Introduction
Osteoarthritis (OA) is a chronic degenerative
disease of the joints, characterized by cartilage degeneration,
chronic inflammation and decreased normal joint function (1). Its clinical manifestations are mainly
local pain and limited activity of the joint (2). OA commonly occurs in females over 55
and males over 65 years of age (3).
With economic improvements and the aging of the population, an
increasing number of patients are diagnosed with OA, which is now
the most common cause of disability worldwide. The number of
patients with OA is estimated to be >47 million in the US and is
forecast to increase to ~67 million in 2030 (25% of the adult
population) (4). The quality of
life of patients with OA is significantly lower than that of others
and poses great physical, psychological and financial burdens.
Although the pathogenesis of OA has received much
clinical attention, genetic factors associated with the development
of this disease remain elusive (5).
Previously, OA was thought to be a heritable disease, but later
studies have not determined any clear hereditary factors in the
pathogenesis of OA (6). However,
bioinformatics has advanced the study of OA and has identified
specific genes involved in disease severity (7). Similar advances have been made in the
study of rheumatoid arthritis (8,9). While
hub genes involved in OA have been identified as key genes in its
pathogenesis, no comprehensive genetic analysis has yet been
performed (10). Several studies
have performed gene expression profiling of OA samples and screened
thousands of differentially expressed genes (DEGs) using
high-throughput sequencing technology and other advanced techniques
(11,12). However, a comprehensive analysis of
all gene data collected is still missing. An integrated
bioinformatics approach is able to predict and identify the hub
genes involved in ОA.
In the present study, three datasets,
GSE12021(13), GSE55457(14) and GSE55235(11), were analyzed. Screening of DEGs
between OA patients and normal controls was performed. Gene
Ontology (GO) and DEG pathway enrichment, protein-protein
interaction (PPI) network and functional module analyses were then
performed to explore the underlying molecular mechanisms of the
pathogenesis of OA.
Materials and methods
Microarray data
National Center for Biotechnology Information Gene
Expression Omnibus (NCBI GEO) is a public online repository for
high-throughput gene queries and high-throughput gene expression
detection for the global research community (15). It was used in the present study to
obtain OA-related genes and their expression values were downloaded
for further analysis. The GSE12021, GSE55235 and GSE55457 gene
expression profiles were downloaded from the GEO database.
Subsequently, three datasets from OA patients and normal controls
(GSE55235, GSE55457 and GSE77298) were used for analysis; however,
the data were downloaded without OA stage identification.
Screening of DEGs by GEO2R
The GEO2R online database (https://www.ncbi.nlm.nih.gov/gds/) was used to
identify DEGs between OA samples and normal controls from three
databases (GSE55235, GSE55457 and GSE77298); |log fold change
(FC)|>1 and P<0.01 were considered to be DEGs. Venn diagrams
were used to screen for common significant differences among
DEGs.
GO enrichment analysis and Kyoto
Encyclopedia of Genes and Genomes (KEGG) pathway analysis
For GO analysis, functional terms enriched by the
DEGs were determined in three distinct categories representing
different biological aspects: Molecular function (MF), biological
process (BP) and cellular component (CC) (16). KEGG pathways were determined to
analyze gene functions and link the genetic information in the
genome with the biological functional information of genes. The
Database for Annotation, Visualization and Integrated Discovery
(DAVID; https://david.ncifcrf.gov/) was used
to perform GO analysis and KEGG analysis.
Construction of PPI network
PPI network construction was performed using the
Search Tool for the Retrieval of Interacting Genes/proteins
(STRING) database (https://string-db.org/cgi/network.pl). Subsequently,
the DEGs were imported into Cytoscape software (http://www.cytoscape.org/) for better visualization.
Next, the MCODE plugin was used to screen for key DEGs among all
DEGs.
Reverse transcription-quantitative
(RT-q)PCR verification
RT-qPCR was used to verify the four key genes. The
RNA samples obtained from chondrocytes were extracted and
transcribed into complementary DNA by an RNAiso Plus and
PrimeScript® RT reagent kit (Takara Bio, Inc.). The
DyNAmo SYBR-Green and qPCR systems (Takara Bio, Inc.) were used to
analyze gene expression. Fill the ice box with samples. A ribozyme
free 200 µl EP tube was placed on ice, after which 1,000 ng RNA
samples were added. A total of 1 µl gDNA Eraser and 1 µl 5X gDNA
Eraser Buffer was subsequently added to samples. Finally, RNase
Free ddH2O was added to samples making a total volume of
10 µl. Amplification 2 min at 42˚C. Then at 4˚C, 4 µl 5X
PrimeScript Buffer 2, 4 µl RNase Free ddH2O, 1 µl
1PrimeScrip Enzyme Mix1 and 1 µl RT Prime Mix was added to samples.
Amplification 15 min at 37˚C and 5 sec at 85˚C. A total of 20 µl
cDNA was obtained by reverse transcription. Subsequently, 5 µl SYBR
Premix Ex Taq II with 3.5 µl dd H2O, 0.2 µl ROX, 0.5 µl
cDNA, 0.4 µl PCR Forward Primer (10 µM) and 0.4 µl PCR Reverse
Primer (10 µM) was added 96-well plates. The plates were run on the
fluorescence quantitative PCR instrument, using the following
cycling conditions: 95˚C for 30 sec, followed by 40 cycles at 95˚C
for 5 sec, 60˚C for 30 sec, 90˚C for 15 sec and 60˚C for 60 sec.
The paired primers for the four key genes are listed in Table I. All samples were compared with
GAPDH. The 2-ΔΔCq method was used to quantify the
relative gene expression levels (17).
 | Table IPrimer sequences for four hub genes
(5'-3'). |
Table I
Primer sequences for four hub genes
(5'-3').
| Gene | Forward primer | Reverse primer |
|---|
| BSCL2 |
ATGGTCAACGACCCTCCAGTA |
GCTGACTGTCGGCATATAGGAA |
| FOSL2 |
CAGAAATTCCGGGTAGATATGCC |
GGTATGGGTTGGACATGGAGG |
| CDKN1A |
TGTCCGTCAGAACCCATGC |
AAAGTCGAAGTTCCATCGCTC |
| KTN1 |
AAATGTCTTCGTAGATGAACCCC |
TTTGTCAGTTTCGGTCTTCAGTT |
| GAPDH |
GGCACAGTCAAGGCTGAGAATG |
ATGGTGGTGAAGACGCCAGTA |
Cell culture and immunofluorescence
assays
The collection and culturing of chondrocytes was
performed as described in detail in previous studies by our group
(18-20).
