Introduction
The term endo-periodontal lesion appeared decades
ago in order to describe a specific disease that affects the pulp
and periodontal tissues simultaneously. Patients with diabetes are
more prone to oral infections and periradicular lesions caused by
changes in the immune system, qualitative and quantitative changes
in the normal flora of the oral cavity and poor peripheral blood
irrigation (1).
Furthermore, endo-periodontal lesions can be a risk
factor for severe complications, such as odontogenic sinusitis.
Inflammation and/or disruption of the Schneider membrane results in
the inflammation of the mucosa and impaired mucociliary function in
the maxillary sinus. Impaired mucociliary function results in
altered mucus transport, impaired mucosal defense, blockage of
sinus ostia and consecutive inflammatory processes (2).
Considering that diabetes adversely affects blood
circulation or causes ischemia, sometimes necrotic tissue phenomena
may occur (3). The possible
connection between chronic oral inflammatory processes, such as
apical periodontitis, endodontic status and systemic health,
represents one of the most interesting aspects faced by the medical
and dental scientific community; in order to achieve a proper
healing potential, all parameters must be stabilized, in our case,
inflammation and infection status in a diabetic field.
Glycemic control is essential to prevent
diabetes-related morbidity and mortality. Increased glycated
hemoglobin A1c (HbA1c) has been linked to diabetic microvascular
and macrovascular complications and decreased HbA1c leads to
reduced morbidity and mortality (4).
Doxycycline is an inexpensive, well-tolerated,
broad-spectrum antibiotic that has the added benefit of being a
potent inhibitor of host-derived matrix metalloproteinases (MMPs),
even at subantimicrobial doses. Levels of MMP-class enzymes,
including MMP-9 and MMP-8, have repeatedly been shown to be
decreased in gingival tissues and periodontal lesions by
subantimicrobial doses of doxycycline. Doxycycline in
subantimicrobial doses (SDD) (Periostat™, CollaGenex
Pharmaceuticals, Inc.; now Galderma R&D) has been approved as
an adjuvant to root planning and scaling for the treatment of
periodontitis (5). The additional
benefit of conventional subgingival debridement generated by
doxycycline in subantimicrobial doses is due to the strong
inhibition of extracellular matrix degradation, even in severe
cases of periodontitis. The FDA (Food and Drug Administration)
approved dose for subantimicrobial doxycycline is 20 mg twice daily
for up to 9 months. Antimicrobial action and side effects of
antibiotics (for example, the emergence of antibiotic-resistant
bacteria) do not occur at the recommended therapeutic doses
(6).
Thus, the purpose of the study was to analyze local
and regional changes (in terms of odontogenic sinusitis) in
subjects with endo-periodontal lesions and diabetes mellitus and to
investigate the effect on the level of glycemic control (glycated
hemoglobin) that could be generated by adjunctive therapy with
subantimicrobial doses of doxycycline.
Patients and methods
Patients
This study was performed on 51 subjects with
diabetes mellitus type 2, divided into two therapeutic groups: 31
patients with diabetes (the SDD group) who underwent conventional
endo-periodontal therapy and subantimicrobial doses of doxycycline
and 20 patients with diabetes who followed only conventional
endo-periodontal therapy (the control group). All of these patients
had endo-periodontal lesions.
We excluded from the study patients with systemic
diseases that are not a complication of diabetes mellitus, patients
suffering from cancer, pregnant, breastfeeding or menopausal women,
smokers, patients receiving dental treatment in the last 12 months
or standard antibiotic regime in the last 2 months and those who
had less than 20 remaining teeth.
In conducting the research, the ethical norms set
out in the Declaration of Helsinki were respected. The present
study was approved by the Ethics Committee of ‘Grigore T. Popa’
University of Medicine and Pharmacy (Iasi, Romania). Patients were
informed about the aim of the study and each signed the informed
consent required for study enrollment.
Clinical examination
Patients underwent a complex endodontic and
periodontal clinical examination, which comprised vitality tests
and the determination of the following periodontal parameters:
Probing depth (PD), clinical periodontal attachment loss (CAL) and
bleeding on probing (BOP).
The periodontal probing was performed with both the
manual periodontal probe (CP-12, Hu-Friedy Mfg. Co., LLC) and an
electronic one (Pa-On; Orange Dental GmbH & Co., Germany). The
probing depth, together with the loss of periodontal attachment,
were measured in six points per tooth: Mesial-vestibular,
central-vestibular, distal-vestibular, mesial-oral, central-oral,
distal-oral. The BOP index was assessed by examination after 30 sec
of each probed site. Clinical examinations were conducted at
baseline (T0), and at 3, 6 and 12 months from baseline (T1, T2 and
T3, respectively). All the data collected from the periodontal
measurements are included in the patient's individual
periogram.
