Introduction
Alzheimer's disease (AD) is a progressive
neurodegenerative disease characterized by memory loss, cognitive
dysfunction, personality changes and decreased mobility (1). Furthermore, AD is pathologically
characterized by senile plaques (SP) and neurofibrillary tangles
(1). According to a 2019 report by
the Alzheimer's Association, the risk of developing AD has
increased to 1:10 for individuals older than 65 years and is as
high as 1:2 among individuals older than 85 years in USA (2). Therefore, effective treatments and
controlling the pathological processes underlying AD are of great
concern in the medical field.
The pathogenesis of AD is complex and there are
numerous hypotheses regarding its occurrence, including
neuroinflammation, cholinergic dysfunction, metabolic alterations,
amyloid-β (Aβ) peptide accumulation and abnormalities in τ protein
metabolism (3-5).
Although these hypotheses are controversial, the accumulation of Aβ
is one of the major theories recognized by researchers (6). According to the Aβ cascade hypothesis,
mutations in the amyloid precursor protein (APP), presenilin-1
(PS-1) and PS-2 upregulate Aβ secretion, leading to the
accumulation of Aβ, which forms small molecule oligomers that are
further aggregated into fibers and deposited to form extracellular
SPs (7). Aβ plaques create an
environment that allows for the rapid induction of pathological τ
aggregates from endogenous τ and may enhance or sustain τ
pathologies through additional mechanisms, including intracellular
protein degradation impairment (8).
Furthermore, Aβ plaques induce oxidative stress and mediate damage
to numerous biological macromolecules, including proteins, nucleic
acids and lipids, resulting in changes of neuronal structure and
function, and potential neuronal loss (9). A previous study has indicated an
association between oxidative stress and protein aggregation
processes, which are involved in the development of proteinopathies
(10). Therefore, oxidative stress
can be considered either causative or consecutive in regard to
protein aggregation (11).
Furthermore, recent studies have demonstrated that medications used
to successfully treat AD-like symptoms act by modulating oxidative
stress (12,13).
Autophagy is involved in numerous diseases,
including cancer, diabetes and neurodegenerative disorders
(14). Autophagy consists of a
dynamic pathway, including the formation of surrounding membranes
and the degradation of diverse cellular components, such as
organelles and protein aggregates (15). Additionally, autophagy serves an
important role in Aβ metabolism and τ processing and clearance
(16). Furthermore, autophagy
contributes to endoplasmic reticulum stress and mitophagy in
relation to AD pathology (17,18).
Chlorogenic acid (5-caffeoylquinic acid; CGA) is a
polyphenolic substance that is widely found in the human diet
(19). CGA has various
pharmacologic actions, including antioxidant, anti-inflammatory and
anticarcinogenic activities, that exert hypoglycemic and
hypolipidemic effects (20). A
number of studies have indicated that dietary consumption of CGA
reduces the risk of developing neurodegenerative diseases (21,22).
An in vitro biological study revealed that CGA has an
enhanced inhibitory effect on Aβ40 aggregation and protects PC12
cells from Aβ-aggregation-induced cell death (23). Additionally, a previous study
demonstrated the neuroprotective effects of CGA and its major
metabolites in primary cultures of rat cerebellar granule neurons
(24). Furthermore, numerous
studies have reported that CGA reduces the risk of
neurodegenerative diseases (21,22);
however, the mechanism by which CGA confers this protective effect
remains unclear. To investigate the underlying mechanism, the
present study employed a hydrogen peroxide
(H2O2)-induced SH-SY5Y cell model and
investigated whether CGA regulated autophagic flow and lysosomal
function via the mTOR/transcription factor EB (TFEB) pathway to
protect against H2O2-induced cell damage.
Materials and methods
Cell model and CGA treatment
SH-SY5Y human neuroblastoma cells were purchased
from Shanghai Zhong Qiao Xin Zhou Biotechnology Co., Ltd. (cat. no.
20161116-02) and were authenticated by a short tandem repeat
profiling service provided by American Type Culture Collection. CGA
(purity, ≥98%; assessed by high performance liquid chromatography)
was purchased from Baoji Herbest Bio-Tech Co., Ltd. The details
were as follows: Instrument: Spd-16 High performance liquid
chromatography (Shimadzu Corporation); chromatographic column,
Sepax Bio-C18 [Yuexu Technology (Shanghai) Co., Ltd.; 4.6x250 mm; 5
µl]; temperature, 30˚C; injection volume, 20 µl; mobile phase,
acetonitrile-0.4% acetic acid aqueous solution; velocity of flow:
1.0 ml/min; and detection wavelength, 350 nm.
H2O2 (34.01 g/mol) was purchased from
Sigma-Aldrich; Merck KGaA.
The cell model was established as previously
described (25). Briefly, SH-SY5Y
cells were cultured in DMEM (Gibco; Thermo Fisher Scientific, Inc.)
supplemented with 10% FBS (Zhejiang Tianhang Bio Polytron
Technologies Inc.) and 1% penicillin/streptomycin (Gibco; Thermo
Fisher Scientific, Inc.) and incubated at 37˚C in a humidified
atmosphere containing 5% CO2 (3111 CO2
incubator; Thermo Fisher Scientific, Inc.).
Cells in the logarithmic growth phase were selected
and digested with 0.25% trypsin (Gibco; Thermo Fisher Scientific,
Inc.). Subsequently, cells were seeded into 96-well plates (seeding
density, 3x104 cells/ml) for 16 h. To determine the
optimal concentration and duration of H2O2
treatment to elicit toxic effects on SH-SY5Y cells, cells were
treated with 50, 100, 200, 400, 500, 600, 700 or 800 µM
H2O2 for 4, 8, 12 or 48 h. After 12 h of
plating, the medium was replaced with CGA (100, 50, 25, 12.5, 6.25
and 3.125 µM) in the CGA groups or fresh DMEM in the normal and
model groups. These cells were incubated for an additional 24 h
prior to analyses.
Cell viability assessment using a Cell
Counting Kit-8 (CCK-8) assay
Cell viability was measured using CCK-8 assays
according to the manufacturer's protocol. Briefly, cells were
treated with different concentrations of H2O2
and viability was measured at 12, 24 and 36 h. Cells were treated
with 10 µl CCK-8 reagent (cat. no. JE914; Dojindo Molecular
Technologies, Inc.) for 2 h at 37˚C. Following this, optical
density (OD) values were measured at a wavelength of 450 nm using
an automatic microplate reader (Thermo Fisher Scientific, Inc.).
The SH-SY5Y cells were treated with different concentrations of CGA
(0, 100, 200, 300, 400, 500, 600, 700 and 800 µM) for 12, 24 and 36
h to determine the optimal concentration, and cell viability was
detected using the CCK-8 assay. Cell viability (%) was calculated
as follows: ODvalue of the administration well -
ODvalue of the zero well/ODvalue of the
normal well - ODvalue of the zero well.
Morphologic changes were observed using phase-contrast microscopy
(x400), and analysis of the length of cell processes and numbers of
SH-SY5Y cells were analyzed using Image-Pro Plus software (version
6.0; Media Cybernetics, Inc.).
Monodansylcadaverine (MDC) and Hoechst
fluorescence staining
MDC (Sigma-Aldrich; Merck KGaA) is a lysosomotropic
compound used for the identification of autophagic vesicles via
fluorescence microscopy and for the assessment of autophagy
induction via the accumulation of MDC-labeled vacuoles (26). Hoechst staining was used to test for
cell apoptosis. CGA (50, 25, 12.5 and 6.25 µM) and/or
H2O2 (500 µM) was added to each well at 37˚C.
