Chronic kidney disease (CKD) represents a public
health concern as it affects over 50 million people worldwide, and
is more and more commonly encountered, especially due to the
increased incidence of high blood pressure (HBP) and diabetes
mellitus (DM). Over 1 million CKD patients require renal
replacement therapy (RRT) through dialysis and renal
transplantation (1). DM, HBP and a
family history of kidney failure are all major risk factors for CKD
(2-4).
Official data in the USA reported over 661,000 patients with
advanced stage CKD, out of which 468,000 receive RRT through
dialysis and 193,000 have a functional kidney transplant (5-7).
The acute complications occur quite frequently during dialysis, and
are caused by complex mechanisms, which are insufficiently known
(8). Among these, the most
important are cardiovascular complications, which influence the
morbidity and mortality rates in this group of patients (8).
Advanced stage CKD is associated with the increased
risk of cardiovascular affectation. Thus, in the case of chronic
dialysis patients, cardiovascular disease is identified in a large
percentage of patients. An important role in its onset, in addition
to factors such as mineral bone disease and patient comorbidities
(e.g., HBP, hyperlipidemia, hyperglycemia, homocysteine,
hypeuricemia), is chronic inflammation (9-12).
Research particularly describe a smaller total
antioxidant capacity (TAC) in healthy controls than in diabetic
hemodialysis patients; oxidative stress is one of the main factors
leading to the onset of CKD in this group of patients (13).
Acute intradyalitic cardiovascular complications
besides chronic cardivascular affectation are identified in chronic
hemodialysis patients. These are summarized in Table I and are: intradialytic hypotension
(IDH), HBP, arrhythmias, acute coronary syndrome (unstable
angina/myocardial infarction) and sudden death (1).
IDH is quite commonly encountered. It has an impact
on the quality of lives of these patients, on the cost of dialysis
and is associated with mortality. There is no clear definition of
IDH; two factors are taken into account in clinical practice: The
decrease in systolic pressure under 90 mmHg (14) or the symptomatic intradialytic
decrease in systolic pressure by more than 20 mmHg compared to the
value from the beginning of dialysis (15). Studies have demonstrated the strong
association between the decrease in systolic pressure under 90 mmHg
during dialysis in over 30% of treatments and an increase in
mortality (15).
IDH reporting differs according to the defining
criteria. Thus, IDH episodes can vary between 5 and 30% of all
hemodialysis treatments (16-18).
A study that analyzed a number of 44,801 hemodialysis treatments
performed on 1,137 patients found IDH present in 75% (16).
There are several groups of risk factors for IDH
onset, and they concern the patient, the dialysis machine or the
medical manoeuvres (iatrogenic factors). Hemodialysis patients with
direct or indirect cardiovascular affectation, that is to say
elderly patients undergoing dialysis for a long time, diabetic
patients, patients with low arterial pressure prior to dialysis,
patients with systemic infections, arrhythmias, valvulopathy,
myocardial infarction, hemorrhage, or patients with hypoalbuminemia
are predisposed to IDH (16,17,19,20)
(Fig. 1).
Dialysis parameters, such as acetate dialysis, the
dialysate composition and temperature (20-22),
the ultrafiltration rate and the total ultrafiltration volume
(23), the rapid reduction in
plasma osmolality, incorrect determination of the dry weight,
antihypertensive medication before dialysis and food ingestion pre-
or intradialysis can also represent risk factors for IDH onset.
IDH may occurs in the case of patients with acute
hemodialysis, air embolism or allergic reaction to the dialysate
(17,18,20,24).
Table II summarizes the factors
leading to IDH.
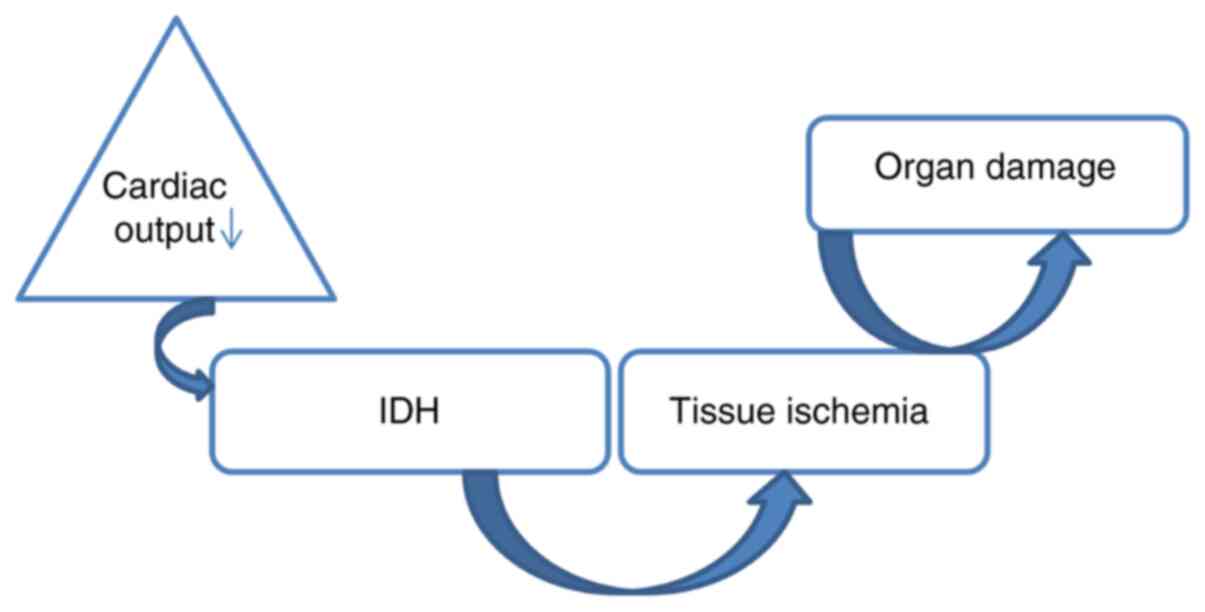
Intradialytic ultrafiltration causes the decrease in
venous return, with the subsequent decrease in cardiac flow. This
phenomenon is emphasized in patients with cardiac damage in whom
the ventricular allure or the myocardial contractility do not
increase to compensate (25,26).
Several studies have shown that the optimal ultrafiltration rate is
10 ml/kg/h; an ultrafiltration rate higher than 13 ml/kg/h is
associated with an increased IDH risk and an elevation in mortality
(27).
The decrease in blood volume in hemodialysis
patients takes place along with peripheral vasodilation (25,28-30).
There are several mechanisms which may produce this phenomenon,
such as the release of adenosine in response to tissue ischemia,
the increase in the synthesis of the vasodilating endogenous
substances (nitric oxide) and the inadequate decrease in the
vasopressin plasma levels (31-37).
IDH patients can be asymptomatic or can suffer from
dizziness, muscle cramps, nausea, vomiting and dyspnea at rest.
Vagal symptoms such as yawning, ‘sighing’ or hoarseness can occur
before a decrease in BP (17).
IDH management involves two aspects: Emergency
treatment and the prevention of relapses.
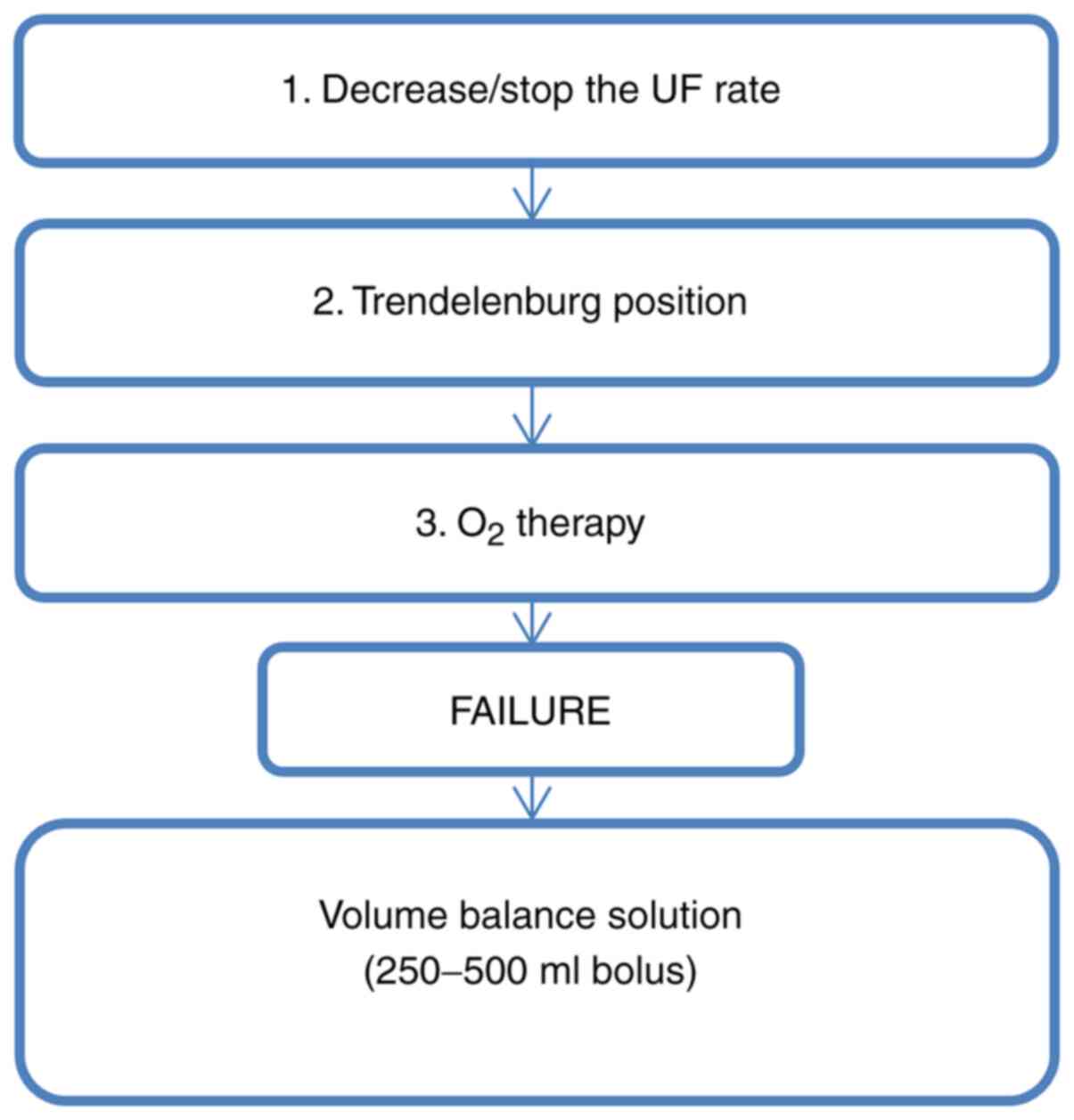
In emergency IDH treatment, the rate of
ultrafiltration is decreased/stopped, the patient is placed in the
Trendelenburg position, and oxygen (O2) is administered
(38,39). If blood pressure does not increase
following these techniques, hyperosmolar solutions or albumin is
administered in bolus; these include isotonic saline
solutions (40,41) (Fig.
2).