Clinical examinations were supplemented with serial
retro-dental-alveolar radiographs and CBCT examinations for the
areas indicating radiological signs of odontogenic sinusitis.
Therapeutic procedure
All patients underwent non-surgical periodontal
therapy, consisting of manual and ultrasonic scaling and root
planning, with the help of curettes (Hu-Friedy Mfg. Co., LLC). For
the mechanical instrumentation of root canals, the access cavity
was made, the canals were permeabilized with Kerr needles (Kerr
Corp., USA) no. 10 or 15. The instrumentation was performed with
the manual ProTaper system (Dentsply Sirona), using the crown-down
technique. Each patient was trained on the appropriate oral hygiene
means.
These therapeutic procedures were performed for all
subjects included in the study. In addition, patients in the SDD
group underwent adjunct therapy to modulate the host's inflammatory
response to bacterial aggression with subantimicrobial doses of
doxycycline, 20 mg twice daily, for 3 months. Adverse events were
monitored and recorded throughout the study.
Each subject performed, at home, oral rinses with
0.10% chlorhexidine solution and 0.50% chlorobutanol
(Eludril®), twice/daily after dental brushing, for 2
weeks, starting on the first day of endo-periodontal treatment.
For patients with poor glycemic control, infection
prophylaxis was also performed, with oral amoxicillin 2 g, taken as
a single dose, 1 h before each treatment session. The patients that
required this type of prophylaxis treatment were excluded from the
study.
Analysis of glycated hemoglobin
For each patient, glycated hemoglobin A1c (HbA1c)
was determined. The method of determining HbA1c was
immunoturbidimetric. This test does not interfere with other forms
of pathological hemoglobin, such as carbamylated hemoglobin in
uremic patients or acetylated hemoglobin caused by aspirin
treatment; this is due to the high specificity of the anti-HbA1c
antibodies for a 4 amino acid sequence at the N-terminus of the β
chain in the glycated state. Therefore, this test determines ‘real’
HbA1c, as defined by the International Federation of Clinical
Chemistry (IFCC) (7).
The quantification of glycated hemoglobin in total
hemolyzed blood was based on a turbidimetric inhibition reaction.
In a first step, glycated hemoglobin from the collected sample
reacted with anti-HbA1c antibodies, with the formation of soluble
antigen-antibody complexes. In the second step, polyhaptenes were
added, which reacted with excess anti-HbA1c, by forming
antibody-polyhaptene complexes, which were determined by
immunoturbidimetry. The total hemoglobin concentration was
determined in a separate channel. In the hemolyzed blood sample,
the released hemoglobin was converted into a derivative, with a
characteristic absorption spectrum; it was measured in two
colors.
The percentage calculation of glycated hemoglobin
was performed according to the Diabetes Controls and Complications
Trial/National Glycohemoglobin Standardization Program (DCCT/NGSP)
protocol (8), to which a correction
formula was applied:
% HbA1c = (HbA1c/Hb) x 91.5 + 2.15
This evaluation was performed at the beginning of
the study and 3, 6 and 12 months after baseline.
Statistical analysis
The data obtained during the course and at the end
of the 12 months of the study were analyzed and statistically
processed. The average values for the bleeding index, the probing
depth and the level of clinical attachment loss per patient and at
group and subgroup level were calculated. The Mann-Whitney test was
used in order to detect significant differences between groups at
different time points. The Wilcoxon test was used to evaluate
changes over time. Values of P<0.025 were considered
statistically significant. The Mann-Whitney test with a
significance level P<0.05 was used to determine the significant
differences between groups.
Results
The mean age of the 51 subjects was 52.97±10.21
years. The group consisted of 30 female subjects (58.82%) and 21
male subjects (41.18%). Regarding the environment of origin, 33
subjects came from urban areas (64.71%) and 18 from rural areas
(35.29%). Demographic data by study group are presented in Table I. Moreover, a significant percentage
of patients, both in the study group and in the control group,
showed radiological signs of odontogenic sinusitis, totaling 29
patients (56.86%).
 | Table IDemographic data of the study
groups. |
Table I
Demographic data of the study
groups.
| Parameters | SDD group (n=31) | Control group
(n=20) | Total (n=51) |
|---|
| Age (years) (mean ±
SD) | 52.17±9.72 | 53.23±8.38 | 52.97±10.21 |
| Sex, n (%) |
|
Male | 13 (41.94%) | 8 (40.00%) | 21 (41.18%) |
|
Female | 18 (58.06%) | 12 (60.00%) | 30 (58.82%) |
| Provenance, n
(%) |
|
Urban | 21 (67.74%) | 12 (60.00%) | 33 (64.71%) |
|
Rural | 10 (32.26%) | 8 (40.00%) | 18 (35.29%) |
| Odontogenic sinusitis
prevalence, n (%) | 18 (58.06%) | 11 (55.00%) | 29 (56.86%) |
In terms of patient compliance, 34 subjects were
initially included in the SDD group, but 3 of them (8.82%) did not
complete the regimen with subantimicrobial doses of doxycycline.
