Introduction
Benign prostatic hyperplasia (BPH) is an irregular
benign hyperplasia of smooth muscle cells and other stromal cells
in the transitional area of the prostate gland (1,2). BPH
may be induced by a variety of risk factors, including obesity,
hyperlipidemia, type 2 diabetes and certain androgenic hormone
disorders, which may lead to a series of lower urinary tract
symptoms (LUTS) (3,4). BPH with LUTS is a common disease that
is associated with androgen among older men (4). According to statistical data in the
USA, the incidence of BPH in men aged >50 years is ~50%, where
the incidence in men aged >80 years can reach as high as 80%
(5). Accumulating evidence suggest
that BPH/LUTS is also closely associated with the occurrence of
erectile dysfunction (ED), where severe bladder outlet obstruction
may induce sexual dysfunction (6-8).
Since BPH/LUTS can significantly reduce the life quality of older
men (5,6,9), a
safe and effective therapeutic strategy for this condition is
warranted.
Phosphodiesterases (PDEs) by regulate the
intracellular concentrations of cyclic nucleotides by catalyzing
the hydrolysis of both cyclic adenosine monophosphate (cAMP) and
cyclic guanosine monophosphate (cGMP) (10). The PDE superfamily consists of 11
gene families (PDE1-11), each presenting distinct affinities for
cAMP and cGMP (11). Among them,
phosphodiesterase 5 (PDE5) is a specific cGMP hydrolase, such that
the inhibition of PDE5 activity can block cGMP hydrolysis and
reduce intracellular Ca2+ concentration to promote
smooth muscle relaxation (12).
This suggests that PDE5 may serve as a potentially promising target
for the treatment of BPH/LUTS (13,14).
However, since PDE5 is ubiquitously expressed in the human body and
participates in a number of physiological and pathological
processes in numerous tissues or organs, inhibition of PDE5 using
traditional inhibitors may induce various adverse side effects
(15,16). Therefore, an effective inhibitor
targeting PDE5 that causes fewer adverse side effects is
required.
PDE5 can be divided into three subtypes based on the
different terminal sequences, namely PDE5A1, PDE5A2 and
PDE5A3(17). In particular, PDE5A3
is mainly found in human smooth muscle tissues but is absent in a
number of essential organs and tissues, including the brain, lung,
liver, kidney and skeletal muscle (15). Short-hairpin RNA (shRNA) is a
versatile tool that has the potential to modulate cell gene
expression in a stable manner (18). Pan et al (19) previously revealed the effects of
shRNA-mediated downregulation of PDE5A3 on cGMP in smooth muscle
cells of human corpus cavernosum. However, this study of PDE5A3 was
mainly performed on the penis, whilst the role of PDE5A3 in human
prostate smooth muscle cells and patients with BPH/LUTS remain
poorly understood.
The aim of the present study was to effectively
silence the expression of the PDE5A3 gene stably by shRNA
transfection and observe its effects on the intracellular levels of
cGMP and free Ca2+ in human prostate smooth muscle cells
(HPSMCs), with specific focus on HPSMC proliferation. The purpose
of the present study was to identify a potential target for the
treatment of BPH/LUTS.
Materials and methods
Tissue collection and cell
culture
Prostate tissues from six male patients with BPH
(67.3±8.4 years old) who underwent transurethral resection of the
prostate were initially collected. Surgery was performed at the
Department of Urology, Nanjing First Hospital, Nanjing Medical
University (Nanjing, China). The present study was approved by the
Ethics Committee of Nanjing Medical University and written informed
consents were collected from all participants. The BPH tissues were
placed in sterile tubes containing PBS before being immediately
transported on ice to the laboratory. After being briefly washing
three times using fresh sterile PBS, BPH tissues were sliced into
small pieces and digested using collagenase (type I, 0.075%;
Sigma-Aldrich; Merck KGaA) at 37˚C on an orbital shaker. After 30
min enzymatic digesting, the cell suspension was strained using a
75-µm mesh (Corning, Inc.) and subsequently centrifuged at 400 x g
for 5 min at room temperature (RT). The cell pellet was then
resuspended in fresh DMEM containing 10% FBS and centrifuged once
more (400 x g for 5 min at RT) to remove any residual collagenase
and thereafter cultured on new dishes. Cells in all dishes were
cultured at 37˚C in an incubator with 5% CO2 and 95%air
(20). After continuous culture to
passage 3 and differential velocity adherent (smooth muscle cells
require longer adherence time compared with fibroblasts), the
stromal cells (except smooth muscle cells) were gradually removed
and homogeneous HPSMCs were finally obtained. Cellular assays were
performed in 24-well plates or 96-well microplates (Corning, Inc.)
with an initial seeding density of 1x104 cells per well
or 2,000 cells per well, respectively.
Cell identification
Freshly isolated HPSMCs at passage 3 were gathered,
washed three times with PBS containing 1% bovine serum albumin
(BSA; Beijing Solarbio Science & Technology Co., Ltd.) and
centrifuged at 400 x g for 5 min at RT. Subsequently, cells were
fixed at RT in 4% buffered paraformaldehyde for 20 min before 0.1%
Triton X-100 was utilized to permeabilize the cell membranes for 10
min at RT. After rinsing twice, blocking solution (1% BSA in PBS)
was applied for 30 min at RT and the cells were subsequently
incubated with primary antibodies against α-smooth muscle actin
(α-SMA; cat. no. ab32575; 1:100; Abcam), trasngelin-1 (SM22α; cat.
no. ab212857, 1:200; Abcam), Desmin (cat. no. ab271829; 1:100;
Abcam), Calponin (cat. no. MABT1504; 1:300; Sigma-Aldrich; Merck
KGaA) and smooth muscle myosin heavy chain (SMMHC; cat. no.
ab133567; 1:100; Abcam) at 4˚C overnight. The cells were then
washed three times with PBS and incubated with FITC-conjugated
secondary antibodies (cat. nos. ab7086 and ab7064, 1:300; Abcam) at
RT for 1 h. Thereafter, the cells were incubated with DAPI (5
mg/ml, Beyotime Institute of Biotechnology) at RT for 10 min and
rinsed three times with PBS. Fluorescent signals were detected at
x200 magnification using a Nikon A1R confocal microscope (Nikon
Corporation) with the NIS-Elements AR 4.0 software package (Nikon
Corporation).
In addition to immunofluorescence staining,
collected and washed HPSMCs (1x106/100 µl) were also
incubated with conjugated primary antibodies against CD31 (FITC;
cat. no. 557508; 1:200; BD Biosciences), CD34 (phycoerythrin (PE);
cat. no. NBP2-34713PE, 1:200; Novus Biologicals, LLC), CD45 (PE;
cat. no. 560975; 1:200; BD Biosciences), CD14 (FITC; cat. no.
561712, 1:200; BD Biosciences), kinase insert domain receptor (KDR;
PE; cat. no. FAB357P; 1:200; R&D Systems, Inc.), CD29 (PE; cat.
no. 557332, 1:200; BD Biosciences), platelet-derived growth factor
receptor β (PDGFR-β; PE; cat. no. 558429, 1:200; BD Biosciences)
and CD90 (PE; cat. no. 561970, 1:200; BD Biosciences) at RT for 40
min in the dark. After washing twice, the collected cells were
resuspended in PBS and further investigated using a BD
FACSCalibur™ flow cytometer (BD Biosciences). All flow
cytometry data were analyzed using FlowJo (version 10.0.7; FlowJo
LLC). IgG-matched isotypes (PE; Mouse IgG, cat. no. 555749, 1:200;
cat. no. 554679, FITC-Mouse IgG 1:200; BD Biosciences) were
utilized for each procedure.
shRNA transfection
According to the design principles for the RNA
interference target sites, PDE5A3 shRNA sequences were designed
based on the PDE5A3 sequence (GenBank accession number: NM_033437;
https://www.ncbi.nlm.nih.gov/nuccore/NM_033437.4),
where four of which were eventually selected. The corresponding
sequences are shown in Table I. The
target non-homologous shRNA was also selected as a negative
control. The shRNA lentiviral particles were constructed as
previously described (21).
