Introduction
Glucocorticoids (GCs) are one of the most widely
used classes of drugs due to their remarkable anti-inflammation and
immunosuppressive function and it is estimated that 1-2% of the
population is undergoing long-term GC therapy worldwide (1-4).
However, extensive GC therapy leads to various adverse effects, and
one of the most severe of them is GC-induced osteoporosis (GIO),
characterized by systemic damage of the bone mass and the
microarchitecture that induces fragility fractures (5). Previous studies have demonstrated that
the lack of balance between bone formation and resorption induced
by osteoblast dysfunction, such as inhibited proliferation and
differentiation and enhanced apoptosis of osteoblasts, is involved
in GIO (5-7).
However, the underlying mechanism of GIO has not been fully
elucidated.
MicroRNAs (miRs) are a class of small (~22
nucleotides), highly conserved noncoding RNAs that
post-transcriptionally regulate the expression of their target
genes and exert pivotal roles in multiple diseases, including
chronic lymphocytic leukemia, Huntington's disease and myocardial
ischemia-reperfusion injury (8-10).
An increasing number of studies have demonstrated that miRs are
involved in the pathogenesis and development of osteoporosis
(11-13).
Shi et al (11) reported
that miR-17/20a suppressed GC-induced osteoclast differentiation
and dysfunction by targeting the expression of receptor activator
of NFκΒ ligand (RANKL) in osteoblastic cells. In addition, Zhang
et al (12) demonstrated
that miR-221 participated in the process of osteoporosis by
regulating the protein expression of runt-related transcription
factor 2 and osteoblast differentiation. Notably, a previous study
demonstrated that miR-22 is a negative modulator of osteogenesis
(13). Additionally, oxidative
stress triggered by excessive reactive oxygen species (ROS)
production was reported to contribute to dexamethasone
(DEX)-induced osteoporosis (7,14,15),
and miR-22 facilitated myocardial damage following
ischemia/reperfusion insult and hypoxia/reoxygenation-induced
dysfunction in cultured cardiomyocytes by inducing mitochondrial
oxidative stress (9). However,
whether miR-22 is involved in the development of DEX-induced
osteoporosis by targeting the oxidative stress process remains to
be determined. Notably, Zhang et al (16) demonstrated that caveolin-3 (CAV3) is
a direct target of miR-22, containing two seed binding sites in its
3'-untranslated region (3'-UTR). As a member of the caveolin
proteins (CAV1, 2 and 3) with six subsets, CAV3 is involved not
only in the formation of caveolae, but also bone formation and
osteoporosis progression (14,17).
Yang et al (14)
demonstrated that upregulation of CAV3 enhances bone formation and
inhibits the progression of osteoporosis by activating the Wnt
signaling pathway in a rat model initially induced by means of
ovariotomy, which suggested that CAV3 may be a potential biomarker
and osteoporosis treatment target. Therefore, the present
hypothesized that miR-22 may also be involved in osteoporosis
progression by regulating the expression of CAV3.
Previous studies have demonstrated that GC-induced
osteoblast apoptosis contributed to the development and progression
of osteoporosis (7,15). For instance, Gohel et al
(18) reported that GC treatment
resulted in osteoblast apoptosis in vivo and in
vitro. In addition, upregulation of CAV3 prevented hypoxia or
TNF-α treatment-induced cardiomyocyte apoptosis (19,20).
The present study aimed to examine the expression of
miR-22 in DEX-treated MC3T3-E1 cells and the effects of miR-22
mimic on DEX-induced osteoblast damage and apoptosis, and to
investigate the effects of miR-22 on CAV3 expression in osteoblasts
to determine whether miR-22 may be involved in DEX-induced
osteoblast dysfunction and apoptosis through the regulation of CAV3
expression.
Materials and methods
Cell culture and drug treatments
The murine osteoblastic MC3T3-E1 cell line was
purchased from the American Type Culture Collection and cultured in
α-MEM (Gibco; Thermo Fisher Scientific, Inc.) containing 10% FBS
(Thermo Fisher Scientific, Inc.) and 1% penicillin-streptomycin
(cat. no. 10378016; Gibco; Thermo Fisher Scientific, Inc.) at 37˚C
with 5% CO2 and 95% air. DEX (cat. no. D4902;
Sigma-Aldrich; Merck KGaA) was dissolved in absolute ethanol and
the cells were incubated with 1 µM DEX in the culture medium for 72
h (21). N-acetyl L-cysteine (NAC;
cat. no. A9165; Sigma-Aldrich; Merck KGaA) and p53 inhibitor
pifithrin-α (cat. no. P4359; Sigma-Aldrich; Merck KGaA) were
dissolved in saline and DMSO, respectively. Cells were incubated
with 1 mM NAC or 20 µM pifithrin-α for 48 h (10).
miR-22 mimic, inhibitor and small
interfering RNA (siRNA) transfection
miR-22 mimic, inhibitor and siRNA (200 µM)
transfections were performed using Xfect™ RNA transfection reagent
(cat. no. 631450; Takara Bio, Inc.). At 37˚C, osteoblastic MC3T3-E1
cells were firstly transfected with control or CAV3 siRNA for 24 h
and then transfected with miR control or miR-22 inhibitor for
another 24 h. Immediately after miR control or miR-22 inhibitor
transfection, cells were treated with DEX for 72 h. The sequences
were as follows: miR-22 mimic sense, 5'-AAGCUGCCAGUUGAAGAACUGU-3'
and antisense, 5'-AGUUCUUCAACUGGCAGCUUUU-3'; miR mimic control
sense, 5'-UUCUUCGAACGUGUCACGUTT-3' and antisense,
5'-ACGUGACACGUUCGGAGAATT-3'; miR-22 inhibitor,
5'-ACAGUUCUUCAACUGGCAGCUU-3' miR inhibitor control,
5'-CAGUACUUUUGUGUAGUACAA-3', CAV3 siRNA sense,
5'-GCAGCAACAUUAAGGUGGUTT-3' and antisense,
5'-ACCACCUUAAUGUUGCUGCTT-3' and control siRNA sense,
5'-UUCUUCGAACGUGUCACGUTT-3' and antisense,
5'-ACGUGACACGUUCGGAGAATT-3'.
