Introduction
Triple negative breast cancer (TNBC) is a
heterogeneous subclass of breast cancer, characterized by the lack
of expression of epidermal growth factor receptor, estrogen
receptor and progesterone receptor (1-3).
Compared with other types of breast cancer, patients with TNBC
present a higher risk of metastasis and recurrence, as well as a
poorer prognosis (3). Due to the
absence of a specific biomarker, the current strategies for
early-stage or advanced TNBC remain chemotherapy or radiotherapy,
and the clinical outcomes for patients with TNBC are still
uncertain; however, patients tend to exhibit more aggressive
features compared with other forms of breast cancer (4). The genomic and molecular aberrations
that contribute to the initiation and progression of TNBC remain
largely unknown. Therefore, identifying novel factors and
characterizing the related functional mechanism of actions are
critical for the diagnosis and treatment of patients with TNBC.
MicroRNAs (miRNAs) are small non-coding, single
stand RNA molecules that negatively modulate gene expression at the
post-transcriptional level by binding to the 3'-untranslated region
(UTR) of target mRNAs (5-7).
Previous studies have reported that miRNAs play a pivotal role in
cancer prognoses and drug resistance (8-12).
Moreover, aberrant expression of miRNA (miR) has been demonstrated
in patients with TNBC, which is correlated with the clinical
outcomes of the patient (13,14). A
recent study revealed that miR-890 was downregulated in TNBC and
that it inhibited the proliferation and invasion of TNBC cells
(15). Inhibition of miR-214 also
significantly attenuates the migration and invasion of TNBC cells
(16). Furthermore, previous
studies have identified the downregulation and tumor suppressive
functions of miR-598 in multiple cancer types, including gastric
cancer, non-small cell lung cancer (NSCLC) and colorectal cancer
(17-19).
Overexpression of miR-598 also suppresses the malignant features of
cancer cells (17-19).
Therefore, developing miRNA-based therapeutics may improve the
treatment of cancer, particularly for patients with TNBC who show
early relapse and poor survival. However, the expression and
functional mechanism of action of miR-598 in TNBC remains largely
unknown.
Jagged 1 (JAG1) is a canonical ligand that functions
primarily in the highly conserved Notch signaling pathway (20). Notch signaling plays important roles
in the determination of cellular fate and organ development
(21). The classic interaction
between JAG1 and Notch leads to a cascade of proteolytic cleavages
that induces the transportation of Notch intracellular domain into
the nucleus to activate the transcription of target genes (22-24).
Previous studies have shown that frequent upregulation of JAG1 in
various types of cancer is associated with a poor survival rate
(21,25). However, downregulation of JAG1
inhibits the progression of cancer types, suggesting the clinical
significance of JAG1 as a potential target for cancer treatment
(25). Therefore, the present study
aimed to detect the expression of miR-598 in TNBC and characterize
the functional mechanism of miR-598 in the malignancy of TNBC.
Materials and methods
Cell lines
The TNBC cell lines MDA-MB-231, HCC-1937, MDA-MB-468
and BT-549 and normal human breast cell line MCF-10A were purchased
from The Cell Bank of Type Culture Collection of the Chinese
Academy of Sciences. Cells were maintained in RPMI-1640 medium
(Thermo Fisher Scientific, Inc.) supplemented with 10% FBS (Thermo
Fisher Scientific, Inc.). Cells were cultured at 37˚C with 5%
CO2.
Tissue samples
A total of 50 patients (age range, 44-70 years) who
were diagnosed as TNBC with negative expressions of estrogen
receptor, progesterone receptor and human epidermal growth factor
receptor were enrolled in this study. TNBC tissues and adjacent
healthy tissues were obtained via surgical resection at Shanxi
Provincial Cancer Hospital (Taiyuan, Shanxi, China) between
November 2012 and September 2014. Patients who were subjected to
neoadjuvant chemotherapy or radiotherapy before the surgery, or
those without adjacent healthy tissues were excluded in this study.
The lymph node metastasis of patients was determined via
hematoxylin and eosin staining by three independent pathologists.
All tissues were frozen immediately and stored at -80˚C until use.
Written informed consents were received from all patients. The
study was approved by the Ethics Committee of Shanxi Provincial
Cancer Hospital. The relevant clinical characteristics of the
patients enrolled in the present study are provided in Table SI.
Cell transfection
miR-598 mimics (5'-UACGUCAUCGUUG UCAUCGUCA-3') and
miR-negative control (NC; 5'-GUUC GUACGUACACUGUUCA-3') were
purchased from Shanghai GenePharma Co., Ltd. The overexpression
plasmid of JAG1 was generated by amplifying the cDNA of JAG1 and
inserting it into the pcDNA-Myc vector (Addgene, Inc.). For cell
transfection, MDA-MB-231 and BT-549 cells (1x105
cells/well) were plated into 6-well plates. After culturing
overnight, miRNA (50 nM) was transfected using
Lipofectamine® 2000 (Thermo Fisher Scientific, Inc.),
according to the manufacturer's instructions. After transfection
for 48 h, cells were harvested for further analysis.
Cell proliferation assay
The Cell Counting Kit-8 (CCK-8) assay was performed
to determine the cell viability according to the manufacturer's
protocol (Beyotime, Institute of Biotechnology). TNBC cells
transfected with miR-598 mimics or miR-NC were plated into 96-well
plates with 2,000 cells per well. Following incubation with 10 µl
CCK-8 regent at 37˚C for 4 h at the indicated time points (1, 2, 3,
4 and 5 days), the absorbance of each well at 450 nm was measured
using the microplate reader (Roche Diagnostics).