After 24 h, cells (1x106/well) were washed twice with
PBS and treated with 500 µl MDC solution (25 µM) or Hoechst
solution (50 µM) and incubated for 30 min at 37˚C. Fluorescence
images were captured using an inverted fluorescence microscope
(DMI3000; Leica Microsystems GmbH) and analyzed using Image-Pro
Plus software (version 6.0; Media Cybernetics, Inc.).
Autophagic flow test
mCherry-green fluorescent protein (GFP)-LC3
adenoviruses (cat. no. 030217170302; Beyotime Institute of
Biotechnology) were infected into SH-SY5Y cells, according to the
manufacturer's protocol. SH-SY5Y cells were inoculated into 24-well
plates (2.5x105/well) and cultured for 24 h at 37˚C.
Subsequently, the culture medium was removed and a virus solution
at MOI=5 was added. Cells that were not exposed to the virus served
as the control group. Cells were cultured for 12 h at 37˚C with 5%
CO2. After 24 h of treatment with CGA (50 µM) and/or
H2O2 (500 µM), mean fluorescence intensities
were measured using Image-Pro Plus software.
Western blotting
The primary antibodies used in the present study
were as follows: Anti-rabbit-sequestosome 1 (SQSTM1)/p62 (1:1,000;
Cell Signaling Technology, Inc.), anti-rabbit-Lamin A/C (1:1,000;
Cell Signaling Technology, Inc.), anti-rabbit-phosphorylated-mTOR
Ser 2448 (P-mTOR; 1:1,000; Cell Signaling Technology, Inc.),
anti-rabbit-TFEB (1:1,000; Cell Signaling Technology, Inc.),
anti-rabbit-mTOR (1:1,000; Cell Signaling Technology, Inc.),
anti-rabbit-LC3B (1:500; Cell Signaling Technology, Inc.),
anti-rabbit-Beclin 1 (1:500; BIOSS), anti-rabbit-autophagy protein
5 (Atg5)/autophagy related 5 (1:1,000; BIOSS), anti-rabbit GAPDH
(1:5,000; Wuhan Servicebio Technology Co., Ltd.),
anti-rabbit-cathepsin D (CTSD; 1:500; Beyotime Institute of
Biotechnology), anti-rabbit-Bcl-2 (1:500; Wuhan Sanying
Biotechnology), anti-rabbit-Bax (1:500; Wuhan Sanying Biotechnology
Co., Ltd.). Cells were washed with PBS and subjected to lysis at
4˚C in RIPA lysis buffer (Servicebio Technology Co., Ltd.)
supplemented with phenyl methylsulfonyl fluoride [Hangzhou Multi
Sciences (Lianke) Biotech Co., Ltd.], phosphatase inhibitors
(Boster Biological Technology) at a ratio of 100:1:1 and a cocktail
of protease inhibitors [Hangzhou Multi Sciences (Lianke) Biotech
Co., Ltd.]. Cell suspensions were centrifuged at 800 x g for 10 min
at 4˚C and protein concentrations were quantified using a BCA kit
(Sichuan Mike Biological Technology Co., Ltd.). Protein extracts
(30 µg/lane) were separated and fractionated via 8-15% SDS-PAGE and
transferred to a PVDF membrane (Merck KGaA). The membranes were
blocked with 5% BSA (Guangzhou Saiguo Biotech Co., Ltd.) for 90 min
at room temperature. Cells were incubated with primary antibodies
overnight at 4˚C. After washing with TBS with 0.1% Tween-20,
membranes were incubated with horseradish peroxidase-conjugated
goat anti-rabbit secondary antibodies (1:5,000; BIOSS) for 2 h at
room temperature. The best blocking reagent was 5% BSA at 90 min.
Following washing, bands were visualized using an ECL kit (Beijing
4A Biotech Co., Ltd.). Quantity One software (v4.6.6; Bio-Rad
Laboratories, Inc.) was used for quantification.
LysoTracker Red and LysoSensor™ Green
staining
Lysosomal staining was performed using LysoTracker
Red and LysoSensor Green staining assays in order to assess
lysosomal acidification. Following 16 h of treatment with CGA (50
µM) at 37˚C, Earle's Balanced Salt Solution (EBSS; cat. no.
354655A; incubation, 30 min; Beyotime Institute of Biotechnology),
chloroquine (CQ; 25 µM; incubation, 2 h; cat. no. J0102A; Dalian
Meilun Biology Technology Co., Ltd.) and H2O2
(500 µM; incubation, 12 h) were added. Cells
(2.5x105/well) were subsequently incubated with
LysoTracker Red (5 µl) and LysoSensor Green (10 µl) for 30 min at
37˚C, washed with PBS and examined using fluorescence microscopy
(Leica Microsystems GmbH). The mean fluorescence intensity was
measured using Image-Pro Plus software (version 6.0; Media
Cybernetics, Inc.).
Statistical analysis
Statistical analysis was performed using SPSS
software (version 21.0; IBM Corp.). Quantitative data are presented
as the mean ± SD. All experiments were repeated at least three
times Non-parametric tests were used for data that were not
normally distributed. Comparisons among different groups were
performed using one-way ANOVA. If the datasets contained more than
three groups, Tukey's multiple comparison analysis was used.
P<0.05 was considered to indicate a statistically significant
difference.
Results
Effect of CGA on SH-SY5Y cells
SH-SY5Y cells were treated with different
concentrations of H2O2 to determine the
optimal concentration for subsequent experiments, as assessed by
CCK-8 assays. Different concentrations of
H2O2 (100-800 µM) affected cells differently
at 12 and 24 h compared with the normal group (Fig. 1A). Treatment with
H2O2 decreased cell viability in a dose- and
time-dependent manner, and the IC50 of 500 µM
H2O2 regarding cell injury at 24 h was 973.90
µM. The cell viability was 69.25±3.17% following treatment with 500
µM H2O2 for 24 h. Based on the results of
this experiment and previous studies, 500 µM
H2O2 and 24 h were selected as the optimal
damage conditions for subsequent experiments (27,28).