MTT assay
Cell viability was evaluated by an MTT assay
(10). Briefly, 250 mg MTT (cat.
no. M5655; Sigma-Aldrich; Merck KGaA) was dissolved in 50 ml PBS to
produce a 5 g/l solution and filtered through a 0.22-µm filter. A
48-well plate was used to incubate cells at a density of
2x105 cells/ml in 200 µl culture media, and the cells
were exposed to MTT (0.5 g/l) for 2-4 h following drug treatments.
The generated formazan crystals were dissolved in DMSO, and the
absorbance at a wavelength of 550 nm was measured by a microplate
reader (Tecan Group, Ltd.).
Alkaline phosphatase (ALP) activity
assay
Osteoblastic MC3T3-E1 cells were cultured in a
96-well plate at a density of 1x106 cells/ml in 100 µl
α-MEM with 10% FBS. Subsequently, 1% Triton X-100 was added to the
medium and centrifuged for 20 min (14,000 x g, 4˚C) to recover the
supernatant. The change in absorbance at a wavelength of 405 nm was
measured by the method previously described by Bowers and McComb
(22).
Dual luciferase reporter assay
The wild-type 3'-UTR and the miR-22 ‘seed’ mutant
3'-UTR of CAV3 were synthesized in vitro and cloned into the
psiCHECK2 luciferase reporter plasmid (cat. no. C8021; Promega
Corporation). Osteoblastic MC3T3-E1 cells were co-transfected with
the psiCHECK-2 plasmid containing the wild-type or mutant CAV3
3'-UTR and miRNA control or miR-22 mimic using Xfect™ Transfection
Reagent (cat. no. 631317; Takara Bio, Inc.) and Xfect™ RNA
transfection reagent (cat. no. 631450; Takara Bio, Inc.)
respectively. Cell lysates were collected 24 h after transfection,
and reporter activity was determined by measuring firefly
luciferase activity by a dual luciferase reporter system
(Dual-Luciferase Reporter Assay System; cat. no. E1910; Promega
Corporation) and normalized to Renilla luciferase
activity.
Reverse transcription-quantitative PCR
(RT-qPCR)
TRIzol® reagent (Invitrogen; Thermo
Fisher Scientific, Inc.) was used to extract total RNA from
osteoblasts, which was reverse-transcribed to generate cDNA using
SuperScript™ II reverse transcriptase (Thermo Fisher Scientific,
Inc.) with a specific stem-loop primer for miR-22 and miR-16 and
oligodeoxythymidine for mRNAs. The temperature protocol used was
25˚C for 10 min, 42˚C for 1 h and finally 72˚C 10 min. qPCR was
performed using a MiniOpticon Real-Time PCR Detection system
(Bio-Rad Laboratories, Inc.). The following primer pairs were used
for the qPCR: CAV3 (GenBank accession no. NM_007617) sense,
5'-TCAACGATACCAGCCACAAG-3' and antisense,
5'-ACACCGTCGAAGCTGTAGGT-3'; GAPDH (GenBank accession no. NM_017008)
sense, 5'-TCTACATGTTCCAGTATGACTC-3' and antisense,
5'-ACTCCACGACATACTCAGCACC-3' (16);
miR-22 sense, 5'-GGGGGAGCTGCCAGTTGAAG-3' and antisense,
5'-GTGCAGGGTCCGAGGT-3' (10) and
miR-16 sense, 5'-GCCCCTTCGTCGTTAGA-3' and antisense,
5'-GTGCAGGGTCCGAGGT-3' (10). The
reaction solution consisted of 5.0 µl diluted cDNA, 0.2 µM/l of
each paired primer, 1X SYBR-Green qPCR Mix buffer (Toyobo Life
Science) and 4.9 µl diethyl pyrocarbonate-treated water. The
annealing temperature was set at 58-62˚C and amplification was set
at 40 cycles. The temperature range to detect the melting
temperature of the PCR product was set between 60-95˚C. To
determine the relative quantitation of gene expression, the
comparative Ct (threshold cycle) method with arithmetic formula
(2-ΔΔCq) was used (23).
mRNA levels and miRNA levels were normalized relative to the
housekeeping gene GAPDH and miR-16, respectively.
Western blot analysis
Osteoblastic MC3T3-E1 cells proteins were lysed
using cold RIPA buffer (Beyotime Institute of Biotechnology)
containing 1% Protease Inhibitor Cocktail (Thermo Fisher
Scientific, Inc.) and BCA assays were used to calculate the protein
concentrations. Subsequently, 30 µg proteins per lane were
separated using 10% SDS-PAGE and transferred to PVDF membranes.
Following blocking with non-fat dry milk dissolved in TBS
containing 0.05% Tween-20 for 1-2 h at room temperature, the
membranes were incubated with diluted primary antibodies against
CAV3 (cat. no. sc-5310; Santa Cruz Biotechnology, Inc.), Bax (cat.
no. ab32503; Abcam), Bcl-2 (cat. no. ab182858; Abcam), pro- and
cleaved caspase-3 (cat. no. sc-56053; Santa Cruz Biotechnology,
Inc.), p53 (cat. no. 10422-1-AP; ProteinTech Group, Inc.), p21
(cat. no. 27296-1-AP; ProteinTech Group, Inc.) or β-actin (cat. no.
sc-81178; Santa Cruz Biotechnology, Inc.) in primary antibody
dilution buffer (Beyotime Institute of Biotechnology) at 4˚C
overnight, all at a dilution of 1:1,000. Following washing with
TBST buffer (0.05% Tween-20, 0.15 M NaCl, 50 mM Tris-HCI, pH 7.5),
the membranes were incubated with a horseradish
peroxidase-conjugated secondary antibody (cat. nos. SA00001-1 and
SA00001-2; ProteinTech Group, Inc.) for 1-2 h at room temperature
at a dilution of 1:2,000. The Enhanced Chemiluminescence Western
Blotting Detection system (Santa Cruz Biotechnology, Inc.) and a
GeneGnome HR scanner (SynGene Europe) were used to visualize the
immunoreactive proteins and detect chemiluminescent signals from
the membranes using GeneSnap software version 7.12 (Syngene).
