Introduction
A healthy skin barrier can be attributed to
well-differentiated corneocytes, correctly arranged extracellular
lipid bilayers, balanced activities of antimicrobial peptides and
enzymes, and a physiologically weak acidic pH environment on the
skin surface (1). Inflammatory skin
conditions, such as psoriasis and atopic dermatitis, present with
impaired skin barrier function (1).
Psoriasis is a chronic, recurrent immune-mediated disease and
affects people of all ages, most commonly in individuals aged
between 15-30 years old (2-4).
Epidermal hyperplasia, acanthosis, hyperparakeratosis, angiogenesis
with blood vessel dilatation and excess T helper type-1 (Th-1) and
Th-17 lymphocyte infiltration are the main histopathological
features of psoriasis (2-5).
Furthermore, the interleukin (IL)-23/IL-17 axis model for psoriasis
proposes that IL-23 activates Th17 lymphocytes, resulting in the
subsequent release of proinflammatory cytokines, including IL-17,
leading to the psoriatic phenotype (5,6).
Although genetic, immunological and environmental factors have been
proposed as being the cause of this condition, the exact cause of
psoriasis has yet to be elucidated, and the mechanisms underlying
psoriasis continue to be poorly understood (3,4,7).
Magnolol (5,5'-diallyi-2,2'-dihydroxy biphenyl;
C18H18O2, MW=266.33 Da) is one of
the major active polyphenolic ingredients isolated from Magnolia
officinalis (known as houpu magnolia) (8,9). The
considerable efficacy of magnolol has been confirmed through an
assessment of its anti-inflammatory, antiproliferative,
anti-photoaging and anti-free radical activity (8,10-14).
Magnolol has also been indicated to be an agonist of peroxisome
proliferator-activated receptor-γ (PPAR-γ) (15-17).
The key functions of PPAR-γ in the epidermis include maintenance of
skin barrier homeostasis, regulation of the stratum corneum surface
pH and water-holding capacity, controlling cell differentiation and
responding to inflammatory responses via PPAR-γ activation, thus
resulting in increased cell survival and reduced apoptosis in
UV-induced damage studies (18,19).
However, the effect of magnolol on psoriasis has been less well
reported, although it may be hypothesized that magnolol could
contribute towards permeability barrier homeostasis via PPAR-γ in
psoriatic skin. Therefore, the present study aimed to investigate
the therapeutic effects, as well as the effect on the skin barrier,
of magnolol on an imiquimod (IMQ)-induced psoriatic-like dermatitis
model. The underlying mechanisms governing this interaction were
also investigated.
Materials and methods
Materials
Magnolol was purchased from Merck KGaA. Esperson
(0.25% Desoximetasone ointment) was purchased form Sanofi S.A. All
other chemicals were of analytical grade.
Animals
A total of 15 male BALB/c mice (8-12 weeks old;
purchased from National Laboratory Animal Center, Tainan, Taiwan),
weighing 22±2 g, were housed under standard laboratory conditions
with sufficient food and water that was accessible at all times, as
well as with minimized handling, odors, noises and vibrations in
the Laboratory Animal Center of the Cathay General Hospital (12 h
light/dark cycles and 24±2˚C ambient temperature). A total of three
mice were placed in each group. The duration of the experiment was
11 days. The animal health and behavior were monitored every day
via body weight and food intake measurement. All animal experiments
were performed and approved by the Institutional Animal Care and
Use Committee (IACUC) of Cathay General Hospital (IACUC
registration no. 107-028). During the experimental period, each
animal was housed in a separate cage with wooden bar toys,
complying with the IACUC regulations. To minimize distress during
hair shaving, mice were placed in the induction anesthesia chamber
(10x10x20 cm) with 4% isoflurane in oxygen (flow rate=0.5 l/min),
followed by 2% isoflurane in oxygen (flow rate=0.2 l/min) for
maintenance of anesthesia via a facemask. During the experimental
period, the mice were euthanized via excessive isoflurane exposure
followed by cervical dislocation to confirm successful euthanasia
as a humane endpoint for this study, when cachexia led to a
body-weight loss of 10% or more. No mice suffered spontaneous
mortality or were euthanized because of a body-weight loss of 10%
or more during the experiment. For euthanasia, mice were placed in
the induction anesthesia chamber with 5% isoflurane in oxygen (flow
rate=0.5 l/min) exposure continued for at least 1 min after
respiratory arrest, followed by cervical dislocation to confirm
successful euthanasia. All animals were sacrificed on day 11 at the
end of the experiment to obtain the skin samples for further
investigation.
Establishment of the IMQ-induced
psoriasis-like skin animal model
Psoriasiform dermatitis was induced in mice
following a widely used protocol (20-23)
through the topical application of a dose of 62.5 mg 5% Aldara IMQ
cream (3M Pharmaceuticals) on the shaved dorsal skin for six
consecutive days, once daily, prior to the experimental period
(days 0-6) (20-23).
Experimental protocols
A total of 18 mice were used in the present study.
Three untreated (normal) mice were used as negative control
specimens for morphology, PPAR-γ and cytokine array studies.
