Introduction
Coronary artery disease can cause imbalance between
coronary artery blood flow and myocardial demand, leading to
myocardial injury, resulting in myocardial ischemia and hypoxia or
necrosis, which is clinically known as ‘coronary heart disease’
(CHD). Acute myocardial infarction (AMI) is one of the common
manifestations of CHD (1). AMI is a
kind of myocardial ischemic necrosis caused by coronary artery
occlusion and interruption of blood flow. The clinical
manifestations of AMI include persistent post-sternal pain, shock,
arrhythmia and even death caused by severe heart failure (2). Early diagnosis and treatment of AMI
can significantly reduce the mortality and improve the prognosis of
patients (3). According to whether
there is ST-segment elevation in the ECG, AMI can be divided into 6
types of infarction: Coronary artery bypass graft-related
infarction, coronary stent thrombosis-related infarction,
percutaneous coronary intervention-related infarction, infarction
leading to sudden death without biomarkers, infarction caused by
imbalance between supply and demand in blood flow due to causes
other than acute atherosclerotic thrombosis, and infarction caused
by coronary atherosclerotic thrombosis (1). Epidemiological surveys show that there
are approximately 550,000 new AMI and 200,000 relapsed AMI patients
in the United States each year, and the global burden of the
disease has begun to shift to low- and middle-income countries
(4). Approximately 80% of deaths
from cardiovascular diseases come from low- and middle-income
countries (4).
Early detection, diagnosis, and treatment are the
keys to effectively preventing and reducing the occurrence and
death of AMI. Cardiac troponin (cTn) is the first-choice biomarker
for AMI detection (2). It has high
value in distinguishing unstable angina pectoris, diseases other
than acute coronary syndrome and non-ST-segment elevation AMI. The
cTn test is highly sensitive to AMI and can improve the sensitivity
of diagnosis, but its specificity is low. Its diagnostic
performance is affected by diseases such as myocarditis, kidney
damage, respiratory failure, and intracranial hemorrhage.
Therefore, multi-index combined diagnosis can improve the diagnosis
of AMI. Ischemia-modified albumin (ische-mia-modified albumin, IMA)
is an ideal ischemia marker. It is the first myocardial ischemia
marker approved for sale by the US FDA (5). A previous study has confirmed that
when myocardial necrosis indicators are negative, IMA can show
extremely high sensitivity. For example, IMA can be detected in the
blood within 5-10 min of myocardial ischemia. After it reaches a
peak value in 1-2 h, IMA returns to the basic level in 3-6(5). In addition, plasma D-dimer (D-D) and
inflammatory cytokine monocyte chemoattractant protein-1 (MCP-1)
were detected. As a specific degradation product of cross-linked
fibrin, the increase of D-D level reflects the enhancement of
secondary fibrinolytic activity, and it can be used as a specific
molecular marker for hypercoagulability and fibrinolytic
hyperactivity in vivo (6).
It has been shown that plasma D-dimer has diagnostic significance
in vascular diseases and can be used as one of the auxiliary
diagnoses for the determination of AMI (7). MCP-1 is mainly secreted and released
by endothelial cells, vascular smooth muscle cells and macrophages.
It can induce the expression of certain inflammatory factors,
chemokines, matrix metalloproteinases and tissue factors in plaque
cells, converting the originally stable plaques into vulnerable
plaques, and rupture and secondary thrombosis lead to the
occurrence of AMI (8).
The aim of this study was to investigate the
application value of the combined detection of IMA, D-D, and MCP-1
for early diagnosis of AMI. The results showed that, the levels of
IMA, D-D and MCP-1 were positively correlated with CTnT and hs-CRP
levels in AMI patients. Combined detection of IMA, D-D, and MCP-1
can improve the accuracy.
Materials and methods
General information
A total of 87 patients with AMI who met the
diagnostic criteria of AHA/ACC (9)
from January 2017 to January 2018 were enrolled in the AMI group,
including 53 males and 34 females, with the age of 64.35±11.90
years. Another 82 patients who were hospitalized at the same time
and confirmed by coronary angiography without coronary artery
disease were enrolled in a control group, including 50 males and 32
females, with the age of 59.04±12.13 years. The general data of
patients in the two groups were compared, and there was no
statistical difference, as detailed in Table I.
 | Table IGeneral information. |
Table I
General information.
| Indicators | AMI group (n=87) | Control group
(n=82) | t/χ2 | P-value |
|---|
| Age | 64.35±11.90 | 59.04±12.13 | 2.872 | >0.05 |
| Sex | | | 0.191 | >0.05 |
|
Male | 53 (60.92) | 50 (60.98) | | |
|
Female | 34 (39.08) | 32 (39.02) | | |
| Body mass index | 23.67±3.75 | 22.74±3.40 | 1.590 | 0.113 |
| Academic level | | | | |
|
>high
school | 58 (66.67%) | 55 (67.07%) | 0.003 | 0.955 |
|
≤high
school | 29 (33.33%) | 27 (32.93%) | | |
| Place of
domicile | | | 0.001 | 0.999 |
|
Rural | 52 (59.77%) | 49 (59.76%) | | |
|
City | 35 (40.23%) | 33 (40.24%) | | |
| Smoking | 34 (39.08%) | 30 (36.59%) | 0.112 | 0.738 |
| Family history of
CHD | 45 (51.72%) | 40 (48.78%) | 0.146 | 0.702 |
The study was approved by the Medical Ethics
Committee of the China-Japan Union Hospital of Jilin University,
and informed consent was signed by all the selected subjects.
Inclusion and exclusion criteria
Inclusion criteria were: i) Patients with AMI,
diagnosed by AHA/ACC diagnostic criteria; ii) age ≥30 years.
Exclusion criteria were: i) Patients with severe liver and kidney
insufficiency, tumors and rheumatic diseases; ii) acute or chronic
infections; iii) trauma and sports injury.
