Introduction
Breast cancer is the second leading cause of
cancer-associated mortality after lung cancer in women worldwide
(1). One of the breast cancer
subtypes, triple-negative breast cancer (TNBC), which is
characterized by the lack of estrogen and progesterone receptors
and HER-2 expression, accounts for 15-20% of all breast cancers
(2,3). TNBC typically exhibits aggressive
behaviors, including a high recurrence rate and early metastasis,
resulting in poor prognoses (4,5).
Neoadjuvant chemotherapy (NACT) can facilitate breast conservation,
render inoperable tumors operable and provide important prognostic
information based on the response to therapy (6,7). In
addition, NACT is considered to be a treatment option for patients
with early operable TNBC (6). The
criterion for determining the response to NACT is the tumor
pathological complete response (pCR), which is defined as the
absence of residual cancer in the primary breast tumor and lymph
nodes (8). Achievement of pCR
following NACT is associated with good long-term outcomes (9-11).
Although TNBC is initially sensitive to chemotherapy, it rapidly
develops drug resistance such that only 10-40% of patients can
achieve pCR (12,13). A number of clinical trials have
previously attempted to apply novel agents, such as immunotherapy
and anti-angiogenetic agents to increase the pCR but adverse side
effects have limited their potential application in clinical
practice (14,15). Additionally, not all increases in
pCR lead to an improvement of long-term outcomes (11,16).
Therefore, development of novel specific biomarkers may help to
identify patients who would benefit from NACT.
Glucosylceramide synthase (GCS) is a
glycosyltransferase that transfers a glucose group from uridine
diphospho-glucose to ceramide to produces glucosylceramide
(17,18). GCS expression is upregulated in a
number of multidrug resistance (MDR) cancer cell lines, including
breast cancer (18,19), leukemia (20) and renal cell cancer (21) cell lines. Previous studies have
reported that GCS was associated with MDR to anthracycline drugs in
breast cancer, where GCS expression was upregulated in 30% of
patients with TNBC (22,23). Although there is at present no
standard regimen for NACT, anthracycline and taxanes are commonly
used for NACT treatment (24).
Among a number of factors which can induce drug
resistance, one proposed mechanism is the activation or
inactivation of drug-metabolizing enzymes. The cytochrome P450
(CYP) family is a multigene family of enzymes that has been
implicated in the metabolism of a diverse range of drugs (25). In particular, CYP family 1 subfamily
A1 (CYP1A1) belongs to the CYP family, which is an enzyme that is
involved in the bioactivation of endogenous and environmentally
reactive compounds, such as dimethylbenz(a)anthracene and
heterocyclic amine,
2-amino-1-methyl-6-phenylimidazo[4,5-b]pyridine. In addition,
CYP1A1 can also bind to DNA to mediate carcinogenesis (26). CYP1A1 colocalizes with
P-glycoprotein and contributes to tumor cell chemoresistance by
enhancing the metabolism of numerous drugs (27-30).
Therefore, the present study explored the
association between GCS and CYP1A1 expression in TNBC, by analyzing
their association with clinicopathological parameters, pCR and
disease-free survival (DFS) following NACT. In addition, the
present study also focused on the possibility that GCS is
associated with responses to NACT and that in can be used to
predict prognosis following anthracycline- or taxanes-based NACT
regimen in TNBC.
Materials and methods
Patients
In total, 80 female patients with a median age of 56
years (range, 24-72 years), who met the following inclusion
criteria between January 1, 2012 and February 31, 2014 at
Yuhuangding Hospital Affiliated to Qingdao University (Yantai,
China) were eligible for the present study: i) Age ≥18 years, core
needle biopsy diagnosis of invasive breast cancer and
immunohistochemistry-confirmed estrogen receptor (ER) expression to
be <1% positive, progesterone receptor (PR) expression to be
<1% positive and HER-2 score 0 or 1-2+, if a Her-2 score of 2+
was found, fluorescence in situ hybridization was used to
further test for Her-2 negativity as previously described (31); ii) patients underwent ≥2 cycles of
NACT and were demonstrated to have operable breast cancer (stage
IIA-IIIB); iii) all tumors of the patients were deemed by at least
a CT scan as having one measurable lesion; iv) Eastern Cooperative
Oncology Group score of 0-2(32);
v) normal routine blood tests reporting hemoglobin levels of ≥100
g/l, leukocyte count ≥4x109/l, neutrophil count
≥1.5x109/l, thrombocyte count ≥100x109/l and
liver and kidney functions within ≤1.5X of the normal range; vi) no
previous therapy, including chemotherapy, radiotherapy, endocrine
therapy, immunotherapy or surgery, for breast cancer; and vii) life
expectancy >6 months.
The exclusion criteria were as follows: i) Patients
with active concomitant malignancy; ii) patients with active
infection and serious concomitant diseases, including heart
failure, severe diabetes, liver failure, severe peripheral
neuropathy or severe drug allergy; and iii) pregnant or lactating.
The characteristics of all patients are presented in Table I.
 | Table IClinicopathological characteristics
of patients with triple-negative breast cancer in the present
study. |
Table I
Clinicopathological characteristics
of patients with triple-negative breast cancer in the present
study.
| Clinical
characteristics | Number (%) |
|---|
| Age, years | |
|
<35 | 6 (7.50) |
|
35-60 | 46 (57.50) |
|
>60 | 28 (35.00) |
| Grade | |
|
I | 9 (11.25) |
|
II | 45 (56.25) |
|
III | 26 (32.50) |
| Node | |
|
0 | 25 (31.25) |
|
0-3 | 19 (23.75) |
|
4 | 36 (45.00) |
| Tumor size | |
|
T1-2 | 44 (55.00) |
|
T3-T4 | 36 (45.00) |
| Ki67 | |
|
<14% | 16 (20.00) |
|
≥14% | 64 (80.00) |
| TNM stage | |
|
IIA-B | 29 (36.25) |
|
IIIA-B | 51 (63.75) |
Written informed consent was obtained from all
individual participants included in the present study before
treatment. The present study was conducted in accordance with the
Declaration of Helsinki. The present study was approved by the
Institutional Review Board, Medical Ethics Committee of Yantai
Yuhuangding Hospital (Yantai, China).
NACT treatment
NACT (33-36)
was administered every 21 days. The present study used an AT
regimen (doxorubicin 50 mg/m2 + docetaxel 75
mg/m2 or paclitaxel 175 mg/m2) in the
majority of the cases. An AC regimen (doxorubicin 60
mg/m2 + cyclophosphamide 600 mg/m2), TC
regimen (docetaxel 75 mg/m2 + cyclophosphamide 600
mg/m2) or AC-Follow T regimen (four cycles of
doxorubicin 60 mg/m2 + cyclophosphamide 600
mg/m2, followed by four cycles of docetaxel 75
mg/m2 or paclitaxel 175 mg/m2) was used in
the remaining cases. Most of patients received 6 cycles of
chemotherapy.
Response to NACT assessment
Tumor staging was performed according to the Eighth
Edition of the guidelines of the American Joint Committee on Cancer
(37). The primary objective was to
evaluate the pCR rate, which is defined as no histological evidence
of residual invasive tumor cells in the breast and axillary lymph
nodes (ypT0/TisypN0) (38).
Residual tumors were defined as non-pCR and were classified using a
pathological TNM system (36). The
secondary objective was to evaluate the clinical response rate and
DFS. Clinical tumor response was assessed according to the Response
Evaluation Criteria in Solid Tumors version 1.1(39): i) Complete response (cCR) was
defined as the disappearance of all tumor foci after chemotherapy;
ii) partial response was defined as ≥30% decline in the maximum
tumor diameters; iii) progressive disease was defined as ≥20%
increase in the cumulative measurement of all tumor diameters from
the baseline; and iv) stable disease was confirmed when complete
response, partial response or progressive disease was not noted.