Lentiviruses were generated by transfecting 293T cells with 1.5 µg
shRNA-encoding plasmids (pGLV3/H1/GFP + Puro; Shanghai Jikai Gene
Chemical Technology Co., Ltd.). For transduction, the HPSMCs were
passaged to 60% confluence in a 24-well plate before Opti-MEM
medium (0.5 ml per well; Gibco; Thermo Fisher Scientific, Inc.)
with 5 µg/ml polybrene (Sigma-Aldrich; Merck KGaA) was added to the
cells. Subsequently, the viral particles were added at a
multiplicity of infection of 10. After 24 h incubation in 5%
CO2 at 37˚C, the viral particle-containing medium was
removed and fresh DMEM with 10% FBS was added for further
incubation. After 2 days, cells were screened using 3 µg/ml
puromycin (Sigma-Aldrich; Merck KGaA) for 4 days at 37˚C. Cells
were then assigned to the control group (no transfection), NC group
(transfected with NC shRNA), shPDE5A3-1 group (transfected with
PDE5A3-shRNA1), shPDE5A3-2 group (transfected with PDE5A3-shRNA2),
shPDE5A3-3 group (transfected with PDE5A3-shRNA3) and shPDE5A3-4
group (transfected with PDE5A3-shRNA4). Transfection efficiency was
monitored using flow cytometric analysis and fluorescence
microscopy, which was verified further by reverse
transcription-quantitative PCR (RT-qPCR) and western blot analysis.
For flow cytometric analysis, the transfected cells were gathered,
washed three times with PBS containing 1% BSA, centrifuged at 400 x
g for 5 min at RT and investigated further using a BD
FACSCalibur™ flow cytometer (BD Biosciences) using the
FITC channel. All flow cytometry data were analyzed using Flow Jo
(version 10.0.7; FlowJo LLC). For fluorescence detection, the
transfected cells were washed twice using PBS and observed using
fluorescence microscopy at x100 magnification. The transfection
efficiency was decided by the proportion of GFP-positive cells.
 | Table ISequences of shRNAs used for the
present study. |
Table I
Sequences of shRNAs used for the
present study.
| Name | Sequence |
|---|
| shPDE5A3-1 |
5'-GCTCAAGACTCTTGGAATTAG-3' |
| shPDE5A3-2 |
5'-GCATATCCATGGACTGATATC-3' |
| shPDE5A3-3 |
5'-GCTCAGCTCTATGAGACTTCA-3' |
| shPDE5A3-4 |
5'-GGATGAAGATTGCTCCGATTC-3' |
| Negative
control |
5'-TTCTCCGAACGTGTCACGT-3' |
RT-qPCR
The extraction of total RNA in cells was carried out
using the TRIzol™ Plus RNA purification kit (cat. no.
12183555; Invitrogen; Thermo Fisher Scientific, Inc.) on the basis
of manufacturer's protocol. The purified RNA was then reverse
transcribed to cDNA using High-Capacity cDNA Reverse Transcription
kit (cat. no. 4368814; Applied Biosystems,; Thermo Fisher
Scientific, Inc.). To perform reverse transcription, the conditions
were set as following: 25˚C for 10 min, 37˚C for 120 min and 85˚C
for 5 min. LightCycler 480 System (Roche Applied Science) was
utilized to carry out the RT-qPCR experiments. According to the
specifications, the qPCR reaction mixture volume was 20 µl and
included 10 µl 2X SYBR™ Green PCR Master Mix (cat. no.
4309155; Applied Biosystems; Thermo Fisher Scientific, Inc.), 1 µl
cDNA template, 1 µl forward primer (10 µM), 1 µl reverse primer (10
µM) and 7 µl sterile water. The qPCR conditions were set as
following: 95˚C for 10 min, followed by 40 cycles at 95˚C for 20
sec and 60˚C for 1 min. Relative expression of PDE5A3 mRNA was
expressed as 2-ΔΔCq (22). GAPDH was utilized as the control for
normalization. The specific primer sequences of PDE5A3 and GAPDH
are shown in Table II.
 | Table IIPrimer sequences for reverse
transcription-quantitative PCR. |
Table II
Primer sequences for reverse
transcription-quantitative PCR.
| Name | Primer
sequence | Primer length
(bp) |
|---|
| PDE5A3 | F:
5'-GCTTTTGTCATCTTTTGTGGCTT-3' | 139 |
| | R:
5'-GCTCTCTTGTTTCTTCCTCTGCT-3' | |
| GAPDH | F:
5'-CATCTTCTTTTGCGTCGCCA-3' | 115 |
| | R:
5'-TTAAAAGCAGCCCTGGTGACC-3' | |
Western blotting
Total proteins were extracted from HPSMCs in each
treatment groups using a Total Protein Extraction Kit (cat. no.
KGP2100; Nanjing KeyGen Biotech Co., Ltd.). Protein concentration
was quantified using a bicinchoninic acid protein assay kit
(Nanjing KeyGen Biotech Co., Ltd.). SDS-PAGE and immunoblotting
were performed according to the manufacturer's protocols (Bio-Rad
Laboratories, Inc.). Briefly, polyvinylidene fluoride membranes
(EMD Millipore) were blocked using 5% skim milk dissolved in TBS
(pH 7.5)-0.1% Tween-20 (TBS-T) for 2 h at RT after protein transfer
from 12% SDS-PAGE gels (50 mg/lane). Subsequently, the membranes
were incubated overnight at 4˚C with either the rabbit polyclonal
anti-PDE5A3 antibody (cat. no. ab64179; 1:1,000; Abcam) or rabbit
polyclonal anti-cGMP antibody (cat. no. ab12416; 1:2,000; Abcam).
The membranes were then washed with TBS-T and incubated with
horseradish peroxidase (HRP)-conjugated anti-rabbit secondary
antibody (1:5,000; cat. no. KGAA35; Nanjing KeyGen Biotech Co.,
Ltd.) at RT for 2 h. Enhanced Chemiluminescence Detection Kit
(Bio-Rad Laboratories, Inc.) and the ImageJ software (version 1.5i;
National Institutes of Health) were used to detect and
semi-quantitatively analyze the immunoreactive proteins. This
experiment was performed three times.
Immunocytochemical staining
The pretreatment process was identical to that of
immunofluorescence staining aforementioned. After and blocking,
HPSMCs were incubated with the rabbit anti-cGMP antibody (cat. no.
ab12416; 1:100; Abcam) at 4˚C overnight. On the following day, each
sample was washed three times with PBS for 5 min each time, before
incubation with HRP labeled goat anti-rabbit II (cat. no. KGAA35;
1:500; Nanjing KeyGen Biotech Co., Ltd.) for 30 min at RT.
Diaminobenzidine (DAB; cat. no. KGP1045; 1:10; Nanjing KeyGen
Biotech Co., Ltd.) was used for 5 min at RT for coloring, and
nuclear counterstain was performed in hematoxylin (Beijing Solarbio
Science & Technology Co., Ltd., H8070) for 3 min at RT. All
samples were detected by light microscopy at x200 magnification
after re-staining. The level of cGMP was assessed using the Image
Pro Plus software (version 6.0.0.260; Media Cybernetics, Inc.). The
mean density=total density/cell area.
Intracellular free Ca2+
assay
The level of intracellular free Ca2+ in
HPSMCs was determined at 48 h after 4 days puromycin screening
using rhod2-AM (cat. no. R1244; Invitrogen; Thermo Fisher
Scientific, Inc.). In order to remove extracellular Ca2+
and detect only intracellular free Ca2+, the cultured
cells were washed with sterile PBS along with a chelator solution
(10 mM glucose, 10 mM EGTA, 110 mM NaCl, 10 mM HEPES, pH 7.4). The
HPSMCs were then incubated with rhod2-AM (4 µM) in serum-free DMEM
at 37˚C for 30 min. After rinsing the HPSMCs three times using
serum-free DMEM, the signal of intracellular free Ca2+
was detected using fluorescence microscopy at x200 magnification
and assessed further using the Image-Pro Plus software (version
6.0.0.260; Media Cybernetics, Inc.). The mean density=total
fluorescence density/cell area.
