Introduction
Coronavirus disease 2019 (COVID-19), caused by
severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), is
characterized by respiratory tract symptoms with potentially severe
outcomes. It has been reported by epidemiological studies that
COVID-19 presents in the majority of cases with upper respiratory
symptoms (1). Recently, studies
have reported neurological manifestations of COVID-19 in China in
up to 36.4% of hospitalized patients, including alteration of
consciousness, headache, dizziness and delirium (2-4).
The therapy for COVID-19 remains to be established, although
non-pharmacological and preventive treatments have been recommended
(5). Like other coronaviruses,
SARS-CoV-2 is neurotropic (6).
Neurological complications of COVID-19 include cerebrovascular
disease, meningitis/encephalitis, acute necrotizing hemorrhagic
encephalopathy and Guillain-Barré syndrome (7). However, the clinical characteristics
of COVID-19-associated meningitis/encephalitis remain to be
defined. Anosmia/dysgeusia has been reported by COVID-19 patient
(8-10).
This phenomenon provided a hypothesis that COVID-19-associated CNS
infection is manifests through a nasopharyngeal route. The present
study reported on a patient with COVID-19 presenting with
meningio-encephalitis. The clinical characteristics and laboratory
results of the patient were investigated. In order to understand
the features of COVID-19-associated CNS infection, the literature
on COVID-19-associated meningitis/encephalitis cases was reviewed
and summarized.
Case report
Data collection
The patient provided informed written consent for
the publication of the present case report. The
diagnostic/therapeutic procedures applied were in accordance with
institutional and international guidelines for the protection of
human subjects. The study was approved by the ethics committee of
the China-Japan Friendship Hospital (permit no. 2019-183-K124).
Clinical and auxiliary test results were retrieved by the authors.
Viral/bacterial detection was performed by real-time reverse
transcription-PCR (RT-PCR).
Literature search
Entries related to COVID-19-associated
encephalitis/encephalopathy were searched in the PubMed, Chinese
National Knowledge Infrastructure (CNKI) and EMBASE databases.
Articles that were published between December 2019 and 1st June
2020 were specifically screened to ensure the relevance of the
results. The following search terms were used: COVID-19,
SARS-CoV-2, encephalitis, encephalopathy and meningoencephalitis. A
total of 44 articles were retrieved. Case reports or case series
that provided detailed clinical information were included. Cohort
studies, systematic reviews, meta-analyses and clinical trials were
excluded. Of note, no relevant articles were found in the CNKI
database. A total of 13 articles met the criteria (11-23).
Of these, 1 case of COVID-19 diagnosed with herpes encephalitis
(21), 1 study that investigated
patients with COVID-19 who did not regain consciousness after
withdrawal of invasive mechanical ventilation (23) and another study reporting 2 patients
diagnosed with COVID-19 pneumonia complicated by minor neurological
symptoms but without neurological imaging or lumbar puncture data
(22) were excluded. Thus, a total
of 10 articles were finally included in the present study. Of note,
due to the rapid spread of the COVID-19 epidemic, other relevant
articles may have been published during the preparation of the
manuscript of the present study.
Results
Case presentation
The case was a 90-year-old female of Han Chinese
ethnicity who lived in Wuhan city (China) prior to disease onset.
She had a history of cerebral lacunar infarction but with no
neurological deficits. She was not self-reliant and lived in a
health care unit. The patient developed a fluctuatating fever with
temperature range between 37.5-38.5˚C, severe cough, sputum,
fatigue, chest tightness and shortness of breath on the 1st
February 2020. A nasopharyngeal swab was taken and SARS-CoV-2 RNA
was detected by RT-PCR (24). The
patient had a clear consciousness at admission. The patient's
breathing tone was coarse on auscultation and neurological
examinations were negative. The blood test results are presented in
Table I. The patient presented with
elevated CRP and decreased lymphocyte number.
 | Table ILaboratory data of the patient with
COVID-19 pneumonia with subsequent meningoencephalitis. |
Table I
Laboratory data of the patient with
COVID-19 pneumonia with subsequent meningoencephalitis.