DFS was defined as the time from the date of surgery to the first
observation of tumor recurrence (metastatic recurrence and/or local
relapse) or death. Patients who remained alive without recurrence
and/or metastasis were administratively censored at the last
follow-up date. All patients who received chemotherapy (>one
cycle of each regimen) were evaluated. All patients were followed
up for a median period of 68.8 months (range, 33.0-84.0 months)
after surgery.
Immunohistochemistry
Tumor specimens were obtained from patients included
in this study before NACT and after surgery. All specimens were
fixed with 10% formalin for 6-24 h at room temperature, paraffin
embedded and cut into 4-µm sections. The slides were allowed to dry
overnight at room temperature. To deparaffinize the sections, they
were placed in two containers of xylene at room temperature for 5
min each. To start rehydration, the sections were placed in three
containers of 100% ethanol, 95% ethanol and 85% ethanol at room
temperature for 5 min each. Antigen retrieval was performed in a
microwave oven at 100˚C for 15 min in 10 mM citrate buffer (pH
6.0). For all samples, endogenous peroxidase activity was blocked
for 10 min using a 3% H2O2-methanol solution
at room temperature. The slides were blocked with 10% normal goat
serum (Dako; Agilent Technologies, Inc.) at room temperature for 10
min and incubated with appropriately diluted primary antibodies
overnight at 4˚C. The antibodies against ER (ready-to use, no
dilution; cat. no. 790-4325), PR (ready-to use, no dilution; cat.
no. 790-4296), HER-2 (ready-to use, no dilution; cat. no. 790-4493)
and Ki67 (ready-to use, no dilution; cat. no. 790-4286) were
purchased from Roche Diagnostics. CYP1A1 antibody was purchased
from LifeSpan BioSciences, Inc. (dilution, 1:50; cat. no.
LS-C99804). GCS antibody was purchased from BIOSS (dilution, 1:300;
cat. no. bs-0701R). Subsequently, the slides were probed with a
horseradish peroxidase-labeled polymer conjugated to an appropriate
secondary antibody (DAB + substrate chromogen system; cat. no.
GK600505; Dako; Agilent Technologies, Inc.) for 30 min. The slides
were incubated DAB+ (DAB + substrate chromogen system cat. no.
GK600505; Dako; Agilent Technologies, Inc.) at 25˚C for 5-15 min
until there were yellow to brown granules visible when viewed under
a light microscope. The stained sections were then observed under a
light microscope (magnification, x400; Nikon eclipse 80i; Nikon
Corporation).
A dual semi-quantitative scale combining staining
intensity and percentage of positive cells was used to evaluate GCS
and CYP1A1 protein staining. Each index counted 10 high power
microscopic fields, containing at least 1,000 tumor cells. The
staining intensity of the cell plasma was scored as 0 (negative), 1
(weak), 2 (moderate) or 3 (strong). The percentage of positive
cells was scored as follows: i) 0, no staining or staining in
<5% of tumor cells; ii) 1, staining in 5-25% of cells; iii) 2,
staining in 26-50% of cells; iv) 3, staining in 51-75% of cells;
and v) 4, staining in >75% of cells. The staining intensity and
the percentage were then multiplied to obtain a final score (range,
0-12). For GCS and CYP1A1 expression, cytoplasmic staining was
considered positive with a score >4, or negative with an
immunohistochemical score ≤4 (22,23,40).
Transfection with GCS plasmid
The breast cancer cell lines MDA-MB-453 (ATCC
HTB-131; https://www.atcc.org/products/all/HTB-131.aspx) and
MDA-MB-231 (ATCC HTB-26; https://www.atcc.org/products/all/HTB-26.aspx) were
obtained from the ATCC. The full-length GCS vector pcDNA3.1-GCS was
synthesized and purified by Shanghai GenePharma Co., Ltd. Prior to
transfection, cells were cultured in PRMI-1640 with 10% FBS (Gibco;
Thermo Fisher Scientific, Inc.) and seeded into six-well plates at
the density of 1x106 cells per well and incubated at
37˚C in an atmosphere with 5% CO2 for 12 h. For each
well, 10 µl (2 mg/ml) Lipofectamine® 2000 (Invitrogen;
Thermo Fisher Scientific, Inc.) and 5 µl (1 mg/ml) vector were
diluted into 250 µl RPMI-1640 (Gibco; Thermo Fisher Scientific,
Inc.) culture medium without serum. After incubation for 10 min at
room temperature, the diluted vector and Lipofectamine were mixed
together and incubated for 20 min at 25˚C. The mixture was then
added to the cells. The medium was replaced with 1 ml complete
RPMI-1640 culture medium 6 h later, so that the final concentration
of the plasmid was 5 µg/ml. As negative control, 10 µl
Lipofectamine and 5 µl (1 mg/ml) pcDNA3.1 were also transfected
into the two cell lines. Forty-eighrt hours after the transfection,
the subsequent experiments were performed.
RNA extraction and quantitative PCR
(qPCR)
Total RNA from the cell lines was isolated using
TRIzol® reagent (Invitrogen; Thermo Fisher Scientific,
Inc.) according to manufacturer's protocol. Reverse transcription
was performed using a Toyobo First Strand cDNA Synthesis kit (cat.
no. FSQ-201; Toyobo Life Science). A total of 1 µl RNA was added
into 10 µl reaction solutions according to the manufacturer's
protocol and the reaction conditions were as follows: 37˚C for 15
min and 95˚C for 5 min. qPCR was performed using a SYBR®
Green Real-Time PCR Master Mix (Toyobo Life Science). The primers
for GCS were forward, 5'-CCTTTCCTCTCCCCACCTTCCTCT-3' and reverse,
5'-GGTTTCAGAAGAGAGACACCTGGG-3' (41). The primers for CYP1A1 were forward,
5'-CTCAGCTCAGTACCTCAGCCAC-3' and reverse,
5'-CCCCATACTGCTGGCTCATC-3'. The primers for the β-actin were
forward, 5'-ACCCCCACTGAAAAAGATGA-3' and reverse,
5'-ATCTTCAAACCTCCATGATG-3', which was used as an internal control.
The final volume was 25 µl and an iCycler iQ Real-Time PCR
Detection System (Bio-Rad Laboratories, Inc.) was used for qPCR.
The thermocycling conditions for the qPCR reaction were as follows:
Initial denaturation for 5 min at 94˚C; followed by 35 cycles of
denaturation for 30 sec at 94˚C, primer annealing for 30 sec at
60˚C and polymerization for 30 sec at 72˚C; and a final extension
for 10 min at 72˚C. The relative mRNA expressions were calculated
using the 2-ΔΔCq method (42).
Statistical analysis
Data were analyzed using the SPSS software (version
18.0; SPSS, Inc.). χ2 test was used in the table
presented in Tables I and II. In Table
III, P1 represented a comparison between the
GCS+CYP1A1- and
GCS+CYP1A1+ group; P2 represented a
comparison between the GCS-CYP1A1+ and
GCS+CYP1A1+ group; and P3 represented a
comparison between the GCS-CYP1A1- and
GCS+CYP1A1+ group. Spearman's rank
correlation coefficient was used to analyze the correlation between
the immunohistochemical scores of GCS and CYP1A1. DFS curves were
generated according to the Kaplan-Meier method and the survival
between groups was compared using log-rank test. P-values were
adjusted for multiple comparisons using the Bonferroni correction.
For the multiple comparisons of DFS rate, P<0.017 (0.05/3) was
defined as statistically significant. For other tests, P<0.05
was considered to indicate a statistically significant difference.