Inspection indicators Determination of
serum MCP-1, D-D and IMA content
Fasting venous blood (5 ml) was taken from the
selected subjects, and the blood was left to stand for 0.5-1 h at
room temperature. The blood was centrifuged at the speed of 650 x g
at 4˚C for 10 min. After centrifugation, the upper serum was
retained, and the serum levels of MCP-1, D-D and IMA were detected
by ELISA. MCP-1 kit was purchased from ADL Company, and D-D kit was
purchased from Merck Biotechnology Co., Ltd. (product no.: cx20026,
and item no.: 59-20026). IMA kit was purchased from Shanghai
Fusheng Industrial Co., Ltd. (cat. no. A097761-48T). The operation
procedure was performed in accordance with the kit
instructions.
Determination of CTnT, hs-CRP and
blood lipids in patients
Fasting venous blood (5 ml) was taken from the
selected subjects, and the blood was let stand for 0.5-1 h at room
temperature. The blood was centrifuged at the speed of 650 x g at
4˚C for 10 min. After centrifugation, the upper serum was retained,
and the serum levels of CTnT and hs-CRP were detected by ELISA. The
human CTnT ELISA kit (FKO1147B) was purchased from Shanghai Kexing
Biotechnology Co., Ltd., and the human hs-CRP ELISA kit (no.
A09718) was purchased from Shanghai Jining Industrial Co., Ltd..
Surgery was performed in accordance with the kit instructions.
Total cholesterol (TC), triglyceride (TG), high-density lipoprotein
cholesterol (HDL-D) and low-density lipoprotein cholesterol (LDL-C)
were measured in the two groups of patients by AU1000/2700
automatic biochemical analyzer.
Prognosis
Conventional treatment was performed on all patients
with AMI. There was no significant difference in the treatment.
After interventional therapy, oral aspirin enteric-coated tablets
were taken at a dose of 100 mg once a day. Captopril (18 mg) and
valsartan (80 mg) were taken once a day (10). The patients with AMI after treatment
were followed up for one year. Major adverse cardiovascular events
(MACE) in AMI patients after treatment were recorded as the group
with poor prognosis, while those without MACE were classified as
the group with good prognosis. Then serum samples of the two groups
were again extracted to measure the contents of MCP-1, D-D and IMA.
MACE mainly includes recurrent angina pectoris, AMI, severe
arrhythmia, heart failure, death of CHD.
Correlation analysis
Pearson correlation analysis was used to analyze the
correlation between MCP-1, D-D, IMA content and TC, TG, HDL-D,
LDL-C in patients' blood lipids, and the correlation between MCP-1,
D-D, IMA content and prognosis of IMA.
ROC curve analysis
The ROC curve was used to analyze the diagnostic
value of IMA, D-D and MCP-1 alone and combined in AMI.
Statistical analysis
SPSS 18.0 software was used to analyze the data, and
GraphPad Prism 6 was used to draw all the figures. χ2
test was used to compare the counting data. Mean ± standard
deviation was used to express the measurement data. Independent
t-test was used for comparison between the two groups. Paired
t-test was used for analysis of data at different time points.
Pearson's correlation analysis was used to analyze the relationship
between variables. We made the horizontal and vertical co-ordinates
according to the sample size, and used SPSS software to generate
the ROC curves. Then, AUC values were determined according to the
area under the curve. There was statistical difference when
P<0.05.
Results
Determination of serum MCP-1, D-D and
IMA levels in two groups
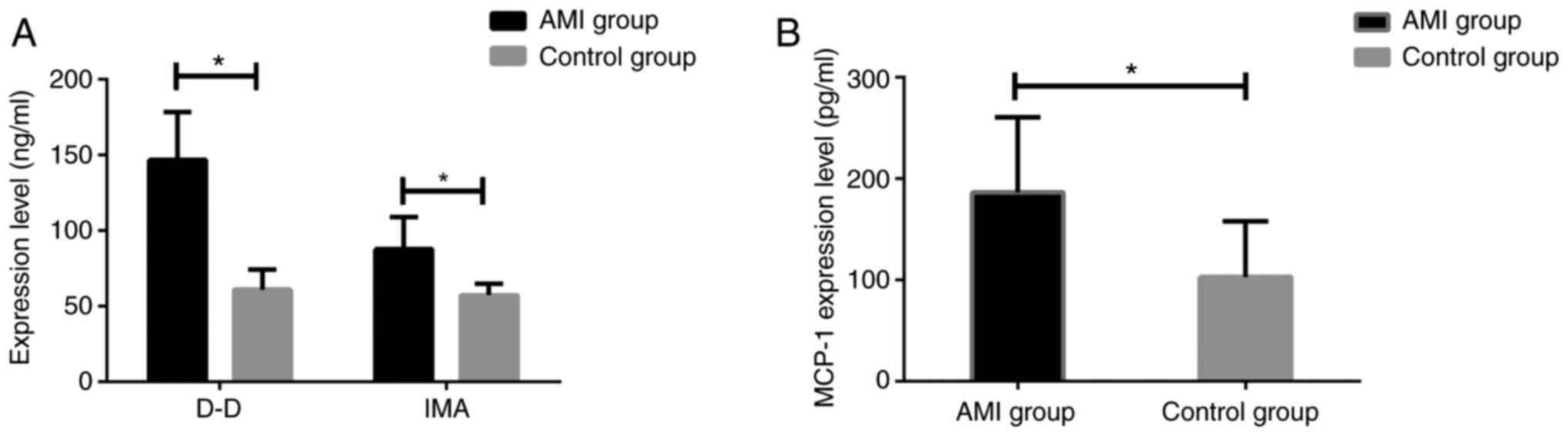
The expression levels of MCP-1, D-D and IMA in serum
of patients in two groups were measured. It was found that the
expression of MCP-1, D-D and IMA in patients with AMI was
significantly higher than that in the control group (all
P<0.001) (Fig. 1).
