Introduction
The reconstruction of critical bone defects caused
by trauma, excision of tumors or deformity remains a significant
challenge in the clinic. Autologous bone tissue grafting has been
considered as the gold standard therapeutic strategy. However, this
is usually limited by the lack of donor tissues that may be
harvested and considerable grafting failure rates (1). In recent years, cell-based bone
regenerative therapy using mesenchymal stem cells (MSCs) has become
a promising bone reconstruction strategy (2). Adipose-derived stem cells (ADSCs)
represent an attractive cell source due to their abundance in the
body, and a less invasive and painful harvesting procedure than
that for other stem cells (1,3).
Furthermore, ADSCs have a self-renewal capacity, high proliferation
(4) and a good ability to
differentiate into osteogenic lineages (5). These features have made ADSCs a
promising candidate for bone tissue engineering.
Since a single-cell suspension lacks cell-to-cell
connections, the rates of cell engraftment, survival and
proliferation on target tissues are frequently insufficient. Cell
sheet technology provides an effective strategy to tackle these
challenges (6). The cell sheets
possess an abundant extracellular matrix (ECM), which may be used
as a natural biological scaffold (7), providing an ambient microenvironment
through which resident cells may communicate with each other
(8). This also provides a channel
for nerves, blood vessels and the diffusion of oxygen and nutrients
in vivo. In addition, the cell sheets easily attach to the
surface of other scaffolds, cell sheets or host tissues in tissue
engineering (9). A previous study
suggested that the cell sheets wrapped around the scaffold
exhibited a more pronounced bone regeneration ability than cells
dispersed in a scaffold (10).
A suitable biomaterial scaffold that accommodates
seeding cells is another approach to induce efficient bone
regeneration for filling small or large bone defects (11). Various materials have been
previously reported to be used for the healing of bone defects
(12). However, the optimization of
biomaterial scaffolds is still an active research field. Most of
the existing scaffolds have several limitations, including
mediating inadequate cell migration, proliferation or nutritive
transportation to secrete a sufficient ECM, establish cell-to-cell
interactions and induce severe inflammatory reactions in
vivo (13). Collagen (COL) is a
major bone component and has excellent biocompatibility with proper
interconnected porous structures for cell proliferation (14). Furthermore, β-tricalcium phosphate
(β-TCP) has been widely used in bone engineering due to its good
osteoconductivity, cellular adhesion, ability to accelerate
differentiation and superior biodegradability (15). In the present study, collagen-I
fibrils integrated with homogeneous β-TCP particles as units were
used to construct a porous β-TCP/COL-I scaffold, simulating the
compositional and microstructural characteristics of natural bone.
This novel porous scaffold had 95% porosity, a pore diameter of
50-100 µm, no reported cytotoxicity and suitable mechanical
properties (14,16). In addition, the porous
microstructure facilitates the ingrowth of local cells and delivery
of nutrients and oxygen, which are crucial for successful bone
regeneration (10,17).
In the present study, a compound of ADSC sheets
wrapped around a β-TCP/COL-I scaffold was established. Its
advantages in osteoinductivity compared with those of merely ADSC
sheets or scattered ADSCs with a β-TCP/COL-I scaffold were
investigated in vitro. The present study aimed to explore an
improved composite of seeded cells and biomaterial, which is
expected to become a novel approach for bone regeneration in the
future.
Materials and methods
ADSC isolation and cultivation
Female Sprague-Dawley rats (age, 3 weeks; body
weight, 70-80 g) were purchased from the Laboratory Animal Center
of Zhejiang Province. All experimental animal procedures were
approved by the Research Ethics Committee of the First Affiliated
Hospital, School of Medicine, Zhejiang University (Hangzhou, China)
and 30 rats were permitted in the current study (permit no.
2019-748). The rats were sacrificed by cervical dislocation after
anesthesia by intraperitoneal injection of 1% pentobarbital sodium
(60 mg/kg). Homologous adipose tissue was obtained from the
inguinal fat pad and washed thrice with PBS (Gibco; Thermo Fisher
Scientific, Inc.). After being minced into a paste with scissors,
the adipose tissue was treated with 0.01 mol/l of type I
collagenase (Sigma-Aldrich; Merck KGaA) at 37˚C for 50 min and then
neutralized with fetal bovine serum (FBS; Sigma-Aldrich; Merck
KGaA). Subsequently, the solution was filtrated using a gauze
strainer (75-µm mesh) and centrifuged at 152 x g, 25˚C for 8 min.
Afterward, these cells were cultured in Dulbecco's modified Eagle's
medium (DMEM), which contained 10% FBS, 100 mg/ml of streptomycin
and 100 U/ml of penicillin (all from Gibco; Thermo Fisher
Scientific, Inc.), and cultured at 37˚C in an atmosphere with 5%
CO2. The medium was replenished every 2-3 days. When the
cultures grew to 70-80% confluence, the cells were washed with PBS,
digested with 0.25% trypsin and 0.02% EDTA (Sigma-Aldrich; Merck
KGaA) and then sub-cultured.
ADSC sheet preparation and osteogenic
differentiation
ADSCs at the 3rd passage were harvested for further
experiments. To create the cell sheets, the ADSCs were cultured at
a density of 1x105 cells/cm2 with regular
medium in a 6-cm cell culture dish until they reached 70-80%
confluence. For osteogenic induction, the regular medium was
replaced by the osteoinductive medium, which contained 0.1 M of
dexamethasone, 10 mM of β-phospherglycerol and 50 mg/l of ascorbic
acid (all from Sigma-Aldrich; Merck KGaA). The medium was
subsequently refreshed every 2 to 3 days. After culturing for 10
days, the ADSC sheets were mechanically scraped from the periphery
and separated from the culture dish with a scraper and forceps.
Three ADSC sheets were fixed with 4% paraformaldehyde and stained
with hematoxylin and eosin for histological observation.
Alizarin red S staining
The cell sheets were continued to be cultured with
osteoinductive medium for 21 days. On days 0, 5, 10, 15, 18 and 21,
the ADSC sheets were subjected to Alizarin red S staining. The cell
sheets were rinsed twice with PBS, fixed with 95% ethanol at 4˚C
for 15 min and washed thrice with double-distilled water. The cell
sheets were then stained with 0.1% Alizarin red S-Tris-Hcl (pH 8.2;
Sigma-Aldrich; Merck KGaA) for 30 min at 37˚C, washed with
distilled water and observed with a phase-contrast microscope
(BX41; Olympus Corp.).
Preparation of the β-TCP/COL-I
scaffold
Porous β-TCP/COL-I composite scaffolds were prepared
as previously described (10). In
brief, calcium chloride, polyethylene glycol (both from Shanghai
Chemical) and trisodium phosphate (Nanjing Chemical, China) was
used to prepare the β-TCP powder. Type-I collagen (Sigma-Aldrich:
Merck KGaA) was disassembled into fibrils in an acid solution.
Subsequently, the β-TCP particles were added to the collagen fibril
suspension and integrated with the fibrils to form bone-like
collagen fibrils. After freeze-drying, the porous β-TCP/COL-I
composites were obtained. The microstructure of the β-TCP/COL-I
scaffold was examined using a field-emission scanning electron
microscope (SEM; FE-SEM SU70; Hitachi, Ltd.). The porosity value of
the scaffold was evaluated by Archimedes' principle (14).
