Introduction
Endothelial cells (ECs) constitute the major cell
type that line the interior surface of blood vessels. Consequently,
ECs are constantly subjected to fluid shear stress (1). Hemodynamic forces generated by blood
flow serve a critical role in maintaining EC function, vascular
structure and homeostasis (1). High
fluid shear stress or high shear stress (HSS) exhibits a protective
effect against atherosclerosis (1).
Previous studies have provided strong evidence for the protective
role of HSS in vascular homeostasis by inhibiting the expression of
proinflammatory and adhesion molecules in ECs (1,2). By
contrast, pathological shear stress is characterized by flow
disturbance and low rates of shear stress (LSS) (1). Accumulating evidence is suggesting
that LSS contributes to the dysregulation of vascular function and
increases susceptibility to atherosclerosis (1,2). HSS
enhances defenses against reactive oxygen species (ROS)-induced
damage by preventing lipid peroxidation induced by high
concentrations of glucose and free fatty acids (FFAs) (3). Therefore, laminar HSS exerts important
effects on EC function, particularly under conditions of metabolic
disorder.
Metabolic disorders, such as type 2 diabetes
mellitus or hyperlipidemia, are frequently accompanied with
dysregulations in the metabolism of glucose or lipids. In
particular, abnormal fatty acid metabolism is a common feature
(4). High plasma levels of FFA are
considered to be reliable indices for the diagnosis of type 2
diabetes (4-6).
Additionally, high concentrations of plasma FFA is a risk factor
for disturbances in EC function for the pathogenesis of vascular
diseases, including atherosclerosis and vascular complications
associated with diabetes (7,8). High
plasma levels of FFA can also induce an inflammatory response and
aggravate oxidative stress, promoting the pathogenesis of
cardiovascular disease (9). An
in vitro study has previously demonstrated that high
concentrations of glucose and FFA can inhibit EC proliferation and
result in cell death (10).
Increased plasma concentrations or treatment with high
concentrations of palmitic acid (PA) impair endothelial progenitor
cell (EPC) bioavailability, reduce the re-endothelialization
ability of EPCs and accelerate EPC senescence (11,12).
In addition, high concentrations of FFAs have been demonstrated to
induce nucleotide-binding oligomerization domain, leucine rich
repeat and pyrin domain containing 3 inflammasome activation, which
further increases endothelial permeability (13-15).
Peroxisome proliferator-activated receptors (PPARs)
belong to the nuclear receptor superfamily of ligand-activated
transcription factors, which are involved in regulating lipid and
glucose metabolism, energy homeostasis and inflammation (16,17).
All three PPAR isoforms have been reported to be involved in
metabolic disorders, including atherosclerosis, dyslipidemia and
diabetes (18). Previous studies
have demonstrated that shear stress acting on ECs induces the
expression and activation of PPAR-α, -δ and -γ, which regulate EC
function (19,20). However, studies reporting the role
of PPARs in the regulation of EC function under metabolic stress
remain insufficient. Increased expression of PPAR-γ ameliorates
PA-induced cytotoxicity in sertoli cells, a non-EC type cell
(21). A previous in vivo
study has demonstrated that a PPAR-δ agonist reversed PA-induced
impairments in endothelium-dependent relaxation (22). These results suggested that PPARs
serve protective roles in different cell types in the presence of a
PA-induced metabolic disturbance within the vasculature.
Although the effect of blood flow-induced
biomechanical force on vascular homeostasis has been extensively
investigated, little is known regarding the tolerance of ECs
against constant shear stress at high concentrations of PA. The
present study utilized a parallel plate flow chamber model to exert
fluid laminar shear stress on ECs. It was subsequently determined
whether different patterns of laminar flow shear stress affected
PPAR expression and cellular energy metabolism regulators in ECs
following treatment with high PA concentrations and exposure to
shear stress.
Materials and methods
Cell culture and treatments
Human aortic ECs (HAECs) (cat. no. 304-05) were
obtained from Cell Applications Inc. and cultured on a glass slide
coated with fibronectin (BD Biosciences). Cells were maintained in
M199 medium (Invitrogen; Thermo Fisher Scientific, Inc.)
supplemented with 20% FBS (Gibco; Thermo Fisher Scientific, Inc.),
EC growth medium (Cell Applications Inc.) and 100 U/ml penicillin
and streptomycin. HAECs were observed under light microscopy and
used between passages 5 and 7 for all experiments. Human aortic
smooth muscle cells (HASMCs; cat. no. 354-05a) were obtained
commercially (Cell Applications Inc.) and maintained in F12K medium
(Gibco; Thermo Fisher Scientific, Inc.) supplemented with 10% FBS.
Cells were maintained in 37˚C and 5% CO2 environment. PA
was purchased from Sigma-Aldrich, Merck KGaA). A solution of 10%
bovine serum albumin (BSA; Gibco; Thermo Fisher Scientific, Inc.)
was used as the solvent control. In the present study, 300 µM PA
was used to treat confluent HAECs for 24 h at 37˚C. Subsequently,
HAECs were subjected to either static or shear stress in a
PA-containing medium for an additional 20 h at 37˚C.
Flow shear stress system
The flow system was constructed by the Department of
Physiology and Biophysics, Graduate Institute of Physiology,
National Defense Medical Center. The system was established based
on the study by Chiu et al (23,24). A
series of studies have been conducted using this flow system
(19,25,26).
Flow system was set up at 37˚C in a 5% CO2 environment
as previously described (19).
Confluent HAECs were seeded onto a fibronectin-coated glass slide
(6x105 cells/cm2) and maintained in culture
medium in a humidified atmosphere with 5% CO2 at 37˚C
overnight prior to flow shear stress exposure. For shear stress
experiments, HAECs were subjected to either static conditions,
exposed to 12 dyn/cm2 (HSS) or 4 dyn/cm2
(LSS) for 20 h at 37˚C.