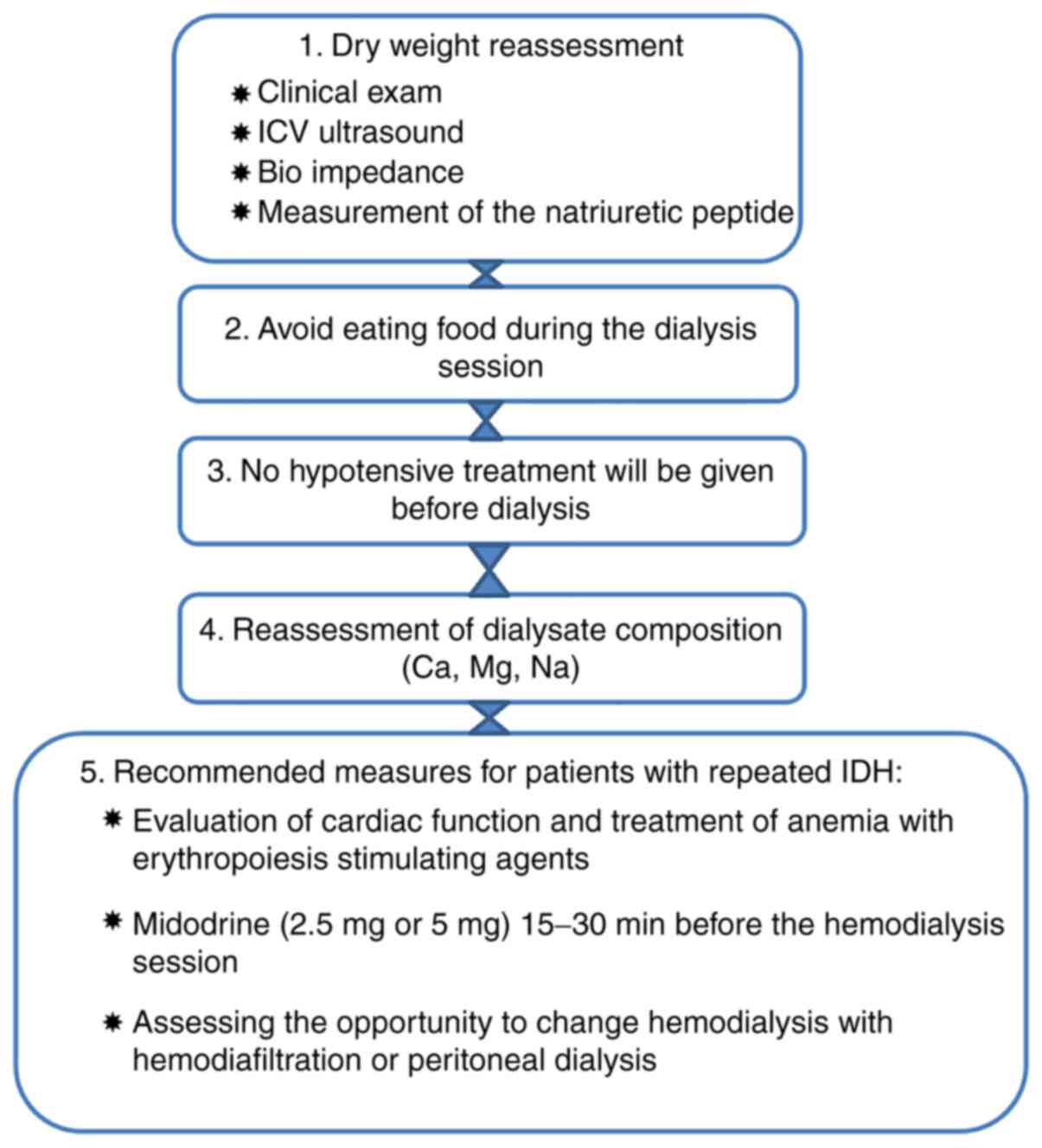
The general measures for the prevention of IDH
relapses involve a re-evaluation of the dry weight (42-46),
avoiding intradialytic food ingestion, avoiding the administration
and diminishing salt intake). Patients should also be advised
regarding caffein consumption as studies have shown that 3 or more
coffees daily increase the risk of a higher diastolic BP, potassium
and interdialytic weight gain (IDWG). IDWG in hemodialysis patients
and caffeine may alter the cardiovascular response even in healthy
people (47-49).
Patients exhibiting recurrent IDH may be
administered midodrine (2.5 or 5 mg) 15-30 min prior to dialysis
(63,69-73).
Sertraline, vasopressine and carnitine can also be administered
(74,75).
The evaluation of the cardiac function and the
treatment of anemia with erythropoiesis-stimulating agents (which
increase cardiac flow) should be performed in patients with
recurrent IDH. It should also be mentioned that chronic dialysis
patients who are prone to recurrent IDH should have a longer
hemodialysis session, and another RRT method, such as
hemodiafiltration or peritoneal dialysis, respectively, should be
considered (76) (Fig. 3).
Intradialytic hypertension (HBP) has been defined as
an increase in intradialytic systolic pressure by ≥10 mmHg compared
to pre-dialysis systolic pressure and it has been confirmed to be
associated with increased mortality in dialysis patients (77). Some patients develop HBP during the
last part of the dialysis session, a moment when the hydric excess
has been ultrafiltered. The frequency of intradialytic HBP varies,
even in the same patient, and the mechanisms are not clear; there
are proofs related to the alteration of the nitric
oxide/endothelin-1 balance and/or endothelial dysfunction (78,79).
Studies have shown that the onset of intradialytic
HBP is associated both with a volume overload between dialysis
sessions and with elevated values of intradialytic pressure
(82,83). Patients with IDH generally have a
smaller dry weight than most hemodialysis patients, have a smaller
IDWG and lower pre-dialysis blood pressure (81). These patients do not have clinical
signs of hyperhydration, which causes the prescription of a smaller
ultrafiltration volume than the one needed, without the lowering of
the arterial pressure (80). The
intensive intradialytic ultrafiltration for several weeks resulted
in the decrease in intradialytic pressure, emphasizing the fact
that the expansion of the extracellular volume, even in the absence
of clinical signs of volemic overload can mediate HBP (82,84).
The intradialytic osmolar changes contribute to the arterial
pressure changes, irrespective of the calculated ultrafiltration
rate (85).
The composition of the dialysate in establishing
blood pressure values plays an important role, alongside volume
overload in the chronic dialysis patients. In this respect, the
clinician focuses on the sodium, potassium and calcium
concentrations of the dialysate (86-88).
A retrospective study undertaken on 113,255
hemodialysis patients over 5 years, highlighted the fact that
patients with intradialytic HBP have a series of common
characteristics, such as malnutrition markers, the lack of correct
feeding or hydration, and lower pre-dialysis values of urea and
creatinine, serum albumine, and normalized protein appearance
(nPNA); they have smaller interdialysis body weight and weight gain
compared to most dialysis patients (81). Sodium in the dialysate represents a
key element in modulating intradialytic blood pressure values; it
has been found that the sodium concentration in the dialysate is
higher compared to the serum concentration of pre-dialysis sodium
in intradialysis HBP patients (increased sodium gradient) (86).
In patients who received a low-level potassium
dialysate, blood pressure values were decreased after the first
hour of the dialysis session (87);
low-level potassium dialysate is also associated with rhythm
disorders in dialysis patients (89).
Calcium levels in the dialysate influence myocardial
contractility and vascular tone (90). A high calcium level in the dialysate
is associated with vascular hyperactivity and possibly with
intradialysis HBP (88,91). Literature data show that
intradialysis hypertension is associated with an increase in
vascular resistance and less with extracellular volume overload
(78,92). An increase in vascular resistance is
likely related to the method of dialysis per se but, on the
other hand, patients with interdialysis HBP have certain common
comorbidities, including ischemic coronary disease, heart failure,
a history of vascular accident, and peripheral vascular disease
(81).
Dialysis patients with intradialysis HBP have
endothelial dysfunction, with an imbalance between vasoconstrictor
substances [endotheline 1 (ET-1) and asymmetric dimethylarginine
(ADMA)] and vasodilator substances [nitric oxide (NO)]. Studies
have shown that ET-1 diminishes or increases during dialysis along
with blood pressure (78,93,94)
and that patients with intradialysis HBP have high levels of ET-1
post dialysis and a low NO/ET-1 ratio (95,96).
The direct involvement of the stimulation of the
sympathetic nervous system in intradialysis HBP has not been
demonstrated; recent research has shown that blood pressure
increases during dialysis when the cardiac rhythm increases and the
baroreflex activity is supressed, indicating an increased activity
of the sympathetic nervous system (80).
Patients experiencing an increase in intradialysis
blood pressure can be asymptomatic or can complain of headaches,
profuse perspiration, thoracic discomfort, dispnea, palpitations or
anxiety (78).
There is no optimal therapeutic approach for HBP.
Taking into account the association of HBP with volemic overload,
it is necessary to accurately establish the dry weight (97).
Considering the role of ET-1 in causing IDH,
carvedilol may play a beneficial role, since it is an inhibitor of
ET-1 release. A pilot study, which lasted for 12 weeks, found that
administration of carvedilol (50 mg twice/day) was asociated with a
decreased frequency of HBP episodes from 77 to 28% during
hemodialysis sessions (98).
Similarly, a reduction in sodium concentration in the dialysate
under the patient's serum sodium level can trigger a decrease in
blood pressure values during dialysis sessions (99).
Hemodialysis patients quite frequently present with
hydroelectrolytic and acid base imbalances both during and between
treatment sessions, which can cause heart rhythm disorders
(100).
In 2013, United States Renal Data System (USDRS)
reported a mortality rate of 198/1,000 patients/year, 40% of the
deaths having a cardiovascular cause. Among the cardiovascular
causes, 26% were cardiac arrhythmias (101). In addition, atrial fibrillation
(AFi) was the most commonly found heart abnormality in clinical
practice and affected more hemodialysis patients than the general
population (102), with
percentages varying between 14% (103) and 27% (104). The Framingham Study reported an
incidence of 0.2% per year for AFi in the general population, for
20 years. In comparison, the Afi incidence in the hemodialysis
patients reaches 1.25 episodes/100 patient-year (105).
Chronic hemodialysis patients have a higher risk to
develop arrhythmias, taking into account the special context of the
disease: The presence of certain structural and functional
myocardial defects (interstitial fibrosis, decrease in coronary
perfusion reserve, endothelial dysfunction), rapid
hydroelectrolytic and blood pressure dynamic changes, as well as
the use of certain drugs (100).
Intradialytic arrhythmias are generated by
hydroelectrolytic and acid base disorders which occur quite
frequently in the dialysis patients; all of these, along with the
composition of the dialysate, create an ‘arrhythmogenic
environment̓ (106). On the other
hand, dialysis patients present cardiovascular comorbidities, such
as myocardial ischemia and secondary anemia, which increase the
risk for intradialytic arrhythmias (107).
A range of acid-base (pH) and electrolytic
(especially in potassium, calcium and magnesium) changes, causing
prolongation of the QT interval and associated with an increased
risk of arrhythmias occur in the dialysis patients, both during and
post-dialysis (108). Dialysis
patients can develop atrial fibrilation during dialysis. The risk
factors for the onset of Afi in these patients include ischemic
coronary disease, old age, enlarged left atrium, the value of
systolic pressure before the beginning of dialysis and the presence
of peripheral vascular disease (104,105).
The clinical picture is influenced by the rapidity
of the onset of rhythm disorder, the cardiac rhythm and the
pre-existing cardiovascular pathology. Patients can be totally
asymptomatic when the rate of ventricular contractions is within
normal limits or can suffer from cardiac failure to collapse. If
the ventricular rhythm is rapid, patients complain of palpitations,
precordial pain, dizziness, nausea, and syncope. Cardiorespiratory
arrest and sudden cardiac death (SCD) may also occur in very severe
cases (100).
It is important to reduce structural myocardial
changes, especially hypertrophy of the left ventricle, which
predisposes to ischemia and arrhythmias. It is also necessary to
optimize the dialysis parameters to ensure hemodynamic and
electrolytic balance, to evaluate the drug treatment and its impact
on the incidence and seriousness of malignant arrhythmias and SCD
(100).
AFi treatment aims mainly to maintain the
ventricular rate by administring antiarrhythmic medication or
cardioversion, to improve symptomatology and to increase effort
tolerance. AFi treatment also focuses on lowering the CVA risk,
discontinuing the anticoagulant treatment, increasing the quality
of life and the survival rate. An accepted alternative, although
often secondary to antiarrhythmic medication is the strategy to
simply control the rate of ventricular response of AFi by using
node blocking agents in association with continuous anticoagulation
(109).
Class 1A and 1C arrhythmic medications can ensure
the rapid conversion of AFi to the sinus rhythm; for example,
propafenone can be administered successfully both for paroxistic
AFi and for the prevention of relapses. Taking into account that it
is eliminated through the liver, it can be safely used to treat
dialysis patients (102). As in
most cases, caution is necessary when administered to patients with
concomitant liver disease (110).
Digoxin is sometimes administered also in patients
with AFi and cardiac failure. Taking into consideration that
digoxin half-time is extended; this can cause arrhythmias in the
presence of arrythmogenic factors such as hypopotassemia or class
1A arrhythmic medication. Digoxin toxicity causes bradycardia,
different degrees of atrioventricular block, junctional
tachycardia, ectopic ventricular activity and ventricular
tachycardia. Oral administration of digoxin in dialysis patients at
doses of 0.125 mg 3 or 4 times a week seems safe and efficient. A
0.125 mg/day dose may easily lead to toxic levels and 0.25 mg/day
can be life threatening. For these reasons, digoxin administration
in dialysis patients should be carried out with extreme care
(100).