| | Day of COVID-19
symptoms |
|---|
| Parameter (normal
range) | 6 | 20 | 27 | 34 |
|---|
| Hemoglobin (g/l,
115-150) | 119 | 119 | 109 | 117 |
| WBC
(109/l, 3.5-9.5) | 3.46 | 5.86 | 11.14 | 9.4 |
| Neutrophils
(109/l, 1.8-6.3) | 1.52 | 4.33 | 10.07 | 8.84 |
| Lymphocytes
(109/l, 1.1-3.2) | 1.37 | 0.94 | 0.81 | 0.34 |
| Monocytes
(109/l, 0.1-0.6) | 0.56 | 52 | 0.23 | 0.17 |
| Platelets
(109/l, 125-350) | 104 | 217 | 225 | 73 |
| Sodium (mmol/l,
133-146) | 138 | 140 | 152 | 146 |
| Potassium (mmol/l,
3.5-5.3) | 3.81 | 3.9 | 3.1 | 2.88 |
| Blood urea nitrogen
(mmol/l, 3.1-8.8) | 4 | 3.5 | 9.18 | 7.34 |
| Creatinine (mol/l,
41-81) | 52 | 37 | 37 | 36 |
| Corrected calcium
(mmol/l, 2.1-2.37) | 2.47 | 2.49 | | 2.18 |
| ALT (U/l, 7-40) | 13 | 11 | | 37 |
| Total bilirubin
(µmol/l, 0-23) | 5.8 | 10.3 | | 24 |
| CRP (mg/l,
0.0-10) | 18.2 | 6 | 22.6 | 12 |
| PCT (ng/ml,
<0.1) | 0.107 | 0.045 | | 0.316 |
| D-dimer (ng/ml,
<500) | 0.63 | | 1.21 | |
| Creatine kinase
(U/l) | 68 | | 25 | 590 |
| TnI (ng/ml,
0-0.04) | | | | 0.382 |
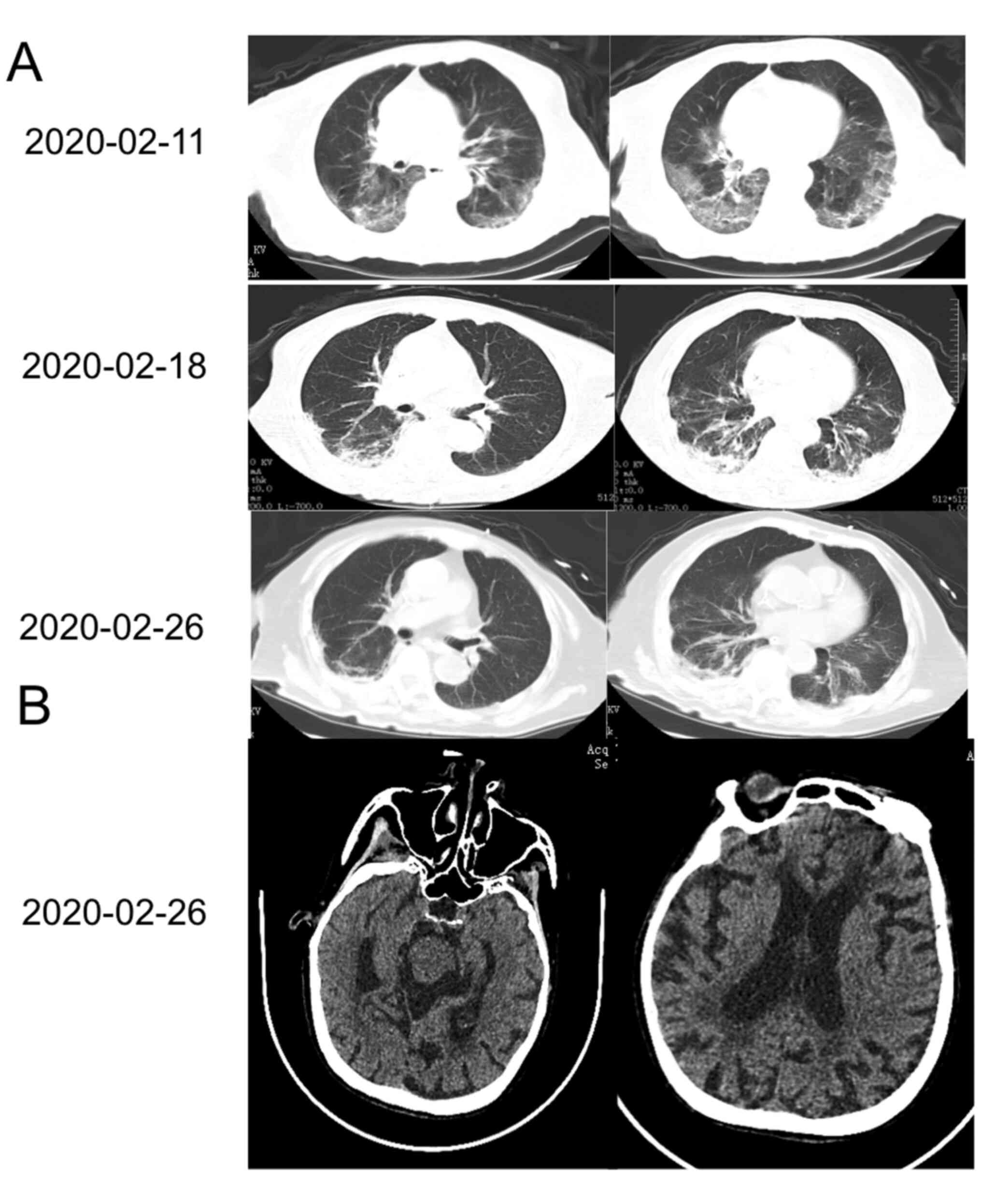
The patient exhibited gradual improvement with
absorption of the ground glass opacity on chest CT (Fig. 1A). 25 days after disease onset, the
patient suddenly became unconscious and the Glasgow Coma Scale
score was determined to be 6. On neurological examination, the
patient was irresponsive to voice and painful stimulation, her
pupils were isochoric (2-mm diameter) and reactive, and there was
no autonomous activity in any limbs. There were significantly
increased muscle tension, positive cortico-spinal tract signs on
both sides, a positive meningeal irritation sign, obvious neck
stiffness, and a positive Kernig sign. Brain CT was unremarkable
compared with a previous brain MRI (Fig. 1B); however, the patient was not
subjected to brain MRI at this time-point.
Considering the probable diagnosis of
meningo-encephalitis, the patient received mannitol and anti-viral
therapy (Ganciclovir). A lumbar puncture on March 6th 2020 (10 days