Western blotting
HAECs were harvested using RIPA buffer (Merck KGaA)
containing a protease inhibitor mixture (Sigma-Aldrich; Merck
KGaA). The protein concentration was determined using a Bradford
assay (Bio-Rad Laboratories, Inc.). The protein supernatant from
the total cell lysate (20 µg) was separated using 10% SDS-PAGE and
transferred onto PVDF membranes. The membrane was blocked with 5%
non-fat milk in TBS-T for 1.5 h at room temperature and further
incubated with primary antibodies with 2.5% BSA in TBS-T (all,
1:1,000) targeting the indicated proteins overnight at 4˚C. The
membrane was hybridized using specific primary rabbit polyclonal
antibodies (from Cell Signaling Technology, Inc.) against
endothelial nitric oxide synthase (eNOS; cat. no. 32027), α-smooth
muscle actin (α-SMA; cat. no. 19245), PPAR-γ (cat. no. 2430),
intercellular adhesion molecule-1 (ICAM-1; cat. no. 4915S),
monocyte chemoattractant protein-1 (MCP-1; cat. no. 2027) and
rabbit polyclonal antibodies against PPAR-α (cat. no. ab24509) and
-δ (cat. no. ab23673; Abcam). The membranes were subsequently
incubated with corresponding Peroxidase-AffiniPure Goat Anti-Mouse
IgG (H+L) (cat. no. 115-035-003; 1:5,000) and Goat Anti-Rabbit IgG
(H+L) (cat. no. 111-035-003; 1:5,000) secondary antibodies diluted
in blocking buffer for 1 h at room temperature (each, Jackson
ImmunoResearch Laboratories, Inc.). Immunoblots were visualized
using the Immobilon™ western chemiluminescent horseradish
peroxidase substrate (EMD Millipore) and the Western-Light
chemiluminescent detection system (Applied Biosystems; Thermo
Fisher Scientific, Inc.) was used for immunodetection. Relative
band intensities were quantified using ImageJ 1.47 software
(National Institutes of Health).
Measurement of 6-keto-prostaglandin
F1α
Perfusion medium was collected from HAECs exposed to
static conditions or HSS in the presence or absence of PA for 20 h
at 37˚C. The medium was collected (supernatant only) and
centrifuged at 270 x g for 10 min at room temperature, after which
the concentration of a stable hydrolyzed metabolite of prostacyclin
(PGI2), 6-keto-prostaglandin F1α (6-keto-PGF1α), was
measured using a 6-keto-PGF1α ELISA kit (cat. no. ADI-900-004)
(Assay designs; Enzo Life Sciences, Inc.) in accordance with the
manufacturer’s protocol.
Statistical analysis
Data were expressed as the mean ± SEM from three or
four independent experiments. Data were analyzed using SigmaPlot
version 12.0 (Systat Software, Inc.). Two-way ANOVA followed by
Bonferroni’ test were used for multiple comparisons.
P<0.05 was considered to indicate a statistically significant
difference.
Results
Identification of HAECs and
morphological changes in response to shear stress and PA
treatment
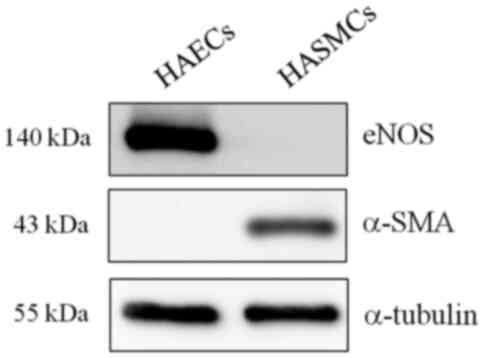
The identity of primary HAECs was confirmed by
measuring the expression of eNOS, a specific marker of endothelial
cells (7), by western blot
analysis. Immunoblotting of the lysates showed no contamination
with other vascular cells such as HASMCs, as the HAECs of Fig. 1 did not present the smooth muscle
cell marker. α-SMA expression was used as the smooth muscle cell
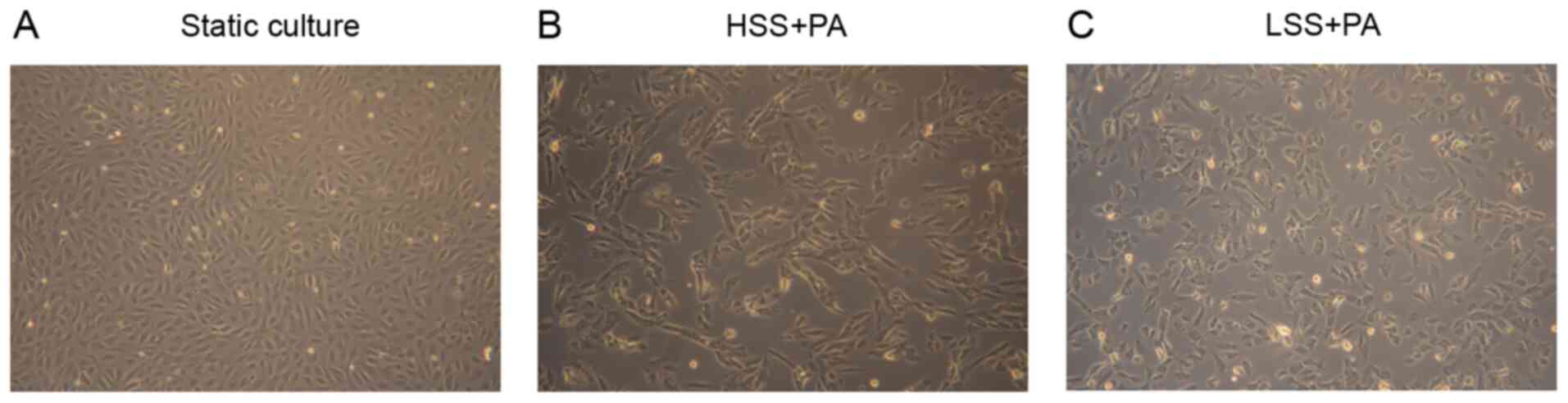
marker (Fig. 1). To investigate the
effect of different types of shear stress with PA treatment on EC
morphology, HAECs were exposed to LSS or HSS for 20 h. ECs exposed
to laminar HSS were elongated and aligned in the direction of flow
with long shapes (27). As
presented in Fig. 2, HAECs
exhibited alignment shapes, which is more physiological, following
HSS with PA treatment. However, the morphology of the HAECs were
shaped more polygonally, which is more pathophysiological, in
response to LSS with PA treatment (Fig.
