Introduction
Periodontitis is one of the leading causes of tooth
loss in the adult population. This disease can be classified into
various categories, and one of the most destructive amongst them is
aggressive periodontitis (AgP). The incidence of AgP is lower than
that of other types of periodontitis. However, it affects young
individuals and may cause severe destruction of tooth-supporting
structures, including tooth loss if left untreated. The current
classification for diagnosing periodontal disease was established
by the American Academy of Periodontology (AAP) in 1999. This
classification provided strict guidelines to aid in AgP diagnosis.
These include three main factors: Systemically healthy individual,
rapid loss of clinical attachment and familial aggregation. AgP is
characterized by generalized or localized extreme periodontal
damage. It is a type of rapid and severe periodontal disease
affecting systemically healthy individuals. This disease is
characterized by an early age of onset, rapid rate of disease
progression and aggregation of family (1).
Fibroblasts serve an important role in chronic
infections, including periodontitis. Human gingival fibroblast (GF)
is a functionally distinct type of fibroblast in periodontal
tissues (2). GFs are subepithelial
and predominantly involved in the maintenance and regeneration of
periodontal tissues (3,4). The inflammatory destruction in
periodontal diseases may be the result of the interactions between
bacterial virulence factors and host defense mechanism (5,6). GFs
respond to periodontopathic organisms or their components by
initiating an inflammatory response, including the production of
various proinflammatory cytokines such as interleukin
(IL)-1, IL-6 and tumor necrosis factor (TNF)-α (7-9).
The results of our previous study demonstrated that GFs may produce
mitochondrial reactive oxygen species (mtROS) following stimulation
with lipopolysaccharides (LPS) of Porphyromonas gingivalis
(P. gingivalis) on the tooth surface, which mediates the
production of IL-1, IL-6 and TNF-α in GFs (10). Furthermore, the increased production
of matrix metalloproteinases (MMPs), including MMP-1, MMP-3 and
MMP-9, which degrade numerous extracellular matrix components, may
be the cause of GFs contributing toward periodontal tissue
destruction (11,12).
Among periodontopathic organisms, P.
gingivalis is an anaerobic Gram-negative bacterium that appears
to be associated with AgP. LPS, located on the outer membrane of
the cell wall of Gram-negative bacteria, is an important bacterial
surface component, and is considered to be a potent immunostimulant
(13). Although periodontitis can
be caused by bacteria such as P. gingivalis, host
susceptibility is crucial to the development of the disease. The
characteristics of the cells interacting with LPS from P.
gingivalis may partly determine susceptibility. For example,
mononuclear cells, neutrophils and platelets in patients with
periodontitis are known to be different from the same cells in
healthy individuals in their interaction with periodontal pathogens
(14-18).
However, little is known regarding whether differences also exist
in GFs between patients with AgP and healthy subjects.
Current evidence has suggested that AgP occurs in
susceptible individuals who have abnormal inflammatory/immune
response to periodontal pathogens (19). Earlier studies have demonstrated
that the expression levels of destruction factors associated with
disease progression were higher in periodontitis tissues in
patients with chronic periodontitis than in healthy subjects
(20,21). At present, there is a lack of data
available for comparison of cytokine profiles between GFs obtained
from patients with AgP and healthy subjects. The present study
tested the hypothesis that GFs in patients with AgP may be more
sensitive to LPS stimulation than GFs in control subjects. The
present study may elucidate the underlying mechanisms contributing
toward the rapid rate of disease progression of AgP.
Materials and methods
The present study was approved by the Review Board
and Ethics Committee of Peking University Health Science Center.
Written informed consent was obtained from all subjects.
Cell culture and treatment
GFs derived from four patients with AgP (AGFs) and
healthy subjects, who sought dental treatment at the Department of
Periodontology, Peking University School and Hospital of
Stomatology between January 2017 and January 2018, were used in the
present study. The test group consisted of 2 males and 2 females,
aged 38-50 years with a mean age of 44. The control group consisted
of 2 males and 2 females, aged 32 to 40 years with a mean age of
36. Exclusion criteria included pregnancy or breastfeeding,
smoking, alcohol abuse, uncontrolled diabetes and other systemic
conditions that could affect the periodontal status. Healthy GFs
(CGFs) were obtained from explants of human normal gingival tissues
as a control group from patients seeking dental treatment at the
Department of Periodontology, Peking University School and Hospital
of Stomatology between January 2017 and January 2018 (Table I; Fig.
1). GF explantation was performed as previously described
(22). Individuals were designated
as having AgP according to the 1999 AAP Classification of
Periodontal Diseases (1). Healthy
gingival tissue samples were collected from periodontal healthy
groups undergoing a crown-lengthening procedure, while inflamed
gingival tissue was harvested from teeth with pockets of 6 mm or
more in patients with chronic periodontitis following flap
debridement (22). The cells were
cultured at 37˚C and 5% CO2 in Dulbecco's modified
Eagle's medium (DMEM; HyClone; Cytiva), containing 10% (v/v) fetal
bovine serum (FBS; HyClone; Cytiva) and 100 U/ml penicillin with
100 µg/ml streptomycin. GFs between passages 3-6 were used. The
cells were treated with 1 µg/ml LPS derived from P.
gingivalis (InvivoGen, cat. no. tlrl-pglps) for 12 h. The
experiments on the 4 AgP patients and 4 healthy control subjects
were repeated 5 times.
 | Table IDemographic characteristics of the
study sample at baseline. |
Table I
Demographic characteristics of the
study sample at baseline.
| Sample | Age, years | Sex | Location | Treatment |
|---|
| Patient 1 | 38 | Female | 15, 16, 17 | Flap
debridement |
| Patient 2 | 43 | Female | 24, 25, 26, 27 | Flap
debridement |
| Patient 3 | 45 | Male | 26, 27 | Flap
debridement |
| Patient 4 | 50 | Male | 44, 45, 46, 47 | Flap
debridement |
| Healthy control
1 | 34 | Male | 16 |
Crown-lengthening |
| Healthy control
2 | 40 | Female | 36 |
Crown-lengthening |
| Healthy control
3 | 38 | Male | 25 |
Crown-lengthening |
| Healthy control
4 | 32 | Female | 26 |
Crown-lengthening |
Multimodal microplate reader
Fluorescence was measured using a multimodal
microplate reader (BioTek Instruments, Inc.). HGFs were trypsinized
and washed with cold phosphate-buffered saline (PBS). The cells
(1.8x105) were resuspended in 1 ml DMEM containing 5 µM
MitoSOX Red and incubated in the dark in a CO2 incubator
for 30 min. The cells were centrifuged at 130 x g for 5 min at room
temperature, washed three times with PBS, and resuspended in 500 µl
PBS. The mtROS content of cells was analyzed based on the
fluorescence intensity of MitoSOX Red. The mtROS content of cells
was analyzed based on the fluorescence intensity of MitoSOX Red
with excitation at 510 nm and emission at 580 nm.