MTT assay
HPSMCs in logarithmic phase were collected before
3,000 cells from each group were inoculated into three duplicated
wells. After cell incubation for 24, 48, 72 and 96 h at 37˚C, 5
mg/ml MTT (20 µl) solution (Beyotime Institute of Biotechnology)
was added to each well at the corresponding time points, followed
by incubation at 37˚C for 3 h. Thereafter, the culture medium and
MTT in each well were removed and 150 µl DMSO (Beyotime Institute
of Biotechnology) was added into each well before the plates were
continuously shaken for 10 min. The optical density value at 570 nm
in each well was detected using a microplate reader.
Assessment of cell proliferation by
EdU assay
The proliferation of HPSMCs were also detected using
BeyoClick™ EdU-555 kit (cat. no. C0075; Beyotime
Institute of Biotechnology) according to the manufacturer's
protocols. HPSMCs were seeded in 96-well plates at 1,000 cells per
well. After 72 h of seeding, HPSMCs were incubated with EdU (10 µM)
for 2 h at 37˚C. After fixation with 4% paraformaldehyde at RT for
10 min, cell nuclei were stained with DAPI (5 mg/ml; Beyotime
Institute of Biotechnology) for 15 min at RT. EdU-positive cells
were detected using fluorescence microscopy at x100 magnification
and automatically quantified using the Image-Pro Plus software
(version 6.0.0.260; Media Cybernetics, Inc.). The proportion of
EdU-positive cells were calculated.
Statistical analysis
Each experiment was repeated three times. All data
were presented as the mean ± SEM. One-way analysis of variance
(ANOVA) was introduced to assess statistical comparisons of the
data among multiple groups. If ANOVA revealed a significant
difference, Tukey's post hoc test was utilized to compare between
each group. P<0.05 was considered to indicate a statistically
significant difference.
Results
HPSMCs identification and
transfection
HPSMCs were isolated from the hyperplastic prostate.
After culture for 2 days, HPSMCs at passage 0 emerged with
shuttle-like or polygon morphologies (Fig. 1A). Subsequently, at passage 3,
HPSMCs became fusiform and arranged themselves into bundles
(Fig. 1A). All spindle cells
