Introduction
Acute spinal cord injury (ASCI) is a fatal central
nervous system disease, which usually causes paralysis below the
contused spinal cord segment (1).
ACSI not only brings great pain to patients, but also causes a
serious socio-economic burden (2),
with ~23 cases per million occurring every year, globally (3). The underlying pathological mechanisms
of action behind ASCI are tissue edema after injury, which
eventually lead to inflammation and apoptosis (4). However, the treatment of ACSI remains
a major problem for researchers and clinicians, and there is no
effective treatment for patients with ASCI (5). ASCI is divided into direct injury and
secondary injury. Secondary injuries result from inflammation,
altered Ca2+ homeostasis, oxidative stress and apoptosis
(6). Apoptosis is one of the most
important causes of spinal cord dysfunction and can dramatically
impact the recovery for patients with ASCI (7). It has been reported that the
expression levels of apoptosis relevant factors, including
caspase-3, Bax and Bcl-2, are altered, accompanied with increasing
neuronal apoptosis, after ASCI (8).
Plantamajoside (PMS;
C29H36O16) belongs to the
phenylpropanoid glycosides family, which is a unique component
identified in Herba plantaginis (9). PMS has numerous beneficial
pharmacological effects. PMS protects advanced glycation
end-induced endothelial cells against inflammatory cellular
dysfunction (10). In addition, PMS
ameliorates lipopolysaccharide (LPS)-induced acute lung injury
through improving pulmonary inflammation (11). PMS also inhibits LPS-induced mucin
5AC expression and inflammation through suppressing the PI3K/Akt
and NF-κB signaling pathways (12).
Moreover, it has been documented that PMS inhibits growth and
metastasis of breast cancer by inhibiting the activity of MMP9 and
MMP2(13). Together, PMS has been
shown to have anti-oxidant, anti-inflammatory, anti-cancer and
anti-proliferative activities (14-16).
However, to the best of our knowledge, there are no studies that
have investigated the effects of PMS on apoptosis in rats after
ASCI. Therefore, the present study aimed to investigate whether PMS
could protect against apoptosis in ASCI rats and to elucidate the
potential anti-apoptosis mechanisms of action that are involved in
the expression of the Bcl-2 and Bax, as well as the caspase-3
signaling pathway.
Materials and methods
Experimental animals
A total of 36 adult male Sprague-Dawley rats
(weight, 200-250 g; age, 9-11 weeks) were purchased from Hubei
Provincial Institute of Science and Technology. Rats were raised in
a suitable environment with 24±3˚C and 12-h light/dark cycle in
separated cages (relative humidity 55-60%). All rats had free
access to food and water and they were allowed to acclimate to the
environment for at least for three days before the experimental
procedure. All of the study protocols were approved by the Ethics
Committee on Animal Experiments of Tongji Medical College, Huazhong
University of Science and Technology.
Rat model of spinal cord injury
Rats were randomly assigned into six groups, namely:
Sham, model, positive, PMS 80 mg/kg, PMS 40 mg/kg and PMS 20 mg/kg
groups. The positive group was treated with methylprednisolone 30
mg/kg as a positive control (17).
The ASCI model of rats was established according to Allen's weight
hit model (18). Drinking was
prohibited until the surgery had been finished. All rats were
anesthetized with 3% chloral hydrate (450 mg/kg) by intraperitoneal
injection and maintained in the prostrate position for surgery. Fur
around the chest and abdomen of these rats was shaved. A 3 cm
incision was performed at the position of the eighth thoracic
vertebrae and subsequently the dura mater was exposed. A 25 g cm
(10 g x 2.5 cm) injury to the spinal cord was set as the injury
gravity, which induces a moderate injury (19). Following induction of the injury,
the wound was sutured. The following standards were used to
evaluate whether a successful rat model was made: i) Spinal cord
ischemia and edema around the wound; ii) flicking of the body and
legs as well as the appearance of the tail sway reflex, and iii)
the above symptoms coupled with sluggish paralysis. To prevent
infection of the wound, liquid ampicillin (8 x 105 U/kg;
Pureone Bio Technology Co., Ltd.) was injected into the back,
exterior muscles once every day, for three days. In order to keep
each cage dry, the padding was changed daily. To establish the
autonomic urinary reflex of the rats, the bladder was massaged
twice per day. Three days after the surgery, all animals were
intraperitoneally injected with pentobarbital (200 mg/kg; Beijing
Huaye Huanyu Chemical Co., Ltd.) for euthanasia prior to further
investigation.
Evaluation of neuronal function
recovery
The neuronal function recovery after injury was
scored in accordance with the 21-point Basso-Beattie-Bresnahan
(BBB) scale, which was scored as 0-21 representing complete
paralysis to normal locomotion, respectively. BBB scores categorize
combinations of rat hindlimb movements; joint movement; weight
support; fore/hindlimb coordination; trunk position and stability;
stepping; paw placement; toe clearance; and tail position,
representing sequential recovery stages that rats attain after ACSI
(20). Rats were allowed to move
randomly and scored over 4 min by two independent observers who
were blinded to the experiments. The hindlimb movement ability was
assessed at 24, 48 and 72 h after surgery. The ability of the
hindlimb joints was firstly assessed with scores between 0-7.
Subsequently the pace and coordination abilities of the hindlimbs
were assessed (0-7 scores) and then the delicate abilities of paws
during movement were assessed (0-7 scores).