Reverse transcription-quantitative PCR
(RT-qPCR)
Total RNA from tissues or cells was extracted using
TRIzol® (Invitrogen; Thermo Fisher Scientific, Inc.) and
quantified using the NanoDrop-2000 spectrophotometer (NanoDrop
Technologies; Thermo Fisher Scientific, Inc.). Then, 1 µg RNA was
reverse transcribed into cDNA using the PrimeScript RT Reagent kit
(Takara Bio, Inc.) at 37˚C for 10 min and 85˚C for 10 sec. The
expression of miR-598 was determined using qPCR assays with the
TaqMan miRNA PCR kit (Applied Biosystems; Thermo Fisher Scientific,
Inc.) on the Option RT-qPCR detection system (ABI 7500; Thermo
Fisher Scientific, Inc.). The PCR cycles were set as follows:
Initial denaturation at 95˚C for 10 min, followed by 40 cycles at
95˚C for 15 sec, 60˚C for 1 min and preservation at 4˚C. The
expression of miR-598 was calculated using the comparative
quantification cycle (2-ΔΔCq) method (26). The expression of GAPDH was used for
normalization. The following primer pairs were used for the qPCR:
miR-598 forward, 5'- TACGTCA TCGTTGTCATCGTCA-3' and reverse,
5'-GCATAGACCTG AATGGCGGTA-3'; U6 forward, 5'-GCTTCGGCAGCACAT
ATACTAAAAT-3' and reverse, 5'-CGCTTCAGAATTT GCGTGTCAT-3'; JAG1
forward, 5'- ATCGTGCTGCCTTTC AGTTT-3' and reverse,
5'-GATCATGCCCGAGTGAGAA-3' and GAPDH forward,
5'-CACCTGCGCTGTGTGGACT-3' and reverse,
5'-GGATGGCTGATGTGTCGGGTGG-3'.
Western blot analysis
The proteins cells were extracted using RIPA lysis
buffer (Beyotime Institute of Biotechnology) containing protease
inhibitor. The protein concentration was assessed using the
bicinchoninic acid Protein Assay kit (Pierce; Thermo Fisher
Scientific, Inc.) according to the manufacturer's protocol. Equal
amounts of protein (20 µg/lane) were separated by 15% SDS-PAGE and
transferred onto the PVDF membrane (EMD Millipore). After blocking
with 5% non-fat milk for 1 h at room temperature, the membrane was
probed with primary antibodies targeting JAG1 (1:1,000; cat. no.
ab109536; Abcam) or GAPDH (1:3,000; cat. no. ab8245; Abcam) at 4˚C
overnight. The membrane was washed three times with PBS-Tween-20
(0.1%) and then incubated with Goat anti-Mouse IgG
(H+L)-horseradish peroxidase (HRP)-conjugated secondary antibodies
(1:5,000; cat. no. 170-6516; Bio-Rad Laboratories, Inc.) or Goat
anti-Rabbit IgG (H+L)-HRP-conjugated secondary antibodies (1:5,000;
cat. no. 170-6515; Bio-Rad Laboratories, Inc.) at room temperature
for 1 h. Following an extensive wash with PBST, the enhanced
chemiluminescence western blotting kit (Pierce; Thermo Fisher
Scientific, Inc.) was used to visualize the bands. Densitometric
analysis was performed using ImageJ software (version 1.8.0;
National Institutes of Health). The expression of GAPDH was used as
the loading control.
Targets prediction
The potential targets of miR-598 were predicted
using the miRDB online database (version 6.0; http://mirdb.org/).
Dual-luciferase reporter assay
The wild-type (WT) or mutant (MT) JAG1 3'-UTR was
amplified and cloned into the pMIR-REPORT Luciferase reporter
vector (Promega Corporation) to generate the pMIR-JAG1-3'-UTR-WT or
pMIR-JAG1-3'-UTR-MT, respectively. Cells (1x104
cells/well) were plated into the 96-well plate and co-transfected
with the luciferase plasmid carrying WT or MT 3'-UTR of JAG1 (100
µg) and miR-598 mimics or miR-NC (50 nM) using
Lipofectamine® 2000 (Invitrogen; Thermo Fisher
Scientific, Inc.). After transfection for 48 h, cells were
harvested and the luciferase activity was assessed using the
Dual-Luciferase Reporter Assay system (Promega Corporation)
according to the manufacturer's instructions. The luciferase
activity of firefly was also detected for normalization.
Colony formation
TNBC cells transfected with miR-598 mimics or miR-NC
were seeded into the 6-well plate with a density of 500 cells per
well. Cells were cultured with RPMI-1640 medium containing 10% FBS
at 37˚C with 5% CO2. Following incubation in the
CO2 incubator for 10 days, cells were washed with PBS
and fixed with 100% methanol at room temperature for 15 min. The
colonies were stained with 0.5% crystal violet (Beyotime Institute
of Biotechnology) at room temperature for 10 min and counted
manually using a light microscope at x40 magnification.
Statistical analysis
Data are presented as the mean ± SD from three
independent experiments. Statistical analysis was performed using
SPSS software 13.0 (SPSS, Inc.). Paired and unpaired Student's
t-tests was used to analyze the significance between two groups.
Difference among multiple groups was determined using one-way ANOVA
followed by Tukey's post hoc test. The correlation between the
expression levels of miR-598 and JAG1 was assessed using the
Pearson's test. P<0.05 was considered to indicate a
statistically significant difference.
