Introduction
With the aging of the population and the quickening
pace of life, the incidence of acute coronary syndrome (ACS) has
continued to increase (1). ACS is a
common cardiovascular disease with a high morbidity and mortality
rates (2). Although the risks of
myocardial ischemia and hypoxia have been improved in patients with
ACS undergoing percutaneous coronary intervention (PCI), some
patients still experience major adverse cardiovascular events
(MACEs) (3). There is an urgent
need for indicators that can predict the occurrence of MACEs in
patients with ACS undergoing PCI with high sensitivity and
specificity.
Protein tyrosine phosphatase receptor-type O
(PTPRO), a protein tyrosine phosphatase receptor, has been reported
to be involved in the pathogenesis and progression of lung squamous
cell carcinoma (4), breast cancer
(5), colorectal cancer (6) and hepatocellular carcinoma (7). However, few studies have focused on
the association between PTPRO and ACS. The Toll-like receptor 4
(TLR4)/nuclear factor B (NF-κB) signaling pathway, which plays an
important role in the inflammatory response, has been reported to
be involved in the development of ACS (8,9).
Through the TLR4/NF-κB signaling pathway, the expression of PTPRO
was shown to aggravate the inflammatory response in ulcerative
colitis (10) and to regulate
oxidative stress and apoptosis (11). Based on this association between
PTPRO expression and the inflammatory response, it was hypothesized
that PTPRO expression might be associated with the progression of
ACS. Therefore, the present study was conducted to explore the
association between the expression level of PTPRO in peripheral
blood mononuclear cells and the prognosis of patients with ACS
undergoing PCI.
Materials and methods
Patients
A total of 185 patients (age range, 31-78 years; 130
males and 55 females) with ACS admitted to Beijing Luhe Hospital,
Capital Medical University (Beijing, China) between April 2016 and
April 2017 were enrolled in this prospective study. ACS was
diagnosed according to the Guidelines for the Rapid Diagnosis and
Treatment of ACS in the Emergency Department (12). The inclusion criteria were as
follows: i) Age ≤80 years; ii) first diagnosis with ACS; iii)
planned treatment with PCI; iv) hospital admission within 24 h
after ACS onset; and v) regular use of anti-platelet aggregation
drugs after PCI. Patients were excluded if they: i) Had
contraindications for PCI; ii) underwent coronary artery bypass
grafting; iii) had lower extremity arteriosclerosis obliterans,
stroke, chronic obstructive pulmonary disease,
hyperhomocysteinemia, heart valvular disease or dilated
cardiomyopathy, cancer, autoimmune diseases or inflammatory
diseases, liver fibrosis, or severe liver or renal dysfunction; or
iv) were lost during the follow-up period. The study was approved
by the medical ethics committee of Beijing Lube Hospital, Capital
Medical University, and all patients included this study provided
written informed consent.
Data collection
The following patient data were collected: Age, sex,
body mass index (BMI), medical history (such as hypertension,
diabetes, hyperlipidemia), family history of ACS, location and
number of vascular lesions, and clinical type of ACS [including
ST-elevation acute myocardial infarction (STEMI), non-ST-elevation
acute myocardial infarction (NSTEMI), and unstable angina
(UA)].
The 36 months of follow-up included examination at 1
month after discharge and once every 3 months thereafter. The
prognosis of patients with ACS undergoing PCI was evaluated by
telephone follow-up survey and readmission. A poor prognosis was
considered when a MACE occurred, including recurrent myocardial
infarction (RMI), recurrent unstable angina (RUA), stent
restenosis, target vessel revascularization (TVR), and cardiac
death. The endpoints were the occurrence of MACEs or the end of
follow-up.
Measurement of PTPRO expression
A total of 10 ml fasting venous blood was extracted
from patients with ACS within 24 h after admission and again 7 days
after PCI. Mononuclear cells were isolated from peripheral blood by
Ficoll density gradient centrifugation (13).
The expression level of PTPRO in mononuclear cells
was detected by western blotting (14). Briefly, radioimmunoprecipitation
assay (RIPA) buffer was added to the test tube containing
mononuclear cells to lyse the cells, and then the proteins were
isolated and quantified using a BCA protein Quantitative kit (cat.
no. KA3718; Abnova). The proteins were separated by 10% sodium
dodecyl sulfate (SDS)-polyacrylamide gel electrophoresis (PAGE) at
120 V until bromophenol blue ran out of the gel surface, and the
PVDF membrane transfer process was performed with 300 mA current
for 60 min after balancing with the membrane buffer three times.
Sealing was achieved by immersion in 5% skim milk for 1 h at room
temperature. The membrane was washed three times with
phosphate-buffered saline containing Tween-20 (PBST) for 5 min each
time, and then incubated with rabbit anti-PTPRO polyclonal
(1:1,000; cat. no. H00005800-W01P; Abnova) or rabbit anti-β-actin
(1:500; cat. no. PAB29054; Abnova) antibodies overnight at 4˚C. The
membrane was again washed three times with PBST for 10 min each
time, after which goat anti-rabbit IgG secondary antibodies
(1:5,000; cat. no. A21020; Yakoyin Biotechnology Co., Ltd.) was
added for incubation for 1 h at room temperature. The membrane was
again washed three times with PBST for 10 min each time, and then
exposure was achieved by enhanced chemiluminescence (ECL).
