Introduction
Colchicine is a traditional natural remedy known for
more than a millennium for its anti-inflammatory properties.
Biochemically, it is a toxic protoaloalkaloid from the group of
tropolon derivatives, that was extracted in 1819 from the bulb of
the autumn crocus (Colchicum autumnale), and in 1833 was
named colchicine by the German pharmacist and chemist Philipp
Lorenz Geiger (1-3).
The pure crystallized form was obtained by the French pharmacist
Alfred Houde (1). The current drug
is the same purified natural substance. The mechanism of action is
still a research topic today. In the years 1950-1960, the main
cellular target of colchicine action, the cytoskeleton, was
identified. Microtubules are major constituents of the cytoskeleton
with a role in cell dynamics, maintaining cell shape through
resistance to compression, intracellular transport and cell
division (3-7).
Colchicine binds to tubulin heterodimers, constituents of
protofilaments in the structure of microtubules, to form
dimer-colchicine complexes that attach to the end of microtubules
causing alterations in their conformation resulting in altered cell
function (3-7).
Its therapeutic action is attributed to the inhibition of
neutrophil chemotaxis, their adhesion and recruitment in
inflammatory lesions as colchicine is more concentrated in
leukocytes than in plasma (3,4,7-9).
Colchicine also suppresses the production of superoxide by
neutrophils and reduces oxidative stress by decreasing the influx
of calcium ions (Ca2+) into neutrophils (10,11).
Other confirmed effects include modulation of hepatic macrophage
secretion of tumor necrosis factor (TNF)α, inhibition of
inflammatory cytokine production [interleukin (IL)-1ß, interferon
(IFN)γ, IL-8, IL-6], promoting dendritic cell maturation and
stimulating the presentation of naive CD4+ lymphocyte
antigens, inhibiting vascular endothelial growth factor (VEGF) and
endothelial proliferation (3-7,11).
Apart from the anti-inflammatory effect, colchicine
also has antifibrotic and cardiovascular protective effects
blocking autoinflammatory pathways, including NLRP3 and IL-1
(3,12). Some pathological conditions such as
gout or rheumatoid arthritis are associated with high
cardiovascular risk due to systemic inflammation. Colchicine is an
immune-modulatory agent able to reduce cardiovascular risk for
these patients considering inflammation an important component for
the development of heart attacks or strokes (13).
After oral administration, colchicine is absorbed in
the jejunum and ileum, is metabolized in the liver by the
cytochrome P450 (CYP450) enzyme and excreted mainly hepatobiliary
and, to a lesser extent, renally (3,4). Drugs
inhibiting CYP450 or P-glycoprotein (intracellular transporter
molecule important for colchicine absorption and pharmacokinetics),
such as erythromycin, clarithromycin, fluconazole, itraconazole,
calcium channel blockers (diltiazem, verapamil), cyclosporine,
tacrolimus or statins enhance the pharmacological effects of
colchicine and increase the risk of its toxic effects (11). The official prescribing guidelines
for colchicine therefore recommend dose adjustments for patients
taking these medications. Colchicine dose reduction is also
recommended for patients with severe renal impairment, including
patients on hemodialysis, as well as for patients with severe
hepatic impairment (14).
Adverse effects are mainly gastrointestinal and are
reversible at dose reduction. They consist of abdominal pain,
diarrhea, nausea, vomiting and occur in 5-10% of cases (6,7).
Increased levels of serum transaminases, myotoxicity and alopecia
are rarely encountered (15-18).
Rarer acute adverse effects include myopathy, rhabdomyolysis and
myelosuppression. A colchicine neuromyopathy may occur with chronic
daily use, particularly in patients whose dose has not been
appropriately adjusted for renal disease. Symptoms of colchicine
toxicity usually resolve within 1 week to several months of
discontinuing the drug (6). The
classic therapeutic indications of colchicine are gout and familial
Mediterranean fever, as well as its complications
(amyloidosis).