Determination of CTnT and hs-CRP in
patients
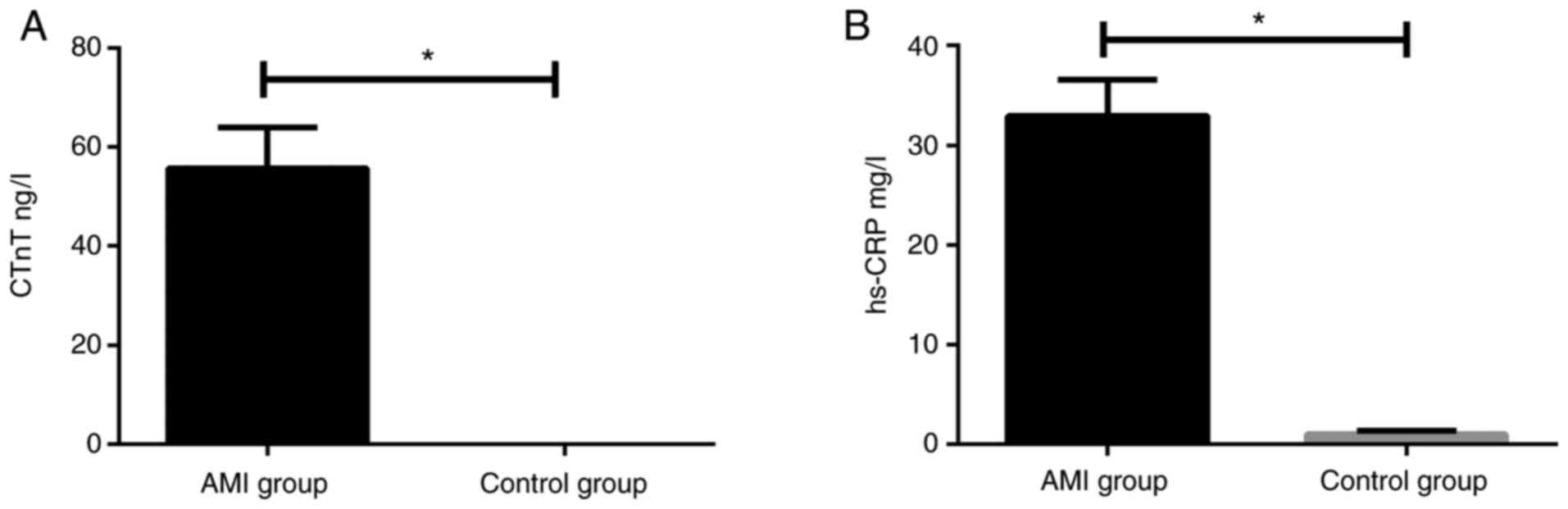
The levels of CTnT (55.72±8.18 ng/l) and hs-CRP
(32.95±3.62 mg/l) in patients with AMI were significantly higher
than those in the control group, with CTnT (0.034±0.01 ng/l) and
hs-CRP (1.02±0.35 mg/l) (all P<0.05) (Fig. 2).
Determination of blood lipids in two
groups of patients
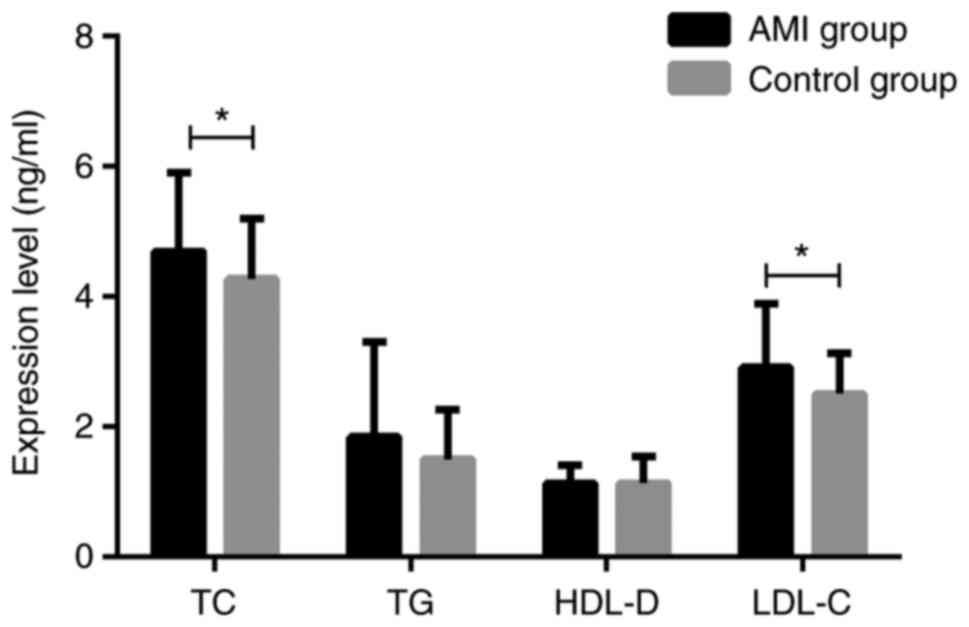
The blood lipids of the two groups were measured.
There were significant differences in TC and LDL-C between AMI
group and control group (both P<0.05), but no significant
differences in TG and HDL-D (both P>0.05) (Fig. 3).