Gray-scale values were calculated using ImageJ 1.8.0 software
(National Institute of Health), and relative expression levels were
calculated by normalization to β-actin expression.
Statistical analysis
R 3.6.3 software (https://www.r-project.org/) was applied for data
analysis, and P<0.05 was considered statistically significant. A
paired sample t-test was used for the intra-group comparison of
normal distribution, and an independent sample t-test was used for
the comparison between two groups. The measurement data with a
normal distribution are expressed as mean ± standard deviation
(x ± s). Mixed ANOVA model was used for comparisons
in PTPRO before and after PCI between groups. The count data were
expressed as rate [n (%)], and the χ2 test was used for
comparison between groups. Receiver operating characteristic (ROC)
curve analysis was applied to estimate the efficacy of PTPRO levels
for predicting the prognosis of patients with ACS undergoing PCI.
Kaplan-Meier analysis was employed to draw disease-free survival
curves for the high PTPRO groups and low PTPRO groups, and the
curves were compared using the log-rank method. Moreover, Cox
regression analysis was performed to analyze risk factors for poor
prognosis among patients with ACS undergoing PCI, and the Cox
regression analysis with restrictive cubic spline model was used to
evaluate the relationship between the expression level of PTPRO and
the prognosis of patients with ACS undergoing PCI.
Results
Prognosis of patients
A total of 185 patients with ACS aged 31-78 years
were enrolled in the present study, including 130 men and 55 women.
During the 36 months of follow-up, 60 patients with ACS suffered
from MACEs (32.43%), including RMI in 26 patients (14.05%), RUA in
14 patients (7.57%), stent restenosis in 12 patients (6.49%), TVR
in 5 patients (2.70%), and cardiac death in 3 patients (1.62%).
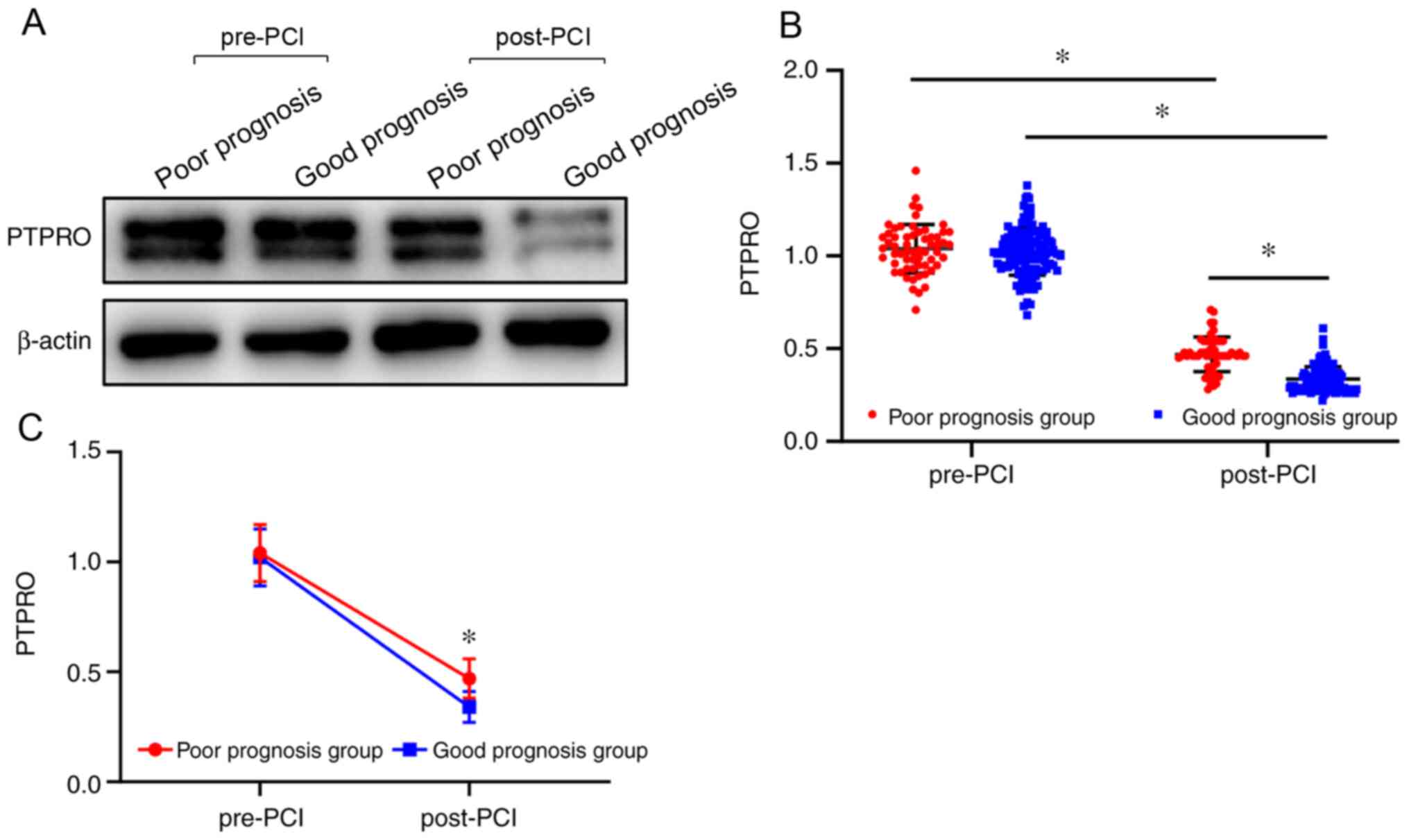
Comparison of PTPRO expression levels