Hematoxylin and eosin (H&E)
staining
A total of 3 days after surgery, 0.9% NaCl solution
was obtained to transcardially perfuse the rats, and subsequently
followed by 4% paraformaldehyde (PFA) for 30 min. In order to
post-fix spinal cords, they were dissected out and placed in 4% PFA
for 12 h at 4˚C. The spinal cords were then further embedded in
paraffin at room temperature and 5-µm thick, serial transverse
sections were made. These slices were subsequently stained with
H&E dye for conventional morphological evaluation to evaluate
the relative changes. Samples were stained with hematoxylin for 10
min and with eosin for 2 min, both at room temperature.
TUNEL staining
A TUNEL detection kit (Roche Diagnostics) was used
to assess DNA fragmentation. The spinal cord specimens were
preserved in 4% paraformaldehyde for 12 h at 4˚C and then washed
with PBS, embed in paraffin and cut into 5-µm thick sections. The
dewaxed sections were then incubated with 1:200 proteinase K for 10
min at 37˚C, followed by rinsing with PBS three times. The slides
were then immersed in TUNEL assay reaction mixture and incubated at
37˚C for 1 h. Subsequently, 50 µl DAB substrate was added to the
tissue and the reaction was carried out at room temperature for 10
min. Cell nuclei of apoptotic cells were distinguished by the
presence of dark brown staining. The positive cells were counted in
five arbitrarily selected fields in each slide (magnification,
x400) using an optic microscope. Cell apoptosis (%) was calculated
using the following formula: (the number of positive cells/the
total cells) x 100%.
Reverse transcription-quantitative PCR
(RT-qPCR)
Total RNA from the spinal cord samples was extracted
using TRIzol® reagent (Invitrogen, Thermo Fisher
Scientific, Inc.) according to the manufacturer's instructions. A
total of 600 ng of RNA was used for cDNA synthesis, at a
temperature of 42˚C for 60 min and 75˚C for 5 min, using a RT First
Strand kit (SA Biosciences LLC). The RT-PCR amplification was
performed using iTaq™ Universal SYBR® Green Supermix
(Bio-Rad Laboratories, Inc.) on an ABI Prism 7500 sequence
detection system (Applied Biosystems; Thermo Fisher Scientific,
Inc.). The thermocycling profiles were as follow: 95˚C for 1 min;
40 cycles of 95˚C for 15 sec, 60˚C for 30 sec and an extension at
72˚C for 30 sec. The primers used in the present study were as
follows: Bcl-2, forward 3'-GCTGGGGATGACTTCTCTCG-5' and reverse
3'-CCACAATCCTCCCCCAGTTC-5'; Bax, forward 3'-CAC
CAAGAAGCTGAGCGAGT-5' and reverse 3'-TAGAAA AGGGCAACCACCCG-5';
caspase-3, forward 3'-CGGACC TGTGGACCTGAAAA-5' and reverse
3'-CGTACAGTT TCAGCATGGCG-5'; caspase-9, forward 3'-TCTTGAGAC
TCGAGGGAGGC-5' and reverse 3'-GGTCGTTCT TCACCTCCACC-5'; poly
(ADP-ribose) polymerase (PARP), forward 3'-AGCCAATGTTCGAGTCGTGT-5'
and reverse 3'-ACAGCATCCTCTTTGGACGG-5'; and GAPDH, forward
3'-TTTCGGTACGGTTAGTAG-5' and reverse 3'-TTTGACCTTGCCTTCCAC-5'. The
relative expression levels of the genes were normalized to GAPDH.
Data were comparatively analyzed using the 2-ΔΔCq method
(21).
Western blot analysis
Proteins from the spinal cord samples were extracted
using RIPA lysis buffer kit (Omega Bio-Tek, Inc.) and the
concentration was detected using a BCA protein assay kit (Bio-Rad
Laboratories, Inc.). Equal amounts of proteins (40 µg per lane)
were loaded into 10% SDS-polyacrylamide gels and transferred onto a
PVDF membrane (EMD Millipore). The membranes were subsequently
blocked with 5% skimmed milk for 1 h at room temperature and
incubated with primary antibodies overnight at 4˚C, including:
Anti-Bcl-2 (sc-56015; 1:1,000), anti-Bax (sc-20067; 1:1,000),
anti-caspase-3 (sc-56053; 1:1,000), anti-caspase-9 (sc-81650;
1:1,000), anti-PARP (sc-56197; 1:1,000) and anti-GAPDH (sc-293335;
1:1,000) antibodies, which were purchased from Santa Cruz
Biotechnology, Inc. The membranes were then incubated with goat
anti-rabbit horseradish peroxidase-conjugated secondary antibodies
(Santa Cruz Biotechnology, Inc.) for 1 h at room temperature.
Enhanced chemiluminescence (Applygen Technologies, Inc.) was used
for visualization. Band intensities were quantified using ImageJ
software (v1.52r; National Institutes of Health). GAPDH was used as
an endogenic control in all samples.
Statistical analysis
All results were confirmed in at least three
independent experiments and analyses were performed using SPSS
v14.0 software (SPPS, Inc.). All quantitative data are presented as
the mean ± SD. The data graded by the scoring system was analyzed
using a Kruskal-Wallis test, with post-hoc Dunn's test. Statistical
comparisons with normally distributed data was made using ANOVAs
followed by Turkey's post hoc tests among the groups. P<0.05 was
considered to indicate a statistically significant difference.
Results
The rat ASCI model assessment
The rat model of ASCI was established using Allen's
weight hit model (18). Rat
functional deficits were evaluated using the BBB score until 28
days after ASCI. It was found that rats in the model group walked
abnormally, with bilateral hind limb paralysis, and the BBB score
of the model group was substantially lower than the sham group
across the observed timeframe (Fig.
