Introduction
Pancreatic cancer (PC), a lethal tumor type
occurring worldwide, is associated with a poor patient prognosis
and a high morbidity rate among elderly patients, with an average
age of 71 years at diagnosis (1,2).
Currently, resection is the most effective treatment to prolong the
survival time of patients with PC (3). However, early-stage diagnosis has not
substantially improved in the past decades (4) and ~80% of patients with PC miss the
optimal time for surgical resection (5). Therefore, it is important to identify
novel biomarkers for early-stage diagnosis of PC.
Long non-coding RNAs (lncRNAs), >200 nucleotides
in length, are a type of transcript that lack translation capacity
and can affect target genes at the transcriptional or
post-transcriptional stages (6). It
has been shown that numerous lncRNAs are involved in PC
progression. For example, lncRNA differentiation antagonizing
non-protein coding RNA (DANCR) promotes cell proliferation and
invasion in PC by sponging microRNA (miRNA/miR)-135a (7). In addition, other lncRNAs, such as
CCDC26 lncRNA (8), Pvt1 oncogene
(PVT1) (9), urothelial cancer
associated 1 (UCA1) (10) and ZEB2
antisense RNA 1 (ZEB2-AS1) (11)
show similar effects in PC. Small nucleolar RNA host gene 7
(SNHG7), located on chromosome 9q34.3 with 984 bp, has been
identified to be aberrantly expressed in several types of tumor,
including lung cancer (12),
gastric cancer (13), glioblastoma
(14) and PC (15). However, to the best of our
knowledge, there have been few studies examining the biological
mechanism of SNHG7 in PC.
miRNAs, ~22 nucleotides in length, are small
non-coding RNAs with no translation ability that constrain gene
expression by inhibiting mRNA translation or mediating mRNA
degradation (16). Previous studies
have shown that several miRNAs, such as miR-135b-5p (17), miR-613(18) and miR-1181(19), are dysregulated and affect PC
progression. It has also been revealed that miR-146b-5p is
implicated in PC progression (20).
Moreover, roundabout homolog 1 (Robo1), located on human chromosome
3p12.3, is related to the processes of tumor progression in PC
(21). Therefore, the aims of the
present study were to investigate the functions and mechanism of
SNHG7 in PC, thus providing a novel therapeutic target for patients
with PC.
Materials and methods
Tissue collection
The study was approved by the Ethics Committee of
Jingzhou Central Hospital (Jingzhou, Hubei, China) and performed
according to the Declaration of Helsinki Principles. The age of the
patients ranged from 20-81 years, including 18 females and 32
males. In total, 50 PC tissue samples, including 20 I-II stage PC
samples and 30 stage III-IV PC samples, were obtained from Jingzhou
Central Hospital between March 2016 and August 2018, as well as 50
corresponding adjacent healthy tissue samples. The staging system
used for PC was the American Joint Committee on Cancer tumor node
metastasis (TNM)system (22). The
collected PC tissue samples were divided into two groups based on
the median value of the expression levels of SNHG7 as determined by
RT-qPCR assays: i) Patients with PC with high SNHG7 expression
(n=25); and ii) Patients with PC with low SNHG7 expression (n=25).
All tissues were frozen at 80˚C until further analysis. Before the
experiments, written informed consent was provided by all patients
with PC.
Cell culture and transfection
In total, four PC cell lines PANC-1, SW1990, BxPC-3
and AsPC-1, as well as the healthy pancreas ductal epithelial cell
line HPDE were purchased from Tong Pai Technology. Cells were
cultured in DMEM [Wokawi (Beijing) Biotechnology Co., Ltd.]
supplemented with 10% FBS (Beijing BioDee Biotechnology Co., Ltd.)
in a 5% CO2 incubator at 37˚C.
Short hairpin RNA (shRNA) targeting SNHG7 (sh-SNHG7)
and its negative control (sh-control), miR-146b-5p mimics
(miR-146b-5p: 5'-UGAGAACUGAAUUCCAUAGGCU-3') and its control
(miR-control: 5'-UUCUCCGAACGUGUCACGUTT-3'), miR-146b-5p inhibitor
(5'-AGCCUAUGGAAUUCAGUUCUCA-3') and its control (inhibitor-control:
5'-CAGUACUUUUGUGUAGUACAA-3') were obtained from Shanghai GenePharma
Co., Ltd. The sequences of SNHG7 (Accession: NR_024543.1) and Robo1
(Accession: GBYX01104758.1) were inserted into pcDNA vectors
(Invitrogen; Thermo Fisher Scientific, Inc.) to construct
overexpression plasmids, referred to as pcDNA-SNHG7 and Robo1,
respectively. SW1990 and AsPC-1 cells were transfected with the
constructed vectors or miRs (final concentration 50 µM) by using
Lipofectamine® 2000 (Invitrogen; Thermo Fisher
Scientific, Inc.) according to the manufacturer's instructions 48 h
after transfection, the cells were used for further assays.
Reverse transcription-quantitative PCR
(RT-qPCR)
RNA from PC tissue samples or cells was extracted
using TRIzol® reagent (Invitrogen; Thermo Fisher
Scientific, Inc.). Specific primers (Takara Biotechnology Co., Ltd)
were used targeting RT SNHG7 and Robo1 with the
PrimeScript™RT Master Mix kit (Takara Biotechnology Co.,
Ltd.). Briefly, the transcription was performed in a 10 µl reaction
mixture, including polyadenylated RNA (100 ng), 5XPrimeScript
Buffer (2 µl), PrimeScript RT Enzyme Mix I (0.5 µl), RT primer
mixture (1 µl) and RNase-free water. The total reaction mixture was
incubated at 50˚C for 15 min and 85˚C for 5 sec.
While RT for miR-146b-5p was conducted using TaqMan
miRNA assays (Applied Biosystems; Thermo Fisher Scientific, Inc.).
qPCR was performed using SYBR Premix Ex Taq II (Takara
Biotechnology Co., Ltd.). The amplification parameters were as
follows: Denaturation at 95˚C for 10 min, followed by 40 cycles of
denaturation at 95˚C for 30 sec, annealing at 60˚C for 30 sec and
extension at 72˚C for 1 min. The relative expression levels of
SNHG7, miR-146b-5p and Robo1 were normalized by GAPDH or small
nuclear RNA U6, and then calculated by the
2-ΔΔCq method (23). The primers were synthesized by
Songon Biotechj Co., Ltd. and are listed in Table I.
 | Table IOligonucleotide sequences used in the
present study. |
Table I
Oligonucleotide sequences used in the
present study.
| Gene | Sequences |
|---|
| SNHG7 | F:
5'-AGGCTGGCTGGAATAAAGGT-3' |
| | R:
5'-TATGAAAAGGGAGGCGTGGT-3' |
|
miR-146b-5p | F:
5'-GATGAGAAGGTATTTCTGCT-3' |
| | R:
5'-GAGAAATTGAAGGTCATAAA-3' |
| Robo1 | F:
5'-GAAACAGCGACAGCAACCT-3' |
| | R:
5'-TGACAAAACGCCCATCCT-3' |
| GAPDH | F:
5'-TGTTCGTCATGGGTGTGAAC-3' |
| | R:
5'-ATGGCATGGACTGTGGTCAT-3' |
| U6 | F:
5'-CTCGCTTCGGCAGCACA-3' |
| | R:
5'-AACGCTTCACGAATTTGCGT-3' |
MTT assay
MTT (Sigma-Aldrich; Thermo Fisher Scientific, Inc.)
was used to assess the viability of SW1990 and AsPC-1 cells. Cells
(6x103 per well) were seeded in 96-well plates and
incubated for 24 h at 37˚C. Following transfection, cells were
cultured for another 0, 24, 48 or 72 h at 37˚C. Subsequently, MTT
(5 mg/ml) was added into each well and incubated at 37˚C for 4 h.
Then, 150 µl DMSO (Sigma-Aldrich; Merck KGaA) was added to
solubilize formazan. The absorbance at 490 nm was measured by a
Multiscan Spectrum (Beijing Putian New Bridge Technology Co.,
Ltd.).
Flow cytometry analysis of cell
apoptosis
An Annexin V-FITC/propidium iodide (PI) apoptosis
detection kit (Beijing Solarbio Science & Technology Co., Ltd.)
was used to evaluate the apoptotic rate. Transfected SW1990 and
AsPC-1 cells (1x105) were re-suspended in binding buffer
(Beijing Solarbio Science & Technology Co., Ltd.), incubated
with 5 µl Annexin V-FITC for 10 min and then with 10 µl PI for 5
min in the dark at room temperature. Cell apoptotic rate was
assessed using flow cytometry (Agilent 2100 Bioanalyzer; Agilent
Technologies, Inc.) and analyzed using CELL Quest 3.0 software (BD
Biosciences).
Transwell assay
Transwell chambers (Corning, Inc.) with inserts of
8-µm pore were used to assess the migratory and invasive abilities
of SW1990 and AsPC-1 cells. For migratory ability, transfected
SW1990 and AsPC-1 cells at a density of 1x105 cells/well
were plated into the upper chamber supplemented with non-FBS DMEM,
while the lower chamber contained DMEM supplemented with 10% FBS.
After 24 h culture, cells were fixed in 4% methanol for 20 min at
room temperature, and stained with 0.1% crystal violet for 30 min
at room temperature. Cells in 10 random fields were analyzed with a
fluorescent microscope (magnification, x100; Olympus Corporation).