after unconsciousness) revealed an opening pressure of 60
mmH2O, cerebrospinal fluid (CSF) cell count with an
increased 25/µl (10 mononuclear and 15 polymorphonuclear cells
without red blood cells) and CSF protein levels were also increased
with 660 mg/l (normal range: 120-600 mg/l), but normal glucose,
chloride and adenosine deaminase levels. Tests for viral/bacterial
pathogens (including varicella zoster virus and herpes simplex
virus) in the CSF were negative. The result of RT-PCR detection of
SARS-CoV-2 RNA in the CSF was negative. However, the patient died
of gastrointestinal bleeding 46 days after disease onset.
Summary of clinical
characteristics
A total of 10 cases from 10 articles were included
in the literature review of the present study. The clinical
characteristics of the 10 cases are listed in Table II. The age of the patients ranged
from 24 to 75 years and the cases included 6 patients aged <60
years. The cases comprised 7 males and 3 females. Only 1 patient
had a negative detection result for SARS-CoV-2 RNA in a
nasopharyngeal swab. Common neurological signs and symptoms
included confusion and coma [patient no. 2 (P2)-5 and P7-9], neck
stiffness (P1 and P8), positive meningeal irritation sign (P1, P7),
mental disturbance (P1, P2, P4 and P8), seizure (P3 and P6),
cognitive impairment (P2 and P4), motor dysfunction (P5 and P10)
and psychotic symptoms (P3). A total of four patients exhibited
abnormalities on brain MRI, which included cerebellar lesions (P5),
lesions of the hippocampus in the temporal lobe (P6), cerebral
hemorrhage, subarachnoid hemorrhage accompanied by bilateral
supratentorial leptomeningeal enhancement (P9) and hyperintensity
in the corpus callosum of splenium (P10).
 | Table IIClinical characteristics of reported
SARS-CoV-2-induced encephalitis/meningitis cases in the
literature. |
Table II
Clinical characteristics of reported
SARS-CoV-2-induced encephalitis/meningitis cases in the
literature.