For the barrier function study, 15 mice were randomly assigned to
the following groups: i) The control group (only induced by IMQ);
ii) vehicle group, treated with ethanol (EtOH; IMQ-induced plus
EtOH treatment); iii) low-dose magnolol group (IMQ-induced, 100
µg/ml magnolol dissolved in EtOH treatment); iv) high-dose magnolol
group (IMQ-induced, 300 µg/ml magnolol dissolved in EtOH treatment)
and v) 0.25% desoximetasone ointment (DXM) group [IMQ-induced plus
Esperson (0.25% Desoximetasone ointment; Sanofi S.A.) treatment as
a positive control]. Following the successful induction of the
psoriasiform skin, the mice continued to receive IMQ application,
followed by their respective treatments until day 11. In the
treatment phase (days 6-11), 3-4 h post-IMQ application, mice were
treated once daily with 100 µl magnolol, EtOH solution or 60 mg DXM
on the dorsal skin.
Assessment of barrier functions
Barrier function parameters, including
transepidermal water loss (TEWL), skin hydration and erythema
values, were measured on the dorsal surface of mice prior to the
application of drugs on day 0 (used as normal barrier functions
value baseline), and subsequently on days 6 and 11 using an MPA-II
system equipped with Tewameter TM300, Corneometer CM825 and
Mexmeter MX18 probes (Courage and Khazaka Electronic GmbH).
Collection of skin specimens
Three untreated mice were sacrificed 48 h after hair
shaving, and the specimens served as negative controls for
inflammatory cytokine analysis. Treated (barrier function study)
mice were sacrificed on day 11 after the final barrier function
assessment. Full-thickness mouse skin was separated into two
samples for histological staining.
Immunohistochemical staining for
PPAR-γ
Skin specimens were fixed in 10% formalin solution
and embedded in paraffin at 4˚C overnight. Sections of 5 µm
thickness were cut and stained with primary antibodies against
PPAR-γ (1:25; cat. no. GTX19481; Rabbit origin; GeneTex, Inc.)
using a Ventana BenchMark XT automated stainer (Ventana Medical
Systems, Inc.). Samples were incubated with the PPAR-γ primary
antibody (1:25; cat. no. GTX19481; Rabbit origin; GeneTex, Inc.) in
universal ready to use blocking reagent (cat. no. 760-050; Ventana
Medical Systems Inc.) for 60 min at 37˚C, and then the incubation
was continued overnight at 4˚C. Subsequently, the samples were
incubated with the universal mouse and rabbit ready to use
secondary biotinylated antibody using an ultraView Universal DAB
ready to use Detection kit (cat. no. 760-500; Ventana Medical
Systems Inc.) for 1 h at room temperature. The levels of
diaminobenzidine (DAB) were subsequently visualized. Samples were
then counterstained with hematoxylin for 4 min at 37˚C, and
examined under a light microscope at a magnification of x200 (BX41;
Olympus Corporation).
Immuno-intensity counting
To objectively evaluate the immunostaining results,
the slides were scanned using a slide scanner (Pannoramic DESK II
DW; 3DHISTECH Ltd.) at a magnification of x200. CellQuant and
PatternQuant computer counting software (version 2.4.0) were used
(all, 3DHISTECH Kft.). PatternQuant was programmed to recognize the
regions of interest, and CellQuant was used to evaluate the
H-Score. Each skin tissue was assigned an annotation, which was 1
mm wide and covered the whole thickness of the skin to the muscle
layer. The H-score was defined in terms of its immune-intensity,
and this was then multiplied by the staining percentage, providing
a range of values from 0-300. The immuno-intensity was recorded as
being 0 for no staining, 1 for faint staining, 2 for moderate
staining, and 3 for intense staining, whereas the staining
percentage was recorded from 0-100%. The immune-intensity and
staining percentages were both determined using computer counting,
as calculated by CellQuant, and counting was only permitted within
the regions of interest recognized by PatternQuant.
Determination of the inflammatory
cytokine protein levels via multiplex cytokines bead array
Studies have reported that IMQ-induced
psoriasis-like skin elicits either the protein or mRNA expression
of IL-17, IL-23, IL-1β, IL-6, tumor necrosis factor-α (TNF-α), and
interferon-γ (IFN-γ) in mice, and successful anti-psoriatic
interventions should therefore inhibit the aforementioned cytokine
expression (5,24-26).
Therefore, in the current study, proteins were extracted from whole
skin of 6 groups in order to determine the levels of IL-17A, IL-23,
IL-1β, IL-6, TNF-α and IFN-γ using the LEGENDplex™ multiplex
cytokines bead array kit (mouse inflammation panel; BioLegend,
Inc.). Aliquots (50 µl) of protein samples were incubated with
labeled microbeads for 2 h at room temperature, and subsequently,
the concentration of each cytokine was determined using flow
cytometry (using an Accuri C6 flow cytometer; BD Biosciences)
according to the manufacturer's instructions. The concentration of
each cytokine was then determined based on a known standard curve
using LEGENDplex™ data analysis software version 8.0 (VigeneTech
Inc.) (23).
Light microscopy
Skin samples were fixed in 10% formalin at 4˚C
overnight. Sample sections (5 µm-thick) were cut, stained with
hematoxylin for 8 min and then eosin for 30 sec both at room
temperature (H&E), and examined under a light microscope at a
magnification of x200 (BX41; Olympus Corporation).
Statistical analysis
Two-way mixed ANOVA followed by Bonferroni's post
hoc test was performed using SPSS 20 software (IBM Corp.). All bar
charts are presented as the mean ± standard deviation using
Sigmaplot 10.0 software (Systat Software, Inc.). P<0.05 was
considered to indicate a statistically significant difference.