Results
miR-598 expression is downregulated in
TNBC tissues and cell lines
To evaluate the potential involvement of miR-598 in
TNBC, RT-qPCR analysis was performed to analyze the expression of
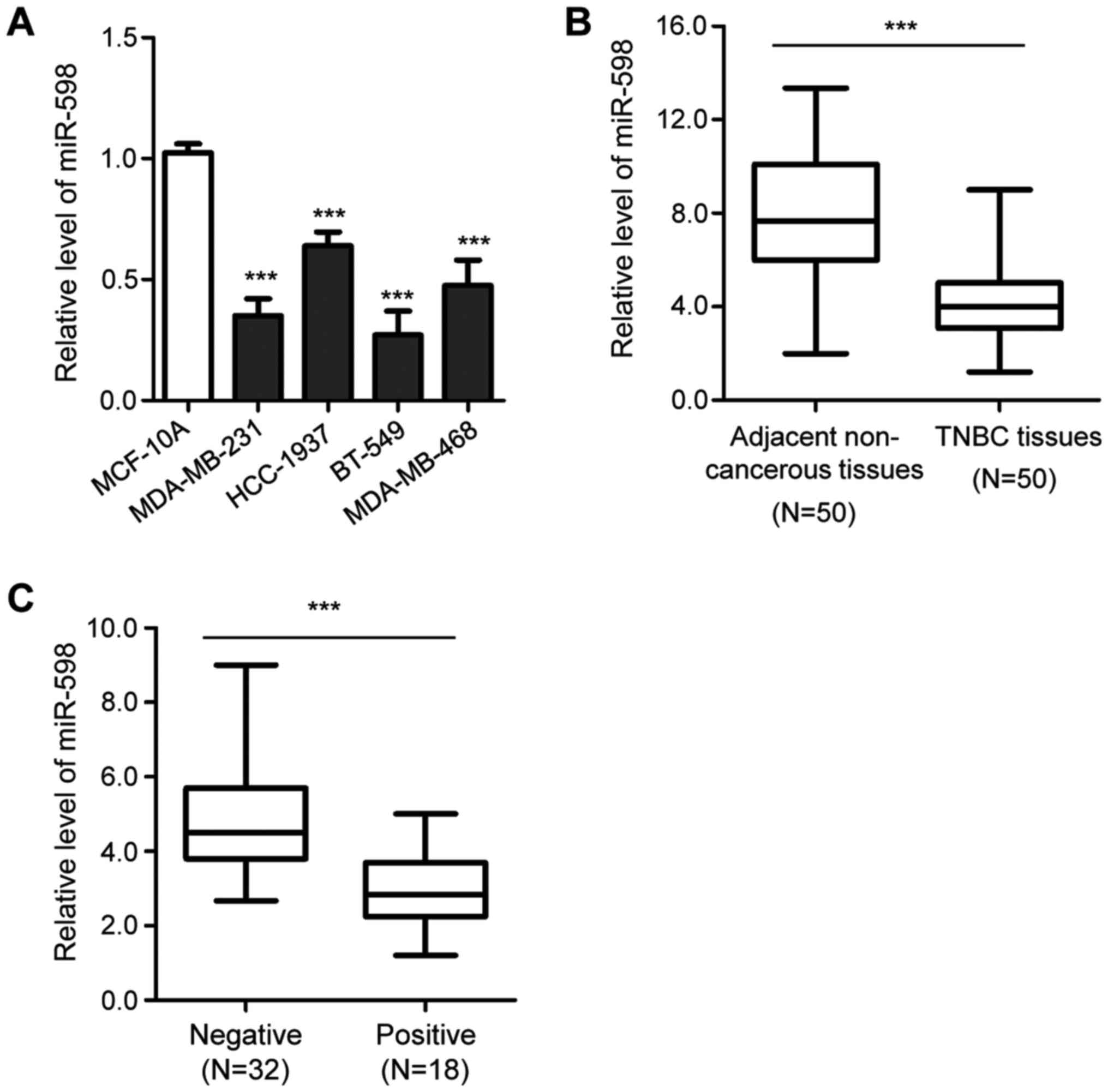
miR-598 in TNBC cells. Compared with the normal cell line, MCF-10A,
the expression of miR-598 was significantly decreased in TNBC cells
(Fig. 1A). Moreover, the expression
of miR-598 in TNBC tissues was evaluated, and was found to be
significantly downregulated in TNBC tissues compared with
corresponding healthy adjacent tissues (Fig. 1B). The results also suggested that
the expression of miR-598 was significantly lower in patients with
lymph node metastasis compared with patients without lymph node
metastasis (Fig. 1C). These
findings indicated that the downregulation of miR-598 may play an
important role in the progression of TNBC.
miR-598 inhibits the viability and
colony formation of TNBC cells
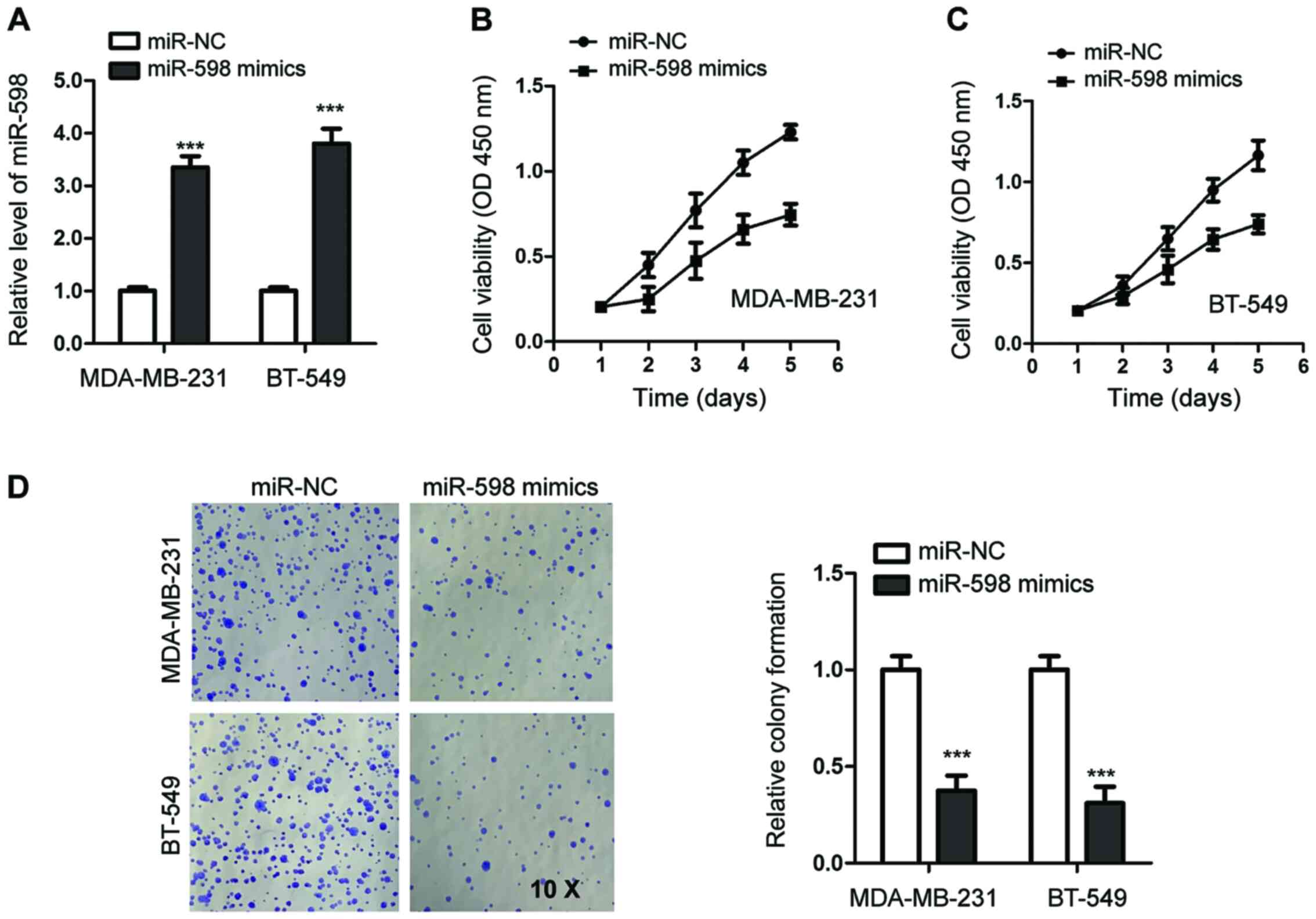
To investigate the function of miR-598 in TNBC,
MDA-MB-231 and BT-549 cells, which expressed lower levels of
miR-598 compared with the other tested TNBC cell lines, were
transfected with miR-598 mimics or miR-NC. The overexpression of
miR-598 was detected using RT-qPCR (Fig. 2A).