There are few studies that show that
angiotensin-converting enzyme (ACE) inhibitor treatment in dialysis
patients is associated with a lower number of AFi episodes as
compared to this incidence in the general population.
Implantable cardioverter defibrillator (ICD)
implantation in dialysis patients who were resuscitated after a
cardiorespiratory arrest significantly improved the survival rate
(the risk of death decreases by 42%) (115). On the other hand, a meta-analysis
of the existing data in the literature showed that the mortality
rate in ICD dialysis patients is 2.7% higher than in non-dialysis
patient (116). The use of ICD in
dialysis patients has a series of adverse effects. It is associated
with an increased risk of bleeding and infection. Positioning the
ICD on the same side with the vascular approach is associated with
a higher rate of stenosis and occlusion of the subclavian vein.
Factors that should be taken into consideration in these patients
include: Performing hemostasis with special care, avoiding
post-implantation anticoagulation, placing of intravascular leads
on the contralateral side of dialysis access and the use of
high-output devices with left-sided prepectoral generator placement
(116).
Cardiovascular diseases account for about 45% of the
death in dialysis patients (123,124). Among these, approximately 10% are
caused by ischemic coronary disease/coronary heart disease (CHD).
Dialysis patients have a higher CHD incidence with a rate of death
through myocardial infarction higher than the general population
(125). The data reported by the
2018 Annual Data Report of the United States Renal Data System
(USRDS) showed a 15.3% prevalence of acute myocardial infarction in
the hemodialysis population (https://adr.usrds.org/2020/end-stage-renal-disease/8-cardiovascular-disease-in-patients-with-esrd)
Similarily, in 2016, the adjusted mortality rate was 166 in 1,000
patients-year for hemodialysis patients; 37% of the deaths had
cardiovascular causes, and 11% were due to myocardial infarction
and CHD (125).
There are several types of CHD risk factors in the
dialysis patients. In this respect, CHD onset can be favored by
‘traditional̓ risk factors or by uremia-related risk factors. The
‘traditional̓ risk factors include: DM (54%), low serum
high-density lipoprotein (HDL) cholesterol (33%), HBP (96%), HVS
diagnosed by electrocardiographic criteria (22%), sedentary life
style (80%), old age (125), and
smoking (126-128).
Chronic hemodialysis patients exhibit the increased
production of nitric oxide (NO) inihibitors, which cause
vasoconstriction and HBP and augument the risk of acute
cardiovascular events. ADMA, an endogenous NO inhibitor, is
significantly elevated in chronic hemodialysis patients and is an
important predictor for cardiovascular mortality in these patients
(140-142).
The amplification of oxidative stress in these patients represents
an extra aggravating factor (143)
and can be evaluated by determining the activity of certain
antioxidant enzymes (144).
Hemodialysis patients present extensive vascular and
valvular calcifications, associated with mineral and bone
anormalities. These patients have been found to have increased
phospho-calcium product, secondary hyperparathyroidism and
increased calcium intake through the treatment with calcium-based
phosphorus binders. In chronic hemodialysis patients, calcium is
identified at the levels of vascular media and the intima, in
atheroma plaque (145,146). The calcification of the vascular
media is associated with an increase in arterial stiffnes, but not
with atherosclerosis or the narrowing of the arterial lumen. Even
in the absence of atherosclerosis or luminal narrowing, coronary
media calcification can cause a decrease in diastolic filling,
while the peripheral medial calcification increases cardiac
afterload (145,146).
Hemodialysis patients are most often asymptomatic or
have atypical symptoms which can delay the diagnosis and choice of
therapeutic approach (147,148).
Angina can occur during dialysis, and is precipitated by the
exchange of fluids and by the episodes of IDH. Myocardial ischemia
and the effort angina are covered in this group of patients because
they are generally sedentary, or the level of their effort is very
low. Patients with serious coronary lesions can suffer from acute
coronary syndrome (ACS)-unstable angina, non-ST elevation
myocardial infarction, or ST elevation myocardial infarction. The
classic diagnostic triad (angina, increased biological markers and
EKG changes) cannot be found in hemodialysis patients (147). EKG can show left ventricle
hypertrophy in HD patients, which can mask the ST segment
depression. The cardiac lesion markers (creatine kinase MB isoform
and troponin I) can be elevated in dialysis patients in the absence
of myocardial necrosis, as a reflection of cellular apoptosis or
small vessel disease (149).
The prognosis of CKD and ACS patients are
unfavorable, in spite of the present medical therapies and the
revascularization techniques (150). Platelet antiagregants in ACS
patients decrease the mortality risk, although they increase minor
bleeding. Thus, clopidogrel administered to non-ST-segment
elevation patients to prevent relapses (CURE Trial) proved
beneficial (151,152). The PLATO Study (Platelet
Inhibition and Patient Outcomes) showed that ticagrelor, an oral
purinergic receptor inhibitor cleared by extrarenal mechanisms,
reduced mortality and major cardiovascular events, being more
efficient than clopidogrel in CKD and ACS patients (153). A recent meta-analysis showed that
antiplatelet agents reduce the probability of myocardial infarction
in CKD patients, but have unclear effects on vascular accidents and
mortality and can increase the risk of bleeding (152).
Glycoprotein IIb/IIIa inhibitors or clopidogrel, in
association with the standard ACS treatment, have a minimal or no
effect on mortality, myocardial infarction or coronary
revascularization and can heighten the risk of major bleeding in
CKD and ACS patients or in patients with high-risk coronary artery
intervention. Aspirin is essential in CKD and ACS patient treatment
(154). The benefits of
antiplatelet agent treatment are not known in CKD and ACS patients
(154).
Statins decrease the risk of cardiovascular events
and cardiovascular death in dialysis patients (155). The results reported in studies
performed to date do not explain the impact of the treatment with
statins in CKD and ACS patients (154).
The therapy of cardiovascular revascularization,
including percutaneous coronary intervention (PCI) and coronary
artery bypass grafting (CABG) is used also in dialysis patients.
Studies have demonstrated that SCA dialysis patients treated with
PCI can have a lower mortality risk compared to those patients who
only receive medication (156).
Comparing various strategies of coronary revascularization,
dialysis patients who received CABG surgery have a prolonged
long-time survival vs. the ones who received PCI (157-159).
Several studies have aimed to ascertain whether dialysis patients
benefit from aggressive SCA therapy more than from conservative
therapy.
Sudden death refers to the sudden arrest of cardiac
activity, with hemodynamic collapse, generally caused by sustained
ventricular arrhythmia (ventricular tachycardia or ventricular
fibrillation). These events occur in patients with preexisting
cardiac diseases, particularly ischemic coronary disease (160).
Data reported by DOOPS (Dialysis Outcomes and
Practice Patterns) show a high SCD prevalence among hemodialysis
patients in the US (33% of all deaths) compared to other countries,
such as Japon (23%), Australia/New Zealand (19%), and Canada (18%)
(161). Hemodialysis patients who
suffered sudden cardiac arrest and were resuscitated present
smaller chances of long-term survival (8%) (162,163).
Hemodialysis patients have a particularity
concerning the predisposition for SCD, because of the myocardial
affectation and due to the risk factors for fatal arrhythmias
(164). In the general population,
the main SCD physiopathologic mechanism is the rupture of atheroma
plaques, with acute secondary ischemia and reduction in the left
ventricle ejection fraction. The association of ventricular
fibrilation causes cardiac arrest and death takes place in about
80% of cases (165,166). The mechanism is different for
hemodialysis patients. Thus, these patients present with arterial
wall stiffening, valvular and vascular calcifications, affecting
especially the vascular media, not the intima (167). A study on 1,200 patients showed
that a reduction in left ventricle ejection fraction occurs in only
13% of the cases (168). On the
other hand, it seems that hemodialysis patients with SCD and left
ventricular hypertrophy present diastolic dysfunction. Studies show
that left ventricular hypertrophy (LVH) is a risk factor for sudden
death in this group of patients (169). More than 70% of SCD patients had
LVH (170,171).
In chronic hemodialysis patients there is a series
of factors which trigger arrhythmias: Low content calcium of the
dialysate, aggressive ultrafiltration, hyperkalemia and rapid
potassium elimination, especially in patients who receive
hemodialysis three times a week, during the session following the
longest interdialysis pause (Monday and Tuesday) (164). The use of a high bicarbonate
concentration in the dialysate causes metabolic alkalosis,
associated with hypocalcemia, hemodynamic instability and the
elongation of the QT interval (164).
Another risk factor for SCD is the overexpression
of angiotensin II. There is a range of angiotensin II mechanisms of
action, such as stimulation of fibrosis and inflammation, increased
activity of the sinus node and of the His-Purkinje system,
alteration of Ca2+, K+ and Na+
exchange at the cell level, increased sympathetic nervous system
activity, and aldosteron release (109).
SCA patients lose consciousness within seconds or
minutes because of insufficient cerebral irrigation. These patients
do not generally have warning symptoms or they may have unspecified
signs, such as discomfort in the thorax, palpitations, dyspnea and
fatigability. Ventricular tachyarrhythmias are the most common and
are associated with cardiorespiratory arrest, both in the general
population and in dialysis patients (172). Studies have shown that the indexed
left ventricular mass is the most powerful predictor for
ventricular arrhytmia in CKD patients (173). A study of 75 chronic hemodialysis
patients who had a portable defibrillator showed that 79% of
cardiac arrests were caused by ventricular tachycardia or
ventricular fibrilation (174).
There are studies showing that supraventricular
rhythm disorders can lead to cardiorespiratory arrest in
hemodialysis patients. SCD patients can suffer from bradycardia
(26.3%), asystole (15.8%) and electromechanical dissociation
(15.8%) (175). There are few data
regarding fatal supraventricular arrythmias, which do not respond
to the classical ressuscitation measures, electrical defibrilation
included. In order to obtain more knowledge in this respect, the
Monitoring in Dialysis Study reports on the use of implantable loop
recorders employed to analyze the type and frequency of arrythmias
on the traces obtained in a 6-month period (176). The final results of the study have
not been published yet, but the preliminary results for 66 enrolled
patients show the presence of atrial arrythmias (57.4%),
bradycardia (15%) and of ventricular arrythmia in only 9.1% of the
cases, mainly in the postdialysis period (177).
Taking into consideration the frequency and the
importance of this phenomenon, identifying the risk factors for SCD
proves to be significant. SCD risk occurs in the first three months
following the onset of hemodialysis and builds up directly
proportional with the period of dialysis, which means both new and
old patients can be considered at risk (164). In addition, at risk for SCD are
hemodialysis patients who suffer from large IDWG, extreme
variations in serum potassium (hypo/hyperpotassemia), uncorrected
mineral or bone deficiencies or malnutrition (89,178).
SCD hemodialysis patients are generally diabetics,
with preexisting cardiac pathology and a history of cardiac
arrythmias (179-181).
There are studies that emphasize the strong association between SCD
and inflammatory markers including interleukin (IL)-6(181), C reactive protein (CRP) (182) and adiponectin (183), but also between SCD and nutrition
markers: Serum albumin (182) and
predialysis serum creatinine (89).
Several drugs have proven useful in lowering the
risk of SCD. In this respect, β adrenergic blockers were found to
reduce SCD risk following myocardial infarction (184). A study of 200 hemodialysis
patients assessed the efficiency of lisinopril vs. atenolol in
reducing left ventricle hypertrophy and reported a significantly
lower number of hospital admissions for cardiovascular events and
cardiac failure in a group of patients who were treated with
atenolol (185). Patients treated
with atenolol had fewer episodes of arrythmia and cardiorespiratory
arrest. On the other hand, the HEMO study did not show an
association between β-blockers and the decrease in SCD risk
(186). However, the initiation of
treatment with β-blockers in hemodialysis patients to prevent SCD
cannot be recommended, based on present data.
There is no clear evidence that treatment with
cholesterol-lowering medication (statin therapy) or
renin-angiotensin-aldosterone system blockers, which is beneficial
in the general population in lowering cardiovascular risk, would
prove equally beneficial in hemodialysis patients (164).