Specimens of the knee joint and femoral head were collected from
patients undergoing joint replacement surgery at the Bone and Joint
Department of Shenzhen Second People's Hospital (Shenzhen, China)
between December 2017 and October 2019. According to the patient's
imageological diagnosis, all OA samples obtained were from patients
with OA of grades III and IV in Kellgren-Lawrence classification
(21). In addition, as the control,
the samples of patients with femoral neck fracture on radiographic
diagnosis were used. The average age of the patients was 78.44±3.42
years in the control group, including 9 patients (2 males and 7
females), and 66.60±4.53 years in the OA group, including 5
patients (5 females). The following procedures were performed under
sterile conditions. The samples were washed with normal saline
three times and a surgical blade was used to cut the cartilage
tissue blocks to final sizes of approximately 1x1x1 mm. The samples
were incubated with collagenase type II (1 mg/ml; cat. no. C6885;
Sigma-Aldrich; Merck KGaA) working liquid and oscillated for
digestion for 8-12 h at 37˚C. The cell suspension was then divided
into two parts. One part was added to complete chondrocyte culture
medium (containing 10% FBS; Gibco; Thermo Fisher Scientific, Inc.),
1% penicillin and streptomycin, 1% HEPES, 1% ascorbic acid, 1%
proline solution and 1% non-essential amino acids) and cultured in
a CO2 incubator. The other part was centrifuged and
resuspended and cells were dropped onto a glass slide for
subsequent experiments. The present study was approved by the
Ethics Committee of Shenzhen Second People's Hospital (Shenzhen,
China) and written informed consent was obtained from all
subjects.
For the immunofluorescence experiment, methods
similar to those of other studies were used (22,23).
The steps of immunofluorescence labeling of target protein were as
follows: After washing the cells with PBS two times, the cells were
fixed with paraformaldehyde for 15 min. The cells were then washed
with PBS six times for 5 min each. Triton X100 (0.5%, diluted with
PBS) was added, and samples were incubated for 15 min at room
temperature. Cells were again washed by PBS six times for 5 min
each. The cells were then blocked with 5% bovine serum albumin
(Amresco LLC) for 2 h at room temperature and then washed with 0.5%
BSA six times for 5 min each. The samples were then incubated with
primary antibody (rabbit anti-Collagen II/FITC conjugated antibody;
cat. no. bs-10589R-FITC; BIOSS) at 4˚C overnight. As these
conjugated fluorescent dye primary antibodies demonstrated a strong
specificity, secondary antibodies were not required. Subsequently,
the cells were washed with 0.5% BSA six times for 5 min each.
Subsequently, the cells were washed by PBS six times for 5 min
each. DAPI (Thermo Fisher Scientific, Inc.) was added for 5 min and
cells were washed with PBS three times for 5 min each. Images of
fluorescently labeled cells were acquired with an LSM800 confocal
microscope (Zeiss AG).
Statistical analysis
Prism 8 (GraphPad Software, Inc.) was used to
generate figures. Experimental data were statistically analyzed
using SPSS 19.0 software (IBM Corp.). Student's t-test was used to
assess differences between groups. P<0.05 was considered to
indicate a statistically significant difference.
Results
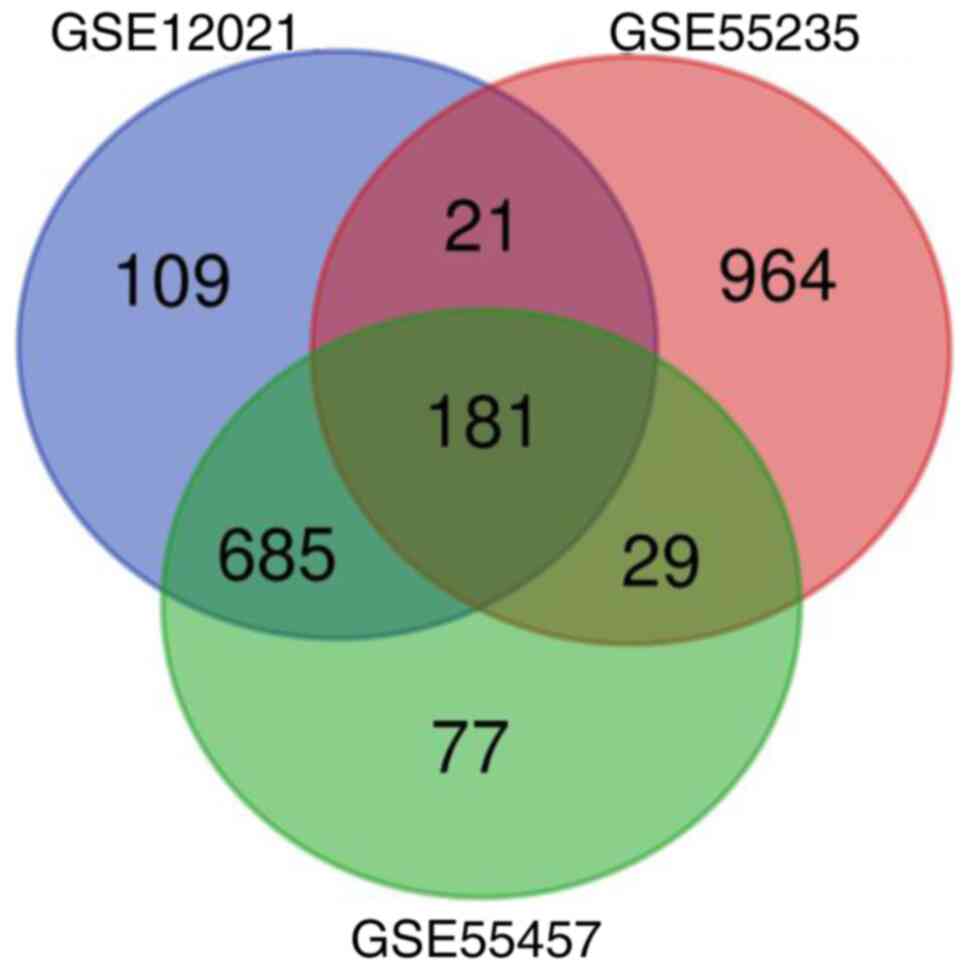
Screening of DEGs
Genes with significant differences in expression
(P<0.051 and |log FC|>1) between the OA samples and normal
controls were screened in the GEO2R website and 181 DEGs, including
48 upregulated and 133 downregulated genes, were identified
(Fig. 1).