Bioinformatics analysis
TargetScan (http://www.targetscan.org/) and miRanda (http://www.microrna.org/microrna/home.do) were used to
perform target prediction. The target genes predicted by TargetScan
and miRanda were screened based on their scoring criteria. In the
TargetScan algorithm, target genes with a context percentile <50
were excluded. In the miRanda algorithm, target genes with
Max-Energy >-10 were excluded. The overlapping prediction
results were selected as the candidate target genes of miR-22
(24,25).
Statistical analysis
The data are presented as the mean ± SEM.
Statistical analysis was performed using SPSS 16.0 (SPSS, Inc.).
Statistical comparisons between two groups were determined by
two-tailed Student's t-test. One-way or two-way ANOVA with
Bonferroni's post hoc test was performed for comparisons among
multiple groups. P<0.05 was considered to indicate a
statistically significant difference.
Results
miR-22 is upregulated in osteoblasts
treated with DEX
To confirm whether miR-22 is involved in DEX-induced
osteoblast dysfunction, miR-22 expression in DEX-treated MC3T3-E1
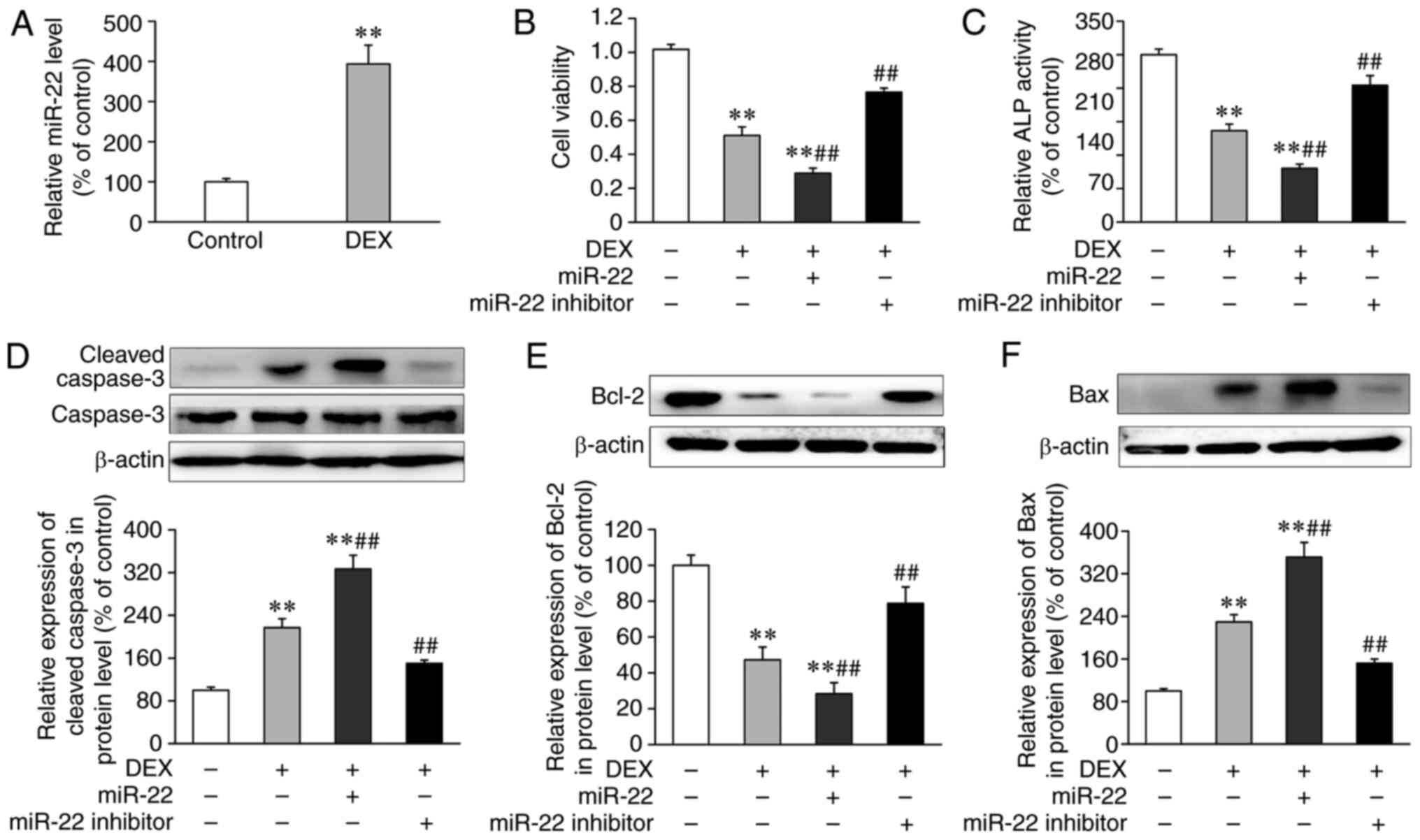
cells was measured by qPCR. As presented in Fig. 1A, miR-22 expression levels in
DEX-treated MC3T3-E1 cells were significantly higher compared with
the control group, suggesting that miR-22 may serve a role in
DEX-induced osteoblast dysfunction.
Transfection of miR-22 mimic
aggravates, whereas miR-22 inhibitor attenuates DEX-induced
dysfunction and apoptosis in osteoblastic MC3T3-E1 cells
The effects of miR-22 on DEX-induced osteoblast
damage were further determined, and MC3T3-E1 cells were transfected
with miR-22 mimic or inhibitor. As demonstrated in Fig. S1A and B, miR-22 levels significantly increased
in the cells transfected miR-22 mimic compared with those
transfected with control mimic; meanwhile, miR-22 levels
significantly decreased in cells transfected with miR-22 inhibitor
compared with those transfected with the miR-22 inhibitor control.