All subjects included in the control group followed the study
methodology.
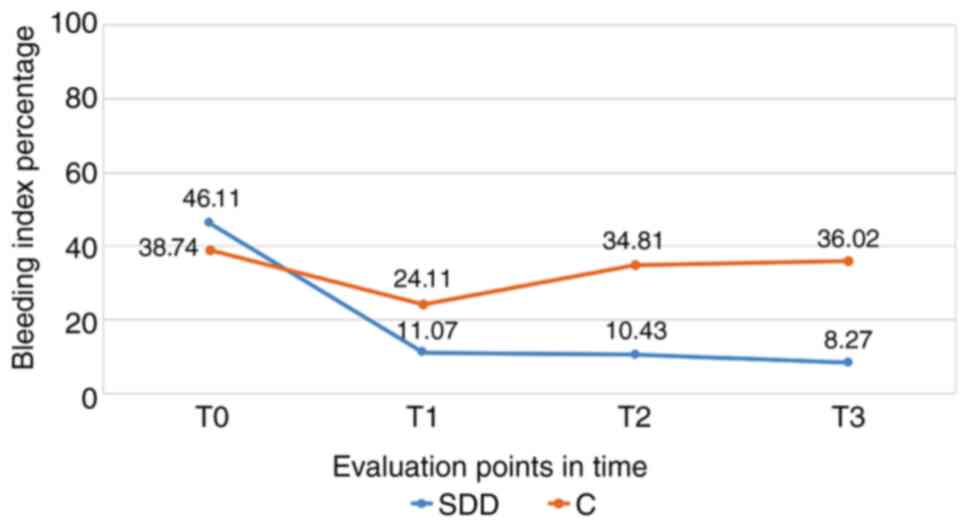
Bleeding index
Following the evaluation of the bleeding index in
the SDD group, we observed a significant decrease at the end of the
therapy with subantimicrobial doses of doxycycline (T1), a decrease
that continued at the 6 (T2) and 12 month (T3) assessments
(P<0.001). For the control group, we noted significant decreases
for the bleeding index after 3 months (T1); however, this followed
an increasing trend at 6 (T2) and 12 months from baseline (T3),
approaching the initial values (Fig.
1).
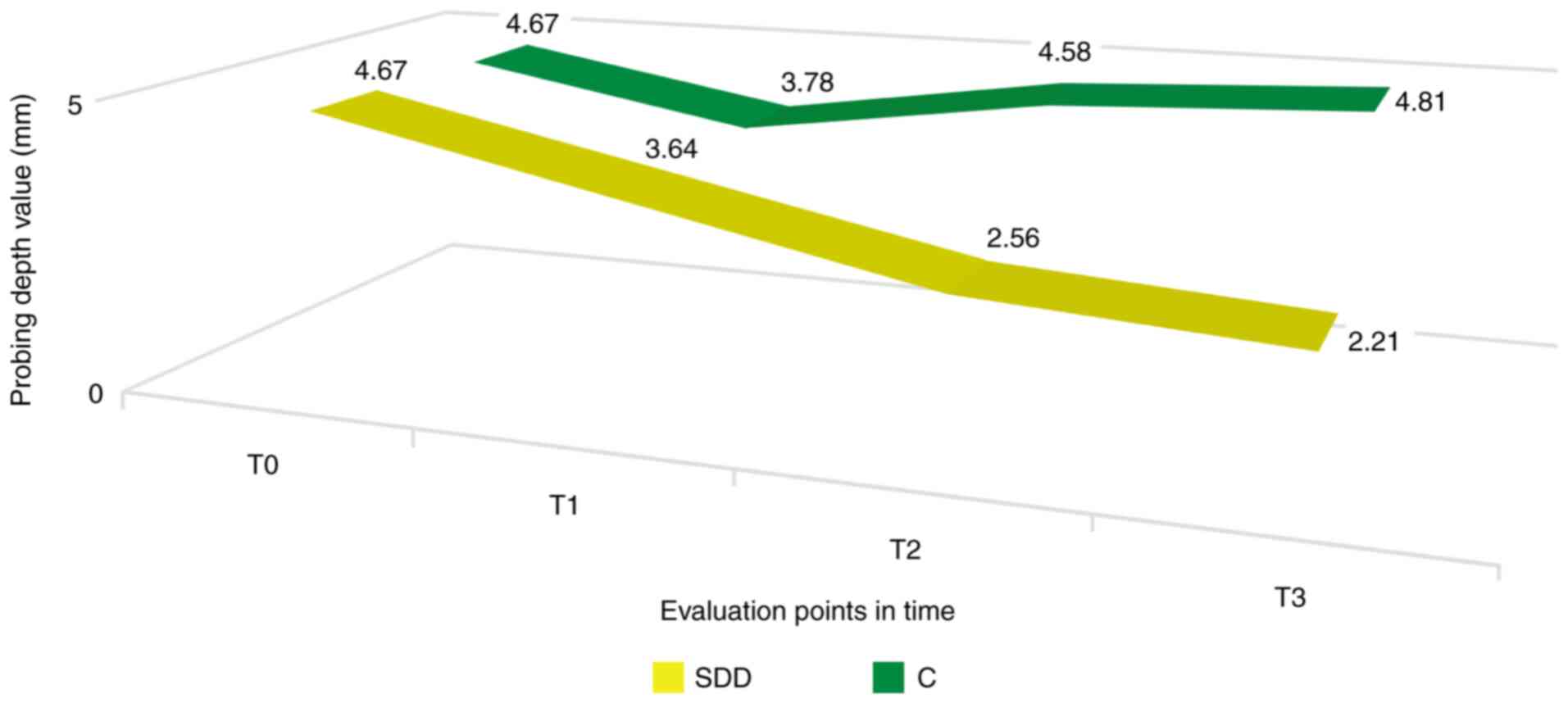
Probing depth
The determination of probing depth at 3 months from