Cell proliferation assay
In 96-well plates, 100-µl suspensions of dispersed
ADSCs were cultured at the center of the β-TCP/COL-I scaffold at a
density of 1x105 cells/cm2 in DMEM containing
10% FBS. From the 2nd day, ADSCs with/without the β-TCP/COL-I
scaffold were serum-starved for 48 h in DMEM, which contained 1%
FBS. On the 4th day, the medium was replaced by DMEM containing 10%
FBS and cells were able to re-enter the cell cycle. After 24 h of
culture, the cell proliferation was determined with an MTT assay
(Sigma-Aldrich; Merck KGaA). At the time-points of 1, 3, 5, 7 and 9
h, 20 µl MTT solution (5 mg/ml) was added to each well, followed by
incubation for 4 h at 37˚C. Next, 150 µl dimethyl sulfoxide was
added to each well, followed by gentle agitation for 10 min. The
optical density of each well was then measured at 450 nm using a
spectrophotometer (Infinite M200; Tecan). The cell proliferation
curve was plotted according to the absorbance values.
Preparation of the composite
material
The ADSC sheets were wrapped around the β-TCP/COL-I
scaffold cylinders (2.0 cm in diameter and 2.5 mm in thickness).
Sheets with or without a scaffold were cultured in osteogenic
medium to facilitate the re-attachment of ADSC sheets to the
surface of the scaffold or culture dish, respectively. The
dispersed ADSCs were cultured under the same laboratory conditions
and seeded on the β-TCP/COL-I scaffolds for comparison with the
sheet protocol and further characterization of cell scaffold
interactions. The β-TCP/COL-I scaffold combined with scattered
ADSCs was fixed with 2.5% glutaraldehyde (Shanghai Pharmaceuticals)
for 2 h, followed by serial dehydration for 15 min in a gradient of
ethanol (30, 50, 70, 85, 90 and 100%). Finally, the specimens were
air-dried for 60 min and gold-sputtered for 60 sec at 10 A (E-1010;
Hitachi, Ltd.). The cell morphology was observed using SEM. After
osteogenic induction for 13 days, the β-TCP/COL-I scaffolds with
ADSC sheet and the β-TCP/COL-I scaffolds with scattered ADSCs were
observed again using SEM according to the above-mentioned
procedures and the relative calcium content on the surface of the
composites was analyzed by energy-dispersive spectrometry (EDS;
FE-SEM SU70; Hitachi, Ltd.).
Experimental groups
Overall, four experimental groups were established:
Group 1, an ADSC sheet was wrapped around a β-TCP/COL-I scaffold
cylinder and continued to be cultured in osteogenic medium as
described above; Group 2, an ADSC sheet was digested with 0.25%
trypsin and 0.02% EDTA into scattered ADSCs and then cultured on
the surface of a β-TCP/COL-I scaffold in osteogenic medium; Group
3, an ADSC sheet alone without any β-TCP/COL-I scaffold was
continued to be cultured in osteogenic medium; Group 4, the
β-TCP/COL-I scaffold alone was immersed in osteogenic medium as a
blank control. The four groups were continued to be cultured at
37˚C with 5% CO2 for 13 days.
ELISA of bone differentiation markers
and alkaline phosphatase (ALP) activity measurement
The activity of ALP is considered as the major bone
regeneration biomarker during the early stages of osteogenesis.
Osteocalcin (OCN) and osteopontin (OPN) are two major
noncollagenous matrix proteins involved in bone matrix synthesis
during the pre-osteoblastic cell stages. ALP, OCN and OPN were
evaluated for the evaluation of osteogenic differentiation. Each
group was cultured in osteogenic medium and the medium was
replenished every 48 h on days 3, 5, 7, 9, 11 and 13. At the
indicated time-points, 2 ml of osteoinductive medium from each
group was collected and the amount of OCN and OPN released into the
medium over the 48 h was examined using an OCN (Rat) ELISA kit
(cat. no. E4764; BioVision. Inc.) and a Rat OPN ELISA kit (cat. no.
ERA46RB; Thermo Fisher Scientific, Inc.) respectively, according to
the manufacturers' protocols. Subsequently, the cell sheets or
scattered ADSCs were washed twice in PBS and precooled on ice.
After freezing-melting twice with 600 µl of 0.05% Triton X-100
(Sigma-Aldrich; Merck KGaA), the mixed sample was centrifuged at
21,130 x g for 15 min at 4˚C. The supernatant was then collected
for measuring ALP activity with an ALP Assay kit (cat. no.
291-58601; Wako LabAssay). The results were normalized relative to
the amount of total protein measured by the BCA Protein Assay kit
(cat. no. 23227; Thermo Fisher Scientific, Inc.).
Reverse transcription-quantitative PCR
(RT-qPCR) analysis
At the indicated time-point, the relative mRNA
expressions of ALP, OCN and OPN in Group 1, Group 2 and Group 3
were measured using RT-qPCR. Total RNA was extracted using RNAiso
plus (Takara Biotechnology, Inc.) according to the manufacturer's
protocol. After quantification by optical density measurement
(NanoDrop 2000; Thermo Fisher Scientific, Inc.), 1 µg total RNA was
reverse-transcribed into random-primed complementary DNA (cDNA)
using a PrimeScript™ RT reagent kit (Takara Biotechnology, Inc.).
The PCR reaction system was then prepared according to the
manufacturer's protocols of SYBR® Premix Ex Taq™ (cat.
no. RR420A; Takara Bio, Inc.) and amplified through real-time PCR
using a CFX96 real-time PCR detection system (Bio-Rad Laboratories,
Inc.) under the following conditions: 2 min of denaturation at
95˚C, 40 rounds of 10 sec of annealing at 95˚C and 30 sec of
extension at 60˚C. The expression levels of the target genes were
normalized relative to the housekeeping gene GAPDH. The primer
sequences were as follows: GAPDH, 5'-TGTGTCCGTCGTGGATCTGA-3' and
5'-TTGCTGTT GAAGTCGCAGGAG-3'; ALP, 5'-ATGGCTCACCTGCTT CACG-3' and
5'-TCAGAACAGGGTGCGTAGG-3'; OCN, 5'-GACCCTCTCTCTGCTCACTCT-3' and
5'-GACCTTACT GCCCTCCTGCTTG-3'; OPN, 5'-TATCCCGATGCC ACAGATGA-3' and
5'-TGAAACTCGTGGCTCTGATG-3'. The results were evaluated using the
2-∆∆Cq method (2,10).
Statistical analysis
The MTT assay was analyzed in three walls and the
independent experiments were performed in triplicate (n=3). The
data of the MTT assay and the ALP, OCN and OPN levels in each group
were expressed as the mean ± standard deviation. All of the data
were normally distributed (according to the
Kolmogorov-Smirnov-Lilliefors test) and evaluated by parametrical
tests (one-way analysis of variance with Scheffe's post-hoc test)
using SPSS 19.0 (IBM Corp.). P<0.05 was considered to indicate
statistical significance.