Since 2009, colchicine has been approved by the Food
and Drug Administration for use in rheumatology, immunology,
cardiology, oncology, dermatology (17). The use of colchicine has been shown
to be beneficial for the treatment of rheumatic diseases,
pericarditis, coronary heart disease, atherosclerosis, and has been
attempted in various dermatological diseases, orally or topically,
with variable efficacy (1,3,5,6).
Severe aphthosis is one of the most documented indications for
colchicine treatment with beneficial effects of oral colchicine
being reported in case studies, case series and less often, in
clinical trials (17-25).
We present 2 cases of chronic recurrent aphthous stomatitis (RAS),
that represented a diagnostic and therapeutic challenge, as
consulted in the Dermatology Clinic of the Railways University
Hospital in Iasi, Romania.
Case 1
A 23-year-old patient, with Turner syndrome treated
with somatotropin and, from the age of 13 with estro-progestins
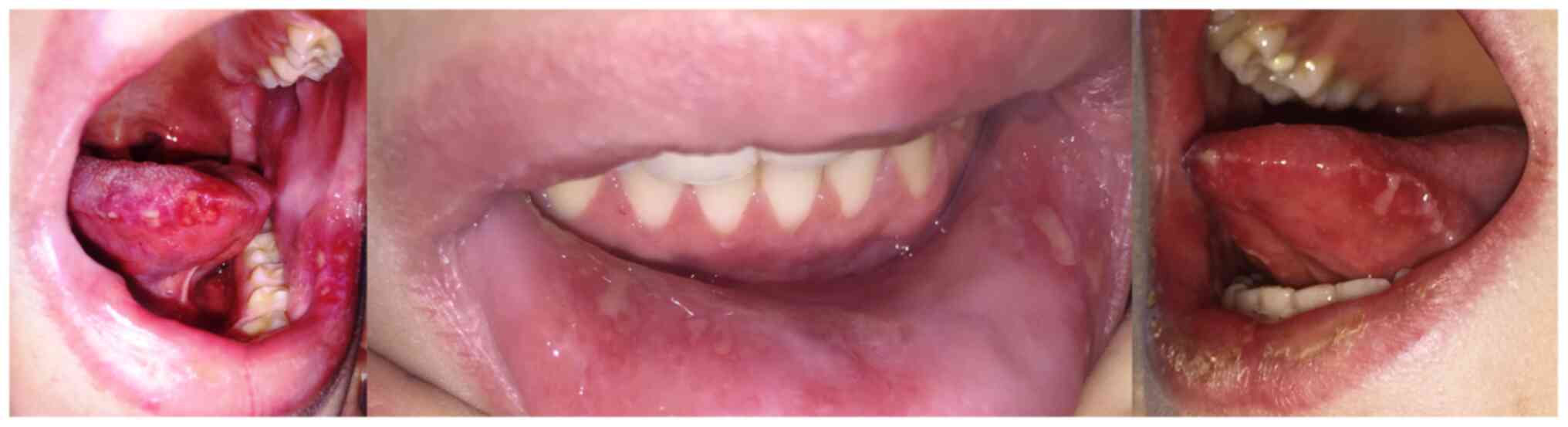
(Zoely), presented with multiple extremely painful oral herpetiform
exulcerations, with a tendency to group in plaques with a
yellowish-gray fibrinous base, persistent for approximately 10 days
(Fig. 1).
Patient interrogation revealed the onset of oral
lesions 4 years prior to presentation, with an episodic, recurrent
course and difficult healing in 2 to 3 weeks, under various topical
treatments with analgesics, borax glycerin and corticosteroids. The
clinical context (numerous miliary exulcerations located on the
non-keratinized labial and lingual mucosa, extremely painful and
recurrent, in a female patient under chronic estrogen-progestative
substitution treatment) led to the diagnosis of RAS with
herpetiform aphthae.