exhibited α-SMA, SM22α, Desmin, Calponin and SMMHC staining whereas
unstained cells were could not be clearly observed (Fig. 1B). Flow cytometric analysis revealed
that HPSMCs were tested positive for CD29, PDGFR-β, and CD90 but
negative for CD31, CD34, CD45, CD14 and KDR (Fig. 1C). These findings suggest that the
cells isolated expressed smooth muscle cell markers.
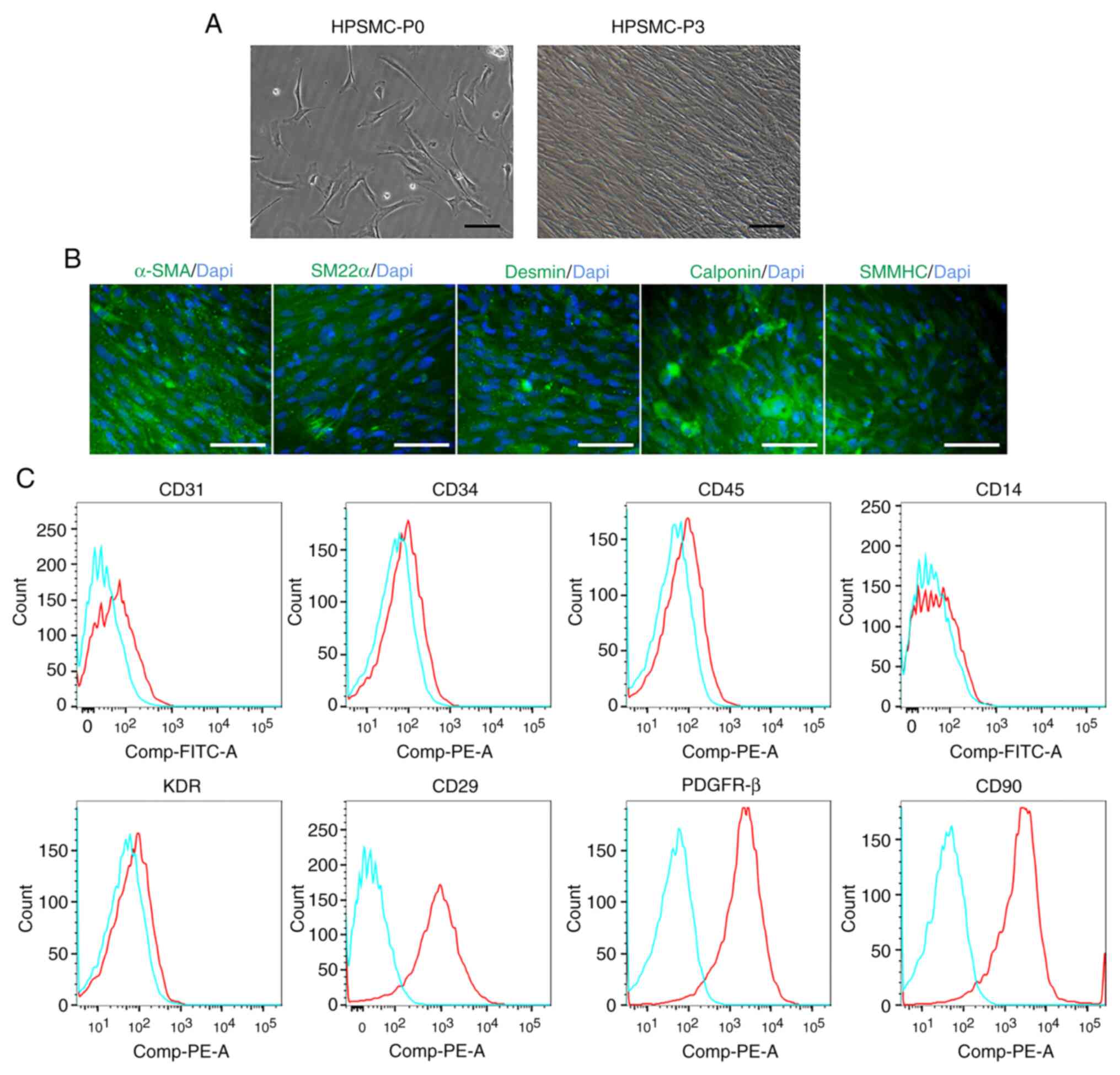 | Figure 1HPSMCs identification. (A) Cell
morphology of freshly isolated and cultured HPSMCs at passages 0
and 3. Scale bar, 50 µm. (B) Immunofluorescence staining of α-SMA,
SM22α, Desmin, Calponin and SMMHC (all green), and cell nucleus
(blue) in HPSMCs at passage 3. Scale bar, 50 µm. (C) Flow
cytometric analysis revealed that HPSMCs were positive for CD29,
PDGFR-β and CD90, whilst negative for CD31, CD34, CD45, CD14 and
KDR. HPSMC, human prostate smooth muscle cells; SMMHC, smooth
muscle myosin heavy chain; PDGFR-β, platelet-derived growth factor
receptor β; KDR, kinase insert domain receptor; SM22α,
transgelin-1. |
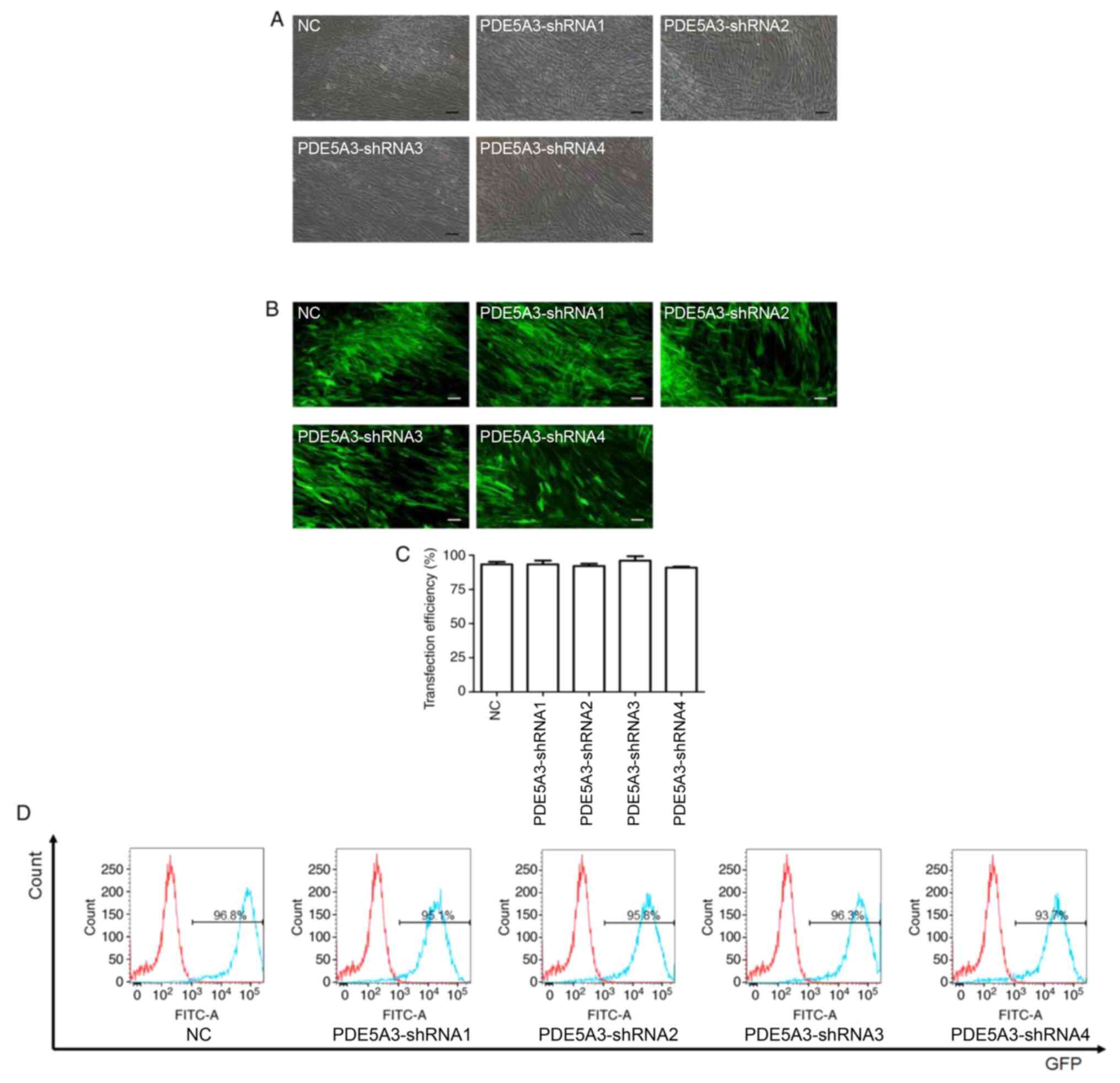
Following transfection with PDE5A3 shRNA, HPSMCs in
each of the groups were observed by light and fluorescence
microscopy (Fig. 2A and B). In addition, flow cytometry was also
utilized to detect the percentage of GFP-positive cells (Fig. 2D). The transfection efficiency of
this lentiviral method in each group was found to be >90%
(Fig. 2C and D) and no statistic significances could be
observed among the five groups (Fig.
2C).
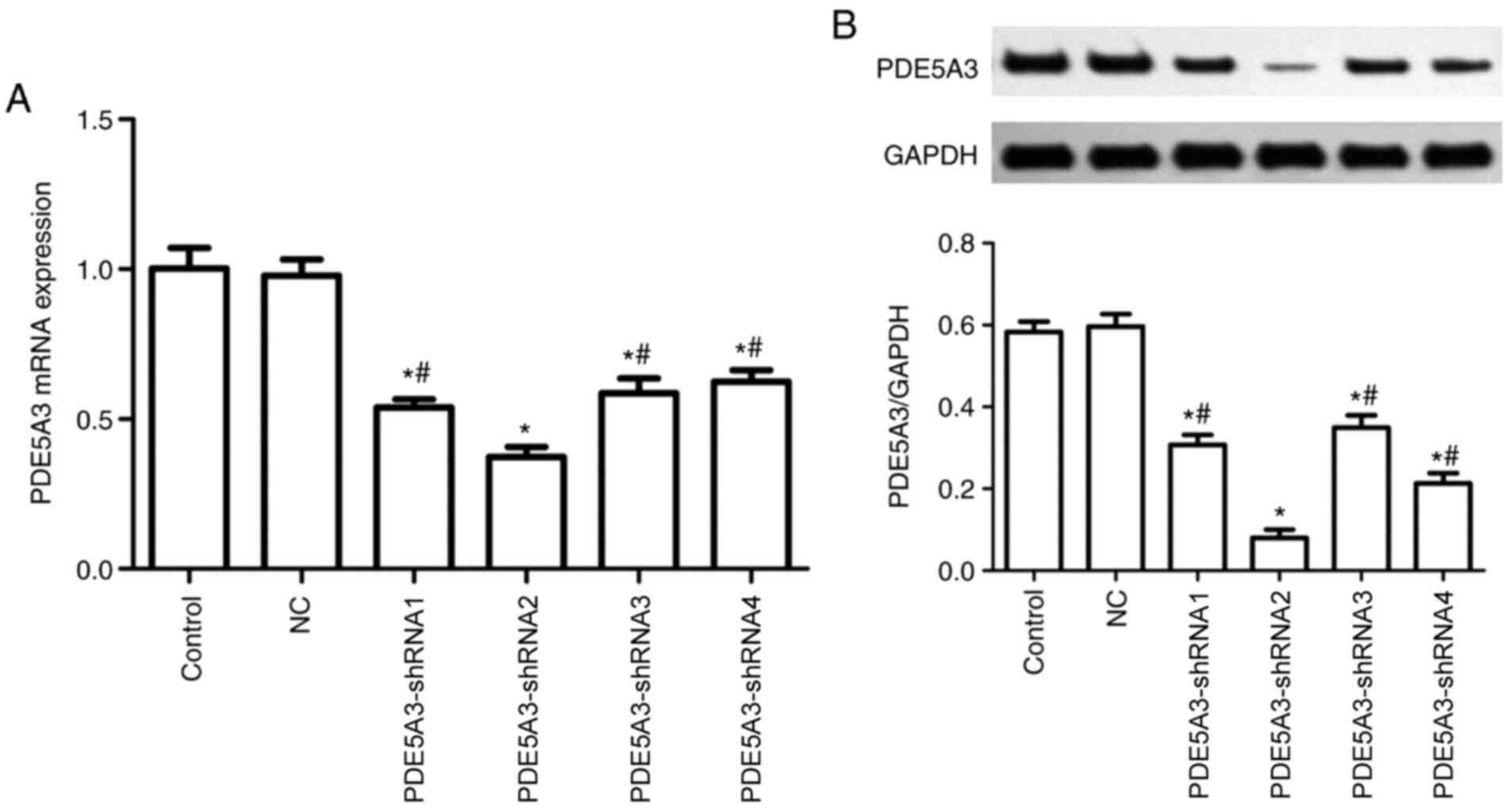
Cells transfected with PDE5A3-shRNA2
exhibits a notable decline in PDE5A3
RT-qPCR and western blotting data demonstrated that
there was no notable difference between the NC and Control group
regarding the mRNA and protein expressions of PDE5A3 after
transfection. By contrast, PDE5A3 expression was significantly
decreased in the PDE5A3-shRNA1-4 groups compared with that in the
Control and NC group (Fig. 3A and
B). In addition, the mRNA and
protein expression of PDE5A3 in PDE5A3-shRNA2 group exhibited
significant reductions compared with that in the other three
transfected groups (Fig. 3A and
B).