1). These results indicated that the rat ASCI model was
established successfully.
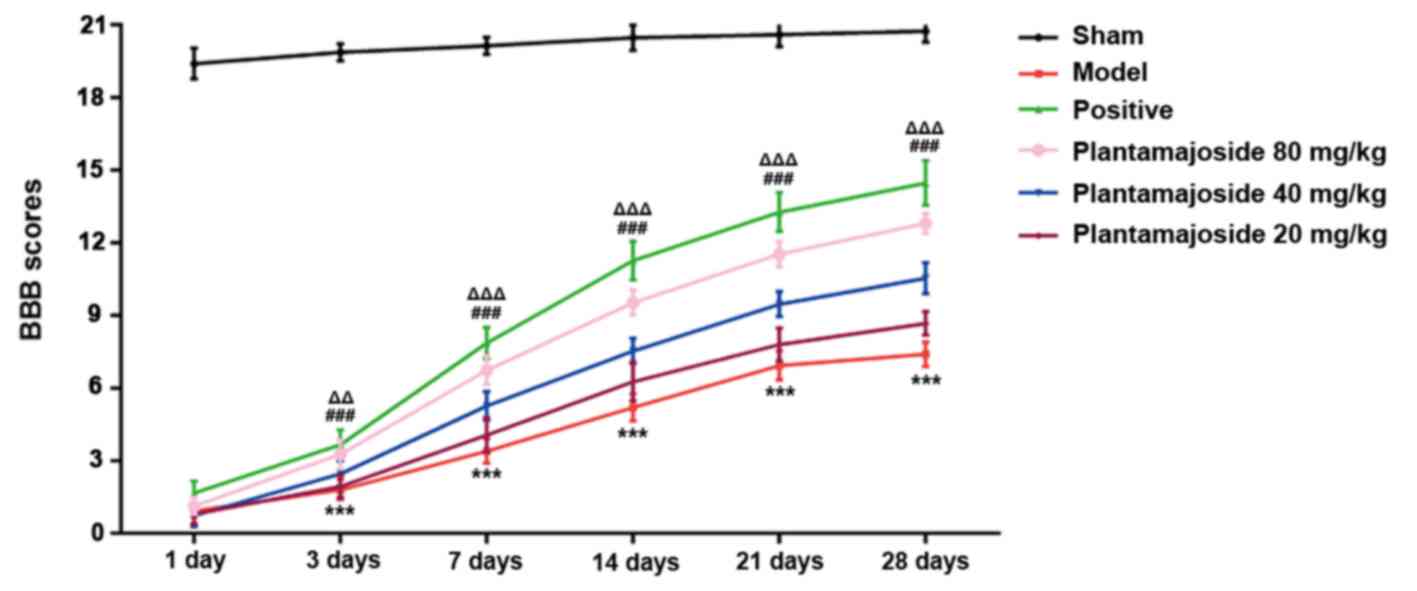 | Figure 1BBB score decreases in the ACSI rat
model group and PMS improves the BBB score of ASCI rats. BBB score
was measured at 1, 3, 7, 14, 21 and 28 days after ASCI among the
six groups (n=6 for each group). ***P<0.001, PMS 80
mg/kg vs. the model group; ###P<0.001, PMS 40 mg/kg
vs. the model group; ΔΔP<0.01,
ΔΔΔP<0.001, PMS 20 mg/kg vs. the model group. ASCI,
acute spinal cord injury; BBB, Basso-Beattie-Bresnahan; PMS,
Plantamajoside. |
PMS improves the behavioral
performance of ASCI rats
According to the experimental result, it was found
that the BBB score in sham group stayed at ~20 points which was the
highest among these groups (Fig.
1). The BBB score of the rest of the groups increased with
time, but the rate of increase varied between them. Overall, the
groups stayed consistently in order, namely, from highest to
lowest: The Sham, positive, PMS 80 mg/kg, PMS 40 mg/kg, PMS 20
mg/kg and lastly, the model group (Fig.
1). This data suggested that PMS can improve the behavioral
performance of ASCI rats in a concentration dependent manner.