between patients with poor and good prognoses
According to the occurrence of MACEs during the
follow-up, patients with ACS were divided into two groups: Good
prognosis group (n=125) and poor prognosis group (n=60). The PTPRO
expression level after PCI in the good prognosis group was
significantly lower compared with that of the poor prognosis group
(P<0.05; Table I; Fig. 1). Moreover, the expression level of
PTPRO before PCI was significantly higher compared with that after
PCI in both groups (P<0.05; Table
I; Fig. 1). A significant
association was observed between prognosis and time (F=3217.922;
P<0.001), namely, the level of reduction about PTPRO in the good
prognosis group was more obvious than that in the poor prognosis
group (P<0.05; Table I and
Fig. 1).
 | Table IComparison of PTPRO expression levels
in patients with ACS according to prognosis. |
Table I
Comparison of PTPRO expression levels
in patients with ACS according to prognosis.
| Group | N | PTPRO before PCI
(x ± s) | PTPRO after PCI
(x ± s) | t | P-value |
|---|
| Poor prognosis
group | 60 | 1.04±0.13 | 0.47±0.09 | 29.133 | <0.001 |
| Good prognosis
group | 125 | 1.02±0.13 | 0.34±0.07 | 56.543 | <0.001 |
| T | | 0.841 | 9.918 | | |
| P-value | | 0.401 | <0.001 | | |
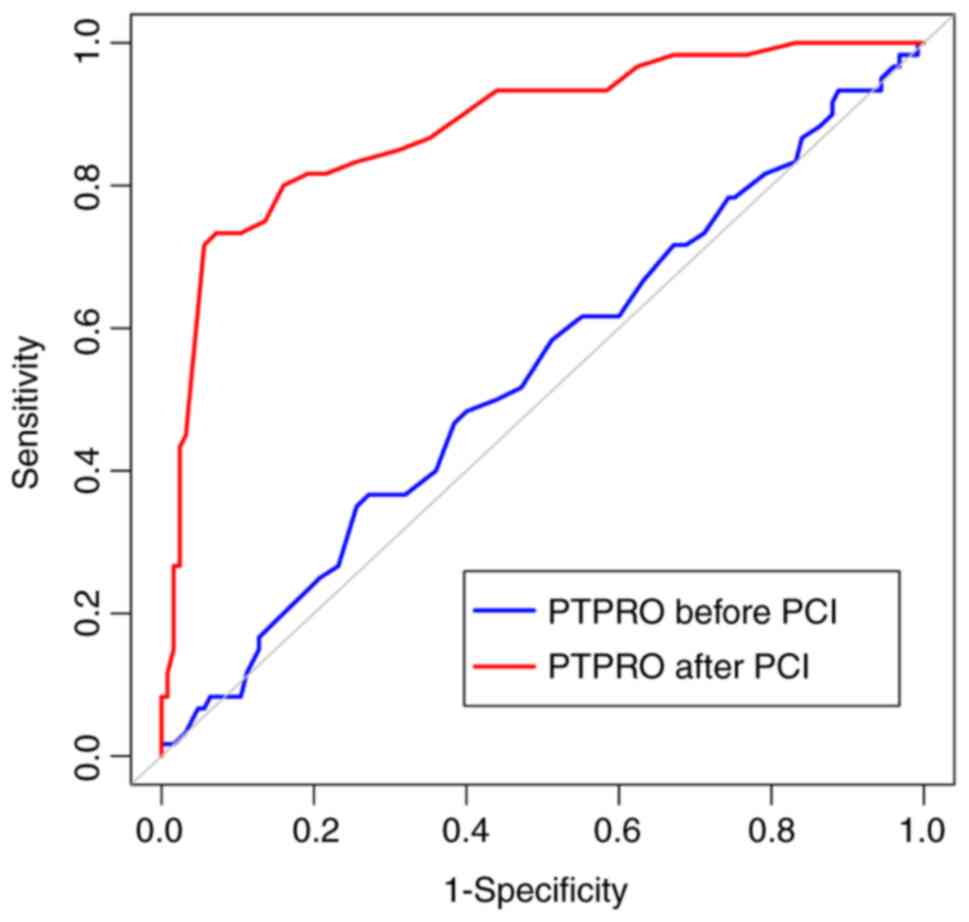
Prognostic efficacy of PTPRO
expression in patients with ACS
The area under the ROC curve (AUC), maximum
approximation index, optimal cut-off point, sensitivity, and
specificity for the ability of the pre-PCI PTPRO expression level
to predict the prognosis of patients with ACS were 0.536 [95%
confidence interval (CI), 0.462-0.610], 0.095, 1.08, 36.67 and
72.80%, respectively. These indexes for the post-PCI PTPRO
expression level were 0.883 (95% CI, 0.828-0.926), 0.661, 0.44,
73.33 and 92.80%, respectively. The AUC value for PTPRO expression
after PCI was significantly higher compared with hat for PTPRO
expression before PCI (Z=6.714; P<0.001; Fig. 2).