baseline (T1) revealed lower values in patients in the SDD group,
even though it did not reach the statistical significance
threshold; these values continued to decrease throughout the study,
the difference being significant at the 6 (T2) and 12 month (T3)
assessments from baseline (P<0.05). Despite the average value of
the probing depth being lower than the baseline at 3 months for the
control group, this difference was not statistically significant.
Moreover, the values increased at T2 and T3 evaluations (Fig. 2).
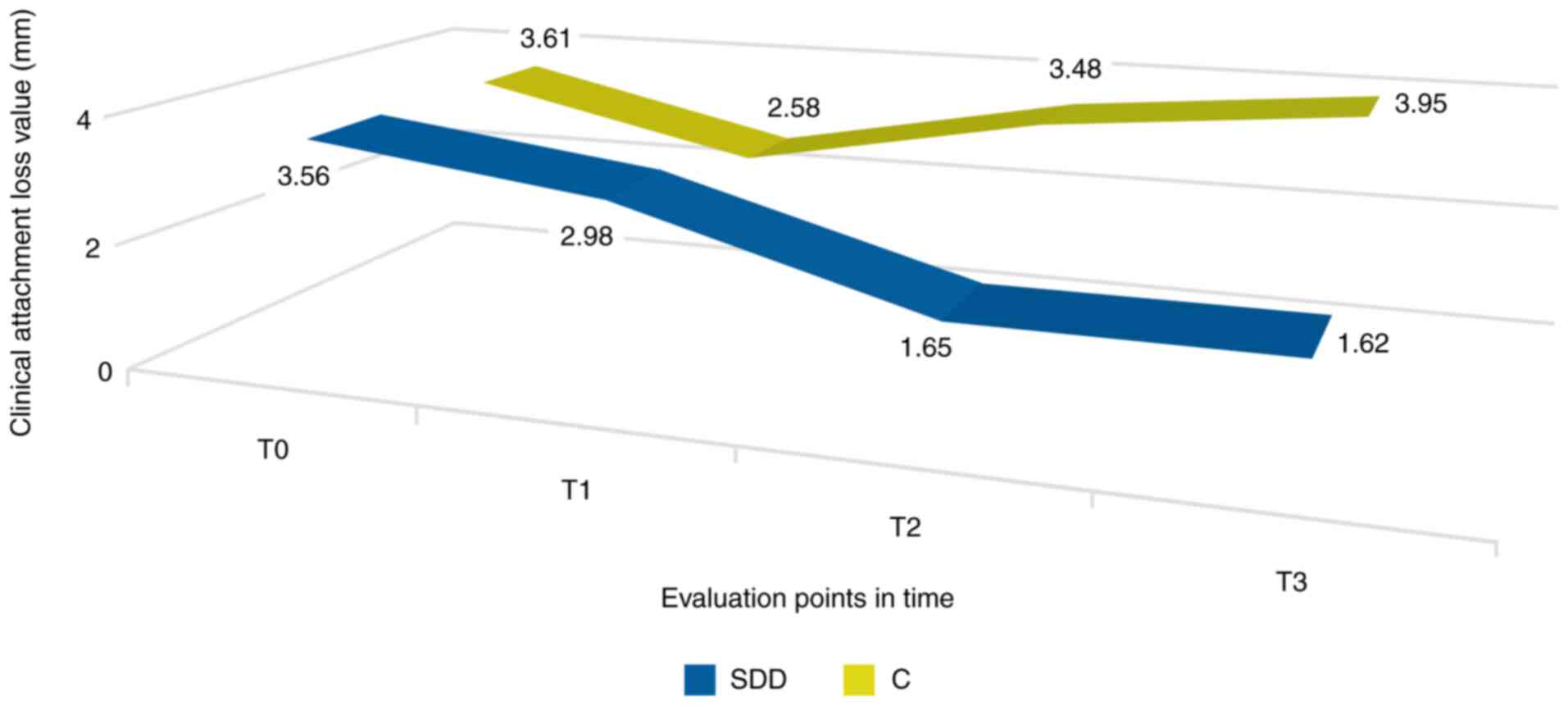
Loss of periodontal clinical
attachment
When assessing the loss of periodontal clinical
attachment after the completion of SDD therapy (T1), the value was
lower, but did not reach a clinical significance threshold.