RNA isolation and reverse
transcription-quantitative polymerase chain reaction (RT-qPCR)
HGFs and AGFs were cultured in 6-well plates
(1x105 per well) followed by the addition of medium with
or without LPS. Total RNA was isolated using TRIzol®
reagent (Invitrogen; Thermo Fisher Scientific, Inc.) according to
the manufacturer's protocol. cDNA was synthesized using Reverse
Transcription Premix (Bioneer Corporation). The thermal profile was
incubation at 70˚C for 5 min before chilling on ice. PCR was
performed using gene-specific primers (Table II) and PCR premix (Kapa Biosystems;
Roche Diagnostics). The PCR conditions were 10 min at 95˚C,
followed by 40 cycles of 95˚C for 15 sec and 60˚C for 60 sec (PCR
machine model: Eppendorf Mastercycler X50h; Eppendorf). All
reactions were performed in triplicate in two separate experiments.
The relative expression levels of the targets in each sample were
calculated using the comparative 2-ΔΔCq method following
normalization against the expression of ACTB (21).
 | Table IIPrimers used for gene
amplification. |
Table II
Primers used for gene
amplification.
| Gene | Primer name | Primer
sequence | Primer length
(bp) |
|---|
| IL-1β | IL-1β-forward |
CTTCAGCCAATCTTCATTGCT | 200 |
| | IL-1β-reverse |
TCGGAGATTCGTAGCTGGAT | |
| IL-6 | IL-6-forward |
GAGGGCTCTTCGGCAAATGTA | 89 |
| | IL-6-reverse |
CCCAGTGGACAGGTTTCTGAC | |
| TNF-α | TNF-α-forward |
GCTCAGACATGTTTTCCGTGAA | 140 |
| | TNF-α-reverse |
GTCACCAAATCAGCATTGTTTAGA | |
| MMP-1 | MMP-1-forward |
TCTGGGGAAAACCTTTCGACT | 136 |
| | MMP-1-reverse |
CACCAACGTATTCAAAAGCACAA | |
| MMP-3 | MMP-3-forward |
AGTCTTCCAATCCTACTGTTGCT | 148 |
| | MMP-3-reverse |
TCCCGTATGGTTACACCAATCC | |
| MMP-9 | MMP-9-forward |
TGTACCGCTATGGTTACACTCG | 180 |
| | MMP-9-reverse |
GGCAGGGACAGTTGCTTCT | |
| ACTB | ACTB-forward |
AGCACAATGAAGATCAAGATCAT | 127 |
| | ACTB-reverse |
ACTCGTCATACTCCTGCTTGC | |
Measurement of various cytokine levels
by ELISA
The GFs were cultured in 96-well plates
(1x104 per well) and then medium with or without 1 µg/ml
LPS was added. The levels of IL-1β, IL-6 and TNF-α in cell culture
supernatants were measured using ELISA kits (R&D Systems China
Co., Ltd.; cat. no. IL-1β, 70-E-EK101B1; IL-6, 70-E-EK1061; TNF-α,
EK1821), according to the manufacturer's protocols.
Western blotting
Whole protein lysates were prepared using PRO-PREP
Protein Extraction Solution (Intron Biotechnology, Inc.). The
nuclear and cytoplasmic fractions were isolated using an NE-PER
Nuclear and Cytoplasmic Extraction Reagent kit (Thermo Fisher
Scientific, Inc.), according to the manufacturer's protocol. Equal
amounts (15 µl) of protein, measured using the BCA protein assay
(Pierce; Thermo Fisher Scientific, Inc.) were loaded into each lane
of the gel. Proteins were separated by electrophoresis on 10-12%
(v/v) sodium dodecyl sulphate-polyacrylamide gels and transferred
onto nitrocellulose membranes (BD Biosciences). The membranes were
then blocked with 5% BSA (Beijing Solarbio Science & Technology
Co., Ltd.; cat. no. SW3015) for 2 h at room temperature and
incubated with rabbit antibodies against MMP-1 (cat. no. Ab137332;
Abcam), MMP-3 (cat. no. Ab2915; Abcam), MMP-9 (cat. no. Ab38898;
Abcam) and actin (cat. no. TA-09; Abcam) at a 1:1,000 dilution for
2 h at room temperature. The membranes were then incubated with
horseradish peroxidase (HRP)-coupled anti-rabbit secondary antibody
(Cell Signaling Technology, Inc. cat. no. 5151, 1:2,000) at room
temperature for 40 min. The membranes were then washed with TBST
(20% Tween-20) 3 times, for 10 min each time. Finally, the bands
were stained with an enhanced chemiluminescence kit (Thermo Fisher
Scientific, Inc.). Rabbit anti-actin was used as a loading control
(Cell Signaling Technology, Inc.). After the image was scanned,
grayscale analysis was performed using Gel Image system ver.4.00
(Tanon Science and Technology Co., Ltd.).
Statistical analysis
Data represent the mean ± standard deviation of five
independent experiments. Data were analyzed using GraphPad Prism
software (version 6; GraphPad Software, Inc.). Variance between the
two groups was analyzed by t-test. P<0.05 was considered to
indicate a statistical difference. P<0.01 was considered to
indicate a statistically significant difference.
Results
Comparison of morphology of CGFs and
AGF
The images of typical morphology of GFs from
patients with AgP and from healthy subjects were observed under a
microscope (Fig. 1). No difference
in morphology or proliferation was identified between CGFs and
AGFs.