Hematological and biochemical investigations,
including dosing of ferritin, folate, zinc, vitamin B12, revealed
normal data. Treatment with colchicine 1 mg/day, after 3 days of
testing digestive tolerance with 0.5 mg/day, in combination with
topical dexamethasone and hyaluronic acid was initiated. The
evolution of the lesions over a follow-up period of 10 months,
under maintenance treatment with colchicine 0.5 mg/day, was towards
faster healing, on average in 10 days. A greater interval of 2
months between recurrent episodes was also achieved. There were no
reported side effects.
Case 2
A non-smoker 67-year-old patient, with no notable
personal medical history, except for pulmonary emphysema, presented
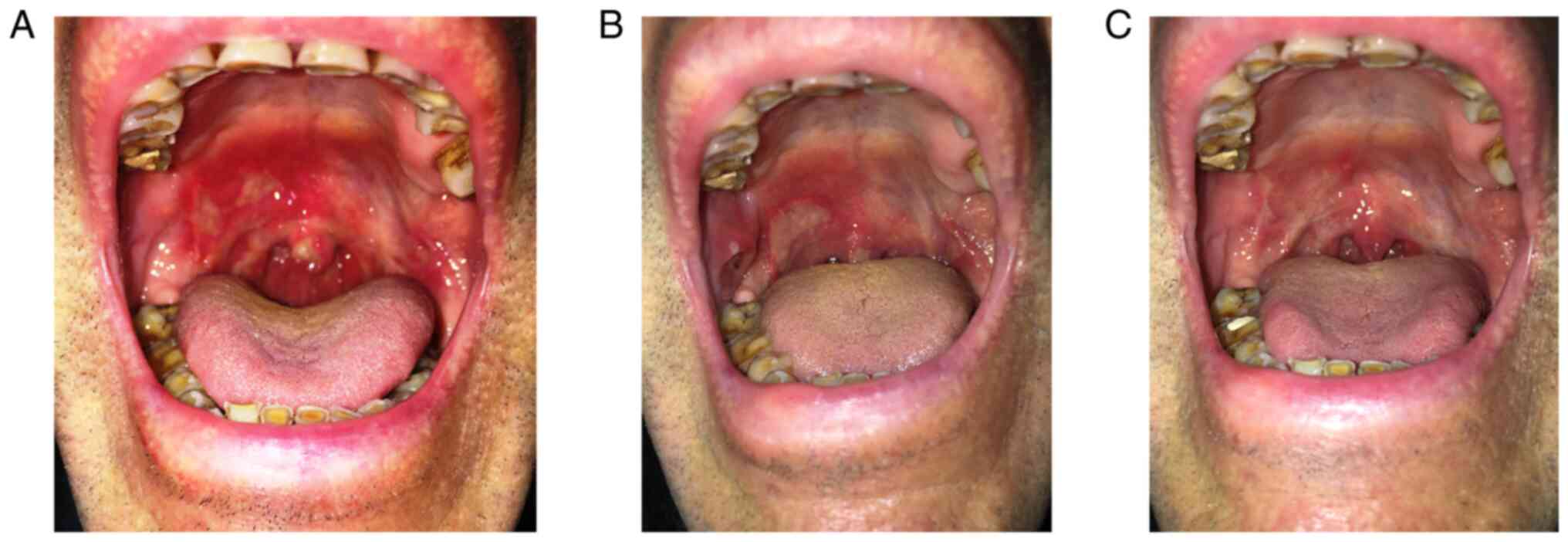
for consultation for extremely painful, clearly delimited oval
ulcerations with a large transverse axis of approximately 2 cm,
with intense erythematous halo and yellowish-gray base, localized
on the soft palate and buccal mucosa, with recurrent episodes for 8
years (Fig. 2A).
He was repeatedly evaluated over time by several
specialists (ENT, infectious disease, gastroenterology, oral
medicine, dermatology). Infectious disease (negative anti-HIV1 and
2 antibodies, AgHbs absent), autoimmune bullous dermatosis
(negative anti-desmoglein 3 antibodies), and a neoplastic process
(repeated biopsies in 2012, 2013, 2015, with nonspecific
inflammatory changes) were excluded. The lesions only partially
responded to topical treatments with corticosteroids, lidocaine,
oral corticosteroids and dapsone (Fig.