As presented in Fig. 1B-E, DEX
treatment and transfection with miR-22 mimic resulted in cell
damage and apoptosis, as evidenced by significantly decreased cell
viability and Bcl-2 protein levels as well as decreased ALP
activity and the expression levels of apoptosis-related proteins
cleaved caspase-3 and Bax when compared with control groups. In
addition, transfection with miR-22 mimic aggravated the DEX-induced
osteoblast damage as the cell viability was significantly
decreased, whereas ALP activity was significantly decreased in the
cells transfected with miR-22 mimic compared with those transfected
with the control group (Fig. 1B and
C). miR-22 mimic also induced a
significant increase in the expression levels of cleaved caspase-3
and Bax and decreased expression of Bcl-2 (Fig. 1D-F). By contrast, transfection with
miR-22 inhibitor mitigated the osteoblast damage and apoptosis
induced by DEX as evidenced by significantly increased cell
viability, Bcl-2 levels and ALP activity as well as decreased
expression levels of cleaved caspase-3 and Bax.
A previous study demonstrated that p53 exerts a
crucial role in inducing miR-22 transcription by binding to the
promoter region of the miR-22 gene (26). In addition, p53 was reported to be
associated with oxidative stress (27). Therefore, the present study
determined whether the p53 signaling pathway was involved in the
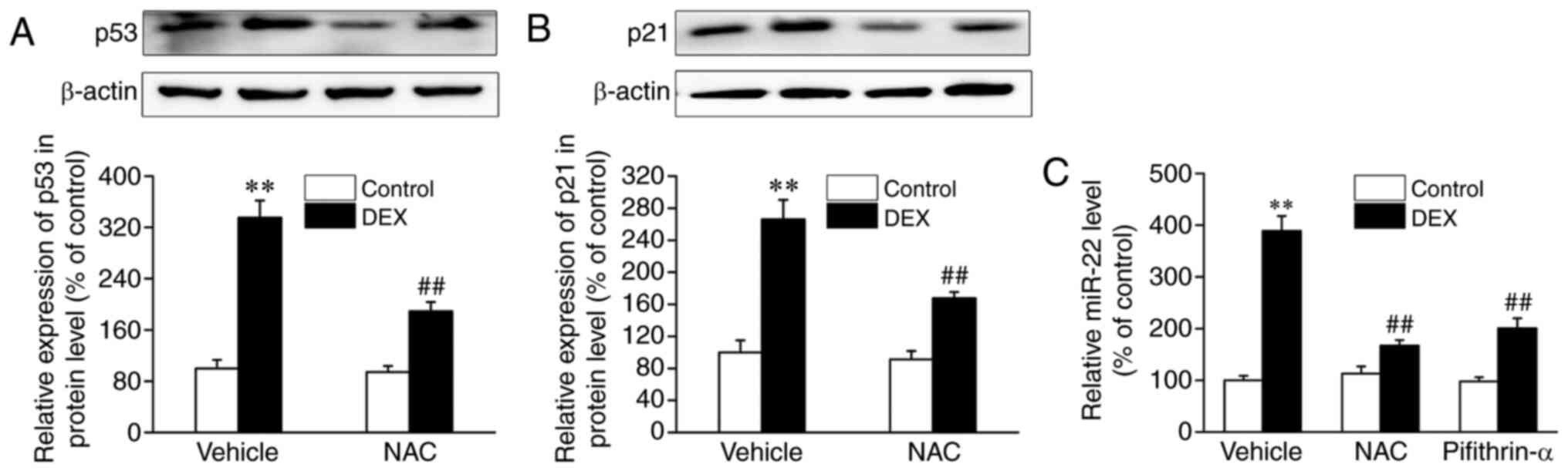
DEX-induced increased expression of miR-22. As presented in
Fig. 2A and B, compared with controls, treatment with
DEX resulted in significant increases in the protein expression
levels of p53 in and its target p21, which were abrogated by the
ROS scavenger NAC. Additionally, the DEX-induced increase in the
expression levels of miR-22 was abolished by NAC and pifithrin-α
(Fig. 2C). Collectively, these
results suggested that the high expression levels of miR-22 in
osteoblasts treated with DEX may be at least partly attributed to
the ROS-induced the activation of the p53 signaling pathway.
miR-22 suppresses CAV3 expression in
osteoblastic MC3T3-E1 cells
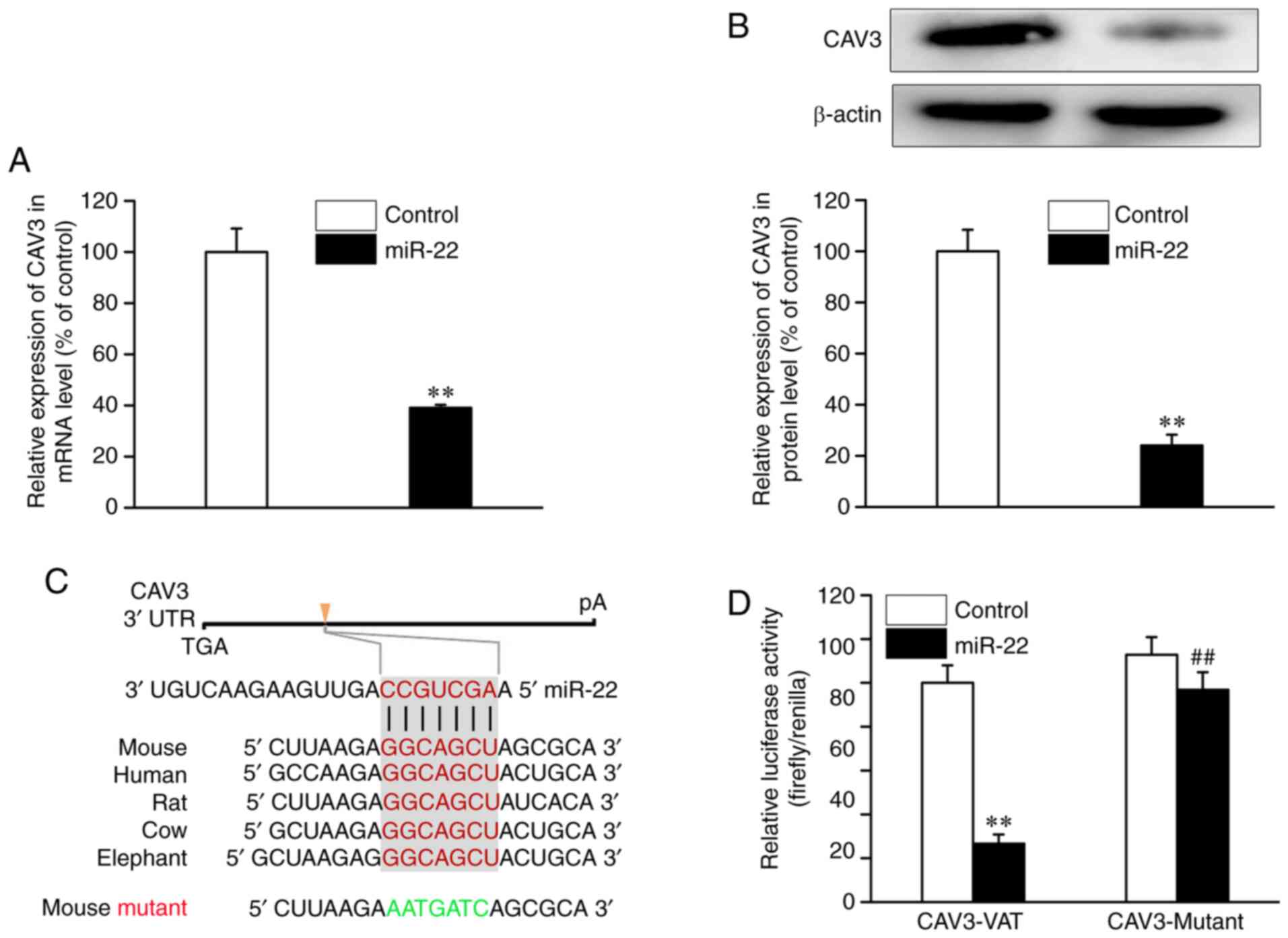
The present bioinformatics analysis identified one
evolutionarily conserved miRNA recognition element that is
partially complementary to miR-22 in the 3'-UTR of the CAV3 gene
(Fig. 3C). As demonstrated in
Fig. 3A and B, transfection with miR-22 mimic
significantly decreased CAV3 expression at the mRNA and protein
levels compared with controls. In order to determine whether miR-22
directly affected CAV3 expression, CAV3 3'-UTR luciferase reporter
constructs and miR-22 mimic were transfected into osteoblasts. The
results revealed significantly lower luciferase activity in the
group co-transfected with miR-22 mimic compared with the miR
control group (Fig. 3D). By
contrast, no decrease in luciferase activity was observed following
co-transfection of miR-22 when the putative miR-22 binding sequence