To investigate the effects of miR-598 on the
viability of TNBC cells, CCK-8 assays were performed. The results
demonstrated that miR-598 overexpression decreased the viability of
MDA-MB-231 cells compared with cells expressing miR-NC (Fig. 2B). The inhibitory function of
miR-598 overexpression on viability was also observed in BT-549
cells (Fig. 2C).
Colony formation assays were performed to evaluate
the influence of miR-598 on the proliferation of TNBC cells.
Compared with the control cells, overexpression of miR-598
significantly decreased the colony-formation ability of both
MDA-MB-231 and BT-549 cells (Fig.
2D). Thus, these results suggested a tumor suppressive role for
miR-598 in TNBC.
miR-598 suppresses cell cycle
progression and induces apoptosis of TNBC cells
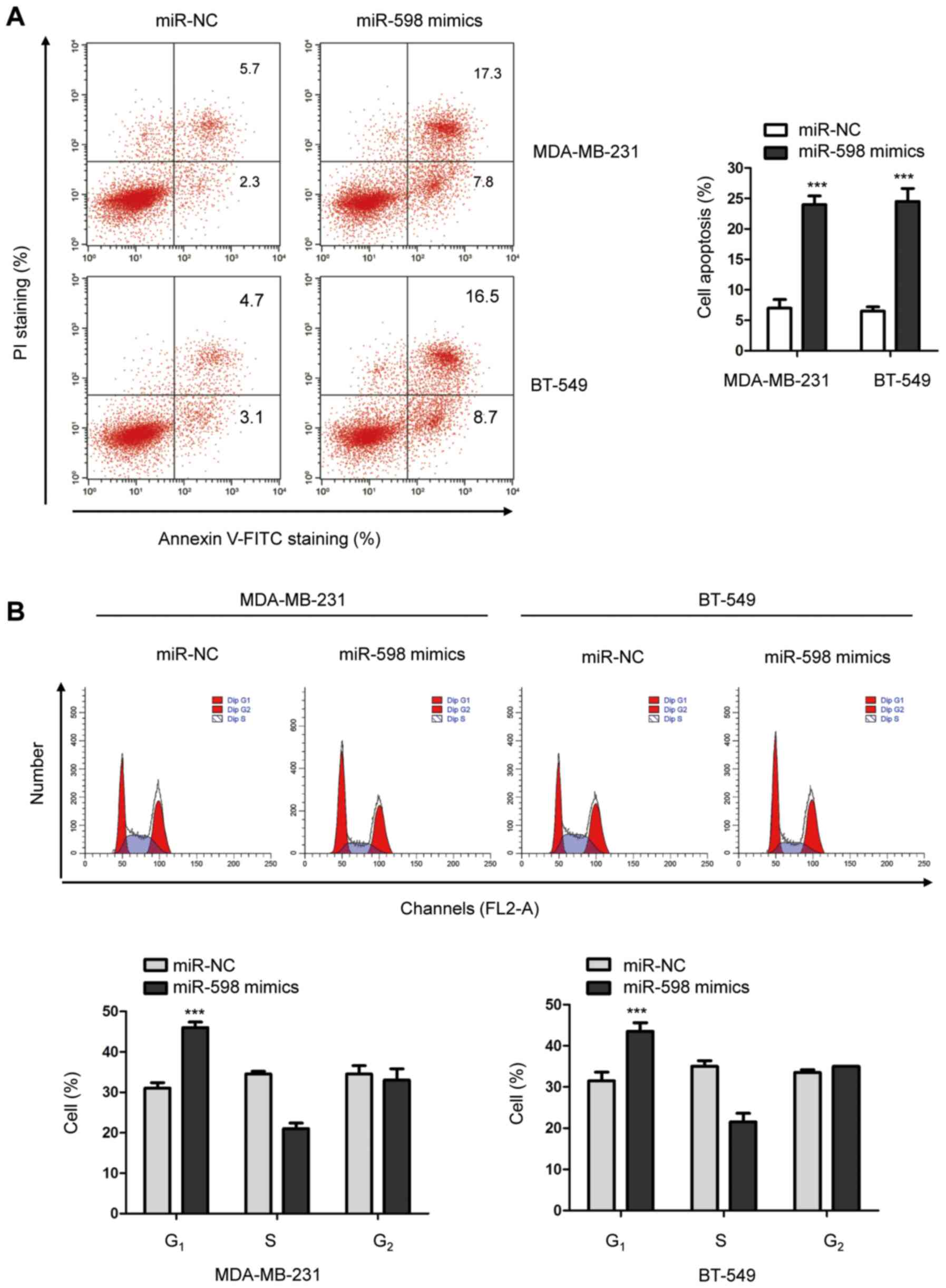
To further illustrate the function of miR-598 in the
progression of TNBC, the cell cycle progression of MDA-MB-231 and
BT-549 cells overexpressing miR-598 was detected using
fluorescence-activated cell sorting (FACS). The results suggested
that overexpression of miR-598 significantly increased the number
of cells in the G1 phase compared with cells expressing miR-NC
(Fig. 3B), which suggested that G1
cell cycle arrest was induced by miR-598.