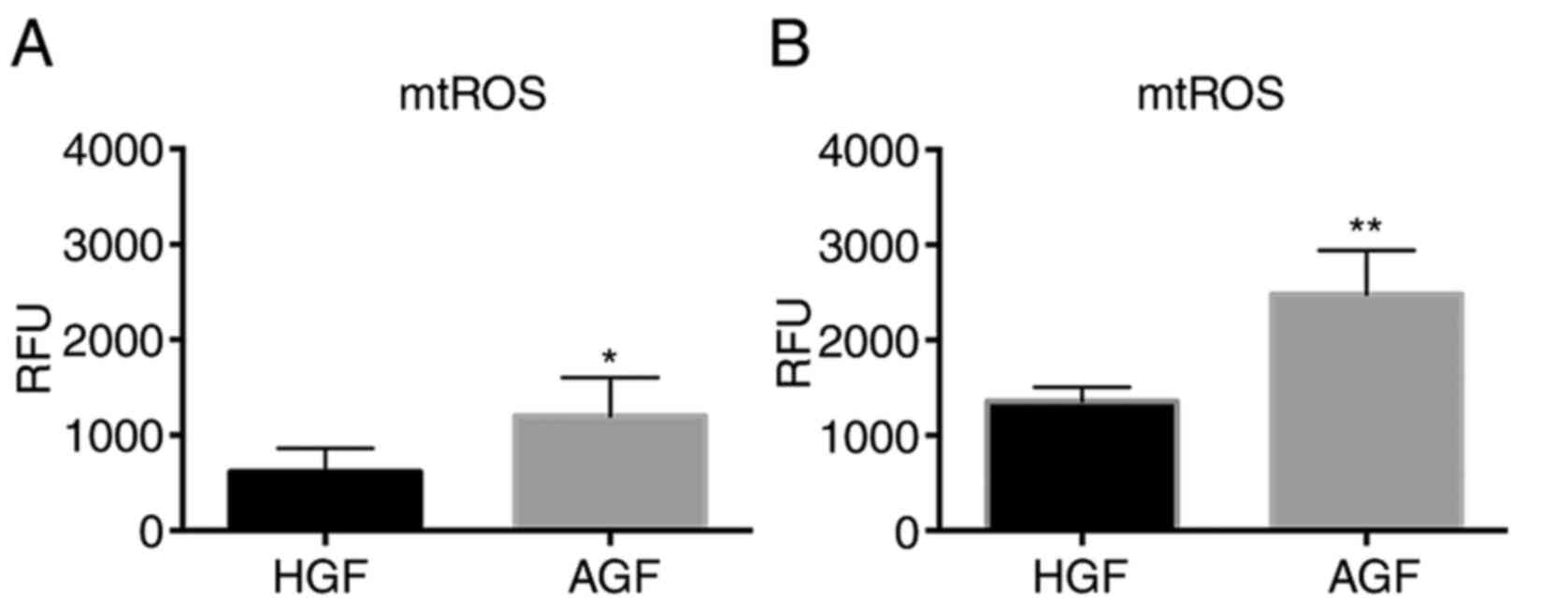
Comparison of basal levels and
augmented mtROS levels stimulated by LPS in CGFs and AGFs
The levels of mtROS in unstimulated or stimulated
GFs were measured and were compared between the heathy and the AgP
groups (Fig. 2). In the
unstimulated cells, the mtROS level was higher in the AgP group
compared with the healthy group (P<0.05). In the stimulated
cells, the mtROS level was significantly higher in the AgP group
than in the healthy group (P<0.01).
Comparison of mRNA expression levels
of proinflammatory genes in HGFs and AGF prior to and following
stimulation by LPS
The mRNA expression levels (relative to the
housekeeping gene) of IL-1β, IL-6, and TNF-α, which encode
proinflammatory cytokines in GFs, were measured in unstimulated or
stimulated GFs. These levels were then compared between the heathy
and the AgP groups (Fig. 3). In the
unstimulated cells, IL-1β and IL-6 mRNA expression was higher in
the AgP group compared with the healthy group (IL-1β, P<0.05;
IL-6, P<0.05; Fig. 3A and
C), while the expression of TNF-α
mRNA did not show a significant difference between the healthy and
AgP groups (TNF-α, P>0.05; Fig.
3E). In stimulated cells, the expression level of IL-1β and
IL-6 mRNA was significantly higher in the AgP group than in the
healthy group (P<0.01; Fig. 3B
and D), and TNF-α mRNA was higher
in the AgP group than in the healthy group (TNF-α, P<0.05;
Fig. 3F).
