Introduction
Organic cation transporters (human: OCT, mouse: Oct)
are membrane transport proteins involved in many metabolic
processes. Recently, we and others found that downregulation of
OCT1 is associated with tumour progression in human hepatocellular
and cholangiocellular carcinoma (1-4).
Furthermore, we demonstrated that the loss of Oct3 (gene: Scl22a3)
leads to enhanced proliferation and hepatocarcinogenesis (5).
OCT expression is regulated via complex mechanisms.
The OCT1 gene SCL22A1 (mouse: Scl22a1) is trans activated by
hepatocyte nuclear factor 4alpha (human: HNF4α, mouse: Hnf4α)
(6). Glucocorticoid receptor
induced expression of HNF4α was found to contribute to indirect
OCT1 gene upregulation in primary human hepatocytes, but not in
hepatocyte-derived tumour cell lines (7).
HNF4α is a master regulator of hepatocyte
differentiation and metabolism, controlling the development of the
hepatic epithelium, liver morphogenesis (8) and hepatic metabolic function (9). This nuclear factor is also known as a
tumour suppressor (10). For
example, HNF4α deletion promotes diethyl nitrosamine-induced
hepatocellular carcinoma in mice (11) and HNF4α inhibition blocks hepatocyte
differentiation and promotes biliary cancer (12). Furthermore, overexpression of HNF4α
in human mesenchymal stem cells suppresses hepatocellular carcinoma
development through downregulation of the Wnt/β-catenin signalling
pathway (13). HNF4α also seems to
play a pivotal role in fibrosis progression, as the downregulation
of HNF4α aggravates hepatic fibrosis in rats (14). Vice versa, Fan et al
described a regression effect of HNF4α on liver cirrhosis in rats
(15) and HNF4α-induced hepatic
stem cells ameliorated chronic liver injury in liver fibrosis
models (16).
Oct3 deficient mice (FVB.Slc22a3tm1Dpb,
Oct3-/-) do not have an obvious phenotype (17). but we have recently shown enhanced
proliferation, hepatocarcinogenesis and fibrosis progression in
these mice (5,18). We studied Oct3-/- mice in
different models of liver damage (DEN/Phenobarbital, bile duct
ligation (BDL), carbon tetrachloride (CCl4) treatment)
in order to analyse Oct1 regulation. The knockout mice showed a
hepatic phenotype with enhanced Ki-67 staining, leucocyte
infiltration and fibrosis quantified by hydroxyproline assay and
Sirius red staining (5,18). Hence, the upstream regulatory
mechanism is still unclear. Surprisingly, we also found differences
in Hnf4α expression in cholestasis and fibrosis in
Oct3-/- mice. Oct1 and Oct3 are both expressed in the
liver (19) and substitute each
other (17,20). To date no data exists on an
interaction between Oct3 and Hnf4α. We hypothesised that loss of
Oct3 has an impact on Hnf4 α expression. Therefore, we analysed
Hnf4α expression in different fibrosis models in Oct3-/-
and wild type (FVB, WT) mice, stably transfected tumour cell lines
and primary murine hepatocytes.
Materials and methods
Animals
Animal care (housing, husbandry conditions) and
animal procedures were performed in accordance with the European
Council Directive of 24 November, 1986 (86/609/EEC), and the
present study was approved by the state animal care commission
(Koblenz; approval number, 23 177-07/G 14-1-010). Mice received
standard food for rodents (Altromin Lage, Nr. 1314) with free
access to food and water. They were kept in groups of five siblings
of the same sex per cage with constant temperatures of 22-24˚C and
humidity of 55±10% as well as a 12-h day and night rhythm. Male
Oct3-knockout (FVB.Slc22a3tm1Dpb, Oct3-/-) (17), their WT littermates (FVB) and
C57BL/6 mice (in total n=51), 4-6 weeks old with an average body
weight of 20 g at the start of the experiment, were used in this
study. Oct3-/- mice were kindly provided by Prof.
Schinkel, Cancer Centre Amsterdam. C57BL/6 and WT mice were bred by
the Translational Animal Research Centre (TARC) of the University
Medical Centre, Johannes Gutenberg-University Mainz. To investigate
the relevance of Oct3 expression and the effects on cholestasis and
fibrosis, two different animal models of fibrosis were analysed: i)
Chemically induced liver fibrosis by the application of
pro-fibrotic carbon tetrachloride (CCl4) or
thioacetamide (TAA) for 6 weeks; and ii) cholestasis-associated
fibrosis after 7 days of bile duct ligation (BDL).
Gene expression analysis
Total RNA was extracted from livers of three
5-week-old untreated WT and Oct3-/- mice using the High
Pure RNA tissue kit (cat. no./ID: 11828665001; Roche Diagnostics)
following the manufacturer's instructions. RNA quantity and purity
were estimated using a NanoDrop ND-1000 Spectrophotometer (NanoDrop
Technologies) and integrity was assessed by Agilent 2100
Bioanalyzer (Agilent Technologies). cDNA libraries were generated
using the QuantSeq 3'mRNA-Seq Library Prep kit for Illumina
(Lexogen, Vienna, Austria) following the manufacturer's
instructions (21). RNA sequencing
was performed using Illumina HiSeq Rapid Mode by the Institute of
Human Genetics, Department of Genomics, Life & Brain Center,
University of Bonn. The sequencing kit was HiSeq 3000/4000 SBS Kit
(single read, 50 cycles) (cat. no./ID: FC-410-1001; Illumina).
Coverage was standard 3' Seq. The loading concentration of DNA was
0.06-0.44 nmol assuming a nucleotide length of 100-300 bp. Data
were deposited at the BioProject database (http://www.ncbi.nlm.nih.gov/bioproject/685115,
BioProject ID PRJNA685115). The read sequences were aligned to the
Mus_musculus.GRCm38.74 reference genome followed by read mapping
and read counting, as described before using the Bioconductor
package Rsubread (V 1.24.2) (22).
Before aligning reads, low quality reads were filtered, reads
containing adapter sequences, and duplicate mapping reads using
Bioconductor package ShortRead (V 1.32.1) (23). For differential expression analysis
(WALD-Test) the Bioconductor package DESeq2 (V 1.14.1) with an
adjusted P-value <0.01 was used (24). All data analysis was performed using
R programming language and related packages.
Functional classification and network analysis were
performed using Ingenuity Pathway Analysis (Ingenuity Systems
Inc.). The significance of each network, function and pathway was
determined by the scoring system provided by Ingenuity Pathway
Analysis tool. Data will be provided on demand.