2).
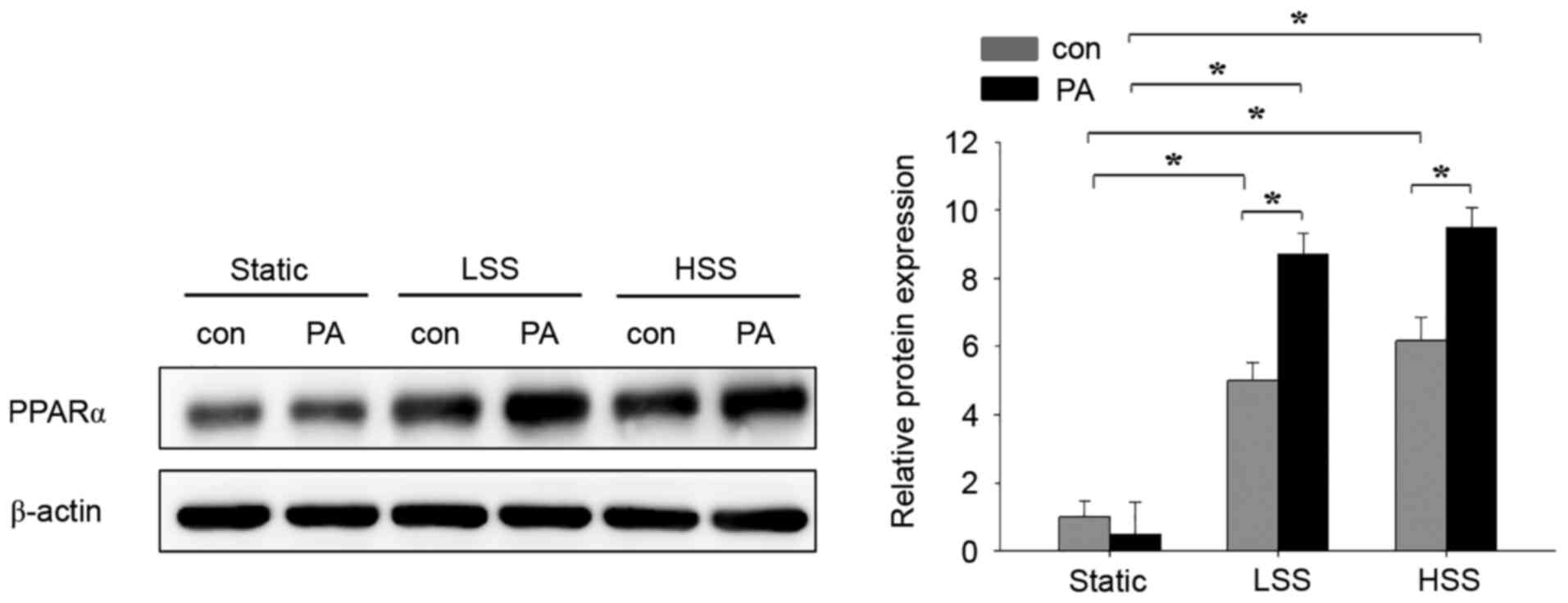
Laminar shear stress in PA-treated
HAECs induces PPAR-α expression
The present study next assessed the effects of
flow-induced shear stress and high PA concentrations on the
expression of PPARα in HAECs. A laminar flow model with
pathological LSS (4 dyn/cm2) or physiological HSS (12
dyn/cm2) (27) was used
to assess HAECs treated with 300 µM PA. There was no difference
between control and PA treatment in the static group (Fig. 3). To investigate the role of shear
stress on PA-treated HAECs, cells were exposed to either LSS or HSS
for 20 h in the presence of PA. Although the expression of PPARα
was similar in PA-untreated HAECs between the LSS and HSS groups,
they remained significantly higher compared with those in
PA-untreated HAECs in the static group (Fig. 3). PPARα expression in the PA-treated
HAECs of the LSS and HSS groups was significantly higher compared
with that in their corresponding PA-untreated HAECs within the same
groups (Fig. 3). Among the three
PA-treated groups, PPAR-α expression was significantly higher after
LSS and HSS compared with that in static conditions (Fig. 3). These results suggest that
PA-treatment in HAECs subjected to LSS and HSS induces the
expression of PPAR-α.