Prognosis and serum levels of MCP-1,
D-D and IMA in patients with AMI after treatment
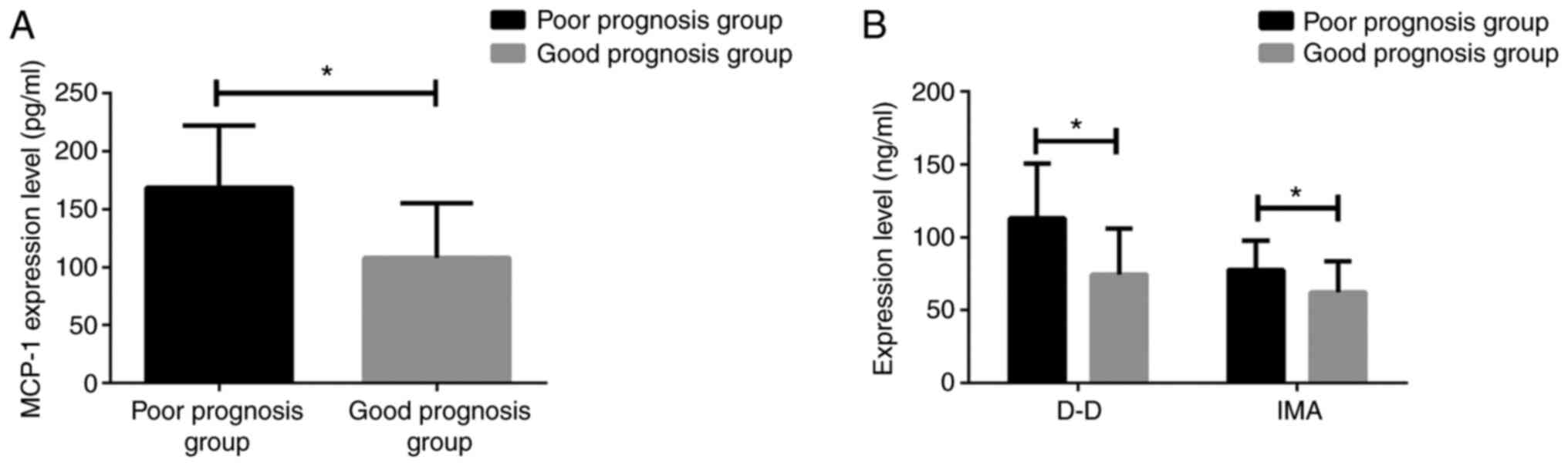
After treatment of 87 patients with AMI, 21 cases
had recurrent angina pectoris, 7 cases had AMI and 14 cases had
severe arrhythmia after one year. After treatment, 42 patients with
MACE were classified as poor prognosis group, while 45 patients
without MACE were classified as good prognosis group. MCP-1, D-D
and IMA in patients with poor prognosis were detected within 48 h
of MACE and the data were retained. At the end of the follow-up
period, MCP-1, D-D and IMA in patients with good prognosis without
MACE were detected, and the data of two groups were compared. The
levels of MCP-1 (168.72±53.31), D-D (113.04±37.47), IMA
(77.61±20.15) in patients with poor prognosis were significantly
higher than those in patients with good prognosis, MCP-1
(108.14±47.20), D-D (74.53±31.48), IMA (62.21±21.47) (all
P<0.05) (Fig. 4).
Correlation analysis of serum MCP-1,
D-D and IMA levels
Pearson's correlation analysis was used to analyze
the correlation between MCP-1, D-D, IMA and TC, TG, HDL-D, LDL-C in
patient's blood lipids. The correlation between MCP-1, D-D, IMA
content and prognosis of IMA was analyzed. The index levels
measured by the AMI group were used as MCP-1, D-D, and IMA
content.
There was no correlation between MCP-1, D-D, IMA