Induction of fibrosis
C57BL/6, WT and Oct3-/- mice, 4-6 weeks
old, were treated with pro-fibrotic thioacetamide (TAA) or
CCl4 for 6 weeks (25).
TAA was injected intraperitoneally three times a week in escalating
doses, starting with 50 mg/kg (doses 1 and 2, week 1), 100 mg/kg
(doses 2 to 5, weeks 1-2), 200 mg/kg (doses 6 to 10, weeks 2-4),
300 mg/kg (doses 11 to 15, weeks 4-5), and 400 mg/kg (dose 16
onwards, week 6). Placebo intraperitoneal injection served as the
control. CCl4 was administered three times a week by
oral gavage in escalating doses 50/50 vol/vol mixed with mineral
oil: 0.875 ml/kg (dose 1 dose, week 1), 1.75 ml/kg (doses 2 to 7,
weeks 1-2), 2.5 ml/kg (doses 8 to 13, weeks 3-4), and 3.25 ml/kg
(after week 4). Oral gavage of mineral oil served as the control.
Animals were culled by cervical dislocation after 6 weeks of
treatment or after 1 to 4 weeks of reversal, death was confirmed by
loss of heartbeat through direct cardiac palpation and tissues were
harvested for qPCR and histological analysis.
Induction of cholestasis
WT and Oct3-/- mice, 7-10 weeks old (body
weight 18-20 g), underwent bile duct ligation (BDL) or placebo
surgery (sham operation) as previously described under anaesthesia
with 100 mg/kg Ketamine and 20 mg/kg Rompun (i.p) (26-28).
Animals were sacrificed by cervical dislocation after 7 days; death
was confirmed by loss of heartbeat and tissues were harvested for
qPCR and histological analysis.
RNA isolation and RT-qPCR
analysis
Total RNA was extracted from liver tissue using the
High Pure RNA Tissue Kit (Roche Diagnostics) and cDNA synthesis was
performed using the iScript cDNA Synthesis kit (Bio-Rad) according
to the manufacturer's recommendations. Quantitative analysis of
Oct1 (Slc22A1) transcripts was performed by quantitative real-time
reverse transcriptase (RT-) polymerase chain reaction (qPCR). The
Quantitect SYBR-Green PCR Kit (Qiagen) and validated primers of a
Quantitect Primer Assay with the primer sets Mm_SLC22A1_2_SG (OCT1;
84 bp fragment), Mm_HNF4α (HNF4α; 100 bp fragment forward,
5'-GGATATGGCCGACTACAGCG-3' and reverse, 5'-AGATGGGGACGTGTCATTGC-3')
and Mm_GAPDH_3_SG (GAPDH; 144 bp fragment) (Qiagen) were used
according to the manufacturer's instructions. For the
amplification, an initial denaturation at 95˚C for 15 min, followed
by 15 sec at 94˚C, 30 sec at 55˚C and 30 sec at 72˚C for 40 cycles
was used. Samples were run on a LightCycler® 480
real-time PCR system (Roche Diagnostics). The relative expression
levels were calculated by normalisation to GAPDH gene expression
using the LightCycler® 480 software Release 1.5.0.
Western blot analysis
Total protein extracts were prepared in sample
buffer pH 8.0 containing 20 mM Tris, 5 mM EDTA, 0.5% Triton X-100
and EDTA-free protease inhibitors (Complete Mini, 1:25; Roche
Diagnostics). For western blot analysis 60 µg total protein was
separated by a 12% SDS-PAGE gel. The gel was transferred onto a
nitrocellulose transfer membrane (OPTITRAN BA-S85/Whatman)
following separation. Rabbit anti-HNF4α monoclonal antibody
(1:1,000; Abcam) or goat anti-actin polyclonal antiserum (1:1,000;
Santa Cruz Biotechnology, Inc.) were used as the primary
antibodies. Horseradish peroxidase (HRP)-conjugated anti-rabbit or
anti-goat IgG (Santa Cruz Biotechnology, Inc.) was used as the
secondary antibody at a 1:10,000 dilution. Protein bands were
visualised using Western Lightning® Plus-ECL enhanced
chemiluminescent substrate (Perkin Elmer).
Immunofluorescence
Primary murine hepatocytes were incubated with
rabbit-polyclonal-anti Hnf4α (Bioss Antibodies Inc.) as the primary
antibody after preincubation with hydrogen peroxide for blocking of
endogenous peroxidase. Endogenous biotin was blocked with the
Avidin-Biotin Blocking kit (Vector Laboratories) and contaminating
proteins were inhibited by ROTI®-Immunoblock solution
(ROTH). After incubation with the secondary antibody (goat
anti-rabbit IgG-Biotin, 1:1,000; Dako Cytomation), the TSA™ Cyanine
system (Perkin Elmer) was added. For the negative control, the
primary antibody was omitted. The images were evaluated under a
fluorescence microscope (Olympus BX51, Olympus U-RFL-T).
Oct inhibition
HepG2 (ATCC® HB-8065™), a human liver
cancer cell line, and HuH7 (RRID: CVCL_0336), a well differentiated
hepatocyte-derived carcinoma cell line, were grown at 37˚C in a
humidified atmosphere (5% CO2) in plastic culture flasks
(Falcon 3112; Becton-Dickinson). The medium was Dulbecco's modified
Eagle's medium (31885-023; Life Technologies) supplemented with 10%
foetal calf serum (Life Technologies). Medium was changed every 2-3
days and the culture was split every 7 days.
The pcDNAOCT1 and pcDNAOCT3 plasmids and an empty
vector (Invitrogen; Thermo Fisher Scientific, Inc.) were stably
transfected into HepG2 and HuH7 cells by mixing with the Attractene
Transfection Reagent (Qiagen) according to the instructions of the
manufacturer. Primary hepatocytes were isolated from
Oct3-/- and WT mice and cultured in collagen-coated
24-well culture plates (2.5x105/ml) as previously
described (29). For functional
inhibition of the transporters, primary murine hepatocytes were
treated with different doses (0, 50, 100 and 150 µM) of the
standard non-selective OCT inhibitor quinine (Sigma-Aldrich; Merck
KGaA) for 48 h (30-35).