PMS reduces the apoptosis of spinal
cord cells
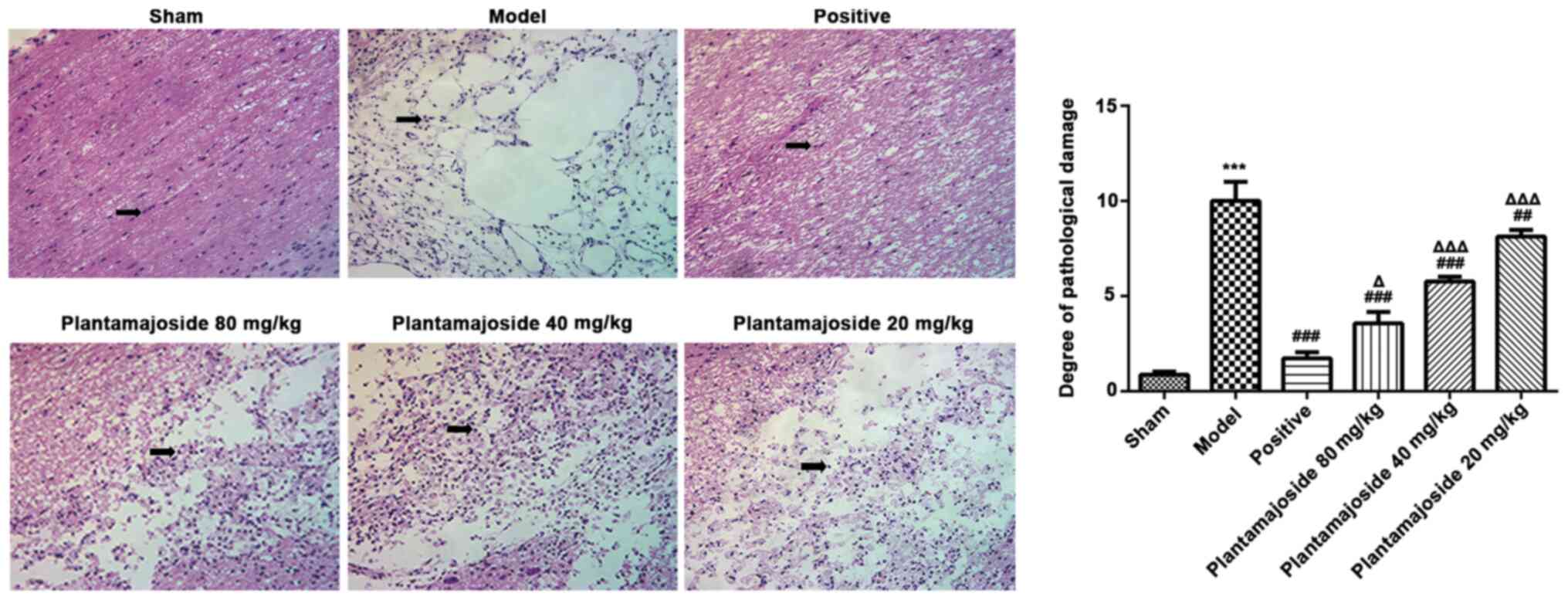
Histopathological alterations were subsequently
investigated in ASCI rats. H&E staining of the spinal cord from
rats at 28 days after ASCI showed that the rats in the model group
had an unclear boundary between white and gray matter. The central
canal displayed an abnormal morphology and some neurons were found
with apoptotic bodies (Fig. 2).
However, PMS improved the morphology in a concentration dependent
manner. The neurons treated with PMS presented a better histologic
characteristics relative to the model group, especially in the PMS
80 mg/kg group (Fig. 2), indicated
that PMS could partially improve the morphology of the spinal cord
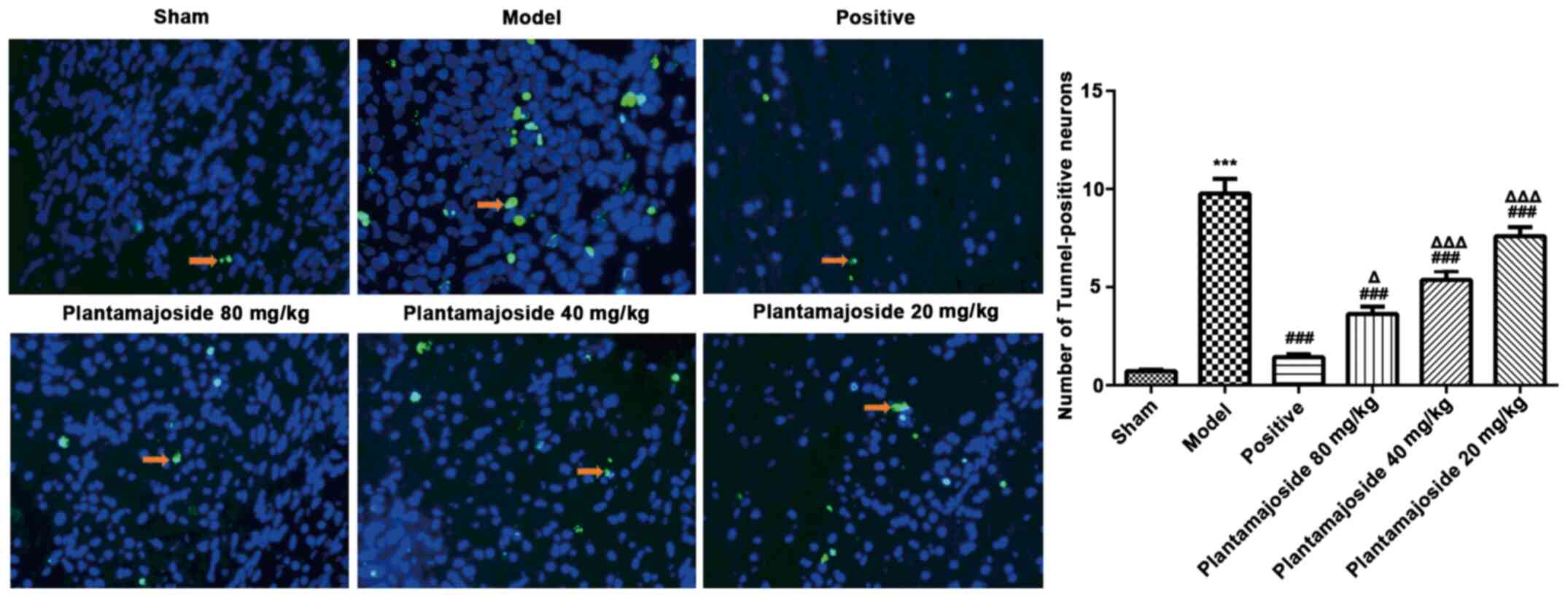
structure. TUNEL staining was also performed which showed the
extent of apoptosis induced neuronal damage. The number of
TUNEL-positive cells notable increased in the model group, compared
with the sham group (Fig. 3).