Results
Characterization of ADSCs and the ADSC
sheet
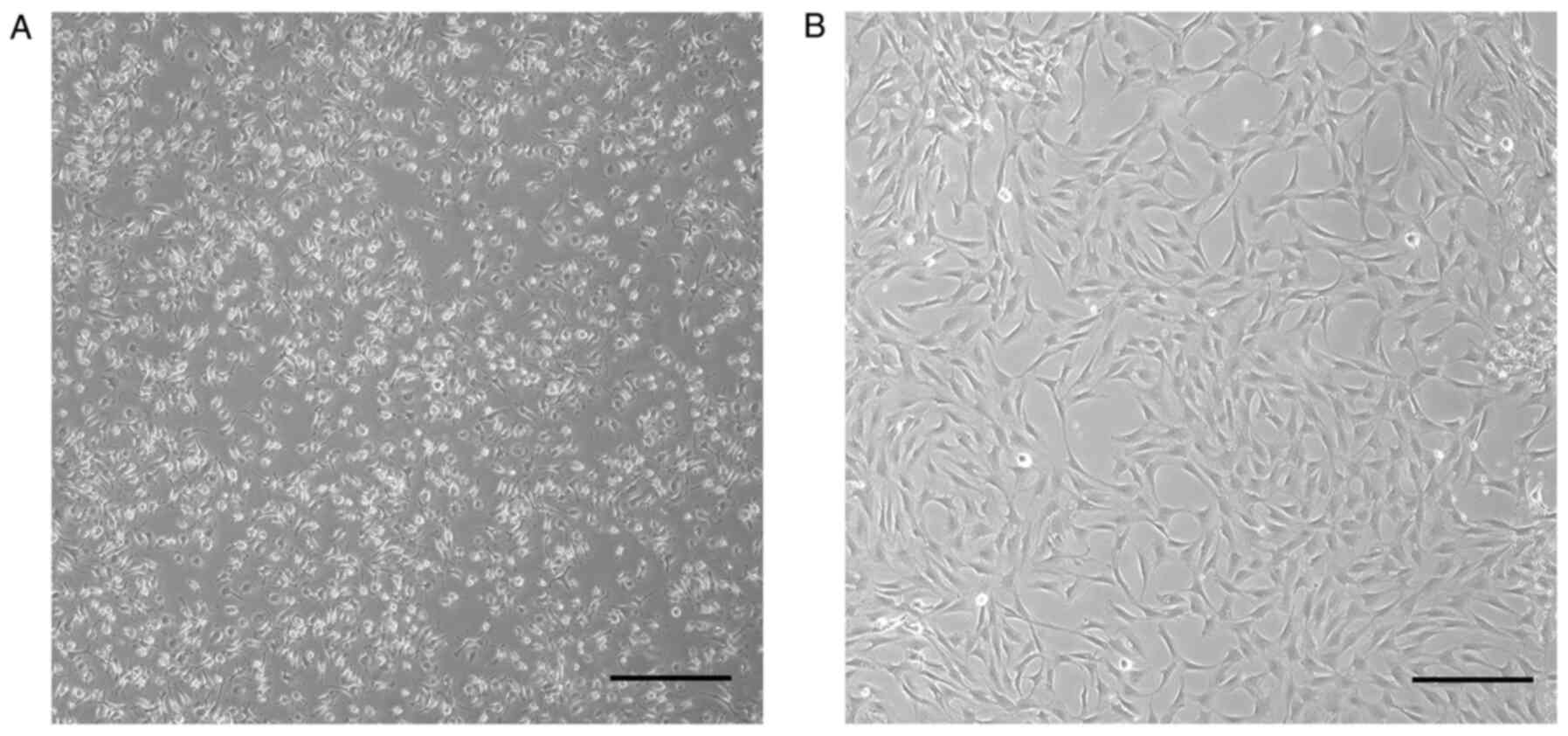
ADSCs exist as polygonal or long spindle-shaped
cells in primary culture (Fig. 1A)
and they became more homogeneous after the third passage (Fig. 1B). When cultured in osteogenic
medium, the ADSCs rapidly proliferated and formed a cell sheet in
10-14 days, and the sheet was easily lifted with a scraper
(Fig. 2A). The cell sheet was
composed of multiple layers of ADSCs with rich ECM wrapping these
cells (Fig. 2B) and the arrangement
of the ADSCs changed into a swirling or radial-shape (Fig. 2C). The calcium nodules stained with
Alizarin Red S in the ADSC sheet were almost absent on day 0 prior
to osteogenic induction and then gradually increased from days 5 to
21 (Fig. 2D), indicating that
osteoblastic differentiation was successful.
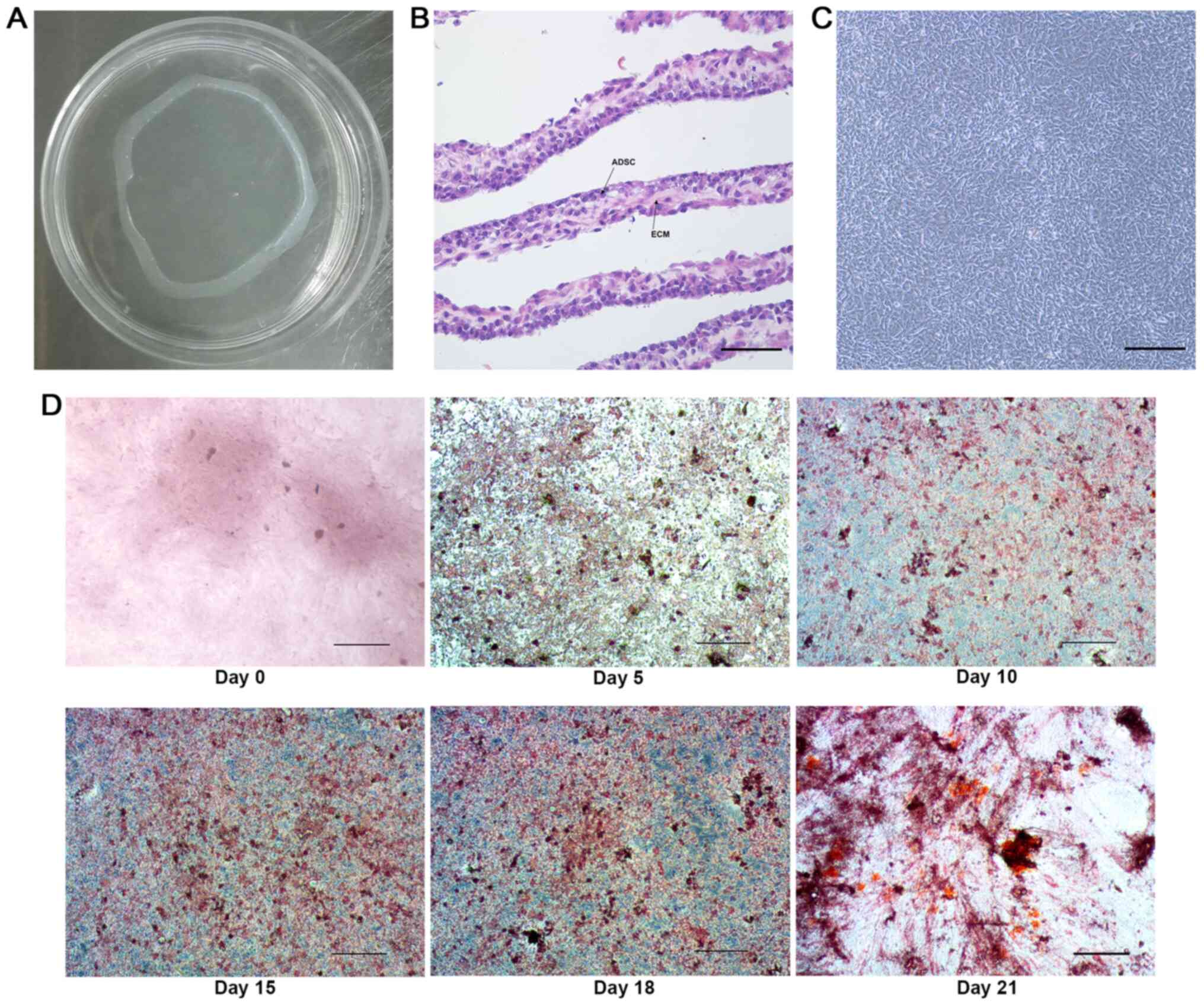 | Figure 2Characterization of the ADSC sheet.