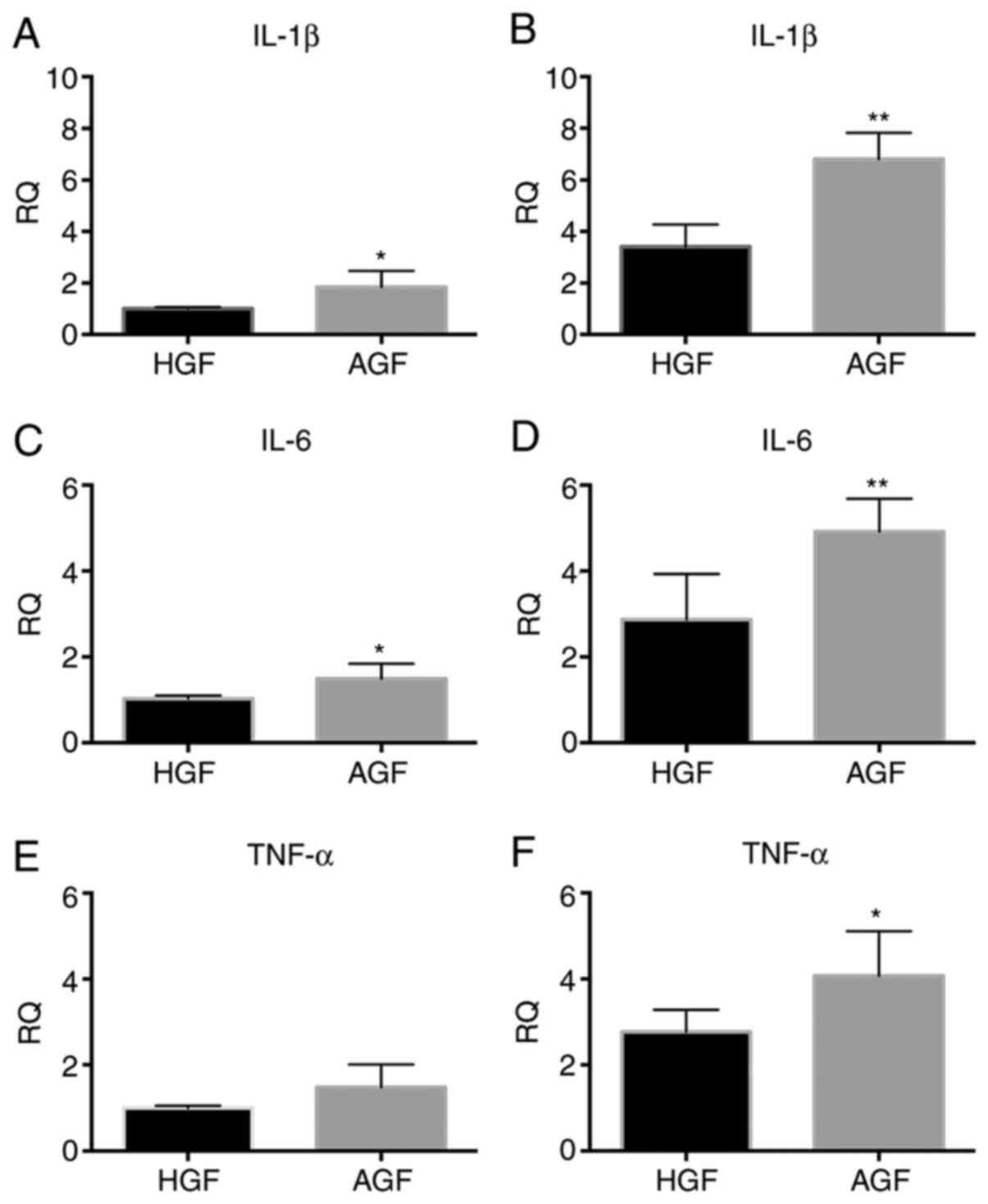 | Figure 3mRNA expression of proinflammatory
cytokines, including IL-1β, IL-6, TNF-α in control subjects and
patients with AgP prior to and following a 12 h challenge with LPS
from P. gingivalis. The mRNA expression of IL-1β in GFs of
control subjects and patients with AgP (A) prior to LPS challenge
and (B) following LPS challenge. The mRNA expression of IL-6 in GFs
of control subjects and patients with AgP (C) prior to LPS
challenge and (D) following LPS challenge. The mRNA expression of
TNF-α in GFs of control subjects and patients with AgP (E) prior to
LPS challenge and (F) following LPS challenge.
*P<0.05, **P<0.01 vs. HGF. IL,
interleukin; TNF-α, tumor necrosis factor-α; GFs, gingival
fibroblasts; AgP, aggressive periodontitis; LPS,
lipopolysaccharide; HGFs, GFs from healthy subjects; AGFs, GFs from
patients with aggressive periodontitis; RQ, relative quantity. |
Comparison of protein levels of
proinflammatory cytokines in HGFs and AGF prior to and following
LPS stimulation
To analyze whether changes in cytokines gene
expression also resulted in increased protein secretion, culture
supernatants of challenged and unchallenged GFs of patients and
control subjects were analyzed for the presence and levels of
IL-1β, IL-6 and TNF-α (Fig. 4).
IL-1β and IL-6 levels in GFs unstimulated with LPS were higher in
the AgP group than in the healthy group (IL-1β, P<0.05; IL-6,
P<0.05 Fig. 4A and C). However, the expression of TNF-α did
not significantly differ between the healthy and AgP groups (TNF-α,
P>0.05; Fig. 4E). With
stimulation, IL-1β, IL-6 and TNF-α levels in GFs were significantly
higher in the AgP group than in the healthy group (IL-1β,
P<0.01; IL-6, P<0.01; TNF-α, P<0.01; Fig. 4B, D
and F). There was a difference in
the ratio of cytokines between GFs from AgP and GFs from healthy
subjects when compared the unstimulated with stimulated states.
Under the stimulated state, the ratio was higher in the AgP group
than in the healthy group (IL-1β, P<0.05; IL-6, P<0.05;
TNF-α, P<0.05; Fig. S1A-C).