Importantly, in the 6 (T2) and 12 month (T3) evaluations, we noted
a decreased tendency of these values in the SDD group. In the
control group, consisting of patients who only followed
conventional therapy, CAL decreased significantly when assessed 3
months (T1) after the initial moment. Nevertheless, similarly to
the other periodontal parameters, it showed an upward trend in the
evaluations from 6 (T2) and 12 months (T3), the last of them
revealing a value even higher than the initial one (Fig. 3).
At the beginning, we did not note significant
differences between groups for any of the analyzed periodontal
parameters. At the end of the SDD therapy (T1), only the bleeding
index showed significantly lower values for the SDD group compared
to the control group (P=0.0311), but 3 (T2) and 9 (T3) months after
the completion of the SDD therapy, we observed significantly lower
values for all the examined periodontal parameters.
Regarding the level of glycated hemoglobin, at T1we
noted significant decreases for both study groups. The differences
between the SDD group and the control group were significant when
compared at the T2 and T3 assessments (P=0.0025 and 0.0002,
respectively). For the group of patients with diabetes who
underwent subantimicrobial doses of doxycycline therapy HbA1c, it
continued to decrease, while for the group of patients who only
followed conventional therapy, these values began to increase,
approaching the baseline values (Table
II).
 | Table IIMean values of glycated hemoglobin in
the study groups. |
Table II
Mean values of glycated hemoglobin in
the study groups.
| | SDD group | Control group | |
|---|
| | T0 | T1 | T2 | T3 | T0 | T1 | T2 | T3 | V0 | V1 | V2 | V3 |
|---|
| HbA1c (%) | 8.8±1.8 | 7.2±1.6a | 7.1±1.7a | 6.8±1.5a | 8.9±1.9 | 7.1±1.5a | 8.3±1.6 | 8.7±1.8 | 0.852 | 0.741 | 0.0025b | 0.000b |
Discussion
Comparisons between patients with diabetes and those
in the control group led to the observation that diabetes
constitutes a risk factor for periodontal disease in general and
for endo-periodontal lesions in particular. Oral disorders, such as
periodontal disease, as well as diabetes, are multifactorial
diseases (9). More obviously,
diabetic patients are susceptible to various forms of periodontal
disease, a particular importance being given to the
diabetes-periodontal disease relationship, with the identification
of patients who are more prone to these types of oral disorders
(10).
Diabetes is known to decrease the host resistance to
infections and diminish wound healing. Insulin is required for
glucose uptake into cells and to provide an energy source for amino
acid amelioration in protein synthesis, as well as for preventing
lipolysis of adipose tissue. If insulin administration is not
sufficient, then the basic cell functions of the body will be
disrupted. Signs of host defense against microbes are well known:
Impaired polymorphonuclear (PMN) leukocyte cell function with
adhesion abnormalities, chemotaxis, phagocytosis, and intercellular
destruction. Type 2 DM is associated with a series of microvascular
complications that most commonly affect the eyes and kidneys, and
histopathological studies have shown internal ear nerve and vessels
damage in subjects with diabetes (11).