2B). Thus, treatment with colchicine 1 mg/day in combination
with pentoxifylline 400 mg x 2/day and topical suspension with
metronidazole, dexamethasone and nystatin was initiated. The
lesions healed significantly in approximately 6 weeks. Treatment
with a maintenance dose of colchicine (0.5 mg/day) was continued
for the next 4 months, and was well tolerated with no recurrent
episodes during this time (Fig.
2C).
Discussion
Efficacious results of colchicine treatment have
been reported in several dermatologic conditions, such as chronic
urticaria unresponsive to antihistamines, urticarial vasculitis,
forms of cutaneous vasculitis (hypocomplementemic urticarial,
leukocytoclastic, nodular, necrotic vasculitis, Henoch-Schonlein
purpura), palmo-plantar pustular psoriasis (applied as a
hydrophilic ointment with colchicine 1%), pyoderma gangrenosum
associated or not with inflammatory bowel disease, Sweet syndrome,
subcorneal pustulosis, acquired bullous epidermolysis with limited
skin lesions, benign mucosal pemphigoid, Behcet's disease, actinic
keratosis (applied as a hydrophilic gel with colchicine 1%) or
granuloma annulare (3,26-34).
Less satisfactory results have been obtained for hidradenitis
suppurativa, acne vulgaris, dermatitis herpetiformis, linear IgA
dermatosis, scleroderma and psoriasis vulgaris (3,35,36).
Recurrent aphthous stomatitis (RAS) is a recurrent
ulcerative stomatitis with an estimated prevalence between 2 and
10%, with an incompletely elucidated etiopathogenesis and,
consequently, with poorly defined treatment (37). In developed countries, the incidence
in the general population reaches 20%, mainly affecting young
adults (38). The
pathophysiological substrate consists of an antigenic stimulation
of oral mucosal keratinocytes in predisposed individuals, followed
by the secretion of proinflammatory cytokines (especially IL-2,
TNFα) and the consequent expression of class I major
histocompatibility complex antigens (MHC). MHC class I
antigen-expressing cells become targets of cytotoxic T lymphocytes
(39,40). The inflammatory process, resulting
in variable epithelial necrosis depending on its histopathological
site, is the consequence of an aberrant immune response, influenced
by an abnormal oral flora (4).
The three modes of clinical expression of the
disorder are common aphthae (a few round or oval exulcerations with
an average diameter of 2-4 mm, with a gray-yellow base and a
characteristic carmine-red areola, with self-limited evolution of
approximately 7-10 days), herpetiform aphthae, the rarest (numerous
yellowish, millimetric exulcerations, with a tendency to coalesce
in erosive patches with micropolycyclicontour, evolving for
approximately two weeks) and major aphthae (Sutton's ulcers or
periadenitis mucosa necrotica recurrens), the most severe clinical
form (crateriform ulcers, with a diameter between 1 and 3 cm, often
solitary, accompanied by satellite adenopathy, with difficult
healing for 1-2 months with sometimes mutilating scars) (8,39,40).
The common clinical features of these ulcerations are intense pain,
location on non-keratinized areas of the oral mucosa, self-limiting
character and recurrences, either spontaneous or correlated with
triggering factors. These factors may be local (e.g. oral trauma,
contact hypersensitivity, sodium lauryl sulfate), nutritional
deficiencies (iron, vitamin B12, folic acid), medications
(angiotensin converting enzyme inhibitors, gold salts,
phenobarbital, diclofenac, piroxicam), inflammatory bowel disease
(gluten-sensitive enteropathy, Crohn's disease, ulcerative
colitis), certain foods (tomatoes, nuts, cocoa, dairy, spices), or
a hormonal context with progesterone deficiency in females. RAS is
also correlated with a genetic predisposition (3,8,39).