In addition, to investigate whether overexpression
of miR-598 regulated the apoptosis of TNBC cells, cells transfected
with miR-598 mimics or miR-NC were stained with Annexin/FITC and
propidium iodide. The FACS analysis indicated that overexpression
of miR-598 significantly enhanced the apoptosis of both MDA-MB-231
and BT-549 cells (Fig. 3A).
Therefore, the results indicated that miR-598 induced cell cycle
arrest and apoptosis of TNBC cells.
JAG1 is a target of miR-598 in
TNBC
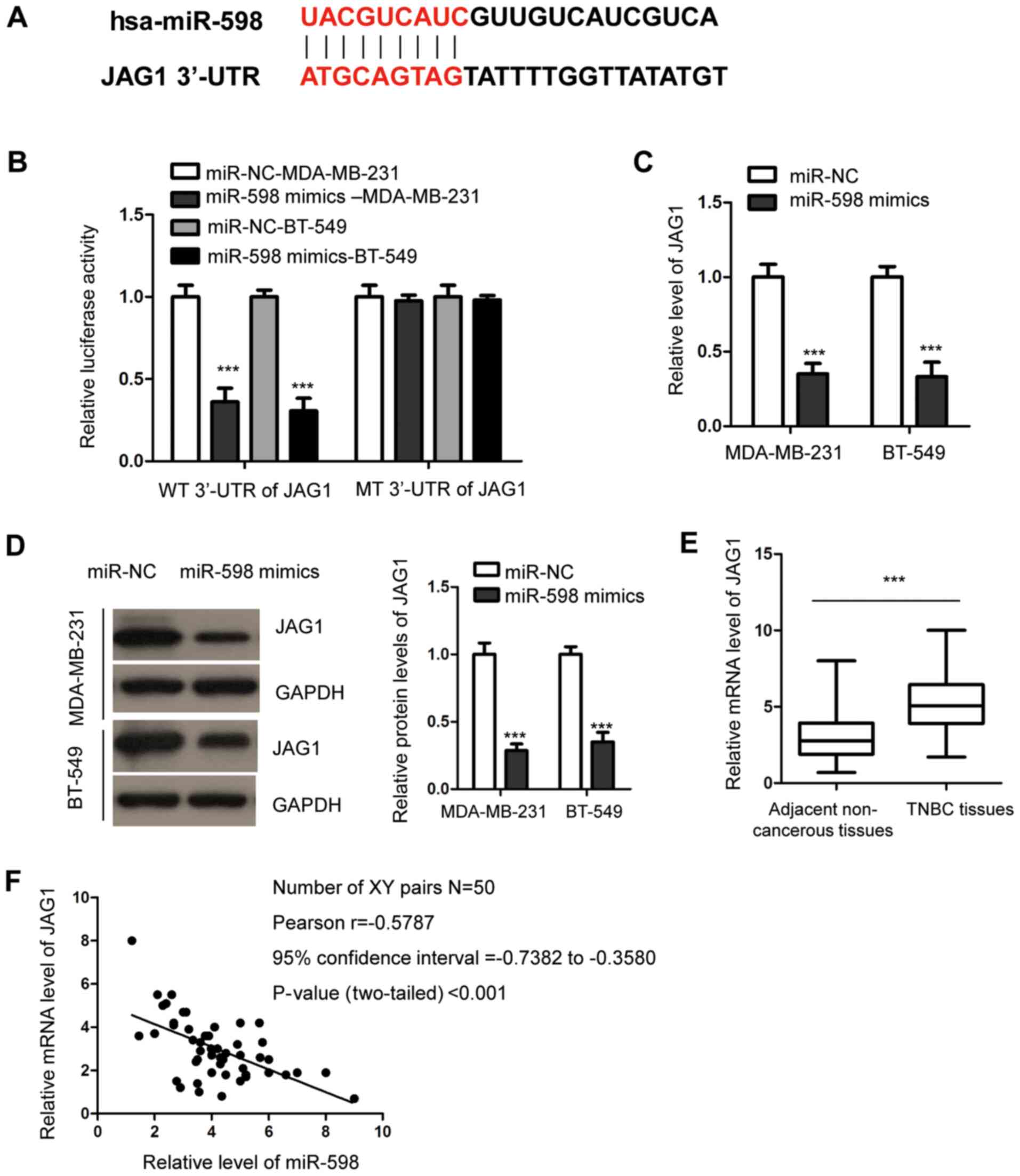
To understand the molecular mechanism of action
underlying the anti-tumor functions of miR-598 in TNBC, the
potential targets of miR-598 were predicted using the miRDB
website. The prediction analysis identified JAG1 as a possible
target of miR-598, as the 3'-UTR of JAG1 contained complementary
binding sites for miR-598 (Fig.
4A). To assess this potential association, dual-luciferase
reporter assays were performed by transfecting luciferase reporter
vectors that harbored WT or MT 3'-UTR of JAG1. The results
indicated that overexpression of miR-598 significantly reduced the
luciferase activity of cells expressing WT, but not MT 3'-UTR of
JAG1 (Fig. 4B), suggesting that
there may be specific binding between miR-598 and the 3'-UTR of
JAG1.
To further analyze the effect of this interaction,
both RT-qPCR and western blotting assays were performed to evaluate
the expression of JAG1 after miR-598 overexpression. It was
demonstrated that transfection of miR-598 mimics led to a
corresponding significant decrease in JAG1 expression in MDA-MB-231
and BT-549 cells (Fig. 4C and
D).
To support the negative regulation of JAG1 by
miR-598, the expression of JAG1 in TNBC tissues was detected using
RT-qPCR. Expression of JAG1 was significantly upregulated in TNBC
tissues compared with the healthy tissues (Fig. 4E). Furthermore, the correlation
between the expression levels of miR-598 and JAG1 was analyzed
using the Pearson's test, which demonstrated that miR-598
expression was moderately inversely correlated to JAG1 expression
in TNBC tissues (Fig. 4F).
