc.) was added to each well, followed
by further incubation for 2 h. Cell proliferation was assessed by
examining the absorbance at 450 nm using a microplate reader.
Migration assay
Transwell chambers with 8.0 µm pore size filter
membranes (Corning, Inc.), without Matrigel coating (for migration
assay), were used to analyze the migration abilities of hHCtASMCs.
Cells in serum-free culture medium were seeded into the upper
chambers and the bottom chambers were filled with medium containing
2% FBS (Gibco; Thermo Fisher Scientific, Inc.). After 48 h of
incubation at 37˚C, cells in the lower chamber were fixed with 4%
paraformaldehyde for 10 min at room temperature and stained with
0.1% crystal violet for 20 min at room temperature. The number of
migrated or invaded cells in five randomly selected fields was
counted under an inverted light microscope (Olympus Corp.).
Luciferase reporter assay
It was found that miR-143-3p contains a binding site
for CDKN2B-AS1 through the use of LncBase Predicted v.2 (http://carolina.imis.athena-innovation.gr/diana_tools/web/index.php?r=lncbasev2%2Findex)
and a luciferase reporter assay was used to confirm the interaction
between miR-143-3p and CDKN2B-AS1(14). The wild-type (WT) sequence of
CDKN2B-AS1 contained the binding site of miR-143-3p was amplified
by PCR, and the mutant-type (MUT) sequence was obtained using a
QuickMutation kit (Beyotime Institute of Biotechnology). The
obtained sequences were combined into a luciferase reporter vector
(pGL3-luciferase basic vector; Promega Corp.). The vectors
constructed were then co-transfected into hHCtASMC with miR-143-3p
mimics or mimics NC using Lipofectamine 3000 (Invitrogen; Thermo
Fisher Scientific, Inc.), according to the manufacturer's protocol.
Changes in luciferase activity, as expected in the WT group with
overexpression of miR-143-3p, were analyzed by a Dual-Luciferase
Reporter Assay System (Promega Corp.) and normalized to
Renilla luciferase activity.
Statistical analysis
Values are expressed as the mean ± standard
deviation and analyzed using SPSS 21.0 (IBM Corp.) and GraphPad 7.0
(GraphPad Software, Inc.). Differences between groups were analyzed
with Student's t-test, the chi-square test or analysis of variance
with Tukey's post-hoc test. Pearson correlation analysis was used
to analyze the correlation between the expression of CDKN2B-AS1 and
miR-143-3p. Logistic regression was used to analyze the association
of factors such as CDKN2B-AS1 and miR-143-3p with the occurrence of
ISR. P<0.05 was considered to indicate statistical
significance.
Results
Baseline characteristics of the study
subjects
The Student's t-test and chi-square test were used
to compare the basic data of the two groups of patients. Among the
patients, there were 26 males and 6 females with a mean age of
64.0±7.3 years in the non ISR group; and 17 males and 3 females
with a mean age of 67.5±8.5 years in the ISR group. The results
indicated that there were no differences in age, sex, BMI, smoking,
drinking, hypertension, hyperlipidemia, TG or HDL-C between the two
groups (all P>0.05). However, more cases of diabetes, higher TC
levels and lower LDL-C levels were present in the ISR group as
compared with those in the non-ISR group (all P<0.05; Table I).
 | Table IBaseline characteristics of the study
subjects. |
Table I
Baseline characteristics of the study
subjects.