Moreover, the number of apoptotic cells in the PMS treated groups
had significantly decreased compared with the model group, with
further decreases in a concentration dependent manner (Fig. 3). These results indicated that PMS
may inhibit apoptosis and protect the spinal cord cells after
ASCI.
The mechanisms of action behind the
inhibition of apoptosis by PMS
To further confirm that PMS can inhibit apoptosis,
the cells lysates of the spinal cords of rats at 28 days after ASCI
were assessed by RT-qPCR and western blot assays, to measure the
mRNA and protein expression levels of apoptosis related proteins,
including caspase-3, caspase-9, PARP, Bax and Bcl-2 (Fig. 4). The RT-qPCR and western blotting
data indicated that PMS significantly downregulated the expression
levels of caspase-3, caspase-9, PARP and Bax compared with the
model group after ASCI. In addition, Bcl-2 levels were
significantly upregulated in the PMS treated rat ASCI models at
both the mRNA and protein level (Fig.
4). These results suggested that PMS plays a protective role
against apoptosis through modulating the expression levels of
apoptotic factors.
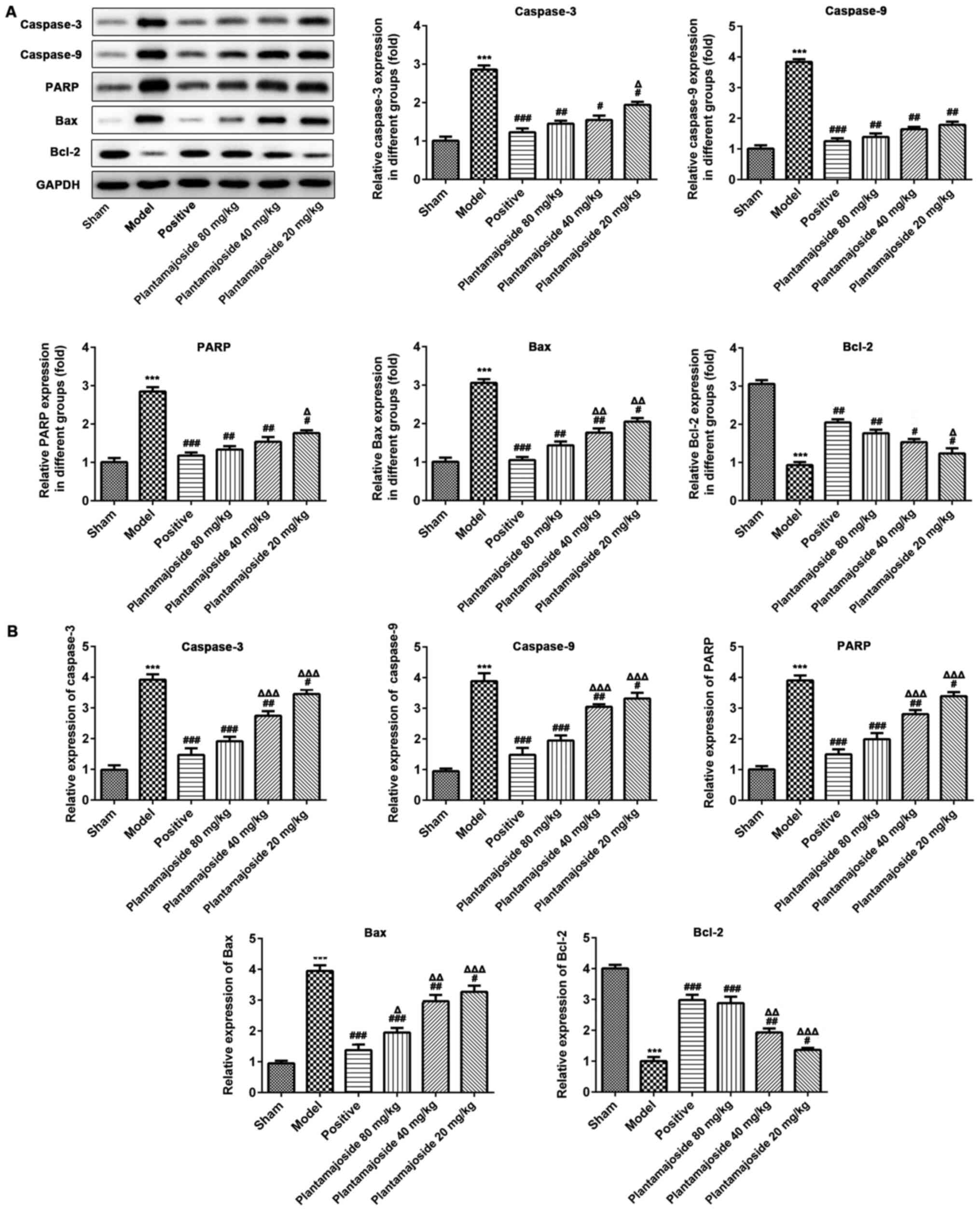 | Figure 4The protein and mRNA expression
levels of apoptosis relevant genes in the various treatment groups
in the spinal cord, 28 days after acute spinal cord injury. (A)
Western blot assays were performed to identify the protein
expression levels of caspase-3, caspase-9, PARP, Bax and Bcl-2. (B)
The mRNA was extracted from the spinal cord samples of each group
and the mRNA expression levels of caspase-3, caspase-9, PARP, Bax
and Bcl-2 were assessed. Data are represented as the mean ± SEM
from three independent experiments. n=3. ***P<0.001
vs. the sham group; #P<0.05, ##P<0.01,
###P<0.001 vs. the model group;
ΔP<0.05, ΔΔP<0.01,
ΔΔΔP<0.001 vs. the positive group. PARP, poly
(ADP-ribose) polymerase. |
Discussion
Damage from ASCI is mainly caused by the direct
injury itself and subsequent secondary injury (22). The secondary injury results in a
pathological change in the normal tissue around the injured tissue
(23). Secondary injury is
accompanied by a series of changes at the molecular and cellular
levels, including an inflammatory reaction, oxidative stress,
internal flow of calcium ions and apoptosis, in which apoptosis is
an important mechanism of action behind the damage observed from
the secondary injury of ASCIs (24). Caspase-3, Bcl-2 and Bax are involved
in apoptosis after spinal cord injury. A number of studies have
suggested that the secondary injury is the key cause of dysfunction
in the central nervous system (25,26).