All P-values were the results of two-sided tests.
 | Table IIAssociation between GCS or CYP1A1
expression and clinical features. |
Table II
Association between GCS or CYP1A1
expression and clinical features.
| Clinical
characteristics | GCS+, n
(%) | GCS-, n
(%) | P-value | CYP1A1+,
n (%) | CYP1A1-,
n (%) | P-value |
|---|
| Age, years |
|
<35 | 4 (5.00) | 2 (2.50) | 0.137 | 3 (3.75) | 3 (3.75) | 0.284 |
|
>35 | 21 (26.25) | 53 (66.25) | | 16 (20.00) | 58 (72.50) | |
| Grade |
|
I | 2 (2.50) | 7 (8.75) | 0.811 | 3 (3.75) | 6 (7.50) | 0.763 |
|
II-III | 23 (28.75) | 48 (60.00) | | 16 (20.00) | 55 (68.75) | |
| Node |
|
N0 | 5 (6.25) | 20 (25.00) | 0.143 | 2 (2.50) | 23 (28.75) | 0.026 |
|
N1-N3 | 20 (25.00) | 35 (43.75) | | 17 (21.25) | 38 (47.50) | |
| Tumor size |
|
T1-T2 | 9 (11.25) | 35 (43.75) | 0.021 | 7 (8.75) | 37 (46.25) | 0.068 |
|
T3-T4 | 16 (20.00) | 20 (25.00) | | 12 (15.00) | 24 (30.00) | |
| Ki67 |
|
<14% | 7 (8.75) | 9 (11.25) | 0.228 | 6 (7.50) | 10 (12.50) | 0.264 |
|
≥14% | 18 (22.50) | 46 (57.50) | | 13 (16.25) | 51 (63.75) | |
| TNM stage |
|
IIA-IIB | 5 (6.25) | 24 (30.00) | 0.042 | 3 (3.75) | 26 (32.50) | 0.034 |
|
IIIA-IIIB | 20 (25.00) | 31 (38.75) | | 16 (20.00) | 35 (43.75) | |
| NACT |
|
Before | 25 (31.25) | 55 (68.75) | 0.024 | 19 (23.75) | 61 (76.25) | 0.027 |
|
After | 39 (48.75) | 41 (51.25) | | 32 (40.00) | 48 (60.00) | |
| pCR |
|
Yes | 9 (11.25) | 15 (18.75) | 0.188 | 6 (7.50) | 18 (22.50) | 0.073 |
|
No | 30 (37.50) | 26 (32.50) | | 26 (32.50) | 30 (37.50) | |
| cCR |
|
Yes | 11 (13.75) | 22 (27.50) | 0.021 | 8 (10.00) | 25 (31.25) | 0.016 |
|
No | 28 (35.00) | 19 (23.75) | | 24 (30.00) | 23 (28.75) | |
 | Table IIIAssociation between GCS and CYP1A1
co-expression and pCR. |
Table III
Association between GCS and CYP1A1
co-expression and pCR.
| Outcome |
GCS+CYP1A1+, n
(%) |
GCS+CYP1A1-, n
(%) | P1-value |
GCS-CYP1A1+, n
(%) | P2-value |
GCS-CYP1A1-, n
(%) | P3-value |
|---|
| pCR | 2 (2.50) | 7 (8.75) | 0.127 | 3 (3.75) | 0.374 | 11 (13.75) | 0.031 |
| Non-pCR | 17 (21.25) | 13 (16.25) | | 10 (12.50) | | 17 (21.25) | |
Results
Association between GCS and CYP1A1 and
the clinicopathologic parameters in TNBC
Cell experiments were performed to assess the
potential association between GCS and CYP1A1 (Fig. S1). A total of 80 patients with TNBC
who had undertaken NACT were enrolled into the present study.
Clinical and pathological TNM classifications of patients were
evaluated according to the Eighth Edition American Joint Committee
on Cancer Staging Criteria (37).
Immunohistochemical staining was performed to detect the expression
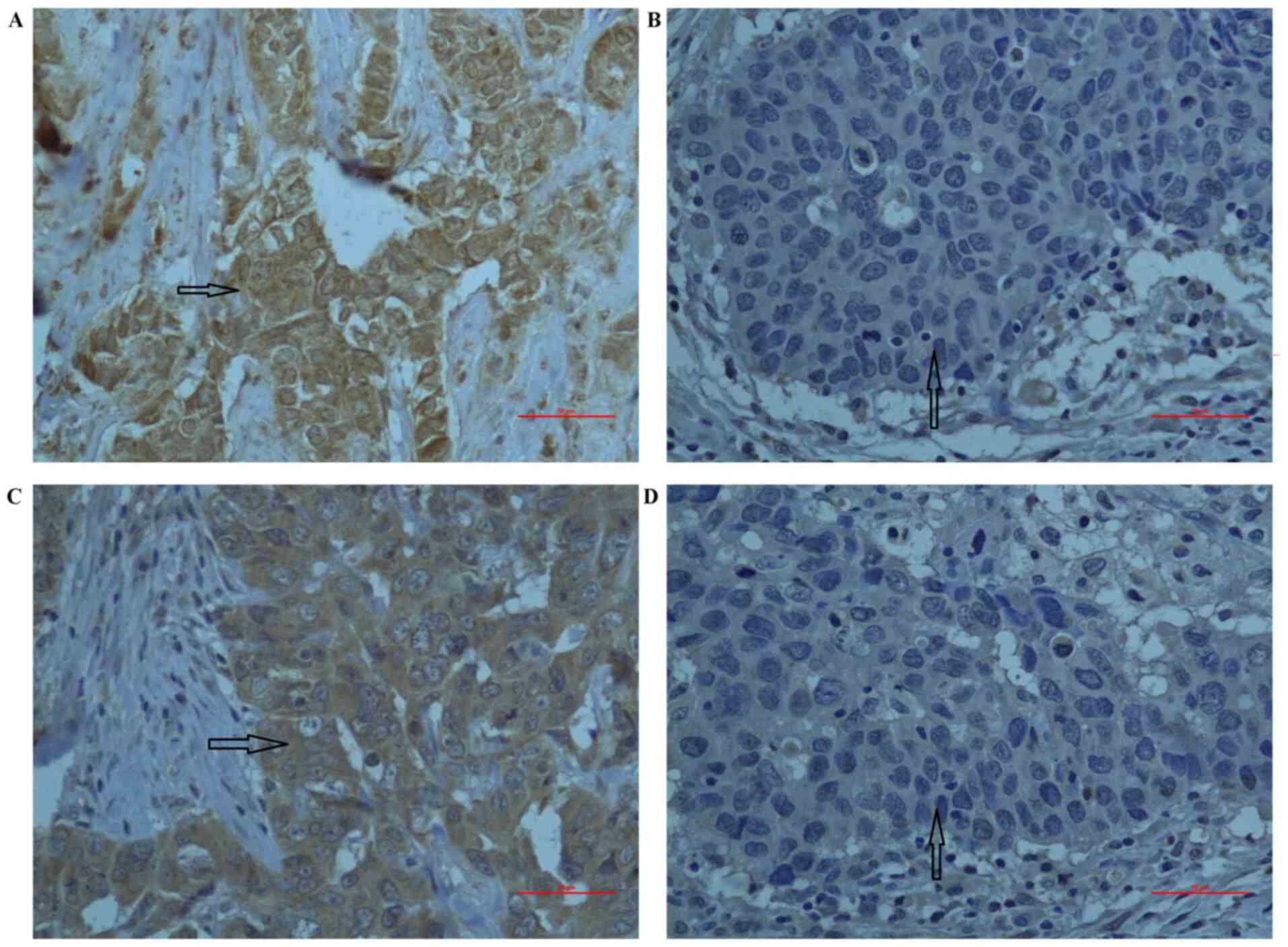
levels of GCS and CYP1A1. GCS and CYP1A1 staining were mainly
observed in the cytoplasm of cancer cells (Fig. 1). The association between GCS or
CYP1A1 expression and each of the clinicopathological parameters
were subsequently analyzed. The expression levels of GCS were found
to be associated with tumor size (P=0.021) and TNM stage (P=0.042).