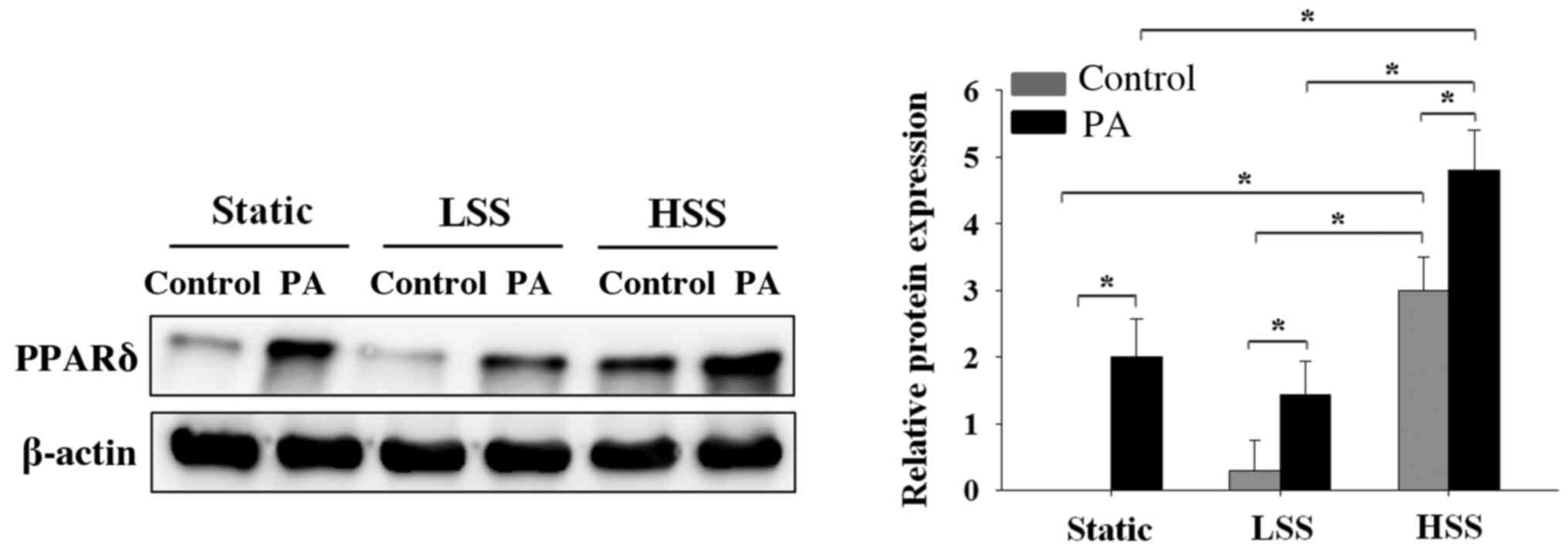
PA-treated HAECs exposed to HSS
exhibit high PPAR-δ expressions
To investigate the effects of the application of
shear stress to HAECs exposed to a high concentration of PA on
PPARδ expression, HAECs were subjected to static conditions, LSS or
HSS in the presence or absence of high PA concentrations for 20 h.
PA-treated HAECs exhibited significantly higher PPAR-δ expression
compared with that in PA-untreated HAECs, irrespective of whether
the HAECs were maintained under static conditions or subjected to
LSS or HSS (Fig. 4). However,
PA-treated HAECs under HSS exhibited significantly higher
expression levels of PPARδ compared with those in PA-treated HAECs
under static or LSS conditions (Fig.
4). These results indicated that only PA treatment sufficiently
increased PPARδ expression. However, among the cell groups that
were not treated with PA, HAECs that were exposed to HSS exhibited
higher levels of PPAR-d expression compared with that in HAECs
under static or LSS conditions (Fig.
4).
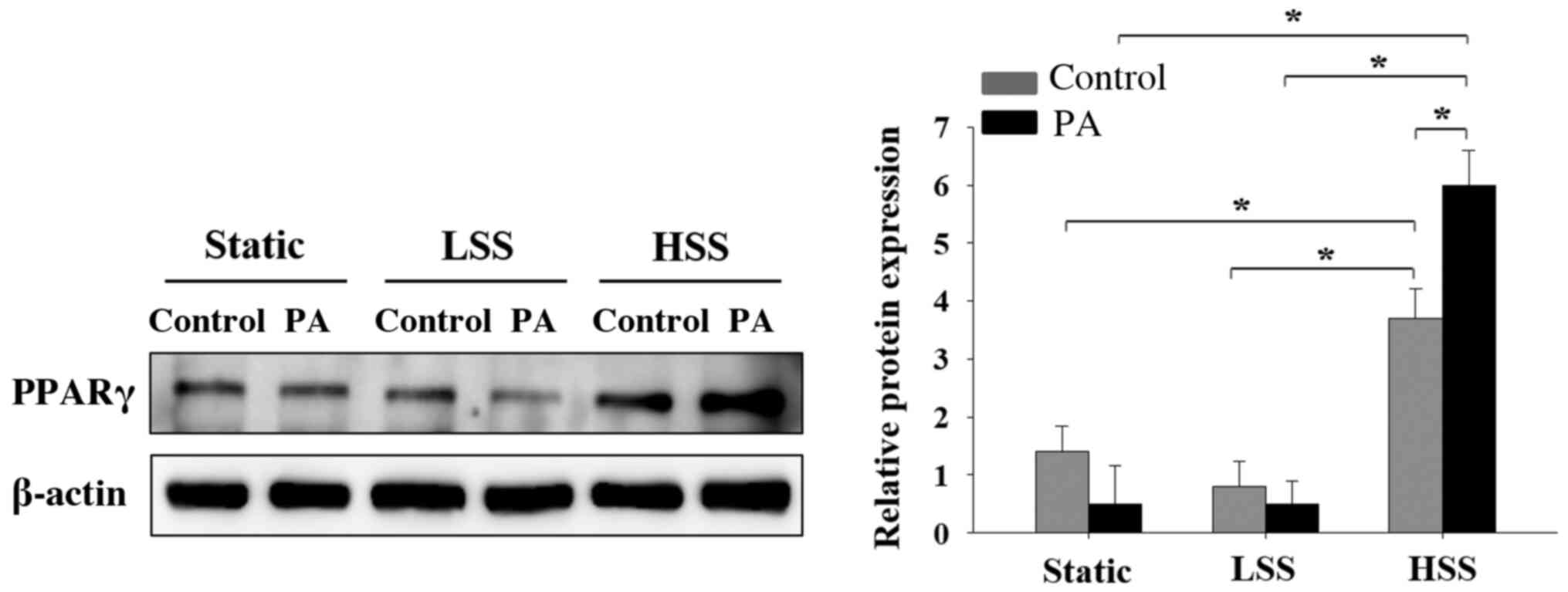
Laminar HSS applied to PA-treated
HAECs increases PPARγ expression
Since it was previously demonstrated that shear
stress induced PPARα and PPARg expression in PA-treated HAECs, the
present study subsequently investigated the effects of shear stress
applied to PA-treated HAECs on PPARγ expression. PPARγ expression
in PA-treated HAECs was lower compared with that in PA-untreated
HAECs in both the static and LSS groups, but neither of these
observations were significant (Fig.
5). In cells exposed to HSS, PA treatment significantly
increased PPARγ expression (Fig.