For cell invasive ability, the upper chamber was coated with
Matrigel matrix (BD Biosciences) at 4˚C, followed by incubation for
5 h at 37˚C.
Dual-luciferase reporter assay
The interaction between miR-146b-5p and SNHG7 or
Robo1 was predicted by starBase v2.0 (http://starbase.sysu.edu.cn/) and TargetScan
(http://www.targetscan.org/vert_72/)
online databases. The wild-type (WT) and mutant (MUT) fragments of
SNHG7 were amplified and then inserted into pGL3 vector (Promega
Corporation) to construct the luciferase reporter, referred to as
SNHG7 WT and SNHG7 MUT, respectively. The luciferase reporter (0.1
µg) and miR-146b-5p mimics (40 nM) or miR-control (40 nM) were
co-transfected into SW1990 and AsPC-1 cells using
Lipofectamine® 2000 (Invitrogen; Thermo Fisher
Scientific, Inc.). The construction of the luciferase reporter
Robo1 3'untranslated region (UTR) WT (GUUCUCA) or Robo1 3'UTR MUT
(ACCAAUG) were similar to that of SNHG7 WT (CAGTTCTC) or SNHG7 MUT
(ATCAAGCA) reporter. The luciferase activity was assessed using a
dual-luciferase assay kit (Beijing Solarbio Science &
Technology Co., Ltd.). Renilla luciferase activities were
used as the internal control for the normalization of firefly
luciferase activity.
RNA immunoprecipitation (RIP)
assay
Following lysis of the transfected SW1990 and AsPC-1
cells, the lysate samples were incubated with magnetic beads
labelled with anti-argonaute-2 (Ago2; BIOSS) or negative control
anti-IgG antibody (BIOSS) for 4 h at 4˚C, and a 10 µl aliquot of
RIP mixture was saved as input. Then, DNAse I (20 U; Sigma-Aldrich;
Merck KGaA) for 15 min at 37˚C, and proteinase K (0.5 mg/ml;
Sigma-Aldrich; Merck KGaA) for 15 min at 55˚C, were used to treat
the immunoprecipitates for 20 min. Input functioned as a positive
control. Subsequently, the expression of SNHG7 was detected by
RT-qPCR as aforementioned.
RNA pull-down assay
Biotin (Bio)-labelled miR-146b-5p (Bio-miR-146b-5p;
forward: 5'-ugagaacuc gccgcgggaccg c-3'; reverse:
5'-gcggucccgcggcgaguucuca-3') and its negative control
(Bio-NC; forward: 5'-uucuccgaacguguc acgutt-3'; reverse:
5'-acgugacacguucgg agaatt-3') were obtained from Sangon Biotech
Co., Ltd. SW1990 and AsPC-1 cells (3x106) were
transfected with Bio-miR-146b-5p or Bio-NCata final concentration
of 100 nM using Lipofectamine® 2000 (Invitrogen; Thermo
Fisher Scientific, Inc.). Subsequently, cells were lysed in
specific lysis buffer (Ambion; Thermo Fisher Scientific, Inc.) for
10 min, and the lysate (the RNA-RNA complex) was conjugated with
streptavidin magnetic beads (Dyna beads M-280 Streptavidin;
Invitrogen; Thermo Fisher Scientific, Inc.), followed by incubation
at 4˚C for 3 h. After elution twice with 500 µl pre-cooled lysis
buffer, thrice with low salt buffer and once with high salt buffer
in succession, the bound RNA was purified using Trizol®
reagent (Invitrogen; Thermo Fisher Scientific, Inc.) and then the
expression of SNHG7 was measured by RT-qPCR.
Western blotting
Protein samples were extracted with RIPA lysis
buffer (Beyotime Institute of Biotechnology) and then quantified
using the BCA Protein Assay kit (Beyotime Institute of
Biotechnology). Next, 50 µg of each protein samples were separated
by 10% SDS-PAGE and then transferred onto a PVDF (Absin Bioscience,
Inc.) membrane. Subsequently, the membrane was blocked in 5%
skimmed milk for 2 h at 37˚C and incubated with primary antibodies
against Robo1 (cat. no. ab7279; 1:1,000; Abcam) and GAPDH (cat. no.
ab8227; 1:5,000; Abcam), which acted as an internal reference, at
4˚C overnight. Then, the membrane was incubated with secondary
antibodies (cat. no. ab205178; 1:10,000; Abcam) for a further 2 h
at 37˚C. Band intensity was detected using an eyoECL Plus kit
(Beyotime Institute of Biotechnology) and analyzed with Quantity
One v4.6.2 software (Bio-Rad Laboratories, Inc.).
Mouse xenograft models
The experiment in nude mice was approved by the
Animal Care Committee of Jingzhou Central Hospital and performed in
accordance with the guidelines of National Institutes of Health
(24). A total of 10 male nude mice
(age, 6 weeks, weight 16-22 g; n=5 per group) were purchased from
Shanghai Laboratory Animal Center, followed by maintained under
specific pathogen-free conditions at a temperature of 25˚C and
relative air humidity between 45 and 50%. Subsequently, 100 µl
SW1990 cells (5x106 cells/ml) stably transfected with
sh-SNHG7 were subcutaneously injected into a single side of the
dorsal flank of nude mice. After inoculation, the tumor volumes
were calculated every 5 days in accordance with the following
formula: Volume (mm3) = width2 x length/2. At
30 days after cell injection, mice were euthanized using 2%
methoxyflurane and cervical dislocation. Subsequently, xenograft
tumor samples were excised for weight measurement, followed by
analysis with RT-qPCR and western blot assays.
Statistical analysis
Data analysis was performed using GraphPad Prism 7
(GraphPad Software, Inc.). All experiments were repeated three
times and data are presented as the mean ± SD. χ2 test
was applied to analyze the correlations between the SNHG7
expression and clinicopathological characteristics of PC patients.
The overall survival curve was plotted using Kaplan-Meier method
and assessed with the log-rank test. Pearson correlation analysis
was used to analyze the expression association. Comparison between
two groups was analyzed via Student's t-test, while comparisons
among multiple groups were calculated by one-way ANOVA followed by
Tukey's test. P<0.05 was considered to indicate a statistically
significant difference.
Results
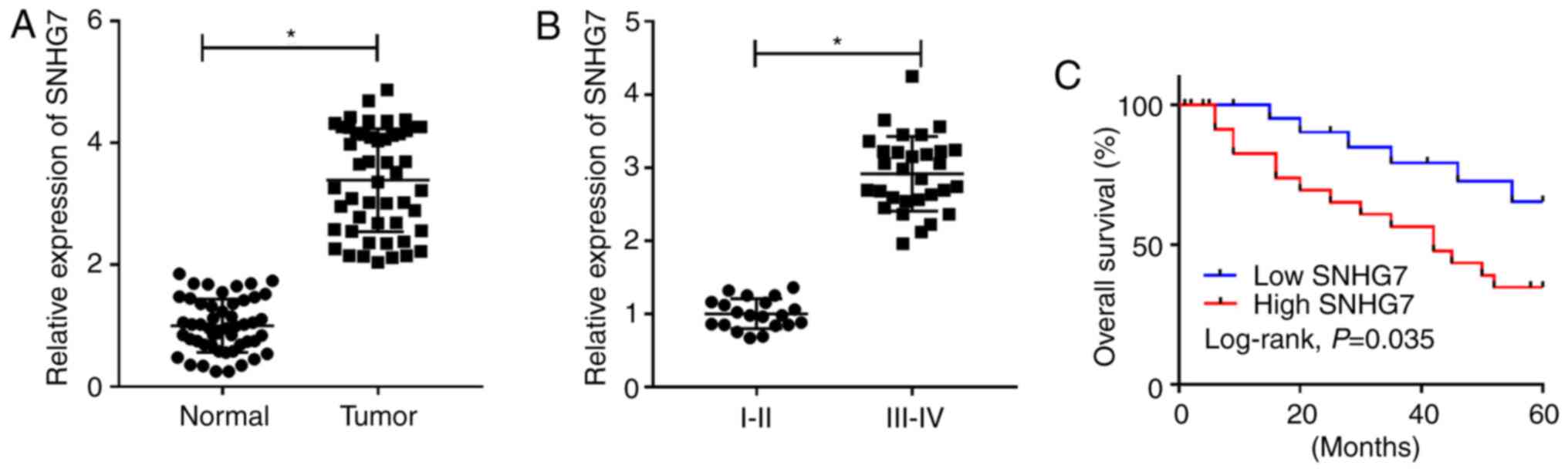
SNHG7 is significantly upregulated in
PC tissues and correlates with the pathological characteristics of
tumor size, distant metastasis, lymph node metastasis and TNM stage
of patients with PC
To assess the role of SNHG7 in PC, the expression of
SNHG7 was detected in 50 PC tissue samples and corresponding
adjacent healthy tissue samples. It was found that the expression
of SNHG7 was significantly enhanced in PC tissues compared with
that noted in the healthy tissue samples (Fig. 1A). Moreover, SNHG7 was more highly
expressed in III-IV stage samples compared with I-II stage samples
(P<0.05; Fig. 1B). Kaplan-Meier
survival analysis results indicated that patients with PC with high
expression of SNHG7 had a low survival rate compared with patients
with PC with low expression of SNHG7 (Fig. 1C). In addition, the χ2
test results identified that high expression of SNHG7 was closely
associated with tumor size (P=0.005), distant metastasis (P=0.011),
lymph node metastasis (P=0.004) and TNM stage (P=0.004), while its
expression was not associated with sex and age (Table II). Therefore, the results
suggested that SNHG7 was significantly elevated in PC tissues and
was associated with the pathological characteristics of tumor size,
distant metastasis, lymph node metastasis and TNM stage in patients
with PC.