The treatment of RAS is a challenge. In the absence
of a clear etiopathogenesis, various topical and systemic therapies
have been attempted over time, with the aim to reduce pain, shorten
the duration of recurrent episodes, to distance them in time, and
in order to improve the quality of life of these patients (40). Severe aphthoses, with very painful
lesions accompanied by functional signs (pain when speaking,
chewing, swallowing), with frequent recurrences and significant
psycho-social impact, raise the issue of systemic therapy.
Prednisone, thalidomide, cyclosporine, azathioprine, methotrexate,
dapsone, pentoxifylline, and colchicine have been administered. The
use of colchicine in the treatment of RAS is well documented. The
mechanism by which colchicine positively influences RAS lesions
could be essentially explained by inhibiting leukocyte chemotaxis
and mobilization, their lysosomal degranulation, by modulating the
interaction between leukocytes and vascular endothelium, and by
decreasing the production of proinflammatory cytokines, including
IL-6 and IL-1ß (19,41). Studies support the effectiveness of
colchicine in RAS, both as a monotherapy and in combination with
other systemic therapies, as an episode treatment and as a
maintenance or prophylactic treatment. The first large
retrospective study (1986-2000), on 54 immunocompetent patients
with RAS followed on average for 4.7 years, concluded that the
administration of colchicine at doses of 1-1.5 mg/day for at least
3 months had beneficial effects on episode frequency, pain
intensity and RAS impact on quality of life in 63% of patients, 37%
of whom maintained results for 5 years (41). Very beneficial effects on reducing
pain and injury were reported in a series of 20 patients with
severe aphthosis, treated with colchicine 0.5 mg three times per
day (20). The mean weekly number
of aphthae and pain score were assessed in patients treated
continuously with colchicine compared to those treated only 2
months after 2 previous months without treatment. This 4-month
study highlighted the obvious prophylactic benefit of uninterrupted
treatment with colchicine (21).
Persistent significant remissions for 3-5 years were also reported
in 2 of a series of 3 patients with severe aphthosis treated with
colchicine. Administration of colchicine at a dose of 0.5 mg three
times per day, 3 consecutive days/week had a similar efficacy to
that of prednisolone administered at a dose of 5 mg/day and to
combination therapy with levamisole 50 mg/day and prednisolone 5
mg/day in a study on 50 patients with RAS followed for 3 weeks
(22). Colchicine can also be used
as a prophylactic treatment. Doses with a prophylactic effect of
0.5-1.8 mg/day have been reported in a study on 9 children with
PFAPA (periodic fever, aphthous stomatitis, pharyngitis, cervical
adenitis) and were found to significantly increase the free
interval between episodes of oral ulceration (23). Another randomized controlled open
label study (18 patients aged 4-11 years) over a period of 6
months, followed the number of active episodes in two groups: One
treated for 3 months after 3 months of surveillance and one
control. The number of episodes were similar in the first 3 months
without treatment in both groups. The number of patients treated
during the follow-up 3 months was significantly lower compared to
the control and compared to the first 3 months of the study - the
authors emphasizing the definite prophylactic role of colchicine.
Daily doses of 0.5-1.5 mg of colchicine alone or in combination
with dapsone (75-100 mg/day) were also shown to be beneficial in
another study of 55 patients with severe aphthosis monitored
between 1998 and 2007, 80% of them with a substantial response
(24,25).
Considering the clinical context of the disease,
with severe episodes, only partially responsive to classic
therapies, the two presented cases are part of the group of complex
aphthoses. The response was favorable to colchicine, starting with
a dose of 1 mg/day until significant remission was obtained, and
continuing with a maintenance dose of 0.5 mg/day for several months
of follow-up (10 and 4 months, respectively, in our cases),
recording only two mild recurrences in the patient with herpetiform
aphthae and no recurrence in the patient with Sutton's ulcers.