5). By contrast, HAECs exposed to HSS significantly increased
the expression of PPAR-γ compared with that in cells in static and
LSS groups, regardless of whether PA was present (Fig. 5). These results suggest that HSS
increased PPARγ expression in HAECs, particularly after PA
treatment.
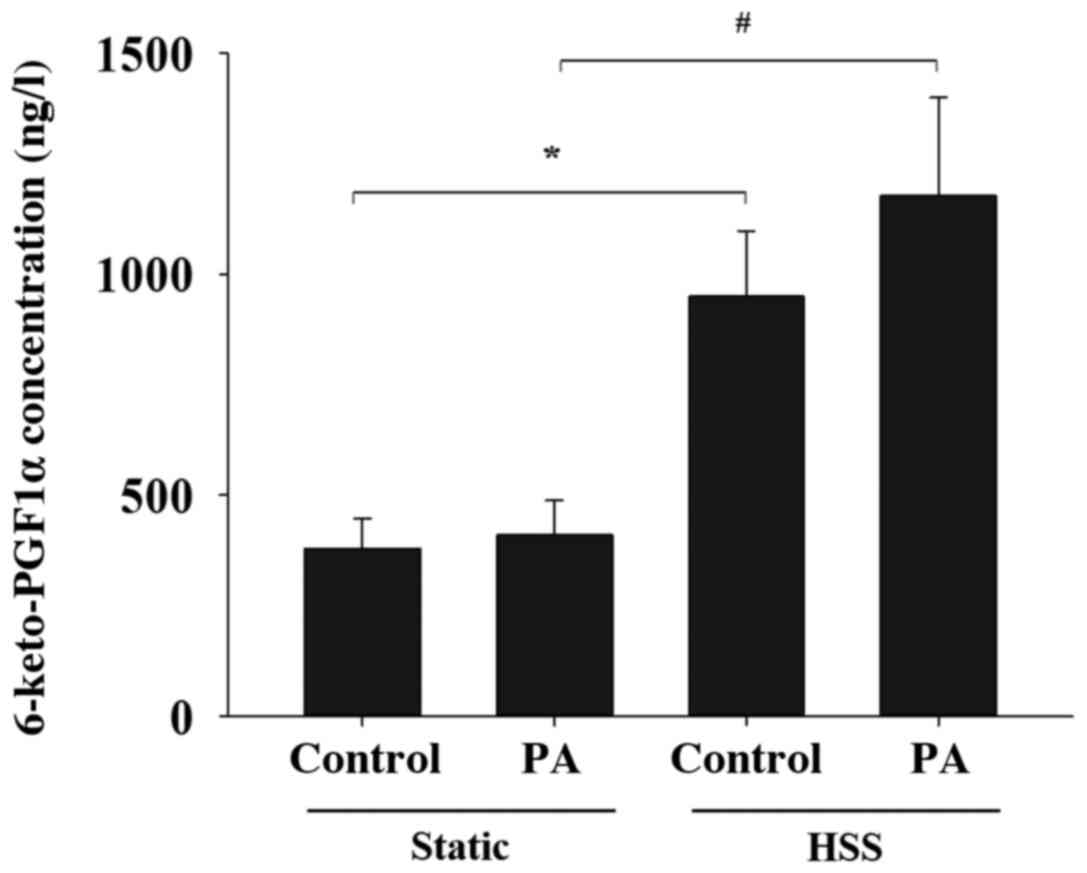
PGI2 is produced by
PA-treated HAECs in the presence of shear stress
PGI2 is a well-known vasoprotector factor
produced by ECs under shear stress and serves as a ligand for PPARα
and δ (19). The present study
therefore investigated the effects of PA treatment on HAEC
PGI2 production in response to shear stress. The levels
of 6-keto-PGF1α, a stable metabolite of PGI2, in the
media of shear stress-stimulated HAECs were significantly higher
compared with those in the HAECs of static control both in the
absence and presence of PA treatment (Fig. 6). These findings suggest that the
application of shear stress to HAECs increases the secretion of
PGI2 in the presence of PA.
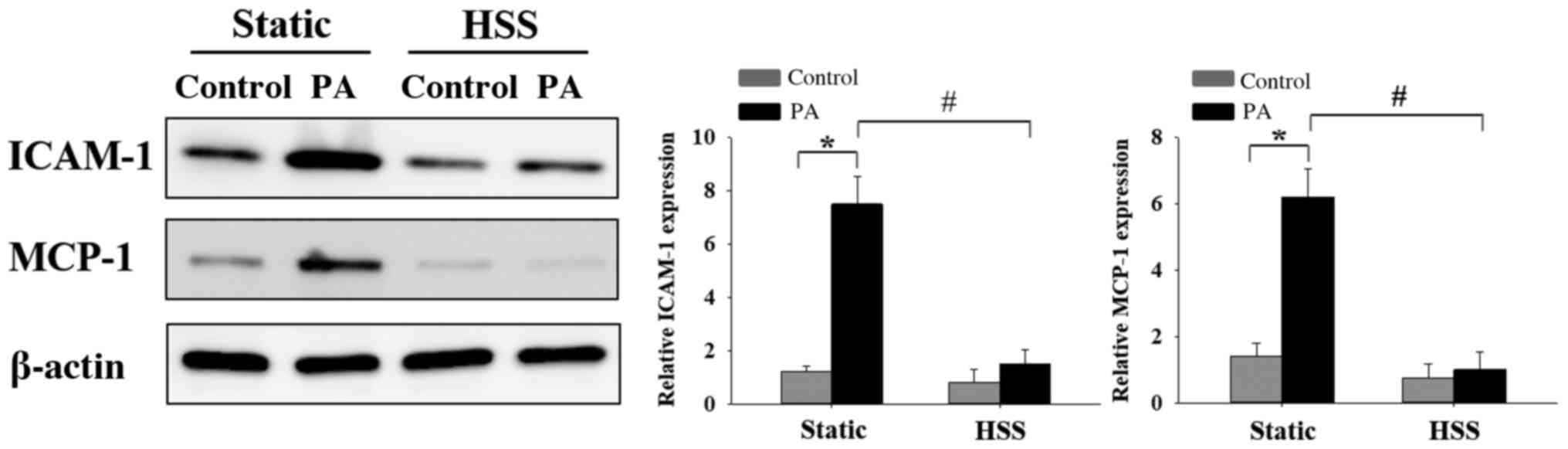
HSS inhibits PA-induced HAEC
inflammation
EC inflammation-induced dysfunction as a result of
high FFA concentrations is a risk factor for the development of
cardiovascular diseases (7,8). To investigate the effects of HSS on
PA-induced EC inflammation, HAECs were subjected to HSS for 20 h,
after which the expression levels of inflammatory markers ICAM-1
and MCP-1(1) were examined by
western blot analysis. High PA concentrations caused significant
increases in the expression of inflammatory markers ICAM-1 and
MCP-1 under static conditions (Fig.