| Variables | non-ISR (n=32) | ISR (n=20) | P-value |
|---|
| Age (years) | 64.0±7.3 | 67.5±8.5 | 0.129 |
| Male sex | 26 | 17 | 0.728 |
| BMI
(kg/m2) | 24.5±2.6 | 25.1±3.1 | 0.449 |
| Smoking history | 13 | 9 | 0.756 |
| Drinking history | 13 | 10 | 0.508 |
| Diabetes | 10 | 10 | 0.041 |
| Hypertension | 21 | 16 | 0.266 |
| Hyperlipidemia | 13 | 11 | 0.312 |
| TC (mg/dl) | 147.6±35.7 | 169.88±36.9 | 0.036 |
| TG (mg/dl) | 140.3±48.4 | 134.6±48.0 | 0.680 |
| HDL-C (mg/dl) | 39.9±6.5 | 41.7±7.1 | 0.367 |
| LDL-C (mg/dl) | 96.6±19.7 | 84.2±18.5 | 0.028 |
Expression of CDKN2B-AS1 and
miR-143-3p in patients with ISR
To further elucidate the roles of CDKN2B-AS1 and
miR-143-3p in ISR, the expression of CDKN2B-AS1 and miR-143-3p in
the serum of patients with ISR was quantified by RT-qPCR. The
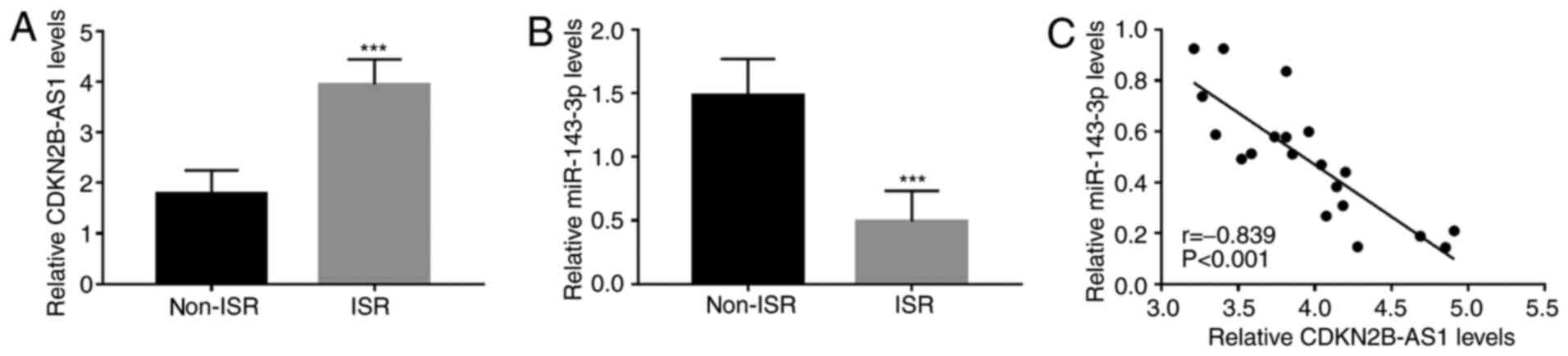
results indicated that compared with those in the non-ISR group,
serum CDKN2B-AS1 was upregulated in the ISR group (P<0.001;
Fig. 1A), while serum miR-143-3p
was downregulated (P<0.001; Fig.
1B). Furthermore, the serum levels of CDKN2B-AS1 and miR-143-3p
were significantly negatively correlated in patients with ISR
(r=-0.839, P<0.001; Fig.
1C).
Association of CDKN2B-AS1 and
miR-143-3p with the occurrence of ISR
The clinical data of all patients and the expression
levels of CDKN2B-AS1 and miR-143-3p were included in the univariate
and multivariate logistic regression analysis. The univariate
analysis results indicated that diabetes, hypertension, TC, LDL-C,
miR-143-3p and CDKN2B-AS1 (all P<0.05) were related with ISR
onset and the further multivariate analysis data demonstrated that
CDKN2B-AS1 (P=0.003), miR-143-3p (P<0.001) and LDL-C (P=0.034)
were independently associated with the occurrence of ISR and were
potential risk factors for the occurrence of ISR (Table II).
 | Table IILogistic regression analysis results
for patients with ISR. |
Table II
Logistic regression analysis results
for patients with ISR.
| | Univariate
analysis | Multivariate
analysis |
|---|
| Variables | OR | 95% CI | P-value | OR | 95% CI | P-value |
|---|
| Age (65.3±7.9) | 1.428 | 0.894-2.016 | 0.228 | 1.229 | 0.812-2.285 | 0.268 |
| Sex (male vs.
female) | 1.094 | 0.639-1.598 | 0.712 | 1.006 | 0.986-1.025 | 0.872 |
| BMI (24.7±2.8) | 1.598 | 0.921-2.228 | 0.114 | 1.612 | 0.809-2.998 | 0.135 |
| Smoking (yes vs.
no) | 1.188 | 0.618-1.849 | 0.662 | 1.126 | 0.525-3.196 | 0.627 |
| Drinking (yes vs.