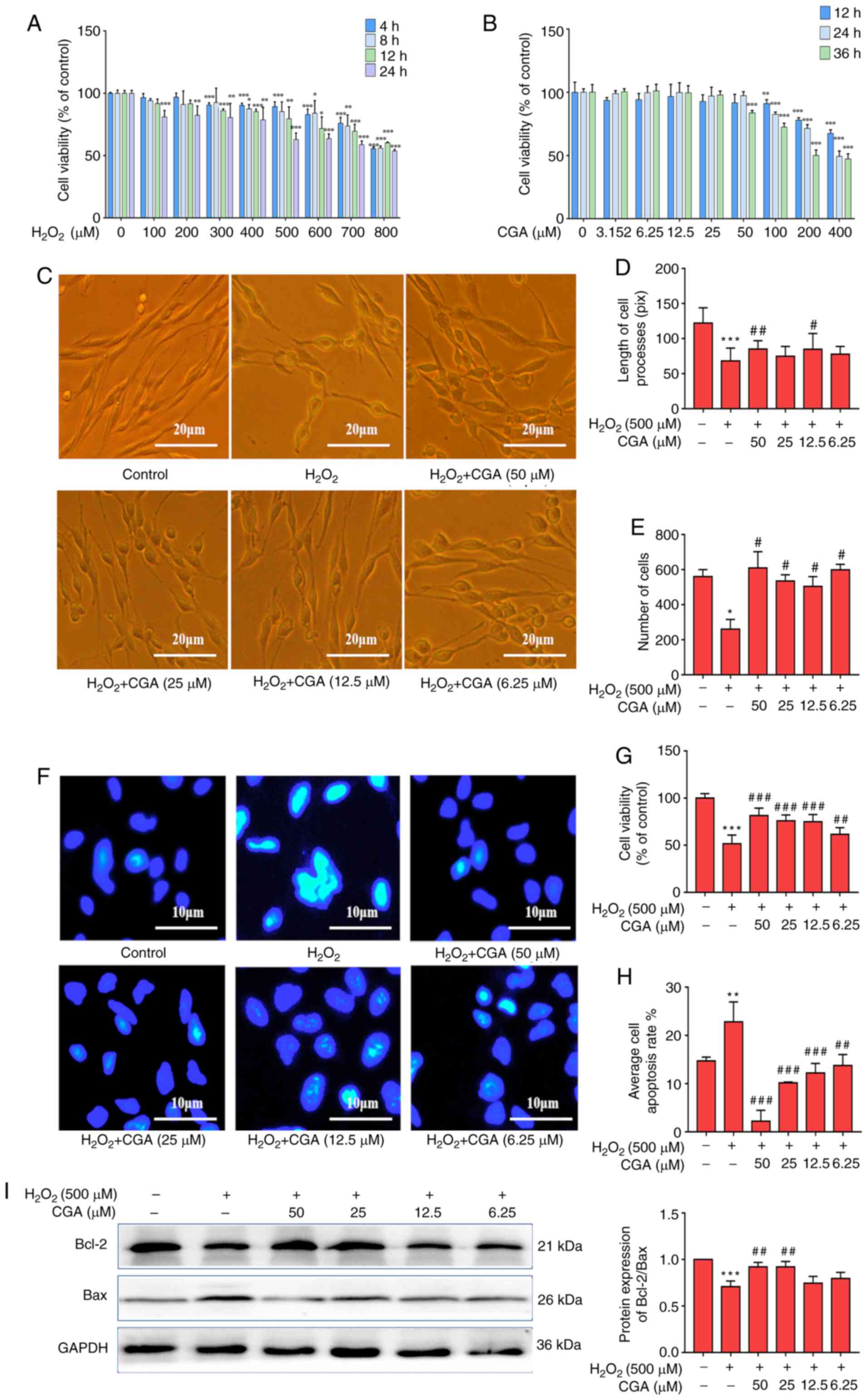 | Figure 1Neuroprotective effects of CGA on
SH-SY5Y cells injured by H2O2. (A) Cell
viability of SH-SY5Y cells damaged by different concentrations of
H2O2 at 4, 8, 12 and 24 h. (B) Cell viability
of SH-SY5Y cells following treatment with different concentrations
of CGA for 12, 24 and 36 h. (C) Morphology of SH-SY5Y cells in the
different groups. Scale bar, 20 µm. (D) Concentrations of 50 and
12.5 µM CGA restored the morphology of SH-SY5Y cells damaged by
H2O2 for 24 h. (E) Numbers of SH-SY5Y cells
were rescued by different concentrations of CGA. (F) Hoechst
staining of SH-SY5Y cells in each group. Scale bar, 10 µm. (G) Cell
viability of SH-SY5Y cells was significantly increased by different
concentrations of CGA compared with control group. (H) Average cell
apoptosis rate was significantly decreased in each CGA group. (I)
Representative western blotting images of Bcl-2 and Bax expression
in SH-SY5Y cells (left panel). GAPDH was used as the loading
control. Relative optical density values of Bcl-2/Bax (right
panel). *P<0.05, **P<0.01 and
***P<0.001 vs. control group. #P<0.05,
##P<0.01 and ###P<0.001 vs.
H2O2 treatment group. CGA, chlorogenic acid;
H2O2, hydrogen peroxide; Bcl-2, B-cell
lymphoma 2; Bax, Bcl-2 associated X. |
According to previous study, CGA has a protective
effect induced by H2O2 (29). However, CGA treatment decreased cell
viability and exhibited drug toxicity at concentrations of 50-400
µM at 12-36 h (Fig. 1B). Therefore,
6.25-50 µM was regarded as the optimum concentration range for CGA
treatment in the present study.
Neuroprotective effect of CGA on
SH-SY5Y cells injured by H2O2
To investigate the effects of CGA on SH-SY5Y cells
injured by H2O2, four concentrations of CGA
(50, 25, 12.5 and 6.25 µM) were used. The morphology of the cells
was altered following 24 h of incubation with CGA (Fig. 1C). Data revealed that the length of
cell processes significantly reduced in the
H2O2 group, luckily, it could be rescue by
CGA, CGA concentrations of 50 and 12.5 µM ameliorated the
morphological alterations (length of cell processes) of the cell
model (Fig. 1D). Similarly, the
numbers and viability of the SH-SY5Y cells were rescued by all
concentrations of CGA (Fig. 1E and
G).
Cell apoptosis was assessed with Hoechst staining
and the average cell apoptosis rate was significantly decreased in
each CGA treatment group compared with the
H2O2 treatment group (Fig. 1F and H). The changes of Bax and Bcl-2 protein
expression may play an important role in cell apoptosis (30). Therefore, the results demonstrated
that CGA upregulated Bcl-2 expression and downregulated Bax
expression to inhibit cell apoptosis (Fig. 1I). It has been suggested that the
Bax/Bcl-2 ratio may be more important than either protein alone in
determining apoptosis (31). These
results indicated that CGA alleviated the morphological damage and
effectively reduced apoptosis in H2O2-treated
SH-SY5Y cells.
Effect of CGA on autophagic flux in
SH-SY5Y cells treated with H2O2
The effects of CGA on the accumulation of autophagic
vacuoles in SH-SY5Y cells injured by H2O2
were assessed. H2O2 treatment increased the
average fluorescence intensity of MDC in a time-dependent manner
when compared with the control group (Fig. 2A and B). Furthermore, the different
concentrations of CGA significantly reduced the average
fluorescence intensity of MDC in the cell model at 12 and 24 h
(Fig. 2A and B).