Colchicine was associated with pentoxifylline in our second
patient, considering literature reports of its beneficial effects
in reducing the severity and frequency of aphthous ulcer episodes.
Pentoxifylline inhibits the production of TNFα and reduces the
migration of neutrophils, but its specific action in aphthous
stomatitis is still unclear (42).
There were no side effects in any of our patients during the
follow-up period.
In conclusion, inhibition of multiple inflammatory
pathways and modulation of the innate immune response are the main
attributes of colchicine exploited in the treatment of several
dermatoses, including RAS. Case studies, series of patients and
clinical trials, although few, provide evidence of the efficacy of
colchicine (level of evidence III) in severe aphthosis, refractory
to classical therapies with topical or systemic corticosteroids,
pentoxifylline, and cyclosporine. There is no consensus on the
ideal therapeutic regimen for colchicine in RAS.
Therapeutic doses of 0.5-1.5 mg/day are usually free
of noticeable side effects, even after 6-9 months of treatment,
provided that drug interactions are avoided and doses are adjusted
in patients with hepatic or renal impairment. The choice of
treatment with colchicine in severe aphthosis should take into
account the severity of the lesions, their chronic nature, the lack
of therapeutic efficacy of other medications and the context of the
patient morbidity. The mechanism of action underlying the efficacy
of colchicine in various dermatoses, as well as the optimal
therapeutic regimen, including RAS, require further extensive
research.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The data that support the findings of this study are
available from the archives of the Railways University Hospital
Iasi, (Iasi, Romania), but restrictions apply to the availability
of these data which are not publicly available. Data are, however,
available from the authors upon reasonable request and with
permission from the Railways University Hospital Iasi.
Author's contributions
TT conceived and supervised the study. MPT, IME and
ST analyzed the data. MPT, TT, MM, IME and ST contributed to data
acquisition and interpretation and wrote the manuscript. All
authors contributed equally to acquisition, analysis and
systematization of data, manuscript writing and critical revision
of it for important intellectual content. All authors reviewed the
results and read and approved the final version of the
manuscript.
Ethics approval and consent to
participate
The Research Ethics Committee of the Railways
University Hospital Iasi (Iasi, Romania) affiliated with ‘Grigore
T. Popa’ University of Medicine and Pharmacy approved the current
study.
Patient consent for publication
Informed written consent was obtained from both
patients for publication of the case reports.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Karamanou M, Tsoucalas G, Pantos K and
Androutsos G: Isolating colchicine in 19th century, an old drug
revisited. Curr Pharm Des. 24:654–658. 2018.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Nerlekar N, Beale A and Harper RW:
Colchicine-a short history of an ancient drug. Med J Aust.
201:687–688. 2014.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Robinson KP and Chan JJ: Colchicine in
dermatology: A review. Australas J Dermatol. 59:278–285.
2018.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Leung YY, Yao Hui LL and Kraus VB:
Colchicine-Update on mechanisms of action and therapeutic uses.
Semin Arthritis Rheum. 45:341–350. 2015.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Slobodnick A, Shah B, Pillinger MH and
Krasnokutsky S: Colchicine: Old and new. Am J Med. 128:461–470.
2015.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Slobodnick A, Shah B, Krasnokutsky S and
Pillinger MH: Update on colchicine, 2017. Rheumatology (Oxford). 57
(Suppl 1):i4–i11. 2018.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Paschke S, Weidner AF, Paust T, Marti O
and Beil M: Ben-Chetrit. Technical advance: Inhibition of
neutrophil chemotaxis by colchicine is modulated through
viscoelastic properties of subcellular compartments. J Leukoc Biol.
94:1091–1096. 2013.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Peter T, Cherian D and Peter T: Recurrent
aphtous stomatitis: Mystery unravelled. J Clin Exp Res. 2:141–145.
2014.
|
|
9
|
Altinor S, Oztürkcan S and Hah MM: The
effects of colchicine on neutrophil function in subjects with
recurrent aphthous stomatitis. J Eur Acad Dermatol Venereol.