no) | 1.213 | 0.685-1.941 | 0.589 | 2.193 | 0.751-4.231 | 0.660 |
| Diabetes (yes vs.
no) | 1.666 | 1.045-2.285 | 0.044 | 1.630 | 0.823-2.512 | 0.254 |
| Hypertension (yes
vs. no) | 1.687 | 1.101-2.318 | 0.038 | 2.507 | 0.912-4.285 | 0.113 |
| Hyperlipidemia (yes
vs. no) | 1.108 | 0.547-1.799 | 0.697 | 1.171 | 0.988-1.842 | 0.895 |
| TC
(156.2±37.4) | 1.691 | 1.112-2.484 | 0.035 | 2.834 | 0.979-5.109 | 0.092 |
| TG
(138.1±47.9) | 1.674 | 0.994-2.485 | 0.051 | 1.948 | 0.894-3.785 | 0.330 |
| HDL-C
(40.6±6.8) | 1.192 | 0.640-1.886 | 0.610 | 1.062 | 0.912-1.285 | 0.803 |
| LDL-C
(91.8±20.0) | 2.085 | 1.397-3.896 | 0.011 | 2.113 | 1.428-4.219 | 0.034 |
| miR-143-3p
(2.6±1.2) | 2.969 | 1.941-4.573 | <0.001 | 3.096 | 1.926-4.899 | <0.001 |
| CDKN2B-AS1
(1.1±0.6) | 2.818 | 1.881-4.141 | 0.002 | 2.854 | 1.701-4.511 | 0.003 |
Knockdown of CDKN2B-AS1 inhibits the
proliferation and migration of hHCtASMCs
The regulatory effects of CDKN2B-AS1 on cell
proliferation and migration as part of the pathology of ISR were
analyzed in hHCtASMCs cells. The expression levels of CDKN2B-AS1 in
hHCtASMCs were confirmed to be significantly downregulated by the
si-CDKN2B-AS1 (P<0.001; Fig.
2A). The results of CCK-8 and Transwell assays suggested that
the proliferation and migration of the cells were significantly
inhibited after silencing CDKN2B-AS1 (all P<0.05; Fig. 2B-D).
CDKN2B-AS1 directly inhibits
miR-143-3p expression in hHCtASMCs
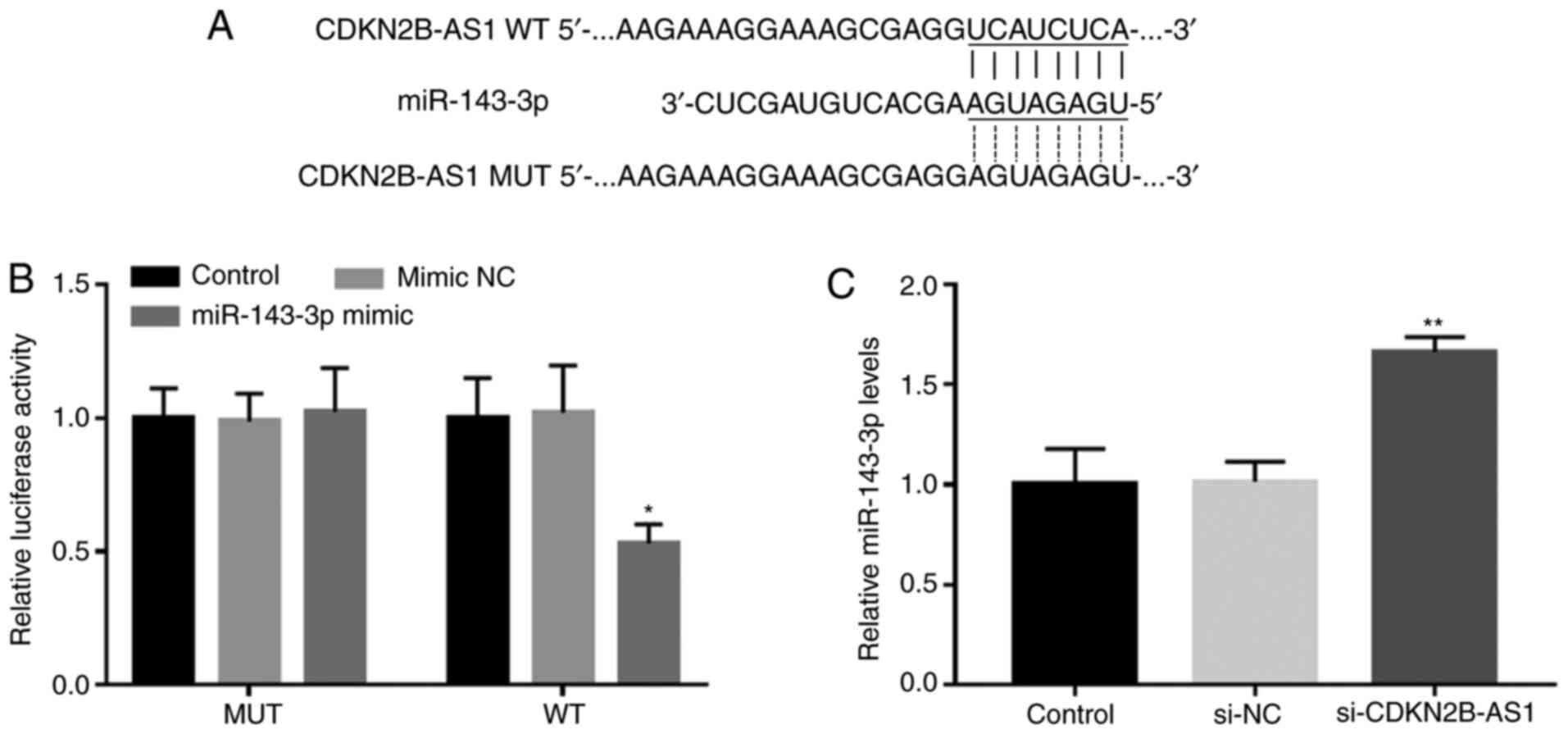
The complementary sequence of miR-143-3p in the