Comparison of characteristics between
patients with high and low PTPRO expression after PCI
According to the optimal cutoff point for evaluating
the prognosis of patients with ACS after PCI, patients with ACS
were divided into a high PTPRO expression group (n=53) and a low
PTPRO expression group (n=132). No statistically significant
differences were observed between the two groups in terms of age,
sex, BMI, medical history (hypertension, diabetes, hyperlipidemia),
family history of ACS, clinical type of ACS, and location and
number of vascular lesions (P>0.05; Table II).
 | Table IIComparison of patient characteristics
between groups according to PTPRO expression after PCI. |
Table II
Comparison of patient characteristics
between groups according to PTPRO expression after PCI.
| Item | High PTPRO group
(n=53) | Low PTPRO group
(n=132) |
t/χ2 | P-value |
|---|
| Age, years | 55.15±9.02 | 55.58±8.71 | 0.297 | 0.767 |
| Sex | | | | |
|
Male | 36 | 94 | 0.196 | 0.658 |
|
Female | 17 | 38 | | |
| BMI,
kg/m2 | 26.88±3.44 | 26.56±2.87 | 0.643 | 0.521 |
| Hypertension | 26 | 61 | 0.123 | 0.726 |
| Hyperlipidemia | 28 | 63 | 0.394 | 0.530 |
| T2DM | 18 | 40 | 0.235 | 0.628 |
| ACS family
history | 7 | 7 | 3.378 | 0.066 |
| ACS type | | | | |
|
STEMI | 14 | 33 | 0.053 | 0.974 |
|
NSTEMI | 26 | 67 | | |
|
UA | 13 | 32 | | |
| Vascular lesion
count | | | | |
|
Single | 17 | 53 | 1.049 | 0.306 |
|
Multiple | 36 | 79 | | |
| Vascular lesion
location | | | | |
|
LMCA | 6 | 9 | 1.238 | 0.744 |
|
LAD | 28 | 78 | | |
|
LCX | 8 | 19 | | |
|
RCA | 11 | 26 | | |