In addition, the expression levels of CYP1A1 were associated with
lymph node metastasis (P=0.026) and TNM stage (P=0.034). No other
clinicopathologic parameters were associated with GCS or CYP1A1
expression (Table II).
Association between GCS and CYP1A1
expression and NACT in TNBC
The present study also measured the expression
levels of GCS and CYP1A1 before NACT and after surgery using
immunohistochemistry. The positive expression of GCS was increased
from 31.25 to 48.75% after NACT. The positive expression of CYP1A1
was also increased from 23.75 to 40.0% after NACT. Upregulation of
both GCS (P=0.024) and CYP1A1 (P=0.027) expression were found to be
associated with NACT (Table II).
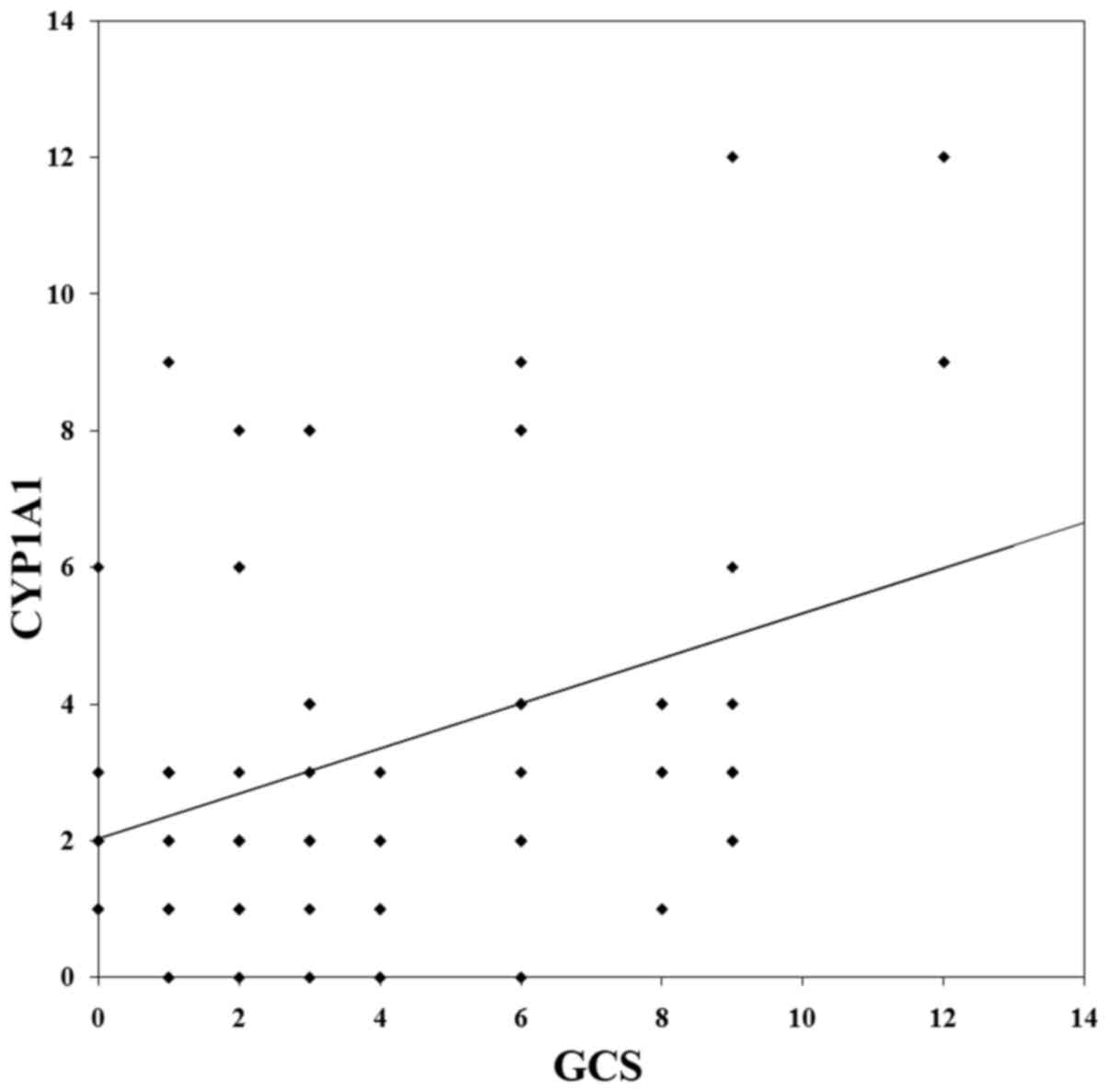
Furthermore, Spearman's rank correlation analysis revealed that
there was a significant but weak correlation between GCS and CYP1A1
expression in the TNBC tissues (P=0.003; r=0.327; Fig. 2).
Association of GCS and CYP1A1
expression with the pathological response to NACT in TNBC
pCR was defined as no histological evidence of
residual invasive tumor cells in the breast and axillary lymph
nodes (ypT0/TisypN0). Due to the upregulation of GCS and CYP1A1
expression, the present study next analyzed the possible
association between the expression levels of GCS or CYP1A1 and pCR.
There was no difference in pCR in the GCS+ (P=0.188) or
CYP1A1+ group (P=0.073) compared with that in the
GCS- or CYP1A1- group (Table II).
cCR was defined as the disappearance of all tumor
foci after chemotherapy. cCR rate in the GCS+ group was
28.20% (11/39), which was lower compared with 53.7% (22/41) in the
GCS- group (P=0.021). The cCR rate in the
CYP1A1+ group was 25.0% (8/32), which was lower compared
with 52.1% (25/48) in the CYP1A1- group (P=0.016;
Table II).
Among the 80 cases of TNBC, 19 cases were
GCS+CYP1A1+, 20 cases were
GCS+CYP1A1-, 13 cases were
GCS-CYP1A1+ and 28 cases were
GCS-CYP1A1-. Compared with that in the
GCS+CYP1A1+ group, incidences of pCR was
increased in the GCS-CYP1A1- group (P=0.031).
However, no significant association was observed between the
incidences of pCR between the GCS+CYP1A1+ and
the GCS+CYP1A1- or
GCS-CYP1A1+ groups (Table III).
Association of GCS and CYP1A1
expression with the prognosis following NACT in TNBC
The association between GCS or CYP1A1 and DFS was
subsequently analyzed by Kaplan-Meier survival analysis. There was
no difference between the DFS of patients in the GCS+
(P=0.301; Fig. 3A) or
CYP1A1+ (P=0.099; Fig.
3B) groups and their corresponding negative groups. DFS of
patients in the GCS-CYP1A1- group was
compared with the other three groups. No statistically significant
difference was observed between the DFS of
GCS-CYP1A1+ and
GCS-CYP1A1- group (61.5 vs. 65.4%; P=0.497).
Similar result was observed between the DFS of
GCS+CYP1A1- and
GCS-CYP1A1- group (65.0 vs. 65.4%; P=0.734).
The DFS of patients in the GCS+CYP1A1+ group
exhibited markedly worse DFS rate compared with that in the
GCS-CYP1A1- group (57.9 vs. 65.4%; P=0.049).
However, after the significance threshold was corrected using
Bonferroni correction, there was no statistical significance
between the two groups (Fig.
3C).