in the CAV3 3'-UTR luciferase reporter construct was mutated. These
results demonstrated that CAV3 expression was repressed in
osteoblasts by the binding of miR-22 to response elements in its
3'-UTR.
Silencing of CAV3 abolishes the
protective effects of miR-22 inhibitor against DEX-induced cell
dysfunction and apoptosis in osteoblastic MC3T3-E1 cells
Next, the effects of miR-22 on CAV3 protein
expression levels were determined in osteoblasts treated with DEX.
As presented in Fig. 4A, CAV3
protein expression levels were reduced in osteoblasts treated with
DEX, and miR-22 inhibitor reversed DEX-suppressed CAV3 expression.
In addition, as shown in Fig. S1C
and D, an 80% reduction in CAV3
expression levels was induced by CAV3 siRNA in osteoblasts compared
with the control siRNA group. The results also demonstrated that
the upregulation of CAV3 expression induced by miR-22 inhibitor in
osteoblasts subjected to DEX was abolished by CAV3 siRNA. Further
experiments were performed to confirm whether CAV3 was involved in
the protective role of miR-22 inhibitor against DEX-induced
osteoblast apoptosis. As presented in Fig. 4B-D, CAV3 siRNA abrogated the
positive effects of miR-22 inhibitor against DEX-induced osteoblast
apoptosis, as evidenced by significantly increased expression
levels of cleaved caspase-3 and Bax and the decreased expression
levels of Bcl-2. In addition, CAV3 siRNA abrogated the positive
effects of miR-22 inhibitor against DEX-induced osteoblast damage
by significantly decreasing cell viability and increasing ALP
activity (Fig. 4E and F).
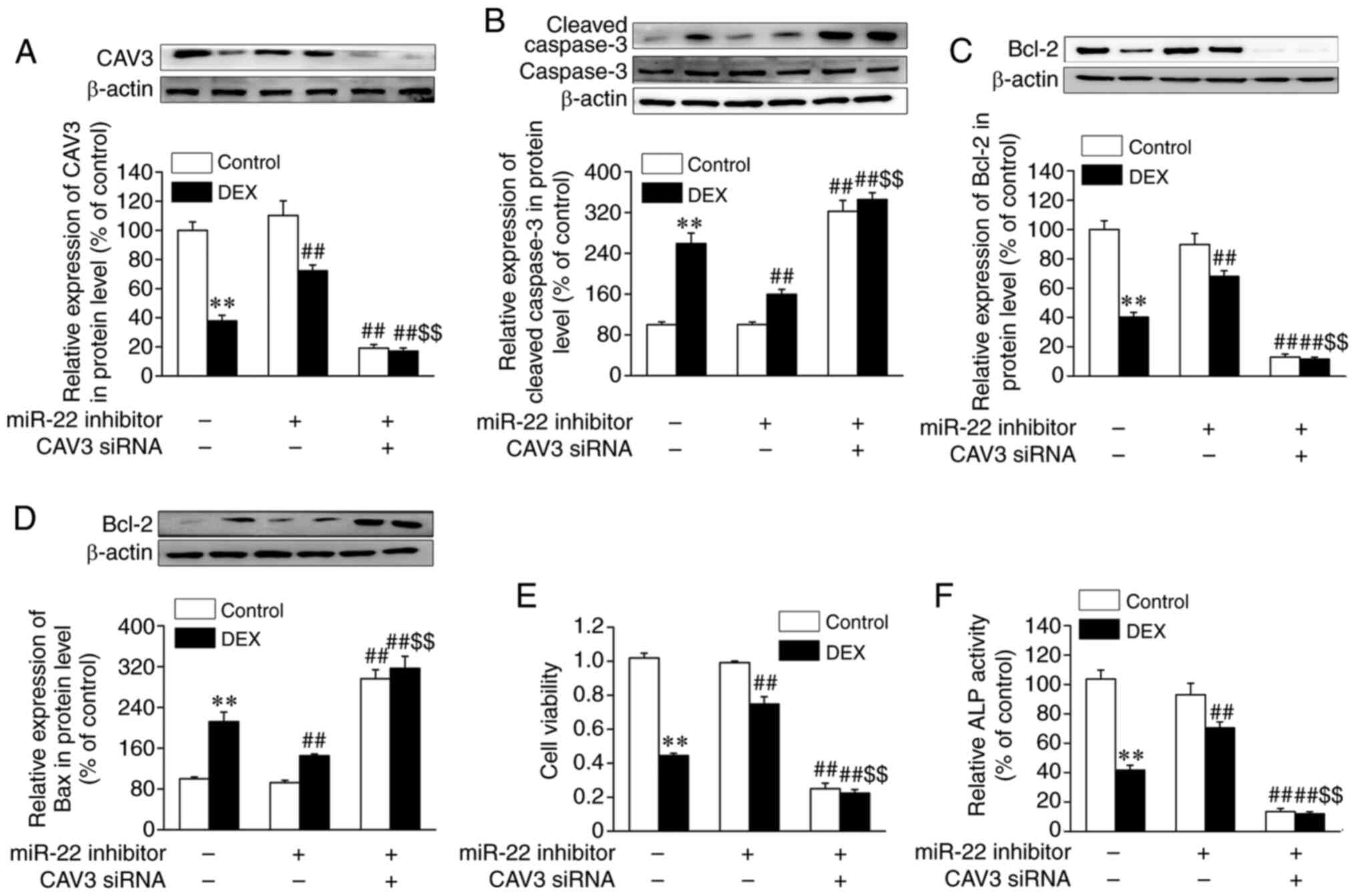 | Figure 4Silencing of CAV3 abolishes the
protective effects of miR-22 inhibitor against DEX-induced cell
damage and apoptosis in osteoblastic MC3T3-E1 cells. Osteoblastic
MC3T3-E1 cells were transfected with control or CAV3 siRNA for 24
h, followed by transfection with miR control or miR-22 inhibitor
for 24 h and subsequent DEX treatment for the following 72 h.
Protein levels of (A) CAV3, (B) caspase-3, (C) Bcl-2, (D) Bax, (E)
cell viability and (F) ALP activity were assessed. Data are
presented as the mean ± standard error of the mean (n=4).
**P<0.01 vs. control; ##P<0.01 vs. DEX;
$$P<0.01 vs. DEX + miR-22 inhibitor. miR, microRNA;
CAV3, caveolin-3; DEX, dexamethasone; siRNA, small interfering RNA;
ALP, alkaline phosphatase. |
Discussion
Osteoporosis is a common complication of long-term
use of GCs and one of its characteristics is osteoblast dysfunction
(5). A previous study has shown
that miR-22 is a negative modulator of osteogenesis (13). The results of the present study also
demonstrated that the levels of miR-22 expression were
significantly upregulated in DEX-treated osteoblasts, and that
upregulation of miR-22 may contribute to DEX-induced osteoblast
dysfunction and apoptosis, as evidenced by decreased cell