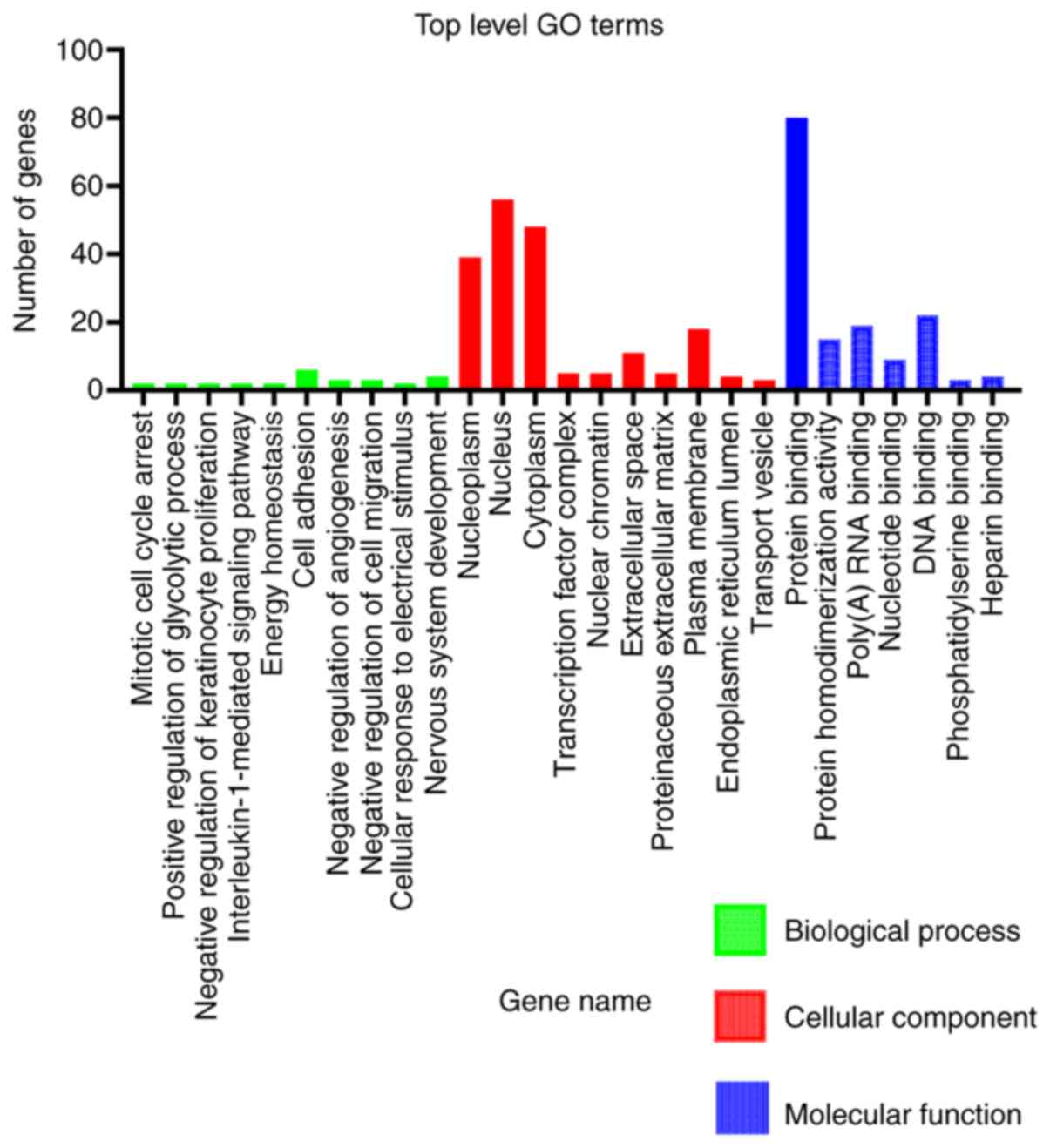
GO functional enrichment analysis
The functions of the 181 DEGs were classified using
DAVID. In the GO category MF, these DEGs were significantly
enriched in protein-DNA binding, RNA binding and protein
homodimerization activity. In addition, in the category CC, the
DEGs were significantly enriched in the nucleoplasm, nucleus,
cytoplasm and plasma membrane. However, in the category BP, there
were fewer genes enriched compared with the other categories
(Fig. 2 and Table II). The upregulated DEGs were
mainly enriched in the extracellular space and plasma membrane. The
downregulated DEGs were significantly enriched in the nucleoplasm,
nucleus and cytoplasm, and were involved in protein binding.
 | Table IISignificant enrichment of DEGs in
osteoarthritis. |
Table II
Significant enrichment of DEGs in
osteoarthritis.
| A, Downregulated
DEGs |
|---|
| Category | Term | Description | Gene count | P-value |
|---|
| BP | GO:0071850 | Mitotic cell cycle
arrest | 2 |
8.41x10-2 |
| BP | GO:0045821 | Positive regulation
of glycolytic process | 2 |
9.02x10-2 |
| BP | GO:0010839 | Negative regulation
of keratinocyte proliferation | 2 |
9.02x10-2 |
| BP | GO:0070498 |
Interleukin-1-mediated signaling
pathway | 2 |
9.02x10-2 |
| BP | GO:0097009 | Energy
homeostasis | 2 |
9.64x10-2 |
| CC | GO:0005654 | Nucleoplasm | 39 |
2.45x10-6 |
| CC | GO:0005634 | Nucleus | 56 |
5.71x10-5 |
| CC | GO:0005737 | Cytoplasm | 48 |
4.78x10-3 |
| CC | GO:0005667 | Transcription
factor complex | 5 |
3.59x10-2 |
| CC | GO:0000790 | Nuclear
chromatin | 5 |
3.59x10-2 |
| MF | GO:0005515 | Protein
binding | 80 |
3.11x10-4 |
| MF | GO:0042803 | Protein
homodimerization activity | 15 |
4.81x10-4 |
| MF | GO:0044822 | poly(A) RNA
binding | 19 |
6.78x10-4 |
| MF | GO:0000166 | Nucleotide
binding | 9 |
2.78x10-3 |
| MF | GO:0003677 | DNA binding | 22 |
4.73x10-3 |
| B, Upregulated
DEGs |
| Category | Term | Description | Gene count | P-value |
| BP | GO:0007155 | Cell adhesion | 6 |
4.97x10-3 |
| BP | GO:0016525 | Negative regulation
of angiogenesis | 3 |
1.00x10-2 |
| BP | GO:0030336 | Negative regulation
of cell migration | 3 |
2.25x10-3 |
| BP | GO:0071257 | Cellular response
to electrical stimulus | 2 |
2.89x10-2 |
| BP | GO:0007399 | Nervous system
development | 4 |
3.27x10-2 |
| CC | GO:0005615 | Extracellular
space | 11 |
6.34x10-4 |
| CC | GO:0005578 | Proteinaceous
extracellular matrix | 5 |
3.02x10-3 |
| CC | GO:0005886 | Plasma
membrane | 18 |
5.37x10-3 |
| CC | GO:0005788 | Endoplasmic
reticulum lumen | 4 |
9.15x10-3 |
| CC | GO:0030133 | Transport
vesicle | 3 |
1.93x10-2 |
| MF | GO:0001786 | Phosphatidylserine
binding | 3 |
2.97x10-3 |
| MF | GO:0008201 | Heparin
binding | 4 |
5.12x10-3 |
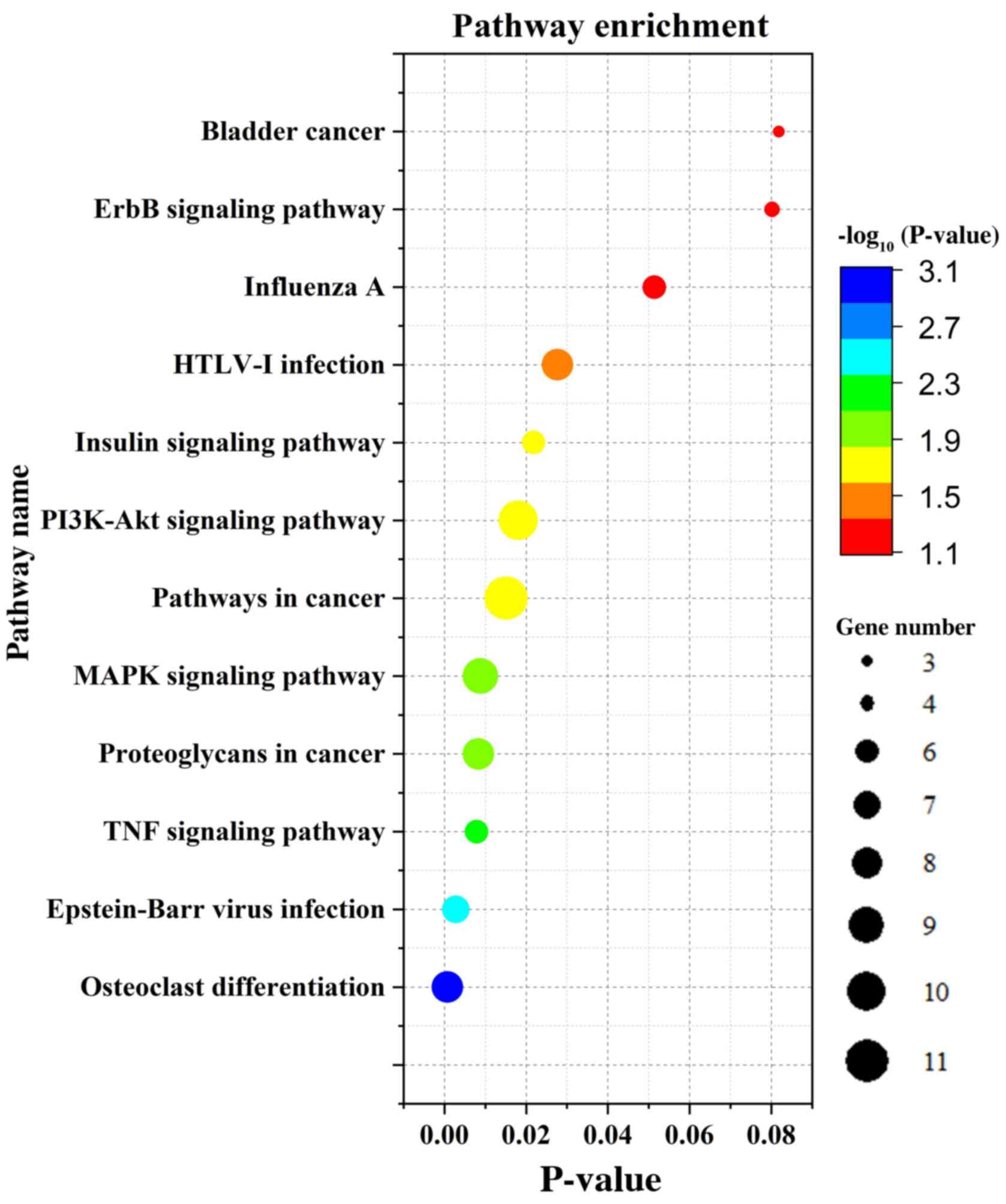
KEGG signaling pathway analysis
DEGs were mainly enriched in the MAPK and PI3K-Akt
signaling pathways (Fig. 3)
according to the KEGG analysis. The signaling pathways of