levels and TC, TG, HDL-D, LDL-C levels in serum lipids in patients
with AMI (all P>0.05) (Fig. 5).
The serum levels of MCP-1, D-D and IMA were positively correlated
with the levels of CTnT and hs-CRP in patients with AMI. Fig. 5A: r=0.6712, P=<0.001; Fig. 5B: r=0.4492, P=<0.001; Fig. 5C: r=0.4547, P=<0.001; Fig. 5D: r=0.5538, P=<0.001; Fig. 5E: r=0.5607, P=<0.001. Fig. 5F: r=0.5070, P=<0.001 (all
P<0.05) (Fig. 6).
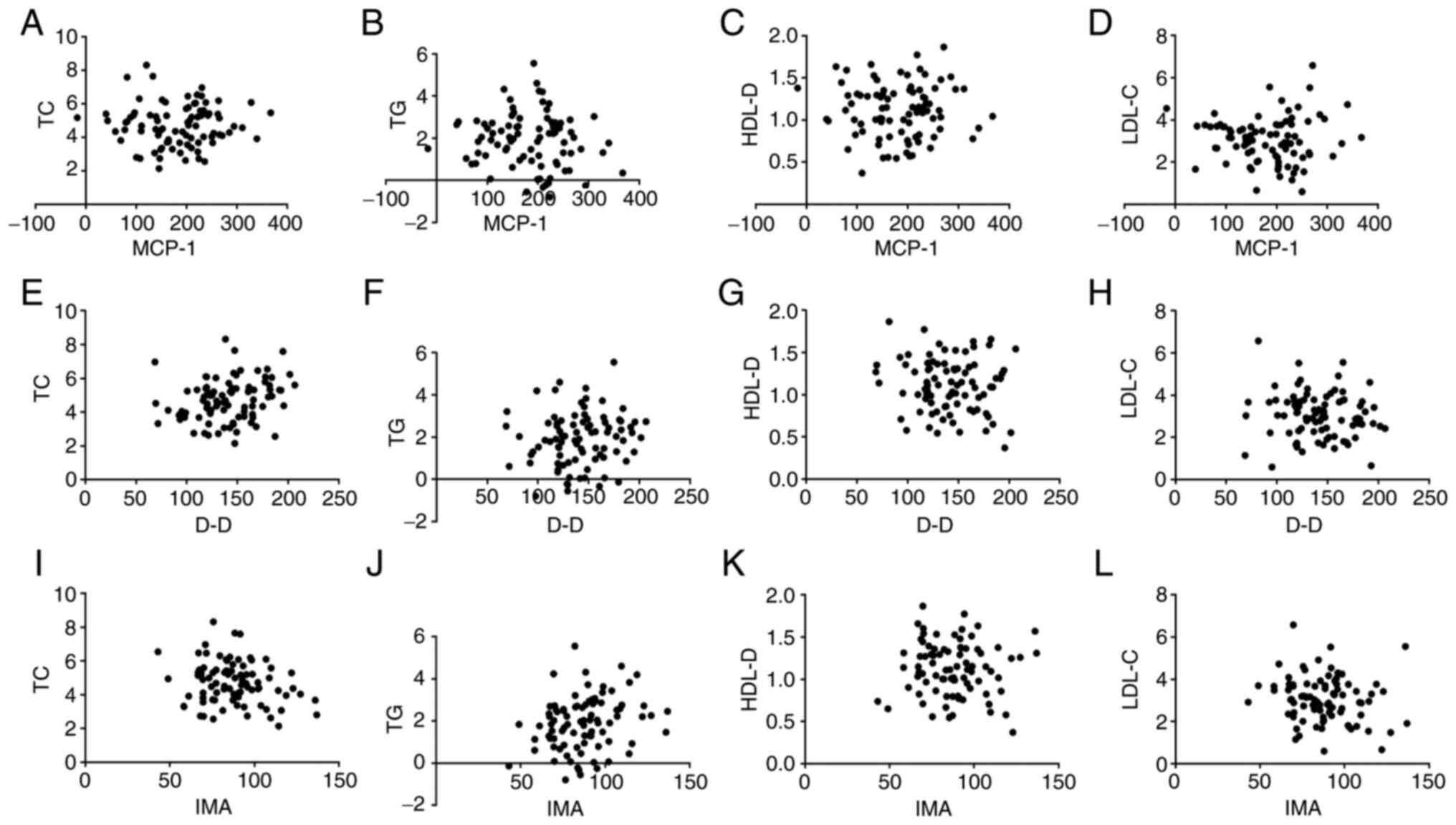 | Figure 5Correlation analysis of serum levels
of MCP-1, D-D, IMA and TC, TG, HDL-D, and LDL-C levels in blood
lipids. Pearson's correlation analysis showed that serum levels of
MCP-1 (A-D), D-D (E-H), and IMA (I-L) were not correlated with TC,
TG, HDL-D, and LDL-C levels in blood lipids (all P>0.05). MCP-1,
monocyte chemoattractant protein-1; D-D, D-dimer; IMA,
ischemia-modified albumin; TC, total cholesterol; TG, triglyceride;
HDL-D, high-density lipoprotein cholesterol; LDL-C, low-density
lipoprotein cholesterol. |
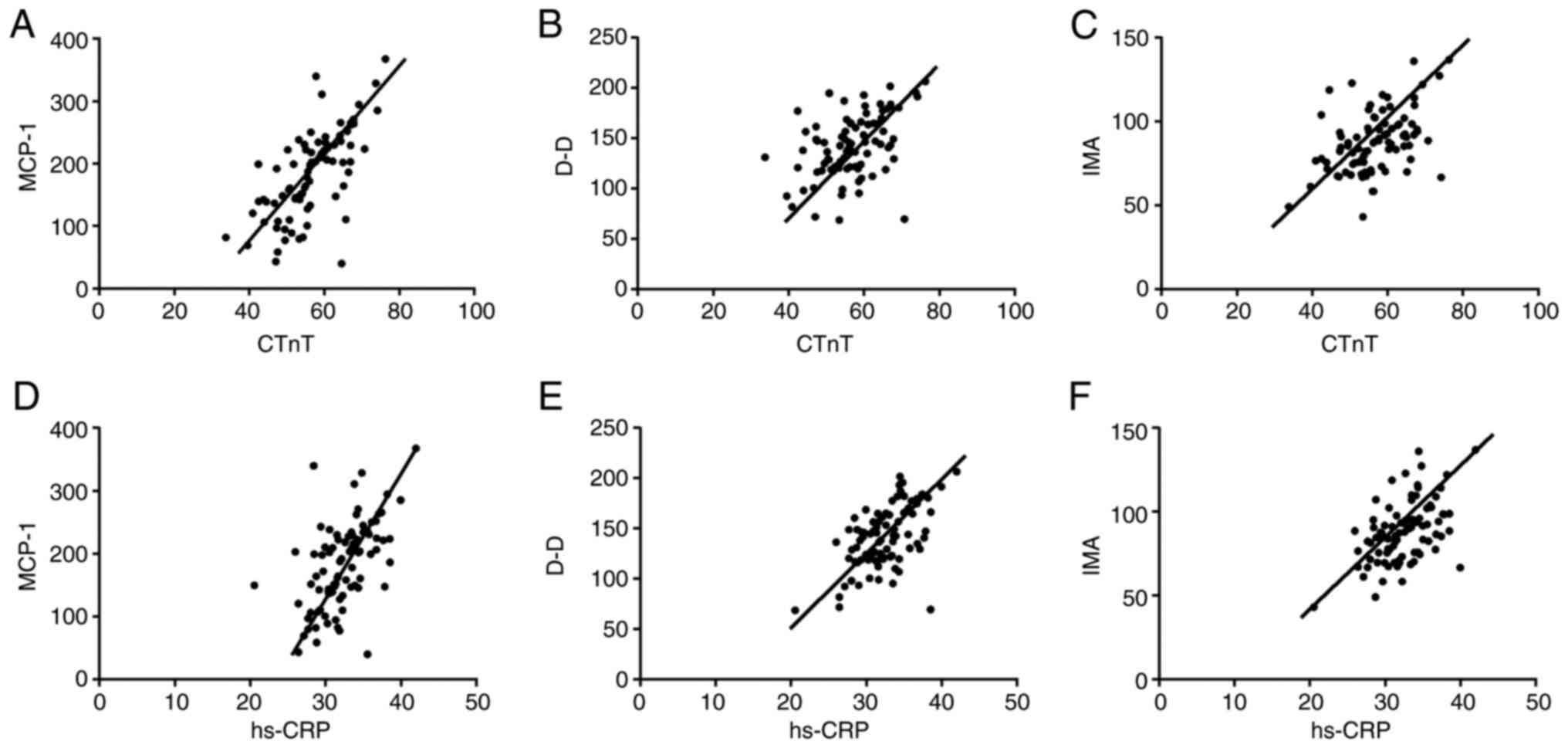 | Figure 6Correlation analysis of MCP-1, D-D,
IMA levels and CTnT, hs-CRP levels. (A) MCP-1 is positively
correlated with CTnT, r=0.6712. (B) D-D is positively correlated
with CTnT, r=0.4492. (C) IMA is positively correlated with CTnT,
r=0.4547. (D) MCP-1 is positively correlated with hs-CRP, r=0.5538.