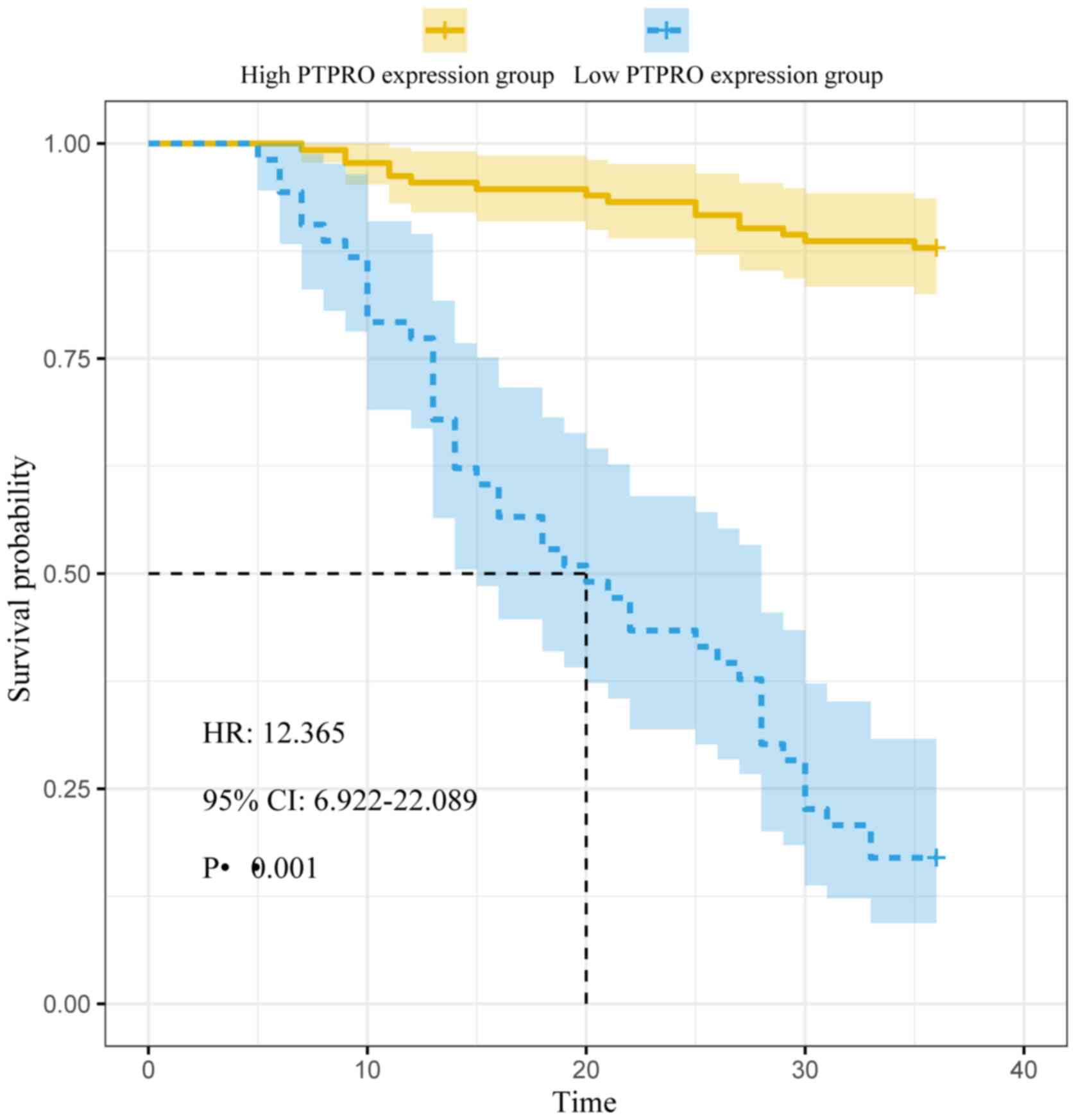
Factors influencing prognosis of
patients with ACS treated with PCI
In the high PTPRO expression group, 44 MACEs
occurred, including 20 cases of RMI, 11 cases of RUA, 8 cases of
stent restenosis, 3 cases of TVR, and 2 cases of cardiac death. In
the low PTPRO expression group, 16 MACEs occurred, including 6
cases of RMI, 3 cases of RUA, 4 cases of stent restenosis, 2 cases
of TVR, and 1 case of cardiac death. The median disease-free
survival time of the high PTPRO expression group (20 months) was
significantly shorter compared with that of the low PTPRO
expression group (36 months; log-rank χ2=113.704;
P<0.001; Fig. 3). For
correlation analysis, age, sex, BMI, medical history (hypertension,
diabetes, hyperlipidemia), family history of ACS, clinical type of
ACS, location and number of vascular lesions, and the expression
level of PTPRO were considered as independent variables, and the
prognosis of patients with ACS was considered as the dependent
variable. Cox univariate regression analysis showed that family
history of ACS and the expression level of PTPRO were significantly
associated with the prognosis of patients with ACS (P<0.05).
Furthermore, Cox multivariate regression analysis identified high
PTPRO expression as an independent risk factor for poor prognosis
in patients with ACS (P<0.05; Table III).
 | Table IIICox regression analysis of factors
affecting the prognosis of patients with ACS. |
Table III
Cox regression analysis of factors
affecting the prognosis of patients with ACS.