downregulated DEGs were mainly enriched in osteoclast
differentiation, MAPK signaling, TNF signaling, Epstein-Barr virus
infection and insulin signaling. Upregulated genes were mainly
enriched in influenza A signaling (Table III).
 | Table IIIKyoto Encyclopedia of Genes and
Genomes analysis result of DEGs. |
Table III
Kyoto Encyclopedia of Genes and
Genomes analysis result of DEGs.
| A, Downregulated
DEGs |
|---|
| Term | Description | Gene count | P-value |
|---|
| hsa04380 | Osteoclast
differentiation | 7 |
9.17x10-4 |
| hsa04010 | MAPK signaling
pathway | 9 |
1.46x10-3 |
| hsa04668 | TNF signaling
pathway | 6 |
2.30x10-3 |
| hsa05169 | Epstein-Barr virus
infection | 6 |
4.05x10-3 |
| hsa04910 | Insulin signaling
pathway | 6 |
6.81x10-3 |
| B, Upregulated
DEGs |
| Term | Description | Gene count | P-value |
| hsa05164 | Influenza A | 3 |
8.21x10-3 |
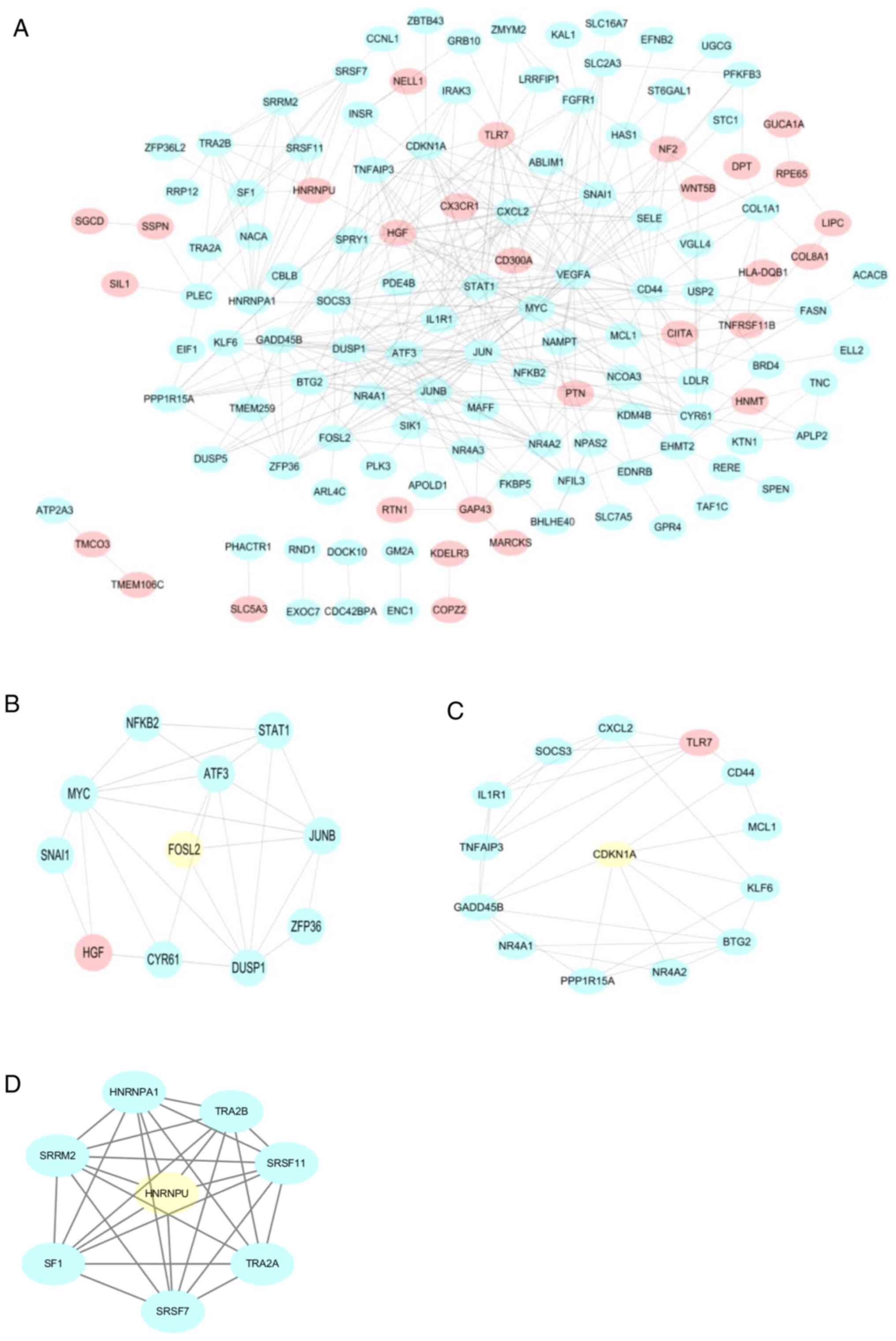
PPI network builds and MCODE
analysis
To further analyze the relationships among all DEGs,
PPI networks were constructed, including 161 nodes and 375 edges.
The network included 181 protein interactions with combined scores
of >0.4 (Fig. 4A). By further
analyzing the PPI networks, four modules were detected using the
MCODE plugin in Cytoscape. According to the MCODE score, key genes
in four modules were screened, including BSCL2 lipid droplet
biogenesis associated, seipin (BSCL2), FOS-like 2, activator
protein-1 transcription factor subunit (FOSL2), cyclin-dependent
kinase inhibitor 1A (CDKN1A) and kinectin 1 (KTN1) (Fig. 4B-D).
Validation of key genes
To verify the results, the expression levels of four
key genes were determined in human articular chondrocytes (Fig. 5). First, normal chondrocytes (Ctrl
group) and osteoarthritis chondrocytes (OA group) were obtained
from patients undergoing joint replacement and subsequently, cells
were cultured and stained with toluidine blue (Fig. 5A and B). To further identify the two types of
cells, immunofluorescence was used to observe differences in the
expression of type II collagen, which was decreased significantly
in OA chondrocytes (Fig. 5C).
Subsequently, the expression levels of the four key genes were
determined by RT-qPCR. The results indicated that the expression
level of BSCL2 in OA samples was increased but with no significant
difference (P>0.05, n=3; Fig.
5F), while the expression levels of FOSL2, CDKN1A and KTN1 were
significantly decreased. The differences between OA and normal
chondrocytes in the expression levels of the four key genes were
consistent with the analytical results of PCR (P<0.05, n=3;
Fig. 5D, E and G).
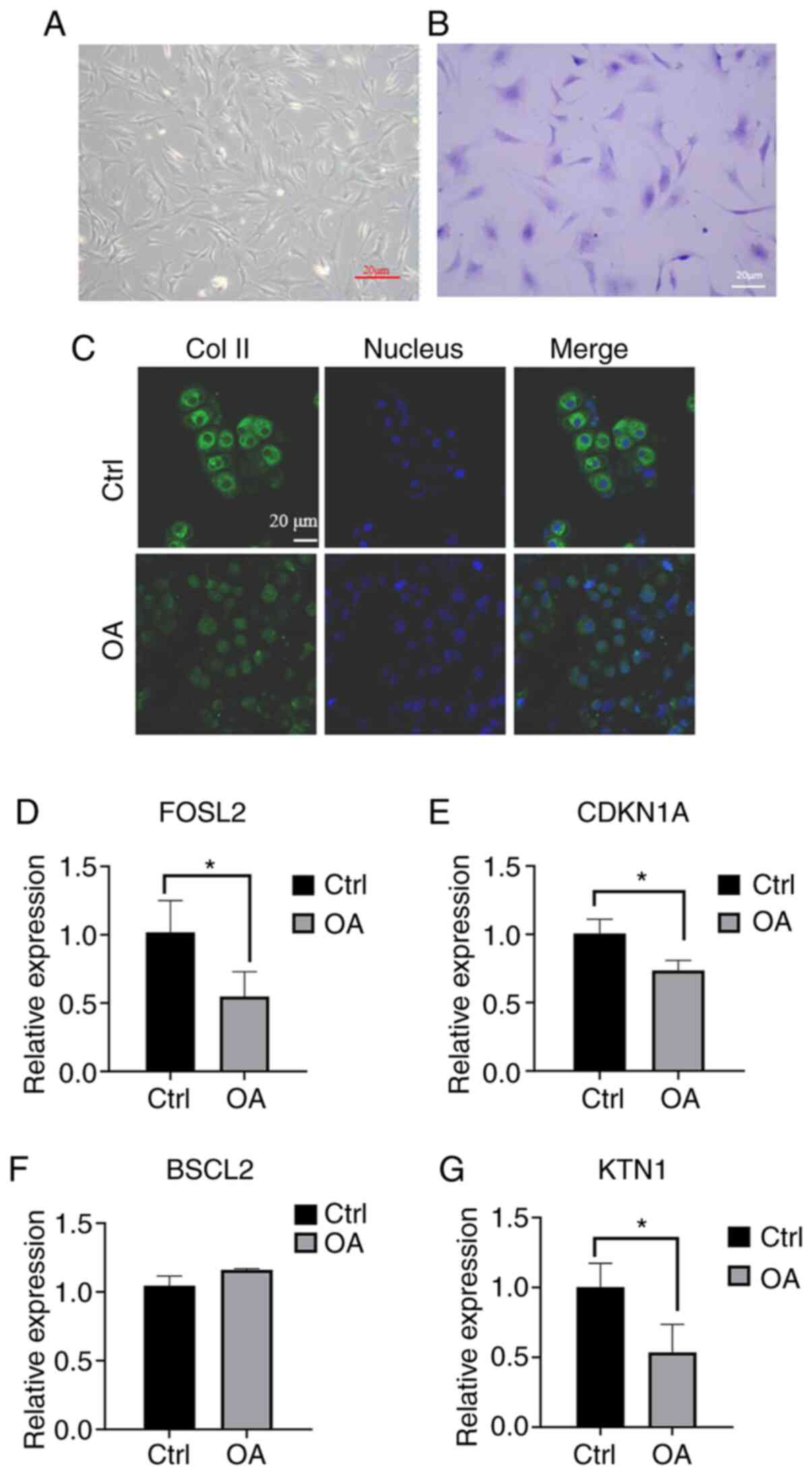 | Figure 5RT-qPCR results of four key genes. (A
and B) Growth of normal chondrocytes (A) under a normal microscope
and (B) staining with toluidine blue (passage number of the cells
is P1; scale bar, 20 µm). (C) Difference in expression of collagen
II (green) between normal chondrocytes and OA chondrocytes under