An important complication related to the poor
glycemic control, with great effect on the quality of life,
bacterial, fungal or viral infections, are common in patients with
diabetes and can affect the skin and soft tissue structure of the
ear and nose. Both hypoglycemia and hyperglycemia have been
associated with internal ear dysfunction and hearing can fluctuate
with the level of glycemic control. The relationship between
diabetes mellitus, sensory hearing loss and vestibular dysfunction
is known, and histopathological changes of the temporal bone have
been clearly documented (12).
The duration of diabetes is an important factor that
causes the occurrence of microvascular complications of diabetes
(13). It seems that the longer
duration of diabetes mellitus predisposes to the development of
deafness in many studies; however, a mild degree of hearing
impairment has been detected in many children with diabetes lasting
more than four years. Such an observation was unusual and may be
explained by poor glycemic control. Elamin et al (14) confirmed the relationship of loss
hearing in children and adolescents with type 1 diabetes mellitus
at medium and high frequencies.
In diabetic patients with endo-periodontal lesions,
periodontal therapy may have beneficial effects on glycemic control
(10). This may be especially true
for patients with relatively poorly controlled diabetes and more
advanced periodontal destruction prior to treatment.
An understanding of the effects of other infections
would be helpful in delimiting the mechanisms by which periodontal
infection influences blood sugar. Acute bacterial and viral
infections have been shown to increase insulin resistance and
worsen metabolic control. This occurs in individuals with or
without diabetes. Systemic infections increase tissue resistance to
insulin, preventing the entry of glucose into the target cells,
leading to an increase in blood sugar and requiring an increase in
insulin production to maintain a normoglycemic state (10).
A systematic review examining the etiology of
odontogenic sinusitis in a group of 674 patients showed that an
iatrogenic etiology accounted for 65.7% of cases, apical
periodontal pathology accounted for 25.1% of cases, and marginal
periodontitis accounted for 8.3% of cases (15). This study further demonstrated that
the most frequently affected maxillary teeth, in order of
frequency, were the first molar (35.6%), the second molar (22%),
the third molar (17.4%) and the second premolar (14.4%) (data not
shown). Thus, there is an increased risk in patients with combined
endo-periodontal lesions, especially if these lesions also affect
the furcation area. In the present study, we noted a significant
percentage of patients with endo-periodontal lesions who had
radiological signs of odontogenic sinusitis, a diagnosis
subsequently confirmed by CBCT examinations. In the context of the
presence of diabetes, patients with endo-periodontal lesions are at
high risk of local and loco-regional complications, including
odontogenic sinusitis; this risk is amplified in cases of poor
glycemic control. Therefore, modulation of the inflammatory
response makes a significant contribution in mitigating these
risks.
Several types of meta-analyses have confirmed that
effective periodontal therapy may result in reduced glycated
hemoglobin A1c (HbA1c). The first reported was performed on 10
interventional studies, with a combined population of 456 patients;
the authors identified a weighted average HbA1c reduction of 0.66%
as a result of periodontal therapy (although this failed to reach
statistical significance) (16). In
2010, a meta-analysis of 5 studies involving 371 patients also
reported a significant weighted average HbA1c reduction of 0.40% at
a 3-9 months follow-up period (17).
The Cochrane collaboration reported studies that
investigated the relationship between periodontal treatment and
glycemic control in people with diabetes. Three studies were
included in this meta-analysis that reported a significant
reduction of 0.40% HbA1c at 3-4 months after conventional
periodontal therapy (18). The
findings of these meta-analyses are supported by a population study
of over 5,000 people with diabetes, reporting that patients who had
at least one periodontal access surgery session had HbA1c levels
that were 0.25% less than patients who had not undergone
periodontal surgery (19).
Taken together, the evidence supports the idea that
improvements in metabolic control can be anticipated following the
effective treatment of periodontitis. The mechanisms by which this
happens is not yet clear, but probably is due to reduced systemic
inflammation (e.g., low serum concentrations of mediators such as
TNF-α and IL-6), after treatment and resolution of periodontal
inflammation (13). These
observations are important as reductions in HbA1c are associated
with a reduced risk of diabetes complications. For example, it was
found that each 1% reduction in HbA1c is associated with a 21% risk
reduction for any diabetes-related complication, 21% for
diabetes-related deaths, 14% for myocardial infarction, and 37% for
microvascular complications (20).
Diabetes affects many functions of the immune system
and is associated with delayed healing and compromised immune
responses. Diabetes-induced changes in immune cell function produce
an inflammatory immune cell phenotype (stimulation of
pro-inflammatory cytokines from monocytes/polymorphonuclear
leukocytes and inhibition of macrophage growth factors). This
predisposes to chronic inflammation, progressive tissue breakdown
and diminished tissue repair capacity (21).