Collectively, these findings indicated that miR-598 targeted JAG1
and inhibited the expression of JAG1 in TNBC cells.
miR-598 suppresses the proliferation
of TNBC cells by targeting JAG1
To further investigate whether miR-598 inhibited the
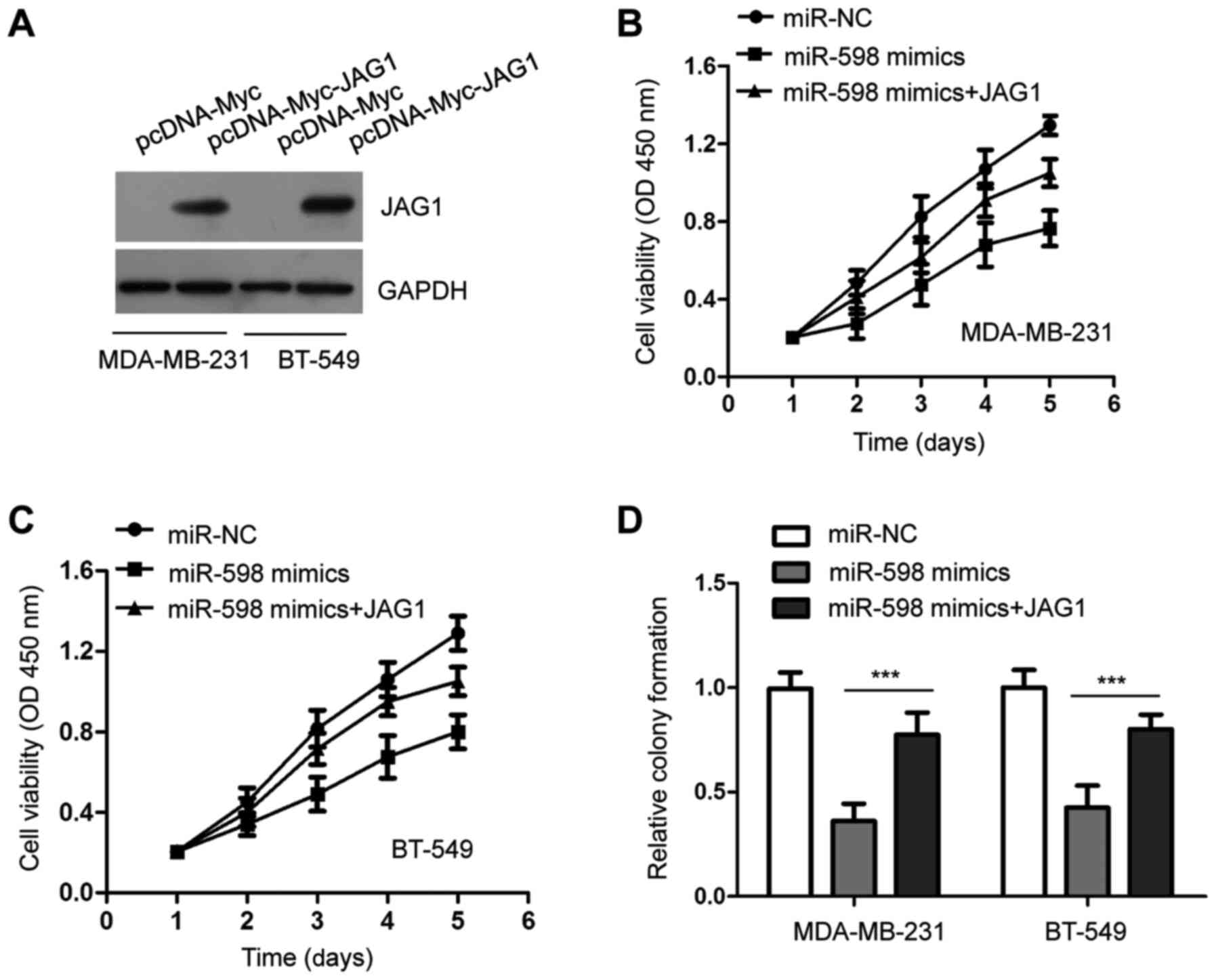
viability of TNBC cells by targeting JAG1, JAG1 was overexpressed
by transfecting pcDNA-Myc-JAG1 into MDA-MB-231 and BT-549 cells
(Fig. 5A). TNBC cells were
co-transfected with miR-598 mimics and JAG1, and the CCK-8 assays
indicated that the viability of TNBC cells was increased following
co-transfection of JAG1 compared with cells transfected with
miR-598 alone (Fig. 5B and C). For the colony formation assay, TNBC
cells formed fewer colonies when overexpressing miR-598. However,
cells co-transfected with miR-598 mimics and pcDNA-JAG1 exhibited
more colonies (Fig. 5D). These
results suggested that JAG1 plays an important role in
miR-598-induced proliferation defects of TNBC cells.
Discussion
Due to the lack of precise targets, chemotherapy has
remained the main therapeutic strategy for the treatment of TNBC
(27-29).
However, increased chemoresistance and worse prognoses have been
reported in patients with TNBC compared with other subtypes of
breast cancer (30,31). Thus, the discovery of novel factors
that can be used as potential targets for the diagnosis and
treatment of TNBC is critical. Previous studies have reported that
frequent aberrant expression of miRNAs is correlated with the
initiation and progression of TNBC (32,33).
In the present study, miR-598 was downregulated in TNBC tissues and
cell lines. Furthermore, highly expressed miR-598 levels
significantly inhibited the malignant features of TNBC cells. Thus,
these findings provided novel insights into the anti-tumor effects
of miR-598 in TNBC.
Abnormal expression of miR-598 is implicated in
multiple cancer types and contributes to the malignant phenotypes
of cancer cells (17-19).
miR-598 inhibits the proliferation and metastasis of ovarian cancer
cell (34). In addition, the
expression of miR-598 is significantly downregulated in NSCLC,
which is negatively correlated with the TNM stage and lymph node
metastasis of patients with NSCLC (18). The tumor suppressive role of miR-598
has also been reported in gastric cancer, which may serve as a
promising anti-cancer target (17).