confocal microscopy (scale bar, 20 µm). (D-G) Validation of the
differential expression of four hub genes between the OA group and
the normal group by RT-qPCR. (D) FOSL2, (E) CDKN1A, (F) BSCL2 and
(G) KTN1. All samples were normalized to the expression of GAPDH
and the relative expression levels of each gene were analyzed using
the 2-ΔΔCq method. *P<0.05. OA,
osteoarthritis; Ctrl, control; BSCL2, BSCL2 lipid droplet
biogenesis associated, seipin; FOSL2, FOS-like 2, activator
protein-1 transcription factor subunit; CDKN1A, cyclin-dependent
kinase inhibitor 1A; KTN1, kinectin 1. |
Discussion
OA is considered to be the most common cause of
disability (24); it seriously
affects the quality of life, creating physical, psychological and
economic burdens for patients. The diagnosis and treatment of OA
require an interdisciplinary approach. Bioinformatics is a tool
widely used to predict potential target genes for numerous
diseases. In the present study, three sets of data from patients
with OA and normal controls were analyzed by using GEO2R. Between
the OA and control groups, 181 DEGs were identified, including 48
upregulated and 133 downregulated genes. The upregulated DEGs were
mainly enriched in the extracellular space and plasma membrane.
Downregulated DEGs were significantly enriched in functional terms
associated with the nucleoplasm, nucleus, cytoplasm and protein
binding, and this explains that the main difference between
patients with OA and individuals without OA is in the cartilage
cells. Cellular changes are an important part of the process of OA.
OA is a chronic degenerative disease characterized by cartilage
defects and chondrocytes are the only cell type in articular
cartilage (25). Cartilage cell
apoptosis is a key part of OA (26). The present study suggested that the
downregulated gene CDKN1A, which regulates the occurrence and
development of apoptosis, may participate in the development of OA.
Further analysis indicated that these DEGs were mainly enriched in
the ErbB, MAPK and PI3K-Akt signaling pathways. These pathways are
associated with chondrocyte apoptosis in OA. MAPK modulates joint
inflammation and joint damage (27). The major pathological changes in OA
are cartilage damage and poor healing (1), so the MAPK pathway is able to promote
the aggravation of OA or rheumatoid arthritis to exert an important
role in the pathogenesis of arthritis (26,28).
Erb activates PI3K to generate
phosphatidylinositol-3,4,5-triphosphate that effectively activates
the Akt pathway. Apoptosis-associated proteins are competitively
inhibited by the Akt pathway. Thus, recruiting this gene may
promote cell survival and inhibit cell apoptosis (29). There is a definite correlation
between the degree of cartilage damage and chondrocyte apoptosis
(26). Therefore, inhibiting
chondrocyte apoptosis may be effective in regulating cartilage
degeneration during OA. Animal experiments have indicated that the
use of MAPK inhibitors effectively improves inflammation and joint