| Patient no. | Author (year) | Sex | Age (years) | NPS | Neurological
symptoms | Brain CT/MRI | CSF | Therapy and
outcome | (Refs.) |
|---|
| 1 | Yin (2020) | M | 64 | + | Lethargic and
irritability; dissociated speech; neck stiffness, meningeal
irritation signs | (-) | Opening pressure, 200
cmH2O; cell count, 1a; protein 275.5b; SARS-CoV2 (-) | Antiviral therapy;
Recovery and discharge | (11) |
| 2 | Pilotto (2020) | M | 60 | + | Irritability;
confusion and asthenia; cognitive fluctuations; akinetic
syndrome | (-) | Lymphocytes,
18a; protein,
696b; viruses (-);
IL-6, IL-8, TNF-α elevated; tau (-); NFL (-) | Methylprednisolone
1 g/day for five days; Normal neurological examination | (12) |
| 3 | Bernard Valnet
(2020) | F | 64 | + | Psychotic symptoms;
seizure; disorientation; attention deficit; bilateral grasping | (-) | cProtein, 466/399b; cells, 17/26a; lymphocyte (%), 97/100;
viruses/bacteria (-); anti-neuronal antibodies (-); SARS-CoV-2
(-) | Antiviral therapy;
Resolution of symptoms 96 h after admission | (13) |
| 4 | Bernard Valnet
(2020) | F | 67 | + | Headache;
drowsiness; confusion, disorientation; bilateral grasping;
aggressiveness; hemianopia; sensory hemineglect | (-) | cProtein, 461/485b; cells, 21/6a; lymphocyte (%), 89/82;
viral/bacterial pathogens (-); anti-neuronal antibodies (-);
SARS-CoV-2 (-) | Antiviral and
anti-bacterial therapy; Symptoms resolved within 24 h, except for a
mild headache | (13) |
| 5 | Wong (2020) | M | 40 | + | Consciousness
disturbance; diplopia, ataxia; altered sensation; hiccups and
dribbling | Lesion in the
inferior cerebellar peduncle and the upper cord | Protein,
423b; no increase in
white cells and negative bacterial culture; SARS-CoV-2 RNA ND | Not mentioned in
therapy; Improvement in hiccups and nystagmus but oscillopsia and
ataxia persisted | (14) |
| 6 | Moriguchi
(2020) | M | 24 | - | Multiple epileptic
seizures; GCS score 6; neck stiffness | Hyperintensity in
the mesial temporal lobe and hippocampus | Opening pressure is
320 mmH2O; mononuclear cells, 10a, polymorphonuclear cells,
2a; other viruses (-);
SARS-CoV-2 (+) | Antiviral and
anti-bacterial therapy, steroids; Impaired consciousness at day
15 | (15) |
| 7 | Ye (2020) | M | Not stated | + | Sudden confusion;
meningeal irritation signs; extensor plantar response | (-) | Opening pressure is
220 mmH2O; protein, 270b; SARS-CoV-2 (-) | Mannitol antiviral
therapy; Consciousness became clear | (16) |
| 8 | Huang 2020 and
Duong (2020) | F | 41 | + | Neck stiffness;
photophobia; confusion, agitation, disorientation and
hallucinations; no respiratory involvement | (-) | White cells,
70a (100%
lymphocytes); protein, 100b; SARS-CoV-2 (+) | Antiviral and
hydroxychloroquine; Mental status gradually improved without
neurological deficits | (17,19) |
| 9 | Al-olama
(2020) | M | 36 | + | Drowsiness and mild
confusion; GCS score 13 | ICH, SAH in frontal
and temporal lobes; bilateral supratentorial leptomeningeal
increased enhancement | Fluid from the
chronic subdural hematoma tested SARS-CoV-2 (+) | Not mentioned in
therapy; Neurologically stable | (18) |
| 10 | Hayashi (2020) | M | 75 | + | Tremor in hand;
walking instability; urinary incontinence; mild ataxic gait | Abnormal
hyperintensity in the SCC on DWI | ND | Corticosteroid
pulse, antiviral and; anti-bacterial therapy Neurologically
recovered but died at last | (20) |
| The present
case | Pu (2020) | F | 90 | + | Consciousness
disturbance; GCS score 6; neck stiffness, meningeal irritation
signs | (-) | Opening pressure is
60 mmH2O; protein, 660b; 10 mononuclear and 15
polymorphonuclear cells; SARS-CoV-2 (-) | Died at last and
without neurological recovery | - |
A total of nine cases received lumbar puncture
examination, with increased intracranial pressure in only 3 cases
(P1, P6 and P7). The CSF protein level was mildly increased in most
cases. CSF cytology indicated a slightly increased proportion of
white blood cells (WBC) and lymphocytes. Only 3 cases presented
with positive SARS-CoV-2 in the CSF (P6, P8 and P9). The patients
were negative for other CSF infections and autoimmune antibodies.