viability, Bcl-2 expression and ALP activity. In addition, the
expression levels of apoptosis-related proteins cleaved caspase-3
and Bax in cells transfected with the miR-22 mimic were increased
compared with control cells, which were reversed by the miR-22
inhibitor. In addition, CAV3 was identified to be a target of
miR-22 in osteoblasts, and CAV3 siRNA attenuated the protective
effects of the miR-22 inhibitor on DEX-induced osteoblast damage
and apoptosis.
The crucial roles of miRs, such as miR-2861,
miR-17/20a, miR-221, miR-133a and miR-338-3p, in GIO have been
validated in a number of studies (11,12,28-31).
Li et al (28) reported that
miR-2861 exerts a physiological effect on osteoblast
differentiation by targeting histone deacetylase 5 and contributes
to primary osteoporosis in two related adolescents. Shi et
al (11) demonstrated that
miR-17/20a represses GC-induced osteoclast differentiation and
function by targeting RANKL expression in osteoblastic cells.
miR-22 is a widely expressed microRNA that is present at high
levels in striated muscle tissues (31); thus, previous studies on miR-22 have
focused on the cardiovascular system. Among them, Liang et
al (13) demonstrated that
miR-22 is involved in the negative regulation of osteogenesis by
inhibiting the Wnt/β-catenin pathway and osteoblast
differentiation. Consistent with this, the results of the present
study demonstrated that transfection with miR-22 mimic not only
resulted in the damage, inhibition of differentiation and apoptosis
in osteoblastic cells, but further aggravated DEX-induced
osteoblast dysfunction and apoptosis.
A previous study demonstrated that p53 exerts a
crucial role in inducing miR-22 transcription by binding to the
promoter region of the miR-22 gene (26). In addition, p53 was reported to be
associated with oxidative stress (27). Li et al (32) demonstrated that DEX treatment
induces p53 activation and inhibits the proliferation of MC3T3-E1
cells. Crochemore et al (33) also revealed that GC administration
leads to rapid p53 nuclear translocation and promotes its
transcriptional activity. The results of the present study
demonstrated that the DEX-induced upregulation of p53 and p21 were
mitigated by the ROS scavenger NAC. In addition, the DEX-induced
increase in the expression levels of miR-22 was abolished by the
ROS scavenger and pifithrin-α. Collectively, these results
demonstrated that high expression of miR-22 in osteoblasts treated
with DEX may be at least partly attributed to the ROS-induced
activation of the p53 signaling pathway.
CAV3, as a primary structural protein of the
caveolae membrane domains, has been reported to be involved in
maintaining the normal physiological cell structure and cell
signaling; CAV3 is predominantly expressed in skeletal muscle, the
diaphragm and the heart, and is selectively induced during the
differentiation of skeletal C2C12 myoblasts (14,34).