Statistical analysis
Data management and statistical analysis were
performed with Prism version 7.0 (GraphPad Software, Inc.). Results
are expressed as means ± SEM and represent data from a minimum of
three independent experiments assessed in triplicates. Three
biological replicates were assumed being the minimum for any
inferential analysis (biological repetition). As sample numbers
were small, normal distribution was assumed. Therefore, no
normality test was necessary. When two groups were compared,
unpaired Student's t-test was used. Data with more than two groups
were analysed by one-way or two-way ANOVA with Dunnett's multiple
comparisons test after one-way ANOVA and Tukey-Kramer test after
two-way ANOVA. For Pearson's correlation analysis SPSS program
(version 23.0; IBM Corp.) was used. P<0.05 was considered
statistically significant.
Results
Hnf4α is one of the top upstream
regulators in Oct3-/- mice
Transcriptome analysis showed that Hnf4α is one of
the top upstream regulators in Oct3-/- mice
(P<0.001), with 110 target molecules. Hnf4α plays a pivotal role
in regulating various transmembrane proteins and enzymes in
Oct3-/- mice (Fig. 1A).
The majority of genes regulated by Hnf4α were upregulated in
Oct3-/- mice (Fig. 1B).
Other significantly upregulated (positive z-score) upstream
regulators were the (proto-)oncogenes myc (P=1.59x10-13;
z=2.21) and kras (P=5.43x10-7; z=0.77), while the tumour
suppressor tp53 was significantly downregulated (negative z-score)
in Oct3-/- mice (P=1.1x10-7; z=-3.15)
(Fig. 1C).
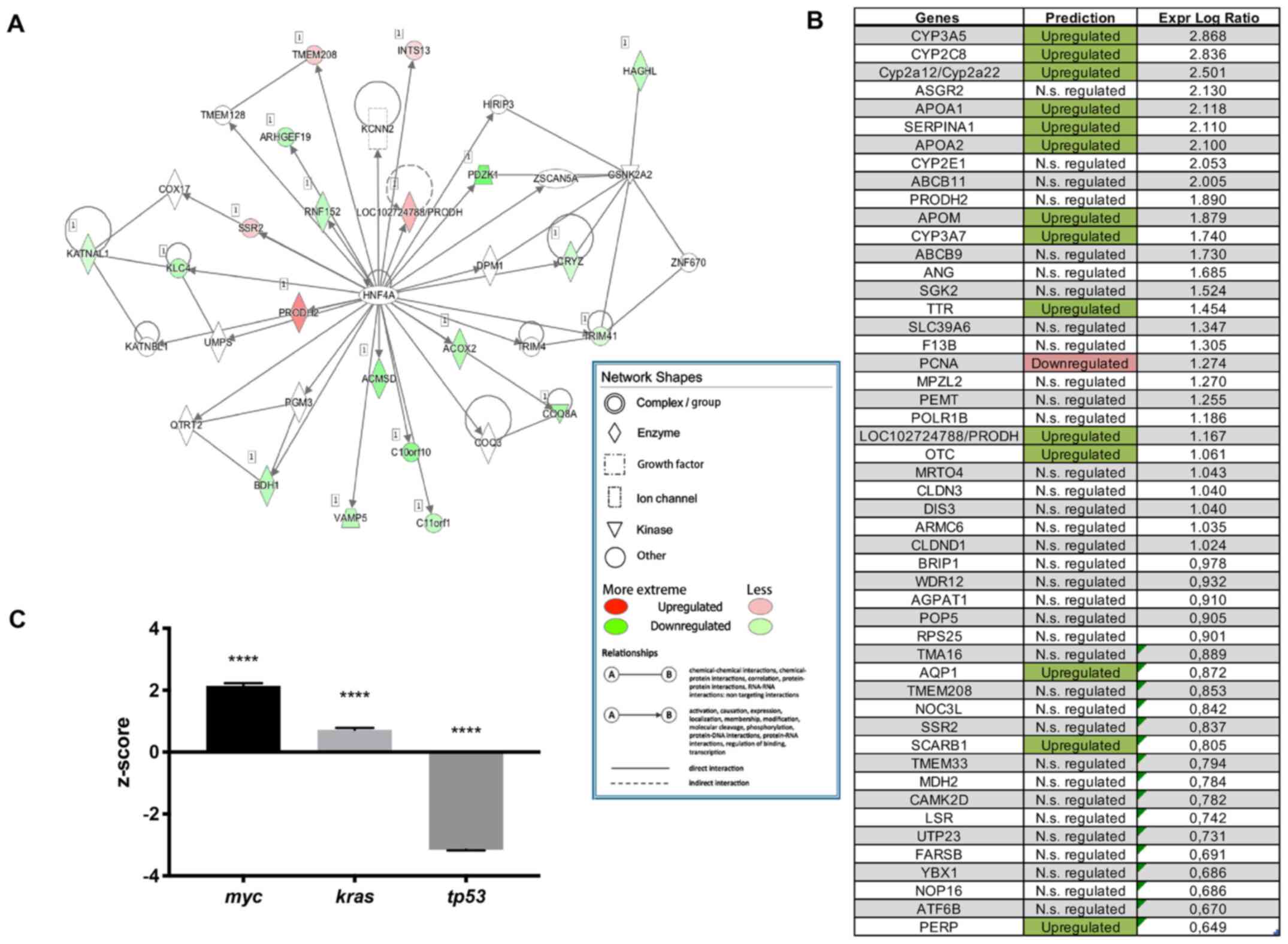 | Figure 1Gene expression analysis. (A) Hnf4α
network in Oct3-/- (n=3) mice. Network shapes: Double
circle, complex/group; diamond, enzyme; square, growth factor; box,
ion channel; triangle, kinase; circle, other; green, upregulated
genes; red, downregulated genes; line, direct interaction; dashed
line, indirect interaction; arrow, causation. (B) Hnf4α dependent
genes in Oct3-/- (n=3) mice. The majority of Hnf4α
dependent genes in Oct3-/- mice is upregulated. (C)
Activation status (z-score) of the three top upstream regulators in
Oct3-/- mice (n=3); while myc and kras are significantly
upregulated, tp53 is significantly downregulated in
Oct3-/- compared to WT mice. Results were normalized to
WT results. ****P<0.0001 vs. WT mice. Hnf4α,
hepatocyte nuclear factor 4α; Oct3-/-, Oct3-knockout
(FVB.Slc22a3tm10pb); N.s., not significant. |
Deletion of Oct3 leads to Hnf4α mRNA
downregulation in cholestasis and fibrosis
Untreated Oct3-/- mice did not show
differences in Hnf4α mRNA expression in comparison to WT
littermates at the age of 4 weeks (Fig.