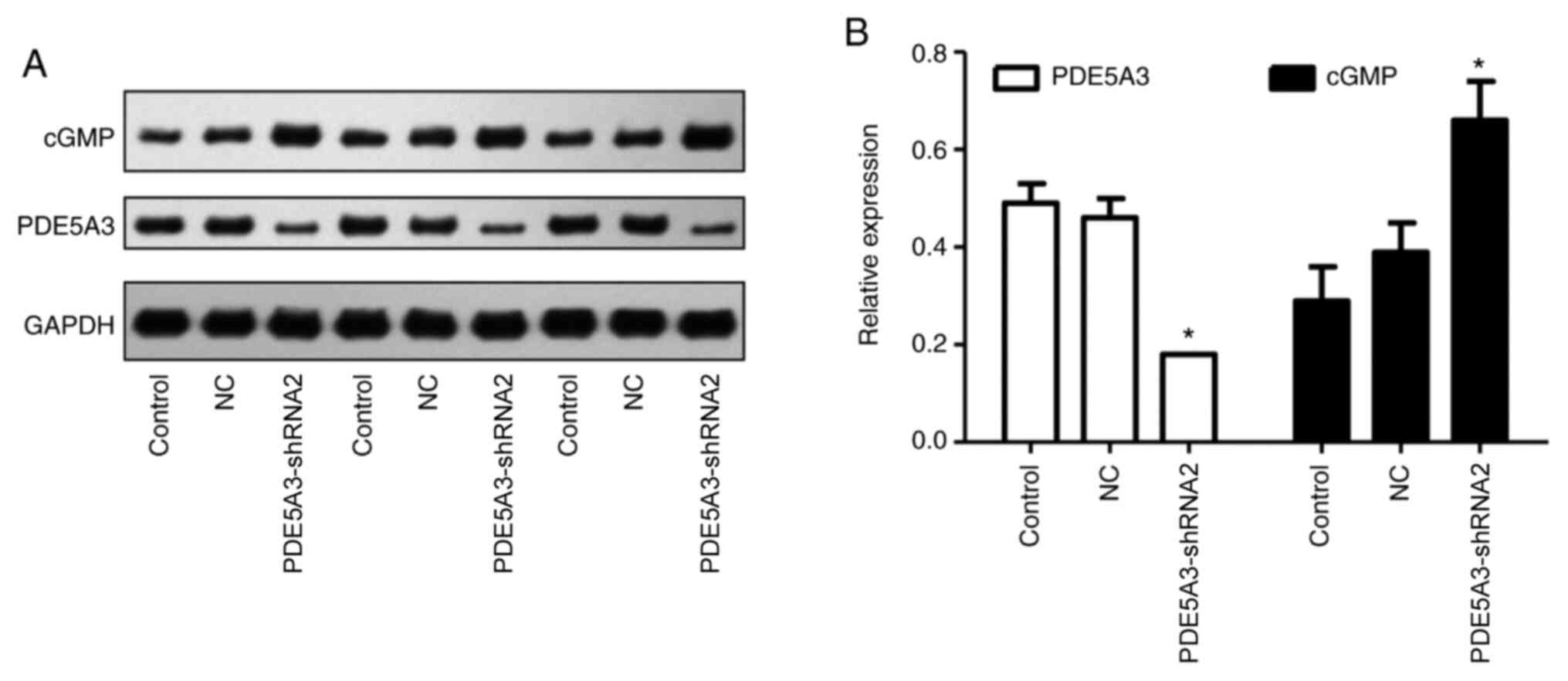
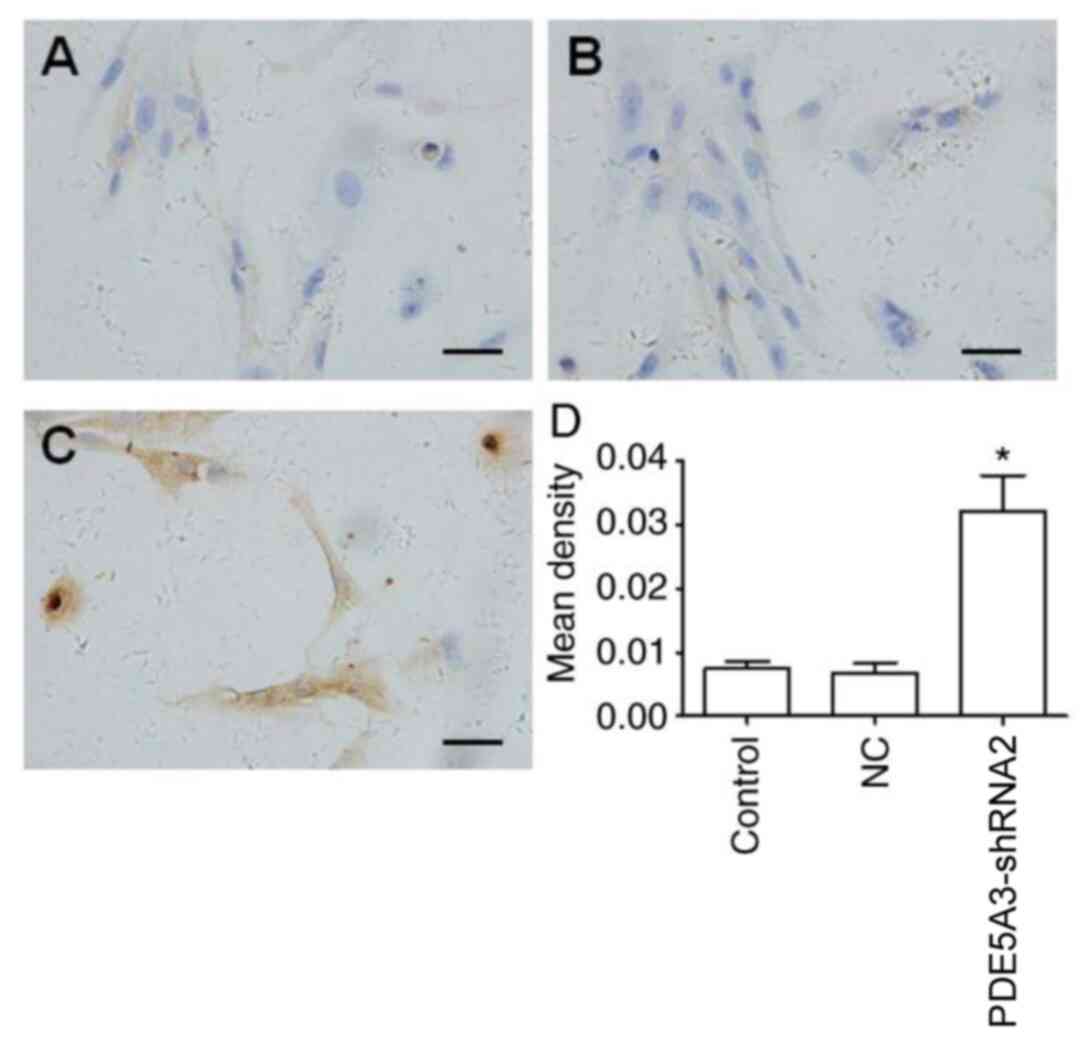
PDE5A3-shRNA2 mediated silencing of
PDE5A3 increases intracellular cGMP levels
PDE5A3-shRNA2 was subsequently utilized to transfect
HPSMCs, which significantly reduced the protein expression of
PDE5A3 compared with that in the Control and NC group (Fig. 4). Western blotting results revealed
that the intracellular level of cGMP was significantly increased in
the PDE5A3-shRNA2 group compared with that in the Control and NC
groups (Fig. 4). This was further
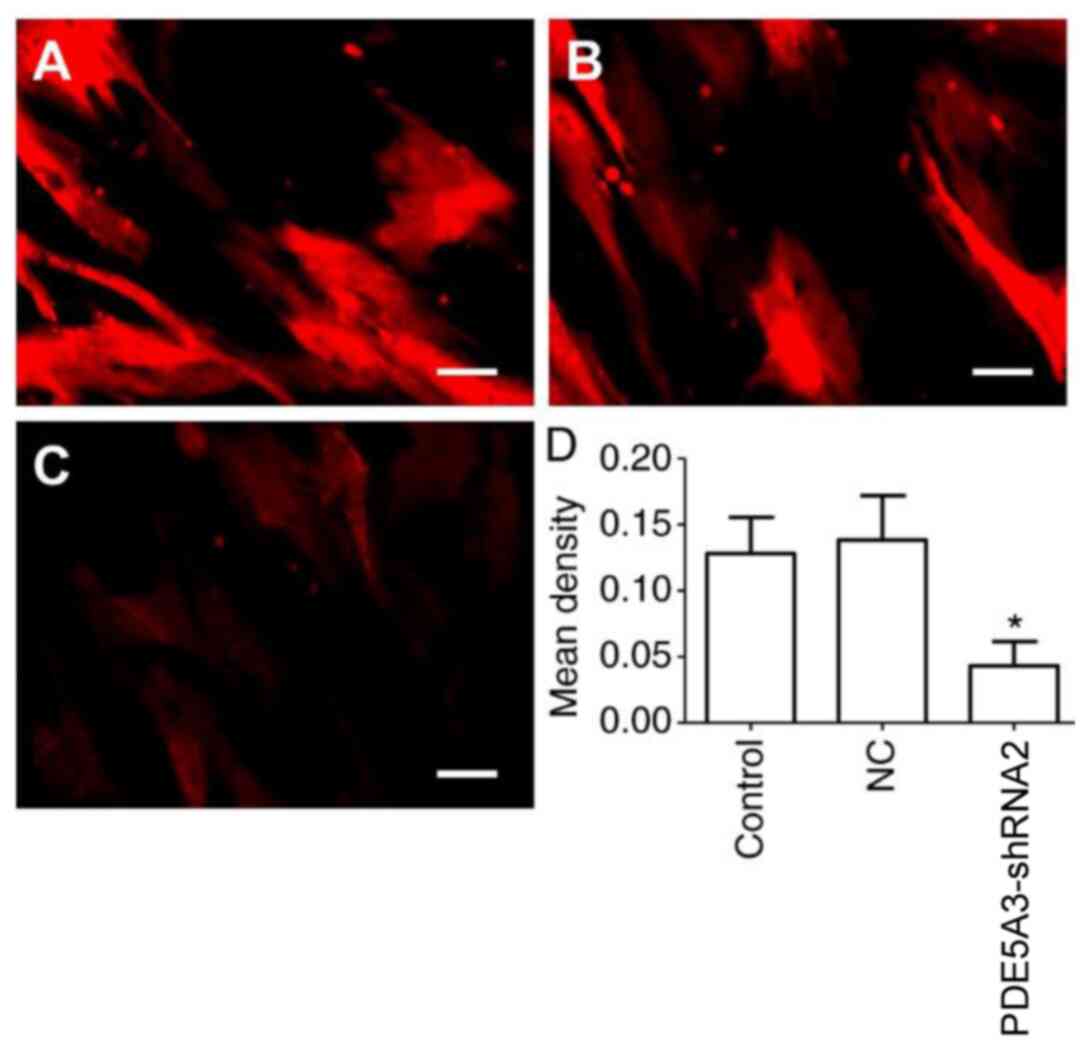
verified by immunocytochemical staining for cGMP (Fig. 5), the mean density of cGMP in the
PDE5A3-shRNA2 group was significantly higher compared with that in
the Control and NC groups.
PDE5A3-shRNA2 mediated silencing of
PDE5A3 reduces intracellular free Ca2+ levels
Extracellular Ca2+ could not be detected