Results
Magnolol improves the barrier function
of IMQ-induced psoriasis-like dermatitis
The chemical structure of magnolol and the study
design are presented in Fig. 1A and
B, respectively. The barrier
function measurement values of the control group on day 0
represented the normal baseline values (TEWL, 7.39±1.20
g/m2/h; skin hydration, 58.25±5.84 arbitrary units (AU);
and erythema, 16.80±1.74 AU). Compared with the values on day 0 (as
normal baseline), the TEWL (40.79±8.05 g/m2/h) and
erythema (31.23±4.17 AU) values in the control group increased
significantly following the establishment of IMQ-induced
psoriasis-like dermatitis over a period of 6 consecutive days (both
P<0.05). By contrast, the skin hydration value (3.69±1.55 AU) of
the control group decreased significantly on day 6 compared with
that of day 0 (P<0.05; Fig.
1C-E).
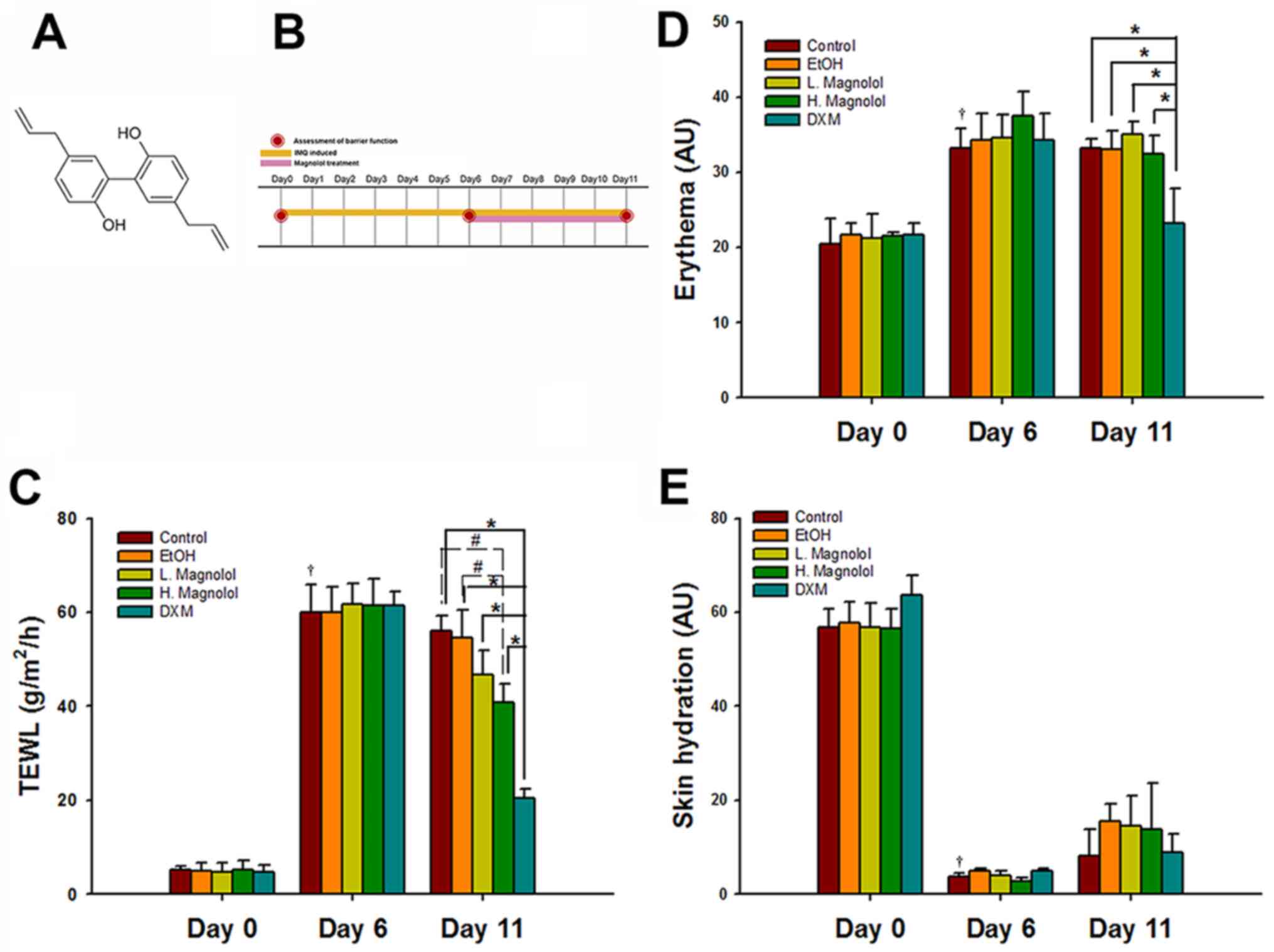 | Figure 1Magnolol improves the barrier function
in IMQ-induced psoriasis-like dermatitis. (A) Chemical structure of
magnolol. (B) Study design. Mice were administered a daily topical
application of a dose of 62.5 mg Aldara IMQ cream (5%) on the
shaved dorsal skin for 6 consecutive days (days 0-6). After the
successful induction of psoriasiform skin, the mice continued to
receive IMQ until day 11. After 3-4 h IMQ application, the mice
subsequently received 100 µl either magnolol, EtOH solution or 60
mg DXM on the dorsal skin once daily (days 6-11). (C) TEWL, (D)
skin hydration and (E) erythema values were measured on the dorsal
surface of mice before the applications of drugs on day 0, and then
monitored on days 6 and 11. (mean ± SD; n=3).