 | Table IICorrelations between SNHG7 expression
and clinicopathological characteristics of patients with PC. |
Table II
Correlations between SNHG7 expression
and clinicopathological characteristics of patients with PC.
| | | SNHG7
expression | |
|---|
|
Characteristics | Total (n=50) | Low (n=25) | High (n=25) |
P-valuea |
|---|
| Sex | | | | 0.556 |
|
Male | 32 | 15 | 17 | |
|
Female | 18 | 10 | 8 | |
| Age | | | | 0.564 |
|
≤60 | 20 | 9 | 11 | |
|
>60 | 30 | 16 | 14 | |
| Tumor size, cm | | | | 0.005b |
|
≤2 | 26 | 18 | 8 | |
|
>2 | 24 | 7 | 17 | |
| Distant
metastasis | | | | 0.011b |
|
Absent | 25 | 17 | 8 | |
|
Present | 25 | 8 | 17 | |
| Lymph node
metastasis | | | | 0.004b |
|
Negative | 30 | 20 | 10 | |
|
Positive | 20 | 5 | 15 | |
| TNM stage | | | | 0.004b |
|
I-II | 28 | 19 | 9 | |
|
III-IV | 22 | 6 | 16 | |
SNHG7 knockdown inhibits
proliferation, migration and invasion of PC cells, and induces cell
apoptosis
Based on the above results, the present study
examined the expression of SNHG7 in PC cells. It was demonstrated
that SNHG7 was significantly upregulated in PANC-1, BxPC-3, SW1990
and AsPC-1 cells compared with that noted in the HPDE cells
(Fig. 2A), most notably in the
SW1990 and AsPC-1 cells. Hence, SW1990 and AsPC-1 cells were chosen
for subsequent experiments. Furthermore, the transfection
efficiencies of sh-SNHG7 and pc-SNHG7 were examined and are
presented in Fig. 2B and C. Subsequently, sh-SNHG7 was transfected
into SW1990 and AsPC-1 cells to investigate the function of SNHG7.
MTT assay results identified that cell viability was significantly
decreased in SW1990 and AsPC-1 cells when SNHG7 was knocked down
(Fig. 2D and E). Moreover, it was found that the
transfection of sh-SNHG7 resulted in a significant increase in the
apoptotic rate of SW1990 and AsPC-1 cells (Fig. 2F and G). In addition, the migratory and invasive
abilities were both significantly reduced in SW1990 and AsPC-1
cells compared with the negative controls (Fig. 2H and I). Collectively, it was demonstrated that
silencing SNHG7 inhibited proliferation, migration and invasion,
and facilitated apoptosis in SW1990 and AsPC-1 cells.
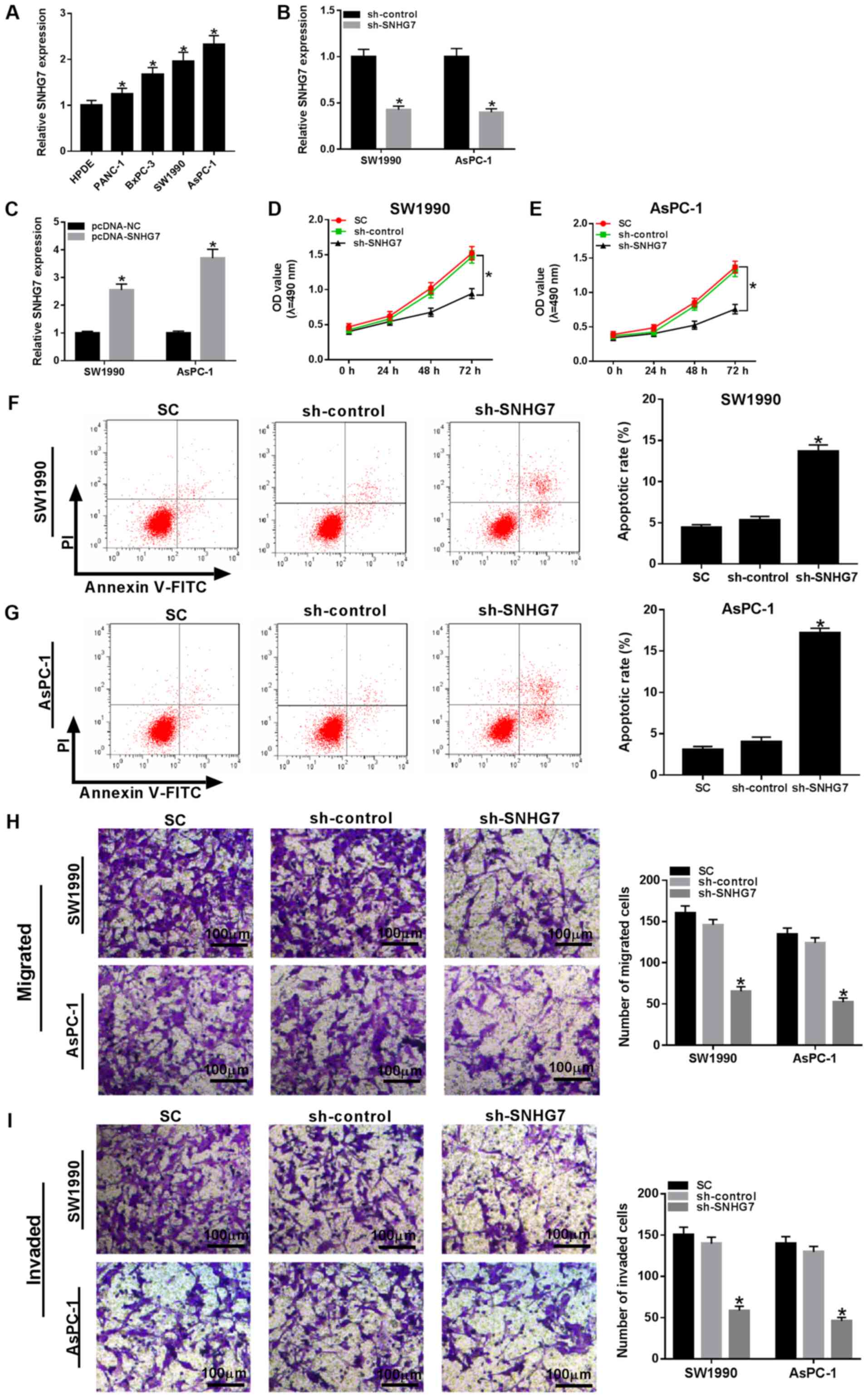 | Figure 2SNHG7 knockdown impairs cell
proliferation, migration and invasion, but induces apoptosis in
SW1990 and AsPC-1 cells. (A) Expression of SNHG7 in human
pancreatic cancer cell lines PANC-1, BxPC-3, SW1990 and AsPC-1, and
human pancreas normal ductal epithelial cell line HPDE was detected
via reverse transcription-quantitative PCR. SNHG7 expression was
detected in SW1990 and AsPC-1 cells transfected with (B) sh-control
and sh-SNHG7, or (C) pcDNA-NC and pcDNA-SNHG7. SW1990 and AsPC-1
cells were transfected with sh-control, sh-SNHG7 or cultured in
normal conditions. Cell viability in (D) SW1990 and (E) AsPC-1
cells was evaluated by MTT assay. Apoptotic rate in (F) SW1990 and
(G) AsPC-1 cells was assessed via flow cytometry. (H) Migratory and
(I) invasive abilities in transfected cells were measured by
Transwell assay. Scale bar, 100 µm. *P<0.05 vs. their
respective normal groups. sh, short hairpin RNA; SC, (standard
conditions); SNHG7, small nucleolar RNA host gene 7; OD, optical
density. |
miR-146b-5p sponges SNHG7
To investigate the biological mechanism of SNHG7 in
PC progression starBase v2.0 (http://starbase.sysu.edu.cn) was used to predict the
putative targets of SNHG7, and it was identified that miR-146b-5p
had complementary sites with SNHG7 (Fig. 3A). Subsequently, dual-luciferase
reporter assay results indicated that the transfection of
miR-146b-5p significantly downregulated the luciferase activity of
the SNHG7 WT reporter in SW1990 and AsPC-1 cells compared with the
miR-control group (Fig. 3B and
C). However, there were no
significant differences in the luciferase activity of the SNHG7 MUT
reporter in any group (Fig. 3B and
C).