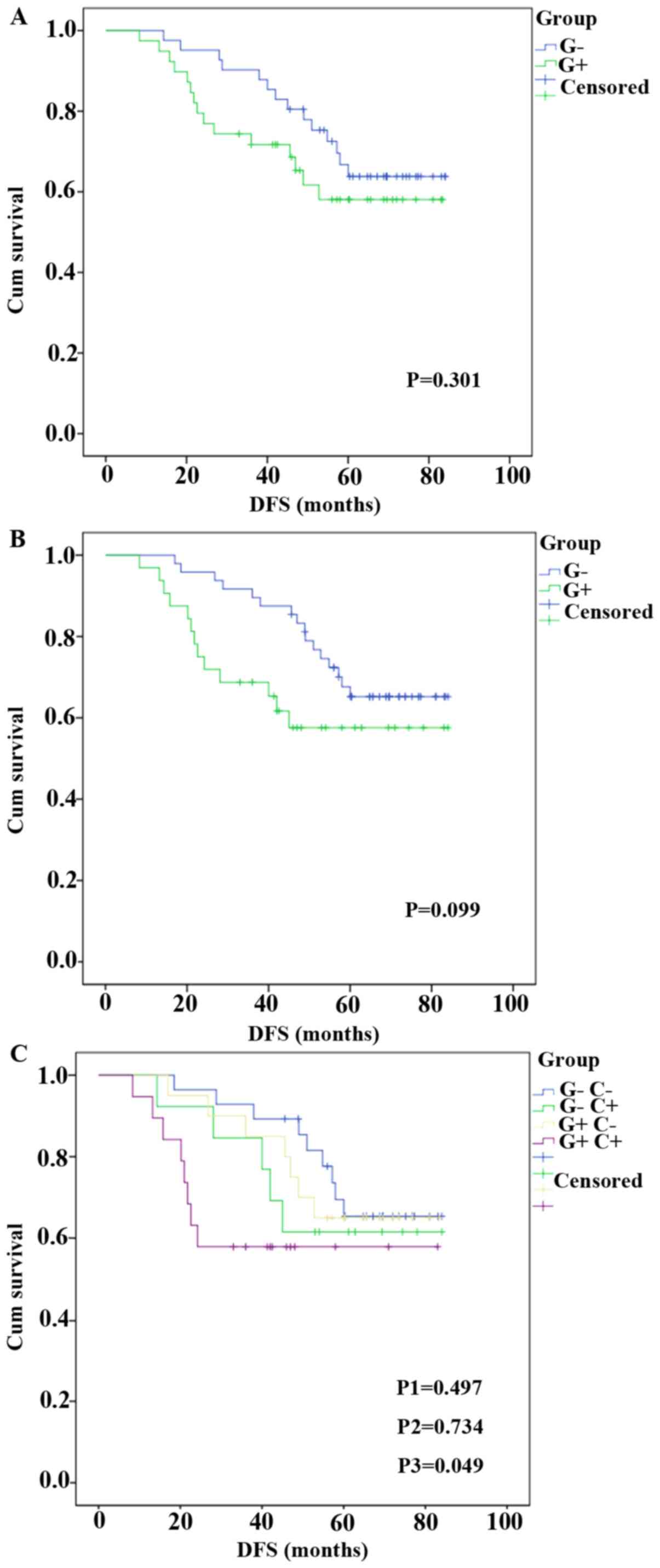 | Figure 3Association between GCS or CYP1A1
expression and DFS. DFS of patients in the (A) GCS+ and
GCS- groups, (B) CYP1A1+ and
CYP1A1- groups, and (C) the
GCS-CYP1A1-,
GCS+CYP1A1+,
GCS-CYP1A1+ and
GCS+CYP1A1- groups. After adjustment using
Bonferroni's correction, P<0.017 (0.05/3) was defined as
statistically significant. P1=0.497,
GCS-CYP1A1+ vs.
GCS-CYP1A1-. P2=0.734,
GCS+CYP1A1- vs.
GCS-CYP1A1-. P3=0.049,
GCS+CYP1A1+ vs. GCS-CYP1A1-.
CYP1A1, cytochrome P450 family 1 subfamily A1; DFS, disease-free
survival; GCS, glucosylceramide synthase. |
Discussion
TNBC represents a heterogeneous group of tumors
based on gene expression profiling (43). Results from the Capecitabine for
Residual Cancer as Adjuvant Therapy (44) and KATERINE (45) clinical trials demonstrated that NACT
has become the preferred treatment strategy for patients with TNBC
and HER-2-positive breast cancer in clinical practice. Numerous
studies have previously highlighted the prognostic significance of
pathological complete response (pCR) (9-11).
A number of drugs, including as poly(ADP-ribose) polymerase
inhibitors (46), vascular
endothelial growth factor inhibitors (47) and immune checkpoint inhibitors
(48,49), have been applied in clinical trials
to improve the pCR rate in breast cancer. Compared with that in
other breast cancer subtypes, TNBC has a relatively high
possibility of achieving pCR. However, this advantage could not be
clearly translated into improved DFS or overall survival (OS) due
to poor outcomes in the non-pCR groups (50,51).
Therefore, early identification of sensitive responders could
provide definitive value for decision making with regards to the
type of therapy for patients with TNBC. There is no universally
approved marker for the prediction of the response to NACT
(52). Therefore, the introduction
of novel biomarkers could expand the repertoire of currently
available clinical options and to accurately predict the response
to NACT for patients with TNBC.
Anthracycline-taxanes are commonly used in clinical
practice due to the lack of a standard treatment regimen for NACT
(24). However, development of drug
resistance to the available treatments is the primary barrier to
TNBC treatment with NACT (53).
Both GCS and CYP1A1 expression levels are associated with
P-glycoprotein expression and upregulate multidrug resistance
protein 1 expression during the regulation of breast cancer drug
resistance via β-catenin signaling (40,54-56).
In a previous study, the expression levels of GCS were found to be
associated with ER-positive (P=0.017) and HER-2-negative (P=0.007)
invasive breast cancer (23). GCS
is more highly expressed in younger patients (<35 years)
(23). However, the present study
did not identify an association between age and GCS upregulation in
patients with TNBC, although GCS upregulation was associated with
tumor size and TNM staging. These differences may be due to the
heterogeneity of TNBC. Zhang et al (22) previously reported that GCS
expression was upregulated after NACT in ER-positive invasive
breast cancer. The present study also detected a change in GCS
expression in TNBC after NACT, the upregulation of which was
associated with NACT in TNBC. CYP encodes enzymes involved in the
metabolism of pharmacological agents (57). By activating or inactivating
carcinogens and anticancer drugs, CYP serves an important role for
the study of cancer and cancer treatments (58). Due to the overlapping substrate
specificity between CYP and P-glycoprotein, numerous drug
interactions can involve both P-glycoprotein and CYP (59). CYP1A1 is one of the most important
isoforms responsible for the metabolic activation of
pre-carcinogens (60). In the
present study, the expression levels of CYP1A1 were associated with
lymph node metastasis and TNM staging. This was consistent with the
results of Wang and Wang (61).
Another report previously revealed that the expression levels of
CYP1A1 are associated with age or tumor grade in breast cancer
(62). The present study did not
identify a relationship between CYP1A1 and these two parameters,
but it did reveal that the upregulation of CYP1A1 were associated
with NACT. This suggested that the upregulation of GCS and CYP1A1
may be the underlying reason for NACT resistance in patients with
TNBC. The mechanisms of GCS- and CYP1A1-induced chemoresistance to
NACT in TNBC need to be confirmed by further in vitro
experiments.
It is of interest to explore whether these two
biomarkers are associated with each other and can provide useful
prognosis information for patients with TNBC after undergoing NACT.
Therefore, the association between GCS and CYP1A1 expression was
next detected. The results revealed that they were positively
correlated. pCR in the primary tumor following NACT is a strong
predictor of freedom from recurrence and long-term survival
(38). cCR is the disappearance of
all lesions with nodes measuring <10 mm and normal tumor
markers. Measuring residual disease after neoadjuvant chemotherapy
could improve the prognostic information that can be obtained from
evaluating the pathologic complete response (pCR) (63). A number of studies have previously
reported factors that affect the prognosis of patients with TNBC
following NACT, including C-X-C motif chemokine ligand 8-C-X-C
motif chemokine receptor 1/2(64),
pre-treatment neutrophil-lymphocyte ratio (65) and tumor-infiltrating lymphocytes
(66). However, it remains to be
difficult to introduce a biomarker into daily clinical use due to
the lack of consistent evidence of clinical significance and
operational barriers to clinical implementation (62,64,65).