†P<0.05, compared with Day 0 and Day 6 in control
group; #P<0.05 and *P<0.05 (using two
way mixed ANOVA, post hoc Bonferroni's test). IMQ, imiquimod; TEWL,
transepidermal water loss; EtOH, ethanol, DMX, 0.25% desoximetasone
ointment. |
High-dose magnolol (300 µg/ml) treatment led to a
restoration of >30% of the TEWL value compared with the control
and vehicle control groups on day 11 (both P<0.05). The topical
application of DXM markedly reduced the TEWL and erythema values
compared with all the other groups on day 11 (all P<0.05;
Fig. 1C and D). However, magnolol treatment did not
significantly affect erythema or skin hydration on psoriasis-like
skin mice compared with the control and vehicle group on day 11
(all P>0.05; Fig. 1D and
E).
DXM inhibits hyperproliferation of
keratinocytes
The morphology of all 5 groups revealed the presence
of scales, erythema and dry skin (Fig.
2A). The results of histopathology using H&E staining
revealed epidermal acanthosis, parakeratosis, tortuous capillary
dilatation in the papillary dermis, and the infiltration of various
types of inflammatory cell in all groups (Fig. 2B). However, the DXM treatment group
exhibited improved clinical and pathological features of the
psoriasis severity compared with all other groups (Fig. 2A and B).
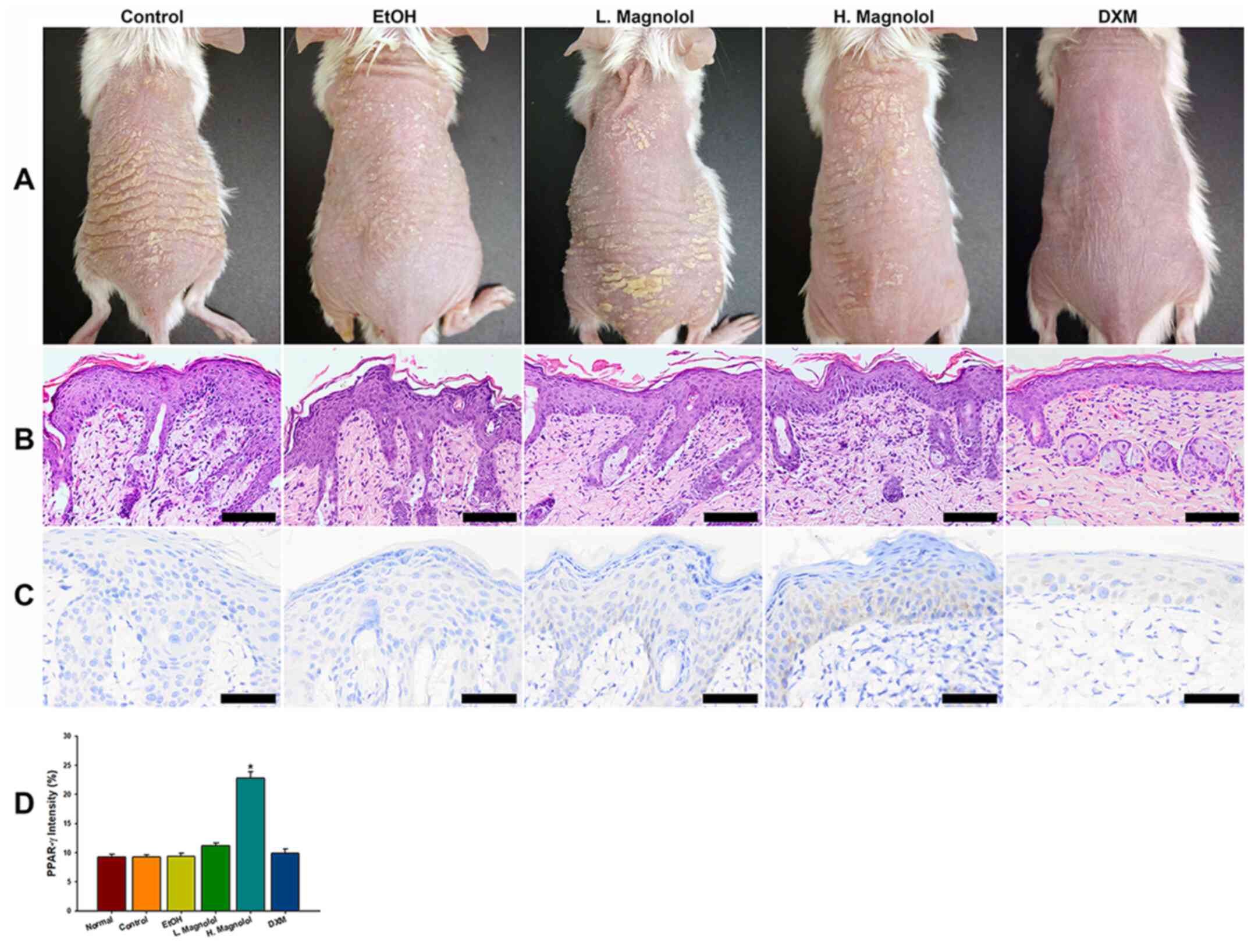 | Figure 2DXM inhibits hyperproliferation of
keratinocytes. (A) Morphology of all the groups, revealing the
presence of scales, erythema and dry skin. (B) Histopathological
staining using H&E revealed epidermal acathotic hyperplasia,
abnormal keratinocyte differentiation, superficial dermal capillary
dilatation and infiltration of various inflammatory cell types in
all groups. However, the Esperson (DMX) treatment group showed much
better gross and pathological features of severity index compared
with all the other groups. (C) Magnolol activates PPAR-γ protein
expression. The high-dose magnolol group showed positive staining
of PPAR-γ. (D) The immuno-intensity of PPAR-γ. The high-dose
magnolol treatment group revealed a higher intensity of PPAR-γ
compared with all the other groups. Scale bar, 50 µm. Yellow arrow
head indicated neutrophil cell. (mean ± standard deviation, n=3);
*P<0.05 (using two-way mixed ANOVA, post hoc
Bonferroni's test). H&E, hematoxylin and eosin; PPAR-γ,
peroxisome proliferator-activated receptor-γ; DMX, 0.25%
desoximetasone ointment. |
Magnolol activates the protein
expression of PPAR-γ
To investigate whether magnolol acts as a PPAR-γ
agonist on the epidermis, PPAR-γ protein expression levels were
evaluated via immunohistochemical staining. The results
demonstrated that magnolol activated PPAR-γ protein expression on
the epidermis, which was more clearly observed in the cytoplasm
(Fig. 2C). Fig. 2D demonstrated the results of the
immune-intensity analysis of PPAR-γ via automatic computer
counting. The high-dose magnolol treatment group exhibited higher
immune-intensities of PPAR-γ compared with all the other groups
(P<0.05).