The parameters of dialysis can be adjusted so as to
prevent SCD. Thus, a low potassium level in the dialysate (<2
mEq/l) in patients with predialysis serum potassium within a normal
limit increases the risk of SCD (89,107,161). A study on 30 hemodialysis patients
who received potassium modeling vs. a fixed potassium dialysate
demonstrated a decrease in ventricular arrythmias, which suggests
that the gradual elimination of potassium excess has a protective
effect compared to its linear elimination, the latter one with
aritmogenous effect. Unfortunately, potassium modeling is not
widely available in hemodialysis centers (164).
In addition to the role of calcium in the
dialysate, further studies are necessary to explain the role of
vitamin D analogues, of phosphate binders and of calcimetics in
SCD. Furthermore, it is necessary to control the phosphate serum
levels, taking into account that the relationship between
hyperphosphatemy and mortality has been demonstrated, probably
because of myocardial calcifications and hemodynamic changes in
microcirculation (193).
In addition to the electrolytic exchanges in
hemodialysis patients, the relationship between cardiovascular
mortality and high rate of ultrafiltration has been demonstrated
(194). An ultrafiltration rate
over 10 ml/kg/h is associated with increased mortality (195). It is necessary to train the
patient to respect dietary recommandations (to limit salt and fluid
intake), to increase the frequency and duration of the dialysis
sessions and to maintain a small gradient between serum sodium and
the sodium in the dialysate (196). The temperature in the dialysate
influences blood pressure and coronary circulation, a decrease in
the dialysate temperature causing a decrease in IDH and myocardial
ischemic injury, and the risk of cardiovascular death (197).
Acute intradialytic cardiovascular complications
are commonly encountered in clinical practice and influence the
quality of life, such as morbidity and the mortality rate of
dialysis patients. In order to have detailed knowledge concerning
the risk factors and the pathogenic mechanisms and to ensure an
optimal management of these complications, more studies must be
conducted.
Not applicable.
No funding was received.
All information included in this review is
documented by relevant references.
DT, MDT, DGB, AT, OS, IAV, AM, PCC, CIC, ME, DM,
RIP and DI designed the review and wrote the manuscript and
performed the literature search and selected the included studies.
DT, MDT, DGB, AT, OS, IAV, AM, PCC, CIC, ME, DM, RIP and DI
critically revised the manuscript. All authors read and approved
the final manuscript. The contributions of all the authors toward
this review are greatly valued and appreciated.
Not applicable.
Not applicable.
The authors declare that they have no competing
interests.
|
1
|
Ozkan G and Ulusoy S: Acute Complications
of Hemodialysis. In: Technical Problems in Patients on
Hemodialysis. Penido MG (ed). InTech, 2011. https://www.intechopen.com/books/technical-problems-in-patients-on-hemodialysis/acute-complications-of-hemodialysis.
Accessed December 7, 2011.
|
|
2
|
Balan DG, Stroescu AEB, Tanasescu MD,
Diaconescu A, Raducu L, Mihai A, Tanase M, Stanescu II and Ionescu
D: Nutritional intervention in patients with diabetic renal
diseases. A brief presentation. Rev Chim. 69:3178–3182. 2018.
|
|
3
|
Balcangiu-Stroescu AE, Tanasescu MD,
Diaconescu AC, Raducu L, Balan DG, Mihai A, Tanase M, Stanescu II
and Ionescu D: Diabetic nephropathy: A concise assessment of the
causes, risk factors and implications in diabetic patients. Rev
Chim. 69:3118–3121. 2018.
|
|
4
|
Mandita A, Timofte D, Balcangiu-Stroescu
AE, Balan DG, Raducu L, Tanasescu MD, Diaconescu AC, Dragos D,
Cosconel CI, Stoicescu SM, et al: Treatment of high blood pressure
in patients with chronic renal disease. Rev Chim. 70:993–995.
2019.
|
|
5
|
National Institute of Diabetes and
Digestive and Kidney Diseases (NIDDK): Kidney Disease Statistics
for the United States. https://www.niddk.nih.gov/health-information/health-statistics/kidney-disease.
Accessed November 2, 2019.
|
|
6
|
Saha M and Allon M: Diagnosis, treatment,
and prevention of hemodialysis emergencies. Clin J Am Soc Nephrol.
12:357–369. 2017.PubMed/NCBI View Article : Google Scholar
|
|
7
|
United States Renal Data System (USRDS):
2009 Annual Report United States Renal Data System. https://www.usrds.org/.
|
|
8
|
Bregman H, Daugirdas JT and Ing TS:
Complications during hemodialysis. In: Handbook of Dialysis.
Dauugirdas JT and Ing TS (eds). Little, Brown, New York, NY, p149,
1994.
|
|
9
|
Paparello J, Kshirsagar A and Batlle D:
Comorbidity and cardiovascular risk factors in patients with
chronic kidney disease. Semin Nephrol. 22:494–506. 2002.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Olariu L, Dumitriu B, Craciun L, Buse E,
Rosoiu N, Bojinca M and Papacocea T: The in vitro influence of a
pharmaceutically active small sea fish extract on apoptosis and
proliferation mechanisms amplified by inflammatory conditions.
Farmacia. 67:140–145. 2019.
|
|
11
|
Balcangiu-Stroescu AE, Tanasescu MD,
Diaconescu AC, Raducu L, Constantin AM, Balan DG, Ţarmure V and
Ionescu D: Cardiovascular comorbidities, inflammation and serum
albumin levels in a group of hemodialysis patients. Rev Chim
Buchar. 69:926–929. 2018.
|
|
12
|
Timofte D, Mandita A, Balcangiu-Stroescu
AE, Balan DG, Raducu L, Tanasescu MD, Diaconescu AC, Dragos D,
Cosconel CI, Stoicescu SM, et al: Hyperuricemia and cardiovascular
diseases-clinical and paraclinical correlations. Rev Chim Buchar.
70:1045–1046. 2019.
|
|
13
|
Totan A, Balcangiu-Stroescu AE, Melescanu
Imre M, Miricescu D, Balan DG, Stanescu II, Ionescu D, Timofte D,
Tanasescu MD and Greabu M: XOR-Possible correlations with oxidative
stress and inflammation markers in the context of diabetic kidney
disease. Rev Chim Buchar. 70:1396–1398. 2019.
|
|
14
|
Sars B, van der Sande FM and Kooman PJ:
Intradialytic Hypotension: Mechanisms and outcome. Blood Purif.
49:158–167. 2020.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Flythe JE, Xue H, Lynch KE, Curhan GC and
Brunelli SM: Association of mortality risk with various definitions
of intradialytic hypotension. J Am Soc Nephrol. 26:724–734.
2015.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Sands JJ, Usvyat LA, Sullivan T, Segal JH,
Zabetakis P, Kotanko P, Maddux FW and Diaz-Buxo JA: Intradialytic
hypotension: Frequency, sources of variation and correlation with
clinical outcome. Hemodial Int. 18:415–422. 2014.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Reilly RF: Attending rounds: A patient
with intradialytic hypotension. Clin J Am Soc Nephrol. 9:798–803.
2014.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Assimon MM and Flythe JE: Definitions of
intradialytic hypotension. Semin Dial. 30:464–472. 2017.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Masani NN, Miyawaki N and Maesaka JK: A
patient with an uncommon etiology of intradialytic hypotension.
Semin Dial. 18:435–439. 2005.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Roy PN and Danziger RS: Dialysate
magnesium concentration predicts the occurrence of intradialytic
hypotension. J Am Soc Nephrol. 7(1496)1996.
|
|
21
|
Van der Sande FM, Cheriex EC, van Kuijk WH
and Leunissen KM: Effect of dialysate calcium concentrations on
intradialytic blood pressure course in cardiac-compromised
patients. Am J Kidney Dis. 32:125–131. 1998.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Alappan R, Cruz D, Abu-Alfa AK,
Mahnensmith R and Perazella MA: Treatment of severe intradialytic
hypotension with the addition of high dialysate calcium
concentration to midodrine and/or cool dialysate. Am J Kidney Dis.
37:294–299. 2001.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Lin CJ, Chen CY, Wu PC, Pan CF, Shih HM,
Huang MY, Chou LH, Tang JS and Wu CJ: Intelligent system to predict
intradialytic hypotension in chronic hemodialysis. J Formos Med
Assoc. 117:888–893. 2018.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Seabra VF and Jaber BL: Acute
complications during hemodialysis. In: Comprehensive Clinical
Nephrology. Floege J, Johnson RJ and Feehally J (eds). Elsevier,
Philadelphia, PA, pp1306-1307, 2010.
|
|
25
|
Kooman JP, Katzarski K, van der Sande FM,
Leunissen KM and Kotanko P: Hemodialysis: A model for extreme
physiology in a vulnerable patient population. Semin Dial.
31:500–506. 2018.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Barth C, Boer W, Garzoni D, Kuenzi T, Ries
W, Schaefer R, Schneditz D, Tsobanelis T, van der Sande F, Wojke R,
et al: Characteristics of hypotension-prone haemodialysis patients:
Is there a critical relative blood volume? Nephrol Dial Transplant.
18:1353–1360. 2003.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Aronoff GR: The effect of treatment time,
dialysis frequency, and ultrafiltration rate on intradialytic
hypotension. Semin Dial. 30:489–491. 2017.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Levin NW, de Abreu MHFG, Borges LE,
Tavares HA, Sarwar R, Gupta S, Hafeez T, Lev S and Williams C:
Hemodynamic response to fluid removal during hemodialysis:
Categorization of causes of intradialytic hypotension. Nephrol Dial
Transplant. 33:1643–1649. 2018.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Feng Y, Zou Y, Zheng Y, Levin NW and Wang
L: The value of non-invasive measurement of cardiac output and
total peripheral resistance to categorize significant changes of
intradialytic blood pressure: A prospective study. BMC Nephrol.
19(310)2018.PubMed/NCBI View Article : Google Scholar
|
|
30
|
van der Sande FM, Dekker MJ, Leunissen KM
and Kooman JP: Novel insights into the pathogenesis and prevention
of intradialytic hypotension. Blood Purif. 45:230–235.
2018.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Assa S, Hummel YM, Voors AA, Kuipers J,
Westerhuis R, de Jong PE and Franssen CFM: Hemodialysis-induced
regional left ventricular systolic dysfunction: Prevalence, patient
and dialysis treatment-related factors, and prognostic
significance. Clin J Am Soc Nephrol. 7:1615–1623. 2012.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Seong EY, Zheng Y, Winkelmayer WC,
Montez-Rath ME and Chang TI: The relationship between intradialytic
hypotension and hospitalized mesenteric ischemia: A case-control
study. Clin J Am Soc Nephrol. 13:1517–1525. 2018.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Magder SA: The highs and lows of blood
pressure: Toward meaningful clinical targets in patients with
shock. Crit Care Med. 42:1241–1251. 2014.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Charytan DM, Skali H, Shah NR, Veeranna V,
Cheezum MK, Taqueti VR, Kato T, Bibbo CR, Hainer J, Dorbala S, et
al: Coronary flow reserve is predictive of the risk of
cardiovascular death regardless of chronic kidney disease stage.
Kidney Int. 93:501–509. 2018.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Burkhardt D, Bartosova M, Schaefer B,
Grabe N, Lahrmann B, Nasser H, Freise C, Schneider A, Lingnau A,
Degenhardt P, et al: Reduced microvascular density in omental
biopsies of children with chronic kidney disease. PLoS One.
11(e0166050)2016.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Mitsides N, Cornelis T, Broers NJ,
Diederen NM, Brenchley P, van der Sande FM, Schalkwijk CG, Kooman
JP and Mitra S: Extracellular overhydration linked with endothelial
dysfunction in the context of inflammation in haemodialysis
dependent chronic kidney disease. PLoS One.
12(e0183281)2017.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Amann K, Wiest G, Zimmer G, Gretz N, Ritz
E and Mall G: Reduced capillary density in the myocardium of uremic
rats-a stereological study. Kidney Int. 42:1079–1085.
1992.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Mancini E, Perazzini C, Gesualdo L,
Aucella F, Limido A, Scolari F, Savoldi S, Tramonti M, Corazza L,
Atti M, et al: Intra-dialytic blood oxygen saturation
(SO2): Association with dialysis hypotension (the SOGLIA
Study). J Nephrol. 30:811–819. 2016.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Campos I, Chan L, Zhang H, Deziel S,
Vaughn C, Meyring-Wösten A and Kotanko P: Intradialytic hypoxemia
in chronic hemodialysis patients. Blood Purif. 41:177–187.