17:469–470. 2003.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Chia EW, Grainger R and Harper JL:
Colchicine suppresses neutrophil superoxide production in a murine
model of gouty arthritis: A rationale for use of low-dose
colchicine. Br J Pharmacol. 153:1288–1295. 2008.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Korkmaz S, Erturan I, Nazıroğlu M, Uğuz
AC, Ciğ B and Övey IS: Colchicine modulates oxidative stress in
serum and neutrophil of patients with Behçet disease through
regulation of Ca²+ release and antioxidant system. J
Membr Biol. 244:113–120. 2011.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Ozkok A: Cholesterol-embolization
syndrome: Current perspectives. Vasc Health Risk Manag. 15:209–220.
2019.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Crowson CS, Liao KP, Davis JM III, Solomon
DH, Matteson EL, Knutson KL, Hlatky MA and Gabriel SE: Rheumatoid
arthritis and cardiovascular disease. Am Heart J. 166:622–628.
2013.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Tarakji B, Gazal G, Al-Maweri SA,
Azzeghaiby SN and Alaizari N: Guideline for the diagnosis and
treatment of recurrent aphthous stomatitis for dental
practitioners. J Int Oral Health. 7:74–80. 2015.PubMed/NCBI
|
|
15
|
Imazio M, Belli R, Brucato A, Cemin R,
Ferrua S, Beqaraj F, Demarie D, Ferro S, Forno D, Maestroni S, et
al: Efficacy and safety of colchicine for treatment of multiple
recurrences of pericarditis (CORP-2): A multicentre, double-blind,
placebo-controlled, randomised trial. Lancet. 383:2232–2237.
2014.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Oh DH, Chan SQ and Wilson AM: Myopathy and
possible intestinal dysfunction in a patient treated with
colchicine and simvastatin. Med J Aus. 197:332–333. 2012.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Dasgeb B, Kornreich D, McGuinn K, Okon L,
Brownell I and Sackett DL: Colchicine: An ancient drug with novel
applications. Br J Dermatol. 178:350–356. 2018.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Dalmau J, Alegre M, Domingo P and Alomar
A: Major oral aphtous ulceration in HIV-1 infection: Successful
response after highly active antiretroviral therapy. J Eur Acad
Dermatol Venereol. 21:126–127. 2007.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Ruah CB, Stram JR and Chasin WD: Treatment
of severe recurrent aphthous stomatitis with colchicine. Arch
Otolaryngol Head Neck Surg. 114:671–675. 1988.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Fontes V, Machet L, Huttenberger B,
Lorette G and Vaillant L: Recurrent aphthous stomatitis: Treatment
with colchicine. An open trial of 54 cases. Ann Dermatol Venereol.
129:1365–1369. 2002.PubMed/NCBI(In French).
|
|
21
|
Katz J, Langevitz P, Shemer J, Barak S and
Livneh A: Prevention of recurrent aphthous stomatitis with
colchicine: An open trial. J Am Acad Dermatol. 31:459–461.
1994.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Pakfetrat A, Mansourian A, Momen-Heravi F,
Delavarian Z, Momen-Beitollahi J, Khalilzadeh O and
Basir-Shabestari S: Comparison of colchicine versus prednisolone in
recurrent aphthous stomatitis: A double-blind randomized clinical
trial. Clin Invest Med. 33:E189–E195. 2010.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Tasher D, Stein M, Dalal I and Somekh E:
Colchicine prophylaxis for frequent periodic fever, aphthous
stomatitis, pharyngitis and adenitis episodes. Acta Paediatr.