Magnolol inhibits the protein
expression levels of IL-23, IL-1β, IL-6, TNF-α and INF-γ in
psoriasis-like skin
The potential underlying mechanisms of magnolol
treatment in psoriasis-like skin were examined using the
inflammatory cytokine panel. The cytokine array results revealed
that the expression of all the cytokines were significantly
increased in the control group (IMQ-induced only) compared with the
normalgroup (untreated skin specimens, P<0.05). High-dose
administration of magnolol led to the inhibition of IL-23, IL-1β,
IL-6, TNF-α and INF-γ protein expression (all P<0.05), although
not of IL-17A (P>0.05), compared with the control group.
Low-dose administration of magnolol led to the inhibition of IL-23,
IL-1β, IL-6 and INF-γ protein expression (all P<0.05) although
not of IL-17A and TNF-α (both P>0.05), compared with the control
group. Both high and low administration of magnolol led to the
inhibition of IL-1β compared with the EtOH group (both P<0.05).
Additionally, DXM inhibited the expression of all inflammatory
cytokines, compared with the normal group (P<0.05; Fig. 3).
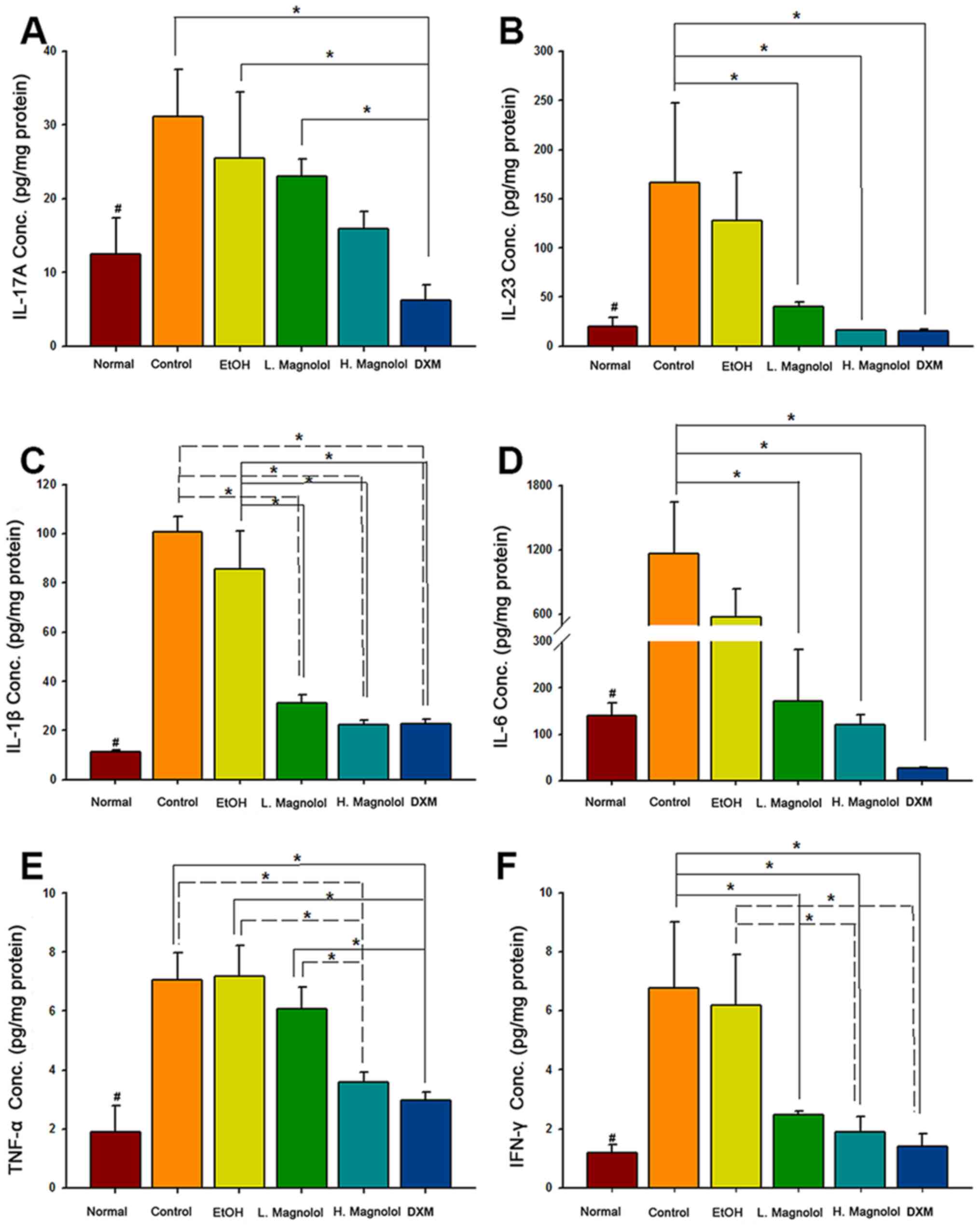 | Figure 3Magnolol inhibits the protein
expression levels of IL-23, IL-1β, IL-6, TNF-α and INF-γ in
psoriasis-like skin. (A) IL-17A, (B) IL-23, (C) IL-1β, (D) IL-6,
(E) TNF-α and (F) IFN-γ protein expression levels were analyzed
using a LEGENDplex™ kit (mean ± standard deviation, n=3);
#P<0.05, compared with control group;
*P<0.05 (using two-way mixed ANOVA, post hoc
Bonferroni's test). IL, interleukin; TNF-α, tumor necrosis
factor-α; IFN-γ, interferon-γ. |
Discussion
The effect of magnolol on psoriasis has been rarely
reported. The present study has demonstrated that magnolol
activates PPAR-γ, and also significantly inhibits the protein
expression of IL-23, IL-1β, IL-6, TNF-α and INF-γ, which may
contribute to skin barrier function. The IL-23/IL-17 axis has been
reported to be a critical regulator for psoriasis and psoriatic
arthritis (5). Previous studies
have reported that IMQ-induced psoriasis-like skin elicits either
protein or mRNA expression of IL-17, IL-23, IL-1β, IL-6, TNF-α and
INF-γ in mice skin (5,24-26),
and successful anti-psoriatic interventions should therefore
inhibit the aforementioned cytokine expression (24-26),
especially with respect to the downregulation of IL-17 and IL-23
expression (23). However, in the
present study, administration of high and low-dose magnolol
treatment did not effectively inhibit IL-17 or lead to any
improvement in the clinical and pathological features of the
psoriasis severity index.
PPARs have been indicated to perform essential roles
in cutaneous homeostasis (18).
PPAR-γ, as one of three PPARs isoforms, has been indicated to exert
anti-inflammatory effects on a variety of cell types, including
macrophages, lymphocytes and connective tissue cells (27). PPAR-γ has been reported to be
localized mainly in the nucleus during a number of cellular
processes (28). It was recently
demonstrated that the downregulation of PPAR-γ by its
mitogen-activated protein kinase-dependent is redistributed from
the nucleus to the cytosol for non-genomic activity (28,29). A