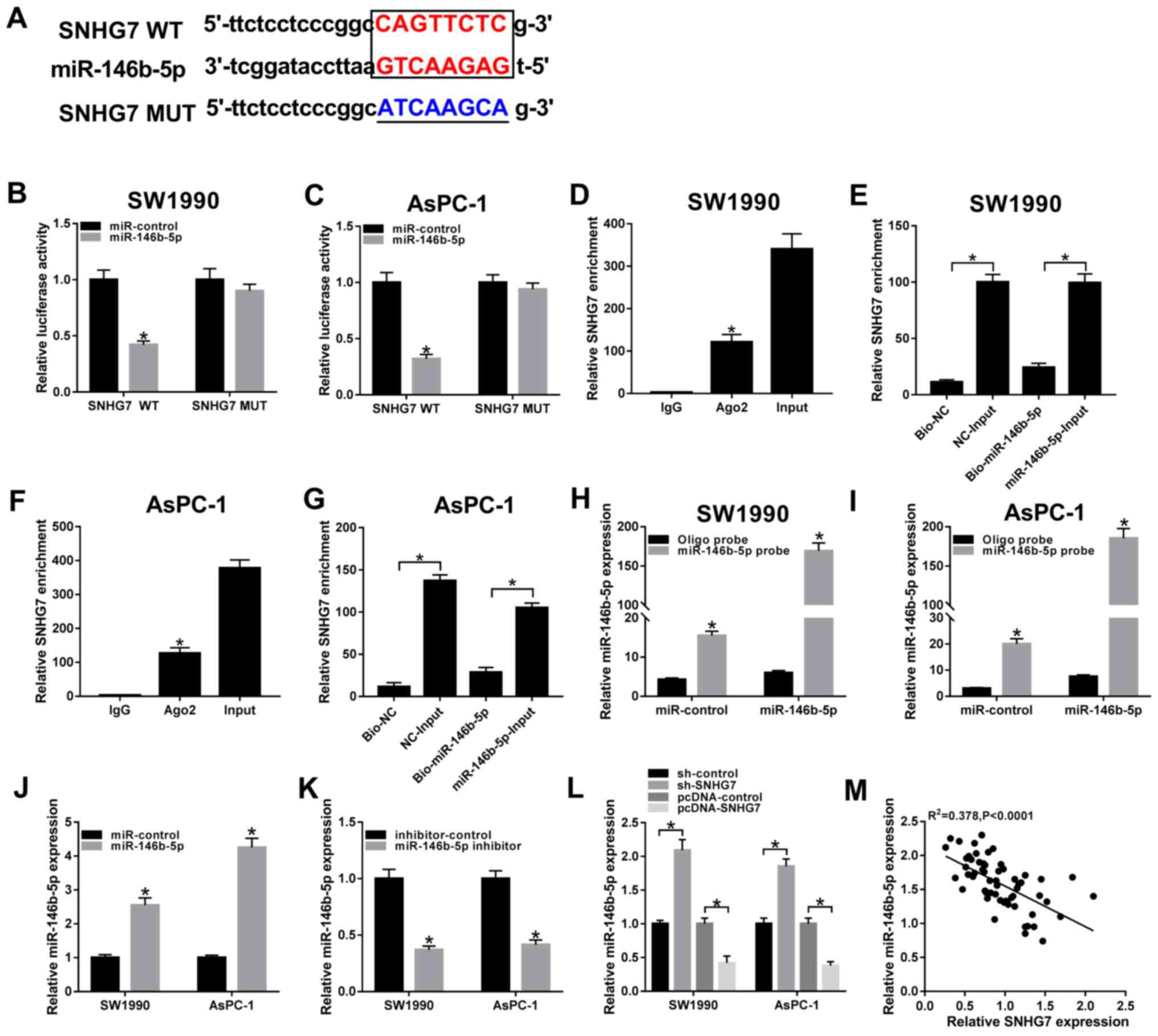 | Figure 3miR-146b-5p is a direct target of
SNHG7. (A) Complementary sequences between miR-146b-5p and SNHG7,
and the MUT sequences of SNHG7. Luciferase activity of SNHG7 WT or
SNHG7 MUT reporter in (B) SW1990 and (C) AsPC-1 cells transfected
with miR-146b-5p or miR-control was assessed via dual-luciferase
reporter. (D) Enrichment of SNHG7 in anti-Ago2 or anti-IgG labeled
SW1990 cells transfected with miR-146b-5p or miR-control was
evaluated by RIP assay. (E) Enrichment of SNHG7 in SW1990 cells
transfected with Bio-miR-146b-5p or Bio-NC was analyzed by RNA
pull-down assay. (F) Enrichment of SNHG7 in anti-Ago2 or anti-IgG
labeled AsPC-1 cells transfected with miR-146b-5p or miR-control
was evaluated by RIP assay. (G) Enrichment of SNHG7 in AsPC-1 cells
transfected with Bio-miR-146b-5p or Bio-NC was analyzed by RNA
pull-down assay. Transfection efficiency of miR-146b-5p probe was
examined in (H) SW1990 and (I) AsPC-1 cells transfected with
miR-control or miR-146b-5p. miR-146b-5p expression was measured in
SW1990 and AsPC-1 cells transfected with (J) miR-control and
miR-146b-5p, or (K) inhibitor-control and miR-146b-5p inhibitor.
(L) Expression of miR-146b-5p in cells transfected with sh-control,
sh-SNHG7, pcDNA-control or pcDNA-SNHG7 were detected via reverse
transcription-quantitative PCR. (M) Correlation between SNHG7 and
miR-146b-5p was analyzed by Pearson test. *P<0.05 vs.
their respective normal groups. WT, wild-type; MUT, mutant; SNHG7,
small nucleolar RNA host gene 7; miR, microRNA; sh, short hairpin
RNA; NC, negative control; Ago2, argonaute-2. |
It was demonstrated that SNHG7 was significantly
enriched by the Ago2 antibody in SW1990 and AsPC-1 cells
transfected with miR-146b-5p compared with the IgG antibody group
(Fig. 3D and F). In addition, the RNA pull-down assay
results indicated that Bio-miR-146b-5p had a lower enrichment of
SNHG7 in SW1990 and AsPC-1 cells compared with the
Bio-miR-146b-5p-Input group, and the Bio-NC group also had a lower
enrichment of SNHG7 in PC cells relative to the NC-Input
group(Fig. 3E and G). It was also found that the transfection
of Bio-miR-146b-5p was successful in SW1990 and AsPC-1 cells
(Fig. 3H and I).
Moreover, the transfection efficiency of the
miR-146b-5p mimic and inhibitor on the upregulation or
downregulation of miR-146b-5p expression, respectively, was
examined (Fig. 3J and K). The results indicated the expression of
miR-146b-5p was significantly elevated in SW1990 and AsPC-1 cells
by silencing of SNHG7, while it was significantly decreased by
overexpression of SNHG7 (Fig. 3L).
It was also identified that the expression of miR-146b-5p was
weakly negatively correlated with SNHG7, based on the
interpretation of correlations (25) (Fig.
3M). Therefore, it was speculated that miR-146b-5p may
negatively interact with SNHG7.
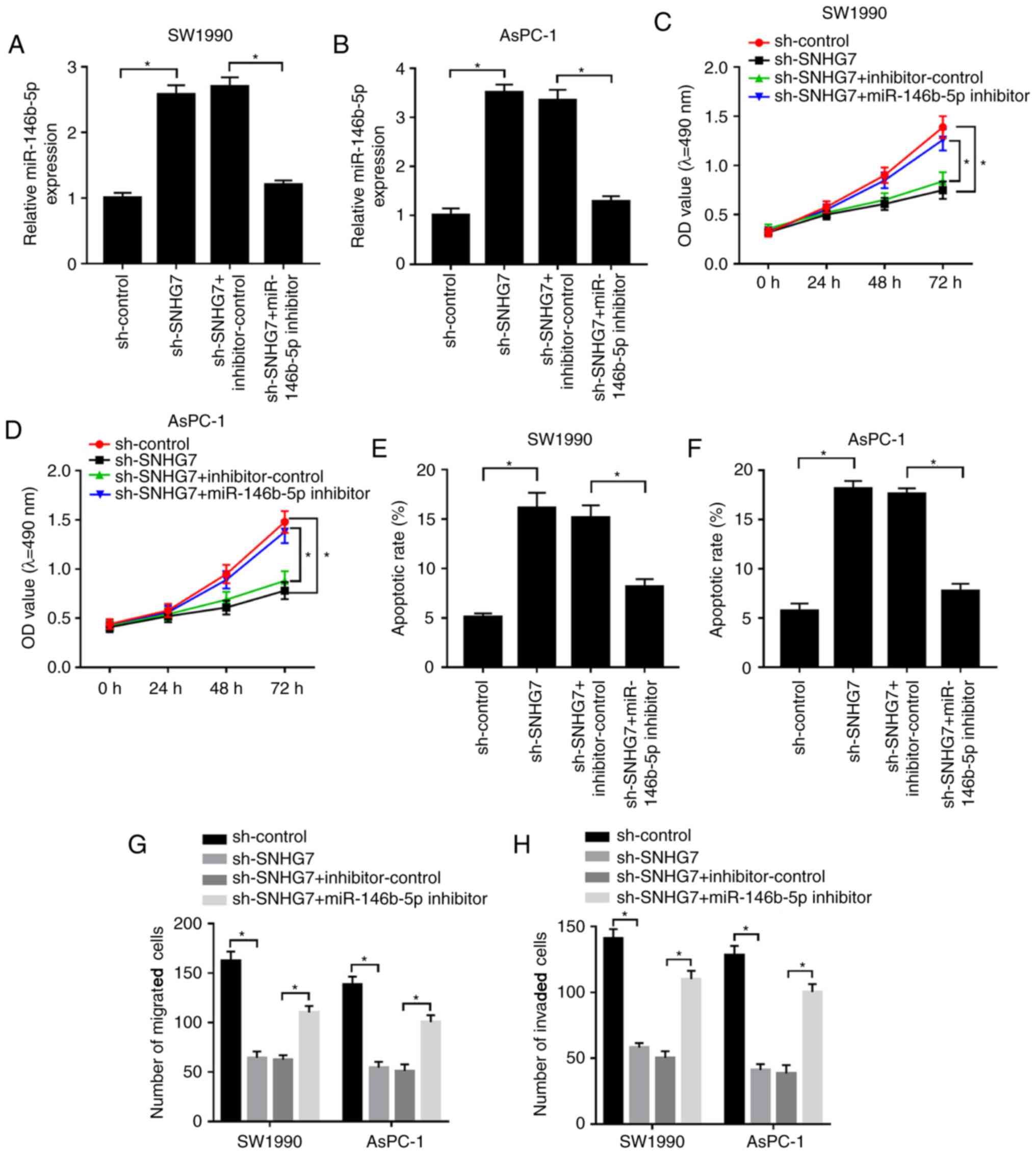
miR-146b-5p inhibitor relieves the
constraint effects on proliferation, migration and invasion, as
well as the promotive effect on cell apoptosis in SW1990 and AsPC-1
cells caused by SNHG7 silencing
To examine whether the effect of SNHG7 on PC
progression was mediated by miR-146b-5p, sh-SNHG7 and the
miR-146b-5p inhibitor were co-transfected into SW1990 and AsPC-1
cells. It was found that the expression of miR-146b-5p was
significantly increased in the sh-SNHG7-transfected SW1990 and
AsPC-1 cells, while it was significantly reduced after miR-146b-5p
inhibitor transfection (Fig. 4A and
B). Furthermore, the miR-146b-5p
inhibitor reduced the suppressive impacts on cell viability and the
migratory and invasive abilities of SW1990 and AsPC-1 cells, which
were inhibited by SNHG7 knockdown (Fig.