2A). Hnf4α mRNA expression was significantly downregulated in
cholestatic Oct3-/- mice (n=6) in comparison to WT mice
(n=8) 7 days after BDL (P<0.01) (Fig. 2B).
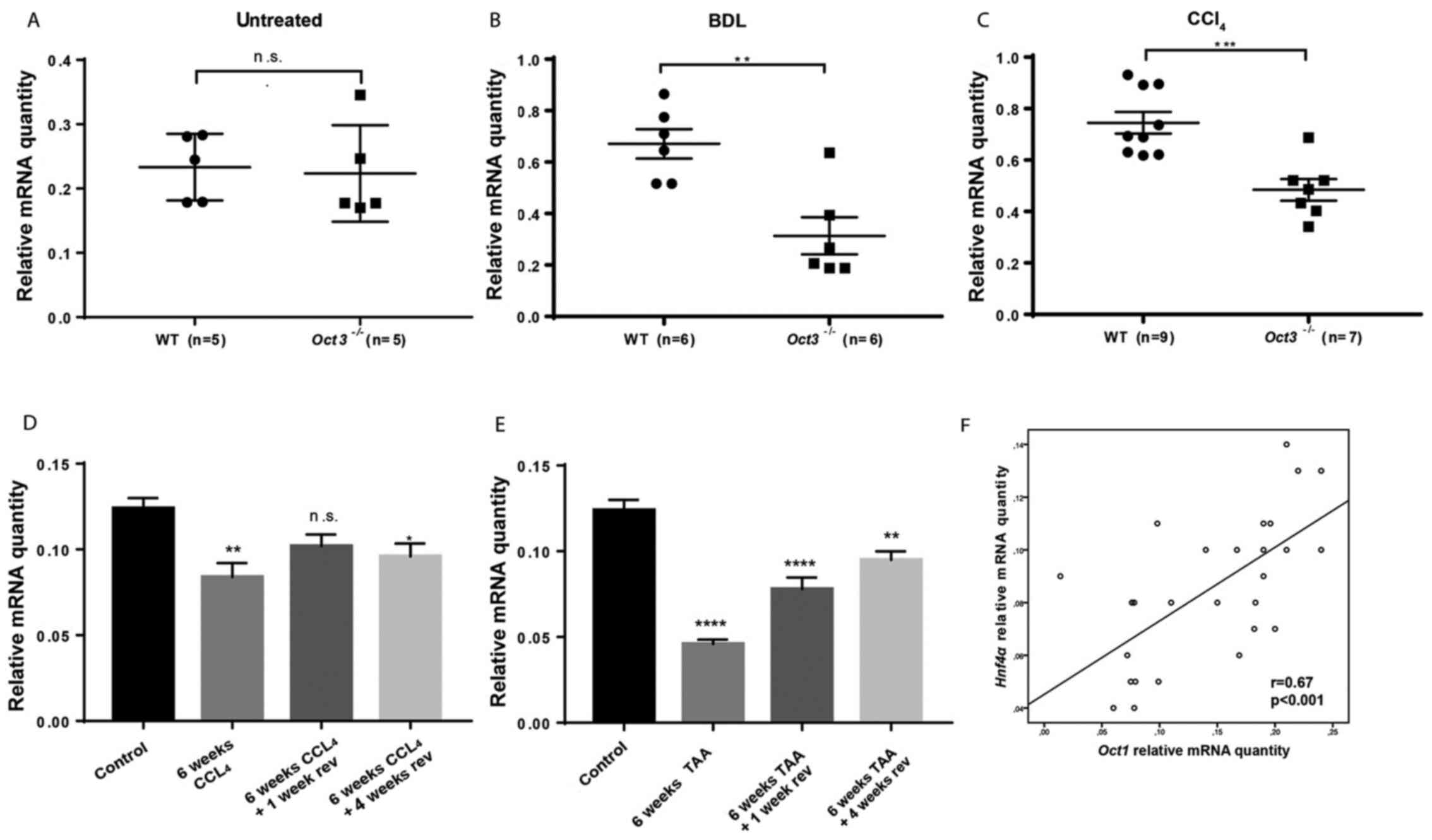 | Figure 2Hnf4α downregulation in cholestasis
and fibrosis. (A) Hnf4α mRNA expression in 4 weeks old untreated
Oct3-/- (n=5) and WT mice (n=5); no significant
difference was detected. (B) Hnf4α mRNA expression in
Oct3-/- (n=6) and WT mice (n=6) 7 days after BDL; Hnf4α
mRNA expression was significantly downregulated in
Oct3-/- mice. Sham operation served as control. Values
are expressed as fold expression relative to the control. (C) Hnf4α
mRNA expression in Oct3-/- (n=7) and WT mice (n=9) after
6 weeks of CCl4 treatment: Hnf4α mRNA expression was
significantly downregulated in Oct3-/- mice. Oral gavage
of mineral oil served as the control. Values are expressed as fold
expression relative to the control. (D) Results of Hnf4α mRNA
expression after induction of fibrosis with TAA for 6 weeks and
after reversal for one and four weeks in C57BL/6 mice (n=5).
Placebo intraperitoneal injection and oral gavage of mineral oil
served as the control. (E) Results of Hnf4α mRNA expression after
induction of fibrosis with CCl4 for 6 weeks and after
reversal for one and four weeks in C57BL/6 mice (n=5). Placebo
intraperitoneal injection and oral gavage of mineral oil served as
the control. (F) Correlation of Hnf4α and Oct1 mRNA expression
after induction of fibrosis with TAA and CCl4 for 6
weeks and after reversal for one and four weeks in C57BL/6 mice
(n=5). *P<0.05, **P<0.01;
***P<0.001; ****P<0.00001 vs. Control.
Hnf4α, hepatocyte nuclear factor 4α; Oct3-/-,
Oct3-knockout (FVB.Slc22a3tm10pb); WT, wild type; TAA,
thioacetamide; CCl4, carbon tetrachloride; BDL, bile
duct ligation; n.s., not significant; w, weeks; rev, reversal. |
Also, after chemical fibrosis induction with 6 weeks
of CCl4 treatment, Hnf4α mRNA expression was
significantly downregulated in Oct3-/- mice (n=7) as
compared to WT mice (n=9) (P<0.001) (Fig. 2C).