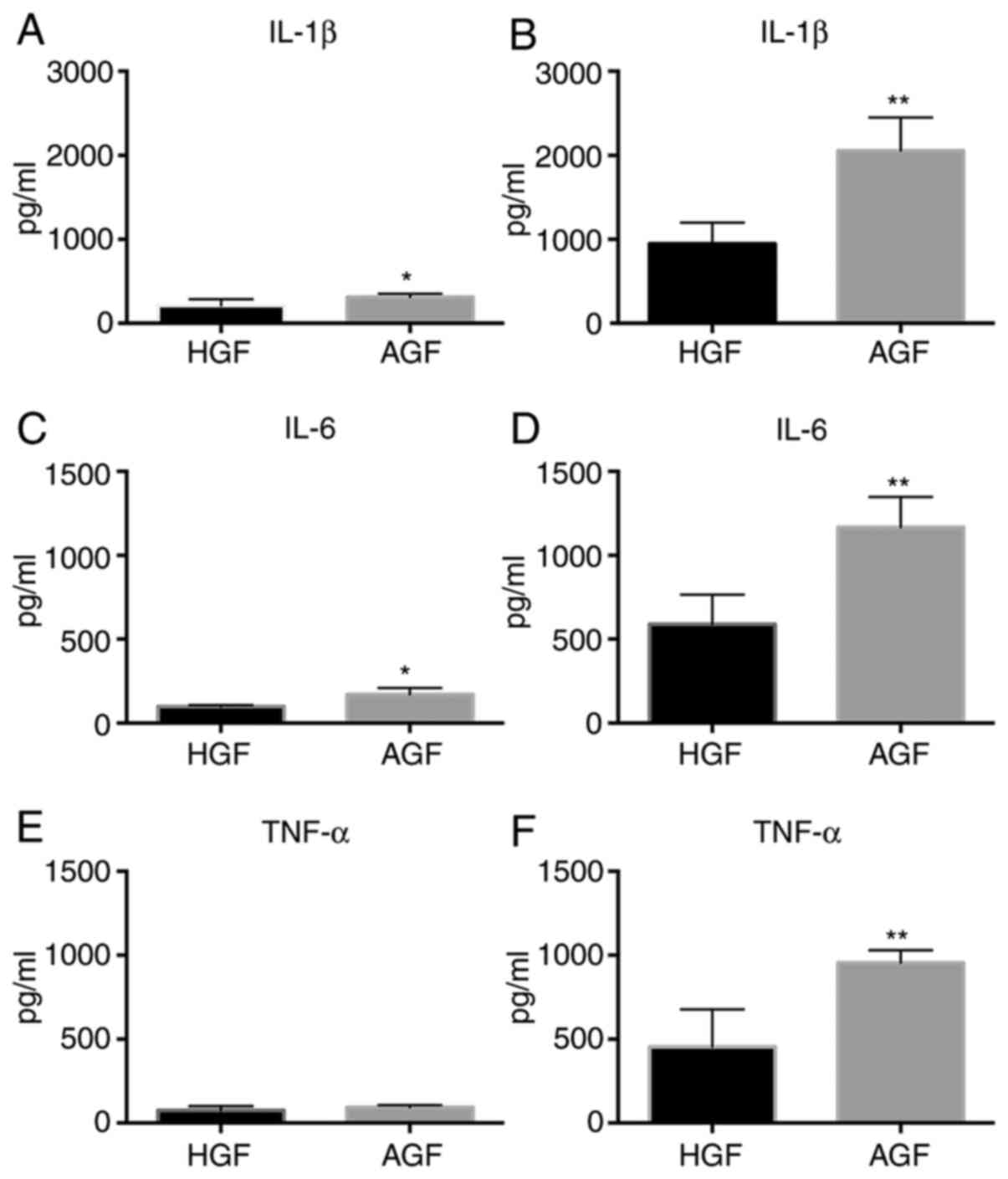 | Figure 4Protein expression of proinflammatory
cytokines, including IL-1β, IL-6 and TNF-α in control subjects and
patients with AgP prior to and following 12 h challenge with LPS
from P. gingivalis. Protein expression of IL-1β in GFs of
control subjects and patients with AgP (A) prior to LPS challenge
and (B) following LPS challenge. Protein expression of IL-6 in GFs
of control subjects and patients with AgP (C) prior to LPS
challenge and (D) following LPS challenge. Protein expression of
TNF-α in GFs of control subjects and patients with AgP (E) prior to
LPS challenge and (F) following LPS challenge.
*P<0.05, **P<0.01 vs. HGF. IL,
interleukin; TNF-α, tumor necrosis factor-α; GFs, gingival
fibroblasts; AgP, aggressive periodontitis; LPS,
lipopolysaccharide; HGFs, GFs from healthy subjects; AGFs, GFs from
patients with aggressive periodontitis. |
Comparison of mRNA levels of genes
associated with matrix degradation in HGFs and AGF with and without
LPS stimulation
Gene expression of MMP-1, MMP-3 and MMP-9 in
unstimulated or stimulated GFs was compared between the healthy and
the AgP groups (Fig. 5). In the
unstimulated cells, MMP-1, MMP-3 and MMP-9 mRNA expression was
higher in the AgP group than in the healthy group (MMP-1,
P<0.05; MMP-3, P<0.05; MMP-9, P<0.05; Fig. 5A, C
and E). In the stimulated cells,
MMP-1, MMP-3 and MMP-9 mRNA expression was significantly higher in
the AgP group than in the healthy group (MMP-1, P<0.01; MMP-3,
P<0.01; MMP-9, P<0.01; Fig.
5B, D and F).
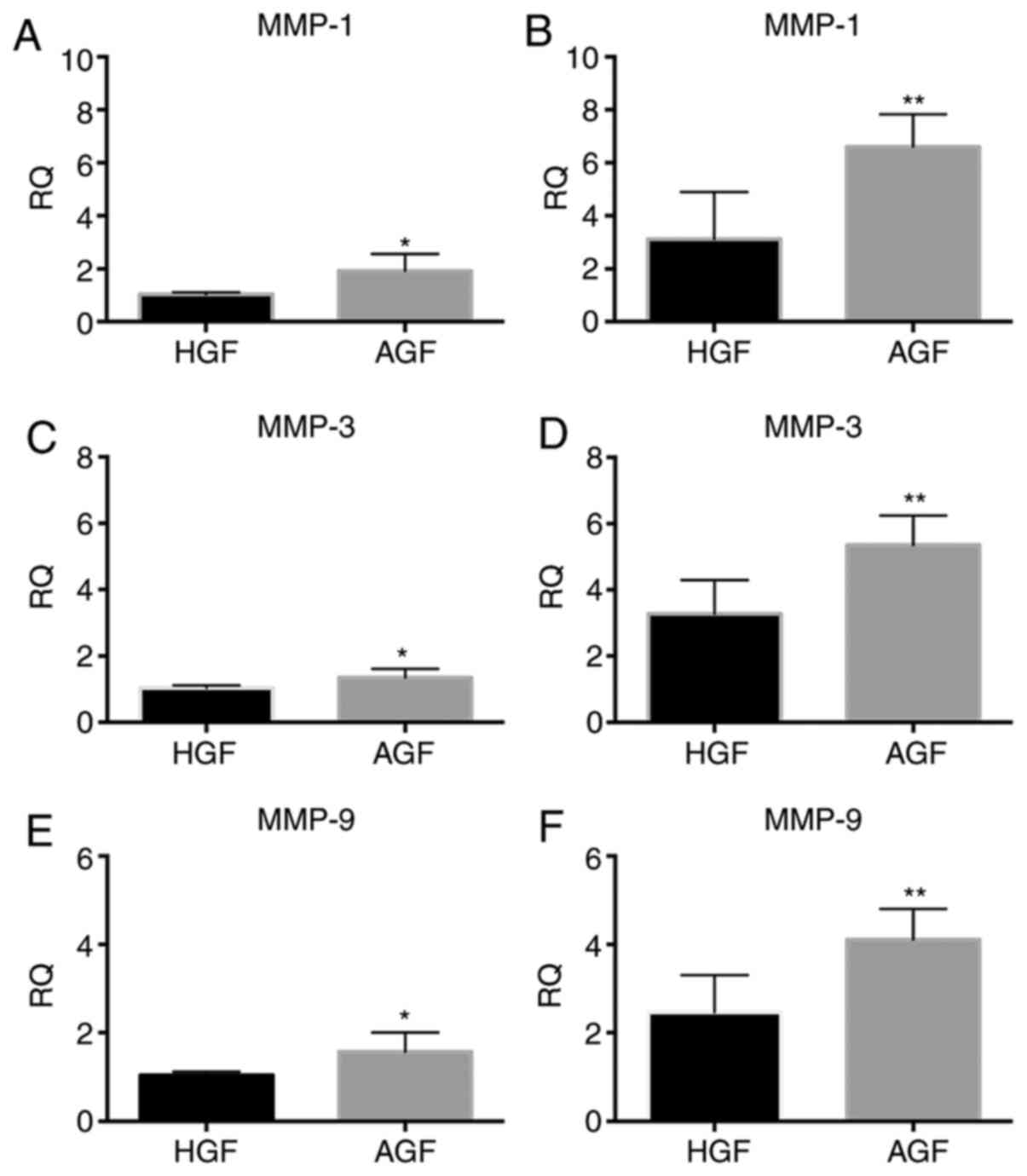 | Figure 5mRNA expression levels of MMP-1, MMP-3
and MMP-9, in control subjects and patients with AgP prior to and
following 12 h challenge with LPS from P. gingivalis. The
mRNA expression of MMP-1 in GFs of control subjects and patients
with AgP (A) prior to LPS challenge and (B) following LPS
challenge. The mRNA expression of MMP-3 in GFs of control subjects
and patients with AgP (C) prior to LPS challenge and (D) following
LPS challenge. The mRNA expression of MMP-9 in GFs of control
subjects and patients with AgP (E) prior to LPS challenge and (F)