4C and D; G and H).
However, transfection of sh-SNHG7 elevated the apoptotic rate of
SW1990 and AsPC-1 cells, but this promotion was attenuated by the
miR-146b-5p inhibitor (Fig. 4E and
F). Collectively, the results
suggested that silencing of SNHG7 reduced cell proliferation,
migration and invasion, but promoted apoptosis in SW1990 and AsPC-1
cells by regulating miR-146b-5p.
Robo1 negatively interacts with
miR-146b-5p
To identify the biological mechanism of miR-146b-5p
in PC, the TargetScan online database (http://www.targetscan.org) was used to predict the
potential candidate targets of miR-146b-5p. The results suggested
that the Robo1 3'UTR had complementary binding sites with
miR-146b-5p (Fig. 5A). Moreover,
dual-luciferase reporter assay results found that the luciferase
activity of Robo1 3'UTR WT reporter was significantly reduced in
SW1990 and AsPC-1 cells transfected with miR-146b-5p, while there
were no significant changes in the miR-control group. However, the
luciferase activity of Robo1 3'UTR MUT reporter had no significant
fluctuations in any group (Fig. 5B
and C).
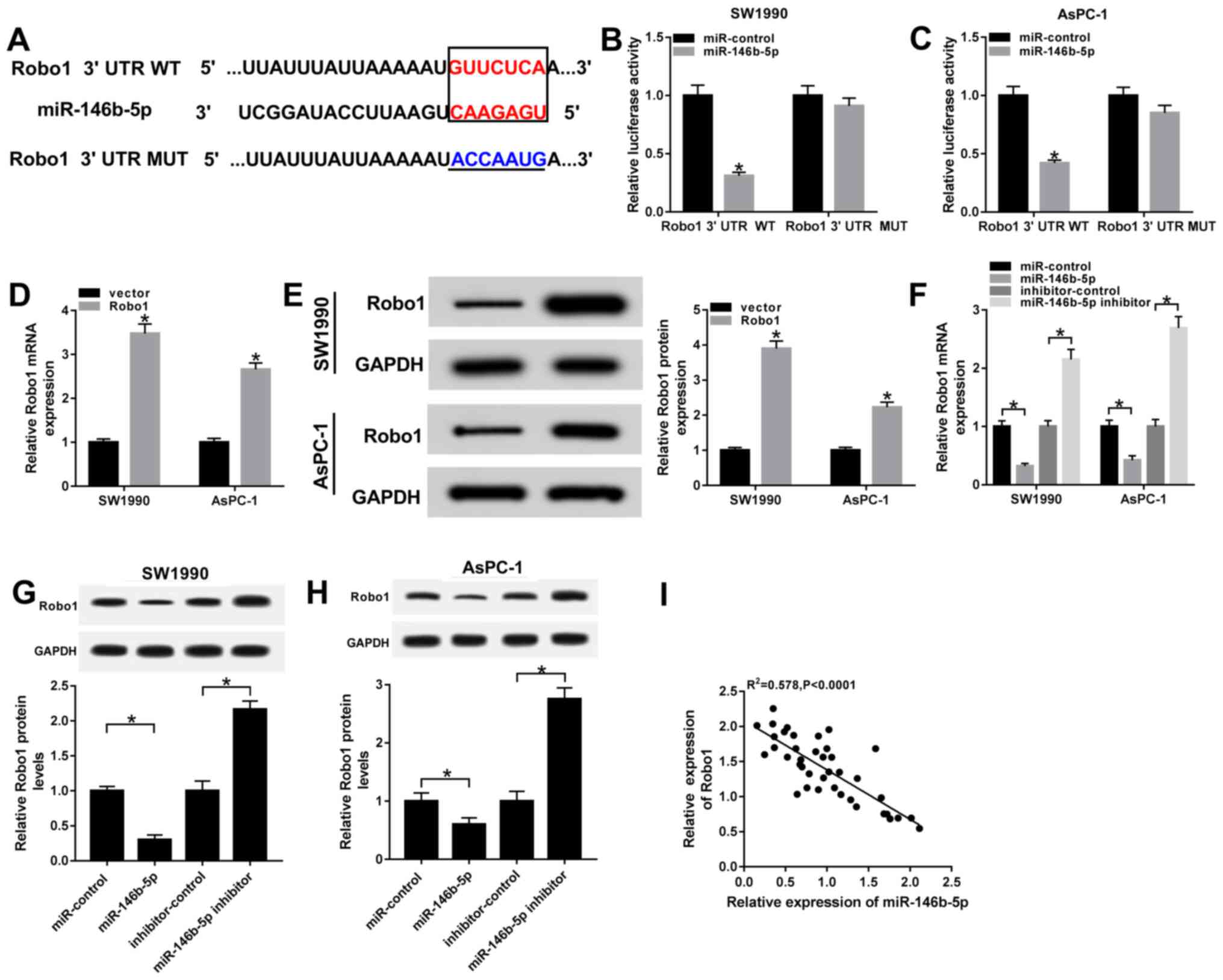 | Figure 5Robo1 negatively interacts with
miR-146b-5p. (A) Complementary binding sites between miR-146b-5p
and Robo1 3'UTR, and the MUT sequences of Robo1 3'UTR. Luciferase
activity of Robo1 3'UTR WT or Robo1 3'UTR MUT reporter in (B)
SW1990 and (C) AsPC-1 cells transfected with miR-146b-5p mimics or
miR-control was evaluated via dual-luciferase reporter assay. (D)
mRNA and (E) protein expression levels of Robo1 were examined in
SW1990 and AsPC-1 cells transfected with vector and Robo1. (F) mRNA
and protein expression levels of Robo1 in (G) SW1990 and (H) AsPC-1
cells transfected with miR-control, miR-146b-5p, inhibitor-control
or miR-146b-5p inhibitor were detected by reverse
transcription-quantitative PCR and western blotting, respectively.
(I) Correlation between Robo1 and miR-146b-5p was assessed with a
Pearson test. *P<0.05 vs. their respective normal
groups. 3'UTR, 3'untranslated region; MUT, mutant; WT, wild-type;
miR, microRNA; Robo1, roundabout homolog 1; SNHG7, small nucleolar
RNA host gene 7. |
Furthermore, the transfection efficiency of Robo1
overexpression in SW1990 and AsPC-1 cells was detected by RT-qPCR
and western blotting (Fig. 5D and
E). It was demonstrated that the
mRNA and protein expression levels of Robo1 were significantly
decreased in SW1990 and AsPC-1 cells transfected with miR-146b-5p
mimics, while both were significant enhanced in the miR-146b-5p
inhibitor group (Fig. 5F-H).
Moreover, the results indicated that the expression of Robo1 was
moderately negatively correlated with miR-146b-5p (Fig. 5I). Thus, it was speculated that
Robo1 may be a direct target of miR-146b-5p.