Doxycycline and other tetracycline analogues have
been shown to reduce tissue protein glycation in animals with
streptozotocin-induced diabetes without apparent changes in serum
glucose levels. Therefore, we hypothesized that doxycycline may be
useful in the treatment of patients with diabetes by reducing
protein glycation. The hypothesis of this study showed that SDD
could play a role in reducing protein glycation in humans.
The implications of this study have far-reaching
potential if the results are confirmed in larger and long-term
studies. Firstly, SDD has already been approved for the adjuvant
treatment of periodontitis. As patients with diabetes have a high
risk of periodontitis, increased use of this type of therapy in the
population will improve the results of periodontal treatment and
may lead to improvements in diabetes outcomes. Secondly, we did not
observe any increased incidence of adverse events in patients with
type 2 diabetes who had SDD for three months (data not shown).
Thirdly, subjects took stable doses of oral hypoglycemic agents
and/or insulin and no adverse events were observed, indicating an
apparent lack of adverse drug interactions between SDD and these
agents.
As described in a larger number of studies on SDD in
non-diabetic populations, no serious adverse events were observed
in this study and SDD appeared to be well tolerated (22-24).
Therefore, the use of SDD appears to be safe and effective for the
treatment of endo-periodontal lesions in subjects with type 2
diabetes. However, these data should be interpreted with caution,
given the small sample size. Clearly, larger studies are needed in
subjects with type 2 diabetes to confirm whether this treatment is
safe and effective for the treatment of endo-periodontal lesions in
patients with type 2 diabetes and to test whether SDD is an
effective adjuvant drug for the treatment of diabetes. It also
remains to be determined whether long-term administration of SDD is
safe and effective in reducing the complications of diabetes.
However, based on these pilot data, longitudinal studies appear to
be warranted.
Therefore, subantimicrobial doses of doxycycline
generated favorable results for the evaluated periodontal
parameters (bleeding index, probing depth and clinical periodontal
attachment loss) and, unlike conventional therapy, these results
were maintained over time.
Moreover, we demonstrated that adjunctive therapy
with SDD had a clear contribution to improving glycemic control in
patients with diabetes and endo-periodontal lesions, an improvement
manifested by significantly reduced glycated hemoglobin levels
throughout the study (12 months). This fact has far-reaching
effects in the sphere of loco-regional complications as well and
the risk of odontogenic sinusitis can be significantly reduced.
In addition, subantimicrobial dose therapy of
doxycycline was well tolerated, with no adverse effects, which
contributes to its recommendation in the therapeutic management of
patients with diabetes mellitus and endo-periodontal lesions.
Acknowledgements
Professional editing, linguistic and technical
assistance were performed by Irina Radu, Individual Service
Provider.
Funding
The study was partially funded by a PhD scholarship
provided to Ms. Anamaria Zaharescu by the ‘Grigore T. Popa’
University of Medicine and Pharmacy (Iasi, Romania).
Availability of data and materials
The data used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
AZ designed the experiments and performed the
interventional study. IM contributed to the design of the study and
data acquisition. AIL contributed to the data analysis and data
interpretation, and edited the manuscript. MAM contributed to the
data analysis and to the design of the study. IGS contributed to
the design of the study and edited the manuscript. CM acquired and
analyzed the data, and wrote the main manuscript text. SMS designed
the study, interpreted the data and provided archive data. All
authors read and approved the manuscript and agree to be
accountable for all aspects of the research in ensuring that the
accuracy or integrity of any part of the work are appropriately
investigated and resolved.
Ethical approval and consent to
participate
The present study was approved by the Ethics
Committee of ‘Grigore T. Popa’ University of Medicine and Pharmacy
(Iasi, Romania). All protocols were in accordance with the
provisions of the Declaration of Helsinki. The purpose and safety
aspects of the study were explained to all patients and written
informed consent was included in the documents of each patient.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Al-Fouzan KS: A new classification of
endodontic periodontal lesions. Int J Dent.
2014(919173)2014.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Gamba P: Odontogenic maxillary cysts
post-dental implant: Proposal of new radiological/clinical
classification. Int J Innovative Res Med Sci. 10:431–438. 2016.
|
|
3
|
Dhoum S, Laslami K, Rouggani F, El
Ouazzani A and Jabri M: Endo-Perio lesion and uncontrolled
diabetes. Case Rep Dent. 2018(7478236)2018.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Khaw KT, Wareham N, Luben R, Bingham S,
Oakes S, Welch A and Day N: Glycated haemoglobin, diabetes, and
mortality in men in Norfolk cohort of European Prospective
Investigation of Cancer and Nutrition (EPIC-Norfolk). BMJ.