previous study demonstrated that PPAR-γ mainly appears to localize
in the cytoplasm in human keratinocytes, whereas it exhibits an
exclusively nuclear localization in the suprabasal layer (30). The immunohistochemistry staining
results presented in the current study exhibited a similar pattern.
Therefore, PPAR-γ may control the cytoplasmatic activity through
the same mechanism in keratinocytes. An in vitro study
revealed that PPAR-γ regulated inflammatory signals by first
inhibiting NF-κB nuclear translocation, and then downregulating the
cytokine protein expression of IL-6, IL-8, IL-12, IL-21, IL-23,
TNF-α and cyclooxygenase-2(31).
PPAR-γ is expressed in keratinocytes, and is also involved in the
regulation of keratinocyte differentiation (18). Thiazolidinediones, a family of
PPAR-γ ligands, have been indicated to reduce epidermal
keratinocyte proliferation and promote epidermal keratinocyte
differentiation in a repeated tape stripping-induced
hyperproliferative animal model (18). These results may suggest that
topical PPAR-γ agonists could be considered as a potential
adjunctive therapeutic agent in hyperproliferative skin diseases,
such as psoriasis (32).
The bark of Magnolia officinalis has been
traditionally used in Asia for the treatment of a number of
diseases, including anxiety, asthma and depression (33). Honokiol is another primary active
compound that is isolated from Magnolia officinalis, and is
also a PPAR-γ agonist (15-17).
A previous study revealed that honokiol could effectively improve
psoriasis treatment by inhibiting the NF-κB pathway in a transgenic
mouse model (34). A previous study
also indicated the effect and mechanism of magnolol on psoriasis
mice induced by imiquimod via oral administration (35). To the best of our knowledge, the
present study has been the first to demonstrate the topical
anti-psoriatic effects as well as skin barrier function improvement
of magnolol on IMQ-induced psoriasis-like dermatitis in mice.
The number of animals used in the current study was
small (n=3). In future studies, the number of animals used should
be increased. Further research is also required to enhance the
topical distribution of magnolol to the skin and to investigate the
therapeutic outcome of this treatment, as well as to clarify the
pharmacological effects of magnolol on psoriasis.
The IL-23/IL-17 axis has been reported to be the
critical regulator for psoriasis and psoriatic arthritis (5,6). In
the present study, it has been demonstrated that magnolol activates
PPAR-γ, and is able to improve barrier function via downregulation
of the IL-23 signaling pathway in an IMQ-induced psoriasis-like
dermatitis mouse model. The results revealed that the
downregulation of IL-23 may contribute to barrier function
improvement, and could possibly serve a role in alleviating
psoriasis-like dermatitis in animals. However, the application of
magnolol alone, when applied topically to inhibit IL-23, may not be
an effective method for psoriasis treatment. The potential of using
systemic magnolol or topical magnolol treatment combined with
topical glucocorticosteroid to treat psoriasis, however, is worth
of further investigation.