degeneration in mice with OA (30),
which demonstrates the potential of inhibiting MAPK in OA
treatment. The PI3K/Akt pathway is associated with TNF-α-induced
activation of OA fibroblast-like synoviocytes, which may be
involved in OA pathogenesis (31).
It promotes cartilage degeneration, subchondral bone function
damage and inflammation during OA (32). The use of MAPK inhibitors reduces
the pathological changes of OA (31). CDKN1A is able to activate the AKT
pathway and induce MAPK8 to participate in the inactivation of
MAPK, further reducing apoptosis (33,34).
It has been reported that CDKN1A is significantly downregulated in
the synovium of arthritis patients and is associated with
inhibition of chondrocyte proliferation (35), suggesting that upregulation of
CDKN1A may have a positive role in the early treatment of OA.
By contrast, the expression of BSCL2 in OA samples
was significantly increased compared with that in normal samples.
BSCL2 has a key role in lipogenesis, lipid metabolism and lipid
droplet synthesis (36). The loss
of BSCL2 may lead to serious disorders in metabolic dysfunction and
a significant reduction in fat (37), whereas upregulation of BSCL2 may
lead to weight gain. Obesity is a major cause of OA (38), and thus, upregulation of BSCL2 may
induce its development. Furthermore, various transforming (FOS)
proteins affect the physiology of chondrocytes, osteoblasts and
osteoclasts (39). FOSL2 is the key
regulator of leptin expression in fat cells (40) and its deficiency is able to promote
obesity. In the present study, its low expression in OA samples was
noted, which is consistent with obesity being a leading cause of OA
(38). Recent studies have
indicated that FOSL2 is suppressed in the early hypertrophy state
of chondrocytes, suggesting that this gene is strongly associated
with the early initiation of OA (41). Therefore, overexpressed BSCL2 and
decreased expression of FOSL2 in OA samples promotes obesity in
patients with OA, and their regulation may have a positive role in
the prevention of OA. Furthermore, KTN1 is a receptor on the
endoplasmic reticulum (42) that
has an important part in adjusting protein biosynthesis in cells
(43). A proteomics analysis of
human mesenchymal stem cells undergoing inhibited chondrogenesis
indicated reduced levels of KTN1(44), suggesting an association of KTN1
with chondrogenesis enhancement. Thus, enhancing KTN1 may induce
chondrocyte proliferation.
Of note, the present study had a limitation: When
NCBI GEO was used to obtain the microarray data, the information on
the OA stage was unclear or the diagnosis of the OA classification
by different doctors was biased. Therefore, it is difficult to
ensure that all patients whose data were downloaded had the same OA
classification. In order to confirm certain key genes that regulate
the occurrence of osteoarthritis identified in bioinformatics
screenings, more data and further experimental verification are
still required.