(E) D-D is positively correlated with hs-CRP, r=0.5607. (F) IMA is
positively correlated with hs-CRP, r=0.5070 (all P<0.05). MCP-1,
monocyte chemoattractant protein-1; D-D, D-dimer; IMA,
ischemia-modified albumin; cTnT, cardiac troponin; hs-CRP,
high-sensitivity C-reactive protein. |
ROC curve analysis
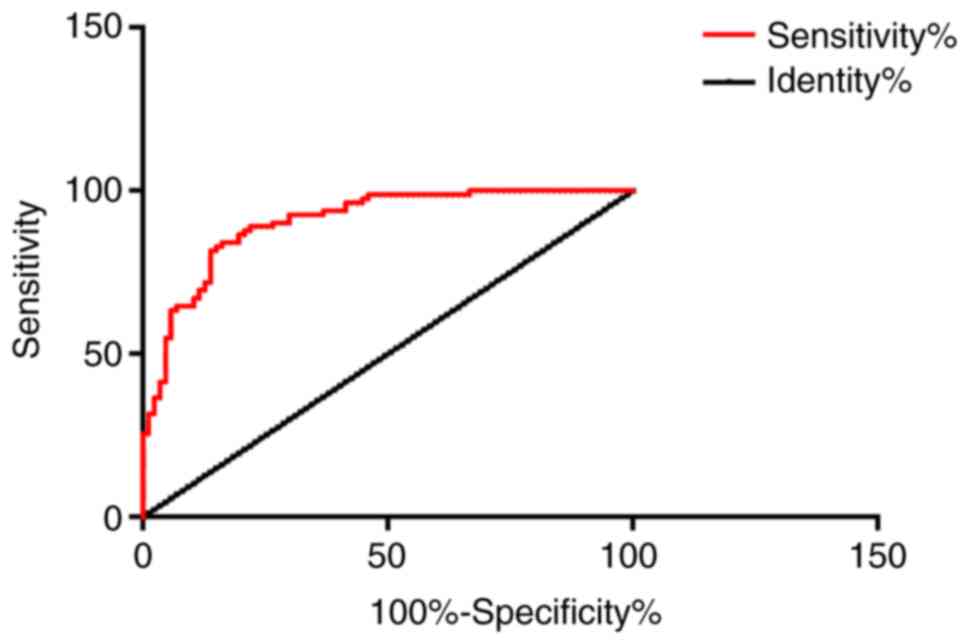
The AUC was 0.8084 for patients with AMI diagnosed
alone with MCP-1, the specificity was 81.61%, and the sensitivity
was 69.51%. The AUC was 0.7302 for patients with AMI diagnosed
alone with D-D, the specificity was 59.77%, and the sensitivity was
81.71%. The AUC was 0.7289 for AMI patients diagnosed alone with
IMA, the specificity was 58.62%, and the sensitivity was 80.49%.
The AUC was 0.9047 in combined detection of MCP-1, D-D and IMA in
patients with AMI, the specificity was 58.62%, and the sensitivity
was 93.90%. Other data are shown in Table II, and Figs. 7 and 8.
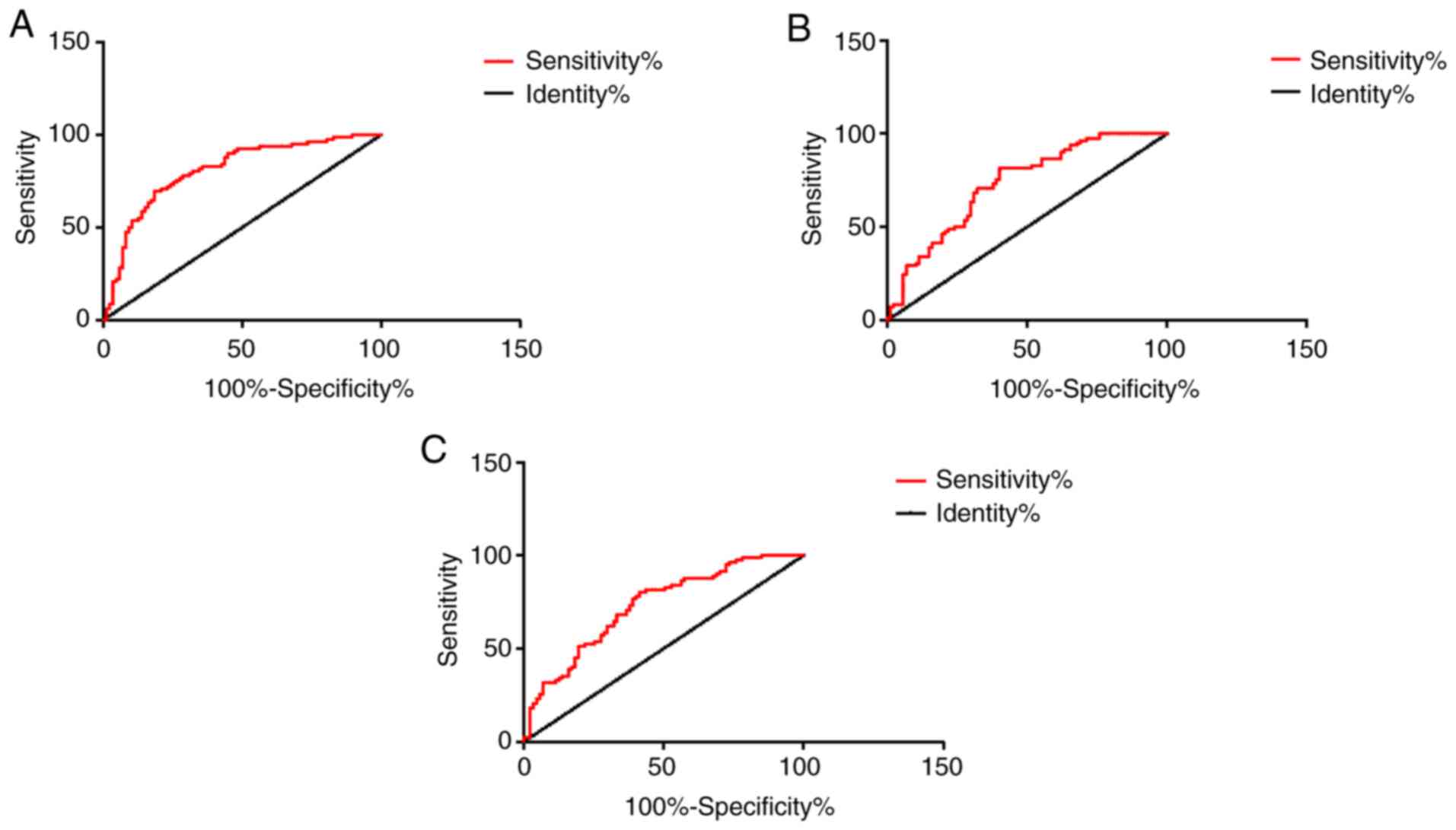 | Figure 7ROC curves of MCP-1, D-D and IMA for
detecting AMI independently. (A) The AUC of patients with AMI
diagnosed alone with MCP-1 was 0.8084, the specificity was 81.61%,
and the sensitivity was 69.51%. (B) The AUC of patients with AMI
diagnosed alone with D-D was 0.7302, the specificity was 59.77%,
and the sensitivity was 81.71%. (C) The AUC of AMI patients
diagnosed alone with IMA was 0.7289, the specificity was 58.62%,
and the sensitivity was 80.49%. MCP-1, monocyte chemoattractant
protein-1; D-D, D-dimer; IMA, ischemia-modified albumin; AMI, acute
myocardial infarction. |
 | Table IIROC curve analysis. |
Table II
ROC curve analysis.