(Fig. 6). Compared with that in the
Control and NC groups, the concentration of Ca2+ in the
cytosol was significantly reduced in the PDE5A3-shRNA2 group
(Fig. 6).
PDE5A3-shRNA2 mediated silencing of
PDE5A3 inhibits HPSMC proliferation
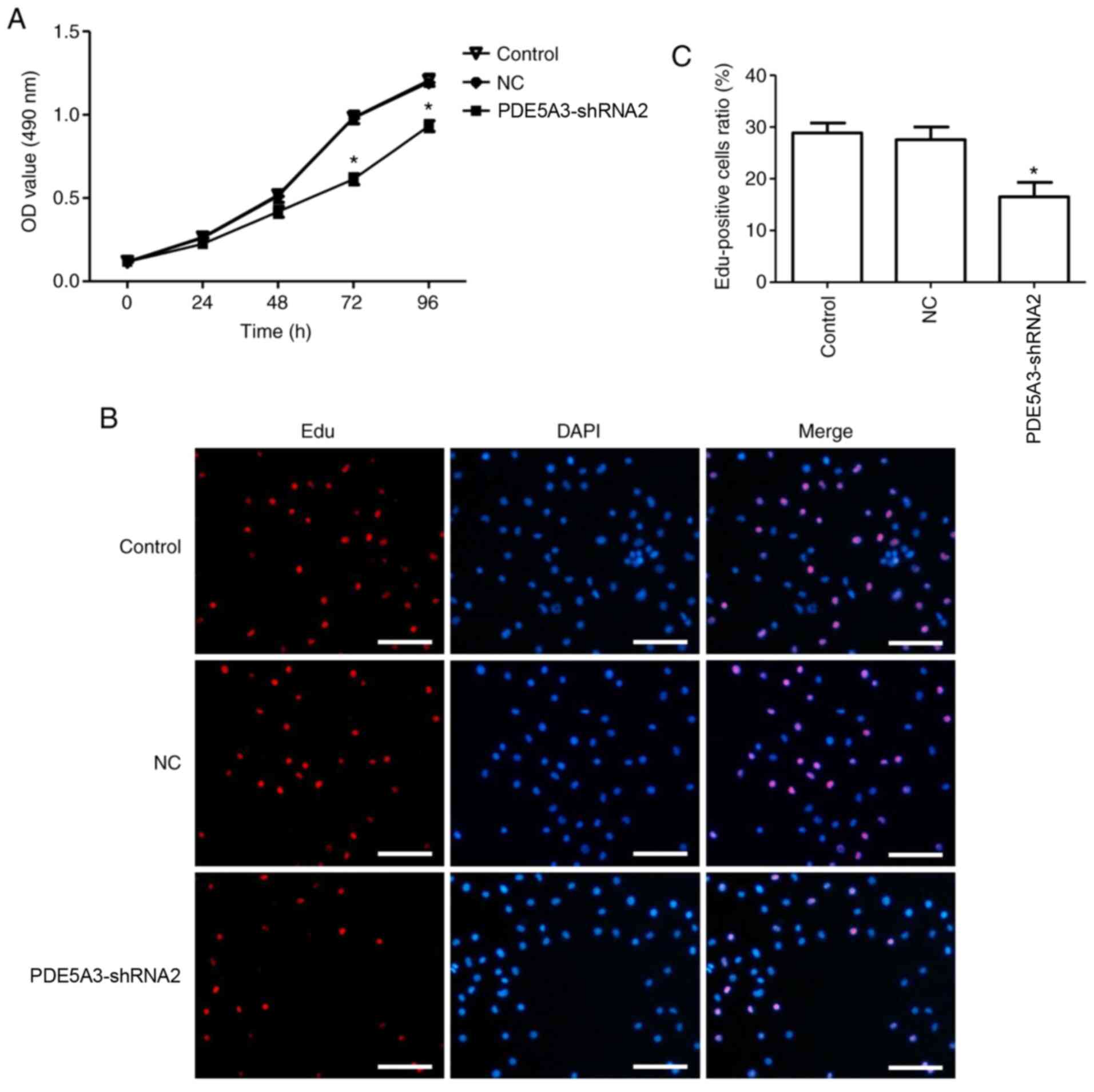
MTT assay, which was used to explore the number of
viable cells, revealed that there were no significant differences
among the three groups within the beginning 2 days (Fig. 7A). Subsequently, the difference
became significant, where the viability of HPSMCs in the
PDE5A3-shRNA2 group was significantly reduced compared with that in
the Control and NC group after 72 and 96 h of culture (Fig. 7A). Similar trends could be observed
after the EdU assay. Specifically, the percentage of EdU-positive
HPSMCs in the PDE5A3-shRNA2 group was significantly decreased
compared with the that in the Control and NC groups after 72 h of
culture (Fig. 7B and C).