Previous studies have demonstrated that CAV3 exerts a critical role
in the progression of osteoporosis. Among them, Yang et al
(14) reported that CAV3
upregulation enhances bone formation and inhibits the progression
of osteoporosis by activating the Wnt signaling pathway in a rat
model initially induced by means of ovariotomy, which suggested
that CAV3 may be a potential target biomarker in osteoporosis
treatment. Additionally, CAV3 has been reported to prevent
apoptosis mediated by hypoxia or TNF-α treatment (20,21).
Consistent with these results, the results of the present study
demonstrated that silencing of CAV3 by siRNA induced osteoblast
cells damage and apoptosis, which were further exacerbated in
DEX-treated osteoblasts.
Further experiments in the present study identified
that CAV3 was a target of miR-22 in osteoblasts, which was
consistent with the prediction of the bioinformatics analysis, as
evidenced by the suppressed mRNA and protein expression levels as
well as decreased luciferase activity of CAV3 3'-UTR luciferase
reporter constructs following transfection with miR-22 mimic.
Although Chen et al (35)
did not report that CAV3 was a target of miR-22, they revealed that
miR-22 was involved in myocardial ischemia and reperfusion by
disrupting CAV-3/endothelial nitric oxide synthase signaling. In
agreement with this, in the present study, the protective effects
of miR-22 inhibitor against DEX-induced osteoblast damage and
apoptosis was also reversed by CAV3 siRNA.
In conclusion, the results of the present study
preliminary clarified that miR-22 may contribute to DEX-induced
osteoblast differentiation inhibition and dysfunction as well as
apoptosis by targeting CAV3 expression in osteoblastic cells.
Supplementary Material
Effects of miR-22 mimic and miR-22
inhibitor transfection and CAV3 siRNA on miR-22 and CAV3 expression
at mRNA and protein levels in osteoblastic MC3T3-E1 cells.
Osteoblasts were transfected with (A) miR control or miR-22 mimic
(200 nM) and (B) miR-22 inhibitor control or miR-22 inhibitor (200
nM) for 24 h. Reverse transcription-quantitative PCR was performed
to measure the expression of miR-22. (C and D) Osteoblasts were
transfected with control siRNA or CAV3 siRNA for 24 h. Reverse
transcription-quantitative PCR and western blot analysis were
performed to determine CAV3 (C) mRNA and (D) protein expression
levels, respectively, in osteoblasts. Data are shown as the mean ±
standard error of the mean (n=4). **P<0.01 vs.
control.
Acknowledgements
Not applicable.
Funding
Funding: This work was supported by the Key Incubation Project
of Li Peng in the Science and Technology Department of Ningxia
Medical University.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
ZL performed the study design and prepared the
manuscript. PL prepared the manuscript, collected data and
performed statistical analysis. WM interpreted the data. SZ, LZ and
ZC performed the literature search and experiments.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
van Staa TP, Leufkens HG, Abenhaim L,
Begaud S, Zhang B and Cooper C: Use of oral corticosteroids in the
United Kingdom. QJM. 93:105–111. 2000.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Fardet L, Petersen I and Nazareth I:
Prevalence of long-term oral glucocorticoid prescriptions in the UK
over the past 20 years. Rheumatology (Oxford). 50:1982–1990.
2011.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Overman RA, Yeh JY and Deal CL: Prevalence
of oral glucocorticoid usage in the United States: A general
population perspective. Arthritis Care Res (Hoboken). 65:294–298.
2013.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Silverman S, Curtis J, Saag K, Flahive J,
Adachi J, Anderson F, Chapurlat R, Cooper C, Diez-Perez A,
Greenspan S, et al: International management of bone health in
glucocorticoidexposed individuals in the observational GLOW study.
Osteoporos Int. 26:419–420. 2015.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Ventura A, Brunetti G, Colucci S, Oranger
A, Ladisa F, Cavallo L, Grano M and Faienza MF:
Glucocorticoid-induced osteoporosis in children with 21-hydroxylase
deficiency. Biomed Res Int. 2013(250462)2013.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Delany AM, Dong Y and Canalis E:
Mechanisms of glucocorticoid action in bone cells. J Cell Biochem.
56:295–302. 1994.PubMed/NCBI View Article : Google Scholar
|
|
7
|
O'Brien CA, Jia D, Plotkin LI, Bellido T,
Powers CC, Stewart SA, Manolagas SC and Weinstein RS:
Glucocorticoids act directly on osteoblasts and osteocytes to
induce their apoptosis and reduce bone formation and strength.
Endocrinology. 145:1835–1841. 2004.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Stamatopoulos B, Meuleman N, Haibe-Kains
B, Saussoy P, Van Den Eric N, Michaux L, Heimann P, Martiat P, Bron
D and Lagneaux L: microRNA-29c and microRNA-223 down-regulation has
in vivo significance in chronic lymphocytic leukemia and improves
disease risk stratification. Blood. 113:5237–5245. 2009.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Lee ST, Chu K, Im WS, Yoon HJ, Im JY, Park
JE, Park KH, Jung KH, Lee SK, Kim M and Roh JK: Altered microRNA
regulation in huntington's disease models. Exp Neurol. 227:172–179.
2011.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Du JK, Cong BH, Yu Q, Wang H, Wang L, Wang
CN, Tang XL, Lu JQ, Zhu XY and Ni X: Upregulation of microRNA-22
contributes to myocardial ischemia-reperfusion injury by
interfering with the mitochondrial function. Free Radic Biol Med.
96:406–417. 2016.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Shi C, Qi J, Huang P, Jiang M, Zhou Q,
Zhou H, Kang H, Qian N, Yang Q, Guo L and Deng L: MicroRNA-17/20a
inhibits glucocorticoid-induced osteoclast differentiation and
function through targeting RANKL expression in osteoblast cells.
Bone. 68:67–75. 2014.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Zhang Y, Gao Y, Cai L, Li F, Lou Y, Xu N,
Kang Y and Yang H: MicroRNA-221 is involved in the regulation of
osteoporosis through regulates RUNX2 protein expression and
osteoblast differentiation. Am J Transl Res. 9:126–135.