| Indicators | AUC | Sensitivity (%) | Specificity (%) | Standard error | 95% CI | P-value |
|---|
| MCP-1 | 0.8084 | 81.61 | 69.51 | 0.03373 | 0.7423-0.8745 | <0.001 |
| D-D | 0.7302 | 59.77 | 81.71 | 0.03832 | 0.6550-0.8053 | <0.001 |
| IMA | 0.7289 | 58.62 | 80.49 | 0.03830 | 0.6538-0.8040 | <0.001 |
| MCP-1, D-D,
IMA | 0.9047 | 58.62 | 93.90 | 0.02249 | 0.8606-0.9488 | <0.001 |
Discussion
Patients with AMI are more likely to have coronary
atherosclerotic stenosis. Due to certain inducements,
atherosclerotic plaque ruptures. Platelets in the blood gather on
the surface of the ruptured plaque, forming blood clots, and
eventually obstruct the coronary artery lumen. In addition, AMI can
also be induced by a sharp increase in myocardial oxygen
consumption or coronary artery spasm. Sudden cold stimulation may
induce myocardial infarction. Approximately half of the patients
often missed diagnosis and delayed the best time of treatment
because there was no obvious changes in early ECG. Early detection
of AMI can effectively improve the treatment and prognosis of
patients (11,12).
MCP-1(13) is a
cellular inflammatory factor secreted by monocytes, with multiple
thiol and hydroxyl groups. It can promote the activation of
downstream monocytes or macrophages and accelerate the formation of
local microvessels on the basis of binding glycoprotein ligands. In
a related study by Niu J (14), it
was concluded that many diseases including cardiovascular diseases
are considered to be inflammatory diseases, and MCP-1 plays a key
role in the development of cardiovascular diseases. MCP-1 also
works in cardiac repair and participates in ischemic angiogenesis.
The results of this experiment showed that MCP-1 is highly
expressed in AMI.
D-D (15,16) is a molecular marker of secondary
fibrinolysis and hypercoagulability. D-D increases due to the
formation of activated thrombosis and the presence of fibrinolysis
activity in the early stage of myocardial infarction. This study
showed that the level of D-D in serum of patients with AMI was
significantly higher than that of the control group, indicating
that there is a potential link between D-D and AMI. However, D-D
was also increased in other diseases (17). ROC curve analysis showed that D-D
was not specific for AMI and it could not be used as an independent
predictor.
IMA (18) was
discovered in the 1990s as a serum albumin damaged by hydroxyl
radicals during myocardial ischemia. Serum albumin is known as IMA
when the 2-4 amino acid residues at the N-terminus of serum albumin
are deleted by N-acetylation and then bound to metallic cobalt.
Sokhanvar S (19) found that IMA
can be used as a diagnostic factor for acute coronary syndrome
(ACS). Although its sensitivity and specificity were not higher
compared with other diagnostic tests, it still has diagnostic value
for coronary disease. The results of this experiment show that the
level of IMA in serum of patients with AMI is significantly higher
than that of control group. These results suggest that IMA can be
used as an independent criterion for increased risk of AMI, which
is consistent with the results of the study by Zhong et al
(20).
At the same time, this paper also selected two
common indicators of AMI: Cardiac troponin (cTnT) and
high-sensitivity C-reactive protein (hs-CRP). CTnT is considered to
be the most ideal early diagnostic index for AMI at present, and it
is also an important index for patients' disease evaluation,
prognosis judgment and curative effect observation (21). hs-CRP is one of the risk indicators
for cardiovascular and cerebrovascular diseases (22). The results of this study showed that
the levels of cTnT and hs-CRP in the patients with AMI were
significantly higher than those in the control group, which was
consistent with the results of Keller and Han (23,24).
It was found that MCP-1, D-D and IMA were highly expressed in
patients with AMI. It was speculated that MCP-1, D-D and IMA were
potentially associated with cTnT and hs-CRP. Pearson correlation
analysis showed that serum MCP-1, D-D, IMA levels were positively
correlated with CTnT and hs-CRP levels in patients with AMI.
Patients were followed up for one year after treatment. Patients
received routine treatment, so there is no difference in the effect
of treatment on prognosis. According to the occurrence of MACE,
patients were divided into group with the poor prognosis and group
with good prognosis, and the levels of MCP-1, D-D and IMA in the
two groups were detected. The results showed that the levels of
MCP-1, D-D and IMA in patients with poor prognosis were
significantly higher than those in patients with good prognosis,
suggesting that detection of MCP-1, D-D and IMA expression levels
can predict the prognosis of patients with AMI.