In the present study, both GCS and CYP1A1 were associated with cCR.
However, neither GCS nor CYP1A1 upregulation was associated with
pCR or DFS. When these two biomarkers were analyzed together, the
results revealed that combined GCS and CYP1A1 upregulation was
associated with pCR (P=0.031). The results also revealed a trend
that patients in the GCS+CYP1A1+ group had a
worse DFS rate, even if there was no statistically significance.
The combination of these two biomarkers could also predict the
prognosis of patients with TNBC undergoing NACT. If successful, it
may differentiate patients who are at high risk of recurrence and
need further therapy from patients with low risk of recurrence,
avoiding the unnecessary long-term toxicity of chemotherapy. This
will be of importance in the clinical setting in the future.
The present study has several limitations. The
sample size was small and the follow-up time was relatively short,
such that there was no statistical significance between the DFS
rate of the GCS+CYP1A1+ group and the
GCS-CYP1A1- group. The present study was a
retrospective single-center study. In addition, different NACT
regimens, which may influence the pCR and DFS (67), were not evaluated. The follow-up
time should be longer and the OS requires further analysis. Whether
the β-catenin signaling pathway induced GCS and CYP1A1 expression
after NACT needs to be explored in further in vitro
experiments. Overall, future prospective studies with a large
sample size and sufficient follow-up times are required to verify
the results found in the present study.
In conclusion, the present study provided evidence
that both GCS and CYP1A1 expression are important in patients with
TNBC treated with NACT. Furthermore, they may help in classifying
TNBC into subtypes with different responses to chemotherapy.
Increased GCS and CYP1A1 expression after NACT could indicate a
poor prognosis in patients with TNBC.
Supplementary Material
Transfection of MDA-MB-453 and
MDA-MB-231 cell with vector or GCS plasmids. Quantitative PCR was
used to detect the expression GCS and CYP1A1 mRNA.
*P<0.05. CYP1A1, cytochrome P450 family 1 subfamily
A1; GCS, glucosylceramide synthase.
Acknowledgements
Not applicable.
Funding
The present study was supported by the Yantai
Science and Technology Project (grant no. 2018SFGY111).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
JL and SW designed the study and analyzed the data
and drafted the manuscript. CW performed the IHC. XK collected the
clinical data. PS conceived and designed the study. All authors
read and approved the final manuscript.
Ethics approval and consent to
participate
Written informed consent was obtained from all
individual participants included in the present study before
treatment. The present study was conducted in accordance with the
Declaration of Helsinki. The present study was approved by the
Institutional Review Board, Medical Ethics Committee of Yantai
Yuhuangding Hospital (Yantai, China).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Allemani C, Weir HK, Carreira H, Harewood
R, Spika D, Wang XS, Bannon F, Ahn JV, Johnson CJ, Bonaventure A,
et al: Global surveillance of cancer survival 1995-2009: Analysis
of individual data for 25,676,887 patients from 279
population-based registries in 67 countries (CONCORD-2). Lancet.
385:977–1010. 2015.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Pareja F and Reis-Filho JS:
Triple-negative breast cancers-a panoply of cancer types. Nat Rev
Clin Oncol. 15:347–348. 2018.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Wolff AC, Hammond MEH, Allison KH, Harvey
BE, Mangu PB, Bartlett JMS, Bilous M, Ellis IO, Fitzgibbons P,
Hanna W, et al: Human epidermal growth factor receptor 2 testing in
breast cancer: American society of clinical oncology/college of
american pathologists clinical practice guideline focused update. J
Clin Oncol. 36:2105–2122. 2018.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Keenan TE and Tolaney SM: Role of
immunotherapy in triple-negative breast cancer. J Natl Compr Canc
Netw. 18:479–489. 2020.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Garrido-Castro AC, Lin NU and Polyak K:
Insights into molecular classifications of triple-negative breast
cancer: Improving patient selection for treatment. Cancer Discov.
9:176–198. 2019.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Chaudhary LN, Wilkinson KH and Kong A:
Triple-negative breast cancer: Who should receive neoadjuvant
chemotherapy? Surg Oncol Clin N Am. 27:141–153. 2018.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Lebert JM, Lester R, Powell E, Seal M and
McCarthy J: Advances in the systemic treatment of triple-negative
breast cancer. Curr Oncol. 25 (Suppl 1):S142–S150. 2018.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Asaoka M, Narui K, Suganuma N, Chishima T,
Yamada A, Sugae S, Kawai S, Uenaka N, Teraoka S, Miyahara K, et al:
Clinical and pathological predictors of recurrence in breast cancer
patients achieving pathological complete response to neoadjuvant
chemotherapy. Eur J Surg Oncol. 45:2289–2294. 2019.PubMed/NCBI View Article : Google Scholar
|
|
9
|
De Mattos-Arruda L, Shen R, Reis-Filho JS
and Cortés J: Translating neoadjuvant therapy into survival
benefits: One size does not fit all. Nat Rev Clin Oncol.
13:566–579. 2016.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Walsh EM, Shalaby A, O'Loughlin M, Keane
N, Webber MJ, Kerin MJ, Keane MM, Glynn SA and Callagy GM: Outcome
for triple negative breast cancer in a retrospective cohort with an
emphasis on response to platinum-based neoadjuvant therapy. Breast
Cancer Res Treat. 174:1–13. 2019.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Telli ML, Timms KM, Reid J, Hennessy B,
Mills GB, Jensen KC, Szallasi Z, Barry WT, Winer EP, Tung NM, et
al: Homologous recombination deficiency (HRD) score predicts
response to platinum-containing neoadjuvant chemotherapy in
patients with triple-negative breast cancer. Clin Cancer Res.
22:3764–3773. 2016.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Ryspayeva D, Lyashenko A, Dosenko I,
Kostryba O, Koshyk O, Krotevych M and Smolanka I: Predictive
factors of pathological response to neoadjuvant chemotherapy in
patients with breast cancer. J BUON. 25:168–175. 2020.PubMed/NCBI
|
|
13
|
von Minckwitz G, Untch M, Blohmer JU,
Costa SD, Eidtmann H, Fasching PA, Gerber B, Eiermann W, Hilfrich
J, Huober J, et al: Definition and impact of pathologic complete
response on prognosis after neoadjuvant chemotherapy in various
intrinsic breast cancer subtypes. J Clin Oncol. 30:1796–1804.
2012.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Miyashita M and Ishida T: Prospect of
immunotherapy in neoadjuvant/adjuvant treatment for early breast
cancer. Chin Clin Oncol. 9(28)2020.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Loibl S, Weber KE, Timms KM, Elkin EP,
Hahnen E, Fasching PA, Lederer B, Denkert C, Schneeweiss A, Braun
S, et al: Survival analysis of carboplatin added to an
anthracycline/taxane-based neoadjuvant chemotherapy and HRD score
as predictor of response-final results from GeparSixto. Ann Oncol.
29:2341–2347. 2018.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Mohammed AA, Elsayed FM, Algazar M, Rashed
HE and Anter AH: Neoadjuvant chemotherapy in triple negative breast
cancer: Correlation between androgen receptor expression and
pathological response. Asian Pac J Cancer Prev. 21:563–568.