following LPS challenge. *P<0.05,
**P<0.01 vs. HGF. MMP, matrix metalloproteinases;
AgP, aggressive periodontitis; LPS, lipopolysaccharide; GFs,
gingival fibroblasts; HGFs, GFs from healthy subjects; AGFs, GFs
from patients with aggressive periodontitis; RQ, relative
quantity. |
Comparison of protein levels of genes
associated with matrix degradation in GFs of HGFs and AGF with and
without LPS stimulation
To investigate whether the difference was also
present at the protein levels, the protein expression of MMP-1,
MMP-3 and MMP-9 was measured through Western blotting. In the
unstimulated cells, the levels of MMP-1 and MMP-9 were higher in
the AgP group than in the healthy group (P<0.05; Fig. 6A-C), but there was no difference in
MMP-3 expression between AgP and healthy groups (P>0.05;
Fig. 6A and D). In the stimulated cells, the protein
expression of MMP-1, MMP-3 and MMP-9 was significantly higher in
the AgP group than in the healthy group (MMP-1, P<0.01;
MMP-3, P<0.01; MMP-9, P<0.01; Fig. 6A-D).
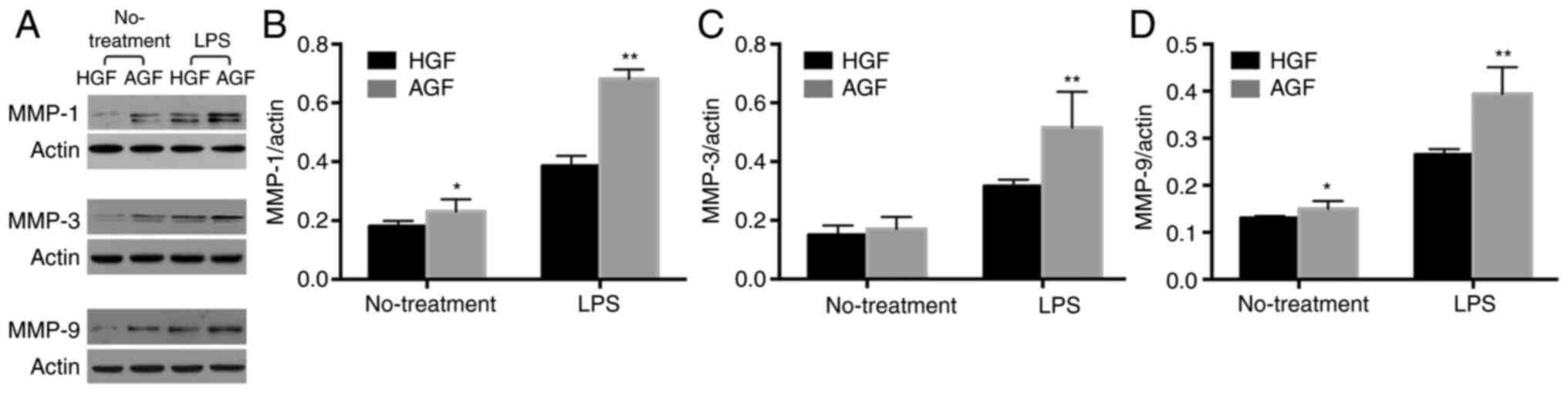 | Figure 6Protein levels of MMP-1, MMP-3 and
MMP-9, in control subjects and patients with AgP prior to and
following 12 h challenge with LPS from P. gingivalis. (A)
The results of Western blotting of MMP-1, MMP-3 and MMP-9 in GFs of
control subjects and patients with AgP prior to and following LPS
challenge. The analysis of the relative content of (B) MMP-1, (C)
MMP-3 and (D) MMP-9. *P<0.05, **P<0.01
vs. treatment matched HGF. MMP, matrix metalloproteinases; AgP,
aggressive periodontitis; LPS, lipopolysaccharide; GFs, gingival
fibroblasts; HGFs, GFs from healthy subjects; AGFs, GFs from
patients with aggressive periodontitis. |
Discussion
Human GFs are the most abundant resident cells in
periodontal tissue. The continuous expression of inflammatory
cytokines by GFs may be involved in the overproduction of lytic
enzymes, apoptotic factors and bone-resorbing mediators, resulting
in periodontal tissue destruction. Excessive production of these
mediators is important for the pathogenesis and progression of
periodontitis.