(A) Macroscopic appearance of the ADSC sheet harvested using a cell
scraper. (B) H&E histological staining of the ADSC sheet,
indicating multiple layers of cells with a rich ECM (scale bar, 25
µm). (C) The appearance of the ADSC sheet was observed using an
inverted microscope (scale bar, 100 µm). (D) Alizarin red S
staining of the ADSC sheet after osteogenic induction on days 0, 5,
10, 15, 18 and 21 (scale bar, 100 µm). ADSC, adipose-derived stem
cell; ECM, extracellular matrix. |
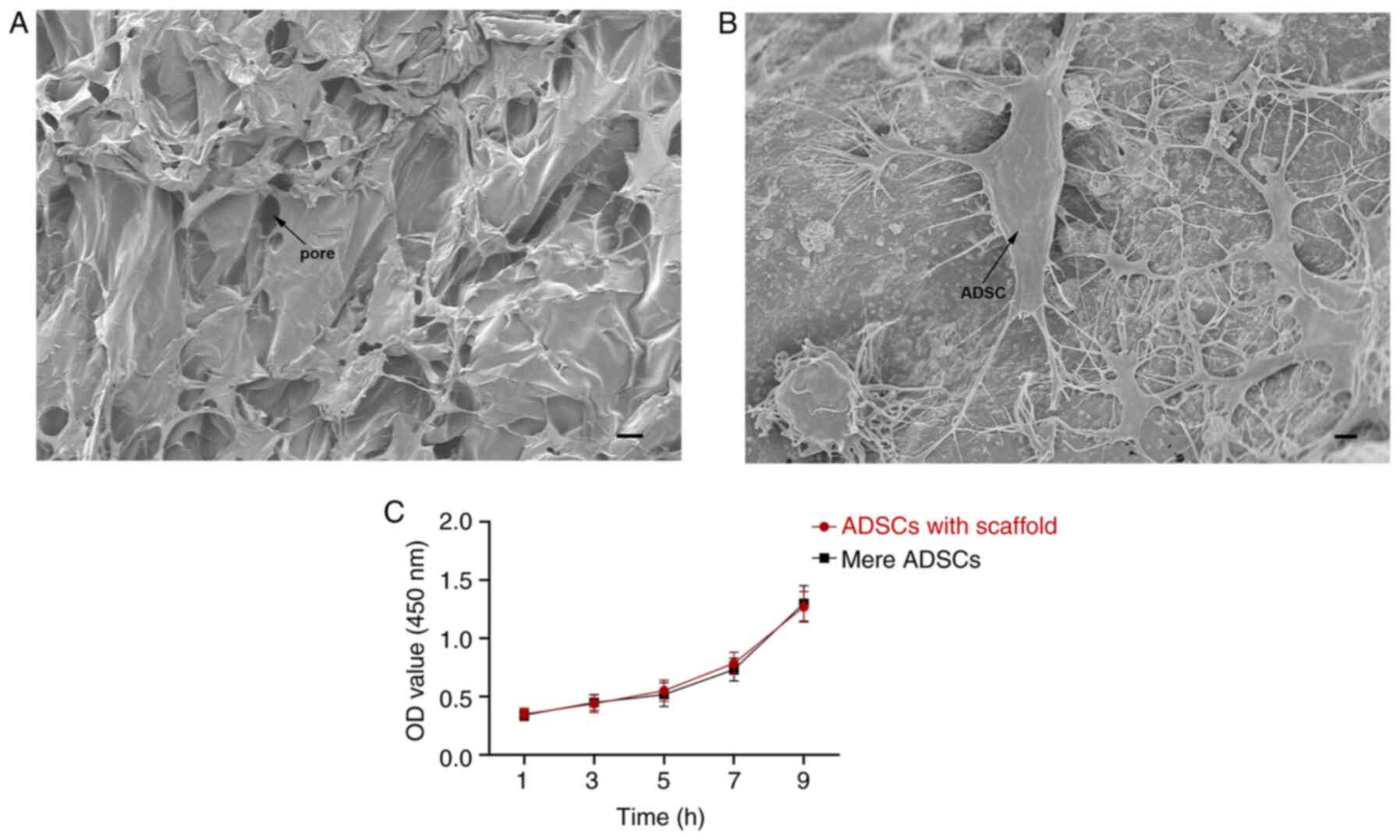
Characterization of the β-TCP/COL-I
scaffold and cell activity of ADSCs on the scaffold
SEM revealed that the β-TCP/COL-I scaffold contained
a 3D porous structure with high porosity (~95%) and appropriate
pore size (nearly 100 µm) (Fig.
3A). The ADSCs became firmly attached to the surface of the
β-TCP/COL-I scaffold and infiltrated into the interconnected pores
of the scaffold. They were connected with each other through
numerous cellular junctions (Fig.
3B). The MTT assay revealed that the cell proliferation curve
of ADSCs on the β-TCP/COL-I scaffold was similar to that of the
conventional culture plates and no significant difference was
obtained between the two groups at any of the time-points assessed
(P>0.05; Fig. 3C), indicating
that the scaffold has no cytotoxicity on ADSCs.
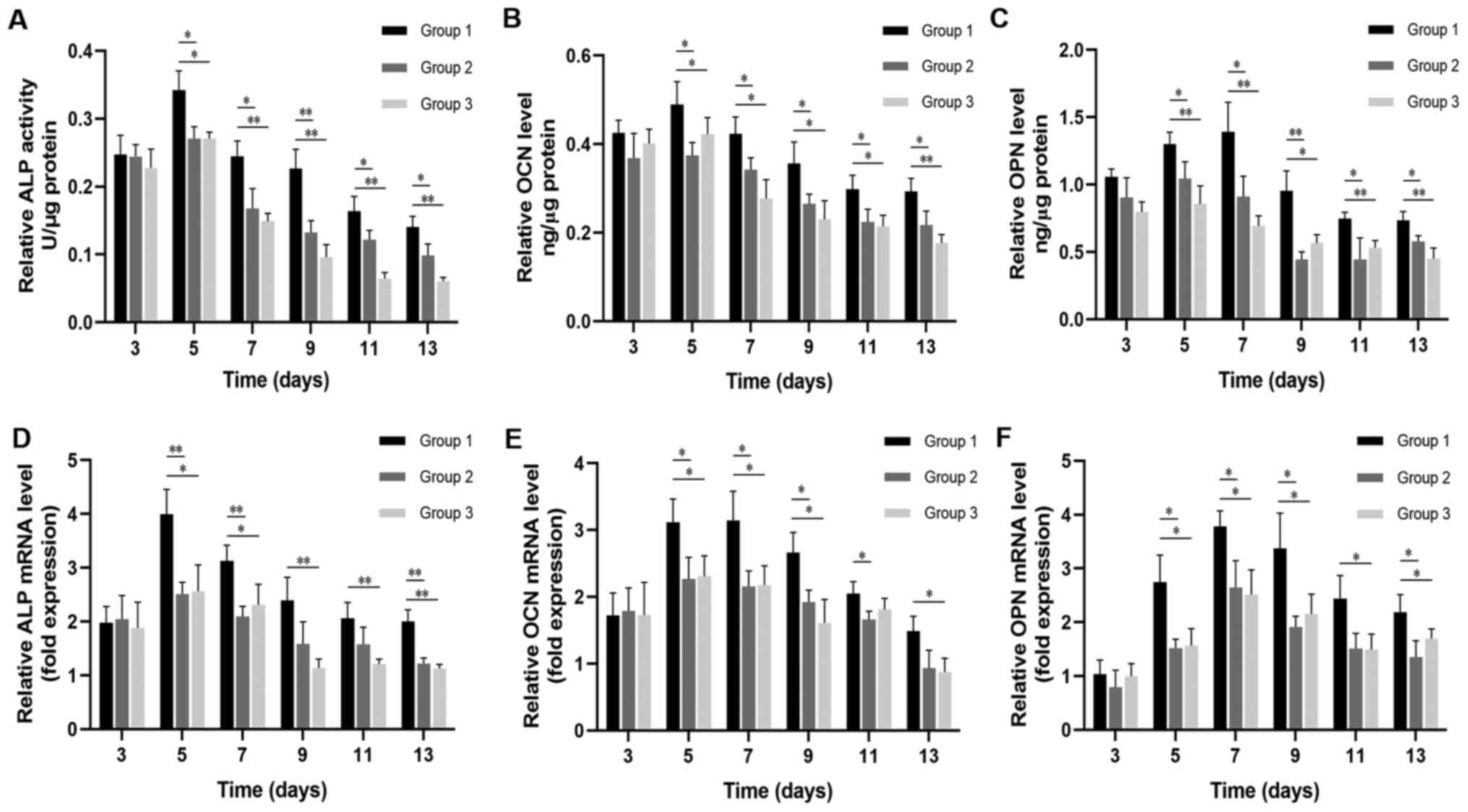
Osteogenic differentiation of the ADSC
sheet on the scaffold
The bone regeneration biomarkers of osteogenic
differentiation in each group were evaluated after osteogenic
induction for 3, 5, 7, 9, 11 and 13 days. The relative ALP activity
and protein expression levels of OCN and OPN in Group 1 (ADSC sheet
combined with a β-TCP/COL-I scaffold), Group 2 (scattered ADSCs
combined with a β-TCP/COL-I scaffold) and Group 3 (ADSC sheet alone
without a β-TCP/COL-I scaffold) reached a peak on day 5 or 7 and
exhibited decreases thereafter (Fig.