2016.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Knoll GA, Grabowski JA, Dervin GF and
O'Rourke K: A randomized, controlled trial of albumin versus saline
for the treatment of intradialytic hypotension. J Am Soc Nephrol.
15:487–492. 2004.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Nette RW, Krepel HP, van den Meiracker AH,
Weimar W and Zietse R: Specific effect of the infusion of glucose
on blood volume during haemodialysis. Nephrol Dial Transplant.
17:1275–1280. 2002.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Kouw PM, Kooman JP, Cheriex EC, Olthof CG,
de Vries PM and Leunissen KM: Assessment of postdialysis dry
weight: A comparison of techniques. J Am Soc Nephrol. 4:98–104.
1993.PubMed/NCBI
|
|
43
|
Donauer J, Kölblin D, Bek M, Krause A and
Bohler J: Ultrafiltration profiling and measurement of relative
blood volume as strategies to reduce hemodialysis-related side
effects. Am J Kidney Dis. 36:115–123. 2000.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Déziel C, Bouchard J, Zellweger M and
Madore F: Impact of hemocontrol on hypertension, nursing
interventions, and quality of life: A randomized, controlled trial.
Clin J Am Soc Nephrol. 2:661–668. 2007.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Basile C, Vernaglione L, Di Iorio B,
Bellizzi V, Chimienti D, Lomonte C, Rubino A and D'Ambrosio N:
Development and validation of bioimpedance analysis prediction
equations for dry weight in hemodialysis patients. Clin J Am Soc
Nephrol. 2:675–680. 2007.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Palmer BF and Henrich WL: Recent advances
in the prevention and management of intradialytic hypotension. J Am
Soc Nephrol. 19:8–11. 2008.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Caetano C, Valente A, Oliveira T and
Garagarza C: Coffee consumption in hemodialysis patients: How many?
Eur J Clin Nutr. 73:924–929. 2018.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Papacocea R, Badarau IA, Ciornei CM,
Burciulescu Lider S and Papacocea MT: The effects of caffeine
intake on cardiovascular in sleep deprived medical residents. Rev
Chim Buchar. 70:1445–1448. 2019.
|
|
49
|
Kearney MT, Cowley AJ, Stubbs TA, Evans A
and Macdonald IA: Depressor action of insulin on skeletal muscle
vasculature: A novel mechanism for postprandial hypotension in the
elderly. J Am Coll Cardiol. 31:209–216. 1998.PubMed/NCBI View Article : Google Scholar
|
|
50
|
Kutlugün AA, Erdem Y, Okutucu S, Yorgun H,
Atalar E and Arici M: Effects of lowering dialysate sodium on
flow-mediated dilatation in patients with chronic kidney disease.
Nephrol Dial Transplant. 26:3678–3682. 2011.PubMed/NCBI View Article : Google Scholar
|
|
51
|
Mc Causland FR, Brunelli SM and Waikar SS:
Dialysate sodium, serum sodium and mortality in maintenance
hemodialysis. Nephrol Dial Transplant. 27:1613–1618.
2012.PubMed/NCBI View Article : Google Scholar
|
|
52
|
Basile C, Pisano A, Lisi P, Rossi L,
Lomonte C and Bolignano D: High versus low dialysate sodium
concentration in chronic haemodialysis patients: A systematic
review of 23 studies. Nephrol Dial Transplant. 31:548–563.
2016.PubMed/NCBI View Article : Google Scholar
|
|
53
|
Marshall MR, Vandal AC, de Zoysa JR,
Gabriel RS, Haloob IA, Hood JC, Irvine JH, Matheson PJ, McGregor
DOR, Rabindranath KS, et al: Effect of low-sodium versus
conventional sodium dialysate on left ventricular mass in home and
self-care satellite facility hemodialysis patients: A randomized
clinical trial. J Am Soc Nephrol. 31:1078–1091. 2020.PubMed/NCBI View Article : Google Scholar
|
|
54
|
Jindal K, Chan CT, Deziel C, Hirsch D,
Soroka SD, Tonelli M and Culleton BF: Canadian Society of
Nephrology Committee for Clinical Practice Guidelines. Hemodialysis
clinical practice guidelines for the Canadian Society of
Nephrology. J Am Soc Nephrol. 17 (3 Suppl 1):S1–S27.
2006.PubMed/NCBI View Article : Google Scholar
|
|
55
|
Maggiore Q, Pizzarelli F, Zoccali C, Sisca
S, Nicolo F and Parlongo S: Effect of extracorporeal blood cooling
on dialytic arterial hypotension. Proc Eur Dial Transplant Assoc.
18:597–602. 1981.PubMed/NCBI
|
|
56
|
Lindholm T, Thysell H, Yamamoto Y,
Forsberg B and Gullberg CA: Temperature and vascular stability in
hemodialysis. Nephron. 39:130–133. 1985.PubMed/NCBI View Article : Google Scholar
|
|
57
|
Sherman RA, Rubin MP, Cody RP and Eisinger
RP: Amelioration of hemodialysis-associated hypotension by the use
of cool dialysate. Am J Kidney Dis. 5:124–127. 1985.PubMed/NCBI View Article : Google Scholar
|
|
58
|
Quereda C, Orofino L, Marcen R, Sabater J,
Matesanz R and Ortuno J: Influence of dialysate and membrane
biocompatibility on hemodynamic stability in hemodialysis. Int J
Artif Organs. 11:259–264. 1988.PubMed/NCBI
|
|
59
|
Orofino L, Marcén R, Quereda C,
Villafruela JJ, Sabater J, Matesanz R, Pascual J and Ortuno J:
Epidemiology of symptomatic hypotension in hemodialysis: Is cool
dialysate beneficial for all patients? Am J Nephrol. 10:177–180.
1990.PubMed/NCBI View Article : Google Scholar
|
|
60
|
Jost CM, Agarwal R, Khair-el-Din T,
Grayburn PA, Victor RG and Henrich WL: Effects of cooler
temperature dialysate on hemodynamic stability in ‘problem̓
dialysis patients. Kidney Int. 44:606–612. 1993.PubMed/NCBI View Article : Google Scholar
|
|
61
|
Schneditz D, Martin K, Krämer M, Kenner T
and Skrabal F: Effect of controlled extracorporeal blood cooling on
ultrafiltration-induced blood volume changes during hemodialysis. J
Am Soc Nephrol. 8:956–964. 1997.PubMed/NCBI
|
|
62
|
Cruz DN, Mahnensmith RL, Brickel HM and
Perazella MA: Midodrine and cool dialysate are effective therapies
for symptomatic intradialytic hypotension. Am J Kidney Dis.
33:920–926. 1999.PubMed/NCBI View Article : Google Scholar
|
|
63
|
Yu AW, Ing TS, Zabaneh RI and Daugirdas
JT: Effect of dialysate temperature on central hemodynamics and
urea kinetics. Kidney Int. 48:237–243. 1995.PubMed/NCBI View Article : Google Scholar
|
|
64
|
Schneditz D, Ronco C and Levin N:
Temperature control by the blood temperature monitor. Semin Dial.
16:477–482. 2003.PubMed/NCBI View Article : Google Scholar
|
|
65
|
Pérgola PE, Habiba NM and Johnson JM: Body
temperature regulation during hemodialysis in long-term patients:
Is it time to change dialysate temperature prescription? Am J
Kidney Dis. 44:155–165. 2004.PubMed/NCBI View Article : Google Scholar
|
|
66
|
Pizzarelli F: From cold dialysis to
isothermic dialysis: A twenty-five year voyage. Nephrol Dial
Transplant. 22:1007–1012. 2007.PubMed/NCBI View Article : Google Scholar
|
|
67
|
Mustafa RA, Bdair F, Akl EA, Garg AX,
Thiessen-Philbrook H, Salameh H, Kisra S, Gihad N, Al-Jaishi A,
Patel P, et al: Effect of lowering the dialysate temperature in
chronic hemodialysis: A Systematic review and meta-analysis. Clin J
Am Soc Nephrol. 11:442–457. 2016.PubMed/NCBI View Article : Google Scholar
|
|
68
|
Flynn JJ III, Mitchell MC, Caruso FS and
McElligott MA: Midodrine treatment for patients with hemodialysis
hypotension. Clin Nephrol. 45:261–267. 1996.PubMed/NCBI View Article : Google Scholar
|
|
69
|
Montagnac R, Clavel P, Delhotal-Landes B,
Flouvat B, Poulain S and Schllinger F: Use of midodrine (Gutron) to
treat permanent hypotension in a chronic hemodialysis patient. Clin
Nephrol. 56:162–168. 2001.PubMed/NCBI
|
|
70
|
Perazella MA: Pharmacologic options
available to treat symptomatic intradialytic hypotension. Am J
Kidney Dis. 38 (4 Suppl 4):S26–S36. 2001.PubMed/NCBI View Article : Google Scholar
|
|
71
|
Prakash S, Garg AX, Heidenheim AP and
House AA: Midodrine appears to be safe and effective for
dialysis-induced hypotension: A systematic review. Nephrol Dial
Transplant. 19:2553–2558. 2004.PubMed/NCBI View Article : Google Scholar
|
|
72
|
Low PA, Gilden JL, Freeman R, Sheng KN and
McElligott MA: Efficacy of midodrine vs. placebo in neurogenic
orthostatic hypotension. A randomized, double-blind multicenter
study. Midodrine Study Group. JAMA. 277:1046–1051. 1997.PubMed/NCBI
|
|
73
|
Dheenan S, Venkatesan J, Grubb BP and
Henrich WL: Effect of sertraline hydrochloride on dialysis
hypotension. Am J Kidney Dis. 31:624–630. 1998.PubMed/NCBI View Article : Google Scholar
|
|
74
|
Imai E, Fujii M, Kohno Y, Kageyama H,
Nakahara K, Hori M and Tsubakihara Y: Adenosine A1 receptor
antagonist improves intradialytic hypotension. Kidney Int.
69:877–883. 2006.PubMed/NCBI View Article : Google Scholar
|
|
75
|
Tattersall J, Martin-Malo A, Pedrini L,
Basci A, Canaud B, Fouque D, Haage P, Kinner K, Kooman J,
Pizzarelli F, et al: EBPG guideline on dialysis strategies. Nephrol
Dial Transplant. 22 (Suppl 2):ii5–ii21. 2007.PubMed/NCBI View Article : Google Scholar
|
|
76
|
Merino JL, Rivera M, Teruel JL, Mercén R
and Ortuño J: CAPD as treatment of chronic debilitating
hemodialysis hypotension. Perit Dial Int. 22(429)2002.PubMed/NCBI
|
|
77
|
Van Buren PN, Kim C, Toto RD and Inrig JK:
The prevalence of persistent intradialytic hypertension in a
hemodialysis population with extended follow-up. Int J Artif
Organs. 35:1031–1038. 2012.PubMed/NCBI View Article : Google Scholar
|
|
78
|
Chou KJ, Lee PT, Chen CL, Chiou CW, Hsu
CY, Chung HM, Liu CP and Fang HC: Physiological changes during
hemodialysis in patients with intradialysis hypertension. Kidney
Int. 69:1833–1838. 2006.PubMed/NCBI View Article : Google Scholar
|
|
79
|
Inrig JK, Van Buren P, Kim C,
Vongpatanasin W, Povsic TJ and Toto RD: Intradialytic hypertension
and its association with endothelial cell dysfunction. Clin J Am
Soc Nephrol. 6:2016–2024. 2011.PubMed/NCBI View Article : Google Scholar
|
|
80
|
Van Buren PN: Pathophysiology and
implications of intradialytic hypertension. Curr Opin Nephrol
Hypertens. 26:303–310. 2017.PubMed/NCBI View Article : Google Scholar
|
|
81
|
Park J, Rhee C, Sim J, Kim YL, Ricks J,
Streja E, Vashistha T, Tolouian R, Kovesdy CP and Zadeh K: A
comparative effectiveness research study of the change in blood
pressure during hemodialysis treatment and survival. Kidney Int.
84:795–802. 2013.PubMed/NCBI View Article : Google Scholar
|
|
82
|
Agarwal R and Light RP: Intradialytic
hypertension is a marker of volume excess. Nephrol Dial Transplant.
25:3355–3361. 2010.PubMed/NCBI View Article : Google Scholar
|
|
83
|
Van Buren PN, Kim C, Toto R and Inrig JK:
Intradialytic hypertension and the association with interdialytic
ambulatory blood pressure. Clin J Am Soc Nephrol. 6:1684–1691.