A limitation of the present study was that cells
were prepared using collagenase II. Further experiments are
therefore required to determine whether this may have affected
results.
In conclusion, bioinformatics and experimental data
suggested that BSCL2, FOSL2, CDKN1A and KTN1 are key DEGs in OA
compared with normal samples. Therapeutic targeting of these genes
may positively contribute to the treatment and prevention of OA and
consequent disability. One limitation of the present study is the
limited amount of collected data. Further analyses and experiments
are required prior to performing some potential targets.
Acknowledgements
Not applicable.
Funding
Funding: The present study was supported by the National Natural
Science Foundation of China (grant nos. 81800785, 81972085 and
81772394), the Natural Science Foundation of Guangdong Province
(grant no. 2018A0303100027), the Sanming Project of Shenzhen Health
and Family Planning Commission (grant no. SZSM201612086), Shenzhen
Science and Technology Planning (grant no. JCYJ20180228163401333),
the Doctor Innovation Project of Shenzhen Health System (grant no.
SZBC2018015) and the Shenzhen Peacock Project (grant no.
KQTD20170331100838136).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
WL and DW conceived and designed the current study.
JX, ZD and MA drafted the manuscript. JX, ZD and MA created the
schematic. JL, MA, ZZ, XC, GW, XH and LD interpreted the data and
revised the manuscript critically for important intellectual
content. JX performed the bioinformatics analysis and laboratory
experiments. All authors read and approved the final version of the
manuscript.
Ethics approval and consent to
participate
The study was reviewed and approved by the First
Affiliated Hospital of Shenzhen University Health Science Center
Research Ethics Committee (Shenzhen, China). All patients provided
written informed consent for publication.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Kraus VB, Blanco FJ, Englund M, Karsdal MA
and Lohmander LS: Call for standardized definitions of
osteoarthritis and risk stratification for clinical trials and
clinical use. Osteoarthritis Cartilage. 23:1233–1241.
2015.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Winter AR, Collins JE and Katz JN: The
likelihood of total knee arthroplasty following arthroscopic
surgery for osteoarthritis: A systematic review. BMC Musculoskelet
Disord. 18(408)2017.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Jimenez G, Cobo-Molinos J, Antich C and
Lopez-Ruiz E: Osteoarthritis: Trauma vs. disease. Adv Exp Med Biol.
1059:63–83. 2018.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Hootman JM and Helmick CG: Projections of
US prevalence of arthritis and associated activity limitations.
Arthritis Rheum. 54:226–229. 2006.PubMed/NCBI View Article : Google Scholar
|
|
5
|
MacGregor AJ, Antoniades L, Matson M,
Andrew T and Spector TD: The genetic contribution to radiographic
hip osteoarthritis in women: Results of a classic twin study.
Arthritis Rheum. 43:2410–2416. 2000.PubMed/NCBI View Article : Google Scholar
|
|
6
|
MacGregor AJ, Li Q, Spector TD and
Williams FM: The genetic influence on radiographic osteoarthritis
is site specific at the hand, hip and knee. Rheumatology (Oxford).
48:277–280. 2009.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Moazedi-Fuerst FC, Hofner M, Gruber G,
Weinhaeusel A, Stradner MH, Angerer H, Peischler D, Lohberger B,
Glehr M, Leithner A, et al: Epigenetic differences in human
cartilage between mild and severe OA. J Orthop Res. 32:1636–1645.
2014.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Lu W and Li G: Identification of key genes
and pathways in rheumatoid arthritis gene expression profile by
bioinformatics. Acta Reumatol Port. 43:109–131. 2018.PubMed/NCBI
|
|
9
|
Duan H, Zhai KF, Khan GJ, Zhou J, Cao TY,
Wu YQ, Zhou YR, Cao WG, Gao GZ and Shan LL: Revealing the
synergistic mechanism of multiple components in compound
fengshiding capsule for rheumatoid arthritis therapeutics by
network pharmacology. Curr Mol Med. 19:303–314. 2019.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Li H, Bai B, Wang J, Xu Z, Yan S and Liu
G: Identification of key mRNAs and microRNAs in the pathogenesis
and progression of osteoarthritis using microarray analysis. Mol
Med Rep. 16:5659–5666. 2017.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Woetzel D, Huber R, Kupfer P, Pohlers D,
Pfaff M, Driesch D, Häupl T, Koczan D, Stiehl P, Guthke R and Kinne
RW: Identification of rheumatoid arthritis and osteoarthritis
patients by transcriptome-based rule set generation. Arthritis Res
Ther. 16(R84)2014.PubMed/NCBI View
Article : Google Scholar
|
|
12
|
Broeren MG, de Vries M, Bennink MB, van
Lent PL, van der Kraan PM, Koenders MI, Thurlings RM and van de Loo
FA: Functional tissue analysis reveals successful cryopreservation
of human osteoarthritic synovium. PLoS One.
11(e0167076)2016.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Huber R, Hummert C, Gausmann U, Pohlers D,
Koczan D, Guthke R and Kinne RW: Identification of intra-group,
inter-individual, and gene-specific variances in mRNA expression
profiles in the rheumatoid arthritis synovial membrane. Arthritis
Res Ther. 10(R98)2008.PubMed/NCBI View
Article : Google Scholar
|
|
14
|
Ye Y, Bao C and Fan W: Overexpression of
miR-101 May target DUSP1 to promote the cartilage degradation in
rheumatoid arthritis. J Comput Biol. 26:1067–1079. 2019.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Barrett T, Wilhite SE, Ledoux P,
Evangelista C, Kim IF, Tomashevsky M, Marshall KA, Phillippy KH,
Sherman PM, Holko M, et al: NCBI GEO: Archive for functional
genomics data sets-update. Nucleic Acids Res. 41:D991–D995.
2013.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Ashburner M, Ball CA, Blake JA, Botstein
D, Butler H, Cherry JM, Davis AP, Dolinski K, Dwight SS, Eppig JT,
et al: Gene ontology: Tool for the unification of biology. The gene
ontology consortium. Nat Genet. 25:25–29. 2000.PubMed/NCBI View
Article : Google Scholar
|
|
17
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408.
2001.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Jia Z, Zhu F, Li X, Liang Q, Zhuo Z, Huang
J, Duan L, Xiong J and Wang D: Repair of osteochondral defects
using injectable chitosan-based hydrogel encapsulated synovial
fluid-derived mesenchymal stem cells in a rabbit model. Mater Sci
Eng C Mater Biol Appl. 99:541–551. 2019.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Liang Y, Duan L, Xiong J, Zhu W, Liu Q and
Wang D, Liu W, Li Z and Wang D: E2 regulates MMP-13 via targeting
miR-140 in IL-1β-induced extracellular matrix degradation in human
chondrocytes. Arthritis Res Ther. 18(105)2016.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Duan L, Liang Y, Ma B, Zhu W and Wang D:
Epigenetic regulation in chondrocyte phenotype maintenance for
cell-based cartilage repair. Am J Transl Res. 7:2127–2140.