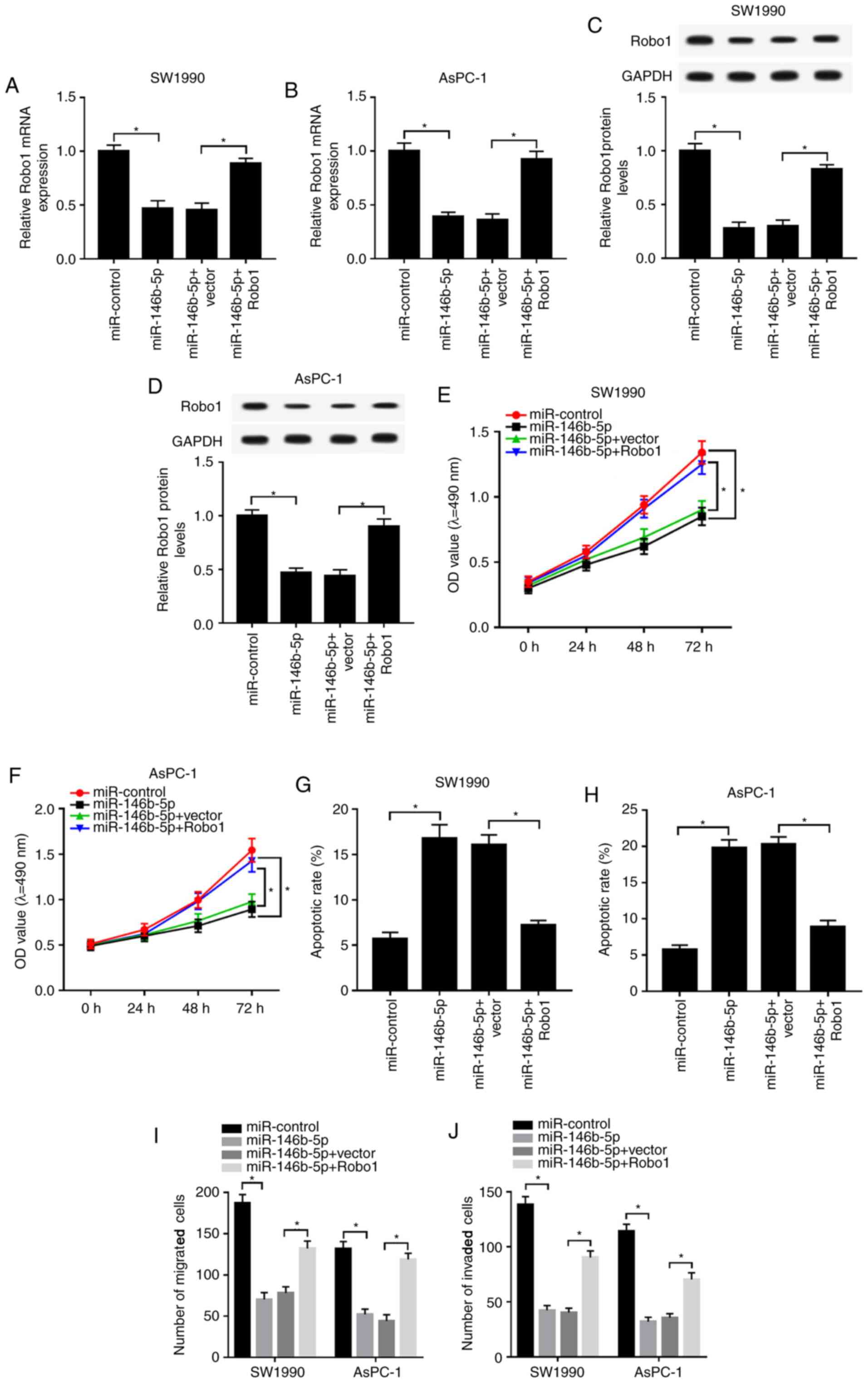
Robo1 overexpression mitigates the
inhibitive effect on proliferation, migration and invasion, as well
as the enhanced effect on apoptosis in SW1990 and AsPC-1 cells
induced by miR-146b-5p mimics
Based on the above results, it was found that Robo1
negatively interacted with miR-146b-5p. Subsequently, the present
study detected the functions of miR-146b-5p and Robo1 in PC. It was
identified that mRNA and protein expression levels of Robo1 were
reduced in miR-146b-5p-transfected SW1990 and AsPC-1 cells, while
they were increased by the overexpression of Robo1 (Fig. 6A-D). Furthermore, overexpression of
Robo1 reduced the inhibitory effect of miR-146b-5p on viability,
migration and invasion in SW1990 and AsPC-1 cells (Fig. 6E and F; I and
J). The flow cytometry results also
demonstrated that the overexpression of Robo1 reversed the
inhibitory effect of miR-146b-5p upregulation on the apoptotic rate
in SW1990 and AsPC-1 cells (Fig. 6G
and H). Therefore, the results
indicated that miR-146b-5p reduced cell proliferation, migration
and invasion, but increased apoptosis in PC cells by modulating
Robo1.
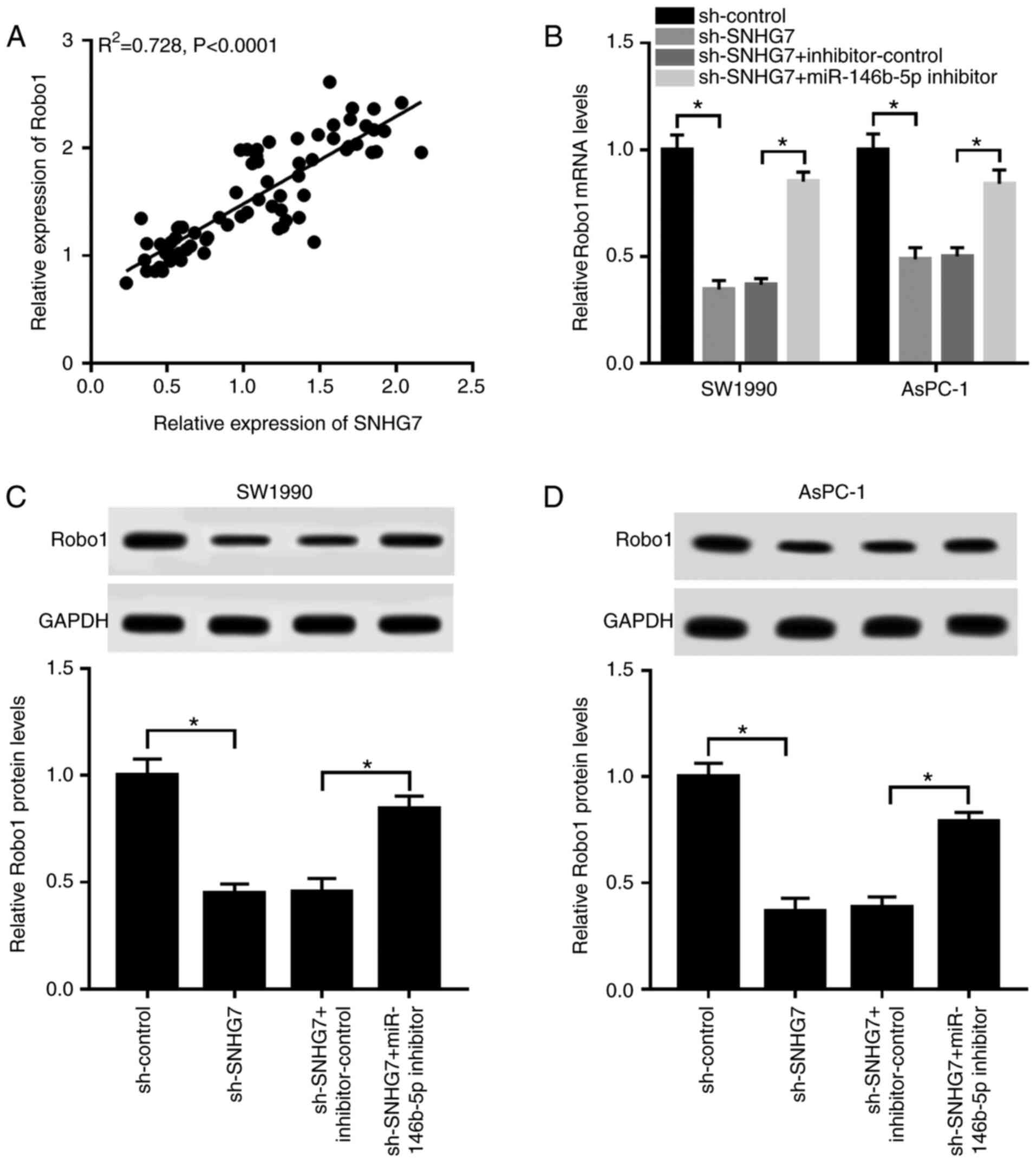
Silencing of SNHG7 downregulates Robo1
expression by sponging miR-146b-5p
The results suggested that the expression of Robo1
was strongly positively correlated with SNHG7 expression (Fig. 7A). To investigate the relationship
between SNHG7, miR-146b-5p and Robo1, sh-SNHG7 and miR-146b-5p
inhibitor were co-transfected into SW1990 and AsPC-1 cells. It was
found that the mRNA and protein expression levels of Robo1 were
significantly reduced in the SW1990 and AsPC-1 cells transfected
with sh-SNHG7, while they were increased after transfection with
the miR-146b-5p inhibitor (Fig.
7B-D). Thus, depletion of SNHG7 reduced Robo1 expression by
acting as a miR-146b-5p sponge.