Discussion
BPH/LUTS is a common disease among older men that
can greatly reduce the quality of life (5,6,9).
Current strategies for the pharmacotherapy of BPH/LUTS, including
α-blockers and 5-α reductase inhibitors, including tamsulosin,
doxazosin and finasteride, have been proven to induce potential
adverse effects on sexual function and increase the incidence of ED
among older patients (23,24). PDE5 inhibitors (PDE5i), including
sildenafil, vardenafil and tadalafil, are currently considered as
the first-line choice for the clinical treatment of ED (25). A preclinical study previously
conducted by Tinel et al (26) revealed that PDE5i could inhibit the
expression of PDE5 in the prostate and urethral smooth muscle
tissues, inhibit the proliferation of prostate stromal cells,
induce the relaxation of lower urinary tract tissue to eventually
alleviate the manifestations of BPH/LUTS. These findings were
verified further by Gacci et al (27) in a clinical study. However, the
application of PDE5i for BPH/LUTS usually requires long-term
administration and high burden that is frequently accompanied with
side effects, including headache and facial flushing (28,29).
In addition, it is difficult for some patients to achieve the
desired therapeutic effect from PDE5i, increasing the difficulty
for promoting PDE5i in a clinical setting (28). PDE5A3 is a subtype of PDE5 that is
mainly found in the human smooth muscle tissue (15) and can serve as a potentially
promising target for the precision therapy of BPH/LUTS. In the
present study, HPSMCs from the prostates of patients with BPH/LUTS
were obtained and a series of experiments were conducted on these
cells isolated from the nidus. shRNA was utilized to transfect
HPSMCs and silence the expression of the PDE5A3 gene, following
which its effects on the level of cGMP and free Ca2+ in
HPSMCs, in addition to their proliferation, were assessed. To the
best of our knowledge, the present study was the first to
investigate the effect of PDE5A3 silencing on HPSMCs from patients
with BPH.
PDE5 is the most important enzyme regulating the
intracellular levels of cGMP (12).
The inhibition of PDE5 activity can block cGMP hydrolysis, where
the increased cGMP can reduce intracellular Ca2+
concentration via the activation of protein kinase G and subsequent
phosphorylation to eventually promote smooth muscle relaxation
(12-14).
The effects of intracellular Ca2+ on smooth muscle
contraction have already been well defined (30,31).
Results from the present study indicated that the specific
inhibition of PDE5A3 expression by shRNA transfection could also
increase the levels of cGMP and reduce the concentration of
Ca2+ in HPSMCs. Increased prostatic smooth muscle tone
and hyperplastic growth contribute to urethral obstruction and
symptoms in BPH (32-34).
Elliot et al (35) also
reported that in addition to other stromal cell proliferation,
increases in prostate smooth muscle cell are also associated with
increasing the prostate volume during BPH. A previous study from
Wharton et al (36) found
that PDE5 inhibition may exert antiproliferative effects on smooth
muscle cells, which is by another study from Chen et al
(37), which reported that PDE5
inhibitors may also exert antiproliferative effects on smooth
muscle cells through the cGMP pathway. The present study revealed
that shRNA-mediated silencing of PDE5A3 could not only alter the
levels of cGMP and Ca2+, but also decrease the
proliferation of HPSMCs. These findings suggested a possibility for
exploring the use of specific PDE5 inhibitors for the treatment of
BPH/LUTS. Future specific inhibitors targeting PDE5A3 may prove to
be more feasible and promising due to their positive effects on
HPSMCs and potential avoidance of the side effects caused by the
traditional inhibitors.
A number of limitations in the present study should
be acknowledged when interpreting the results. The role of PDE5A3
was only detected in vitro, such that evidence from in
vivo experiments was absent, which should be the focus of
future studies. In future studies, PDE5A3 knock-out mice should be
obtained for prostate tissue isolation for tension measurements.
Partial functions of HPSMCs were explored after shRNA transfection
and other alterations in the mechanical contractile force and cell
migration required further exploration. Additionally, the
underlying mechanism during PDE5A3-mediated regulation should be
explored in a future study.
In summary, findings of the present study confirmed
that shRNA-mediated silencing of PDE5A3 could increase the level of
cGMP and reduce the concentration of Ca2+ in HPSMCs,
whilst decreasing the proliferation of HPSMCs. These findings may
provide a novel molecular target for the treatment of BPH/LUTS.
Acknowledgements
Not applicable.
Funding
Funding: The present study was supported by the Nanjing Medical
Science and Technology Development Foundation (grant no. YKK16138),
the Jiangsu Provincial Medical Youth Talent (grant no. QNRC2016072)
and the Jiangsu Provincial Social Development Project (grant no.
BE2017615).
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
RJ and ZX conceptualized and designed the study. ZX,
YG, and CZ performed the experiments and analyzed the data. ZX, KJ
and LX were involved in the data analysis and interpretation. ZX
drafted and revised the manuscript. JZ and LZ drafted the
manuscript, performed the sample collection and the data analysis.
ZX, JZ and LZ confirmed the authenticity of all the raw data. All
authors read and approved the final manuscript.
Ethics approval and consent to
participate
The present study was approved by Ethics Committee
of Nanjing Medical University (Nanjing, China). All patients
provided written informed consent.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Yu ZJ, Yan HL, Xu FH, Chao HC, Deng LH, Xu
XD, Huang JB and Zeng T: Efficacy and side effects of drugs
commonly used for the treatment of lower urinary tract symptoms
associated with benign prostatic hyperplasia. Front Pharmacol.
11(658)2020.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Lee C, Kozlowski JM and Grayhack JT:
Intrinsic and extrinsic factors controlling benign prostatic
growth. Prostate. 31:131–138. 1997.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Yoo S, Oh S, Park J, Cho SY, Cho MC, Jeong
H and Son H: The impacts of metabolic syndrome and lifestyle on the
prevalence of benign prostatic hyperplasia requiring treatment:
Historical cohort study of 130 454 men. BJU Int. 123:140–148.
2019.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Calogero AE, Burgio G, Condorelli RA,
Cannarella R and La Vignera S: Epidemiology and risk factors of
lower urinary tract symptoms/benign prostatic hyperplasia and
erectile dysfunction. Aging Male. 22:12–19. 2019.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Egan KB: The epidemiology of benign
prostatic hyperplasia associated with lower urinary tract symptoms:
Prevalence and incident rates. Urol Clin North Am. 43:289–297.
2016.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Gacci M, Andersson KE, Chapple C, Maggi M,
Mirone V, Oelke M, Porst H, Roehrborn C, Stief C and Giuliano F:
Latest evidence on the use of phosphodiesterase type 5 inhibitors
for the treatment of lower urinary tract symptoms secondary to
benign prostatic hyperplasia. Eur Urol. 70:124–133. 2016.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Speakman MJ: PDE5 inhibitors in the
treatment of LUTS. Curr Pharm Des. 15:3502–3505. 2009.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Broderick GA: Oral pharmacotherapy and the
contemporary evaluation and management of erectile dysfunction. Rev
Urol. 5 (Suppl 7):S9–S20. 2003.PubMed/NCBI
|
|
9
|
Li MK, Garcia L, Patron N, Moh LC, Sundram
M, Leungwattanakij S, Pripatnanont C, Cheng C, Chi-Wai M and
Loi-Cheong N: An Asian multinational prospective observational
registry of patients with benign prostatic hyperplasia, with a
focus on comorbidities, lower urinary tract symptoms and sexual
function. BJU Int. 101:197–202. 2008.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Maurice DH, Ke H, Ahmad F, Wang Y, Chung J
and Manganiello VC: Advances in targeting cyclic nucleotide
phosphodiesterases. Nat Rev Drug Discov. 13:290–314.