Therefore, inhibition of neuronal apoptosis provides an opportunity
for a therapeutic strategy to improve spinal cord function after
ASCI (27-29).
Since its discovery, PMS has been reported to
possess broad pharmacological effects, which may exert beneficial
functions for numerous therapies (14,30).
Studies have indicated that PMS can regulate a variety of
conditions, such as renal damage and breast cancer (13,31).
PMS also possesses anti-oxidant, antibiotic and anti-inflammatory
activities which has been found in a number of previous reports
(32,33). However, the effect of PMS on
apoptosis and its underlying mechanism of action remains
unclear.
Apoptosis is a genetically programmed process
resulting in cell death (34). It
occurs during embryonic development, tissue reconstruction, immune
regulation, and tumor degeneration. Apoptosis is critical for the
development of multicellular organisms, but abnormal apoptosis can
cause a variety of diseases (35).
In damaged spinal cords, apoptosis causes neuronal losses (36). Apoptosis can be divided into two
types of pathways, the external and internal pathways. The external
pathways are induced by death receptors, such as Fas receptors
(37). The internal pathways are
triggered by various factors, such as DNA damage and endoplasmic
reticulum stress (38). The
internal pathway of the cell is regulated by the Bcl-2 protein
family. The main anti-apoptotic members, Bcl-2 and Bcl-xl, play a
key role in the mitochondrial outer membrane to maintain membrane
integrity (39). Bcl-2, is an
anti-apoptotic protein which can prevent apoptosis through
regulating various signaling pathways after spinal cord injury
(40). Bax protein is found in the
cytoplasm of mitochondria. ASCI stimulation can activate Bax
protein and alter the permeability of the mitochondrial membrane,
which in turn can induce neuronal apoptosis (41).
The expression of Bcl-2 and Bax directly affects the
apoptosis of spinal cord neurons (8). Bax disrupts the integrity of the
mitochondrial membrane, causing apoptosis factors such as
cytochrome c to leak into the cytoplasm (42). The cytochrome c complex can promote
the formation and activation of caspase-9, then the activated
caspase-9 is cut off and activates the downstream protease cascade
such as caspase-3(8). Caspase-3 is
a cysteine protease, which can destroy a variety of proteases,
decompose DNA, prevent the normal function of the calcium pump,
cause calcium overload and eventually lead to apoptosis (43). It is considered to be the most
important protease in the process of apoptosis (44). Studies have shown that caspase-3
plays an important role in ASCI and that caspase-3 positive cells
are present in ischemic and traumatic spinal cord injury models
(45,46). Therefore, caspase-3 expression
levels may reflect the degree of apoptosis in spinal cord injuries.
After caspase-3 activation, the ADP ribose polymerase, PARP-1, can
also play a role in apoptosis, resulting in cell death (47,48).
In conclusion, the present study demonstrated that
PMS promotes the recovery of neurological function and protects the
tissue structure of the spinal cord after ASCI. The underlying
mechanisms of action may be due to PMS interrupting apoptosis, thus
enhancing the resistance to further damage of the spinal cord and
rescuing the locomotive activity. Furthermore, it was revealed that
PMS can efficiently inhibit apoptosis by regulating the expression
levels of apoptotic factors, including caspase-3, caspase-9, PARP,
Bax and Bcl-2.
Acknowledgements
The authors would like to thank Professor Xiaofei
Jian of the Department of Orthopedics, The Central Hospital of
Wuhan, Tongji Medical College, Huazhong University of Science and
Technology.
Funding
Funding: No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
HH wrote the manuscript, analyzed the data and
revised the manuscript. HH and XJ performed the literature search,
designed the study and performed experiments. All authors read and
approved the final manuscript.
Ethics approval and consent to
participate
All of the study protocols were approved by the
Ethics Committee on Animal Experiments of Tongji Medical College,
Huazhong University of Science and Technology.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Guan B, Chen R, Zhong M, Liu N and Chen Q:
Protective effect of Oxymatrine against acute spinal cord injury in
rats via modulating oxidative stress, inflammation and apoptosis.
Metab Brain Dis. 35:149–157. 2020.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Witiw CD and Fehlings MG: Acute spinal
cord injury. J Spinal Disord Tech. 28:202–210. 2015.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Hu W, Wang H, Liu Z, Liu Y, Wang R, Luo X
and Huang Y: Neuroprotective effects of lycopene in spinal cord
injury in rats via antioxidative and anti-apoptotic pathway.
Neurosci Lett. 642:107–112. 2017.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Chamberlain JD, Meier S, Mader L, von
Groote PM and Brinkhof MWG: Mortality and longevity after a spinal
cord injury: Systematic review and meta-analysis.