Acknowledgements
The authors would like to thank Dr Tsen-Fang, Tsai
from National Taiwan University Hospital, who provided insights and
expertise that greatly assisted the research project with regards
to the animal study design.
Funding
The present study was supported by grants from the Ministry of
Science and Technology of Taiwan (grant nos.
MOST-107-2314-B-281-010- and MOST-108-2314-B-281-002-) and from
Cathay General Hospital (grant nos. CGH-MR-A106024 and
CGH-MR-A109017).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
The study was conceptualized and designed by JWG,
YPC, and SHJ. JWG wrote the initial version of the manuscript. JWG,
YO, and CYW performed the animal studies and barrier function
study. YPC and SHJ interpreted the animal image data regarding the
severity of psoriasis. JWG and CYL performed the immunostaining and
CYL performed the pathologic diagnosis. JWG performed the cytokines
array and statistical analyses. HYT was responsible for reviewing
and editing the paper. JWG, YPC, and SHJ provided supervision
throughout the study. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
All animal experiments were performed and approved
by the Institutional Animal Care and Use Committee (IACUC) of
Cathay General Hospital (IACUC registration no. 107-028).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Elias PM, Hatano Y and Williams ML: Basis
for the barrier abnormality in atopic dermatitis:
Outside-inside-outside pathogenic mechanisms. J Allergy Clin
Immunol. 121:1337–1343. 2008.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Azfar RS and Gelfand JM: Psoriasis and
metabolic disease: Epidemiology and pathophysiology. Curr Opin
Rheumatol. 20:416–422. 2008.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Gudjonsson JE and Elder JT: Psoriasis. In:
Fitzpatrick's Dermatology in General Medicine. Goldsmith LA, Kazt
SI, Gilchrest BA, Paller AD, Leffell DJ and Wolff K (eds.).
McGraw-Hill Companies, Inc., 2012.
|
|
4
|
Boehncke WH and Schon MP: Psoriasis.
Lancet. 386:983–994. 2015.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Di Cesare A, Di Meglio P and Nestle FO:
The IL-23/Th17 axis in the immunopathogenesis of psoriasis. J
Invest Dermatol. 129:1339–1350. 2009.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Toussirot E: The IL23/Th17 pathway as a
therapeutic target in chronic inflammatory diseases. Inflamm
Allergy Drug Targets. 11:159–168. 2012.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Bowcock AM: The genetics of psoriasis and
autoimmunity. Annu Rev Genomics Hum Genet. 6:93–122.
2005.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Alonso-Castro AJ, Zapata-Bustos R,
Dominguez F, Garcia-Carranca A and Salazar-Olivo LA: Magnolia
dealbata Zucc and its active principles honokiol and magnolol
stimulate glucose uptake in murine and human adipocytes using the
insulin-signaling pathway. Phytomedicine. 18:926–933.
2011.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Dominguez F, Chavez M, Garduno-Ramirez ML,
Chavez-Avila VM, Mata M and Cruz-Sosa F: Honokiol and magnolol
production by in vitro micropropagated plants of Magnolia dealbata,
an endangered endemic Mexican species. Nat Prod Commun. 5:235–240.
2010.PubMed/NCBI
|
|
10
|
Kuo DH, Lai YS, Lo CY, Cheng AC, Wu H and
Pan MH: Inhibitory effect of magnolol on TPA-induced skin
inflammation and tumor promotion in mice. J Agric Food Chem.
58:5777–5783. 2010.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Lee YJ, Lee YM, Lee CK, Jung JK, Han SB
and Hong JT: Therapeutic applications of compounds in the Magnolia
family. Pharmacol Ther. 130:157–176. 2011.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Lin CF, Hwang TL, Al-Suwayeh SA, Huang YL,
Hung YY and Fang JY: Maximizing dermal targeting and minimizing
transdermal penetration by magnolol/honokiol methoxylation. Int J
Pharm. 445:153–162. 2013.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Shen JL, Man KM, Huang PH, Chen WC, Chen
DC, Cheng YW, Liu PL, Chou MC and Chen YH: Honokiol and magnolol as
multifunctional antioxidative molecules for dermatologic disorders.
Molecules. 15:6452–6465. 2010.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Tanaka K, Hasegawa J, Asamitsu K and
Okamoto T: Magnolia ovovata extract and its active component
magnolol prevent skin photoaging via inhibition of nuclear factor
kappaB. Eur J Pharmacol. 565:212–219. 2007.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Dreier D, Latkolik S, Rycek L, Schnürch M,
Dymáková A, Atanasov AG, Ladurner A, Heiss EH, Stuppner H, Schuster
D, et al: Linked magnolol dimer as a selective PPARg
agonist-Structure-based rational design, synthesis, and bioactivity
evaluation. Sci Rep. 7(13002)2017.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Wang L, Waltenberger B, Pferschy-Wenzig
EM, Blunder M, Liu X, Malainer C, Blazevic T, Schwaiger S,
Rollinger JM, Heiss EH, et al: Natural product agonists of
peroxisome proliferator-activated receptor gamma (PPARg): A review.