2017.PubMed/NCBI
|
|
13
|
Liang WC, Fu WM, Wang YB, Sun YX, Xu LL,
Wong CW, Chan KM, Li G, Waye MM and Zhang JF: H19 activates Wnt
signaling and promotes osteoblast differentiation by functioning as
a competing endogenous RNA. Sci Rep. 6(20121)2016.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Yang RB, Lin FF, Yang J, Chen B, Zhang MH,
Lu QP, Xiao B, Liu Y, Zheng K and Qiu YR: Overexpression of CAV3
facilitates bone formation via the Wnt signaling pathway in
osteoporotic rats. Endocrine. 63:639–650. 2019.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Chen F, Zhang L, OuYang Y, Guan H, Liu Q
and Ni B: Glucocorticoid induced osteoblast apoptosis by increasing
E4BP4 expression via up-regulation of Bim. Calcif Tissue Int.
94:640–647. 2014.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Zhang L, Yin HL, Jiao L, Liu TY, Gao YQ,
Shao YC, Zhang YY, Shan HL, Zhang Y and Yang BF: Abnormal
downregulation of caveolin-3 mediates the pro-fibrotic action of
MicroRNA-22 in a model of myocardial infarction. Cell Physiol
Biochem. 45:1641–1653. 2018.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Galbiati F, Engelman JA, Volonte D, Zhang
XL, Minetti C, Li M, Hou H Jr, Kneitz B, Edelmann W and Lisanti MP:
Caveolin-3 null mice show a loss of caveolae, changes in the
microdomain distribution of the dystrophin-glycoprotein complex,
and t-tubule abnormalities. J Biol Chem. 276:21425–21433.
2001.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Gohel A, McCarthy MB and Gronowicz G:
Estrogen prevents glucocorticoid-induced apoptosis in osteoblasts
in vivo and in vitro. Endocrinology. 140:5339–5347. 1999.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Carotenuto F, Minieri M, Monego G,
Fiaccavento R, Bertoni A, Sinigaglia F, Vecchini A, Carosella L and
Di Nardo P: A diet supplemented with ALA-rich flaxseed prevents
cardiomyocyte apoptosis by regulating caveolin-3 expression.
Cardiovasc Res. 100:422–431. 2013.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Zhou Q, Peng X, Liu X, Chen L, Xiong Q,
Shen Y, Xie J, Xu Z, Huang L, Hu J, et al: FAT10 attenuates
hypoxia-induced cardiomyocyte apoptosis by stabilizing caveolin-3.
J Mol Cell Cardiol. 116:115–124. 2018.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Tang YH, Yue ZS, Li GS, Zeng LR, Xin DW,
Hu ZQ and Xu CD: Effect of β-ecdysterone on glucocorticoid-induced
apoptosis and autophagy in osteoblasts. Mol Med Rep. 17:158–164.
2018.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Bowers GN Jr and McComb RB: A continuous
spectrophotometric method for measuring the activity of serum
alkaline phosphatase. Clin Chem. 12:70–89. 1966.PubMed/NCBI
|
|
23
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408.
2001.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Meng SS, Wang H, Xue DB and Zhang WH:
Screening and validation of differentially expressed extracellular
miRNAs in acute pancreatitis. Mol Med Rep. 16:6412–6418.
2017.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Sun F, Yang X, Jin Y, Chen L, Wang L, Shi
M, Zhan C, Shi Y and Wang Q: Bioinformatics analyses of the
differences between lung adenocarcinoma and squamous cell carcinoma
using the cancer genome atlas expression data. Mol Med Rep.
16:609–616. 2017.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Lin J, Huo R, Xiao L, Zhu X, Xie J, Sun S,
He Y, Zhang J, Sun Y, Zhou Z, et al: A novel p53/microRNA-22/Cyr61
axis in synovial cells regulates inflammation in rheumatoid
arthritis. Arthritis Rheumatol. 66:49–59. 2014.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Vurusaner B, Poli G and Basaga H: Tumor
suppressor genes and ROS: Complex networks of interactions. Free
Radic Biol Med. 52:7–18. 2012.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Li H, Xie H, Liu W, Hu R, Huang B, Tan YF,
Xu K, Sheng ZF, Zhou HD, Wu XP and Luo XH: A novel microRNA
targeting HDAC5 regulates osteoblast differentiation in mice and
contributes to primary osteoporosis in humans. J Clin Invest.
119:3666–3677. 2009.PubMed/NCBI View
Article : Google Scholar
|
|
29
|
Li Z, Zhang W and Huang Y: MiRNA-133a is
involved in the regulation of postmenopausal osteoporosis through
promoting osteoclast differentiation. Acta Biochim Biophys Sin
(Shanghai). 50:273–280. 2018.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Guo DW, Han YX, Cong L, Liang D and Tu GJ:
Resveratrol prevents osteoporosis in ovariectomized rats by
regulating microRNA-338-3p. Mol Med Rep. 12:2098–2106.
2015.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Christodoulou F, Raible F, Tomer R,
Simakov O, Trachana K, Klaus S, Snyman H, Hannon GJ, Bork P and
Arendt D: Ancient animal microRNAs and the evolution of tissue
identity. Nature. 463:1084–1088. 2010.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Li H, Qian W, Weng X, Wu Z, Li H, Zhuang
Q, Feng B and Bian Y: Glucocorticoid receptor and sequential P53
activation by dexamethasone mediates apoptosis and cell cycle
arrest of osteoblastic MC3T3-E1 cells. PLoS One.
7(e37030)2012.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Crochemore C, Michaelidis TM, Fischer D,
Loeffler JP and Almeida OF: Enhancement of p53 activity and
inhibition of neural cell proliferation by glucocorticoid receptor
activation. FASEB J. 16:761–770. 2002.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Tang Z, Scherer PE, Okamoto T, Song K, Chu
C, Kohtz DS, Nishimoto I, Lodish HF and Lisanti MP: Molecular
cloning of caveolin-3, a novel member of the caveolin gene family
expressed predominantly in muscle. J Biol Chem. 271:2255–2261.
1996.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Chen Z, Qi Y and Gao C: Cardiac
myocyte-protective effect of microRNA-22 during ischemia and
reperfusion through disrupting the caveolin-3/eNOS signaling. Int J
Clin Exp Pathol. 8:4614–4626. 2015.PubMed/NCBI
|