| | COX single-factor
analysis | COX multi-factor
analysis |
|---|
| Item | HR | 95% CI | P-value | HR | 95% CI | P-value |
|---|
| Age | 1.006 | 0.977-1.036 | 0.691 | | | |
| Sex | 1.108 | 0.634-1.937 | 0.721 | | | |
| BMI | 1.104 | 0.932-1.103 | 0.743 | | | |
| Hypertension | 1.252 | 0.757-2.073 | 0.384 | | | |
| Hyperlipidemia | 0.885 | 0.534-1.466 | 0.636 | | | |
| T2DM | 1.124 | 0.659-1.918 | 0.669 | | | |
| ACS family
history | 2.261 | 1.077-4.746 | 0.032 | 1.291 | 0.611~2.725 | 0.505 |
| ACS type | 1.069 | 0.745-1.534 | 0.718 | | | |
| Vascular lesion
count | 1.075 | 0.638-1.813 | 0.786 | | | |
| Vascular lesion
location | 1.069 | 0.811-1.409 | 0.638 | | | |
| High PTPRO
expression | 12.365 | 6.922~22.089 | <0.001 | 12.084 | 6.733~21.689 | <0.001 |
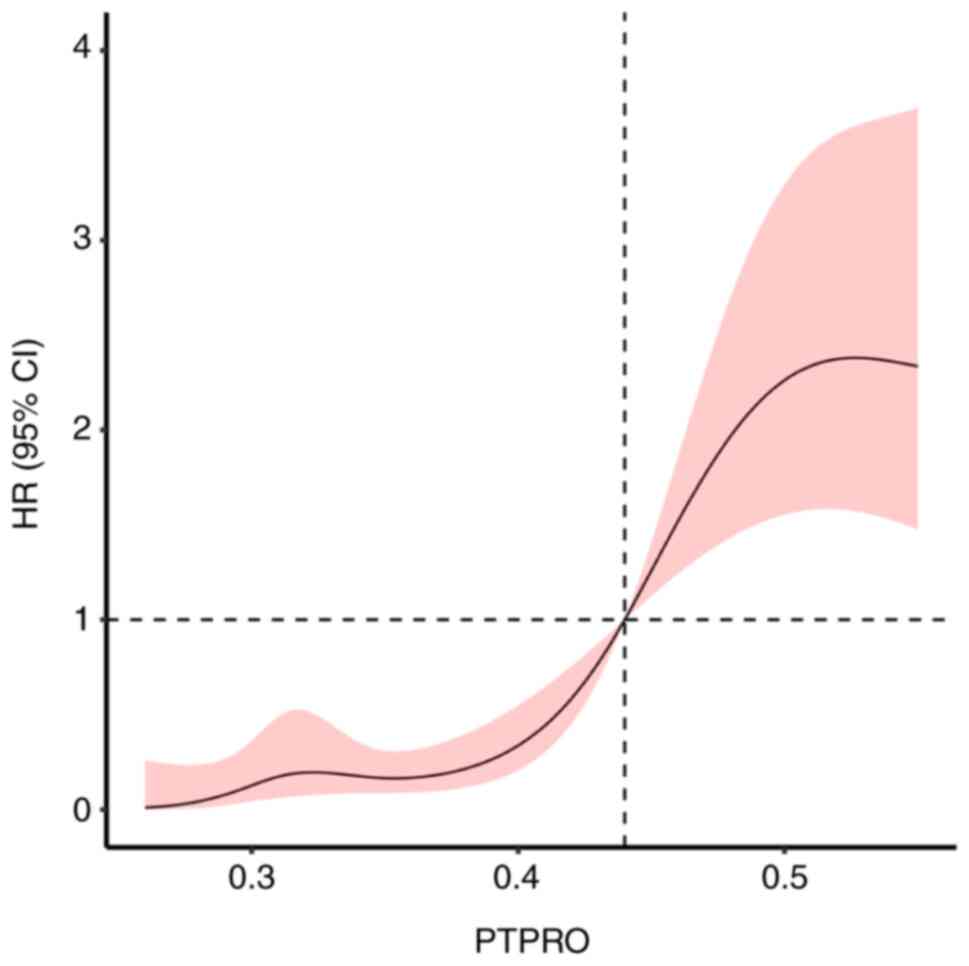
Association between PTPRO expression
and prognosis of patients with ACS undergoing PCI
The Cox regression analysis with restrictive cubic
spline model showed that the expression level of PTPRO was
associated with the prognosis of patients with ACS
(χ2=51.48; P<0.001) via a nonlinear association
(χ2=10.65; P=0.001). According to the cut-off point, the
risk of poor prognosis decreased with a PTPRO expression level
<0.44 but increased with a PTPRO expression level >0.44
(Fig. 4).
Discussion
The present study investigated the expression level
of PTPRO in patients with ACS who underwent PCI and its association
with prognosis. By comparing the expression level of PTPRO in
peripheral blood mononuclear cells between groups with a good
prognosis and a poor prognosis, it was found that the expression
levels of PTPRO in both groups after PCI were lower compared with
those before PCI, and the post-PCI expression level of PTPRO in the
good prognosis group was lower compared with those in the poor
prognosis group. These results indicate that PTPRO is involved in
the pathogenesis and progression of ACS, and a lower expression
level of PTPRO after PCI might be associated with improvement in
the inflammatory response and decreased oxidative stress.
Therefore, the expression level of PTPRO may contribute to the
evaluation of prognosis in these patients.
Atherosclerosis is the pathological basis of ACS.
Recently, several studies have suggested that atherosclerosis is
not a condition of simple lipid deposition but a chronic
inflammatory disease (15,16). Inflammation plays a key role in the
pathogenesis and progression of ACS (15-17),
by mediating intracoronary thrombosis, atherosclerotic plaque
damage, erosion and rupture (18).
Mononuclear cells in peripheral blood also have a crucial role in
the development of atherosclerosis through activating vascular
endothelial cells, as well as releasing inflammatory mediators,
such as interleukin-1 and C-reactive protein (19).