97:1090–1092. 2008.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Butbul Aviel Y, Tatour S, Gershoni Baruch
R and Brik R: Colchicine as a therapeutic option in periodic fever,
aphthous stomatitis, pharyngitis, cervical adenitis (PFAPA)
syndrome. Semin Arthritis Rheum. 45:471–474. 2016.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Lynde CB, Bruce AJ and Rogers RS III:
Successful treatment of complex aphthosis with colchicine and
dapsone. Arch Dermatol. 145:273–276. 2009.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Pho LN, Eliason MJ, Regruto M, Hull CM and
Powell DL: Treatment of chronic urticaria with colchicine. J Drugs
Dermatol. 10:1423–1428. 2011.PubMed/NCBI
|
|
27
|
Jachiet M, Flageul B, Deroux A, Le Quellec
A, Maurier F, Cordoliani F, Godmer P, Abasq C, Astudillo L,
Belenotti P, et al: French vasculitis study group: The clinical
spectrum and therapeutic management of hypocomplementemic
urticarial vasculitis: Data from a French nationwide study of
fifty-seven patients. Arthritis Rheumatol. 67:527–534.
2015.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Goeser MR, Laniosz V and Wetter DA: A
practical approach to the diagnosis, evaluation, and management of
cutaneous small-vessel vasculitis. Am J Clin Dermatol. 15:299–306.
2014.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Kontochristopoulos GJ, Stavropoulos PG,
Gregoriou S and Zakopoulou N: Treatment of pyoderma gangrenosum
with low-dose colchicine. Dermatology. 209:233–236. 2004.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Maillard H, Leclech C, Peria P,
Avenel-Audran M and Verret JL: Colchicine for Sweet's syndrome. A
study of 20 cases. Br J Dermatol. 140:565–566. 1999.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Adisen E, Tekin O and Gulekon and Gürer
MA: A retrospective analysis of treatment responses of palmoplantar
psoriasis in 114 patients. J Eur Acad Dernatol Venereol.
23:814–819. 2009.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Pavithran K: Colchicine in the treatment
of subcorneal pustular dermatosis. Indian J Dermatol Venereol
Leprol. 61:56–57. 1995.PubMed/NCBI
|
|
33
|
Gürcan HM and Ahmed AR: Current concepts
in the treatment of epidermolysis bullosa acquisita. Expert Opin
Pharmacother. 12:1259–1268. 2011.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Chaidemenos G, Sidiropoulos T, Katsioula P
and Koussidou-Eremondi T: Colchicine in the management of mucous
membrane pemphigoid. Dermatol Ther. 24:443–445. 2011.PubMed/NCBI View Article : Google Scholar
|
|
35
|
van der Zee HH and Prens EP: The
anti-inflammatory drug colchicine lacks efficacy in hidradenitis
suppurativa. Dermatology. 223:169–173. 2011.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Schepis C, Siragusa M, Palazzo R and
Guerra AP: Failure of colchicine in the treatment of severe acne
vulgaris. Acta Derm Venereol. 79(491)1999.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Altenburg A, Abdel-Naser MB, Seeber H,
Abdallah M and Zouboulis CC: Practical aspects of management of
recurrent aphthous stomatitis. J Eur Acad Dermatol Venereol.
21:1019–1026. 2007.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Bischoff EW, Uijen A and van der Wel M:
Aphthous ulcers. BMJ. 339(b2382)2009.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Preeti L, Magesh K, Rajkumar K and Karthik
R: Recurrent aphthous stomatitis. J Oral Maxillofac Pathol.
15:252–256. 2011.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Altenburg A and Zouboulis CC: Current
concepts in the treatment of recurrent aphthous stomatitis. Skin
Therapy Lett. 13:1–4. 2008.PubMed/NCBI
|
|
41
|
Mimura MA, Hirota SK, Sugaya NN, Sanches
JA Jr and Migliari DA: Systemic treatment in severe cases of
recurrent aphthous stomatitis: An open trial. Clinics (Sao Paulo).
64:193–198. 2009.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Abdulrahman HS and Mutaz FF: Therapeutic
management of recurrent aphthous stomatitis: A review of the
growing knowledge. Ann Int Med Dent Res. 2:1–9. 2016.
|