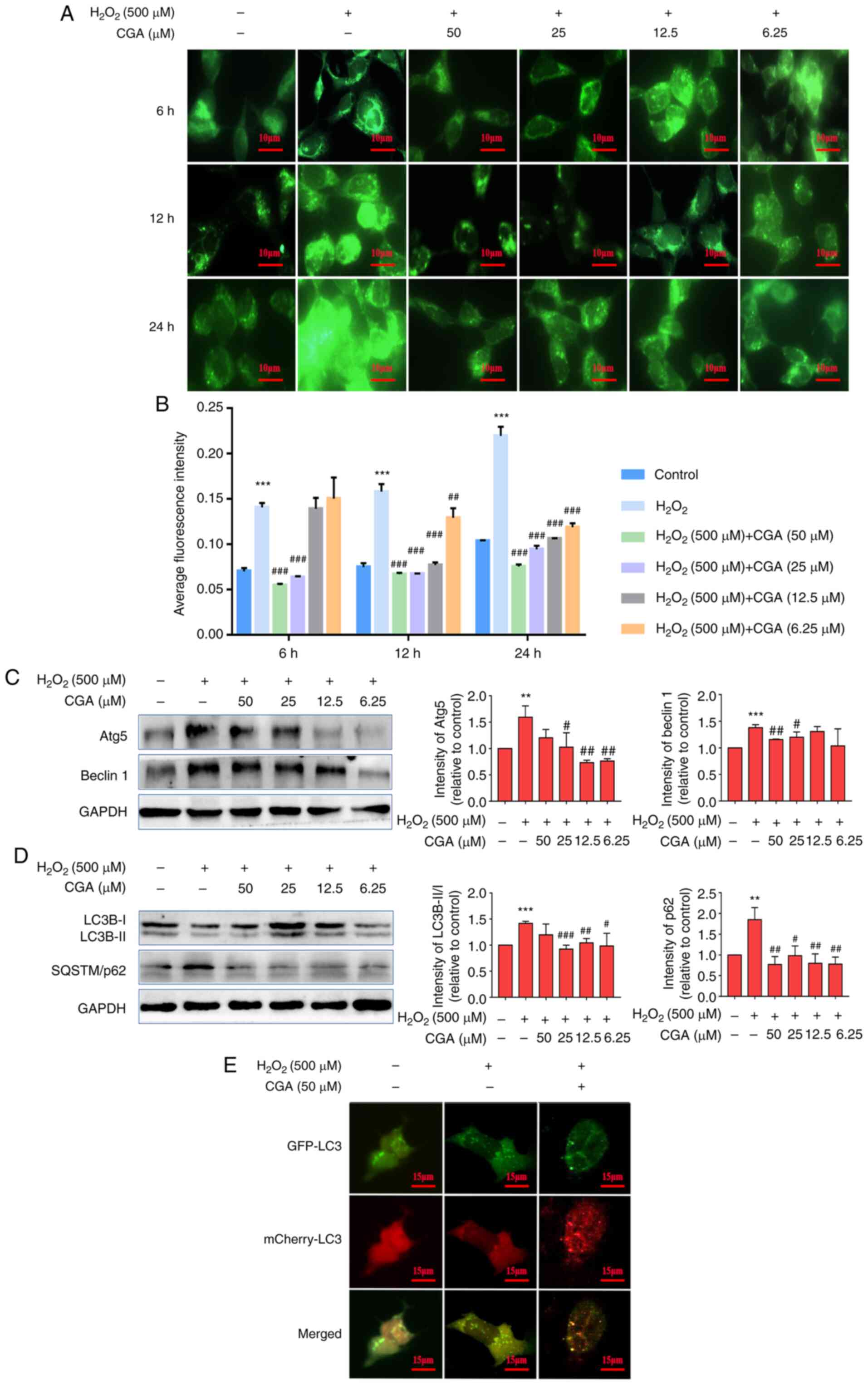 | Figure 2Effects of CGA on autophagic flux in
SH-SY5Y cells injured by H2O2. (A) Effects of
CGA on autophagic vacuole accumulation in SH-SY5Y cells injured by
H2O2. Scale bar, 10 µm. (B) CGA treatments
significantly reduced the average fluorescence intensity of
monodansylcadaverine at 6 (25 and 50 µM), 12 and 24 h compared with
the H2O2 group. (C) Intensity of Beclin-1
bands in SH-SY5Y cells was decreased following incubation with 50
or 25 µM CGA compared with the H2O2 group.
Additionally, the intensity of Atg5 bands was decreased in the 25,
12.5 and 6.25 µM CGA groups. Beclin-1 and Atg5 levels were
normalized to GAPHD. (D) Intensity of LC3B-II/I bands in the
SH-SY5Y cells was decreased following incubation with 25, 12.5 and
6.25 µM CGA compared with the H2O2 group.
Additionally, the intensity of the SQSTM/p62 bands was decreased in
each CGA group. LC3-II/I and p62 levels were normalized to GAPHD.
(E) mCherry-GFP-LC3 adenovirus transfection was used to detect the
effect of CGA on SH-SY5Y cells. mCherry-GFP-LC3 specifically labels
autophagosomes. Scale bar, 15 µm. **P<0.01 and
***P<0.001 vs. control group; #P<0.05,
##P<0.01 and ###P<0.001 vs.
H2O2 treatment group. CGA, chlorogenic acid;
H2O2, hydrogen peroxide; Atg5, autophagy
protein 5; SQSTM, sequestosome 1; GFP, green fluorescent
protein. |
Autophagic flux is a dynamic process and includes
the formation of autophagosomes, fusion of autophagosomes with
lysosomes and cargo degradation. Atg5 and Beclin-1 are involved in
the formation of autophagosomes (32) and the results indicated that
H2O2 increased Atg5 and Beclin-1 levels,
which likely promoted an enhancement of autophagosome formation. To
further characterize the effects of CGA on autophagic flux in the
SH-SY5Y cell model, autophagy-associated proteins were assessed in
each group. Western blotting demonstrated that the expression
levels of Beclin-1 in the 50 and 25 µM CGA groups and Atg5 in the
25, 12.5 and 6.25 µM CGA groups were significantly downregulated
(Fig. 2C). These data indicated
that CGA inhibition of autophagosome production may occur via the
downregulation of Beclin-1 and Atg5 expression.
To determine whether CGA had any effect on
H2O2-mediated autophagy activation, western
blotting was performed. The results indicated that the intensity of
LC3B-II/I bands in the SH-SY5Y cell model was decreased in the 25,
12.5 and 6.25 µM CGA groups compared with the
H2O2 treatment group. P62/SQSTM1 is an
autophagy-selective substrate complex and LC3B-II is localized on
the inner membrane to form the complex (33). The expression levels of P62/SQSTM1
were decreased in each CGA treatment group (Fig. 2D). Therefore, these results
suggested that CGA increased autophagic flux by reducing the levels
of LC3-II/I and P62/SQSTM1.
SH-SY5Y cells successfully transfected with
mCherry-GFP-LC3 were used as vectors to investigate the effect of
CGA on autophagic flow. When autophagy is activated, the
fluorescent GFP signal enters the lysosome. However, during
autophagy flux, lysosomes fuse with autophagosomes, and the green
(GFP) fluorescence is quenched by the acidic microenvironment
whereas the mCherry signal remains stable (34). After entering the lysosome, the
mCherry signal can still be observed (34). The co-localization of GFP and
mCherry (observed as yellow fluorescence) represents the blockage
of autophagic flow. Compared with the H2O2
treatment group, treatment with CGA reduced the occurrence of
mCherry+ GFP+ (yellow) spots induced by
H2O2 and increased the occurrence of
mCherry+ GFP- (red) spots (Fig. 2E).
Effect of CGA on lysosome function in
SH-SY5Y cells injured by H2O2
To investigate how CGA activity may affect
autophagy, lysosome function in the SH-SY5Y cell model was
assessed. CQ is a lysosomal blocker that blocks the
autophagy-lysosomal pathway (ALP) by alkalizing the acidic
environment of lysosome and inhibiting the degradation of lysosomes
(35). Following 25 µM CQ
pretreatment for 2 h to inhibit lysosome activity, the results
indicated that CQ successfully reversed the decreased cell
viability conferred by H2O2 treatment
(Fig. 3A). These results indicated
that H2O2 reduced cell viability, and CGA
significantly relieved the damage caused by
H2O2 in SH-SY5Y cells. However, CQ had an
antagonistic effect on the anti-H2O2 effects
of CGA in regard to cytotoxicity (Fig.
3B).