7). Furthermore, when the ECs were exposed to long-term HSS,
PA-induced protein expression of ICAM-1 and MCP-1 was decreased
significantly. These results suggest that HSS exerted protective
effects against PA-induced EC inflammation.
Discussion
PA is a 16-carbon saturated long chain fatty acid
that is the main constituent of FFAs frequently observed in
metabolic disorders (28). In
healthy individuals, low serum concentrations of FFAs are
maintained (28). However,
concentrations can reach up to 1,000-fold higher compared with that
in healthy individuals in patients with diabetes (28). Elevated plasma FFAs cause metabolic
stress, which has been revealed to disrupt EC function (29). Excessive serum FFAs has been
reported to cause or facilitate EC dysfunction by inducing
inflammation and lipid peroxidation, which are associated oxidative
stress (9,29). In particular, three isoforms of PPAR
are implicated in various physiological processes, including the
suppression of inflammation and oxidative stress (30). It has been demonstrated that laminar
fluid shear stress and PPARγ serve key roles in the regulation of
EC function (20). However, their
role in the presence of high concentrations of FFA remains unclear.
The present study treated HAECs with PA to mimic metabolic
imbalance and serve as a model of metabolic stress. To the best of
our knowledge, the present study was the first to investigate the
effects of varying levels of shear stress on the expression of PPAR
in HAECs when combined with PA. The results revealed that
PA-treated HAECs expressed PPARα, PPARδ and PPARγ in response to
shear stress.
It has been previously demonstrated that perfusion
media obtained from shear-treated HAECs also induces PPAR
ligand-binding activities in smooth muscle cells (19). Previous reports have also
demonstrated that the application of shear stress to ECs can induce
the expression of all three isoforms of PPAR proteins and the
release of PPAR ligands (20,31). A
recent study revealed that activating PPARα and PPARγ ameliorated
total FFA accumulation in macrophages (32). The results of the present study
further demonstrated that all three PPAR isoforms were induced in
HAECs that were treated with HSS and high concentrations of PA.
These findings suggest that activating PPAR signaling may be a
promising therapeutic strategy for the treatment of metabolic
stress-induced vascular complications. Although the present results
demonstrated that HSS serves an important role in the regulation of
PPAR expression under PA-induced metabolic stress, additional
experiments are required to examine the associated mechanisms
involved in this phenomenon.
HSS can suppress inflammatory responses by
inhibiting ROS and control the release of anti-inflammatory factors
from ECs (27). The present study
revealed that the PPARδ and PPARγ expression profiles were similar
in HAECs that were subjected to static and LSS conditions in the
presence of PA. However, HSS was observed to increase PPARδ and
PPARγ protein expression regardless of the presence or absence of
PA stimulation, suggesting that HSS alone can regulate the
biosynthesis of these two PPAR isoforms under metabolic
disturbance. These results may indicate the regulatory role of
shear stress in HAECs under high PA concentrations. In the present
study, in the presence of high PA concentrations, LSS-treated HAECs
exhibited a similar magnitude of increased PPAR-α expression as
that in HSS-treated HAECs. According to previous reports,
PGI2 is one of the endogenous ligands of PPARα, where
only high shear stress could result in high levels of
PGI2 secretion whilst low shear stress could not
(33,34). Therefore, it remains a possibility
that low shear stress can increase the expression of PPARa through
a PGI2-independent pathway. There is insufficient
information regarding the effects of low shear stress on the
expression of PPAR-α, regardless of PA stimulation, on endothelial
cells. Nevertheless, it can be hypothesized that PPARα expression
is more susceptible to shear stress. The molecular mechanism
underlying these effects warrants additional investigation.
Consistent with a previous report (35), shear-stress treated HAECs in the
present study released high levels of 6-keto-PGF1α into the
perfusion medium. Additionally, HAECs conditioned under high PA
concentrations following exposure to HSS produced higher levels of
PGI2 in the perfusion media compared with those in the
static PA-treated HAECs. PGI2 is an important
vasoprotective factor that has been previously characterized as an
anti-inflammatory agent, antioxidant and a potent vasodilator
(36,37). Previous reports have demonstrated
that reductions in PGI2 bioavailability contributes to
EC dysfunction (38,39). Therefore, it was postulated that
increased PGI2 biosynthesis in shear stress-treated
HAECs may serve a protective effect on cells that are under
PA-induced metabolic stress.
The results of the current study suggested
strategies to promote HSS generated by the vessel wall. For
example, continuous exercise may be potentially advantageous for
preventing or ameliorating FFA-induced EC dysfunction. A limitation
of the present study is that the potential regulatory mechanism
underlying the induction of PPAR expression under shear stress and
high PA concentrations was not elucidated. PPARs are critical
factors in sculpting the metabolic phenotype in the vasculature
(17,18). Additional functional experiments are
required to determine whether PPARs exert vasoprotective effects
against high FFA-induced EC dysfunction. The present study further
demonstrated that HSS may serve an essential role by upregulating
PPARs and releasing PGI2 to exert protective effects
against high FFA-induced EC dysfunction.