4). There were no significant differences in these values among
the three groups at day 3 (P>0.05). From the 5th day onwards,
the protein levels of ALP, OCN and OPN in Group 1 were
significantly higher than those in the other two groups (P<0.05
vs. Groups 2 and 3; Fig. 4A-C).
The relative mRNA levels of ALP, OCN and OPN also
demonstrated a similar trend. At all the indicated time-points,
except for the 3rd day, Group 1 persistently exhibited higher
levels of osteogenic mRNA expression than Groups 2 and 3
(P<0.05; Fig. 4D-F). Overall,
the ADSC sheet combined with a β-TCP/COL-I scaffold in Group 1
displayed significantly improved osteogenic activity compared to
the other two groups.
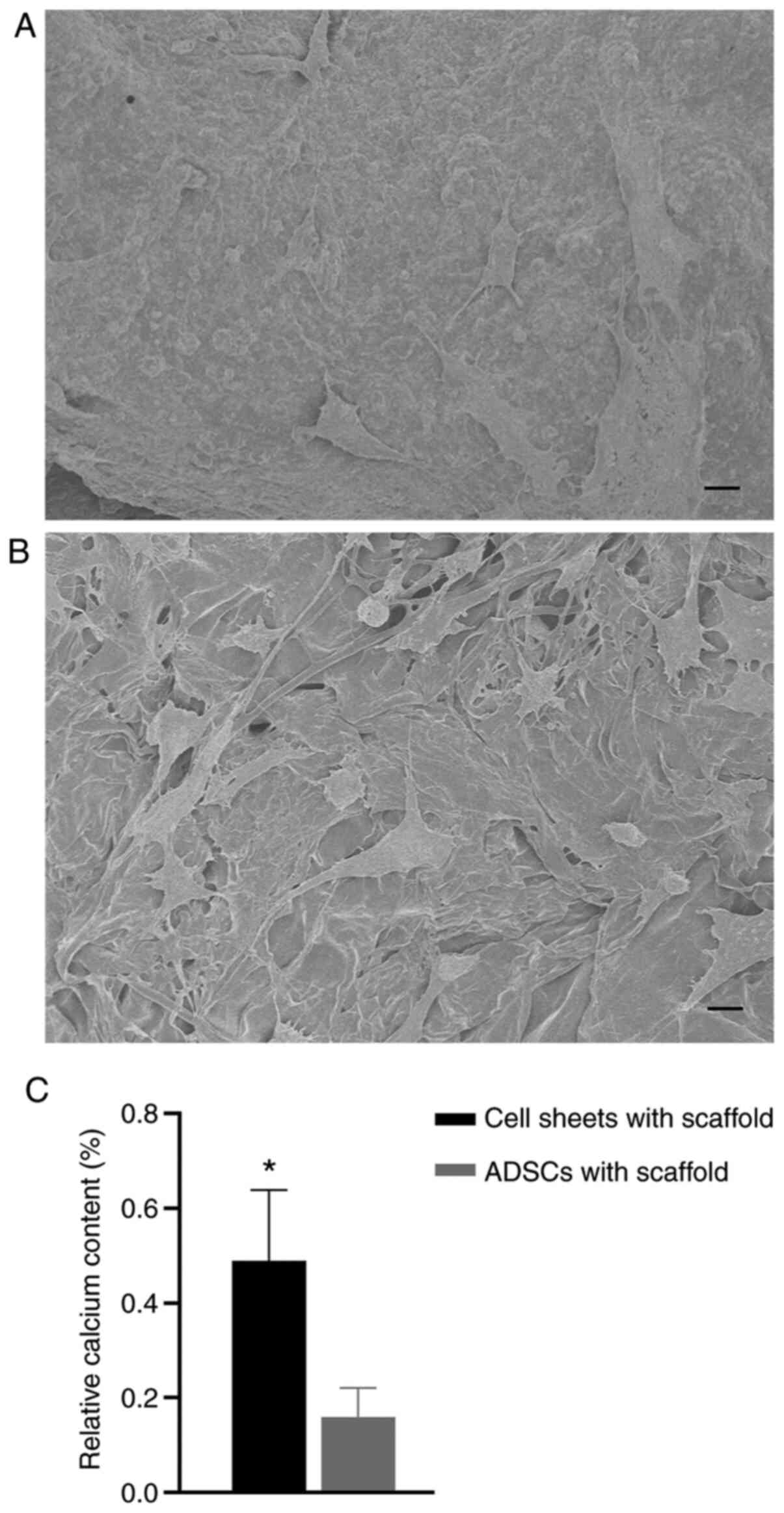
Calcium content on the scaffold with
ADSC sheet
After osteogenic induction for 13 days, the
β-TCP/COL-I scaffold with ADSC sheet (Group 1) contained more
densely populated cells and mineralized nodules than the
β-TCP/COL-I scaffold with scattered ADSCs (Group 2) (Fig. 5A and B, respectively). Furthermore, the relative
calcium content of Group 1 was much higher than that of Group 2 as
determined by EDS (P<0.05 vs. Group 2; Fig. 5C).
Discussion
Millions of patients suffer from critical bone
defects and impaired bone healing, the adequate treatment of which
has remained a longstanding clinical problem worldwide (18). Bone tissue engineering as a
substitute to autogenous bone grafting brought substantial success
in tackling the challenge of repairing bone defects and the use of
biomaterials functionalized with bioactive agents to improve bone
regeneration in osteonecrosis was considered one of the most
promising strategies (12).