Hnf4α mRNA downregulation in fibrosis
is reversible
Fibrosis was induced with TAA and CCl4
treatment for 6 weeks in C57BL/6 mice (n=5), which are susceptible
to conventional toxin-induced fibrosis progression and reversal
models. Hnf4α mRNA expression was quantified by qPCR at the end of
the treatment period and after up to four weeks of reversal. After
6 weeks of TAA and CCl4 treatment, Hnf4α mRNA expression
was significantly downregulated in fibrotic mouse livers (P<0.01
compared to baseline). After reversal for one and four weeks, the
Hnf4α mRNA level increased again (Fig.
2D and E). Hnf4α mRNA
expression correlated well with Oct1 mRNA expression (Fig. 2F).
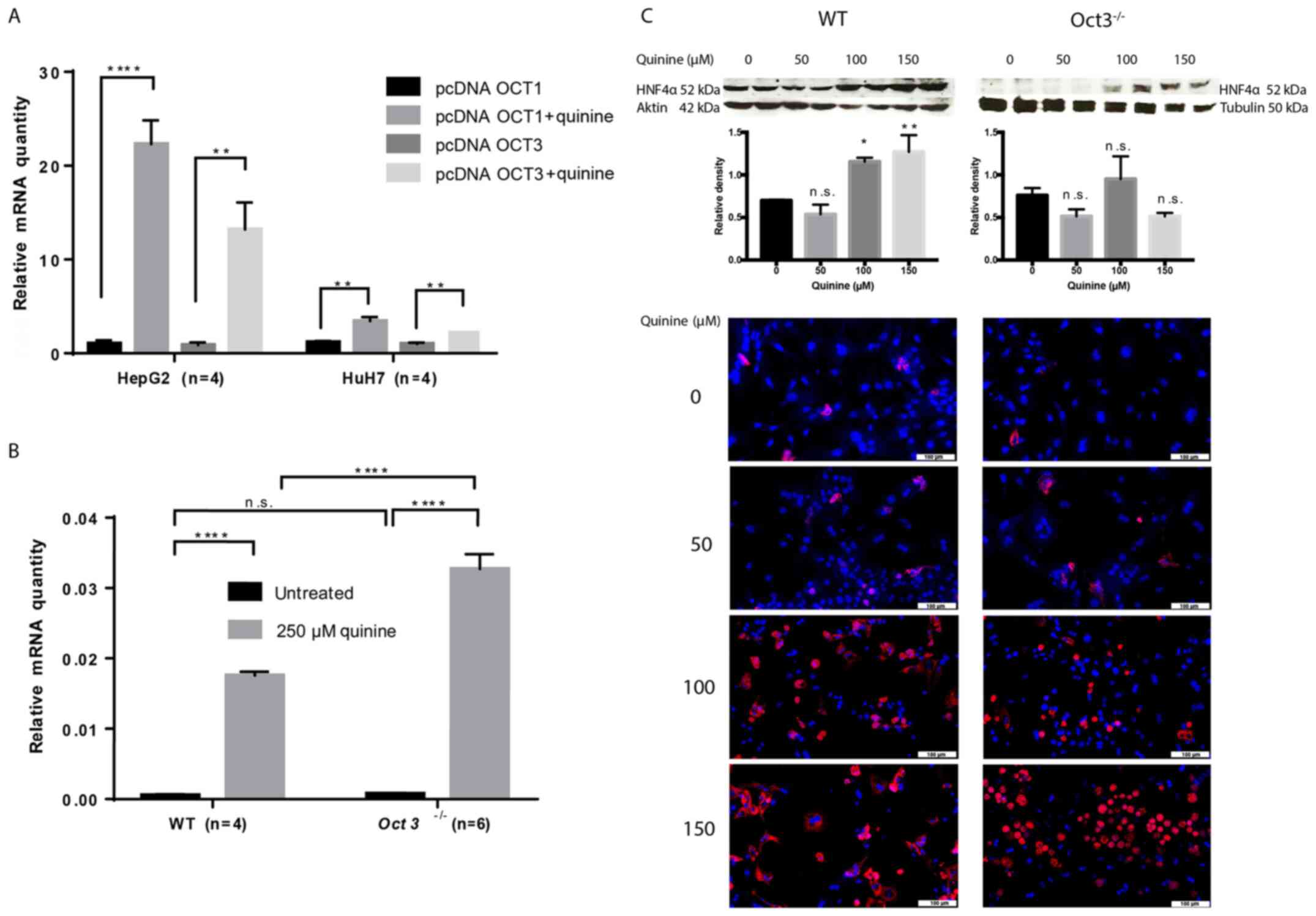
Functional inhibition of Oct induces
Hnf4α mRNA expression
Oct regulation cannot be easily studied, as the
transporters are not relevantly expressed in cell lines (36). Therefore, experiments with stably
OCT1- and OCT3-transfected tumour cell lines (HepG2 and HuH7, n=4)
and primary hepatocytes isolated from Oct3-/- (n=6) and
WT (n=4) mice were performed. Proof that transfection with
pcDNAOCT1 and pcDNAOCT3 induced overexpression of OCT1 and OCT3
compared with the empty vector was provided as Fig. S1. Hnf4α mRNA expression was
significantly upregulated in OCT1- and OCT3-transfected HepG2 and
HuH7 cells compared with in tumour cells transfected with empty
vector (Fig. 3A) and primary
Oct3-/- hepatocytes (Fig.
3B) after treatment with the Oct inhibitor quinine (P<0.01).
Western blots and immunofluorescence in primary WT and
Oct3-/- hepatocytes showed an increase of Hnfα protein
expression with escalating quinine doses (Figs. 3C and S2-4).
These data clearly show that functional loss of Oct induces the
expression of Hnf4α. Interestingly, immunofluorescence of primary
murine hepatocytes showed that Hnf4α was not only increased with
escalating quinine doses, but theHnf4α distribution also differed
between Oct3-/- and WT hepatocytes. While Hnf4α was
located in the cytosol of WT hepatocytes, Oct3-/-
hepatocytes showed nuclear Hnf4α expression, indicating that Oct3
affects Hnf4α in vivo (Figs.
3C and S5).