P2 exhibited increased cytokines (IL-6, IL-8 and TNF-α) in the CSF
and exacerbation clinically. For treatment, corticosteroid therapy
and mannitol infusion along with antiviral therapy were
administered in some of these 10 cases. Good prognosis and
neurological recovery were observed in 6 patients (P1-3 and P7-9),
while 2 patients had mild remaining symptoms (P4 and P5). However,
elderly patients tended to have poor outcomes and the oldest
patient died (P10).
Discussion
The present study reported on a patient who
developed meningoencephalitis after being diagnosed with SARS-CoV-2
infection. The patient gradually recovered from the respiratory
symptoms prior to the onset of neurological symptoms. However, the
patient suddenly became unconscious and developed neck stiffness
and positive cortico-spinal tract signs in all limbs. CSF analysis
suggested infection of the central nervous system (CNS) with
elevated WBC. A screen for usual pathogens (bacterial, fungal and
viral), including SARS-CoV-2, was negative. Based on recently
reported diagnostic criteria (25),
a possible diagnosis of COVID-19-associated encephalitis was made.
Furthermore, published cases of COVID-19-related encephalitis
(until 2020 July) were reviewed and their clinical characteristics
were summarized. Neurological features mostly started from the time
of respiratory symptom onset to 30 days thereafter. The
neurological manifestations included irritability, confusion,
reduced consciousness and seizures.
As other coronaviruses (7), SARS-CoV-2 is neurotropic. SARS-CoV-2
has been postulated to enter the CNS via hematogenous spread from
the systemic circulation to the cerebral circulation, and via
dissemination through the cribriform plate and olfactory bulb
(26). Furthermore, SARS-CoV-2 was
reported to enter the CNS via binding to angiotensin converting
enzyme 2 expressed in the capillary endothelium of the blood-brain
barrier (27). SARS-CoV-2 RNA was
detected in the CSF of 3 patients with COVID-19 reported in the
literature review, of these 3 patients, 2 patients presented with
abnormal neuroimaging findings. SARS-CoV-2 may also reach the CNS
via trans-synaptic propagation through the nasal cavity. This
neuronal approach is consistent with the clinical observation that
certain patients with COVID-19 develop anosmia (28). However, there was no evidence of
anosmia/dysgeusia in the cases reviewed.
Certain cases included in the present literature
review were in a hyperinflammatory state secondary to SARS-CoV-2
infection, with massive release of cytokines and chemokines.
Patient 2 in Table II and another
2 previously reported cases (29)
had obvious elevation of CSF cytokines accompanied by exacerbation
of neurological symptoms. Acute necrotizing encephalopathy was also
recently reported in patients with COVID-19(30). Thus, a hyperinflammatory state
secondary to infection may have an important role in CNS injury by
SARS-CoV-2.
The present analysis indicated that SARS-CoV-2
infected meningitis/encephalitis had a relatively non-fatal process
with complete clinical recovery in the majority of cases.
Specifically, almost 60% of patients exhibited neurological
recovery, but with certain symptoms remaining, including headache.
This suggests that SARS-CoV-2 may induce a viral encephalitis or
aseptic meningitis. With viral clearance and use of
corticosteroids, the CSF pressure was gradually reduced and the
neurological manifestations gradually improved. Immunoinhibition
therapy is effective for reducing CSF cytokines and associated
neurological manifestations. The present analysis suggested a role
for cytokine-mediated neuroinflammation in these patients. Of note,
senior patients (>60 years old), those positive for SARS-CoV-2
RNA in the CSF and those with brain MRI abnormalities tended to
have poor outcomes.