2020.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Xu HB, Xu LZ, Li L, Fu J and Mao XP:
Reversion of P-glycoprotein-mediated multidrug resistance by
guggulsterone in multidrug-resistant human cancer cell lines. Eur J
Pharmacol. 694:39–44. 2012.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Liu YY, Patwardhan GA, Xie P, Gu X,
Giuliano AE and Cabot MC: Glucosylceramide synthase, a factor in
modulating drug resistance, is overexpressed in metastatic breast
carcinoma. Int J Oncol. 39:425–431. 2011.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Sun Y, Zhang T, Gao P, Meng B, Gao Y, Wang
X, Zhang J, Wang H, Wu X, Zheng W and Zhou G: Targeting
glucosylceramide synthase downregulates expression of the multidrug
resistance gene MDR1 and sensitizes breast carcinoma cells to
anticancer drugs. Breast Cancer Res Treat. 121:591–599.
2010.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Itoh M, Kitano T, Watanabe M, Kondo T,
Yabu T, Taguchi Y, Iwai K, Tashima M, Uchiyama T and Okazaki T:
Possible role of ceramide as an indicator of chemoresistance:
Decrease of the ceramide content via activation of glucosylceramide
synthase and sphingomyelin synthase in chemoresistant leukemia.
Clin Cancer Res. 9:415–423. 2003.PubMed/NCBI
|
|
21
|
Boojar MMA, Boojar MMA, Golmohammad S and
Bahrehbar I: Data on cell survival, apoptosis, ceramide metabolism
and oxidative stress in A-494 renal cell carcinoma cell line
treated with hesperetin and hesperetin-7-O-acetate. Data Brief.
20:596–601. 2018.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Zhang X, Wu X, Su P, Gao Y, Meng B, Sun Y,
Li L, Zhou Z and Zhou G: Doxorubicin influences the expression of
glucosylceramide synthase in invasive ductal breast cancer. PLoS
One. 7(e48492)2012.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Liu J, Sun P and Sun Y, Liu A, You D,
Jiang F and Sun Y: Expression of glucosylceramide synthase in
invasive ductal breast cancer may be correlated with high estrogen
receptor status and low HER-2 status. Diagn Pathol.
9(22)2014.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Harbeck N and Gluz O: Neoadjuvant therapy
for triple negative and HER2-positive early breast cancer. Breast.
34 (Suppl 1):S99–S103. 2017.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Michael M and Doherty MM: Tumoral drug
metabolism: Overview and its implications for cancer therapy. J
Clin Oncol. 23:205–229. 2005.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Runge D, Köhler C, Kostrubsky VE, Jäger D,
Lehmann T, Runge DM, May U, Stolz DB, Strom SC, Fleig WE and
Michalopoulos GK: Induction of cytochrome P450 (CYP)1A1, CYP1A2,
and CYP3A4 but not of CYP2C9, CYP2C19, multidrug resistance (MDR-1)
and multidrug resistance associated protein (MRP-1) by prototypical
inducers in human hepatocytes. Biochem Biophys Res Commun.
273:333–341. 2000.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Yan YE, Wang H and Feng YH: Alterations of
placental cytochrome P450 1A1 and P-glycoprotein in tobacco-induced
intrauterine growth retardation in rats. Acta Pharmacol Sin.
26:1387–1394. 2005.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Zhang J, Song J, Liang X, Yin Y, Zuo T,
Chen D and Shen Q: Hyaluronic acid-modified cationic nanoparticles
overcome enzyme CYP1B1-mediated breast cancer multidrug resistance.
Nanomedicine (Lond). 14:447–464. 2019.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Sorf A, Hofman J, Kučera R, Staud F and
Ceckova M: Ribociclib shows potential for pharmacokinetic drug-drug
interactions being a substrate of ABCB1 and potent inhibitor of
ABCB1, ABCG2 and CYP450 isoforms in vitro. Biochem Pharmacol.
154:10–17. 2018.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Chen Y, Huang W, Chen F, Hu G, Li F, Li J
and Xuan A: Pregnane X receptors regulate CYP2C8 and P-glycoprotein
to impact on the resistance of NSCLC cells to Taxol. Cancer Med.
5:3564–3571. 2016.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Yu KD, Liu GY, Zhou XY, Zhou Y, Wu J, Chen
CM, Shen ZZ and Shao ZM: Association of HER-2 copy number and
HER-2/CEP-17 ratio with neoadjuvant taxane-containing chemotherapy
sensitivity in locally advanced breast cancer. Oncologist.
17:792–800. 2012.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Azam F, Latif MF, Farooq A, Tirmazy SH,
AlShahrani S, Bashir S and Bukhari N: Performance status assessment
by using ECOG (Eastern cooperative oncology group) score for cancer
patients by oncology healthcare professionals. Case Rep Oncol.
12:728–736. 2019.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Zaheed M, Wilcken N, Willson ML, O'Connell
DL and Goodwin A: Sequencing of anthracyclines and taxanes in
neoadjuvant and adjuvant therapy for early breast cancer. Cochrane
Database Syst Rev. 2(Cd012873)2019.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Rastogi P, Anderson SJ, Bear HD, Geyer CE,
Kahlenberg MS, Robidoux A, Margolese RG, Hoehn JL, Vogel VG, Dakhil
SR, et al: Preoperative chemotherapy: Updates of national surgical
adjuvant breast and bowel project protocols B-18 and B-27. J Clin
Oncol. 26:778–785. 2008.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Nakatsukasa K, Koyama H, Oouchi Y,
Imanishi S, Mizuta N, Sakaguchi K, Fujita Y, Fujiwara I, Kotani T,
Matsuda T, et al: Docetaxel and cyclophosphamide as neoadjuvant
chemotherapy in HER2-negative primary breast cancer. Breast Cancer.
24:63–68. 2017.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Schneeweiss A, Möbus V, Tesch H, Hanusch
C, Denkert C, Lübbe K, Huober J, Klare P, Kümmel S, Untch M, et al:
Intense dose-dense epirubicin, paclitaxel, cyclophosphamide versus
weekly paclitaxel, liposomal doxorubicin (plus carboplatin in
triple-negative breast cancer) for neoadjuvant treatment of
high-risk early breast cancer (GeparOcto-GBG 84): A randomised
phase III trial. Eur J Cancer. 106:181–192. 2019.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Jang N, Choi JE, Kang SH and Bae YK:
Validation of the pathological prognostic staging system proposed
in the revised eighth edition of the AJCC staging manual in
different molecular subtypes of breast cancer. Virchows Arch.
474:193–200. 2019.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Cortazar P and Geyer CE Jr: Pathological
complete response in neoadjuvant treatment of breast cancer. Ann
Surg Oncol. 22:1441–1446. 2015.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Ghobrial FEI, Eldin MS, Razek AAKA, Atwan
NI and Shamaa SSA: Computed tomography assessment of hepatic
metastases of breast cancer with revised response evaluation
criteria in solid tumors (RECIST) criteria (version 1.1):
Inter-observer agreement. Pol J Radiol. 82:593–597. 2017.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Al-Dhfyan A, Alhoshani A and Korashy HM:
Aryl hydrocarbon receptor/cytochrome P450 1A1 pathway mediates
breast cancer stem cells expansion through PTEN inhibition and
beta-catenin and Akt activation. Mol Cancer. 16(14)2017.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Liu J, Zhang X, Liu A, Zhang D, Su Y, Liu
Y, You D, Yuan L, Kong X, Wang X and Sun P: Altered methylation of
glucosylceramide synthase promoter regulates its expression and
associates with acquired multidrug resistance in invasive ductal
breast cancer. Oncotarget. 7:36755–36766. 2016.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408.
2001.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Yam C, Mani SA and Moulder SL: Targeting
the molecular subtypes of triple negative breast cancer:
Understanding the diversity to progress the field. Oncologist.
22:1086–1093. 2017.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Ozaki A, Takita M and Tanimoto T: A call
for improved transparency in financial aspects of clinical trials:
A case study of the CREATE-X trial in the New England journal of
medicine. invest New Drugs. 36:517–522. 2018.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Pusztai L, Foldi J, Dhawan A, DiGiovanna
MP and Mamounas EP: Changing frameworks in treatment sequencing of
triple-negative and HER2-positive, early-stage breast cancers.