Available seeding cell sources and suitable biomaterials are
currently still being explored and improved (19). In the present study, ADSCs/ADSC
sheets and β-TCP/COL-I scaffold composites were established under
osteoinductive conditions, in which the ascorbic acid promoted
collagen synthesis, the dexamethasone stimulated both osteogenic
differentiation and the glycerol phosphate induced mineral
deposition. SEM and EDS were used to observe and evaluate the
structure of the β-TCP/COL-I scaffold with ADSCs and detect the
relative calcium content on the surface of the composites.
Histological examination, ELISA and RT-qPCR measurements were used
to compare the osteogenic activity of each group. ALP, OCN and OPN
are major biomarkers of the early stages of osteogenesis, while
calcium deposition is considered a later marker (20). The present results demonstrated that
ADSC sheets avoided the cell loss caused by trypsin digestion and
had a superior osteogenic performance after being wrapped around a
β-TCP/COL-I scaffold when compared to scattered ADSCs with a
β-TCP/COL-I scaffold or an ADSC sheet alone. The major advantages
of the method of the present study compared with those of previous
studies in terms of bone tissue engineering were as follows. First,
the ADSCs are easily accessible, abundant and more efficient in
osteoinductivity than other MSCs. Furthermore, the ADSC sheets may
be harvested using a scraper without enzymatic digestion, which
preserved the ECM and cell-cell connections. In addition, the
β-TCP/COL-I scaffold had superior biodegradability,
osteoconductivity and higher compatibility with ADSC sheets. The
composite of ADSC sheets/β-TCP/COL-I scaffold provides a novel
promising candidate for bone regeneration.
In bone tissue engineering, promising seeding cells
for clinical application must be suitable for isolation and exhibit
proliferation efficiency, biocompatibility and formidable
osteogenic capacity (21). In a
previous study by our group, bone marrow-derived MSCs (BMSCs) were
used to construct a stem cell sheet, which had a high bone
regeneration potential when combined with β-TCP/COL-I scaffolds
(10). In clinical practice,
successful repair of huge bone defects via tissue engineering
requires a significant number of BMSCs. However, the quantity of
autologous BMSCs in the body is limited and the extraction process
is frequently painful (21). In
addition, long-term in vitro culture of BMSCs may compromise
their genomic stability (22).
Adipose tissues have been considered as a practical and adequate
source of stromal precursor cells with a high potential for
bone-tissue reconstruction. Compared with BMSCs, ADSCs possess a
higher proliferation and self-renewal capacity (3), and a high quantity of ADSCs may be
easily obtained from the host. In addition, ADSCs have been proven
to possess high efficiency in bone regeneration, in addition to
secreting anti-inflammatory factors and decreasing pro-inflammatory
responses (23). Furthermore, the
differentiation capacity of ADSCs was maintained with aging, thus
having advantages over BMSCs (4).
The present study revealed that ADSCs, alone or combined with a
scaffold, expressed osteogenic biomarkers much earlier than BMSCs
in the previous study by our group under the same conditions
(10).
Since the traditional method of seeding MSCs onto
scaffolds frequently results in a significant loss of cells and the
random dispersal of cells in the biological scaffold frequently
leads to inefficient or inadequate bone regeneration, cell sheet
technology was developed as a promising approach to solve these
problems (2). This avoids applying
trypsin and EDTA to improve the utilization of MSCs to the utmost
and prevents degradation of cell-surface proteins and the ECM,
which is responsible for transmitting biological signals to
regulate biological activity and bone formation (24). In addition, growth factors secreted
by stem cells may be preserved for a longer period of time in the
ECM, suggesting that the cell sheet may act as a controlled release
system (25). Several studies have
demonstrated that through the ECM, stem cells may exert therapeutic
effects via the secretion of trophic factors that provide a
favorable microenvironment for cell survival, renewal and
differentiation, ultimately having a positive role in bone
regeneration (26). Furthermore,
cell sheets always contract when harvested from culture dishes and
shrinkage-generated stress has been considered to specifically
regulate the polarization of MSCs and activate their
differentiation as a biomechanical force (27). In recent years, cell sheet-based
tissue engineering technology has already been applied for treating
various soft-tissue defects, such as the cornea, myocardium and
periodontal ligament (28). This
has also been progressively popular for hard-tissue engineering,
including bone tissues. Furthermore, it has been reported that MSC
sheets have a higher capacity of bone regeneration and
reconstruction for healing bone defects compared to scattered MSCs,
both in vitro and in vivo (2,25).
Consistent with the previous literature, the ADSC sheet in the
present study exhibited better osteogenic ability with enhanced ALP
activity, more calcium deposition and an elevated expression level
of OCN and OPN when compared to suspended ADSCs.
A number of approaches to harvesting intact cell
sheets have been previously reported, including
temperature-responsive culture (29), magnetic force (30), electron beam irradiation (31) and vitamin C application (32). However, these mentioned approaches
require complex procedures and novel materials, which may affect
cell proliferation or differentiation. In the present study, the
ADSC cell sheets were comprised of multiple layers of
osteoprogenitor cells and endogenous ECMs. With the large amount of
ECM and its gradual mineralization, the sheets were sufficiently
robust to be detached while remaining intact with a scraper and
forceps, making it possible to wrap it over the β-TCP/COL-I and
form a 3D compound. In addition, the present procedures are simple
and practical with the common tissue culture dish and cell
scraper.
Although a series of studies reported on harvesting
an osteogenic cell sheet and using it for bone reconstruction
without any scaffold (33), these
scaffold-free cell sheets were unsuitable for large bone defects
due to poor mechanical properties at the early stage of healing
(34). Even for multilayer cell
sheets, manual operation should be performed with care to prevent
the cell sheet from being torn. The biomaterial scaffold may
provide a 3D structure for the seeding cells to form a composite
with a certain shape and this may be transplanted into large bone
defects (35). Bone tissue
engineering requires scaffolds with appropriate architecture and
good osteoconductive activity, including the following (12,36):
i) An appropriate pore size within 50-100 µm; ii) interconnected
porosity for living tissues to grow; iii) sufficient mechanical
properties; iv) controllable degradation efficiency; v) excellent
biocompatibility; and vi) low immunogenicity. Mixtures of organic
and inorganic materials have been frequently used to tackle those
drawbacks of single materials and achieve the optimum desired
physical and chemical properties (37). The inorganic β-TCP has a relative
high biodegradability and osteoconductivity in comparison with
other materials like nano-hydroxyapatite/polymer or polymer-calcium
phosphate cement (17). And the
organic collagen is a major component of bone and has good cell
attachment ability and profound biodegradability (17). They are the commonly used bone
substitute materials that meet bone tissue engineering requirements
to the utmost extent. In addition, β-TCP was proven suitable as an
osteogenic material to repair bone in an animal model, as well as
in a clinical setting (38). While
the mechanical properties of pure collagen (37) or β-TCP (38) are frequently poor, the β-TCP/COL-I
scaffold had a higher compressive modulus (16), which is highly affected by the
material degradation and ECM formation under in vitro
culture conditions (14). Arahira
and Todo (39) reported that the
compressive modulus and strength of the β-TCP/COL scaffold with rat
bone-marrow mesenchymal stem cells (rBMSCs) decreased due to the
local release of β-TCP particles during the early culture period
and then increased as ECM deposition occurred in the late stage,
and finally became higher than those of the β-TCP/COL scaffold
without rBMSCs, which remained constant for the whole experiment.