In the present study, it was hypothesized that as
the host response is important in the pathogenesis of AgP, GFs
respond differently to LPS challenge in patients with AgP than in
healthy controls. Therefore, GFs of patients with AgP and healthy
control subjects were challenged with LPS from P.
gingivalis.
In the present study, AGFs exhibited higher
expression of IL-1β, IL-6, MMP-1, MMP-3 and MMP-9 without LPS than
HGFs. It was not possible to verify the difference in TNF-α mRNA
expression in GFs between patients with AgP and control subjects
without LPS. After 12-h LPS stimulation, AGFs exhibited higher
expression of IL-1β, IL-6, TNF-α, MMP-1, MMP-3 and MMP-9 than HGFs.
A previous study reported that the comparison of mRNA and protein
expression of inflammatory genes was higher in inflamed GFs (IGFs;
GFs isolated from patients with chronic periodontitis) than in HGFs
after stimulation with P. gingivalis LPS (21). The present study was consistent with
this previous report (21,23). To date, few studies have focused on
AGFs and the present study was the first to employ P.
gingivalis LPS to stimulate AGFs and compare the different
responses in HGFs. P. gingivalis LPS mediates inflammation
by inducing the release of proinflammatory cytokines in HGFs
(24). The present study identified
that the expression of inflammatory cytokines and MMPs was elevated
following challenge with P. gingivalis LPS in AGFs.
Furthermore, the differences in factors associated with
inflammation were identified in GFs between the AgP and the healthy
groups with/without LPS stimulation. Additionally, the differences
in GFs between the AgP and the healthy groups with LPS stimulation
were more significant. The results of the present study
demonstrated that GFs of patients with AgP exhibit hyperreactivity
in the presence of LPS. The present study has another limitation in
that expression was analyzed at only one time point. It may take
more time for differences in cytokine and MMP responses to become
detectable. In addition to the inflammatory cytokines and MMPs,
mtROS was higher in GFs in patients with AgP than in control
subjects in unchallenged and challenged cells. LPS-stimulated AGFs
produced inflammatory cytokines more significantly than HGFs.
MtROS are produced in the process of normal aerobic
cell metabolism, serve important physiological roles in maintaining
cell redox status, and are necessary for normal cellular function.
They are generated as by-products of energy production, depending
on the normal structure and function of mitochondrion (25). More mtROS are generated in AGFs,
suggesting a possible dysfunction or a morphological change in
mitochondria (26). Mitochondrial
dysfunction may increase allergic airway inflammation (27), and increase inflammatory response to
cytokines in normal human chondrocytes (28). In the present study, AGFs exhibited
a marked increase in LPS-triggered activation of inflammatory
cytokines, accompanied by MMPs release. Whether the response of GFs
in AgP patients to LPS is aggravated by the increased mtROS will be
a focus of our future studies.
In conclusion, the present study demonstrated that
GFs of patients with AgP display hyperreactivity when challenged
with LPS. Although in vivo analyses are required to verify
these findings, these results explain, in part, the difference in
cellular responses between patients with AgP and healthy subjects.
The results of the present study may help aid understanding of the
pathogenesis of AgP and development of novel strategies to
alleviate the inflammation. There is no clear evidence of a causal
association between mtROS and aggressive periodontitis. However,
increasing data have suggested that it may be an important factor
in the pathogenesis of the disease.
Supplementary Material
The increased multiple of IL-1β, IL-6,
TNF-α stimulated by LPS between GFs from AgP and GFs from healthy
subjects. (A) There is a significant difference in the increased
multiple of IL-1β stimulated by LPS between GFs from AgP and GFs
from healthy subjects (*P<;0.05). (B) There is a
significant difference in the increased multiple of IL-6 stimulated
by LPS between GFs from AgP and GFs from healthy subjects
(*P<0.05). (C) There is a significant difference in
the increased multiple of TNF-α stimulated by LPS between GFs from
AgP and GFs from healthy subjects (*P<0.05). IL,
interleukin; LPS, lipopolysaccharide; GFs, gingival fibroblasts;
AgP, aggressive periodontitis; TNF-α, tumor necrosis factor-α;
HGFs, GFs from healthy subjects; AGFs, GFs from patients with
aggressive periodontitis.
Acknowledgements
Not applicable.
Funding
The present study was funded by the National Natural Science
Foundation of China (grant no. 81271148).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
QXL designed the study; XL and XW performed the
experiments, analyzed the data and prepared the manuscript. QXL
reviewed the manuscript. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
The present study was approved by the Medical
Ethical Committee of the School of Stomatology, Peking University
(approval no. PKUSSIRB-2013017).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
No authors listed. 1999 International
International Workshop for a classification of periodontal diseases
and conditions. Papers. Oak Brook, Illinois, October 30-November 2,
1999. Ann Periodontol 4: i. 1–112. 1999.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Buckley CD, Pilling D, Lord JM, Akbar AN,
Scheel-Toellner D and Salmon M: Fibroblasts regulate the switch
from acute resolving to chronic persistent inflammation. Trends
Immunol. 22:199–204. 2001.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Ara T, Kurata K, Hirai K, Uchihashi T,
Uematsu T, Imamura Y, Furusawa K, Kurihara S and Wang PL: Human
gingival fibroblasts are critical in sustaining inflammation in
periodontal disease. J Periodontal Res. 44:21–27. 2009.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Flavell SJ, Hou TZ, Lax S, Filer AD,
Salmon M and Buckley CD: Fibroblasts as novel therapeutic targets
in chronic inflammation. Br J Pharmacol. 153 (Suppl 1):S241–S246.