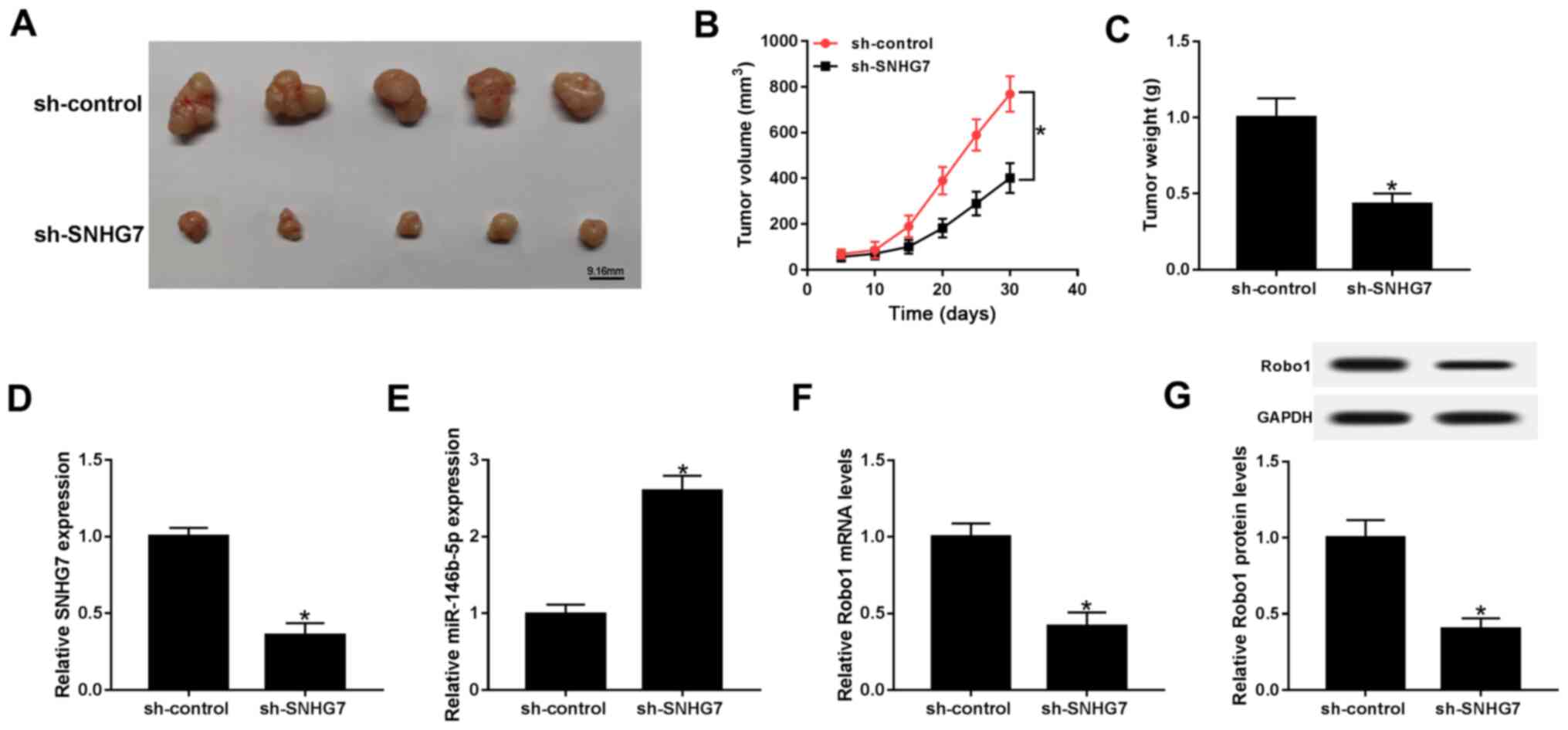
SNHG7 knockdown represses xenograft
tumor growth in vivo
To further assess the function of SNHG7 in PC,
SW1990 cells were transfected with sh-SNHG7 or sh-control and then
injected into nude mice. Following 30 day measurement, the results
indicated that tumor volume and weight were both significantly
decreased in the sh-SNHG7 group compared with the sh-control group
(Fig. 8A-C). Moreover, the maximum
diameter and volume of the tumors were 18.32 mm and 768.56
mm3, respectively. Furthermore, the expression of SNHG7
was significantly downregulated, while the expression of
miR-146b-5p was significantly elevated in the sh-SNHG7 group
(Fig. 8D and E). The results also suggested that the
mRNA and protein expression levels of Robo1 were significantly
decreased in the sh-SNHG7 group (Fig.
8F and G). Collectively, the
results demonstrated that SNHG7 knockdown inhibited xenograft tumor
growth in vivo.
Discussion
PC is a fatal cancer type that occurs worldwide and
is often associated with poor prognosis (1,26).
Previous studies have shown that lncRNAs are implicated in the
progression of various cancers (27-29).
For example, Guo et al (30)
reported that lncRNA HNF1A-AS1 knockdown suppressed cell migration,
invasion and glycolysis through targeting the miR-124/MYO6 in
colorectal cancer. Li et al (31), demonstrated that LncRNA FTX played a
promoting role in gastric cell proliferation and invasion by
regulating the miR144/Axis. Additionally, Feng et al
(32) confirmed that lncRNA NEAT1
acted as a carcinogenic factor through facilitating cell
proliferation and metastasis in PC development. The present study
focused on the function and mechanism of SNHG7 in PC progression,
and it was found that lncRNA SNHG7 knockdown inhibited PC
progression via a miR-146b-5p/Robo1 axis.
Previous studies revealed that aberrant expression
of SNHG1 occurs in various types of cancer, including PC. For
instance, a study in melanoma indicated that SNHG7 is significantly
elevated in melanoma tissues, and its silencing suppresses cell
migration and invasion in vitro (33). Another study in thyroid cancer
showed that SNHG7 is upregulated in thyroid cancer tissues; the
depletion of SNHG7 inhibits cell proliferation, but induces
apoptosis in thyroid cancer cells (34). Xu et al (35), reported that SNHG7 expression is
significantly enhanced in human bladder cancer, and SNHG7 knockdown
represses cell proliferation and metastasis, but induces apoptosis
in SW780, T24, UMUC and 5637 cells. The present results suggested
that the expression of SNHG7 was significantly upregulated in PC
tissues and cell lines, and was increased in high-grade compared
with low-grade tumors. Moreover, the high expression of SNHG7 was
found to be associated with the pathological characteristics of PC.
Furthermore, functional experiment results identified that SNHG7
silencing reduced cell proliferation, migration and invasion, while
it promoted apoptosis in PC cells. It was also demonstrated that
the depletion of SNHG7 decreased xenograft tumor growth in
vivo. Thus, these results of SNHG7 are in line with a previous
study (15) and indicated that
SNHG7 may promote PC progression.
lncRNA can function as a competing endogenous RNA to
recruit miRNA and further affected target gene expression. For
example, a previous study showed that SNHG7 sponges miR-186 to
promote breast cancer progression (36). Another study in breast cancer
revealed that SNHG7 increases cell proliferation, invasion and
epithelial-mesenchymal transition by sponging miR-34a (37). Cheng et al (15), reported that SNHG7 knockdown blocks
cell proliferation, migration and invasion via miR-342-3p in PC. In
the present study, it was found that miR-146b-5p was sponged to
SNHG7. Moreover, the restoration experimental results indicated
that the expression of miR-146b-5p was reduced in SW1990 and AsPC-1
cells transfected with sh-SNHG7 induced by the miR-146b-5p
inhibitor. It was also found that SNHG7 silencing inhibited PC
progression by regulating miR-146b-5p. Therefore, it was speculated
that SNHG7 accelerated PC progression by sponging miR-146b-5p.
It has been shown that the dysregulation of Robo1 is
associated with cancer progression. For example, a previous study
in hepatocellular carcinoma showed that Robo1 is significantly
increased in hepatocellular carcinoma tissues and cells, and its
overexpression reduces hepatocellular carcinoma progression
regulated by miR-490-5p in vitro (38). The present results identified an
interaction between miR-146b-5p and Robo1 by dual luciferase
reporter assay. Moreover, it was demonstrated that Robo1
overexpression attenuated the restraint impacts on cell viability,
migration and invasion, and the promoting action on apoptosis
caused by miR-146b-5p mimics. The present results relating to Robo1
in PC were consistent with those of a previous study (21). Furthermore, it was found that SNHG7
increased Robo1 expression by sponging miR-146b-5p in PC cells.
Thus, the present results indicated that SNHG7 modulated Robo1
expression to promote cell proliferation, migration and invasion,
as well as inhibit apoptosis in PC by sponging miR-146b-5p.
In conclusion, the expression of SNHG7 was
significantly increased in PC tissues and cells. Based on the
results of the functional and mechanical experiments, it was
speculated that SNHG7 positively regulated Robo1 expression by
sponging miR-146b-5p, which contributed to the promotion of PC
progression. Therefore, this novel regulatory network may provide a
new therapeutic target for patients with PC.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
Conceptualization of the research design and the
execution of the experiment were carried out by YJ. Acquisition of
data was conducted by YJ. Writing and review of the manuscript were
done by YJ and QF. Study supervision was carried out by YJ. Formal
analysis and data curation was carried out by QF. All authors read
and approved the manuscript and agree to be accountable for all
aspects of the research in ensuring that the accuracy or integrity
of any part of the work are appropriately investigated and
resolved.