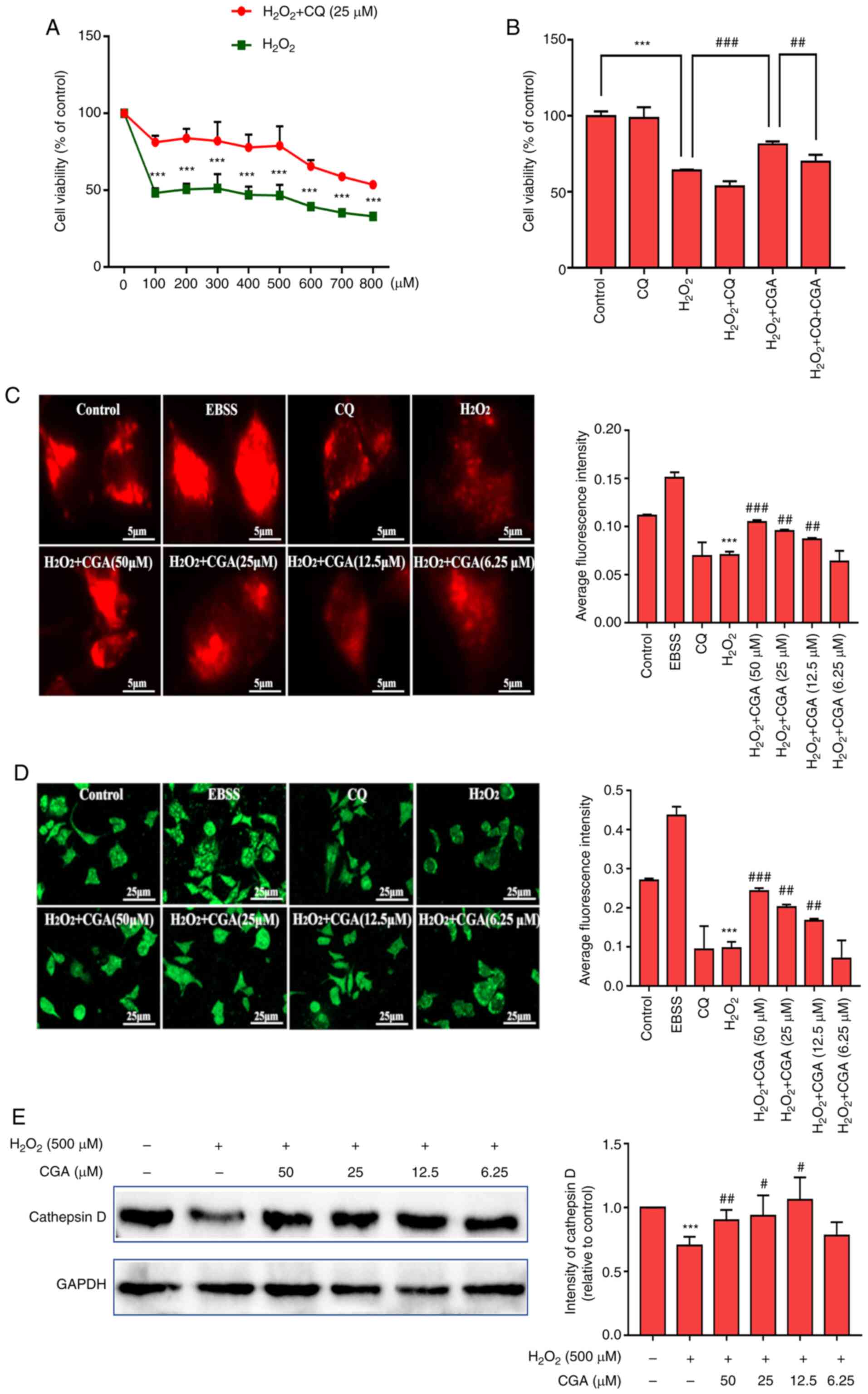 | Figure 3Effect of CGA on lysosome function in
SH-SY5Y cells injured by H2O2. (A) CQ
pretreatment prevented damage to SH-SY5Y cells as the cell
viability of SH-SY5Y cells was significantly decreased in the
H2O2 + CQ group. ***P<0.001 vs.
H2O2 group. (B) Cell viability of SH-SY5Y
cells was reduced by H2O2 treatment; however,
cell viability was rescued by CGA treatment. However, cell
viability was lower in the H2O2 + CQ + CGA
group compared with the H2O2 + CGA group.
***P<0.001, ##P<0.05 and
###P<0.01, as indicated. (C) LysoTracker Red was used
to specifically label intracellular lysosomes. Scale bar, 5 µm.
***P<0.001 vs. control group; ##P<0.01
and ###P<0.001 vs. H2O2
treatment group. (D) Lysosomes were labeled with LysoSensor Green
and observed by fluorescence microscopy. Scale bar, 25 µm.
***P<0.001 vs. control group; ##P<0.01
and ###P<0.001 vs. H2O2
treatment group. (E) Representative western blotting images of CTSD
in SH-SY5Y cells (left panel). ***P<0.001 vs. control
group; #P<0.05 and ##P<0.01 vs.
H2O2 treatment group. GAPDH was used as the
internal control. Relative optical density values of CTSD in the
experimental groups (right panel). CGA, chlorogenic acid;
H2O2, hydrogen peroxide; CQ, chloroquine;
CTSD, cathepsin D; EBSS, Earle's Balanced Salt Solution. |
Following this, the effects of CGA on the lysosomal
acidity of H2O2-treated SH-SY5Y cells were
investigated. LysoTracker Red specifically labels intracellular
lysosomes and these are presented as red fluorescent spots under a
fluorescence microscope. LysoSensor Green exhibits a pH-dependent
increase in fluorescence intensity following acidification, which is
used to detect the effect of CGA on the lysosomes. EBSS is a
balanced salt solution that induces autophagosome formation via
starvation while rapidly upregulating the acidic environment of the
lysosomes and, therefore, promoting lysosomal degradation and
enhancing the ALP (36).
H2O2 treatment significantly decreased the
average fluorescence intensity of LysoTracker Red (Fig. 3C). Compared with the
H2O2 treatment group, the 50, 25 and 12.5 µM
CGA groups exhibited significant upregulation of the mean
fluorescence intensity of LysoTracker Red induced by
H2O2. The acidic intracellular compartments
(lysosomes) in SH-SY5Y cells were measured using LysoSensor Green.
H2O2 (500 µM) exposure significantly reduced
the fluorescence signal compared with the control group (Fig. 3D), indicating a
H2O2-mediated reduction of intracellular
acidic components. Furthermore, treatment with CGA significantly
increased LysoSensor Green fluorescence compared with the
H2O2 treatment group. Finally, the expression
levels of CTSD in the different CGA groups were determined. These
data revealed that the levels of CTSD were significantly enhanced
in the 50, 25 and 12.5 µM CGA + H2O2 groups
compared with the H2O2 treatment group
(Fig. 3E).
Effect of CGA on the mTOR-TFEB
signaling pathway in SH-SY5Y cells treated with
H2O2
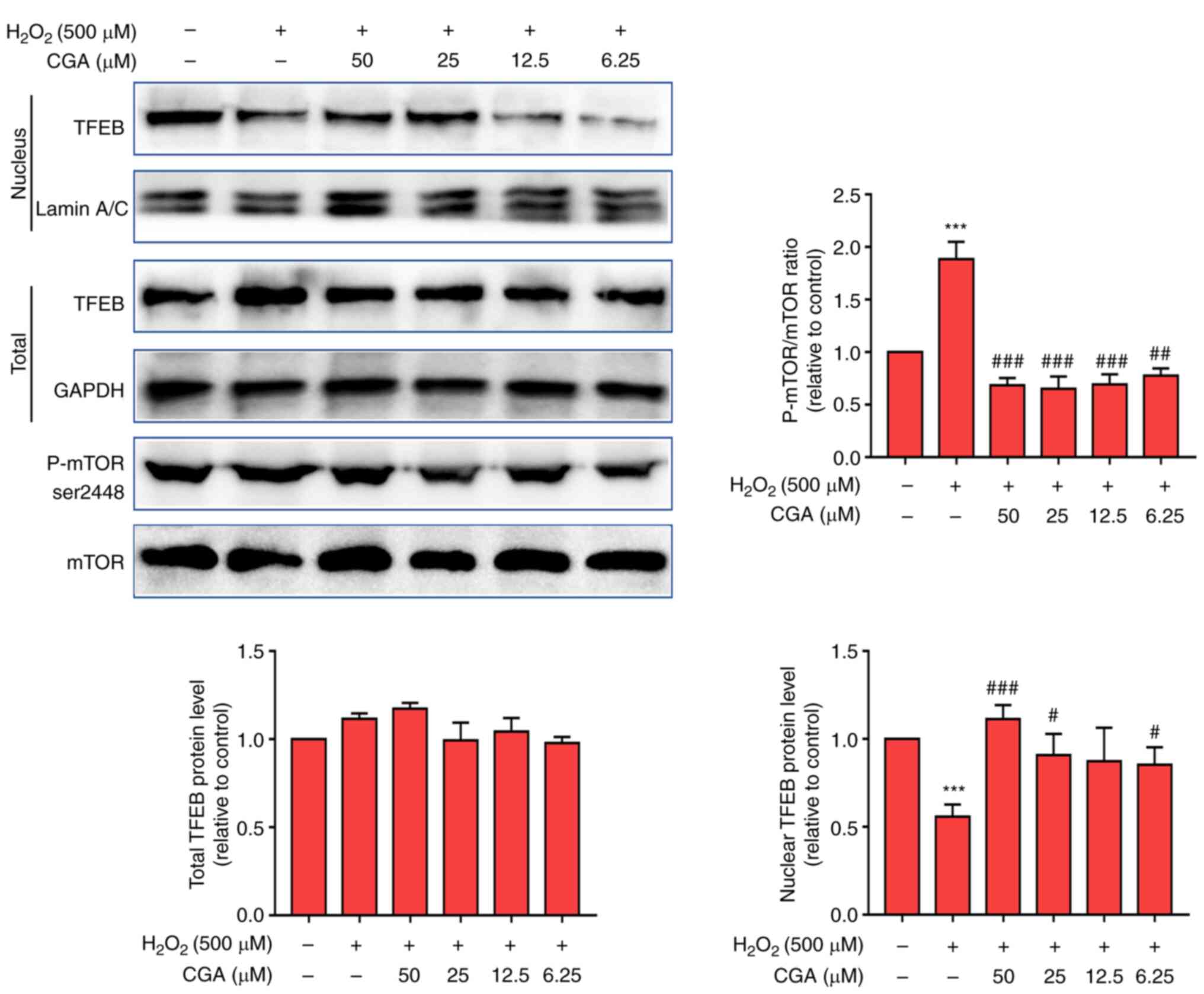
Western blotting was used to determine total protein
and TFEB levels in cell nuclei. The results indicated that
H2O2 had no significant effect on the overall
levels of TFEB in cells (Fig. 4).