The previous study by our group demonstrated that β-TCP/COL-I
scaffolds were able to enhance the bone regeneration potential of
rBMSC sheets in vitro and in vivo (10). In the present study, bone-like
collagen fibrils integrated with homogeneous β-TCP particles were
used as units to construct the β-TCP/COL-I scaffold in order to
mimic the constituent and structural properties of natural bone.
The porous structure of this composite facilitates the attachment
and ingrowth of local cells (37)
and provides a sufficient supply of nutrients and oxygen due to the
neovascularization (17,40). The present study indicated that the
ADSCs were able to tightly adhere and grow on the surface of the
β-TCP/COL-I scaffold without any impairment of cell viability
compared to ADSCs only, indicating that this material had good
biocompatibility. Furthermore, ADSC sheets wrapped around a
β-TCP/COL-I scaffold expressed higher levels of ALP, OCN and OPN
when compared to a mere ADSC sheet, suggesting that the scaffold
was able to significantly enhance the osteogenic activity of the
ADSC sheet, rather than just providing structural support. The
possible mechanism is that the mechanical properties of the
scaffold may help the ADSC sheet to resist the inherent retraction
tendency of the cell sheet, which is unfavorable for cell
proliferation and differentiation.
The present study provided a basic and novel
approach for bone engineering. Further studies should focus on the
development of new materials, alone or with bioactive factors
(41-44)
to enhance the osteogenic capacity of ADSC sheets. In addition,
various preparation methods should be tested to make the
composition, morphology and mechanical properties of the scaffold
closer to natural bone, and improve the practicability of the use
of ADSC sheets in regenerating bone tissues (45,46).
There are two shortcomings of the current study,
which may be addressed in the future. ADSCs should be
comprehensively compared with other MSCs, including rBMSCs, in
terms of osteoconductive activity when combined with a β-TCP/COL-I
scaffold in vitro and in vivo, to determine which is
more effective in bone engineering. In addition, the mechanical
properties of the β-TCP/COL-I scaffold with and without ADSCs
should be tested to prove their durability.
In conclusion, the present study demonstrated that
ADSC sheets combined with a β-TCP/COL-I scaffold have more
substantial osteogenic potential when compared to scattered ADSCs
with a β-TCP/COL-I scaffold or an ADSC sheet alone. The synergistic
effect of the ECM in the cell sheet and the mechanical properties
of the scaffold may have a vital role in the improved osteogenic
potential. The combination of the ADSC sheet and β-TCP/COL-I
scaffold provides an advanced strategy for treating bone defects,
which may be used to enhance treatment outcomes in the future.
Acknowledgements
Not applicable.
Funding
The present study was supported by two projects from the
National Natural Science Foundation of China (grant nos. 81873937
and 81970978).
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
JX designed all of the experiments. YW wrote the
manuscript. YW, XJS, RL, NZ and LZ performed the experiments. WX
and JL analyzed the data. YW and JX checked and confirmed the
authenticity of the raw data. All authors have discussed the data
and commented on the manuscript. All the authors have read and
approved the final version of this manuscript.
Ethics approval and consent to
participate
All animal experiments and the protocol of the
present study were conducted according to the guidelines of the
Ethics Committee of the Laboratory Animal Center, the First
Affiliated Hospital, School of Medicine, Zhejiang University
(Hangzhou, China).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Han Y, Li X, Zhang Y, Han Y, Chang F and
Ding J: Mesenchymal stem cells for regenerative medicine. Cells.
8(886)2019.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Xu X, Fang K, Wang L, Liu X, Zhou Y and
Song Y: Local application of semaphorin 3A combined with
adipose-derived stem cell sheet and anorganic bovine bone granules
enhances bone regeneration in type 2 diabetes mellitus rats. Stem
Cells Int. 2019(2506463)2019.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Jin HJ, Bae YK, Kim M, Kwon S-J, Jeon HB,
Choi SJ, Kim SW, Yang YS, Oh W and Chang JW: Comparative analysis
of human mesenchymal stem cells from bone marrow, adipose tissue,
and umbilical cord blood as sources of cell therapy. Int J Mol Sci.
14:17986–18001. 2013.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Mazini L, Rochette L, Amine M and Malka G:
Regenerative capacity of adipose derived stem cells (ADSCs),
comparison with mesenchymal stem cells (MSCs). Int J Mol Sci.
20(E2523)2019.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Ebrahimian TG, Pouzoulet F, Squiban C,
Buard V, André M, Cousin B, Gourmelon P, Benderitter M, Casteilla L
and Tamarat R: Cell therapy based on adipose tissue-derived stromal
cells promotes physiological and pathological wound healing.
Arterioscler Thromb Vasc Biol. 29:503–510. 2009.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Li M, Ma J, Gao Y and Yang L: Cell sheet
technology: A promising strategy in regenerative medicine.
Cytotherapy. 21:3–16. 2019.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Chen M, Xu Y, Zhang T, Ma Y, Liu J, Yuan
B, Chen X, Zhou P, Zhao X, Pang F, et al: Mesenchymal stem cell
sheets: A new cell-based strategy for bone repair and regeneration.
Biotechnol Lett. 41:305–318. 2019.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Maruthamuthu V, Sabass B, Schwarz US and
Gardel ML: Cell-ECM traction force modulates endogenous tension at
cell-cell contacts. Proc Natl Acad Sci USA. 108:4708–4713.
2011.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Sasagawa T, Shimizu T, Sekiya S, Haraguchi
Y, Yamato M, Sawa Y and Okano T: Design of prevascularized
three-dimensional cell-dense tissues using a cell sheet stacking
manipulation technology. Biomaterials. 31:1646–1654.
2010.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Lin J, Shao J, Juan L, Yu W, Song X, Liu
P, Weng W, Xu J and Mehl C: Enhancing bone regeneration by
combining mesenchymal stem cell sheets with β-TCP/COL-I scaffolds.
J Biomed Mater Res B Appl Biomater. 106:2037–2045. 2018.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Hutmacher DW, Schantz JT, Lam CX, Tan KC
and Lim TC: State of the art and future directions of
scaffold-based bone engineering from a biomaterials perspective. J
Tissue Eng Regen Med. 1:245–260. 2007.PubMed/NCBI View
Article : Google Scholar
|
|
12
|
Zhu T, Cui Y, Zhang M, Zhao D, Liu G and
Ding J: Engineered three-dimensional scaffolds for enhanced bone
regeneration in osteonecrosis. Bioact Mater. 5:584–601.
2020.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Pereira HF, Cengiz IF, Silva FS, Reis RL
and Oliveira JM: Scaffolds and coatings for bone regeneration. J
Mater Sci Mater Med. 31(27)2020.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Arahira T and Todo M: Effects of
proliferation and differentiation of mesenchymal stem cells on
compressive mechanical behavior of collagen/β-TCP composite
scaffold. J Mech Behav Biomed Mater. 39:218–230. 2014.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Kang Y, Kim S, Bishop J, Khademhosseini A
and Yang Y: The osteogenic differentiation of human bone marrow
MSCs on HUVEC-derived ECM and β-TCP scaffold. Biomaterials.
33:6998–7007. 2012.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Baheiraei N, Nourani MR, Mortazavi SMJ,
Movahedin M, Eyni H, Bagheri F and Norahan MH: Development of a
bioactive porous collagen/β-tricalcium phosphate bone graft
assisting rapid vascularization for bone tissue engineering
applications. J Biomed Mater Res A. 106:73–85. 2018.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Zou C, Weng W, Deng X, Cheng K, Liu X, Du
P, Shen G and Han G: Preparation and characterization of porous
beta-tricalcium phosphate/collagen composites with an integrated
structure. Biomaterials. 26:5276–5284. 2005.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Li JJ, Akey A, Dunstan CR, Vielreicher M,
Friedrich O, Bell DC and Zreiqat H: Effects of material-tissue
interactions on bone regeneration outcomes using baghdadite
implants in a large animal model. Adv Healthc Mater.