CDKN2B-AS1 sequence is presented in Fig. 3A. The results of the luciferase
assay demonstrated that CDKN2B-AS1 and miR-143-3p were able to
directly interact, which manifested as a significantly decreased
luciferase activity following miR-143-3p overexpression in the WT
CDKN2B-AS1 group (P<0.05; Fig.
3B). Furthermore, the RT-qPCR results indicated that the
silencing of CDKN2B-AS1 in hHCtASMCs significantly promoted the
expression of miR-143-3p, indicating that CDKN2B-AS1 is able to
directly interfere with the expression of miR-143-3p in hHCtASMCs
(P<0.01; Fig. 3C).
miR-143-3p mediates the effects of
CDKN2B-AS1 on hHCtASMC proliferation and migration
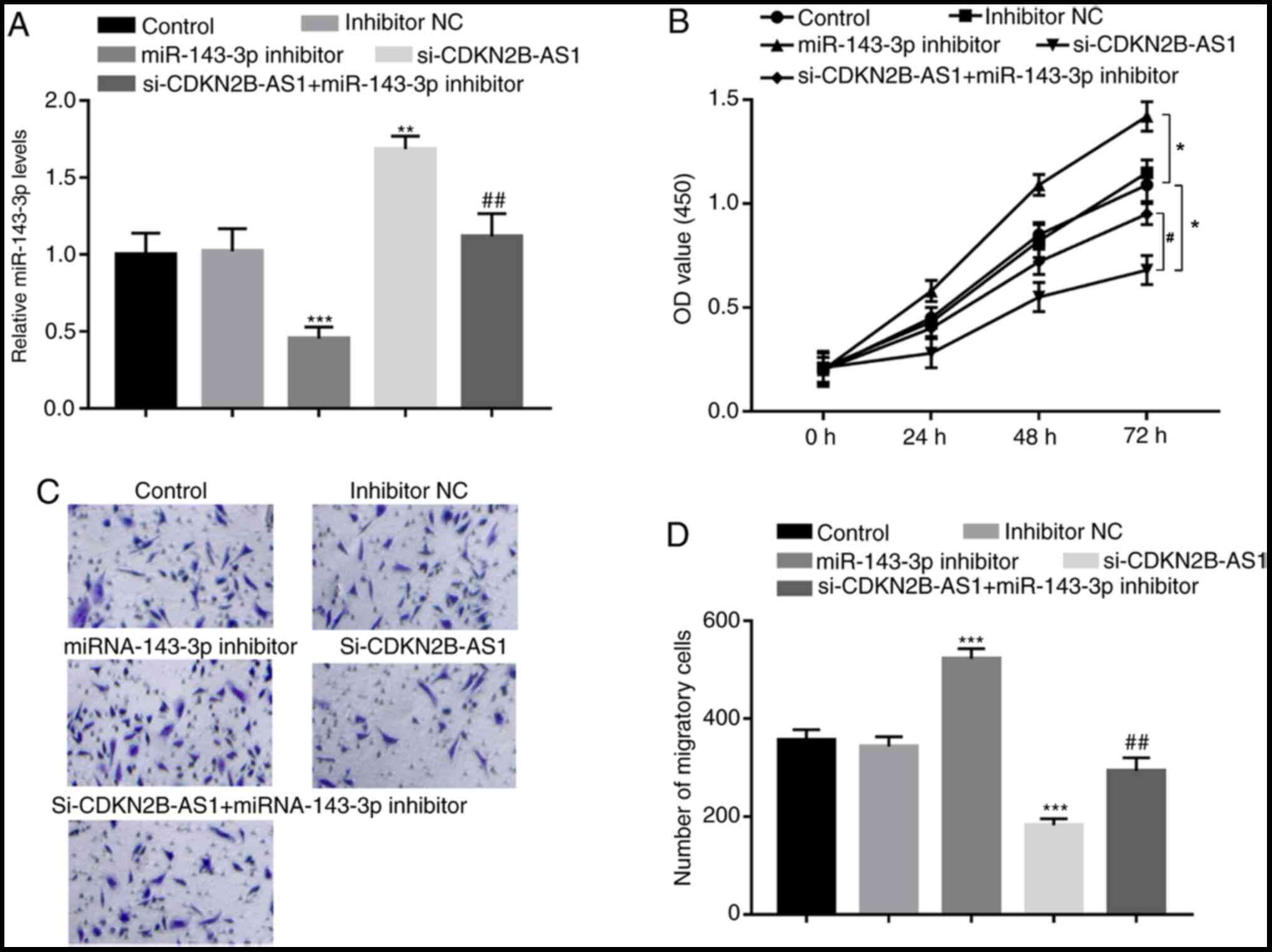
To further confirm the regulatory effect of
miR-143-3p on the biological function of CDKN2B-AS1, the expression
levels of CDKN2B-AS1 and miR-143-3p were co-regulated in hHCtASMCs.
miR-143-3p inhibitor was confirmed to significantly inhibit the
expression of miR-143-3p in hHCtASMCs (P<0.001; Fig. 4A), which led to marked increases in
hHCtASMC proliferation and migration (all P<0.05; Fig. 4B-D). Furthermore, in cells
co-transfected with si-CDKN2B-AS1 and miR-143-3p inhibitor, the
increase in miR-143-3p expression induced by CDKN2B-AS1 knockdown
alone was significantly decreased (P<0.01; Fig. 4A). In addition, CDKN2B-AS1
knockdown-induced inhibition of hHCtASMC proliferation and
migration were abrogated by simultaneous downregulation of
miR-143-3p (all P<0.05; Fig.