A recent study also revealed an anti-cancer function of miR-598 in
glioblastomas by directly targeting MET transcriptional regulator
MACC1(35). In the present study,
miR-598 was decreased in TNBC tissues and cell lines. Furthermore,
downregulation of miR-598 was significantly correlated with lymph
node metastasis of patients with TNBC, suggesting a potential
involvement of miR-598 in the development of TNBC. However, further
research with larger samples size is required to assess the
correlation between miR-598 expression and the 5-year overall
survival of patients with TNBC to highlight the clinical
significance of miR-598. Overexpression of miR-598 suppressed the
viability, colony formation and induced apoptosis of TNBC cells.
Based on the key roles of metastasis and invasion in the
development of TNBC, the effects of miR-598 on the migration of
TNBC cells should be further examined in future studies. Consistent
with the role of miR-598 in other types of cancer, the present
results indicated that miR-598 acted as a tumor suppressor in TNBC
and may be a possible target to inhibit the development of TNBC. To
support this possibility, the tumor suppressive function of miR-598
should be investigated in in vivo studies with mice models. In
addition, the complexity of tumor microenvironment, the side
effects of miR-598 introduction, as well as the delivery of miR-598
into the tumor sites require further examination.
JAG1 mediates multiple signaling pathways and is
involved in both physiological and pathological conditions
(21). As an oncogene, upregulation
of JAG1 has been identified in various cancer types and is
associated with the malignant progression and a poor prognosis
(36). JAG1 has also been reported
to be the target of miRNAs in several types of cancer. For example,
miR-186 suppresses the proliferation of myelomas by targeting
JAG1(37). A recent study showed
that JAG1 was sponged by miR-377-3p and that JAG1 inhibited the
proliferation of ovarian cancer cells (38). Additionally, it has been reported
that miR-34a attenuated the paclitaxel resistance by directly
suppressing JAG1 in prostate cancer (39). In the present study, JAG1 was
identified as a downstream target of miR-598 and was inhibited by
miR-598. Decreased miR-598 expression was significantly inversely
correlated with the expression of JAG1 in TNBC tissues. As a cell
surface ligand, JAG1 activates the Notch signaling pathway by
interacting with Notch receptors (20). The Notch pathway promotes the
metastasis of various types of cancer cells. Moreover, a recent
study showed that miR-598 regulated the epithelial-mesenchymal
transitions of colorectal cancer cells via directly targeting JAG1
to inactivate the Notch signaling (19). However, further investigation is
required to assess the influence of miR-598 on the Notch pathway to
explain how decreased JAG1 expression is involved in TNBC. As
miRNAs usually have multiple targets, the involvement of other
targets along with JAG1 in the progression of TNBC should also be
further elucidated.
In conclusion, the present results suggested that
miR-598 was downregulated in TNBC tissues and cells. miR-598
exerted its anti-cancer effects on the proliferation of TNBC cells,
at least partially, by targeting JAG1. Thus, these findings
identified a novel mechanism of action for miR-598 in the
malignancy of TNBC, suggesting miR-598 may be a potential target
for the treatment of TNBC.
Supplementary Material
Disease staging of the patients
enrolled in this study.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
GHH, XDB and QH conceived and designed the study,
and drafted the manuscript. GHH, XDB and HCJ collected and analyzed
the data. All authors have revised and approved the final
manuscript.
Ethics approval and consent to
participate
This study was approved by the Ethics Committee of
Shanxi Provincial Cancer Hospital. Written informed consents were
received from all patients.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Tariq K and Rana F: TNBC vs. Non-TNBC: A
five-year retrospective review of differences in mean age, family
history, smoking history and stage at diagnosis at an Inner City
University Program. World J Oncol. 4:241–247. 2013.PubMed/NCBI View
Article : Google Scholar
|
|
2
|
Bianchini G, Balko JM, Mayer IA, Sanders
ME and Gianni L: Triple-negative breast cancer: Challenges and
opportunities of a heterogeneous disease. Nat Rev Clin Oncol.
13:674–690. 2016.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Jhan JR and Andrechek ER: Triple-negative
breast cancer and the potential for targeted therapy.
Pharmacogenomics. 18:1595–1609. 2017.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Nakhjavani M, Hardingham JE, Palethorpe
HM, Price TJ and Townsend AR: Druggable Molecular Targets for the
Treatment of Triple Negative Breast Cancer. J Breast Cancer.
22:341–361. 2019.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Bartel DP: MicroRNAs: Genomics,
biogenesis, mechanism, and function. Cell. 116:281–297.
2004.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Mohr AM and Mott JL: Overview of microRNA
biology. Semin Liver Dis. 35:3–11. 2015.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Fabian MR, Sonenberg N and Filipowicz W:
Regulation of mRNA translation and stability by microRNAs. Annu Rev
Biochem. 79:351–379. 2010.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Qu H, Xu W, Huang Y and Yang S:
Circulating miRNAs: Promising biomarkers of human cancer. Asian Pac
J Cancer Prev. 12:1117–1125. 2011.PubMed/NCBI
|
|
9
|
Momtazi AA, Shahabipour F, Khatibi S,
Johnston TP, Pirro M and Sahebkar A: Curcumin as a microRNA
regulator in cancer: A review. Rev Physiol Biochem Pharmacol.
171:1–38. 2016.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Iorio MV and Croce CM: MicroRNA
dysregulation in cancer: Diagnostics, monitoring and therapeutics.
A comprehensive review. EMBO Mol Med. 9(852)2017.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Kwak PB, Iwasaki S and Tomari Y: The
microRNA pathway and cancer. Cancer Sci. 101:2309–2315.
2010.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Rupaimoole R and Slack FJ: MicroRNA
therapeutics: Towards a new era for the management of cancer and
other diseases. Nat Rev Drug Discov. 16:203–222. 2017.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Piasecka D, Braun M, Kordek R, Sadej R and
Romanska H: MicroRNAs in regulation of triple-negative breast
cancer progression. J Cancer Res Clin Oncol. 144:1401–1411.
2018.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Petrovic N, Davidovic R, Bajic V,
Obradovic M and Isenovic RE: MicroRNA in breast cancer: The
association with BRCA1/2. Cancer Biomark. 19:119–128.