2011.PubMed/NCBI View Article : Google Scholar
|
|
84
|
Agarwal R, Alborzi P, Satyan S and Light
RP: Dry-weight reduction in hypertensive hemodialysis patients
(DRIP): A randomized, controlled trial. Hypertension. 53:500–507.
2009.PubMed/NCBI View Article : Google Scholar
|
|
85
|
McCausland F and Waikar S: Association of
predialysis calculated plasma osmolarity with intradialytic blood
pressure decline. Am J Kidney Dis. 66:499–506. 2015.PubMed/NCBI View Article : Google Scholar
|
|
86
|
Movilli E, Camerini C, Gaggia P, Zubani R,
Feller P, Poiatti P, Pola A, Carli O, Valzorio B and Cancarini G:
Role of dialysis sodium gradient on intradialytic hypertension: An
observational study. Am J Nephrol. 38:413–419. 2013.PubMed/NCBI View Article : Google Scholar
|
|
87
|
Dolson GM, Ellis KJ, Bernardo MV, Prakash
R and Adrogué HJ: Acute decreases in serum potassium augment blood
pressure. Am J Kidney Dis. 26:321–326. 1995.PubMed/NCBI View Article : Google Scholar
|
|
88
|
Gabutti L, Bianchi G, Soldini D, Marone C
and Burnier M: Haemodynamic consequences of changing bicarbonate
and calcium concentrations in haemodialysis fluids. Nephrol Dial
Transplant. 24:973–981. 2009.PubMed/NCBI View Article : Google Scholar
|
|
89
|
Pun PH, Lehrich RW, Honeycutt EF, Herzog
CA and Middleton JP: Modifiable risk factors associated with sudden
cardiac arrest within hemodialysis clinics. Kidney Int. 79:218–227.
2011.PubMed/NCBI View Article : Google Scholar
|
|
90
|
Fellner SK, Lang RM, Neumann A, Spencer
KT, Bushinsky DA and Borow KM: Physiological mechanisms for
calcium-induced changes in systemic arterial pressure in stable
dialysis patients. Hypertension. 13:213–218. 1989.PubMed/NCBI View Article : Google Scholar
|
|
91
|
LeBeouf A, Mac-Way F, Utescu MS, Chbinou
N, Douville P, Desmeules S and Agharazii M: Effects of acute
variation of dialysate calcium concentrations on arterial stiffness
and aortic pressure waveform. Nephrol Dial Transplant.
24:3788–3794. 2009.PubMed/NCBI View Article : Google Scholar
|
|
92
|
Van Buren PN, Zhou Y, Neyra JA, Xiao G,
Vongpatanasin W, Inrig J and Toto R: Extracellular volume overload
and increased vasoconstriction in patients with recurrent
intradialytic hypertension. Kidney Blood Press Res. 41:802–814.
2016.PubMed/NCBI View Article : Google Scholar
|
|
93
|
Raj DSC, Vincent B, Simpson K, Sato E,
Jones KL, Welbourne TC, Levi M, Shah V, Blandon P, Zager P and
Robbins RA: Hemodynamic changes during hemodialysis: Role of nitric
oxide and endothelin. Kidney Int. 61:697–704. 2002.PubMed/NCBI View Article : Google Scholar
|
|
94
|
El-Shafey EM, El-Nagar GF, Selim MF,
El-Sorogy HA and Sabry AA: Is there a role for endothelin-1 in the
hemodynamic changes during hemodialysis? Clin Exp Nephrol.
12:370–375. 2008.PubMed/NCBI View Article : Google Scholar
|
|
95
|
Gutierrez-Adrianzen OA, Moraes ME, Almeida
AP, Lima JW, Marinho MF, Marques AL, Madeiro JP, Nepomuceno L, da
Silva JM Jr, Silva GB Jr, et al: Pathophysiological, cardiovascular
and neuroendocrine changes in hypertensive patients during the
hemodialysis session. J Hum Hypertens. 29:366–372. 2015.PubMed/NCBI View Article : Google Scholar
|
|
96
|
Teng J, Tian J, Lv WL, Zhang XY, Zou JZ,
Fang Y, Yu J, Shen B, Liu ZH and Ding XQ: Inappropriately elevated
endothelin-1 plays a role in the pathogenesis of intradialytic
hypertension. Hemodial Int. 19:279–286. 2015.PubMed/NCBI View Article : Google Scholar
|
|
97
|
Sinha AD, Light RP and Agarwal R: Relative
plasma volume monitoring during hemodialysis AIDS the assessment of
dry weight. Hypertension. 55:305–311. 2010.PubMed/NCBI View Article : Google Scholar
|
|
98
|
Inrig J, Van Buren P, Kim C, Vongpatanasin
W, Povsic TJ and Toto R: Probing the mechanisms of intradialytic
hypertension: A pilot study targeting endothelial cell dysfunction.
Clin J Am Soc Nephrol. 7:1300–1309. 2012.PubMed/NCBI View Article : Google Scholar
|
|
99
|
Inrig JK, Molina C, D'Silva K, Kim C, Van
Buren P, Allen J and Toto R: Effect of low versus high dialysate
sodium concentration on blood pressure and endothelial-derived
vasoregulators during hemodialysis: A randomized crossover study.
Am J Kidney Dis. 65:464–473. 2015.PubMed/NCBI View Article : Google Scholar
|
|
100
|
Voroneanu L and Covic A: Arrhythmias in
hemodialysis patients. J Nephrol. 22:716–725. 2009.PubMed/NCBI
|
|
101
|
United States Renal Data System (USRDS):
USRDS 2013 Annual Data Report: Atlas of End-Stage Renal Disease in
the United States. National Institutes of Health, National
Institute of Diabetes and Digestive and Kidney Diseases, Bethesda,
MD, 2013. https://www.usrds.org/atlas13.aspx Accessed June 25,
2020.
|
|
102
|
Zebe H: Atrial fibrillation in dialysis
patients. Nephrol Dial Transplant. 15:765–768. 2000.PubMed/NCBI View Article : Google Scholar
|
|
103
|
Vazquez E, Sanchez-Perales C, Borrego F,
Garcia-Cortes MJ, Lozano C, Guzman M, Gil JM, Borrego MJ and Perez
V: Influence of atrial fibrillation on the morbido-mortality of
patients on hemodialysis. Am Heart J. 140:886–890. 2000.PubMed/NCBI View Article : Google Scholar
|
|
104
|
Genovesi S, Vincenti A, Rossi E, Pogliani
D, Acquistapace I, Stella A and Valsecchi MG: Atrial fibrillation
and morbidity and mortality in a cohort of long-term hemodialysis
patients. Am J Kidney Dis. 51:255–262. 2008.PubMed/NCBI View Article : Google Scholar
|
|
105
|
Abbott KC, Trespalacios FC, Taylor AJ and
Agodoa LY: Atrial fibrillation in chronic dialysis patients in the
United States: Risk factors for hospitalization and mortality. BMC
Nephrol. 4(1)2003.PubMed/NCBI View Article : Google Scholar
|
|
106
|
Hecking E, Bragg-Gresham JL, Rayner HC,
Pisoni RL, Andreuci VE, Combe C, Greenwood R, McCullough Feldman
HI, Young EW, et al: Haemodialysis prescription, adherence and
nutritional indicators in five European countries: Results from the
dialysis Outcomes and practice patterns Study (DOPPS). Nephrol Dial
Transplant. 19:100–107. 2004.PubMed/NCBI View Article : Google Scholar
|
|
107
|
Karnik JA, Young BS, Lew NL, Herget M,
Dubinsky C, Lazarus JM and Chertow GM: Cardiac arrest and sudden
death in dialysis units. Kidney Int. 60:350–357. 2001.PubMed/NCBI View Article : Google Scholar
|
|
108
|
Genovessi S, Pogliani D, Faini A,
Valsecchi MG, Riva A, Stefani F, Acquistapace I, Stella A, Bonforte
G, DeVecchi A, et al: Prevalence of atrial fibrillation and
associated factors in a population of long-term hemodialysis
patients. Am J Kidney Dis. 46:897–902. 2005.PubMed/NCBI View Article : Google Scholar
|
|
109
|
Fischer R, Dechend R, Gapelyuk A,
Shagdarsuren E, Gruner K, Gratze A, Gratze P, Qadri F, Wellner M,
Fiebeler A, et al: Angiotensin II-induced sudden arrhythmic death
and electrical remodeling. Am J Physiol Heart Circ Physiol.
293:H1242–H1253. 2007.PubMed/NCBI View Article : Google Scholar
|
|
110
|
Fierbințeanu-Braticevici C, Papacocea R,
Tribus L and Băicuş C: Role of 13C Methacetin breath
test for noninvasive staging of liver fibrosis in patients with
chronic hepatitis C. Indian J Med Res. 140:123–129. 2014.PubMed/NCBI
|
|
111
|
Takeda K, Nakamoto M, Baba M, Tanaka T,
Yasunaga C, Nishihara G, Matsuo K and Urabe M: Echocardiographic
evaluation in long-term continuous ambulatory peritoneal dialysis
compared with the hemodialysis patients. Clin Nephrol. 49:308–312.
1998.PubMed/NCBI
|
|
112
|
Tica OA, Tica O, Antal L, Hatos A, Popescu
MI, Pantea Stoian A, Bratu OG, Găman MA, Pituru SM and Diaconu CC:
Modern oral anticoagulant treatment in patients with atrial
fibrillation and heart failure: Insights from the clinical
practice. Farmacia. 66:972–976. 2018.
|
|
113
|
Laslo C, Pantea Stoian A, Socea B,
Paduraru D, Bodean O, Socea L, Neagu TP, Stanescu AMA, Marcu D and
Diaconu CC: New oral anticoagulants and their reversal agents. J
Mind Med Sci. 5:195–201. 2018.
|
|
114
|
Routledge HC, Chowdhary S and Townend JN:
Heart rate variability: A therapeutic target? J Clin Pharm Ther.
27:85–92. 2002.PubMed/NCBI View Article : Google Scholar
|
|
115
|
Herzog CA: Don't forget the defibrillator
in the dialysis unit. Nephrol Dial Transplant. 19:2959–2960.
2004.PubMed/NCBI View Article : Google Scholar
|
|
116
|
Sakhuja R, Keebler M, Lai TS, McLaughli
Gavin C, Thakur R and Bhatt DL: Meta-analysis of mortality in
dialysis patients with an implantable cardioverter defibrillator.
Am J Cardiol. 103:735–741. 2009.PubMed/NCBI View Article : Google Scholar
|
|
117
|
Anavekar NS, McMurray JJ, Velazquez EJ,
Solomon SD, Kober L, Rouleau JL, White HD, Nordlander R, Maggioni
A, Dickstein K, et al: Relation between renal dysfunction and
cardiovascular outcomes after myocardial infarction. N Engl J Med.
351:1285–1295. 2004.PubMed/NCBI View Article : Google Scholar
|
|
118
|
Go AS, Chertow GM, Fan D, McCulloch CE and
Hsu C: Chronic kidney disease and the risks of death,
cardiovascular events, and hospitalization. N Engl J Med.
351:1296–1305. 2004.PubMed/NCBI View Article : Google Scholar
|
|
119
|
Collins AJ, Foley RN, Herzog C, Chavers B,
Gilbertson D, Ishani A, Kasiske B, Liu J, Mau LW, McBean M, et al:
United States renal data system 2008 annual data report abstract.
Am J Kidney Dis. 53 (1 Suppl):S1–S374. 2009.PubMed/NCBI View Article : Google Scholar
|
|
120
|
Ix JH, Shlipak MG, Liu HH, Schiller NB and
Whooley MA: Association between renal insufficiency and inducible
ischemia in patients with coronary artery disease: The heart and
soul study. J Am Soc Nephrol. 14:3233–3238. 2003.PubMed/NCBI View Article : Google Scholar
|
|
121
|
Sarnak MJ, Levey AS, Schoolwerth AC,
Coresh J, Culleton B, Hamm LL, McCullough PA, Kasiske BL,
Kelepouris E, Klag MJ, et al: Kidney disease as a risk factor for
development of cardiovascular disease: A statement from the
American Heart Association Councils on Kidney in Cardiovascular
Disease, High Blood Pressure Research, Clinical Cardiology, and
Epidemiology and Prevention. Circulation. 108:2154–2169.