An elevated serum hypersensitive C-reactive protein
level is an important predictor of cardiovascular events (20). After treatment with
anti-interleukin-1β in patients with atherosclerosis, the serum
C-reactive protein level was significantly decreased, and the
incidence of cardiovascular events were subsequently decreased
(21). However, the efficacy of
C-reactive protein for predicting the prognosis of patients with
ACS seemed to be limited, according to the finding that the AUC
value for the prognostic value of C-reactive protein was only
0.634(22). In the present study,
the prognostic value of the PTPRO expression level in peripheral
blood mononuclear cells in patients with ACS was investigated, and
the results suggest that PTPRO expression in peripheral blood
mononuclear cells after PCI can be used for the prediction of
prognosis in patients with ACS.
To further clarify the association between the
expression level of PTPRO and the prognosis of patients with ACS,
the median disease-free survival times was compared between
patients with high and low PTPRO expression according to the
calculated cutoff value. The results showed that the median
disease-free survival time was shorter in those with high PTPRO
expression compared with those with low PTPRO expression, which
further indicates that the expression level of PTPRO is associated
with the prognosis of patients with ACS. Cox regression analysis
identified high PTPRO expression as an independent risk factor for
poor prognosis in patients with ACS. In addition, Cox regression
analysis with the restrictive cubic spline model showed an
increased risk of poor prognosis with a PTPRO expression level
>0.44, using a cutoff value of PTPRO expression=0.44. All of the
results from the present study indicated that PTPRO expression
after PCI is associated with the prognosis of patients with
ACS.
Previous studies showed that PTPRO is involved in
the inflammatory response and oxidative stress. For example, PTPRO
was shown to activate NF-κB in the regulation of hepatic
ischemia-reperfusion injury through feedback mechanisms (23) and to promote the oxidative stress
and cellular apoptosis by inducing oxidized low-density lipoprotein
via the TLR4/NF-κB signaling pathway (11). It was speculated that the underlying
reason might be that PTPRO participates in the atherosclerotic
inflammation and oxidative stress response through the TLR4/NF-κB
signal transduction pathway, which further promotes the damage,
erosion and rupture of atherosclerosis plaques, and subsequently
induces ACS. The expression level of PTPRO in patients with ACS
could be detected following PCI, in order to understand the status
of oxidative stress and inflammation, which further reflects the
prognosis of patients with ACS.
In summary, the expression level of PTPRO in
peripheral blood mononuclear cells after PCI was shown to be
associated with the prognosis of patients with ACS. High PTPRO
expression indicated a high risk of poor prognosis in patients with
ACS undergoing PCI. Further prospective, multi-center, large-scale
trials are needed to verify the results of the present study.
Acknowledgements
None.
Funding
No funding was received.
Availability of data and materials
The datasets generated and/or analyzed during the
current study are available from the corresponding author on
reasonable request.
Authors' contributions
MH designed the study, analyzed the data and wrote
the manuscript; MG diagnosed the disease, supervised the study and
revised the manuscript; RY collected the data, isolated mononuclear
cells and detected protein tyrosine phosphatase. All authors read
and approved the final manuscript. MH and MG confirm the
authenticity of all the raw data.
Ethics approval and consent to
participate
The study was approved by the medical ethics
committee of Beijing Lube Hospital, Capital Medical University
(approval no. 2016-LHKY-012-013). All procedures performed in
studies involving human participants were in accordance with the
ethics standards of the institutional and national research
committee and with the 1964 Helsinki Declaration and its later
amendments or comparable ethics standards. Written informed consent
was obtained from all individual participants included in the
present study.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Lin F, Yang Y, Guo Q, Xie M, Sun S, Wang
X, Li D, Zhang G, Li M, Wang J and Zhao G: Analysis of the
molecular mechanism of acute coronary syndrome based on
circRNA-miRNA network regulation. Evid Based Complement Alternat
Med. 2020(1584052)2020.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Chandiramani R, Cao D and Mehran R:
Periprocedural anticoagulation in non-ST-segment elevation acute
coronary syndrome: Time to reassess? Ann Transl Med.
8(556)2020.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Huang ZS, Zheng ZD, Zhang JW, Tang LL,
Zhou LL, Li SH, Xie XJ, Dong RM, Zhu JM and Liu JL: Association of
major adverse cardiovascular events and cardiac troponin-I levels
following percutaneous coronary intervention: A three-year
follow-up study at a single center. Eur Rev Med Pharmacol Sci.
24:3981–3992. 2020.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Ming F and Sun Q: Epigenetically silenced
PTPRO functions as a prognostic marker and tumor suppressor in
human lung squamous cell carcinoma. Mol Med Rep. 16:746–754.
2017.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Dong H, Ma L, Gan J, Lin W, Chen C, Yao Z,
Du L, Zheng L, Ke C, Huang X, et al: PTPRO represses ERBB2-driven
breast oncogenesis by dephosphorylation and endosomal
internalization of ERBB2. Oncogene. 36:410–422. 2017.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Yan S, Cheng M, Duan Q, Wang Z, Gao W, Ren
B and Xu D: MiR-6803-5p promotes cancer cell proliferation and
invasion via PTPRO/NF-κB axis in colorectal cancer. Mediators
Inflamm. 2019(8128501)2019.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Xu D, Wang X, Yan S, Yin Y, Hou J, Wang X
and Sun B: Interaction of PTPRO and TLR4 signaling in
hepatocellular carcinoma. Tumour Biol. 35:10267–10273.