In conclusion and to the best of our knowledge, the
present study was the first to demonstrate that sustained HSS
induced the expression of PPARs in HAECs during PA-induced
metabolic stress. Metabolic stress is a critical risk factor for
vascular disease and therefore results from the present study may
provide a potential target for future therapeutic strategies.
Acknowledgements
Not applicable.
Funding
The present study was supported by grants from the Cheng Hsin
General Hospital-National Defense Medical Center cooperative
research project (grant no. CH-NDMC-108-7), Chi Mei Medical
Center-National Defense Medical Center cooperative research project
(grant no. CM-NDMC-108-12) and Ministry of National Defense-Medical
Affairs Bureau (grant nos. MAB-106-102 and MAB-109-069).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors’ contributions
MCT and YLW performed the experiments. MCT and YLW
conceived and designed the present study. YLW, CTC and MCT acquired
and analyzed the data, and drafted the manuscript. YLW, CTC and MCT
revised and edited the manuscript and CTC performed the additional
experiments of Figs. 1 and 2. CST conceived the current study and
interpreted the data. YLW, CTC, CST and MCT contributed
substantially to the manuscript preparation. All authors discussed
the results, analyzed the data and commented on the manuscript. All
authors read and gave the final approval of this manuscript. MCT
and YLW confirm the authenticity of all the raw data.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Morigi M, Zoja C, Figliuzzi M, Foppolo M,
Micheletti G, Bontempelli M, Saronni M, Remuzzi G and Remuzzi A:
Fluid shear stress modulates surface expression of adhesion
molecules by endothelial cells. Blood. 85:1696–1703.
1995.PubMed/NCBI
|
|
2
|
Surapisitchat J, Hoefen RJ, Pi X,
Yoshizumi M, Yan C and Berk BC: Fluid shear stress inhibits
TNF-alpha activation of JNK but not ERK1/2 or p38 in human
umbilical vein endothelial cells: Inhibitory crosstalk among MAPK
family members. Proc Natl Acad Sci USA. 98:6476–6481.
2001.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Mun GI, An SM, Park H, Jo H and Boo YC:
Laminar shear stress inhibits lipid peroxidation induced by high
glucose plus arachidonic acid in endothelial cells. Am J Physiol
Heart Circ Physiol. 295:H1966–H1973. 2008.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Boden G: Free fatty acids, insulin
resistance, and type 2 diabetes mellitus. Proc Assoc Am Physicians.
111:241–248. 1999.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Liu L, Li Y, Guan C, Li K, Wang C, Feng R
and Sun C: Free fatty acid metabolic profile and biomarkers of
isolated post-challenge diabetes and type 2 diabetes mellitus based
on GC-MS and multivariate statistical analysis. J Chromatogr B
Analyt Technol Biomed Life Sci. 878:2817–2825. 2010.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Yi LZ, He J, Liang YZ, Yuan DL and Chau
FT: Plasma fatty acid metabolic profiling and biomarkers of type 2
diabetes mellitus based on GC/MS and PLS-LDA. FEBS Lett.
580:6837–6845. 2006.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Ghosh A, Gao L, Thakur A, Siu PM and Lai
CWK: Role of free fatty acids in endothelial dysfunction. J Biomed
Sci. 24(50)2017.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Pankow JS, Duncan BB, Schmidt MI,
Ballantyne CM, Couper DJ, Hoogeveen RC and Golden SH:
Atherosclerosis Risk in Communities Study. Fasting plasma free
fatty acids and risk of type 2 diabetes: The atherosclerosis risk
in communities study. Diabetes Care. 27:77–82. 2004.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Inoguchi T, Li P, Umeda F, Yu HY, Kakimoto
M, Imamura M, Aoki T, Etoh T, Hashimoto T, Naruse M, et al: High
glucose level and free fatty acid stimulate reactive oxygen species
production through protein kinase C-dependent activation of NAD(P)H
oxidase in cultured vascular cells. Diabetes. 49:1939–1945.
2000.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Su J, Tian H, Liu R and Liang J:
Inhibitive effects of glucose and free fatty acids on proliferation
of human vascular endothelial cells in vitro. Chin Med J (Engl).
115:1486–1490. 2002.PubMed/NCBI
|
|
11
|
Trombetta A, Togliatto G, Rosso A,
Dentelli P, Olgasi C, Cotogni P and Brizzi MF: Increase of palmitic
acid concentration impairs endothelial progenitor cell and bone
marrow-derived progenitor cell bioavailability: Role of the
STAT5/PPARγ transcriptional complex. Diabetes. 62:1245–1257.
2013.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Song X, Yang B, Qiu F, Jia M and Fu G:
High glucose and free fatty acids induce endothelial progenitor
cell senescence via PGC-1α/SIRT1 signaling pathway. Cell Biol Int.
41:1146–1159. 2017.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Legrand-Poels S, Esser N, L'homme L,
Scheen A, Paquot N and Piette J: Free fatty acids as modulators of
the NLRP3 inflammasome in obesity/type 2 diabetes. Biochem
Pharmacol. 92:131–141. 2014.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Qi Y, Du X, Yao X and Zhao Y: Vildagliptin
inhibits high free fatty acid (FFA)-induced NLRP3 inflammasome
activation in endothelial cells. Artif Cells Nanomed Biotechnol.
47:1067–1074. 2019.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Wang L, Chen Y, Li X and Zhang Y, Gulbins
E and Zhang Y: Enhancement of endothelial permeability by free
fatty acid through lysosomal cathepsin B-mediated Nlrp3
inflammasome activation. Oncotarget. 7:73229–73241. 2016.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Evans RM, Barish GD and Wang YX: PPARs and
the complex journey to obesity. Nat Med. 10:355–361.
2004.PubMed/NCBI View
Article : Google Scholar
|
|
17
|
Chinetti G, Fruchart JC and Staels B:
Peroxisome proliferator-activated receptors (PPARs): Nuclear
receptors at the crossroads between lipid metabolism and
inflammation. Inflamm Res. 49:497–505. 2000.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Marx N, Duez H, Fruchart JC and Staels B:
Peroxisome proliferator-activated receptors and atherogenesis:
Regulators of gene expression in vascular cells. Circ Res.