7(e1800218)2018.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Wubneh A, Tsekoura EK, Ayranci C and
Uludağ H: Current state of fabrication technologies and materials
for bone tissue engineering. Acta Biomater. 80:1–30.
2018.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Birmingham E, Niebur GL, McHugh PE, Shaw
G, Barry FP and McNamara LM: Osteogenic differentiation of
mesenchymal stem cells is regulated by osteocyte and osteoblast
cells in a simplified bone niche. Eur Cell Mater. 23:13–27.
2012.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Wu V, Helder MN, Bravenboer N, Ten
Bruggenkate CM, Jin J, Klein-Nulend J and Schulten EAJM: Bone
tissue regeneration in the oral and maxillofacial region: a review
on the application of stem cells and new strategies to improve
vascularization. Stem Cells Int. 2019(6279721)2019.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Tropel P, Noël D, Platet N, Legrand P,
Benabid A-L and Berger F: Isolation and characterisation of
mesenchymal stem cells from adult mouse bone marrow. Exp Cell Res.
295:395–406. 2004.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Si Z, Wang X, Sun C, Kang Y, Xu J, Wang X
and Hui Y: Adipose-derived stem cells: Sources, potency, and
implications for regenerative therapies. Biomed Pharmacother.
114(108765)2019.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Martino MM, Mochizuki M, Rothenfluh DA,
Rempel SA, Hubbell JA and Barker TH: Controlling integrin
specificity and stem cell differentiation in 2D and 3D environments
through regulation of fibronectin domain stability. Biomaterials.
30:1089–1097. 2009.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Liu Y, Ming L, Luo H, Liu W, Zhang Y, Liu
H and Jin Y: Integration of a calcined bovine bone and BMSC-sheet
3D scaffold and the promotion of bone regeneration in large
defects. Biomaterials. 34:9998–10006. 2013.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Pensak M, Hong S, Dukas A, Tinsley B,
Drissi H, Tang A, Cote M, Sugiyama O, Lichtler A, Rowe D, et al:
The role of transduced bone marrow cells overexpressing BMP-2 in
healing critical-sized defects in a mouse femur. Gene Ther.
22:467–475. 2015.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Yang J, Yamato M, Nishida K, Ohki T,
Kanzaki M, Sekine H, Shimizu T and Okano T: Cell delivery in
regenerative medicine: The cell sheet engineering approach. J
Control Release. 116:193–203. 2006.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Hasegawa M, Yamato M, Kikuchi A, Okano T
and Ishikawa I: Human periodontal ligament cell sheets can
regenerate periodontal ligament tissue in an athymic rat model.
Tissue Eng. 11:469–478. 2005.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Okano T, Yamada N, Sakai H and Sakurai Y:
A novel recovery system for cultured cells using plasma-treated
polystyrene dishes grafted with poly(N-isopropylacrylamide). J
Biomed Mater Res. 27:1243–1251. 1993.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Penland N, Choi E, Perla M, Park J and Kim
DH: Facile fabrication of tissue-engineered constructs using
nanopatterned cell sheets and magnetic levitation. Nanotechnology.
28(075103)2017.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Kwon OH, Kikuchi A, Yamato M, Sakurai Y
and Okano T: Rapid cell sheet detachment from
poly(N-isopropylacrylamide)-grafted porous cell culture membranes.
J Biomed Mater Res. 50:82–89. 2000.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Nakamura A, Akahane M, Shigematsu H,
Tadokoro M, Morita Y, Ohgushi H, Dohi Y, Imamura T and Tanaka Y:
Cell sheet transplantation of cultured mesenchymal stem cells
enhances bone formation in a rat nonunion model. Bone. 46:418–424.
2010.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Ma D, Ren L, Liu Y, Chen F, Zhang J, Xue Z
and Mao T: Engineering scaffold-free bone tissue using bone marrow
stromal cell sheets. J Orthop Res. 28:697–702. 2010.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Ma D, Yao H, Tian W, Chen F, Liu Y, Mao T
and Ren L: Enhancing bone formation by transplantation of a
scaffold-free tissue-engineered periosteum in a rabbit model. Clin
Oral Implants Res. 22:1193–1199. 2011.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Yuan J, Cui L, Zhang WJ, Liu W and Cao Y:
Repair of canine mandibular bone defects with bone marrow stromal
cells and porous beta-tricalcium phosphate. Biomaterials.
28:1005–1013. 2007.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Li X, Wang L, Fan Y, Feng Q, Cui FZ and
Watari F: Nanostructured scaffolds for bone tissue engineering. J
Biomed Mater Res A. 101:2424–2435. 2013.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Zhang Y, Liu X, Zeng L, Zhang J, Zuo J,
Zou J, Ding J and Chen X: Polymer Fiber Scaffolds for Bone and
Cartilage Tissue Engineering. Adv Funct Mater. 29(1903279)2019.
|
|
38
|
Luvizuto ER, Queiroz TP, Margonar R,
Panzarini SR, Hochuli-Vieira E, Okamoto T and Okamoto R:
Osteoconductive properties of β-tricalcium phosphate matrix,
polylactic and polyglycolic acid gel, and calcium phosphate cement
in bone defects. J Craniofac Surg. 23:e430–e433. 2012.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Arahira T and Todo M: Variation of
mechanical behavior of β-TCP/collagen two phase composite scaffold
with mesenchymal stem cell in vitro. J Mech Behav Biomed Mater.
61:464–474. 2016.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Zhou Y, Chen F, Ho ST, Woodruff MA, Lim TM
and Hutmacher DW: Combined marrow stromal cell-sheet techniques and
high-strength biodegradable composite scaffolds for engineered
functional bone grafts. Biomaterials. 28:814–824. 2007.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Zhang Y, Ding J, Qi B, Tao W, Wang J, Zhao
C, Peng H and Shi J: Multifunctional fibers to shape future
biomedical devices. Adv Funct Mater. 29(1902834)2019.
|
|
42
|
Cui L, Zhang J, Zou J, Yang X, Guo H, Tian
H, Zhang P, Wang Y, Zhang N, Zhuang X, et al: Electroactive
composite scaffold with locally expressed osteoinductive factor for
synergistic bone repair upon electrical stimulation. Biomaterials.
230(119617)2020.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Qiu H, Guo H, Li D, Hou Y, Kuang T and
Ding J: Intravesical hydrogels as drug reservoirs. Trends
Biotechnol. 38:579–583. 2020.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Wang Y, Jiang Z, Xu W, Yang Y, Zhuang X,
Ding J and Chen X: Chiral polypeptide thermogels induce controlled
inflammatory response as potential immunoadjuvants. ACS Appl Mater
Interfaces. 11:8725–8730. 2019.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Ding J, Xiao H, Li J, Zhuang X, Zhang J,
Di Li XC, Xiao C and Yang H: Electrospun polymer biomaterials. Prog
Polym Sci. 90:1–34. 2019.
|
|
46
|
Feng X, Li J, Zhang X, Liu T, Ding J and
Chen X: Electrospun polymer micro/nanofibers as pharmaceutical
repositories for healthcare. J Control Release. 302:19–41.
2019.PubMed/NCBI View Article : Google Scholar
|