2003.PubMed/NCBI View Article : Google Scholar
|
|
122
|
Go AS, Bansal N, Chandra M, Lathon PV,
Fortmann SP, Iribarren C, Hsu CY and Hlatky M: ADVANCE Study
Investigators. Chronic kidney disease and risk for presenting with
acute myocardial infarction versus stable exertional angina in
adults with coronary heart disease. J Am Coll Cardiol.
58:1600–1607. 2011.PubMed/NCBI View Article : Google Scholar
|
|
123
|
Collins AJ, Foley RN, Herzog C, Chavers
BM, Gilbertson D, Ishani A, Kasiske BL, Liu J, Mau LW, McBean M, et
al: Excerpts from the US renal data system 2009 annual data report.
Am J Kidney Dis. 55 (1 Suppl 1):S1–S420, A6-A7. 2010.PubMed/NCBI View Article : Google Scholar
|
|
124
|
United States Renal Data System (USRDS):
USRDS 2013 annual data report: atlas of chronic kidney disease and
end-stage renal disease in the United States. National Institutes
of Health, National Institute of Diabetes and Digestive and Kidney
Diseases, Bethesda, MD, 2013. https://www.usrds.org/atlas13.aspx. Accessed August
19, 2016.
|
|
125
|
Herzog CA and Passman R: Evaluation of
sudden cardiac arrest and sudden cardiac death in dialysis
patients. UpToDate, 2020. https://www.uptodate.com/contents/evaluation-of-sudden-cardiac-arrest-and-sudden-cardiac-death-in-dialysis-patients.
Accessed June 2, 2020.
|
|
126
|
Muntner P, He J, Hamm L, Loria C and
Whelton PK: Renal insufficiency and subsequent death resulting from
cardiovascular disease in the United States. J Am Soc Nephrol.
13:745–753. 2002.PubMed/NCBI
|
|
127
|
Di Benedetto A, Marcelli D, D'Andrea A,
Cice G, D'Isa S, Cappabianca F, Pacchiano G, D'Amato R, Oggero AR,
Bonanno D, et al: Risk factors and underlying cardiovascular
diseases in incident ESRD patients. J Nephrol. 18:592–598.
2005.PubMed/NCBI
|
|
128
|
Shah DS, Polkinghorne KR, Pellicano R and
Kerr PG: Are traditional risk factors valid for assessing
cardiovascular risk in end-stage renal failure patients? Nephrology
(Carlton). 13:667–671. 2008.PubMed/NCBI View Article : Google Scholar
|
|
129
|
Levey AS, Coresh J, Balk E, Kausz AT,
Levin A, Steffes MW, Hogg RJ, Perrone RD, Lau J and Eknoyan G:
National Kidney Foundation. National kidney foundation practice
guidelines for chronic kidney disease: Evaluation, classification,
and stratification. Ann Intern Med. 139:137–147. 2003.PubMed/NCBI View Article : Google Scholar
|
|
130
|
Antman EM, Anbe DT, Armstrong PW, Bates
ER, Green LA, Hand M, Hochman JS, Krumhols H, Kushner FG, Lamas GA,
et al: ACC/AHA guidelines for the management of patients with
ST-elevation myocardial infarction-executive summary: A report of
the American College of Cardiology/American Heart Association Task
Force on Practice Guidelines (Writing Committee to Revise the 1999
Guidelines for the Management of Patients With Acute Myocardial
Infarction). Circulation. 110:588–636. 2004.PubMed/NCBI View Article : Google Scholar
|
|
131
|
Van der Velde M, Matsushita K, Coresh J,
Astor BC, Woodward M, Levey A, de Jong P and Gansevoort RT: Chronic
Kidney Disease Prognosis Consortium. van der Velde M, et al: Lower
estimated glomerular filtration rate and higher albuminuria are
associated with all-cause and cardiovascular mortality. A
collaborative meta-analysis of high-risk population cohorts. Kidney
Int. 79:1341–1352. 2011.PubMed/NCBI View Article : Google Scholar
|
|
132
|
Hörl WH, Cohen JJ, Harrington JT, Madias
NE and Zusman CJ: Atherosclerosis and uremic retention solutes.
Kidney Int. 66:1719–1731. 2004.PubMed/NCBI View Article : Google Scholar
|
|
133
|
Becker BN, Himmelfarb J, Henrich WL and
Hakim RM: Reassessing the cardiac risk profile in chronic
hemodialysis patients: A hypothesis on the role of oxidant stress
and other non-traditional cardiac risk factors. J Am Soc Nephrol.
8:475–486. 1997.PubMed/NCBI
|
|
134
|
Harper SJ and Bates DO: Endothelial
permeability in uremia. Kidney Int Suppl. 63:S84–S44.
2003.PubMed/NCBI View Article : Google Scholar
|
|
135
|
Stenvinkel P, Pecoits-Filho R and Lindholm
B: Coronary artery disease in end-stage renal disease: No longer a
simple plumbing problem. J Am Soc Nephrol. 14:1927–1939.
2003.PubMed/NCBI View Article : Google Scholar
|
|
136
|
Buzello M, Törnig J, Faulhaber J, Ehmke H,
Ritz E and Amann K: The apolipoprotein e knockout mouse: A model
documenting accelerated atherogenesis in uremia. J Am Soc Nephrol.
14:311–316. 2003.PubMed/NCBI View Article : Google Scholar
|
|
137
|
Bro S, Bentzon JF, Falk E, Andersen CB,
Olgaard K and Nielsen LB: Chronic renal failure accelerates
atherogenesis in apolipoprotein E-deficient mice. J Am Soc Nephrol.
14:2466–2474. 2003.PubMed/NCBI View Article : Google Scholar
|
|
138
|
Bro S, Moeller F, Andersen CB, Olgaard K
and Nielsen LB: Increased expression of adhesion molecules in
uremic atherosclerosis in apolipoprotein-E-deficient mice. J Am Soc
Nephrol. 15:1495–1503. 2004.PubMed/NCBI View Article : Google Scholar
|
|
139
|
Deicher R, Ziai F, Bieglmayer C,
Schillinger M and Horl WH: Low total vitamin C plasma level is a
risk factor for cardiovascular morbidity and mortality in
hemodialysis patients. J Am Soc Nephrol. 16:1811–1818.
2005.PubMed/NCBI View Article : Google Scholar
|
|
140
|
Antoniades C, Demosthenous M, Tousoulis D,
Antonopoulos AS, Vlachopoulos C, Toutouza M, Marinou K, Bakogiannis
C, Mavragani K, Lazaros G, et al: Role of asymmetrical
dimethylarginine in inflammation-induced endothelial dysfunction in
human atherosclerosis. Hypertension. 58:93–98. 2011.PubMed/NCBI View Article : Google Scholar
|
|
141
|
Juonala M, Viikari JS, Alfthan G, Marniemi
J, Kahonen M, Taittonen L, Laitinen T and Raitakari OT: Brachial
artery flow-mediated dilation and asymmetrical dimethylarginine in
the cardiovascular risk in young Finns study. Circulation.
116:1367–1373. 2007.PubMed/NCBI View Article : Google Scholar
|
|
142
|
Zoccali C, Bode-Böger S, Mallamaci F,
Benedetto F, Tripepi G, Malatino L, Cataliotti A, Bellanuova I,
Fermo I, Frolich J and Böger R: Plasma concentration of
asymmetrical dimethylarginine and mortality in patients with
end-stage renal disease: A prospective study. Lancet.
358:2113–2117. 2001.PubMed/NCBI View Article : Google Scholar
|
|
143
|
Nuhu F and Bhandari S: Oxidative stress
and cardiovascular complications in chronic kidney disease, the
impact of anaemia. Pharmaceuticals (Basel). 11(103)2018.PubMed/NCBI View Article : Google Scholar
|
|
144
|
Papacocea T, Buraga I, Papacocea R,
Badarau AI, Buraga M, Ciornei C, Mihai G, Stoian I and Adam D:
Antioxidant enzymes-potential targets in intracerebral haemorrhage.
Farmacia. 62:1118–1125. 2014.
|
|
145
|
Vervloet M and Cozzolino M: Vascular
calcification in chronic kidney disease: Different bricks in the
wall? Kidney Int. 91:808–817. 2017.PubMed/NCBI View Article : Google Scholar
|
|
146
|
Jablonski KL and Chonchol M: Vascular
calcification in end-stage renal disease. Hemodial Int. 17 (Suppl
1):S17–S21. 2013.PubMed/NCBI View Article : Google Scholar
|
|
147
|
Sosnov J, Lessard D, Goldberg RJ,
Yarzebski J and Gore JM: Differential symptoms of acute myocardial
infarction in patients with kidney disease: A community-wide
perspective. Am J Kidney Dis. 47:378–384. 2006.PubMed/NCBI View Article : Google Scholar
|
|
148
|
Herzog CA, Littrell K, Arko C, Frederick
PD and Blaney M: Clinical characteristics of dialysis patients with
acute myocardial infarction in the United States: A collaborative
project of the United States Renal Data System and the National
Registry of Myocardial Infarction. Circulation. 116:1465–1472.
2007.PubMed/NCBI View Article : Google Scholar
|
|
149
|
Freda BJ, Tang WH, Van Lente F, Peacock WF
and Francis GS: Cardiac troponins in renal insufficiency: Review
and clinical implications. J Am Coll Cardiol. 40:2065–2071.
2002.PubMed/NCBI View Article : Google Scholar
|
|
150
|
Bonello L, De Labriolle A, Roy P,
Steinberg DH, Okabe T, Pinto Slottow TL, Xue Z, Torguson R, Suddath
WO, Satler LF, et al: Impact of optimal medical therapy and
revascularization on outcome of patients with chronic kidney
disease and on dialysis who presented with acute coronary syndrome.
Am J Cardiol. 102:535–540. 2008.PubMed/NCBI View Article : Google Scholar
|
|
151
|
Keltai M, Tonelli M, Mann JF, Sitkei E,
Lewis BS, Hawken S, Mehta SR and Yusuf S: CURE Trial Investigators.
Renal function and outcomes in acute coronary syndrome: Impact of
clopidogrel. Eur J Cardiovasc Prev Rehabil. 14:312–318.
2007.PubMed/NCBI View Article : Google Scholar
|
|
152
|
Palmer SC, Di Micco L, Razavian M, Craig
JC, Perkovic V, Pellegrini F, Copetti M, Graziano G, Tognoni G,
Jardine M, et al: Effects of antiplatelet therapy on mortality and
cardiovascular and bleeding outcomes in persons with chronic kidney
disease: A systematic review and meta-analysis. Ann Intern Med.
156:445–459. 2012.PubMed/NCBI View Article : Google Scholar
|
|
153
|
James S, Budaj A, Aylward P, Buck KK,
Cannon CP, Cornel JH, Harrington RA, Horrow J, Katus H, Keltai M,
et al: Ticagrelor versus clopidogrel in acute coronary syndromes in
relation to renal function: Results from the Platelet Inhibition
and Patient Outcomes (PLATO) trial. Circulation. 122:1056–1067.
2010.PubMed/NCBI View Article : Google Scholar
|
|
154
|
Huang CC and Chen JW: Contemporary
management of coronary artery disease and acute coronary syndrome
in patients with chronic kidney disease and end-stage renal
disease. Acta Cardiol Sin. 29:132–141. 2013.PubMed/NCBI
|
|
155
|
Strippoli GF, Navaneethan SD, Johnson DW,
Perkovic V, Pellegrini F, Nicolucci A and Craig JC: Effects of
statins in patients with chronic kidney disease: Meta-analysis and
metaregression of randomised controlled trials. BMJ. 336:645–651.
2008.PubMed/NCBI View Article : Google Scholar
|
|
156
|
Chou MT, Wang JJ, Sun YM, Sheu MJ, Chu CC,
Weng SF, Chio CC, Kan WC and Chien CC: Epidemiology and mortality
among dialysis patients with acute coronary syndrome: Taiwan
National Cohort Study. Int J Cardiol. 167:2719–2723.
2012.PubMed/NCBI View Article : Google Scholar
|
|
157
|
Herzog CA, Ma JZ and Collins AJ:
Comparative survival of dialysis patients in the United States
after coronary angioplasty, coronary artery stenting, and coronary
artery bypass surgery and impact of diabetes. Circulation.