4B-D), indicating that miR-143-3p may mediate the regulatory
effects of CDKN2B-AS1 on hHCtASMC proliferation and migration.
Discussion
Carotid stenosis refers to the narrowing or
contraction of the internal surface of the carotid artery (21). It may result in brain tissue injury,
which is associated with intracranial arterial flow disturbances,
as well as microembolic complications (22). Carotid stenosis seriously threatens
human health. CAS has become one of the important means of treating
carotid stenosis (23), but there
is a certain proportion of ISR after stent implantation that causes
problems in the clinic (24). ISR
seriously affects the efficacy of CAS. Of note, abnormal expression
of certain miRNAs and lncRNAs in ISR has been discovered. For
instance, miR-93-5p was reported to have cardiac biomarker
potential as a predictor of ISR (25). Furthermore, Jiang et al
(24) indicated that miR-17 is
abnormally expressed in patients with ISR and has potential as a
biomarker for ISR. The expression of lncRNA antisense non-coding
RNA in the INK4 locus (ANRIL) was reported to be associated with
ISR, indicating that ANRIL may be a suitable prognostic factor for
ISR (26). lncRNA CDKN2B-AS1 was
previously reported to affect cancer progression by regulating
miRNAs. For instance, overexpression of CDKN2B-AS1 resulted in the
promotion of cell proliferation, invasion, migration and inhibition
of apoptosis and senescence of cervical cancer cells by regulating
the miR181a-5p/TGFβ1 axis (27).