Discussion
HNF4α has been extensively studied in many tissues
and tumour cell lines, but few data exist about an interaction with
OCTs. According to previous findings, Hnf4α is downregulated in
fibrosis (14). Chemically induced
fibrogenesis with two different agents (CCl4 and TAA)
resulted in Hnf4α mRNA downregulation. Interestingly, the mRNA of
this nuclear factor was re-expressed after stopping administration
of TAA and CCl4 when fibrosis reversal occurred,
indicating that the Hnf4α downregulation in fibrotic tissue is
reversible (Fig. 2A and B). This means that the effect is real,
reproducible and relevant. To date, no data exist on the
reversibility of Hnf4α downregulation in fibrosis, emphasising that
confounders do not falsify previous findings. Moreover, the
activation of the (proto-) oncogenes myc and kras and the
inhibition of the tumour suppressor tp53 in Oct3-/- mice
(Fig. 1D) are in line with previous
findings of enhanced proliferation and hepatocarcinogenesis with
the loss of Oct3(5). However, the
upstream regulatory mechanism is still unclear.
To date, no data exist on a link between OCT3 and
HNF4α. The OCT1 gene is transactivated by HNF4α (6), and chemosensitivity to oxaliplatin and
5-FU mediated by OCT1 is induced by HNF4α in renal cell carcinoma
(37). Therefore, differences in
Hnf4α expression between Oct3-/- and WT mice are likely.
There was no difference in Hnf4α mRNA expression between untreated
Oct3-/- and WT mice (Fig.
2D), but upon induction of fibrosis or cholestasis, the
downregulation of Hnf4α mRNA was more intense in Oct3-/-
mice (Fig. 2E and F). This clearly shows that Hnf4α
regulation is affected in cholestasis and fibrosis in
Oct3-/- mice. Because Hnf4α is a master regulator of
hepatocyte differentiation (8) and
fibrosis progression (14), these
findings may contribute to identify Hnf4α as an upstream regulator
involved in the promotion of enhanced proliferation, inflammation
and fibrosis progression in Oct3-/- mice, as recently
published (5,18). Also, gene expression analyses
revealed that the majority of genes regulated by Hnf4α are
activated in untraded Oct3-/- mice. But these data
represent a pilot study and have to be evaluated critically. To
further study the effect of loss of OCT function on HNF4α, Hnf4α
mRNA expression was induced in stably OCT1- and OCT3-transfected
tumour cell lines (HepG2 and HuH7) and primary Oct3-/-
and WT hepatocytes after treatment with the non-selective OCT
inhibitor quinine (P<0.01), showing n upregulation of Hnf4α mRNA
expression with the loss of Oct function (Fig. 2A and B). Due to the transactivation of the OCT1
gene by HNF4α (6), a feedback
mechanism is possible, but not identified yet. Interestingly,
immunofluorescence of primary murine hepatocytes showed that Hnf4α
was not only increased with escalating quinine doses, but the Hnf4α
distribution also differed between Oct3-/- (nuclear) and
WT (cytosol) hepatocytes (Fig. 2C),
indicating that not only transcriptional loss of Oct3 but also
functional loss of Oct affect Hnf4α. The fact that not only
transcriptional but also functional factors play a relevant role in
OCT regulation is in line with a previous characterisation of OCT3
as a cellular mechanism underlying rapid, non-genomic
glucocorticoid regulation of monoaminergic neurotransmission,
physiology and behaviour (38). OCT
expression is regulated by transcriptional as well as complex
epigenetic (39,40) and metabolic (41,42)
factors. There is not a distinct pathway to explain the function
and mechanism of Oct3 in the context of liver damage. Therefore,
the role of transcriptional and functional loss of Oct3 in Hnf4α
regulation and finding a mechanistic link between Oct3 and Hnf4α
needs further investigation.
For the first time, we show that Oct3 and Hnf4α
regulation might be associated, with crucial effects on
proliferation and fibrosis progression in the liver. Our results
suggest that these transporters are key regulators of
Hnf4α-dependent pathways. Further efforts are necessary to
understand the complex regulation of Oct in the context of Hnf4α.
Clinical relevance remains open. OCTs are emerged via gene
duplication and substitute each other (39,40,43).
Potentially a complete loss of Oct function is not compatible with
life. This needs further studies
In conclusion, Hnf4α is downregulated in cholestasis
and fibrosis and functional inhibition of OCT leads to the
upregulation of Hnf4α. Thus, we present a novel link between the
transporters and the Hnf4α network.
Supplementary Material
Proof of successful transfection. OCT1
(SLC22A1) and OCT3 (SLC22A3) mRNA expression levels in stably OCT1
(pcDNAOCT1)- and OCT3 (pcDNAOCT3)-transfected HepG2 (n=4) and HuH7
(n=4) cells in comparison to empty vector-transfected tumour cells
after 48 h. *P<0.05, **P<0.01.
Uncropped western blot No. 1.
Uncropped western blots in primary murine hepatocytes of
Oct3-/- and WT mice after 48 h treatment with escalating
quinine doses (0, 50, 100 and 150 μM). Oct3-/-,
Oct3-knockout (FVB.Slc22a3tm10pb), WT, wild-type.
Uncropped western blot No. 2.
Uncropped western blots in primary murine hepatocytes of
Oct3-/- and WT mice after 48 h treatment with escalating
quinine doses (0, 50, 100 and 150 μM). Oct3-/-,
Oct3-knockout (FVB.Slc22a3tm10pb), WT, wild-type.
Uncropped western blots Nos. 3 and 4.
Uncropped western blots in primary murine hepatocytes of Oct3-/-
and WT mice after 48 h treatment with escalating quinine doses (0,
50, 100 and 150 μM). Oct3-/-, Oct3-knockout
(FVB.Slc22a3tm10pb), WT, wild-type.
Magnified immunofluorescence.
Magnified immunofluorescence (magnification, x40) in primary murine
hepatocytes of Oct3-/- and WT mice after 48 h treatment
with 150 μM quinine. Oct3-/-, Oct3-knockout
(FVB.Slc22a3tm10pb), WT, wild-type.
Acknowledgements
The authors would like to thank Mrs. Larissa Herbel
(1st Department of Internal Medicine, Gastroenterology and
Hepatology, University Medical Centre, Johannes
Gutenberg-University Mainz, Mainz, Germany) and Mrs. Kim (Institute
of Translational Immunology, Fibrosis and Metabolism Centre,
Johannes Gutenberg-University Mainz, Mainz, Germany) for excellent
technical support.
Funding
Funding: This work was supported by a MAIFOR grant from the
Johannes Gutenberg University of Mainz to TZ. The funder only
provided financial support.
Availability of data and materials
The sequencing datasets generated and/or analysed
during the current study are available in the Gene Expression
Omnibus repository under BioProject no. PRJNA685115 (http://www.ncbi.nlm.nih.gov/bioproject/685115). All
other data are available on request.