106:2207–2211. 2002.PubMed/NCBI View Article : Google Scholar
|
|
158
|
Szczech LA, Reddan DN, Owen WF, Racz M,
Jones RH and Hannan EL: Differential survival after coronary
revascularization procedures among patients with renal
insufficiency. Kidney Int. 60:292–299. 2001.PubMed/NCBI View Article : Google Scholar
|
|
159
|
Koyanagi T, NisIDHa H, Kitamura M, Endo M,
Koyanagi H, Kawaguchi M, Magosaki N, Suiyoshi T and Hosoda S:
Comparison of clinical outcomes of coronary artery bypass grafting
and percutaneous transluminal coronary angioplasty in renal
dialysis patients. Ann Thorac Surg. 61:1793–1796. 1996.PubMed/NCBI View Article : Google Scholar
|
|
160
|
Podrid PJ: Pathophysiology and etiology of
sudden cardiac arrest. UpToDate, 2020. https://www.uptodate.com/contents/pathophysiology-and-etiology-of-sudden-cardiac-arrest
Accessed June 2, 2020.
|
|
161
|
Jadoul M, Thumma J, Fuller DS, Tentori F,
Li Y, Morgenstern H, Mendekssohn D, Tomo T, Ethier J, Port F and
Robinson BM: Modifiable practices associated with sudden death
among hemodialysis patients in the dialysis outcomes and practice
patterns study. Clin J Am Soc Nephrol. 7:765–774. 2012.PubMed/NCBI View Article : Google Scholar
|
|
162
|
Pun PH, Lehrich RW, Smith SR and Middleton
JP: Predictors of survival after cardiac arrest in outpatient
hemodialysis clinics. Clin J Am Soc Nephrol. 2:491–500.
2007.PubMed/NCBI View Article : Google Scholar
|
|
163
|
Kudenchuk PJ, Stuart R, Husain S,
Fahrenbruch C and Eisenberg M: Treatment and outcome of
out-of-hospital cardiac arrest in outpatient health care
facilities. Resuscitation. 97:97–102. 2015.PubMed/NCBI View Article : Google Scholar
|
|
164
|
Makar MS and Pun HP: Sudden cardiac death
among hemodialysis patients. Am J Kidney Dis. 69:684–695.
2017.PubMed/NCBI View Article : Google Scholar
|
|
165
|
Huikuri HV, Castellanos A and Myerburg RJ:
Sudden death due to cardiac arrhythmias. N Engl J Med.
345:1473–1482. 2001.PubMed/NCBI View Article : Google Scholar
|
|
166
|
Bayes de Luna A, Coumel P and Leclercq JF:
Ambulatory sudden cardiac death: Mechanisms of production of fatal
arrhythmia on the basis of data from 157 cases. Am Heart J.
117:151–159. 1989.PubMed/NCBI View Article : Google Scholar
|
|
167
|
Ohtake T, Kobayashi S, Moriya H, Negishi
K, Okamoto K, Maesato K and Saito S: High prevalence of occult
coronary artery stenosis in patients with chronic kidney disease at
the initiation of renal replacement therapy: An angiographic
examination. J Am Soc Nephrol. 16:1141–1148. 2005.PubMed/NCBI View Article : Google Scholar
|
|
168
|
Yamada S, Ishii H, Takahashi H, Aoyama T,
Morita Y, Kasuga H, Kimura K, Ito Y, Takahashi R, Toriyama T, et
al: Prognostic value of reduced left ventricular ejection fraction
at start of hemodialysis therapy on cardiovascular and all-cause
mortality in end-stage renal disease patients. Clin J Am Soc
Nephrol. 5:1793–1798. 2010.PubMed/NCBI View Article : Google Scholar
|
|
169
|
Kalantar-Zadeh K, Block G, Humphreys MH
and Kopple JD: Reverse epidemiology of cardiovascular risk factors
in maintenance dialysis patients. Kidney Int. 63:793–808.
2003.PubMed/NCBI View Article : Google Scholar
|
|
170
|
Bleyer AJ, Hartman J, Brannon PC,
Reeves-Daniel A, Satko SG and Russell G: Characteristics of sudden
death in hemodialysis patients. Kidney Int. 69:2268–2273.
2006.PubMed/NCBI View Article : Google Scholar
|
|
171
|
Mangrum AJ, Lin D, Dimarco JP, Bolton WK
and Mangrum JM: Sudden cardiac death and left ventricular function
in hemodialysis patients. Heart Rhythm. 2 (Suppl)(S33)2005.
|
|
172
|
Keller SP and Halperin HR: Cardiac arrest:
The changing incidence of ventricular fibrillation. Curr Treat
Options Cardiovasc Med. 17(392)2015.PubMed/NCBI View Article : Google Scholar
|
|
173
|
Bonato FO, Lemos MM, Cassiolato JL and
Canziani ME: Prevalence of ventricular arrhythmia and its
associated factors in nondialyzed chronic kidney disease patients.
PLoS One. 8(e66036)2013.PubMed/NCBI View Article : Google Scholar
|
|
174
|
Wan C, Herzog CA, Zareba W and Szymkiewicz
SJ: Sudden cardiac arrest in hemodialysis patients with wearable
cardioverter defibrillator. Ann Noninvasive Electrocardiol.
19:247–257. 2014.PubMed/NCBI View Article : Google Scholar
|
|
175
|
Lafrance JP, Nolin L, Senecal L and
Leblanc M: Predictors and outcome of cardiopulmonary resuscitation
(CPR) calls in a large haemodialysis unit over a seven-year period.
Nephrol Dial Transplant. 21:1006–1012. 2006.PubMed/NCBI View Article : Google Scholar
|
|
176
|
Charytan DM, Foley R, McCullough PA,
Rogers JD, Zimetbaum P, Herzog CA and Tumlin JA: MiD Investigators
and Committees. Arrhythmia and sudden death in hemodialysis
patients: Protocol and baseline characteristics of the monitoring
in dialysis study. Clin J Am Soc Nephrol. 11:721–734.
2016.PubMed/NCBI View Article : Google Scholar
|
|
177
|
Roy-Chaudhury P, Tumlin JA, Koplan BA,
Costea AI, Kher V, Williamson D, Pokhariyal S and Charytan DM: MiD
investigators and committees. Primary outcomes of the Monitoring in
Dialysis Study indicate that clinically significant arrhythmias are
common in hemodialysis patients and related to dialytic cycle.
Kidney Int. 93:941–951. 2018.PubMed/NCBI View Article : Google Scholar
|
|
178
|
Ganesh SK, Stack AG, Levin NW,
Hulbert-Shearon T and Port FK: Association of elevated serum PO(4),
Ca x PO(4) product, and parathyroid hormone with cardiac
mortality risk in chronic hemodialysis patients. J Am Soc Nephrol.
12:2131–2138. 2001.PubMed/NCBI
|
|
179
|
Herzog CA: Cardiac arrest in dialysis
patients: Approaches to alter an abysmal outcome. Kidney Int Suppl.
63:S197–S200. 2003.PubMed/NCBI View Article : Google Scholar
|
|
180
|
Pun PH, Smarz TR, Honeycutt EF, Shaw LK,
Al-Khatib SM and Middleton JP: Chronic kidney disease is associated
with increased risk of sudden cardiac death among patients with
coronary artery disease. Kidney Int. 76:652–658. 2009.PubMed/NCBI View Article : Google Scholar
|
|
181
|
Diaconu C: Treatment of diabetes in
patients with heart failure. In: Proceedings of the 3rd
International Conference on Interdisciplinary Management of
Diabetes Mellitus and its Complications-Diabetes mellitus as
cardiovascular disease. INTERDIAB, Bucharest, pp170-177, 2017.
|
|
182
|
Parekh RS, Plantinga LC, Kao WHL, Meoni
LA, Jaar BG, Fink NE, Powe NR, Coresh J and Klag MJ: The
association of sudden cardiac death with inflammation and other
traditional risk factors. Kidney Int. 74:1335–1342. 2008.PubMed/NCBI View Article : Google Scholar
|
|
183
|
Drechsler C, Krane V, Winkler K, Dekker FW
and Wanner C: Changes in adiponectin and the risk of sudden death,
stroke, myocardial infarction, and mortality in hemodialysis
patients. Kidney Int. 76:567–575. 2009.PubMed/NCBI View Article : Google Scholar
|
|
184
|
Domanski MJ, Zipes DP and Schron E:
Treatment of sudden cardiac death. Current understandings from
randomized trials and future research directions. Circulation.
95:2694–2699. 1997.PubMed/NCBI View Article : Google Scholar
|
|
185
|
Agarwal R, Sinha AD, Pappas MK, Abraham TN
and Tegegne GG: Hypertension in hemodialysis patients treated with
atenolol or lisinopril: A randomized controlled trial. Nephrol Dial
Transplant. 29:672–681. 2014.PubMed/NCBI View Article : Google Scholar
|
|
186
|
Tangri N, Shastri S, Tighiouart H, Beck
GJ, Cheung AK, Eknoyan G and Sarnak MJ: β-Blockers for prevention
of sudden cardiac death in patients on hemodialysis: A propensity
score analysis of the HEMO Study. Am J Kidney Dis. 58:939–945.
2011.PubMed/NCBI View Article : Google Scholar
|
|
187
|
Beaubien ER, Pylypchuk GB, Akhtar J and
Biem HJ: Value of corrected QT interval dispersion in identifying
patients initiating dialysis at increased risk of total and
cardiovascular mortality. Am J Kidney Dis. 39:834–842.
2002.PubMed/NCBI View Article : Google Scholar
|
|
188
|
Di Iorio B, Torraca S, Piscopo C, Sirico
ML, Micco LD, Pota A, Tartaglia D, Berardino L, Morrone LF and
Russo D: Dialysate bath and QTc interval in patients on chronic
maintenance hemodialysis: Pilot study of single dialysis effects. J
Nephrol. 25:653–660. 2012.PubMed/NCBI View Article : Google Scholar
|
|
189
|
Pun PH, Horton JR and Middleton JP:
Dialysate calcium concentration and the risk of sudden cardiac
arrest in hemodialysis patients. Clin J Am Soc Nephrol. 8:797–803.
2013.PubMed/NCBI View Article : Google Scholar
|
|
190
|
Timofte D, Ionescu D, Medrihan L, Mandita
A, Rasina A and Damian L: Vascular calcification and bone disease
in hemodialysis patients assessment, association and risk factors.
Nephrol Dialysis Transplantation. 22:325–326. 2007.
|
|
191
|
Timofte D, Dragos D, Balcangiu-Stroescu
AE, Tanasescu MD, Balan DG, Raducu L, Tulin A, Stiru O and Ionescu
D: Abdominal aortic calcification in predialysis patients:
Contribution of traditional and uremia-related risk factors. Exp
Ther Med. 20:97–102. 2020.PubMed/NCBI View Article : Google Scholar
|
|
192
|
Kim ED and Parekh RS: Calcium and sudden
cardiac death in end-stage renal disease. Semin Dial. 28:624–635.
2015.PubMed/NCBI View Article : Google Scholar
|
|
193
|
Di Lullo L, Rivera R, Barbera V, Bellasi
A, Cozzolino M, Russo D, De Pascalis A, Banerjee D, Floccari F and
Ronco C: Sudden cardiac death and chronic kidney disease: From
pathophysiology to treatment strategies. Int J Cardiol. 217:16–27.
2016.PubMed/NCBI View Article : Google Scholar
|
|
194
|
Flythe JE and Brunelli SM: The risks of
high ultrafiltration rate in chronic hemodialysis: Implications for
patient care. Semin Dial. 24:259–265. 2011.PubMed/NCBI View Article : Google Scholar
|
|
195
|
Flythe JE, Kimmel SE and Brunelli SM:
Rapid fluid removal during dialysis is associated with
cardiovascular morbidity and mortality. Kidney Int. 79:250–257.
2011.PubMed/NCBI View Article : Google Scholar
|
|
196
|
Mendoza JM, Bayes LY, Sun S, Doss S and
Schiller B: Effect of lowering dialysate sodium concentration on
interdialytic weight gain and blood pressure in patients undergoing
thrice-weekly in-center nocturnal hemodialysis: A quality
improvement study. Am J Kidney Dis. 58:956–963. 2011.PubMed/NCBI View Article : Google Scholar
|
|
197
|
Jefferies HJ, Burton JO and McIntyre CW:
Individualised dialysate temperature improves intradialytic
haemodynamics and abrogates haemodialysis-induced myocardial
stunning, without compromising tolerability. Blood Purif. 32:63–68.
2011.PubMed/NCBI View Article : Google Scholar
|