Lancet Oncol. 20:e390–e396. 2019.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Diana A, Carlino F, Franzese E,
Oikonomidou O, Criscitiello C, De Vita F, Ciardiello F and Orditura
M: Early triple negative breast cancer: Conventional treatment and
emerging therapeutic landscapes. Cancers (Basel).
12(819)2020.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Barton MK: Bevacizumab in neoadjuvant
chemotherapy increases the pathological complete response rate in
patients with triple-negative breast cancer. CA Cancer J Clin.
64:155–156. 2014.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Schmid P, Salgado R, Park YH,
Muñoz-Couselo E, Kim SB, Sohn J, Im SA, Foukakis T, Kuemmel S, Dent
R, et al: Pembrolizumab plus chemotherapy as neoadjuvant treatment
of high-risk, early-stage triple-negative breast cancer: Results
from the phase 1b open-label, multicohort KEYNOTE-173 study. Ann
Oncol. 31:569–581. 2020.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Garufi G, Palazzo A, Paris I, Orlandi A,
Cassano A, Tortora G, Scambia G, Bria E and Carbognin L:
Neoadjuvant therapy for triple-negative breast cancer: Potential
predictive biomarkers of activity and efficacy of platinum
chemotherapy, PARP- and immune-checkpoint-inhibitors. Expert Opin
Pharmacother. 21:687–699. 2020.PubMed/NCBI View Article : Google Scholar
|
|
50
|
Wang RX, Chen S, Jin X and Shao ZM: Value
of Ki-67 expression in triple-negative breast cancer before and
after neoadjuvant chemotherapy with weekly paclitaxel plus
carboplatin. Sci Rep. 6(30091)2016.PubMed/NCBI View Article : Google Scholar
|
|
51
|
Carey LA, Dees EC, Sawyer L, Gatti L,
Moore DT, Collichio F, Ollila DW, Sartor CI, Graham ML and Perou
CM: The triple negative paradox: Primary tumor chemosensitivity of
breast cancer subtypes. Clin Cancer Res. 13:2329–2334.
2007.PubMed/NCBI View Article : Google Scholar
|
|
52
|
Sano H, Wada S, Eguchi H, Osaki A, Saeki T
and Nishiyama M: Quantitative prediction of tumor response to
neoadjuvant chemotherapy in breast cancer: Novel marker genes and
prediction model using the expression levels. Breast Cancer.
19:37–45. 2012.PubMed/NCBI View Article : Google Scholar
|
|
53
|
Nøhr-Nielsen A, Bagger SO, Brünner N,
Stenvang J and Lund TM: Pharmacodynamic modelling reveals
synergistic interaction between docetaxel and SCO-101 in a
docetaxel-resistant triple negative breast cancer cell line. Eur J
Pharm Sci. 148(105315)2020.PubMed/NCBI View Article : Google Scholar
|
|
54
|
Zhang X, Li J, Qiu Z, Gao P, Wu X and Zhou
G: Co-suppression of MDR1 (multidrug resistance 1) and GCS
(glucosylceramide synthase) restores sensitivity to multidrug
resistance breast cancer cells by RNA interference (RNAi). Cancer
Biol Ther. 8:1117–1121. 2009.PubMed/NCBI View Article : Google Scholar
|
|
55
|
Weiss J, Gajek T, Köhler BC and Haefeli
WE: Venetoclax (ABT-199) might act as a perpetrator in
pharmacokinetic drug-drug interactions. Pharmaceutics.
8(5)2016.PubMed/NCBI View Article : Google Scholar
|
|
56
|
Liu YY, Gupta V, Patwardhan GA, Bhinge K,
Zhao Y, Bao J, Mehendale H, Cabot MC, Li YT and Jazwinski SM:
Glucosylceramide synthase upregulates MDR1 expression in the
regulation of cancer drug resistance through cSrc and beta-catenin
signaling. Mol Cancer. 9(145)2010.PubMed/NCBI View Article : Google Scholar
|
|
57
|
Kivistö KT, Kroemer HK and Eichelbaum M:
The role of human cytochrome P450 enzymes in the metabolism of
anticancer agents: Implications for drug interactions. Br J Clin
Pharmacol. 40:523–530. 1995.PubMed/NCBI View Article : Google Scholar
|
|
58
|
Reed L, Arlt VM and Phillips DH: The role
of cytochrome P450 enzymes in carcinogen activation and
detoxication: An in vivo-in vitro paradox. Carcinogenesis.
39:851–859. 2018.PubMed/NCBI View Article : Google Scholar
|
|
59
|
Wilson A, Urquhart BL, Ponich T, Chande N,
Gregor JC, Beaton M and Kim RB: Crohn's disease is associated with
decreased CYP3A4 and P-glycoprotein protein expression. Mol Pharm.
16:4059–4064. 2019.PubMed/NCBI View Article : Google Scholar
|
|
60
|
Stiborová M, Martínek V, Rýdlová H, Koblas
T and Hodek P: Expression of cytochrome P450 1A1 and its
contribution to oxidation of a potential human carcinogen
1-phenylazo-2-naphthol (Sudan I) in human livers. Cancer Lett.
220:145–154. 2005.PubMed/NCBI View Article : Google Scholar
|
|
61
|
Wang H and Wang WJ: Relationship between
CYP1A1 polymorphisms and invasion and metastasis of breast cancer.
Asian Pac J Trop Med. 6:835–838. 2013.PubMed/NCBI View Article : Google Scholar
|
|
62
|
Hafeez S, Ahmed A, Rashid AZ and Kayani
MA: Down-regulation of CYP1A1 expression in breast cancer. Asian
Pac J Cancer Prev. 13:1757–1760. 2012.PubMed/NCBI View Article : Google Scholar
|
|
63
|
Symmans WF, Peintinger F, Hatzis C, Rajan
R, Kuerer H, Valero V, Assad L, Poniecka A, Hennessy B, Green M, et
al: Measurement of residual breast cancer burden to predict
survival after neoadjuvant chemotherapy. J Clin Oncol.
25:4414–4422. 2007.PubMed/NCBI View Article : Google Scholar
|
|
64
|
Wang RX, Ji P, Gong Y, Shao ZM and Chen S:
Value of CXCL8-CXCR1/2 axis in neoadjuvant chemotherapy for
triple-negative breast cancer patients: A retrospective pilot
study. Breast Cancer Res Treat. 181:561–570. 2020.PubMed/NCBI View Article : Google Scholar
|
|
65
|
Dan J, Tan J, Huang J, Zhang X, Guo Y,
Huang Y and Yang J: The dynamic change of neutrophil to lymphocyte
ratio is predictive of pathological complete response after
neoadjuvant chemotherapy in breast cancer patients. Breast Cancer.
27:982–988. 2020.PubMed/NCBI View Article : Google Scholar
|
|
66
|
Ochi T, Bianchini G, Ando M, Nozaki F,
Kobayashi D, Criscitiello C, Curigliano G, Iwamoto T, Niikura N,
Takei H, et al: Predictive and prognostic value of stromal
tumour-infiltrating lymphocytes before and after neoadjuvant
therapy in triple negative and HER2-positive breast cancer. Eur J
Cancer. 118:41–48. 2019.PubMed/NCBI View Article : Google Scholar
|
|
67
|
Sikov WM, Berry DA, Perou CM, Singh B,
Cirrincione CT, Tolaney SM, Kuzma CS, Pluard TJ, Somlo G, Port ER,
et al: Impact of the addition of carboplatin and/or bevacizumab to
neoadjuvant once-per-week paclitaxel followed by dose-dense
doxorubicin and cyclophosphamide on pathologic complete response
rates in stage II to III triple-negative breast cancer: CALGB 40603
(Alliance). J Clin Oncol. 33:13–21. 2015.PubMed/NCBI View Article : Google Scholar
|