Furthermore, CDKN2B-AS1 promoted hepatocellular carcinoma growth
and metastasis by promoting nucleosome assembly protein 1 like
1-mediated PI3K/AKT/mTOR signaling via acting as a molecular sponge
of let-7c-5p (17). However, the
biological functions of CDKN2B-AS1 and miRNA-143-3p in ISR remain
unclear. In the present study, it was determined that CDKN2B-AS1
and miR-143-3p were abnormally expressed in ISR. The results
suggested that compared to patients in the non-ISR group, patients
in the ISR group had upregulated serum levels of CDKN2B-AS1 and
downregulated serum levels of miR143-3p. Furthermore, the
expression levels of CDKN2B-AS1 and miR-143-3p in serum were
significantly negatively correlated in patients with ISR. The
results of the logistic multivariate regression analysis indicated
that CDKN2B-AS1 and miR-143-3p were independently associated with
the occurrence of ISR and are potential risk factors for the
occurrence of ISR.
VSMCs are the major cell type in blood vessels
(28). Abnormal proliferation and
migration of VSMCs are related to ISR (29). Certain studies have indicated that
lncRNA may affect the proliferation and migration of VSMCs. For
instance, lncRNA taurine-upregulated gene 1/miR-145-5p/fibroblast
growth factor 10 was reported to promote the proliferation and
migration of VSMCs in the hypertensive state by activating the
Wnt/β-catenin pathway (30). Tang
et al (31) demonstrated for
the first time that lncRNA growth arrest specific 5 negatively
impacts VSMC survival via activation of the p53 pathway during
vascular remodeling. However, the relationship between CDKN2B-AS1
and miR-143-3p and VSMCs had remained elusive. In the present
study, RT-qPCR was used to measure the expression levels of
CDKN2B-AS1 in hHCtASMCs and it was confirmed that CDKN2B-AS1
expression was downregulated by si-CDKN2B-AS1. It was determined
that silencing of CDKN2B-AS1 inhibited the migration and
proliferation of hHCtASMCs cells. Therefore, it was speculated that
CDKN2B-AS1 has a promoting role in the development of ISR. It was
identified that miR-143-3p is able to bind with CDKN2B-AS1(14). A luciferase reporter assay confirmed
that CDKN2B-AS1 is able to specifically associate with miR-143-3p
in hHCtASMCs. Furthermore, it was demonstrated that inhibition of
miR-143-3p was able to abrogate the effect of CDKN2BAS1 knockdown
to inhibit the proliferation and migration of hHCtASMCs. Thus, it
was suggested that CDKN2B-AS1 may directly regulate miR-143-3p in
hHCtASMCs.
In conclusion, in the present study, CDKN2B-AS1
expression levels were determined to be upregulated while
miR-143-3p expression levels were downregulated in hHCtASMCs, and
CDKN2B-AS1 and miR-143-3p were independently associated with the
occurrence of ISR, which were thus suggested to be potential risk
factors for the occurrence of ISR. In addition, silencing of
CDKN2B-AS1 inhibited the proliferation and migration of hHCtASMC by
targeting miR-143-3p. Thus, aberrant CDKN2B-AS1 and miR-143-3p
expression may be involved in the development and progression of
ISR via regulating biological functions of VSMCs. Overall, the
present study may provide novel non-invasive biomarkers for ISR
prevention and treatment and novel insight into the pathogenesis of
ISR development. However, there are still certain limitations to
the present study. For instance, changes of the
CDKN2B-AS1/miR-143-3p axis in the lesion tissue samples were not
assessed. Therefore, further studies are required in the future to
confirm the role of CDKN2B-AS1/miR-143-3p in ISR lesion tissue, and
the exact mechanisms remain to be explored.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
HM analyzed and interpreted the data. HM and AD
performed the cell experiments and blood analysis, wrote and
revised the manuscript, and checked and confirm the authenticity of
the raw data. Both authors read and approved the final
manuscript.