However, H2O2 significantly decreased the
levels of nuclear TFEB and increased the ratio of P-mTOR/mTOR
compared with the normal group. Furthermore, CGA treatment (50, 25
and 6.25 µM) significantly increased the levels of nuclear TFEB and
decreased the ratio of P-mTOR/mTOR compared with the
H2O2 treatment group.
Discussion
A previous study has reported that natural compounds
reduce the risk of developing neurodegenerative conditions,
including vitamins A, C, and E and β-carotene (37). The present study provided additional
evidence for the pivotal role of CGA in AD pathogenesis. CGA
attenuated cell injury and apoptosis, reduced autophagy
overactivation, increased autophagic flux and enhanced lysosomal
function in SH-SY5Y cells injured by H2O2.
Additionally, the results demonstrated that CGA may inhibit
excessive autophagy by regulating the mTOR-TFEB signaling pathway
and decreasing TFEB nuclear translocation.
AD is a neurodegenerative disorder with no known
cure (1). The progression of Aβ
plaque accumulation and hyperphosphorylation of τ proteins are
enhanced by oxidative stress (38).
The risk of AD increases significantly with age and, based on the
hypotheses proposed to explain the causes of aging and AD, it has
been reported that oxidative stress serves a central role in this
occurrence (37). A previous study
has indicated that brain tissues of patients with AD are exposed to
oxidative stress during the development of the disease (39). Over time, oxidative stress
contributes to a range of aging-related degenerative diseases,
including cancer, diabetes, macular degeneration and AD (37). Furthermore, oxidative stress is one
of the main causes of neuronal degeneration and loss-of-function in
patients with AD and it can interact with β-amyloid peptides and
activate signaling pathways to promote the occurrence and
progression of AD (37).
Neuroblastoma cell lines, including SH-SY5Y, are the most
frequently utilized models in neurodegenerative studies and their
use has advanced the understanding of the pathology of
neurodegeneration over the past few decades (40).
CGA is a phenylpropanoid compound produced in plants
via the shikimic acid pathway during aerobic respiration (22). Polyphenols are secondary plant
metabolites, comprising several antioxidant compounds and are
generally considered to be involved in the defense against numerous
chronic human diseases, including prostate cancer among others,
cardiovascular diseases, diabetes mellitus and neurodegenerative
diseases, such as Alzheimer's and Parkinson's disease (41). CGA is a polyphenolic substance
widely found in the human diet (22). CGA has various pharmacologic
actions, including antioxidant, anti-inflammatory and
anticarcinogenic functions, and exerts hypoglycemic and
hypolipidemic effects (20).
Furthermore, CGA is mainly found in Eucommia ulmoides,
sunflowers, coffee, burdock, honeysuckle and cocoa, and contains a
plurality of phenolic hydroxyl groups that form hydrogen radicals
and possesses strong antioxidant properties (19). Previous studies have demonstrated
that CGA reduces the risk of developing neurodegenerative
conditions, such as Alzheimer's and Parkinson's disease, via its
inhibitory effect on Aβ40 aggregation (21,23).
Additionally, a clinical study has demonstrated that patients who
exhibit subjective memory loss of composite and verbal memory show
improved complex attention, cognitive flexibility, executive
function, motor speed domains of the central nervous system
following a 6-month intake of a test beverage containing CGAs prior
to bedtime (42). The present study
observed that H2O2 decreased cell viability
and affected the morphology of SH-SY5Y cells. Importantly, CGA
ameliorated these morphological alterations and reduced the
occurrence of cell apoptosis in a dose- and time-dependent manner
following damage by H2O2.
To further clarify the mechanism of CGA action in
this neuroprotective role, the ALP in
H2O2-treated SH-SY5Y cells was assessed. The
data indicated that CGA treatment reduced the average fluorescence
intensity of MDC within the cells and inhibited autophagosome
production by downregulating the levels of Beclin-1 and Atg5.
Additionally, CGA enhanced lysosomal function in SH-SY5Y cells.
Defects of the ALP are strongly associated with AD and the ALP has
been reported to regulate APP turnover and Aβ metabolism (43). The accumulation of proinflammatory
cytokines and dysfunction of the ALP may damage hippocampal
neuronal cells, which is associated with the pathogenesis of AD
(44). Autophagy influences the
secretion of Aβ to the extracellular space, thereby directly
affecting Aβ plaque formation, a pathological hallmark of AD
(45). During autophagy, LC3-I is
conjugated with phosphatidylethanolamine to form LC3-II, recruited
to the autophagosomal membranes and degraded by lysosomal
hydrolases following the fusion of autophagosomes with lysosomes
(46). Therefore, the LC3-II/I
ratio is directly proportional to the number of autophagosomes and
is considered to be an important measure of autophagosome levels
(46). Cytoplasmic components are
selected and tagged prior to being sequestered into an
autophagosome by p62/SQSTM1 during selective autophagy and the
transcription of p62 is markedly increased during conditions in
which selective autophagy substrates have accumulated (47). Therefore, H2O2
treatment induced the levels of LC3-II/I and P62/SQSTM1, while CGA
prevented this, preventing the accumulation of autophagic vacuoles
and the increase of autophagy-related proteins. Furthermore,
defects in lysosomal function appear at the earliest stages of AD
development and progress to a widespread failure of intraneuronal
waste clearance, neuritic dystrophy and neuronal cell death, as
well as inhibition of autophagic flow and decreased levels of
autophagosome degradation (48).
The results of the present study indicated that CGA enhanced
lysosomal function by increasing the levels of CTSD. CTSD
upregulation may be an adaptive response to AD-related processes,
leading to neurofibrillary degeneration (49). Additionally, the activity of CTSD
has been associated with the metabolism of cholesterol and
glycosaminoglycans, which accounts for its involvement in neuronal
plasticity (50). Therefore, CGA
likely serves a vital neuroprotective role during the progression
of AD.