322:15–18. 2001.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Caton J and Ryan ME: Clinical studies on
the management of periodontal diseases utilizing subantimicrobial
dose doxycycline (SDD). Pharmacol Res. 63:114–120. 2011.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Engebretson SP and Hey-Hadavi J:
Sub-antimicrobial doxycycline for periodontitis reduces hemoglobin
A1c in subjects with type 2 diabetes: A pilot study. Pharmacol Res.
64:624–629. 2011.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Groche D, Hoeno W, Hoss G, Vogt B,
Herrmann Z and Witzigmann A: Standardization of two immunological
HbA1c routine assays according to the new IFCC reference method.
Clin Lab. 49:657–661. 2003.PubMed/NCBI
|
|
8
|
Little R, Rohlfing C, Wiedmeyer H, Myers
G, Sacks D and Goldstein D: The National Glycohemoglobin
standardization program: A five-year progress report. Clin Chem.
47:1985–1992. 2001.PubMed/NCBI
|
|
9
|
Liccardo D, Cannavo A, Spagnulo G, Ferrara
N, Cittadini A, Rengo C and Rengo G: Periodontal disease: A risk
factor for diabetes and cardiovascular disease. Int J Mol Sci.
20(1414)2019.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Mealey BL and Oates TW: Diabetes mellitus
and periodontal diseases. J Periodontol. 77:1289–1303.
2006.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Graves DT, Ding Z and Yang Y: The impact
of diabetes on periodontal disease. Periodontol. 82:214–224.
2020.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Gazzaz ZJ, Makhdom MN, Dhafar KO, Maimini
O, Farooq MU and Rasheed A: Patterns of otorhinolaryngological
disorders in patients with diabetes. Int Med J Malaysia. 10:13–16.
2011.
|
|
13
|
American diabetes association. Diagnosis
and classification of diabetes mellitus. Diabetes Care. 37:581–590.
2014.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Elamin A, Fadlallah M and Tuvemo T:
Hearing loss in children with type 1 diabetes. Indian Pediatr.
42:15–21. 2005.PubMed/NCBI
|
|
15
|
Lechien JR, Filleul O, de Araujo PC, Hsieh
JW, Chantrain G and Saussez S: Chronic maxillary rhinosinusitis of
dental origin: A systematic review of 674 patient cases. Int J
Otolaryng. 2014(465173)2014.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Janket SJ, Wightman A, Baird AE, Van Dyke
TE and Jones JA: Does periodontal treatment improve glycemic
control in diabetic patients? A meta-analysis of intervention
studies. J Dent Res. 84:1154–1159. 2005.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Teeuw WJ, Gerdes VEA and Loos BG: Effect
of periodontal treatment on glycemic control of diabetic patients:
A systematic review and meta-analysis. Diabetes Care. 33:421–427.
2010.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Simpson TC, Needleman I, Wild SH, Moles DR
and Mills EJ: Treatment of periodontal disease for glycaemic
control in people with diabetes. Cochrane Database Syst Rev.
5(CD004714)2010.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Spangler L, Reid RJ, Inge R, Newton KM,
Hujoel P, Chaudhari M, Genco RJ and Barlow WE: Cross-sectional
study of periodontal care and glycosylated hemoglobin in an insured
population. Diabetes Care. 33:1753–1758. 2010.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Stratton IM, Adler AI, Neil HA, Matthews
DR, Manley SE, Cull CA, Hadden D, Turner RC and Holman RR:
Association of glycaemia with macrovascular and microvascular
complications of type 2 diabetes (UKPDS 35): Prospective
observational study. BMJ. 321:405–412. 2000.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Nayak AU, Nevill AM, Bassett P and Singh
BM: Association of glycation gap with mortality and vascular
complications in diabetes. Diabetes Care. 36:3247–3253.
2013.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Caton JC, Ciancio SG, Blieden TM, Bradshaw
M, Crout RJ, Hefti AF, Massaro JM, Polson AM, Thomas J and Walker
C: Treatment with subantimicrobial dose doxycycline improves the
efficacy of scaling and root planing in patients with adult
periodontitis. J Periodontol. 71:521–532. 2000.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Walker C, Puumala S, Golub LM, Stoner JA,
Reinhardt RA, Lee H and Payne JB: Subantimicrobial dose doxycycline
effects on osteopenic bone loss: Microbiologic results. J
Periodontol. 78:1590–1601. 2007.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Payne JB, Golub LM, Stoner JA, Lee H,
Reinhardt RA, Sorsa T and Slepian MJ: The effect of
subantimicrobial-dose-doxycycline periodontal therapy on serum
biomarkers of systemic inflammation: A randomized, double-masked,
placebo-controlled clinical trial. J Am Dent Assoc. 142:262–273.
2011.PubMed/NCBI View Article : Google Scholar
|