Ethics approval and consent to
participate
Written informed consent was obtained from each
patient and the experimental procedures were approved by the Ethics
Committee of Cangzhou Central Hospital (Cangzhou, China; approval
no. CZCH14h0283).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Meschia JF, Klaas JP, Brown RD Jr and
Brott TG: Evaluation and management of atherosclerotic carotid
stenosis. Mayo Clin Proc. 92:1144–1157. 2017.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Sarikas A, Carrier L, Schenke C, Doll D,
Flavigny J, Lindenberg KS, Eschenhagen T and Zolk O: Impairment of
the ubiquitin-proteasome system by truncated cardiac myosin binding
protein C mutants. Cardiovasc Res. 66:33–44. 2005.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Cheng SF, Brown MM, Simister RJ and
Richards T: Contemporary prevalence of carotid stenosis in patients
presenting with ischaemic stroke. Br J Surg. 106:872–878.
2019.PubMed/NCBI View Article : Google Scholar
|
|
4
|
De Reuck JL: Pathophysiology of carotid
artery disease and related clinical syndromes. Acta Chir Belg.
104:30–34. 2004.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Sherman DG: The carotid artery and stroke.
Am Fam Phys. 40 (5 Suppl):41S–44S, 7S-9S. 1989.PubMed/NCBI
|
|
6
|
Fanelli F, Boatta E, Cannavale A, Corona
M, Lucatelli P, Wlderk A, Cirelli C and Salvatori FM: Carotid
artery stenting: Analysis of a 12-year single-center experience. J
Endovasc Ther. 19:749–756. 2012.PubMed/NCBI View Article : Google Scholar
|
|
7
|
AbuRahma AF, Abu-Halimah S, Hass SM,
Nanjundappa A, Stone PA, Mousa A, Lough E and Dean LS: Carotid
artery stenting outcomes are equivalent to carotid endarterectomy
outcomes for patients with post-carotid endarterectomy stenosis. J
Vasc Surg. 52:1180–1187. 2010.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Dangas GD, Claessen BE, Caixeta A, Sanidas
EA, Mintz GS and Mehran R: In-stent restenosis in the drug-eluting
stent era. J Am Coll Cardiol. 56:1897–1907. 2010.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Zhang H, Ren KF, Chang H, Wang JL and Ji
J: Surface-mediated transfection of a pDNA vector encoding short
hairpin RNA to downregulate TGF-β1 expression for the prevention of
in-stent restenosis. Biomaterials. 116:95–105. 2017.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Bagyura Z, Kiss L, Berta B, Szilágyi Á,
Hirschberg K, Széplaki G, Lux Á, Szelid Z, Soós P and Merkely B:
High rate of in-stent restenosis after coronary intervention in
carriers of the mutant mannose-binding lectin allele. BMC
Cardiovasc Disord. 17(4)2017.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Santulli G: microRNAs distinctively
regulate vascular smooth muscle and endothelial cells: Functional
implications in angiogenesis, atherosclerosis, and in-stent
restenosis. Adv Exp Med Biol. 887:53–77. 2015.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Yuan Y, Liu X, Hao S, He Q and Shen Z:
Plasma levels of miR-143 and miR-145 are associated with coronary
in-stent restenosis within 1 year of follow-up after drug-eluting
stent implantation. Ann Transl Med. 8(756)2020.PubMed/NCBI View Article : Google Scholar
|
|
13
|
He M, Gong Y, Shi J, Pan Z, Zou H, Sun D,
Tu X, Tan X, Li J, Li W, et al: Plasma microRNAs as potential
noninvasive biomarkers for in-stent restenosis. PLoS One.
9(e112043)2014.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Xu C, Zhai J and Fu Y: LncRNA CDKN2B-AS1
promotes the progression of ovarian cancer by miR-143-3p/SMAD3 axis
and predicts a poor prognosis. Neoplasma. 67:782–793.
2020.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Li H, Han S, Sun Q, Yao Y, Li S, Yuan C,
Zhang B, Jing B, Wu J, Song Y and Wang H: Long non-coding RNA
CDKN2B-AS1 reduces inflammatory response and promotes cholesterol
efflux in atherosclerosis by inhibiting ADAM10 expression. Aging
(Albany NY). 11:1695–1715. 2019.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Ou M, Li X, Zhao S, Cui S and Tu J: Long
non-coding RNA CDKN2B-AS1 contributes to atherosclerotic plaque
formation by forming RNA-DNA triplex in the CDKN2B promoter.