Neuroepidemiology. 44:182–198. 2015.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Rouanet C, Reges D, Rocha E, Gagliardi V
and Silva GS: Traumatic spinal cord injury: Current concepts and
treatment update. Arq Neuropsiquiatr. 75:387–393. 2017.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Zhong ZX, Feng SS, Chen SZ, Chen ZM and
Chen XW: Inhibition of MSK1 promotes inflammation and apoptosis and
inhibits functional recovery after spinal cord injury. J Mol
Neurosci. 68:191–203. 2019.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Varma AK, Das A, Wallace G IV, Barry J,
Vertegel AA, Ray SK and Banik NL: Spinal cord injury: A review of
current therapy, future treatments, and basic science frontiers.
Neurochem Res. 38:895–905. 2013.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Luo Y, Fu C, Wang Z, Zhang Z, Wang H and
Liu Y: Mangiferin attenuates contusive spinal cord injury in rats
through the regulation of oxidative stress, inflammation and the
Bcl-2 and Bax pathway. Mol Med Rep. 12:7132–7138. 2015.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Li Y, Gan L, Li GQ, Deng L, Zhang X and
Deng Y: Pharmacokinetics of plantamajoside and acteoside from
Plantago asiatica in rats by liquid chromatography-mass
spectrometry. J Pharm Biomed Anal. 89:251–256. 2014.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Son WR, Nam MH, Hong CO, Kim Y and Lee KW:
Plantamajoside from Plantago asiatica modulates human
umbilical vein endothelial cell dysfunction by
glyceraldehyde-induced AGEs via MAPK/NF-κB. BMC Complement Altern
Med. 17(66)2017.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Wu H, Zhao G, Jiang K, Chen X, Zhu Z, Qiu
C, Li C and Deng G: Plantamajoside ameliorates
lipopolysaccharide-induced acute lung injury via suppressing NF-κB
and MAPK activation. Int Immunopharmacol. 35:315–322.
2016.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Ma C and Ma W: Plantamajoside inhibits
lipopolysaccharide-induced MUC5AC expression and inflammation
through suppressing the PI3K/Akt and NF-κB signaling pathways in
human airway epithelial cells. Inflammation. 41:795–802.
2018.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Pei S, Yang X, Wang H, Zhang H, Zhou B,
Zhang D and Lin D: Plantamajoside, a potential anti-tumor herbal
medicine inhibits breast cancer growth and pulmonary metastasis by
decreasing the activity of matrix metalloproteinase-9 and -2. BMC
Cancer. 15(965)2015.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Liu F, Huang X, He JJ, Song C, Peng L,
Chen T and Wu BL: Plantamajoside attenuates inflammatory response
in LPS-stimulated human gingival fibroblasts by inhibiting PI3K/AKT
signaling pathway. Microb Pathog. 127:208–211. 2019.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Yin WZ, Xu J, Li C, Dai XK, Wu T and Wen
JF: Plantamajoside inhibits the proliferation and
epithelial-to-mesenchymal transition in hepatocellular carcinoma
cells via modulating hypoxia-inducible factor-1α-dependent gene
expression. Cell Biol Int. 44:1616–1627. 2020.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Wang Y and Yan D: Plantamajoside exerts
antifibrosis effects in the liver by inhibiting hepatic stellate
cell activation. Exp Ther Med. 18:2421–2428. 2019.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Li Y, Gu R, Zhu Q and Liu J: Changes of
spinal edema and expression of aquaporin 4 in
methylprednisolone-treated rats with spinal cord injury. Ann Clin
Lab Sci. 48:453–459. 2018.PubMed/NCBI
|
|
18
|
Wang B, Dai W, Shi L, Teng H, Li X, Wang J
and Geng W: Neuroprotection by Paeoniflorin against Nuclear Factor
Kappa B-Induced Neuroinflammation on Spinal Cord Injury. BioMed Res
Int. 2018(9865403)2018.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Yune TY, Lee JY, Jung GY, Kim SJ, Jiang
MH, Kim YC, Oh YJ, Markelonis GJ and Oh TH: Minocycline alleviates
death of oligodendrocytes by inhibiting pro-nerve growth factor
production in microglia after spinal cord injury. J Neurosci.
27:7751–7761. 2007.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Basso DM, Beattie MS and Bresnahan JC: A
sensitive and reliable locomotor rating scale for open field
testing in rats. J Neurotrauma. 12:1–21. 1995.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) Method. Methods. 25:402–408.
2001.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Oyinbo CA: Secondary injury mechanisms in
traumatic spinal cord injury: A nugget of this multiply cascade.
Acta Neurobiol Exp (Wars). 71:281–299. 2011.PubMed/NCBI
|
|
23
|
Tator CH and Benzel EC (eds): Contemporary
Management of Spinal Cord Injury: From Impact to Rehabilitation.
Thieme for the American Association of Neurological Surgeons,
Illinois, 2000.
|
|
24
|
Dumont RJ, Okonkwo DO, Verma S, Hurlbert
RJ, Boulos PT, Ellegala DB and Dumont AS: Acute spinal cord injury,
part I: Pathophysiologic mechanisms. Clin Neuropharmacol.
24:254–264. 2001.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Jin X and Yamashita T: Microglia in
central nervous system repair after injury. J Biochem. 159:491–496.
2016.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Hall ED, Wang JA, Bosken JM and Singh IN:
Lipid peroxidation in brain or spinal cord mitochondria after
injury. J Bioenerg Biomembr. 48:169–174. 2016.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Li G, Shen F, Fan Z, Wang Y, Kong X, Yu D,
Zhi X, Lv G and Cao Y: Dynasore improves motor function recovery
via inhibition of neuronal apoptosis and astrocytic proliferation
after spinal cord injury in rats. Mol Neurobiol. 54:7471–7482.