Authors' contributions
JV and TZ designed research, performed experiments,
collected and analysed data, and wrote the manuscript. JUM
conducted array data analysis. JV and TZ confirm the authenticity
of all the raw data. PRG and DS made substantial contributions to
interpretation of data. DS, JUM and PRG performed a critical review
of the manuscript. YOK performed data analysis and provided
methodological support. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
Animal care (housing, husbandry conditions) and
animal procedures were performed in accordance with the European
Council Directive of 24 November, 1986 (86/609/EEC). This study was
approved by the state animal care commission (23 177-07/G
14-1-010). The study was not submitted to the institutional ethics
committee/review board, but rather to the state animal care
commission, because living mice and cell lines were used. No
patient material was used.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Heise M, Lautem A, Knapstein J,
Schattenberg JM, Hoppe-Lotichius M, Foltys D, Weiler N, Zimmermann
A, Schad A, Gründemann D, et al: Downregulation of organic cation
transporters OCT1 (SLC22A1) and OCT3 (SLC22A3) in human
hepatocellular carcinoma and their prognostic significance. BMC
Cancer. 12(109)2012.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Lautem A, Heise M, Gräsel A,
Hoppe-Lotichius M, Weiler N, Foltys D, Knapstein J, Schattenberg
JM, Schad A, Zimmermann A, et al: Downregulation of organic cation
transporter 1 (SLC22A1) is associated with tumor progression and
reduced patient survival in human cholangiocellular carcinoma. Int
J Oncol. 42:1297–1304. 2013.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Grimm D, Lieb J, Weyer V, Vollmar J,
Darstein F, Lautem A, Hoppe-Lotichius M, Koch S, Schad A,
Schattenberg JM, et al: Organic Cation Transporter 1 (OCT1) mRNA
expression in hepatocellular carcinoma as a biomarker for sorafenib
treatment. BMC Cancer. 16(94)2016.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Herraez E, Lozano E, Macias RI, Vaquero J,
Bujanda L, Banales JM, Marin JJ and Briz O: Expression of SLC22A1
variants may affect the response of hepatocellular carcinoma and
cholangiocarcinoma to sorafenib. Hepatology. 58:1065–1073.
2013.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Vollmar J, Lautem A, Closs E, Schuppan D,
Kim YO, Grimm D, Marquardt JU, Fuchs P, Straub BK, Schad A, et al:
Loss of organic cation transporter 3 (Oct3) leads to enhanced
proliferation and hepatocarcinogenesis. Oncotarget.
8:115667–115680. 2017.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Saborowski M, Kullak-Ublick GA and
Eloranta JJ: The human organic cation transporter-1 gene is
transactivated by hepatocyte nuclear factor-4alpha. J Pharmacol Exp
Ther. 317:778–785. 2006.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Rulcova A, Krausova L, Smutny T, Vrzal R,
Dvorak Z, Jover R and Pavek P: Glucocorticoid receptor regulates
organic cation transporter 1 (OCT1, SLC22A1) expression via
HNF4alpha upregulation in primary human hepatocytes. Pharmacol Rep.
65:1322–1335. 2013.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Parviz F, Matullo C, Garrison WD, Savatski
L, Adamson JW, Ning G, Kaestner KH, Rossi JM, Zaret KS and Duncan
SA: Hepatocyte nuclear factor 4alpha controls the development of a
hepatic epithelium and liver morphogenesis. Nat Genet. 34:292–296.
2003.PubMed/NCBI View
Article : Google Scholar
|
|
9
|
Sasaki S, Urabe M, Maeda T, Suzuki J, Irie
R, Suzuki M, Tomaru Y, Sakaguchi M, Gonzalez FJ and Inoue Y:
Induction of hepatic metabolic functions by a novel variant of
hepatocyte nuclear factor 4γ. Mol Cell Biol. 8:e00213–18.
2018.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Ning BF, Ding J, Yin C, Zhong W, Wu K,
Zeng X, Yang W, Chen YX, Zhang JP, Zhang X, et al: Hepatocyte
nuclear factor 4 alpha suppresses the development of hepatocellular
carcinoma. Cancer Res. 70:7640–7651. 2010.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Walesky C, Edwards G, Borude P,
Gunewardena S, O'Neil M, Yoo B and Apte U: Hepatocyte nuclear
factor 4 alpha deletion promotes diethylnitrosamine-induced
hepatocellular carcinoma in rodents. Hepatology. 57:2480–2490.
2013.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Saha SK, Parachoniak CA, Ghanta KS,
Fitamant J, Ross KN, Najem MS, Gurumurthy S, Akbay EA, Sia D,
Cornella H, et al: Mutant IDH inhibits HNF-4alpha to block
hepatocyte differentiation and promote biliary cancer. Nature.
513:110–114. 2014.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Wu N, Zhang YL, Wang HT, Li DW, Dai HJ,
Zhang QQ, Zhang J, Ma Y, Xia Q, Bian JM and Hang HL: Overexpression
of hepatocyte nuclear factor 4α in human mesenchymal stem cells
suppresses hepatocellular carcinoma development through
Wnt/β-catenin signaling pathway downregulation. Cancer Biol Ther.
17:558–565. 2016.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Yue HY, Yin C, Hou JL, Zeng X, Chen YX,
Zhong W, Hu PF, Deng X, Tan YX, Zhang JP, et al: Hepatocyte nuclear
factor 4alpha attenuates hepatic fibrosis in rats. Gut. 59:236–246.
2010.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Fan TT, Hu PF, Wang J, Wei J, Zhang Q,
Ning BF, Yin C, Zhang X, Xie WF, Chen YX and Shi B: Regression
effect of hepatocyte nuclear factor 4alpha on liver cirrhosis in
rats. J Dig Dis. 14:318–327. 2013.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Park MR, Wong MS, Araúzo-Bravo MJ, Lee H,
Nam D, Park SY, Seo HD, Lee SM, Zeilhofer HF, Zaehres H, et al:
Oct4 and Hnf4α-induced hepatic stem cells ameliorate chronic liver
injury in liver fibrosis model. PLoS One.
14(e0221085)2019.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Zwart R, Verhaagh S, Buitelaar M,
Popp-Snijders C and Barlow DP: Impaired activity of the
extraneuronal monoamine transporter system known as uptake-2 in
Orct3/Slc22a3-deficient mice. Mol Cell Biol. 21:4188–4196.