2014.PubMed/NCBI View
Article : Google Scholar
|
|
11
|
Keravis T and Lugnier C: Cyclic nucleotide
phosphodiesterase (PDE) isozymes as targets of the intracellular
signalling network: Benefits of PDE inhibitors in various diseases
and perspectives for future therapeutic developments. Br J
Pharmacol. 165:1288–1305. 2012.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Murthy KS: Signaling for contraction and
relaxation in smooth muscle of the gut. Annu Rev Physiol.
68:345–374. 2006.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Wroński S: The new horizons of
pharmacotherapy. Unexpected pharmacological actions and a new
therapeutic strategy of phosphodiesterase-5 inhibitors. Cent
European J Urol. 67:314–318. 2014.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Gacci M, Corona G, Salvi M, Vignozzi L,
McVary KT, Kaplan SA, Roehrborn CG, Serni S, Mirone V, Carini M and
Maggi M: A systematic review and meta-analysis on the use of
phosphodiesterase 5 inhibitors alone or in combination with
α-blockers for lower urinary tract symptoms due to benign prostatic
hyperplasia. Eur Urol. 61:994–1003. 2012.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Lin CS, Lin G, Xin ZC and Lue TF:
Expression, distribution and regulation of phosphodiesterase 5.
Curr Pharm Des. 12:3439–3457. 2006.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Zucchi A, Costantini E, Scroppo FI,
Silvani M, Kopa Z, Illiano E, Petrillo MG, Cari L and Nocentini G:
The first-generation phosphodiesterase 5 inhibitors and their
pharmacokinetic issue. Andrology. 7:804–817. 2019.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Lin CS, Chow S, Lau A, Tu R and Lue TF:
Human PDE5A gene encodes three PDE5 isoforms from two alternate
promoters. Int J Impot Res. 14:15–24. 2002.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Aguiar S, van der Gaag B and Cortese FAB:
RNAi mechanisms in Huntington's disease therapy: siRNA versus
shRNA. Transl Neurodegener. 6(30)2017.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Pan YG, Liu JH, Zhan Y, Wang T, Wan ZH, Li
ZY and Liu Y: Impact of rAd5-shRNA-PDE5A3 on cGMP in the smooth
muscle cells of human corpus cavernosum. Zhonghua Nan Ke Xue.
15:689–692. 2009.PubMed/NCBI(In Chinese).
|
|
20
|
Henry GH, Malewska A, Joseph DB, Malladi
VS, Lee J, Torrealba J, Mauck RJ, Gahan JC, Raj GV, Roehrborn CG,
et al: A cellular anatomy of the normal adult human prostate and
prostatic urethra. Cell Rep. 25:3530–3542.e5. 2018.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Li C, Ye L, Yang L, Yu X, He Y, Chen Z, Li
L and Zhang D: Rapamycin promotes the survival and adipogenesis of
ischemia-challenged adipose derived stem cells by improving
autophagy. Cell Physiol Biochem. 44:1762–1774. 2017.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408.
2001.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Gacci M, Ficarra V, Sebastianelli A,
Corona G, Serni S, Shariat SF, Maggi M, Zattoni F, Carini M and
Novara G: Impact of medical treatments for male lower urinary tract
symptoms due to benign prostatic hyperplasia on ejaculatory
function: A systematic review and meta-analysis. J Sex Med.
11:1554–1566. 2014.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Fwu CW, Eggers PW, Kirkali Z, McVary KT,
Burrows PK and Kusek JW: Change in sexual function in men with
lower urinary tract symptoms/benign prostatic hyperplasia
associated with long-term treatment with doxazosin, finasteride and
combined therapy. J Urol. 191:1828–1834. 2014.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Carvalheira A, Forjaz V and Pereira NM:
Adherence to phosphodiesterase type 5 inhibitors in the treatment
of erectile dysfunction in long-term users: How do men use the
inhibitors? Sex Med. 2:96–102. 2014.PubMed/NCBI View
Article : Google Scholar
|
|
26
|
Tinel H, Stelte-Ludwig B, Hütter J and
Sandner P: Pre-clinical evidence for the use of phosphodiesterase-5
inhibitors for treating benign prostatic hyperplasia and lower
urinary tract symptoms. BJU Int. 98:1259–1263. 2006.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Gacci M, Salvi M, Sebastianelli A,
Vignozzi L, Corona G, McVary KT, Kaplan SA, Maggi M, Carini M and
Oelke M: The use of a single daily dose of tadalafil to treat signs
and symptoms of benign prostatic hyperplasia and erectile
dysfunction. Res Rep Urol. 5:99–111. 2013.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Chrysant SG: Effectiveness and safety of
phosphodiesterase 5 inhibitors in patients with cardiovascular
disease and hypertension. Curr Hypertens Rep. 15:475–483.
2013.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Rashid A: The efficacy and safety of PDE5
inhibitors. Clin Cornerstone. 7:47–56. 2005.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Paul M, Murphy SF, Hall C, Schaeffer AJ
and Thumbikat P: Protease-activated receptor 2 activates
CRAC-mediated Ca2+ influx to cause prostate smooth
muscle contraction. FASEB Bioadv. 1:255–264. 2019.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Brozovich FV, Nicholson CJ, Degen CV, Gao
YZ, Aggarwal M and Morgan KG: Mechanisms of vascular smooth muscle
contraction and the basis for pharmacologic treatment of smooth
muscle disorders. Pharmacol Rev. 68:476–532. 2016.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Shi YF, Yu DJ, Jiang CY, Wang XJ, Zhu YP,
Zhao RZ, Lv Z and Sun XW: TRAF6 regulates proliferation of stromal
cells in the transition and peripheral zones of benign prostatic
hyperplasia via Akt/mTOR signaling. Prostate. 78:193–201.
2018.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Chen P, Yin J, Guo YM, Xiao H, Wang XH,
DiSanto ME and Zhang XH: The expression and functional activities
of smooth muscle myosin and non-muscle myosin isoforms in rat
prostate. J Cell Mol Med. 22:576–588. 2018.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Wang X, Wang Y, Gratzke C, Sterr C, Yu Q,
Li B, Strittmatter F, Herlemann A, Tamalunas A, Rutz B, et al:
Ghrelin aggravates prostate enlargement in rats with
testosterone-induced benign prostatic hyperplasia, stromal cell
proliferation, and smooth muscle contraction in human prostate
tissues. Oxid Med Cell Longev. 2019(4748312)2019.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Elliot SJ, Zorn BH, McLeod DG, Moul JW,
Nyberg L, Striker LJ and Striker GE: Pentosan polysulfate decreases
prostate smooth muscle proliferation and extracellular matrix
turnover. Prostate Cancer Prostatic Dis. 6:138–142. 2003.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Wharton J, Strange JW, Møller GM, Growcott
EJ, Ren X, Franklyn AP, Phillips SC and Wilkins MR:
Antiproliferative effects of phosphodiesterase type 5 inhibition in
human pulmonary artery cells. Am J Respir Crit Care Med.
172:105–113. 2005.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Chen L, Daum G, Chitaley K, Coats SA,
Bowen-Pope DF, Eigenthaler M, Thumati NR, Walter U and Clowes AW:
Vasodilator-stimulated phosphoprotein regulates proliferation and
growth inhibition by nitric oxide in vascular smooth muscle cells.
Arterioscler Thromb Vasc Biol. 24:1403–1408. 2004.PubMed/NCBI View Article : Google Scholar
|