94:1168–1178. 2004.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Tsai MC, Chen L, Zhou J, Tang Z, Hsu TF,
Wang Y, Shih YT, Peng HH, Wang N, Guan Y, et al: Shear stress
induces synthetic-to-contractile phenotypic modulation in smooth
muscle cells via peroxisome proliferator-activated receptor
alpha/delta activations by prostacyclin released by sheared
endothelial cells. Circ Res. 105:471–480. 2009.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Liu Y, Zhu Y, Rannou F, Lee TS, Formentin
K, Zeng L, Yuan X, Wang N, Chien S, Forman BM and Shyy JY: Laminar
flow activates peroxisome proliferator-activated receptor-gamma in
vascular endothelial cells. Circulation. 110:1128–1133.
2004.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Ge X, Pan P, Jing J, Hu X, Chen L, Qiu X,
Ma R, Jueraitetibaike K, Huang X and Yao B: Rosiglitazone
ameliorates palmitic acid-induced cytotoxicity in TM4 Sertoli
cells. Reprod Biol Endocrinol. 16(98)2018.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Zhang Z, Xie X, Yao Q, Liu J, Tian Y, Yang
C, Xiao L and Wang N: PPARδ agonist prevents endothelial
dysfunction via induction of dihydrofolate reductase gene and
activation of tetrahydrobiopterin salvage pathway. Br J Pharmacol.
176:2945–2961. 2019.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Chiu JJ, Lee PL, Chen CN, Lee CI, Chang
SF, Chen LJ, Lien SC, Ko YC, Usami S and Chien S: Shear stress
increases ICAM-1 and decreases VCAM-1 and E-selectin expressions
induced by tumor necrosis factor-(alpha) in endothelial cells.
Arterioscler Thromb Vasc Biol. 24:73–79. 2004.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Chiu JJ, Chen LJ, Chang SF, Lee PL, Lee
CI, Tsai MC, Lee DY, Hsieh HP, Usami S and Chien S: Shear stress
inhibits smooth muscle cell-induced inflammatory gene expression in
endothelial cells: Role of NF-kappaB. Arterioscler Thromb Vasc
Biol. 25:963–969. 2005.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Chiu JJ, Chen LJ, Lee CI, Lee PL, Lee DY,
Tsai MC, Lin CW, Usami S and Chien S: Mechanisms of induction of
endothelial cell E-selectin expression by smooth muscle cells and
its inhibition by shear stress. Blood. 110:519–528. 2007.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Chang SF, Chang CA, Lee DY, Lee PL, Yeh
YM, Yeh CR, Cheng CK, Chien S and Chiu JJ: Tumor cell cycle arrest
induced by shear stress: Roles of integrins and Smad. Proc Natl
Acad Sci USA. 105:3927–3932. 2008.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Chiu JJ and Chien S: Effects of disturbed
flow on vascular endothelium: Pathophysiological basis and clinical
perspectives. Physiol Rev. 91:327–387. 2011.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Vessby B: Dietary fat, fatty acid
composition in plasma and the metabolic syndrome. Curr Opin
Lipidol. 14:15–19. 2003.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Guerci B, Böhme P, Kearney-Schwartz A,
Zannad F and Drouin P: Endothelial dysfunction and type 2 diabetes.
Part 2: Altered endothelial function and the effects of treatments
in type 2 diabetes mellitus. Diabetes Metab. 4:436–447.
2001.PubMed/NCBI
|
|
30
|
Ndisang JF: Cross-talk between heme
oxygenase and peroxisome proliferator-activated receptors in the
regulation of physiological functions. Front Biosci (Landmark Ed).
19:916–935. 2014.PubMed/NCBI View
Article : Google Scholar
|
|
31
|
Taba Y, Sasaguri T, Miyagi M, Abumiya T,
Miwa Y, Ikeda T and Mitsumata M: Fluid shear stress induces
lipocalin-type prostaglandin D(2) synthase expression in vascular
endothelial cells. Circ Res. 86:967–973. 2000.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Ye G, Gao H, Wang Z, Lin Y, Liao X, Zhang
H, Chi Y, Zhu H and Dong S: PPARα and PPARγ activation attenuates
total free fatty acid and triglyceride accumulation in macrophages
via the inhibition of Fatp1 expression. Cell Death Dis.
10(39)2019.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Moraes LA, Piqueras L and Bishop-Bailey D:
Peroxisome proliferator-activated receptors and inflammation.
Pharmacol Ther. 110:371–385. 2006.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Doroudi R, Gan LM, Selin Sjögren L and
Jern S: Effects of shear stress on eicosanoid gene expression and
metabolite production in vascular endothelium as studied in a novel
biomechanical perfusion model. Biochem Biophys Res Commun.
269:257–264. 2000.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Frangos JA, Eskin SG, McIntire LV and Ives
CL: Flow effects on prostacyclin production by cultured human
endothelial cells. Science. 227:1477–1479. 1985.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Vane J and Corin RE: Prostacyclin: A
vascular mediator. Eur J Vasc Endovasc Surg. 26:571–578.
2003.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Rolland PH, Jouve R, Pellegrin E, Mercier
C and Serradimigni A: Alteration in prostacyclin and prostaglandin
E2 production. Correlation with changes in human aortic
atherosclerotic disease. Arteriosclerosis. 4:70–78. 1984.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Shimokawa H: Primary endothelial
dysfunction: Atherosclerosis. J Mol Cell Cardiol. 31:23–37.
1999.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Di Francesco L, Totani L, Dovizio M,
Piccoli A, Di Francesco A, Salvatore T, Pandolfi A, Evangelista V,
Dercho RA, Seta F and Patrignani P: Induction of prostacyclin by
steady laminar shear stress suppresses tumor necrosis factor-alpha
biosynthesis via heme oxygenase-1 in human endothelial cells. Circ
Res. 104:506–513. 2009.PubMed/NCBI View Article : Google Scholar
|