2017.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Schwartz M and Hauben E: T cell-based
therapeutic vaccination for spinal cord injury. Prog Brain Res.
137:401–406. 2002.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Young W: Spinal cord contusion models.
Prog Brain Res. 137:231–255. 2002.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Han AR, Nam MH and Lee KW: Plantamajoside
inhibits UVB and advanced glycation end products-induced MMP-1
expression by suppressing the MAPK and NF-κB pathways in HaCaT
cells. Photochem Photobiol. 92:708–719. 2016.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Jung HY, Seo DW, Hong CO, Kim JY, Yang SY
and Lee KW: Nephroprotection of plantamajoside in rats treated with
cadmium. Environ Toxicol Pharmacol. 39:125–136. 2015.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Ravn HW, Mondolot L, Kelly MT and Lykke
AM: Plantamajoside-A current review. Phytochem Lett. 12:42–53.
2015.
|
|
33
|
Xiao D, Yang R, Gong L, Zhang Y, Xie Y and
Ni S: Plantamajoside inhibits high glucose-induced oxidative
stress, inflammation, and extracellular matrix accumulation in rat
glomerular mesangial cells through the inactivation of Akt/NF-κB
pathway. J Recept Signal Transduct Res: doi.org/10.1080/10799893.2020.1784939.
|
|
34
|
Wang C, Zhang L, Ndong JC, Hettinghouse A,
Sun G, Chen C, Zhang C, Liu R and Liu CJ: Progranulin deficiency
exacerbates spinal cord injury by promoting neuroinflammation and
cell apoptosis in mice. J Neuroinflammation. 16(238)2019.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Thompson CB: Apoptosis in the pathogenesis
and treatment of disease. Science. 267:1456–1462. 1995.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Lu X, Xue P, Fu L, Zhang J, Jiang J, Guo
X, Bao G, Xu G, Sun Y, Chen J, et al: HAX1 is associated with
neuronal apoptosis and astrocyte proliferation after spinal cord
injury. Tissue Cell. 54:1–9. 2018.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Zhang J, Cui Z, Shen A, Li W, Xu G, Bao G,
Sun Y, Wang L, Gu H, Zhou Y, et al: Upregulation of myelin and
lymphocyte protein (MAL) after traumatic spinal cord injury in
rats. J Mol Histol. 44:125–134. 2013.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Timmins JM, Ozcan L, Seimon TA, Li G,
Malagelada C, Backs J, Backs T, Bassel-Duby R, Olson EN, Anderson
ME, et al: Calcium/calmodulin-dependent protein kinase II links ER
stress with Fas and mitochondrial apoptosis pathways. J Clin
Invest. 119:2925–2941. 2009.PubMed/NCBI View
Article : Google Scholar
|
|
39
|
Lan WB, Lin JH, Chen XW, Wu CY, Zhong GX,
Zhang LQ, Lin WP, Liu WN, Li X and Lin JL: Overexpressing
neuroglobin improves functional recovery by inhibiting neuronal
apoptosis after spinal cord injury. Brain Res. 1562:100–108.
2014.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Edlich F: BCL-2 proteins and apoptosis:
Recent insights and unknowns. Biochem Biophys Res Commun.
500:26–34. 2018.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Tang P, Hou H and Zhang L, Lan X, Mao Z,
Liu D, He C, Du H and Zhang L: Autophagy reduces neuronal damage
and promotes locomotor recovery via inhibition of apoptosis after
spinal cord injury in rats. Mol Neurobiol. 49:276–287.
2014.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Hou Q, Cymbalyuk E, Hsu SC, Xu M and Hsu
YT: Apoptosis modulatory activities of transiently expressed Bcl-2:
Roles in cytochrome C release and Bax regulation. Apoptosis.
8:617–629. 2003.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Ying X, Tu W, Li S, Wu Q, Chen X, Zhou Y,
Hu J, Yang G and Jiang S: Hyperbaric oxygen therapy reduces
apoptosis and dendritic/synaptic degeneration via the BDNF/TrkB
signaling pathways in SCI rats. Life Sci. 229:187–199.
2019.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Mueller THJ, Kienle K, Beham A, Geissler
EK, Jauch KW and Rentsch M: Caspase 3 inhibition improves survival
and reduces early graft injury after ischemia and reperfusion in
rat liver transplantation. Transplantation. 78:1267–1273.
2004.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Yuan B, Pan S and Zhang WW: Effects of
gangliosides on expressions of caspase-3 and NGF in rats with acute
spinal cord injury. Eur Rev Med Pharmacol Sci. 21:5843–5849.
2017.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Yuan B, Pan S and Zhang WW: Effects of
gangliosides on expressions of caspase-3 and NGF in rats with acute
spinal cord injury. Eur Rev Med Pharmacol Sci. 21:5843–5849.
2017.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Yamasaki K, Setoguchi T, Takenouchi T,
Yone K and Komiya S: Stem cell factor prevents neuronal cell
apoptosis after acute spinal cord injury. Spine. 34:323–327.
2009.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Decker P and Muller S: Modulating poly
(ADP-ribose) polymerase activity: Potential for the prevention and
therapy of pathogenic situations involving DNA damage and oxidative
stress. Curr Pharm Biotechnol. 3:275–283. 2002.PubMed/NCBI View Article : Google Scholar
|