2017.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Wang C, Xu C, Niu R, Hu G, Gu Z and Zhuang
Z: miR-890 inhibits proliferation and invasion and induces
apoptosis in triple-negative breast cancer cells by targeting
CD147. BMC Cancer. 19(577)2019.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Zhang Y, Zhao Z, Li S, Dong L, Li Y, Mao
Y, Liang Y, Tao Y and Ma J: Inhibition of miR 214 attenuates the
migration and invasion of triple negative breast cancer cells. Mol
Med Rep. 19:4035–4042. 2019.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Ma Y, Yan F, Wei W, Deng J, Li L, Liu L
and Sun J: MicroRNA-598 inhibits the growth and maintenance of
gastric cancer stem-like cells by down-regulating RRS1. Cell Cycle.
18:2757–2769. 2019.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Tong X, Su P, Yang H, Chi F, Shen L, Feng
X, Jiang H, Zhang X and Wang Z: MicroRNA-598 inhibits the
proliferation and invasion of non-small cell lung cancer cells by
directly targeting ZEB2. Exp Ther Med. 16:5417–5423.
2018.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Chen J, Zhang H, Chen Y, Qiao G, Jiang W,
Ni P, Liu X and Ma L: miR-598 inhibits metastasis in colorectal
cancer by suppressing JAG1/Notch2 pathway stimulating EMT. Exp Cell
Res. 352:104–112. 2017.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Shimizu K, Chiba S, Saito T, Kumano K and
Hirai H: Physical interaction of Delta1, Jagged1, and Jagged2 with
Notch1 and Notch3 receptors. Biochem Biophys Res Commun.
276:385–389. 2000.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Grochowski CM, Loomes KM and Spinner NB:
Jagged1 (JAG1): Structure, expression, and disease associations.
Gene. 576:381–384. 2016.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Choi K, Ahn YH, Gibbons DL, Tran HT,
Creighton CJ, Girard L, Minna JD, Qin FX and Kurie JM: Distinct
biological roles for the notch ligands Jagged-1 and Jagged-2. J
Biol Chem. 284:17766–17774. 2009.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Zavadil J, Cermak L, Soto-Nieves N and
Böttinger EP: Integration of TGF-beta/Smad and Jagged1/Notch
signalling in epithelial-to-mesenchymal transition. EMBO J.
23:1155–1165. 2004.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Chen X, Stoeck A, Lee SJ, Shih IeM, Wang
MM and Wang TL: Jagged1 expression regulated by Notch3 and
Wnt/β-catenin signaling pathways in ovarian cancer. Oncotarget.
1:210–218. 2010.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Steg AD, Katre AA, Goodman B, Han HD, Nick
AM, Stone RL, Coleman RL, Alvarez RD, Lopez-Berestein G, Sood AK,
et al: Targeting the notch ligand JAGGED1 in both tumor cells and
stroma in ovarian cancer. Clin Cancer Res. 17:5674–5685.
2011.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) Method. Methods. 25:402–408.
2001.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Nagini S: Breast Cancer: Current Molecular
Therapeutic Targets and New Players. Anticancer Agents Med Chem.
17:152–163. 2017.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Wein L and Loi S: Mechanisms of resistance
of chemotherapy in early-stage triple negative breast cancer
(TNBC). Breast. 34 (Suppl 1):S27–S30. 2017.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Lehmann BD, Jovanović B, Chen X, Estrada
MV, Johnson KN, Shyr Y, Moses HL, Sanders ME and Pietenpol JA:
Refinement of Triple-Negative Breast Cancer Molecular Subtypes:
Implications for Neoadjuvant Chemotherapy Selection. PLoS One.
11(e0157368)2016.PubMed/NCBI View Article : Google Scholar
|
|
30
|
O'Reilly EA, Gubbins L, Sharma S, Tully R,
Guang MH, Weiner-Gorzel K, McCaffrey J, Harrison M, Furlong F, Kell
M, et al: The fate of chemoresistance in triple negative breast
cancer (TNBC). BBA Clin. 3:257–275. 2015.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Das S: Identification and targeting of
microRNAs modulating acquired chemotherapy resistance in Triple
negative breast cancer (TNBC): A better strategy to combat
chemoresistance. Med Hypotheses. 96:5–8. 2016.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Tang Q, Ouyang H, He D, Yu C and Tang G:
MicroRNA-based potential diagnostic, prognostic and therapeutic
applications in triple-negative breast cancer. Artif Cells Nanomed
Biotechnol. 47:2800–2809. 2019.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Gupta I, Sareyeldin RM, Al-Hashimi I,
Al-Thawadi HA, Al Farsi H, Vranic S and Al Moustafa AE: Triple
Negative Breast Cancer Profile, from Gene to microRNA, in Relation
to Ethnicity. Cancers (Basel). 11(363)2019.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Xing F, Wang S and Zhou J: The Expression
of MicroRNA-598 Inhibits Ovarian Cancer Cell Proliferation and
Metastasis by Targeting URI. Mol Ther Oncolytics. 12:9–15.
2018.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Wang N, Zhang Y and Liang H: MicroRNA-598
Inhibits Cell Proliferation and Invasion of Glioblastoma by
Directly Targeting Metastasis Associated in Colon Cancer-1 (MACC1).
Oncol Res. 26:1275–1283. 2018.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Li D, Masiero M, Banham AH and Harris AL:
The notch ligand JAGGED1 as a target for anti-tumor therapy. Front
Oncol. 4(254)2014.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Liu Z, Zhang G, Yu W, Gao N and Peng J:
miR-186 inhibits cell proliferation in multiple myeloma by
repressing Jagged1. Biochem Biophys Res Commun. 469:692–697.
2016.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Tang L, Yang B, Cao X, Li Q, Jiang L and
Wang D: MicroRNA-377-3p inhibits growth and invasion through
sponging JAG1 in ovarian cancer. Genes Genomics. 41:919–926.
2019.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Duan K, Ge YC, Zhang XP, Wu SY, Feng JS,
Chen SL, Zhang LI, Yuan ZH and Fu CH: miR-34a inhibits cell
proliferation in prostate cancer by downregulation of SIRT1
expression. Oncol Lett. 10:3223–3227. 2015.PubMed/NCBI View Article : Google Scholar
|