2001.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Vollmar J, Kim YO, Marquardt JU, Becker D,
Galle PR, Schuppan D and Zimmermann T: Deletion of organic cation
transporter Oct3 promotes hepatic fibrosis via upregulation of
TGFβ. Am J Physiol Gastrointest Liver Physiol. 317:G195–G202.
2019.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Jonker JW and Schinkel AH: Pharmacological
and physiological functions of the polyspecific organic cation
transporters: OCT1, 2, and 3 (SLC22A1-3). J Pharmacol Exp Ther.
308:2–9. 2004.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Jonker JW, Wagenaar E, Van Eijl S and
Schinkel AH: Deficiency in the organic cation transporters 1 and 2
(Oct1/Oct2 [Slc22a1/Slc22a2]) in mice abolishes renal secretion of
organic cations. Mol Cell Biol. 23:7902–7908. 2003.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Moll P, Ante M, Seitz A and Reda T:
QuantSeq 3'mRNA sequencing for RNA quantification. Nat Methods.
12:2014.
|
|
22
|
Liao Y, Smyth GK and Shi W: The Subread
aligner: Fast, accurate and scalable read mapping by seed-and-vote.
Nucleic Acids Res. 41(e108)2013.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Morgan M, Anders S, Lawrence M, Aboyoun P,
Pagès H and Gentleman R: ShortRead: A bioconductor package for
input, quality assessment and exploration of high-throughput
sequence data. Bioinformatics. 25:2607–2608. 2009.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Love MI, Huber W and Anders S: Moderated
estimation of fold change and dispersion for RNA-seq data with
DESeq2. Genome Biol. 15(550)2014.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Kim YO, Popov Y and Schuppan D: Optimized
mouse models for liver fibrosis. Methods Mol Biol. 1559:279–296.
2017.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Nies AT, Koepsell H, Winter S, Burk O,
Klein K, Kerb R, Zanger UM, Keppler D, Schwab M and Schaeffeler E:
Expression of organic cation transporters OCT1 (SLC22A1) and OCT3
(SLC22A3) is affected by genetic factors and cholestasis in human
liver. Hepatology. 50:1227–1240. 2009.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Denk GU, Soroka CJ, Mennone A, Koepsell H,
Beuers U and Boyer JL: Down-regulation of the organic cation
transporter 1 of rat liver in obstructive cholestasis. Hepatology.
39:1382–1389. 2004.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Tag CG, Sauer-Lehnen S, Weiskirchen S,
Borkham-Kamphorst E, Tolba RH, Tacke F and Weiskirchen R: Bile duct
ligation in mice: induction of inflammatory liver injury and
fibrosis by obstructive cholestasis. J Vis Exp: 52438, 2015.
|
|
29
|
Li WC, Ralphs KL and Tosh D: Isolation and
culture of adult mouse hepatocytes. Methods Mol Biol. 633:185–196.
2010.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Arndt P, Volk C, Gorboulev V, Budiman T,
Popp C, Ulzheimer-Teuber I, Akhoundova A, Koppatz S, Bamberg E,
Nagel G and Koepsell H: Interaction of cations, anions, and weak
base quinine with rat renal cation transporter rOCT2 compared with
rOCT1. Am J Physiol Renal Physiol. 281:F454–F468. 2001.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Müller J, Lips KS, Metzner L, Neubert RH,
Koepsell H and Brandsch M: Drug specificity and intestinal membrane
localization of human organic cation transporters (OCT). Biochem
Pharmacol. 70:1851–1860. 2005.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Koepsell H: Polyspecific organic cation
transporters: Their functions and interactions with drugs. Trends
Pharmacol Sci. 25:375–381. 2004.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Koepsell H, Lips K and Volk C:
Polyspecific organic cation transporters: structure, function,
physiological roles, and biopharmaceutical implications. Pharm Res.
24:1227–1251. 2007.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Keller T, Elfeber M, Gorboulev V,
Reiländer H and Koepsell H: Purification and functional
reconstitution of the rat organic cation transporter OCT1.
Biochemistry. 44:12253–12263. 2005.PubMed/NCBI View Article : Google Scholar
|
|
35
|
van der Velden M, Bilos A, van den Heuvel
JJMW, Rijpma SR, Hurkmans EGE, Sauerwein RW, Russel FGM and
Koenderink JB: Proguanil and cycloguanil are organic cation
transporter and multidrug and toxin extrusion substrates. Malar J.
16(422)2017.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Hilgendorf C, Ahlin G, Seithel A,
Artursson P, Ungell AL and Karlsson J: Expression of thirty-six
drug transporter genes in human intestine, liver, kidney, and
organotypic cell lines. Drug Metab Dispos. 35:1333–1340.
2007.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Hagos Y, Wegner W, Kuehne A, Floerl S,
Marada VV, Burckhardt G and Henjakovic M: HNF4α induced
chemosensitivity to oxaliplatin and 5-FU mediated by OCT1 and CNT3
in renal cell carcinoma. J Pharm Sci. 103:3326–3334.
2014.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Gasser PJ and Lowry CA: Organic cation
transporter 3: A cellular mechanism underlying rapid, non-genomic
glucocorticoid regulation of monoaminergic neurotransmission,
physiology, and behavior. Horm Behav. 104:173–182. 2018.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Sleutels F, Tjon G, Ludwig T and Barlow
DP: Imprinted silencing of Slc22a2 and Slc22a3 does not need
transcriptional overlap between Igf2r and Air. EMBO J.
22:3696–3704. 2003.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Sleutels F, Zwart R and Barlow DP: The
non-coding Air RNA is required for silencing autosomal imprinted
genes. Nature. 415:810–813. 2002.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Chen L, Shu Y, Liang X, Chen EC, Yee SW,
Zur AA, Li S, Xu L, Keshari KR, Lin MJ, et al: OCT1 is a
high-capacity thiamine transporter that regulates hepatic steatosis
and is a target of metformin. Proc Natl Acad Sci USA.
111:9983–9988. 2014.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Chen L, Hong C, Chen EC, Yee SW, Xu L,
Almof EU, Wen C, Fujii K, Johns SJ, Stryke D, et al: Genetic and
epigenetic regulation of the organic cation transporter 3, SLC22A3.
Pharmacogenomics J. 13:110–120. 2013.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Nagano T, Mitchell JA, Sanz LA, Pauler FM,
Ferguson-Smith AC, Feil R and Fraser P: The Air noncoding RNA
epigenetically silences transcription by targeting G9a to
chromatin. Science. 322:1717–1720. 2008.PubMed/NCBI View Article : Google Scholar
|