EBioMedicine. 55(102694)2020.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Huang Y, Xiang B, Liu Y, Wang Y and Kan H:
LncRNA CDKN2B-AS1 promotes tumor growth and metastasis of human
hepatocellular carcinoma by targeting let-7c-5p/NAP1L1 axis. Cancer
Lett. 437:56–66. 2018.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Zhuang H, Cao G, Kou C and Li D:
Overexpressed lncRNA CDKN2B-AS1 is an independent prognostic factor
for liver cancer and promotes its proliferation. J BUON.
24:1441–1448. 2019.PubMed/NCBI
|
|
19
|
Gasecki AP, Hachinski VC, Mendel T and
Barnett HT: Endarterectomy for symptomatic carotid stenosis. Review
of the European and North American symptomatic carotid surgery
trials. Nebr Med J. 77:121–123. 1992.PubMed/NCBI
|
|
20
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408.
2001.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Chen KP, Wang JJ, Wang LJ, Lu J, Qi P, Hu
S, Yang XM, Wang HF and Wang DM: Study of correlation between
carotid artery tortuosity and atherosclerotic carotid artery
stenosis. Zhonghua Wai Ke Za Zhi. 55:608–612. 2017.PubMed/NCBI View Article : Google Scholar : (In Chinese).
|
|
22
|
Puz P, Lasek-Bal A, Urbanek T and
Kazibutowska Z: Assessment of cerebral embolism and vascular
reserve parameters in patients with carotid artery stenosis. Neurol
Neurochir. 50:356–362. 2016.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Wang T, Mei B and Zhang J: Atherosclerotic
carotid stenosis and cognitive function. Clin Neurol Neurosurg.
146:64–70. 2016.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Jiang F, Zhang X, Lu YM, Li YG, Zhou X and
Wang YS: Elevated level of miR-17 along with decreased levels of
TIMP-1 and IL-6 in plasma associated with the risk of in-stent
restenosis. Biosci Trends. 13:423–429. 2019.PubMed/NCBI View Article : Google Scholar
|
|
25
|
O'Sullivan JF, Neylon A, Fahy EF, Yang P,
McGorrian C and Blake GJ: MiR-93-5p is a novel predictor of
coronary in-stent restenosis. Heart Asia.
11(e011134)2019.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Wang F, Su X, Liu C, Wu M and Li B:
Prognostic value of plasma long noncoding RNA ANRIL for in-stent
restenosis. Med Sci Monit. 23:4733–4739. 2017.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Zhu L, Zhang Q, Li S, Jiang S, Cui J and
Dang G: Interference of the long noncoding RNA CDKN2B-AS1
upregulates miR-181a-5p/TGFβI axis to restrain the metastasis and
promote apoptosis and senescence of cervical cancer cells. Cancer
Med. 8:1721–1730. 2019.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Frismantiene A, Philippova M, Erne P and
Resink TJ: Smooth muscle cell-driven vascular diseases and
molecular mechanisms of VSMC plasticity. Cell Signal. 52:48–64.
2018.PubMed/NCBI View Article : Google Scholar
|
|
29
|
He Y, Zou P, Lu Y, Jia D, Li X, Yang H,
Tang L, Zhu Z, Tu T, Tai S, et al: Osteoprotegerin promotes intimal
hyperplasia and contributes to in-stent restenosis: Role of an
αVβ3/FAK dependent YAP pathway. J Mol Cell Cardiol. 139:1–13.
2020.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Shi L, Tian C, Sun L, Cao F and Meng Z:
The lncRNA TUG1/miR-145-5p/FGF10 regulates proliferation and
migration in VSMCs of hypertension. Biochem Biophys Res Commun.
501:688–695. 2018.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Tang R, Mei X, Wang YC, Cui XB, Zhang G,
Li W and Chen SY: LncRNA GAS5 regulates vascular smooth muscle cell
cycle arrest and apoptosis via p53 pathway. Biochim Biophys Acta
Mol Basis Dis. 1865:2516–2525. 2019.PubMed/NCBI View Article : Google Scholar
|