Ethics approval and consent to
participate
The current study was approved by the Ethics
Committee of Jingzhou Central Hospital. A written informed consent
form was obtained from each patient.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2016. CA Cancer J Clin. 66:7–30. 2016.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Surveillance Epidemiology and End Results
(SEER) Fact Sheets: Pancreas. Web site. http://seer.cancer.gov/. Accessed July 15, 2016.
|
|
3
|
Aarnink A, Richard C, Truntzer C, Vincent
J, Bengrine L, Vienot A, Borg C and Ghiringhelli F: Baseline
splenic volume as a surrogate marker of FOLFIRINOX efficacy in
advanced pancreatic carcinoma. Oncotarget. 9:25617–25629.
2018.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Paulson AS, Tran Cao HS, Tempero MA and
Lowy AM: Therapeutic advances in pancreatic cancer.
Gastroenterology. 144:1316–1326. 2013.PubMed/NCBI View Article : Google Scholar
|
|
5
|
De Luca R, Blasi L, Alù M, Gristina V and
Cicero G: Clinical efficacy of nab-paclitaxel in patients with
metastatic pancreatic cancer. Drug Des Devel Ther. 12:1769–1775.
2018.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Yang G, Lu X and Yuan L: LncRNA: A link
between RNA and cancer. Biochim Biophys Acta. 1839:1097–1109.
2014.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Tang Y, Cao G, Zhao G, Wang C and Qin Q:
LncRNA differentiation antagonizing non-protein coding RNA promotes
proliferation and invasion through regulating miR-135a/NLRP37 axis
in pancreatic cancer. Invest New Drugs. 38:714–721. 2019.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Peng W and Jiang A: Long noncoding RNA
CCDC26 as a potential predictor biomarker contributes to
tumorigenesis in pancreatic cancer. Biomed Pharmacother.
83:712–717. 2016.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Zhang X, Feng W, Zhang J, Ge L, Zhang Y,
Jiang X, Peng W, Wang D, Gong A and Xu M: Long non-coding RNA PVT1
promotes epithelial-mesenchymal transition via the TGF-β/Smad
pathway in pancreatic cancer cells. Oncol Rep. 40:1093–1102.
2018.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Zhang M, Zhao Y, Zhang Y, Wang D, Gu S,
Feng W, Peng W, Gong A and Xu M: LncRNA UCA1 promotes migration and
invasion in pancreatic cancer cells via the hippo pathway. Biochim
Biophys Acta Mol Basis Dis. 1864:1770–1782. 2018.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Gao H, Gong N, Ma Z, Miao X, Chen J, Cao Y
and Zhang G: LncRNA ZEB2-AS1 promotes pancreatic cancer cell growth
and invasion through regulating the miR-204/HMGB1 axis. Int J Biol
Macromol. 116:545–551. 2018.PubMed/NCBI View Article : Google Scholar
|
|
12
|
She K, Huang J, Zhou H, Huang T, Chen G
and He J: lncRNA-SNHG7 promotes the proliferation, migration and
invasion and inhibits apoptosis of lung cancer cells by enhancing
the FAIM2 expression. Oncol Rep. 36:2673–2680. 2016.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Wang MW, Liu J, Liu Q, Xu QH, Li TF, Jin S
and Xia TS: LncRNA SNHG7 promotes the proliferation and inhibits
apoptosis of gastric cancer cells by repressing the P15 and P16
expression. Eur Rev Med Pharmacol Sci. 21:4613–4622.
2017.PubMed/NCBI
|
|
14
|
Ren J, Yang Y, Xue J, Xi Z, Hu L, Pan SJ
and Sun Q: Long noncoding RNA SNHG7 promotes the progression and
growth of glioblastoma via inhibition of miR-5095. Biochem Biophys
Res Commun. 496:712–718. 2018.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Cheng D, Fan J, Ma Y, Zhou Y, Qin K, Shi M
and Yang J: LncRNA SNHG7 promotes pancreatic cancer proliferation
through ID4 by sponging miR-342-3p. Cell Biosci.
9(28)2019.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Esteller M: Non-coding RNAs in human
disease. Nat Rev Genet. 12:861–874. 2011.PubMed/NCBI View
Article : Google Scholar
|
|
17
|
Zhang Z, Che X, Yang N, Bai Z, Wu Y, Zhao
L and Pei H: MiR-135b-5p Promotes migration, invasion and EMT of
pancreatic cancer cells by targeting NR3C2. Biomed Pharmacother.
96:1341–1348. 2017.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Cai H, Yao J, An Y, Chen X, Chen W, Wu D,
Luo B, Yang Y, Jiang Y, Sun D and He X: LncRNA HOTAIR acts a
competing endogenous RNA to control the expression of notch3 via
sponging miR-613 in pancreatic cancer. Oncotarget. 8:32905–32917.
2017.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Wang J, Guo XJ, Ding YM and Jiang JX:
MiR-1181 inhibits invasion and proliferation via STAT3 in
pancreatic cancer. World J Gastroenterol. 23:1594–1601.
2017.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Lin F, Wang X, Jie Z, Hong X, Li X, Wang M
and Yu Y: Inhibitory effects of miR-146b-5p on cell migration and
invasion of pancreatic cancer by targeting MMP16. J Huazhong Univ
Sci Technolog Med Sci. 31(509)2011.PubMed/NCBI View Article : Google Scholar
|
|
21
|
He H, Hao SJ, Yao L, Yang F, Di Y, Li J,
Jiang YJ, Jin C and Fu DL: MicroRNA-218 inhibits cell invasion and
migration of pancreatic cancer via regulating ROBO1. Cancer Biol
Ther. 15:1333–1339. 2014.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Cuccurullo V and Mansi L: AJCC cancer
staging handbook: From the AJCC cancer staging manual (7th
edition). Eur J Nucl Med Mol Imaging. 38(408)2011.
|
|
23
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408.
2001.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Harden VA and Hannaway C: National
Institutes of Health (NIH). In: Encyclopedia of Life Sciences. New
York, John Wiley & Sons, Ltd, 2001.
|
|
25
|
Schober P, Boer C and Schwarte LA:
Correlation coefficients: Appropriate use and interpretation.
Anesth Analg. 126:1763–1768. 2018.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Bray F, Ferlay J, Soerjomataram I, Siegel
RL, Torre LA and Jemal A: Global cancer statistics 2018: GLOBOCAN
estimates of incidence and mortality worldwide for 36 cancers in
185 countries. CA Cancer J Clin. 68:394–424. 2018.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Xu W, Chang J, Du X and Hou J: Long
non-coding RNA PCAT-1 contributes to tumorigenesis by regulating
FSCN1 via miR-145-5p in prostate cancer. Biomed Pharmacother.
95:1112–1118. 2017.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Huo X and Wang H, Huo B, Wang L, Yang K,
Wang J, Wang L and Wang H: FTX contributes to cell proliferation
and migration in lung adenocarcinoma via targeting miR-335-5p/NUCB2
axis. Cancer Cell Int. 20(89)2020.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Sun J, Zhang P, Yin T, Zhang F and Wang W:
Upregulation of LncRNA PVT1 facilitates pancreatic ductal
adenocarcinoma cell progression and glycolysis by regulating
MiR-519d-3p and HIF-1A. J Cancer. 11:2572–2579. 2020.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Guo X, Zhang Y, Liu L, Yang W and Zhang Q:
HNF1A-AS1 regulates cell migration, invasion and glycolysis via
modulating miR-124/MYO6 in colorectal cancer cells. Onco Targets
Ther. 13:1507–1518. 2020.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Li H, Yao G, Zhai J, Hu D and Fan Y:
LncRNA FTX promotes proliferation and invasion of gastric cancer
via miR-144/ZFXAxis. Onco Targets Ther. 12:11701–11713.
2019.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Feng Y, Gao L, Cui G and Cao Y: LncRNA
NEAT1 facilitates pancreatic cancer growth and metastasis through
stabilizing ELF3 mRNA. Am J Cancer Res. 10:237–248. 2020.PubMed/NCBI
|
|
33
|
Zhang C, Zhu B, Li XB, Cao YQ, Yang JC, Li
X, Liu YX and Wang YB: Long non-coding RNA SNHG7 promotes migration
and invasion of melanoma via upregulating SOX4. Eur Rev Med
Pharmacol Sci. 23:4828–4834. 2019.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Wang YH, Huo BL, Li C, Ma G and Cao W:
Knockdown of long noncoding RNA SNHG7 inhibits the proliferation
and promotes apoptosis of thyroid cancer cells by downregulating
BDNF. Eur Rev Med Pharmacol Sci. 23:4815–4821. 2019.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Xu C, Zhou J, Wang Y, Wang A, Su L, Liu S
and Kang X: Inhibition of malignant human bladder cancer phenotypes
through the down-regulation of the long non-coding RNA SNHG7. J
Cancer. 10:539–546. 2019.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Luo X, Song Y, Tang L, Sun DH and Ji DG:
LncRNA SNHG7 promotes development of breast cancer by regulating
microRNA-186. Eur Rev Med Pharmacol Sci. 22:7788–7797.
2018.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Sun X, Huang T, Liu Z, Sun M and Luo S:
LncRNA SNHG7 contributes to tumorigenesis and progression in breast
cancer by interacting with miR-34a through EMT initiation and the
notch-1 pathway. Eur J Pharmacol. 856(172407)2019.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Chen W, Ye L, Wen D and Chen F: MiR-490-5p
inhibits hepatocellular carcinoma cell proliferation, migration and
invasion by directly regulating ROBO1. Pathol Oncol Res. 25:1–9.
2019.PubMed/NCBI View Article : Google Scholar
|