2015.PubMed/NCBI
|
|
21
|
Kohn MD, Sassoon AA and Fernando ND:
Classifications in Brief: Kellgren-Lawrence classification of
osteoarthritis. Clin Orthop Related Res. 474:1886–1893.
2016.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Zhai KF, Duan H, Khan GJ, Xu H, Han FK,
Cao WG, Gao GZ, Shan LL and Wei ZJ: Salicin from Alangium Chinense
ameliorates rheumatoid arthritis by modulating the Nrf2-HO-1-ROS
pathways. J Agric Food Chem. 66:6073–6082. 2018.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Zhai KF, Duan H, Chen Y, Khan GJ, Cao WG,
Gao GZ, Shan LL and Wei ZJ: Apoptosis effects of imperatorin on
synoviocytes in rheumatoid arthritis through
mitochondrial/caspase-mediated pathways. Food Funct. 9:2070–2079.
2018.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Wight L, Owen D, Goldbloom D and Knupp M:
Pure ankle dislocation: A systematic review of the literature and
estimation of incidence. Injury. 48:2027–2034. 2017.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Fassbender HG: Role of chondrocytes in the
development of osteoarthritis. Am J Med. 83:17–24. 1987.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Hwang HS and Kim HA: Chondrocyte apoptosis
in the pathogenesis of osteoarthritis. Int J Mol Sci.
16:26035–26054. 2015.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Sundarrajan M, Boyle DL, Chabaud-Riou M,
Hammaker D and Firestein GS: Expression of the MAPK kinases MKK-4
and MKK-7 in rheumatoid arthritis and their role as key regulators
of JNK. Arthritis Rheum. 48:2450–2460. 2003.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Zhai KF, Duan H, Cui CY, Cao YY, Si JL,
Yang HJ, Wang YC, Cao WG, Gao GZ and Wei ZJ: Liquiritin from
Glycyrrhiza uralensis attenuating rheumatoid arthritis via reducing
inflammation, suppressing angiogenesis, and inhibiting MAPK
signaling pathway. J Agric Food Chem. 67:2856–2864. 2019.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Park HK, Kim IH, Kim J and Nam TJ:
Induction of apoptosis and the regulation of ErbB signaling by
laminarin in HT-29 human colon cancer cells. Int J Mol Med.
32:291–295. 2013.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Maudens P, Seemayer CA, Pfefferle F,
Jordan O and Allemann E: Nanocrystals of a potent p38 MAPK
inhibitor embedded in microparticles: Therapeutic effects in
inflammatory and mechanistic murine models of osteoarthritis. J
Control Release. 276:102–112. 2018.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Liu S, Cao C, Zhang Y, Liu G, Ren W, Ye Y
and Sun T: PI3K/Akt inhibitor partly decreases TNF-α-induced
activation of fibroblast-like synoviocytes in osteoarthritis. J
Orthop Surg Res. 14(425)2019.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Sun K, Luo J, Guo J, Yao X, Jing X and Guo
F: The PI3K/AKT/mTOR signaling pathway in osteoarthritis: A
narrative review. Osteoarthritis Cartilage. 28:400–409.
2020.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Kleinsimon S, Longmuss E, Rolff J, Jäger
S, Eggert A, Delebinski C and Seifert G: GADD45A and CDKN1A are
involved in apoptosis and cell cycle modulatory effects of viscumTT
with further inactivation of the STAT3 pathway. Sci Rep.
8(5750)2018.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Yarushkin AA, Mazin ME, Yunusova AY,
Korchagina KV, Pustylnyak YA, Prokopyeva EA and Pustylnyak VO:
CAR-mediated repression of Cdkn1a(p21) is accompanied by the Akt
activation. Biochem Biophys Res Commun. 504:361–366.
2018.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Gang X, Xu H, Si L, Zhu X, Yu T, Jiang Z
and Wang Y: Treatment effect of CDKN1A on rheumatoid arthritis by
mediating proliferation and invasion of fibroblast-like
synoviocytes cells. Clin Exp Immunol. 194:220–230. 2018.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Mori E, Fujikura J, Noguchi M, Nakao K,
Matsubara M, Sone M, Taura D, Kusakabe T, Ebihara K, Tanaka T, et
al: Impaired adipogenic capacity in induced pluripotent stem cells
from lipodystrophic patients with BSCL2 mutations. Metabolism.
65:543–556. 2016.PubMed/NCBI View Article : Google Scholar
|
|
37
|
McIlroy GD, Mitchell SE, Han W,
Delibegovic M and Rochford JJ: Female adipose tissue-specific Bscl2
knockout mice develop only moderate metabolic dysfunction when
housed at thermoneutrality and fed a high-fat diet. Sci Rep.
8(17863)2018.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Francisco V, Perez T, Pino J, López V,
Franco E, Alonso A, Gonzalez-Gay MA, Mera A, Lago F, Gómez R and
Gualillo O: Biomechanics, obesity, and osteoarthritis. The role of
adipokines: When the levee breaks. J Orthop Res. 36:594–604.
2018.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Bozec A, Bakiri L, Jimenez M, Schinke T,
Amling M and Wagner EF: Fra-2/AP-1 controls bone formation by
regulating osteoblast differentiation and collagen production. J
Cell Biol. 190:1093–1106. 2010.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Wrann CD, Eguchi J, Bozec A, Xu Z,
Mikkelsen T, Gimble J, Nave H, Wagner EF, Ong SE and Rosen ED:
FOSL2 promotes leptin gene expression in human and mouse
adipocytes. J Clin Invest. 122:1010–1021. 2012.PubMed/NCBI View Article : Google Scholar
|
|
41
|
He X, Ohba S, Hojo H and McMahon AP: AP-1
family members act with Sox9 to promote chondrocyte hypertrophy.
Development. 143:3012–3023. 2016.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Abe E, Okawa S, Sugawara M, Watanabe S and
Toyoshima I: Identification of ER membrane targeting signal of
kinectin. Neurosci Lett. 413:238–240. 2007.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Ong LL, Lin PC, Zhang X, Chia SM and Yu H:
Kinectin-dependent assembly of translation elongation factor-1
complex on endoplasmic reticulum regulates protein synthesis. J
Biol Chem. 281:33621–33634. 2006.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Martínez Sánchez AH, Omidi M, Wurlitzer M,
Wurlitzer M, Fuh MM, Feyerabend F, Schlüter H, Willumeit-Römer R
and Luthringer BJ: Proteome analysis of human mesenchymal stem
cells undergoing chondrogenesis when exposed to the products of
various magnesium-based materials degradation. Bioact Mater.
4:168–188. 2019.PubMed/NCBI View Article : Google Scholar
|