The present study aimed to identify the signaling
pathway by which CGA affected the progression of AD. TFEB is a
transcription factor that upregulates lysosomal function when
translocated into the nucleus. mTOR is a key protein involved in
regulating factors involved upstream of TFEB nuclear transcription
and these factors mainly function to promote TFEB entry into the
nucleus by inhibiting phosphorylation of mTOR at the serine 2448
phosphorylation site (51). The
results of the present study demonstrated that CGA treatment
enhanced the levels of nuclear TFEB and decreased the ratio of
P-mTOR/mTOR. It was hypothesized that CGA serves an indispensable
neuroprotective role in the process of AD, likely via the mTOR-TFEB
signaling pathway. Prior research has revealed that alterations of
mTOR signaling and autophagy occur at the early stages of AD and
are associated with autophagy impairment (52). Additionally, autophagy is strictly
regulated by the mTOR signaling pathway, and regulation of the
functional status of autophagy via the mTOR signaling pathway
during physical activity is used for the prevention and treatment
of AD (53).
In summary, the present study demonstrated that CGA
inhibited the activation of the mTOR-TFEB signaling pathway by
upregulating lysosomal function and promoting autophagic flux to
exert neuroprotective effects.
Acknowledgements
Not applicable.
Funding
The present study was supported by the e National Natural
Science Foundation of China (No. 82074150), Key Research and
Development Project of Sichuan Province (Major Science and
Technology Projects; grant no. 19ZDYF0600) and the Chengdu
Technological Innovation R & D project (grant no.
2019-YF05-01332-SN).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
SJX conceived, designed the experiments and
critically revised the manuscript. LJG devised the methodology,
performed the experiments and wrote the original draft. XQL
performed the data analyses and participated in the revision of the
manuscript. YD and ZQZ contributed the sample assays and revised
the manuscript. SM performed data analysis and assisted in
preparing the figures. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Pugazhenthi S: Metabolic syndrome and the
cellular phase of Alzheimer's disease. Prog Mol Biol Transl Sci.
146:243–258. 2017.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Ryu JC, Zimmer ER, Rosa-Neto P and Yoon
SO: Consequences of metabolic disruption in Alzheimer's disease
pathology. Neurotherapeutics. 16:600–610. 2019.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Lee M, McGeer E and McGeer PL: Activated
human microglia stimulate neuroblastoma cells to upregulate
production of beta amyloid protein and tau: Implications for
Alzheimer's disease pathogenesis. Neurobiol Aging. 36:42–52.
2015.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Hampel H, Mesulam MM, Cuello AC,
Khachaturian AS, Vergallo A, Farlow MR, Snyder PJ, Giacobini E and
Khachaturian ZS: Revisiting the cholinergic hypothesis in
Alzheimer's disease: Emerging evidence from translational and
clinical research. J Prev Alzheimers Dis. 6:2–15. 2019.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Manyevitch R, Protas M, Scarpiello S,
Deliso M, Bass B, Nanajian A, Chang M, Thompson SM, Khoury N,
Gonnella R, et al: Evaluation of metabolic and synaptic dysfunction
hypotheses of Alzheimer's disease (AD): A meta-analysis of CSF
markers. Curr Alzheimer Res. 15:164–181. 2018.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Haapasalo A, Pikkarainen M and Soininen H:
Alzheimer's disease: A report from the 7th Kuopio Alzheimer
symposium. Neurodegener Dis Manag. 5:379–382. 2015.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Chen XQ and Mobley WC: Exploring the
pathogenesis of Alzheimer disease in basal forebrain cholinergic
neurons: Converging insights from alternative hypotheses. Front
Neurosci. 13(446)2019.PubMed/NCBI View Article : Google Scholar
|
|
8
|
He Z, Guo JL, McBride JD, Narasimhan S,
Kim H, Changolkar L, Zhang B, Gathagan RJ, Yue C, Dengler C, et al:
Amyloid-β plaques enhance Alzheimer's brain tau-seeded pathologies
by facilitating neuritic plaque tau aggregation. Nat Med. 24:29–38.
2018.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Swomley AM and Butterfield DA: Oxidative
stress in Alzheimer disease and mild cognitive impairment: Evidence
from human data provided by redox proteomics. Arch Toxicol.
89:1669–1680. 2015.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Korovila I, Hugo M, Castro JP, Weber D,
Höhn A, Grune T and Jung T: Proteostasis, oxidative stress and
aging. Redox Biol. 13:550–567. 2017.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Lévy E, El Banna N, Baïlle D,
Heneman-Masurel A, Truchet S, Rezaei H, Huang ME, Béringue V,
Martin D and Vernis L: Causative links between protein aggregation
and oxidative stress: A review. Int J Mol Sci.
20(3896)2019.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Chu Q, Zhu Y, Cao T and Zhang Y, Chang Z,
Liu Y, Lu J and Zhang Y: Studies on the neuroprotection of osthole
on glutamate-induced apoptotic cells and an Alzheimer's disease
mouse model via modulation oxidative stress. Appl Biochem
Biotechnol. 190:634–644. 2020.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Alvariño R, Alonso E, Abbasov ME, Chaheine
CM, Conner ML, Romo D, Alfonso A and Botana LM: Gracilin A
derivatives target early events in Alzheimer's disease: In vitro
effects on neuroinflammation and oxidative stress. ACS Chem
Neurosci. 10:4102–4111. 2019.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Bagherniya M, Butler AE, Barreto GE and
Sahebkar A: The effect of fasting or calorie restriction on
autophagy induction: A review of the literature. Ageing Res Rev.
47:183–197. 2018.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Castillo K, Valenzuela V, Oñate M and Hetz
C: A molecular reporter for monitoring autophagic flux in nervous
system in vivo. Methods Enzymol. 588:109–131. 2017.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Reddy PH and Oliver DM: Amyloid beta and
phosphorylated tau-induced defective autophagy and mitophagy in
Alzheimer's disease. Cells. 8(488)2019.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Correia SC, Resende R, Moreira PI and
Pereira CM: Alzheimer's disease-related misfolded proteins and
dysfunctional organelles on autophagy menu. DNA Cell Biol.
34:261–273. 2015.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Damme M, Suntio T, Saftig P and Eskelinen
EL: Autophagy in neuronal cells: General principles and
physiological and pathological functions. Acta Neuropathol.
129:337–362. 2015.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Gao L, Li X, Meng S, Ma T, Wan L and Xu S:
Chlorogenic acid alleviates Aβ25-35-induced autophagy
and cognitive impairment via the mTOR/TFEB signaling pathway. Drug
Des Devel Ther. 14:1705–1716. 2020.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Farhood HB, Balas M, Gradinaru D, Margina
D and Dinischiotu A: Hepatoprotective effects of chlorogenic acid
under hyperglycemic conditions. Rom Biotechnol Lett. 1–10.
2017.
|
|
21
|
Heitman E and Ingram DK: Cognitive and
neuroprotective effects of chlorogenic acid. Nutr Neurosci.
20:32–39. 2017.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Tajik N, Tajik M, Mack I and Enck P: The
potential effects of chlorogenic acid, the main phenolic components
in coffee, on health: A comprehensive review of the literature. Eur
J Nutr. 56:2215–2244. 2017.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Yang L, Wang N and Zheng G: Enhanced
effect of combining chlorogenic acid on selenium nanoparticles in
inhibiting amyloid β aggregation and reactive oxygen species
formation in vitro. Nanoscale Res Lett. 13(303)2018.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Taram F, Winter AN and Linseman DA:
Neuroprotection comparison of chlorogenic acid and its metabolites
against mechanistically distinct cell death-inducing agents in
cultured cerebellar granule neurons. Brain Res. 1648:69–80.