2008.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Dongari-Bagtzoglou AI and Ebersole JL:
Increased presence of interleukin-6 (IL-6) and IL-8 secreting
fibroblast subpopulations in adult periodontitis. J Periodontol.
69:899–910. 1998.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Pathirana RD, O'Brien-Simpson NM and
Reynolds EC: Host immune responses to Porphyromonas
gingivalis antigens. Periodontol 2000. 52:218–237.
2010.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Wilson M: Biological activities of
lipopolysaccharides from oral bacteria and their relevance to the
pathogenesis of chronic periodontitis. Sci Prog. 78:19–34.
1995.PubMed/NCBI
|
|
8
|
Garlet GP: Destructive and protective
roles of cytokines in periodontitis: A re-appraisal from host
defense and tissue destruction viewpoints. J Dent Res.
89:1349–1363. 2010.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Liu J, Wang Y and Ouyang X: Beyond
toll-like receptors: Porphyromonas gingivalis induces IL-6,
IL-8, and VCAM-1 expression through NOD-mediated NF-KB and ERK
signaling pathways in periodontal fibroblasts. Inflammation.
37:522–533. 2014.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Li X, Wang X, Zheng M and Luan QX:
Mitochondrial reactive oxygen species mediate the
lipopolysaccharide-induced pro-inflammatory response in human
gingival fibroblasts. Exp Cell Res. 347:212–221. 2016.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Goncalves PF, Huang H, McAninley S, Alfant
B, Harrison P, Aukhil I, Walker C and Shaddox LM: Periodontal
treatment reduces matrix metalloproteinase levels in localized
aggressive periodontitis. J Periodontol. 84:1801–1808.
2013.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Seguier S, Gogly B, Bodineau A, Godeau G
and Brousse N: Is collagen breakdown during periodontitis linked to
inflammatory cells and expression of matrix metalloproteinases and
tissue inhibitors of metalloproteinases in human gingival tissue? J
Periodontol. 72:1398–1406. 2001.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Henderson B, Poole S and Wilson M:
Bacterial modulins: A novel class of virulence factors which cause
host tissue pathology by inducing cytokine synthesis. Microbiol
Rev. 60:316–341. 1996.PubMed/NCBI
|
|
14
|
Gustafsson A, Ito H, Asman B and Bergstrom
K: Hyper-reactive mononuclear cells and neutrophils in chronic
periodontitis. J Clin Periodontol. 33:126–129. 2006.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Nicu EA, Van der Velden U, Everts V, Van
Winkelhoff AJ, Roos D and Loos BG: Hyper-reactive PMNs in
FcgammaRIIa 131 H/H genotype periodontitis patients. J Clin
Periodontol. 34:938–945. 2007.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Matthews JB, Wright HJ, Roberts A, Cooper
PR and Chapple IL: Hyperactivity and reactivity of peripheral blood
neutrophils in chronic periodontitis. Clin Exp Immunol.
147:255–264. 2007.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Zhan Y, Lu R, Meng H, Wang X and Hou J:
Platelet activation and platelet-leukocyte interaction in
generalized aggressive periodontitis. J Leukoc Biol. 100:1155–1166.
2016.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Nicu EA, Van der Velden U, Nieuwland R,
Everts V and Loos BG: Elevated platelet and leukocyte response to
oral bacteria in periodontitis. J Thromb Haemost. 7:162–170.
2009.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Ogawa T, Ozaki A, Shimauchi H and Uchida
H: Hyporesponsiveness of inflamed human gingival fibroblasts from
patients with chronic periodontal diseases against cell surface
components of Porphyromonas gingivalis. FEMS Immunol Med
Microbiol. 18:17–30. 1997.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Scheres N, Laine ML, Sipos PM,
Bosch-Tijhof CJ, Crielaard W, de Vries TJ and Everts V: Periodontal
ligament and gingival fibroblasts from periodontitis patients are
more active in interaction with Porphyromonas gingivalis. J
Periodontal Res. 46:407–416. 2011.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Kang W, Hu Z and Ge S: Healthy and
inflamed gingival fibroblasts differ in their inflammatory response
to Porphyromonas gingivalis lipopolysaccharide.
Inflammation. 39:1842–1852. 2016.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Zdarilova A, Svobodova A, Simanek V and
Ulrichova J: Prunella vulgaris extract and rosmarinic acid suppress
lipopolysaccharide-induced alteration in human gingival
fibroblasts. Toxicol In Vitro. 23:386–392. 2009.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Wang PL, Ohura K, Fujii T, Oido-Mori M,
Kowashi Y, Kikuchi M, Suetsugu Y and Tanaka J: DNA microarray
analysis of human gingival fibroblasts from healthy and
inflammatory gingival tissues. Biochem Biophys Res Commun.
305:970–973. 2003.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Imatani T, Kato T and Okuda K: Production
of inflammatory cytokines by human gingival fibroblasts stimulated
by cell-surface preparations of Porphyromonas gingivalis.
Oral Microbiol Immunol. 16:65–72. 2001.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Kowaltowski AJ, de Souza-Pinto NC,
Castilho RF and Vercesi AE: Mitochondria and reactive oxygen
species. Free Radic Biol Med. 47:333–343. 2009.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Yu T, Robotham JL and Yoon Y: Increased
production of reactive oxygen species in hyperglycemic conditions
requires dynamic change of mitochondrial morphology. Proc Natl Acad
Sci USA. 103:2653–2658. 2006.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Aguilera-Aguirre L, Bacsi A,
Saavedra-Molina A, Kurosky A, Sur S and Boldogh I: Mitochondrial
dysfunction increases allergic airway inflammation. J Immunol.
183:5379–5387. 2009.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Vaamonde-Garcia C, Riveiro-Naveira RR,
Valcarcel-Ares MN, Hermida-Carballo L, Blanco FJ and Lopez-Armada
MJ: Mitochondrial dysfunction increases inflammatory responsiveness
to cytokines in normal human chondrocytes. Arthritis Rheum.
64:2927–2936. 2012.PubMed/NCBI View Article : Google Scholar
|