In conclusion, COVID-19 infection may be associated
with meningitis/encephalitis. The initial symptoms vary, although
changes in consciousness, seizures and meningeal irritation signs
were most frequent. Furthermore, CSF protein and white cell levels
were typically elevated, with positive SARS-CoV RNA and elevated
cytokine levels in the CSF in certain patients. Of note, most cases
had favorable outcomes, except for older patients. A limitation of
the present study was the lack of brain MRI in certain patients,
including the present case. However, MRI was difficult to perform
in certain patients due to medical isolation during the COVID-19
pandemic. Another limitation was that inflammatory cytokines in the
CSF were not examined in the present case, which would have been
required to confirm the disease pathogenesis.
Acknowledgements
Not applicable.
Funding
Funding: The present study was supported by the China-Japan
Friendship Hospital funding for Youth (grant no. 2019-1-QN-23 and
2018-2-QN-34).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
PL and XPW contributed to the conception and design
of the study. FP contributed to the acquisition and analyses of
data. PL performed the literature review. YZ, LZ, LS, YW, NL, PH
and TH contributed to analyzing and drafting the manuscript, PL,
YZ, LZ and LS contributed to preparing the figures. PL and XPW
check and confirm the raw data of the study. All authors read and
approved the final version of the manuscript.
Ethics approval and consent to
participate
The study was approved by the ethics committee of
the China-Japan Friendship Hospital (Beijing, China).
Patient consent for publication
The patient's guardians provided informed written
consent for the case report.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Marini JJ and Gattinoni L: Management of
COVID-19 respiratory distress. JAMA. 323:2329–2330. 2020.PubMed/NCBI View Article : Google Scholar
|
|
2
|
The Lancet Neurology. The neurological
impact of COVID-19. Lancet Neurol. 19(471)2020.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Favas TT, Dev P, Chaurasia RN, Chakravarty
K, Mishra R, Joshi D, Mishra VN, Kumar A, Singh VK, Pandey M and
Pathak A: Neurological manifestations of COVID-19: A systematic
review and meta-analysis of proportions. Neurol Sci. 41:3437–3470.
2020.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Harapan BN and Yoo HJ: Neurological
symptoms, manifestations, and complications associated with severe
acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and
coronavirus disease 19 (COVID-19). J Neurol: Jan 21, 2021 (Epub
ahead of print).
|
|
5
|
Lesser IA and Nienhuis CP: The impact of
COVID-19 on physical activity behavior and well-being of Canadians.
Int J Environ Res Public Health. 17(3899)2020.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Nunnari G, Sanfilippo C, Castrogiovanni P,
Imbesi R, Li Volti G, Barbagallo I, Musumeci G and Di Rosa M:
Network perturbation analysis in human bronchial epithelial cells
following SARS-CoV2 infection. Exp Cell Res.
395(112204)2020.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Zhou Z, Kang H, Li S and Zhao X:
Understanding the neurotropic characteristics of SARS-CoV-2: From
neurological manifestations of COVID-19 to potential neurotropic
mechanisms. J Neurol. 267:2179–2184. 2020.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Lechien JR, Barillari MR, Jouffe L and
Saussez S: Anosmia is a key symptom of COVID-19 infection and
should be used as a diagnostic tool. Ear Nose Throat J. 99:577–578.
2020.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Chiu A, Fischbein N, Wintermark M,
Zaharchuk G, Yun PT and Zeineh M: COVID-19-induced anosmia
associated with olfactory bulb atrophy. Neuroradiology. 63:147–148.
2021.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Altundag A, Saatci O, Sanli DET, Duz OA,
Sanli AN, Olmuscelik O, Temirbekov D, Kandemirli SG and Karaaltin
AB: The temporal course of COVID-19 anosmia and relation to other
clinical symptoms. Eur Arch Otorhinolaryngol: Nov 25, 2020 (Epub
ahead of print).
|
|
11
|
Yin R, Feng W, Wang T, Chen G, Wu T, Chen
D, Lv T and Xiang D: Concomitant neurological symptoms observed in
a patient diagnosed with coronavirus disease 2019. J Med Virol.
92:1782–1784. 2020.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Pilotto A, Odolini S, Masciocchi S,
Comelli A, Volonghi I, Gazzina S, Nocivelli S, Pezzini A, Focà E,
Caruso A, et al: Steroid-responsive encephalitis in coronavirus
disease 2019. Ann Neurol. 88:423–427. 2020.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Bernard Valnet R, Pizzarotti B, Anichini
A, Demars Y, Russo E, Schmidhauser M, Cerutti-Sola J, Rossetti AO
and Du Pasquier R: Two patients with acute meningoencephalitis
concomitant with SARS-CoV-2 infection. Eur J Neurol. 27:e43–e44.