2014.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Qi C, Shao Y, Liu X, Wang D and Li X: The
cardioprotective effects of icariin on the isoprenaline-induced
takotsubo-like rat model: Involvement of reactive oxygen species
and the TLR4/NF-κB signaling pathway. Int Immunopharmacol.
74(105733)2019.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Tang YL, Jiang JH, Wang S, Liu Z, Tang XQ,
Peng J, Yang YZ and Gu HF: TLR4/NF-κB signaling contributes to
chronic unpredictable mild stress-induced atherosclerosis in
ApoE-/- mice. PLoS One. 10(e0123685)2015.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Zhao J, Yan S, Zhu X, Bai W, Li J and
Liang C: PTPRO exaggerates inflammation in ulcerative colitis
through TLR4/NF-κB pathway. J Cell Biochem. 121:1061–1071.
2020.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Liang C, Wang X, Hu J, Lian X, Zhu T,
Zhang H, Gu N and Li J: PTPRO promotes oxidized low-density
lipoprotein induced oxidative stress and cell apoptosis through
toll-like receptor 4/nuclear factor κB pathway. Cell Physiol
Biochem. 42:495–505. 2017.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Chinese Medical Association Emergency
Physicians Branch, Chinese Medical Association Cardiovascular
Medicine Branch and Chinese Medical Association Laboratory Medicine
Branch. Guidelines for the rapid diagnosis and treatment of acute
coronary syndrome in the emergency department. Chin J Emerg Med.
25:397–404. 2016.
|
|
13
|
Zhao JW, Shi G, Ping JD and Ming L: Study
on the negative regulation of TIPE2 from peripheral blood
mononuclear cells on tissue factor in patients with bronchial
asthma. Zhonghua Yi Xue Za Zhi. 98:2889–2893. 2018.PubMed/NCBI View Article : Google Scholar : (In Chinese).
|
|
14
|
Ye G, Qin Y, Wang S, Pan D, Xu S, Wu C,
Wang X, Wang J, Ye H and Shen H: Lamc1 promotes the Warburg effect
in hepatocellular carcinoma cells by regulating PKM2 expression
through AKT pathway. Cancer Biol Ther. 20:711–719. 2019.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Gowdak LHW: Atherosclerosis, inflammation,
and genetics- and you thought it was just LDL-cholesterol. Arq Bras
Cardiol. 114:273–274. 2020.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Frodermann V, Rohde D, Courties G, Severe
N, Schloss MJ, Amatullah H, McAlpine CS, Cremer S, Hoyer FF, Ji F,
et al: Exercise reduces inflammatory cell production and
cardiovascular inflammation via instruction of hematopoietic
progenitor cells. Nat Med. 25:1761–1771. 2019.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Zhang Y, Zhang C and Zhang M: A new era of
anti-inflammatory therapy for atherosclerosis. Zhonghua Xin Xue
Guan Bing Za Zhi. 46:332–337. 2018.PubMed/NCBI View Article : Google Scholar : (In Chinese).
|
|
18
|
Sun YF and Guo DL: Research progress of
the relationship between inflammatory factor and coronary heart
disease. China Mod Med. 24:12–15. 2017.
|
|
19
|
Szekely Y and Arbel Y: A review of
interleukin-1 in heart disease: Where do we stand today? Cardiol
Ther. 7:25–44. 2018.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Gao S, Shu S, Wang L, Zhou J and Yuan Z:
Pro-inflammatory and anti-inflammatory cytokine responses of
peripheral blood mononuclear cells in apparently healthy subjects.
Nan Fang Yi Ke Da Xue Xue Bao. 34:1589–1593. 2014.PubMed/NCBI(In Chinese).
|
|
21
|
Ridker PM, Everett BM, Thuren T, MacFadyen
JG, Chang WH, Ballantyne C, Fonseca F, Nicolau J, Koenig W, Anker
SD, et al: Antiinflammatory therapy with canakinumab for
atherosclerotic disease. N Engl J Med. 377:1119–1131.
2017.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Dongxu H, Yundi J, Jia S, Zongyu W, Jiake
W, Weili D, Na S, Tongtong Y, Zhijun S and Zaoqing S: The effects
of C-reactive protein and albumin ratio on the prognoses in
patients with acute coronary syndrome. Chin J Cardiovasc Res.
16:1090–1094. 2018.
|
|
23
|
Hou J, Xia Y, Jiang R, Chen D, Xu J, Deng
L, Huang X, Wang X and Sun B: PTPRO plays a dual role in hepatic
ischemia reperfusion injury through feedback activation of NF-κB. J
Hepatol. 60:306–312. 2014.PubMed/NCBI View Article : Google Scholar
|