2016.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Wang XJ, Wang LY, Fu Y, Wu J, Tang XC,
Zhao WM and Zhang HY: Promising effects on ameliorating
mitochondrial function and enhancing Akt signaling in SH-SY5Y cells
by (M)-bicelaphanol A, a novel dimeric podocarpane type
trinorditerpene isolated from Celastrus orbiculatus. Phytomedicine.
20:1064–1070. 2013.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Zheng Y, Ma L, Liu N, Tang X, Guo S, Zhang
B and Jiang Z: Autophagy and apoptosis of porcine ovarian granulosa
cells during follicular development. Animals (Basel).
9(1111)2019.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Shi C, Zhao L, Zhu B, Li Q, Yew DT, Yao Z
and Xu J: Dosage effects of EGb761 on hydrogen peroxide-induced
cell death in SH-SY5Y cells. Chem Biol Interact. 180:389–397.
2009.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Wang W, Sun F, An Y, Ai H, Zhang L, Huang
W and Li L: Morroniside protects human neuroblastoma SH-SY5Y cells
against hydrogen peroxide-induced cytotoxicity. Eur J Pharmacol.
613:19–23. 2009.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Yu BW, Li JL, Guo BB, Fan HM, Zhao WM and
Wang HY: Chlorogenic acid analogues from Gynura nepalensis protect
H9c2 cardiomyoblasts against H2O2-induced
apoptosis. Acta Pharmacol Sin. 37:1413–1422. 2016.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Chao X, Ni HM and Ding WX: Insufficient
autophagy: A novel autophagic flux scenario uncovered by impaired
liver TFEB-mediated lysosomal biogenesis from chronic
alcohol-drinking mice. Autophagy. 14:1646–1648. 2018.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Aghdaei HA, Kadijani AA, Sorrentino D,
Mirzaei A, Shahrokh S, Balaii H, Geraci M and Zali MR: An increased
Bax/Bcl-2 ratio in circulating inflammatory cells predicts primary
response to infliximab in inflammatory bowel disease patients.
United European Gastroenterol J. 6:1074–1081. 2018.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Zhao T, Fu Y, Sun H and Liu X:
Ligustrazine suppresses neuron apoptosis via the Bax/Bcl-2 and
caspase-3 pathway in PC12 cells and in rats with vascular dementia.
IUBMB Life. 70:60–70. 2018.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Katsuragi Y, Ichimura Y and Komatsu M:
p62/SQSTM1 functions as a signaling hub and an autophagy adaptor.
FEBS J. 282:4672–4678. 2015.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Ahsan A, Zheng Y, Ma S, Liu M, Cao M, Li
Y, Zheng W, Zhou X, Xin M, Hu WW, et al: Tomatidine protects
against ischemic neuronal injury by improving lysosomal function.
Eur J Pharmacol. 882(173280)2020.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Schaaf MB, Houbaert D, Meçe O, To SK,
Ganne M, Maes H and Agostinis P: Lysosomal pathways and autophagy
distinctively control endothelial cell behavior to affect tumor
vasculature. Front Oncol. 9(171)2019.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Pierzyńska-Mach A, Janowski PA and
Dobrucki JW: Evaluation of acridine orange, LysoTracker Red, and
quinacrine as fluorescent probes for long-term tracking of acidic
vesicles. Cytometry A. 85:729–737. 2014.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Thapa A and Carroll NJ: Dietary modulation
of oxidative stress in Alzheimer's disease. Int J Mol Sci.
18(1583)2017.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Pohanka M: Alzheimer's disease and
oxidative stress: A review. Curr Med Chem. 21:356–364.
2014.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Gubandru M, Margina D, Tsitsimpikou C,
Goutzourelas N, Tsarouhas K, Ilie M, Tsatsakis AM and Kouretas D:
Alzheimer's disease treated patients showed different patterns for
oxidative stress and inflammation markers. Food Chem Toxicol.
61:209–214. 2013.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Lee HJ, Spandidos DA, Tsatsakis A, Margina
D, Izotov BN and Yang SH: Neuroprotective effects of Scrophularia
buergeriana extract against glutamate-induced toxicity in SH-SY5Y
cells. Int J Mol Med. 43:2144–2152. 2019.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Costa C, Tsatsakis A, Mamoulakis C,
Teodoro M, Briguglio G, Caruso E, Tsoukalas D, Margina D, Dardiotis
E, Kouretas D and Fenga C: Current evidence on the effect of
dietary polyphenols intake on chronic diseases. Food Chem Toxicol.
110:286–299. 2017.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Kato M, Ochiai R, Kozuma K, Sato H and
Katsuragi Y: Effect of chlorogenic acid intake on cognitive
function in the elderly: A pilot study. Evid Based Complement
Alternat Med. 2018(8608497)2018.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Martini-Stoica H, Xu Y, Ballabio A and
Zheng H: The autophagy-lysosomal pathway in neurodegeneration: A
TFEB perspective. Trends Neurosci. 39:221–234. 2016.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Wang D, Zhang J, Jiang W, Cao Z, Zhao F,
Cai T, Aschner M and Luo W: The role of NLRP3-CASP1 in
inflammasome-mediated neuroinflammation and autophagy dysfunction
in manganese-induced, hippocampal-dependent impairment of learning
and memory ability. Autophagy. 13:914–927. 2017.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Ntsapi C, Lumkwana D, Swart C, du Toit A
and Loos B: New insights into autophagy dysfunction related to
amyloid beta toxicity and neuropathology in Alzheimer's disease.
Int Rev Cell Mol Biol. 336:321–361. 2018.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Tanida I and Waguri S: Measurement of
autophagy in cells and tissues. Methods Mol Biol. 648:193–214.
2010.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Lamark T, Svenning S and Johansen T:
Regulation of selective autophagy: The p62/SQSTM1 paradigm. Essays
Biochem. 61:609–624. 2017.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Colacurcio DJ, Pensalfini A, Jiang Y and
Nixon RA: Dysfunction of autophagy and endosomal-lysosomal
pathways: Roles in pathogenesis of down syndrome and Alzheimer's
disease. Free Radic Biol Med. 114:40–51. 2018.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Chai YL, Chong JR, Weng J, Howlett D,
Halsey A, Lee JH, Attems J, Aarsland D, Francis PT, Chen CP and Lai
MKP: Lysosomal cathepsin D is upregulated in Alzheimer's disease
neocortex and may be a marker for neurofibrillary degeneration.
Brain Pathol. 29:63–74. 2019.PubMed/NCBI View Article : Google Scholar
|
|
50
|
Vidoni C, Follo C, Savino M, Melone MA and
Isidoro C: The role of cathepsin D in the pathogenesis of human
neurodegenerative disorders. Med Res Rev. 36:845–870.
2016.PubMed/NCBI View Article : Google Scholar
|
|
51
|
Ye B, Wang Q, Hu H, Shen Y, Fan C, Chen P,
Ma Y, Wu H and Xiang M: Restoring autophagic flux attenuates
cochlear spiral ganglion neuron degeneration by promoting TFEB
nuclear translocation via inhibiting MTOR. Autophagy. 15:998–1016.
2019.PubMed/NCBI View Article : Google Scholar
|
|
52
|
Tramutola A, Triplett JC, Di Domenico F,
Niedowicz DM, Murphy MP, Coccia R, Perluigi M and Butterfield DA:
Alteration of mTOR signaling occurs early in the progression of
Alzheimer disease (AD): Analysis of brain from subjects with
pre-clinical AD, amnestic mild cognitive impairment and late-stage
AD. J Neurochem. 133:739–749. 2015.PubMed/NCBI View Article : Google Scholar
|
|
53
|
Kou X, Chen D and Chen N: Physical
activity alleviates cognitive dysfunction of Alzheimer's disease
through regulating the mTOR signaling pathway. Int J Mol Sci.
20(1591)2019.PubMed/NCBI View Article : Google Scholar
|