Biochem Pharmacol. 92:73–89. 2014.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Dreier D, Resetar M, Temml V, Rycek L,
Kratena N, Schnürch M, Schuster D, Dirsch VM and Mihovilovic MD:
Magnolol dimer-derived fragments as PPARg-selective probes. Org
Biomol Chem. 16:7019–7028. 2018.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Mao-Qiang M, Fowler AJ, Schmuth M, Lau P,
Chang S, Brown BE, Moser AH, Michalik L, Desvergne B, Wahli W, et
al: Peroxisome-proliferator-activated receptor (PPAR)-gamma
activation stimulates keratinocyte differentiation. J Invest
Dermatol. 123:305–312. 2004.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Flori E, Mastrofrancesco A, Kovacs D,
Bellei B, Briganti S, Maresca V, Cardinali G and Picardo M: The
activation of PPARg by 2,4,6-Octatrienoic acid protects human
keratinocytes from UVR-induced damages. Sci Rep.
7(9241)2017.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Horvath S, Komlodi R, Perkecz A, Pinter E,
Gyulai R and Kemeny A: Methodological refinement of Aldara-induced
psoriasiform dermatitis model in mice. Sci Rep.
9(3685)2019.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Chong HT, Yang GN, Sidhu S, Ibbetson J,
Kopecki Z and Cowin AJ: Reducing Flightless I expression decreases
severity of psoriasis in an imiquimod-induced murine model of
psoriasiform dermatitis. Br J Dermatol. 176:705–712.
2017.PubMed/NCBI View Article : Google Scholar
|
|
22
|
van der Fits L, Mourits S, Voerman JS,
Kant M, Boon L, Laman JD, Cornelissen F, Mus AM, Florencia E, Prens
EP and Lubberts E: Imiquimod-induced psoriasis-like skin
inflammation in mice is mediated via the IL-23/IL-17 axis. J
Immunol. 182:5836–5845. 2009.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Guo JW, Cheng YP, Liu CY, Thong HY, Huang
CJ, Lo Y, Wu CY and Jee SH: Salvianolic acid B in microemulsion
formulation provided sufficient hydration for dry skin and
ameliorated the severity of imiquimod-induced psoriasis-like
dermatitis in mice. Pharmaceutics. 12(457)2020.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Lin ZM, Ma M, Li H, Qi Q, Liu YT, Yan YX,
Shen YF, Yang XQ, Zhu FH, He SJ, et al: Topical administration of
reversible SAHH inhibitor ameliorates imiquimod-induced
psoriasis-like skin lesions in mice via suppression of
TNF-α/IFN-g-induced inflammatory response in keratinocytes and T
cell-derived IL-17. Pharmacol Res. 129:443–452. 2018.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Li YL, Du ZY, Li PH, Yan L, Zhou W, Tang
YD, Liu GR, Fang YX, Zhang K, Dong CZ and Chen HX:
Aromatic-turmerone ameliorates imiquimod-induced psoriasis-like
inflammation of BALB/c mice. Int Immunopharmacol. 64:319–325.
2018.PubMed/NCBI View Article : Google Scholar
|
|
26
|
OuYang Q, Pan Y, Luo H, Xuan C and Liu J
and Liu J: MAD ointment ameliorates imiquimod-induced psoriasiform
dermatitis by inhibiting the IL-23/IL-17 axis in mice. Int
Immunopharmacol. 39:369–376. 2016.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Ramot Y, Mastrofrancesco A, Camera E,
Desreumaux P, Paus R and Picardo M: The role of PPARγ-mediated
signalling in skin biology and pathology: New targets and
opportunities for clinical dermatology. Exp Dermatol. 24:245–251.
2015.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Burgermeister E and Seger R: MAPK kinases
as nucleo-cytoplasmic shuttles for PPARgamma. Cell Cycle.
6:1539–1548. 2007.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Burgermeister E, Chuderland D, Hanoch T,
Meyer M, Liscovitch M and Seger R: Interaction with MEK causes
nuclear export and downregulation of peroxisome
proliferator-activated receptor gamma. Mol Cell Biol. 27:803–817.
2007.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Westergaard M, Henningsen J, Svendsen ML,
Johansen C, Jensen UB, Schrøder HD, Kratchmarova I, Berge RK,
Iversen L, Bolund L, et al: Modulation of keratinocyte gene
expression and differentiation by PPAR-selective ligands and
tetradecylthioacetic acid. J Invest Dermatol. 116:702–712.
2001.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Mastrofrancesco A, Kovacs D, Sarra M,
Bastonini E, Cardinali G, Aspite N, Camera E, Chavatte P,
Desreumaux P, Monteleone G and Picardo M: Preclinical studies of a
specific PPARg modulator in the control of skin inflammation. J
Invest Dermatol. 134:1001–1011. 2014.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Demerjian M, Man MQ, Choi EH, Brown BE,
Crumrine D, Chang S, Mauro T, Elias PM and Feingold KR: Topical
treatment with thiazolidinediones, activators of peroxisome
proliferator-activated receptor-gamma, normalizes epidermal
homeostasis in a murine hyperproliferative disease model. Exp
Dermatol. 15:154–160. 2006.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Poivre M and Duez P: Biological activity
and toxicity of the Chinese herb magnolia officinalis rehder &
E. Wilson (Houpo) and its constituents. J Zhejiang Univ Sci B.
18:194–214. 2017.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Wen J, Wang X, Pei H, Xie C, Qiu N, Li S,
Wang W, Cheng X and Chen L: Anti-psoriatic effects of Honokiol
through the inhibition of NF-kappaB and VEGFR-2 in animal model of
K14-VEGF transgenic mouse. J Pharmacol Sci. 128:116–124.
2015.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Tsai ZX, Mo SY and Zhu SG: Effect and
mechanism of Magnolol on psoriasis mice induced by imiquimod. Chin
J Clin Pharmacol. 35:56–59. 2019.(In Chinese).
|

