2020.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Wong PF, Craik S, Newman P, Makan A,
Srinivasan K, Crawford E, Dev D, Moudgil H and Ahmad N: Lessons of
the month 1: A case of rhombencephalitis as a rare complication of
acute COVID-19 infection. Clin Med (Lond). 20:293–294.
2020.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Moriguchi T, Harii N, Goto J, Harada D,
Sugawara H, Takamino J, Ueno M, Sakata H, Kondo K, Myose N, et al:
A first case of meningitis/encephalitis associated with
SARS-Coronavirus-2. Int J Infect Dis. 94:55–58. 2020.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Ye M, Ren Y and Lv T: Encephalitis as a
clinical manifestation of COVID-19. Brain Behav Immun. 88:945–946.
2020.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Huang YH, Jiang D and Huang JT: SARS-CoV-2
detected in cerebrospinal fluid by PCR in a case of COVID-19
encephalitis. Brain Behav Immun. 87(149)2020.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Al-Olama M, Rashid A and Garozzo D:
COVID-19-associated meningoencephalitis complicated with
intracranial hemorrhage: A case report. Acta Neurochir (Wien).
162:1495–1499. 2020.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Duong L, Xu P and Liu A:
Meningoencephalitis without respiratory failure in a young female
patient with COVID-19 infection in downtown Los Angeles, early
April 2020. Brain Behav Immun. 87(33)2020.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Hayashi M, Sahashi Y, Baba Y, Okura H and
Shimohata T: COVID-19-associated mild encephalitis/encephalopathy
with a reversible splenial lesion. J Neurol Sci.
415(116941)2020.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Lovati C, Osio M and Pantoni L: Diagnosing
herpes simplex-1 encephalitis at the time of COVID-19 pandemic.
Neurol Sci. 41:1361–1364. 2020.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Zayet S, Ben Abdallah Y, Royer PY, Toko L,
Gendrin V and Klopfenstein T: Encephalopathy in patients with
COVID-19: ‘Causality or. coincidence?.’ J Med Virol.
93(1193)2020.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Dogan L, Kaya D, Sarikaya T, Zengin R,
Dincer A, Akinci IO and Afsar N: Plasmapheresis treatment in
COVID-19-related autoimmune meningoencephalitis: Case series. Brain
Behav Immun. 87:155–158. 2020.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Chen CJ, Hsieh LL, Lin SK, Wang CF, Huang
YH, Lin SY and Lu PL: Optimization of the CDC protocol of molecular
diagnosis of COVID-19 for timely diagnosis. Diagnostics (Basel).
10(333)2020.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Ellul MA, Benjamin L, Singh B, Lant S,
Michael BD, Easton A, Kneen R, Defres S, Sejvar J and Solomon T:
Neurological associations of COVID-19. Lancet Neurol. 19:767–783.
2020.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Montalvan V, Lee J, Bueso T, De Toledo J
and Rivas K: Neurological manifestations of COVID-19 and other
coronavirus infections: A systematic review. Clin Neurol Neurosurg.
194(105921)2020.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Khaleeq A, Ali U and Syeda H: Evidence of
the COVID-19 virus targeting the CNS: Tissue distribution,
host-virus interaction, and proposed neurotropic mechanisms. ACS
Chem Neuroscience. 11:995–998. 2020.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Guan WJ, Ni ZY, Hu Y, Liang WH, Ou CQ, He
JX, Liu L, Shan H, Lei CL, Hui DSC, et al: Clinical characteristics
of coronavirus disease 2019 in China. N Engl J Med. 382:1708–1720.
2020.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Bodro M, Compta Y, Llansó L, Esteller D,
Doncel-Moriano A, Mesa A, Rodríguez A, Sarto J, Martínez-Hernandez
E, Vlagea A, et al: Increased CSF levels of IL-1β, IL-6, and ACE in
SARS-CoV-2-associated encephalitis. Neurol Neuroimmunol
Neuroinflamm. 7(e821)2020.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Elkady A and Rabinstein AA: Acute
necrotizing encephalopathy and myocarditis in a young patient with
COVID-19. Neurol Neuroimmunol Neuroinflamm. 7(e801)2020.
|