The ROC curves of patients with MCP-1, D-D and IMA
were analyzed. The AUC of single detection of AMI was 0.8084,
0.7302 and 0.7289, respectively. However, the AUC of three combined
detection was 0.9047, and the specificity and sensitivity were
significantly improved, suggesting that combined detection can
improve the accuracy of diagnosis of AMI, so misdiagnosis and
missed diagnosis could be reduced. However, this study also has
certain limitations, such as the small sample size. Moreover, the
combined detection of MCP-1, D-D and IMA in this study can only be
used as a complementary diagnosis, but not as a substitute for
coronary angiography. This experiment failed to make a deeper
exploration of the pathogenesis of AMI, and hope that in-depth
research will be conducted in the future. We did not collect
multicenter data in this study, but multicenter data will be
collected in the future.
In conclusion, combined detection of MCP-1, D-D and
IMA can effectively improve the diagnostic rate of AMI, detect the
levels of IMA, D-D and MCP-1 to predict the prognosis of patients,
and suggest routine joint detection in clinical departments.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
MD and ML conceived and designed the study, and
drafted the manuscript. MD, ML and HY collected, analyzed and
interpreted the experimental data. HY revised the manuscript for
important intellectual content. All authors read and approved the
final manuscript.
Ethics approval and consent to
participate
The study was approved by the Ethics Committee of
China-Japan Union Hospital of Jilin University. Signed written
informed consents were obtained from the patients and/or
guardians.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Boersma E, Mercado N, Poldermans D,
Gardien M, Vos J and Simoons ML: Acute myocardial infarction.
Lancet. 361:847–858. 2003.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Rumana N, Kita Y, Turin TC, Murakami Y,
Sugihara H, Morita Y, Tomioka N, Okayama A, Nakamura Y, Abbott RD
and Ueshima H: Trend of increase in the incidence of acute
myocardial infarction in a Japanese population: Takashima AMI
registry, 1990-2001. Am J Epidemiol. 167:1358–1364. 2008.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Ferrières J, Cambou JP, Ruidavets JB and
Pous J: Trends in acute myocardial infarction prognosis and
treatment in southwestern France between 1985 and 1990 (the MONICA
Project-Toulouse). Am J Cardiol. 75:1202–1205. 1995.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Anderson JL and Morrow DA: Acute
myocardial infarction. N Engl J Med. 376:2053–2064. 2017.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Açıkgöz Ş, Edebali N, Barut F, Can M,
Tekin İÖ, Büyükuysal Ç and Açıkgöz B: Ischemia modified albumin
increase indicating cardiac damage after experimental subarachnoid
hemorrhage. BMC Neurosci. 15(33)2014.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Tripodi A: D-dimer testing in laboratory
practice. Clin Chem. 57:1256–1262. 2011.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Andreescu AC, Cushman M and Rosendaal FR:
D-dimer as a risk factor for deep vein thrombosis: The Leiden
thrombophilia study. Thromb Haemost. 87:47–51. 2002.PubMed/NCBI
|
|
8
|
Lloyd CM, Minto AW, Dorf ME, Proudfoot A,
Wells TN, Salant DJ and Gutierrez-Ramos JC: RANTES and monocyte
chemoattractant protein-1 (MCP-1) play an important role in the
inflammatory phase of crescentic nephritis, but only MCP-1 is
involved in crescent formation and interstitial fibrosis. J Exp
Med. 185:1371–1380. 1997.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Ryan TJ, Antman EM, Brooks NH, Califf RM,
Hillis LD, Hiratzka LF, Rapaport E, Riegel B, Russell RO, Smith EE
III, et al: 1999 update: ACC/AHA guidelines for the management of
patients with acute myocardial infarction. A report of the American
college of cardiology/American heart association task force on
practice guidelines (committee on management of acute myocardial
infarction). J Am Coll Cardiol. 34:890–911. 1999.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Gong X, Zhou R and Li Q: Effects of
captopril and valsartan on ventricular remodeling and inflammatory
cytokines after interventional therapy for AMI. Exp Ther Med.
16:3579–3583. 2018.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Rubinfeld GD, Smilowitz NR, Berger JS and
Newman JD: Association of thrombocytopenia, revascularization, and
in-hospital outcomes in patients with acute myocardial infarction.
Am J Med. 132:942–948.e5. 2019.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Rasmussen MB, Stengaard C, Sørensen JT,
Riddervold IS, Hansen TM, Giebner M, Rasmussen CH, Bøtker HE and
Terkelsen CJ: Predictive value of routine point-of-care cardiac
troponin T measurement for prehospital diagnosis and
risk-stratification in patients with suspected acute myocardial
infarction. Eur Heart J Acute Cardiovasc Care. 8:299–308.
2019.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Gruber HE, Hoelscher GL, Ingram JA, Bethea
S, Cox M and Hanley EN Jr: Proinflammatory cytokines modulate the
chemokine CCL2 (MCP-1) in human annulus cells in vitro: CCL2
expression and production. Exp Mol Pathol. 98:102–105.
2015.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Niu J and Kolattukudy PE: Role of MCP-1 in
cardiovascular disease: Molecular mechanisms and clinical
implications. Clin Sci (Lond). 117:95–109. 2009.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Buitrago L, Zafar H, Zhang Y, Li J, Walz T
and Coller BS: Dominant role of αIIbβ3 in platelet interactions
with cross-linked fibrin fragment D-dimer. Blood Adv. 4:2939–2949.
2020.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Simes J, Robledo KP, White HD, Espinoza D,
Stewart RA, Sullivan DR, Zeller T, Hague W, Nestel PJ, Glasziou PP,
et al: D-dimer predicts long-term cause-specific mortality,
cardiovascular events, and cancer in patients with stable coronary
heart disease: LIPID Study: LIPID Study. Circulation. 138:712–723.
2018.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Shaw E, Massaro J, Levy D, O'Donnell CJ,
D'Agostino R and Tofler G: D-dimer and the risk of cardiovascular
disease: The Framingham heart study. Heart Lung Circ. 20 (Suppl
2)(S30)2011.
|
|
18
|
Kim KS, Shin SD, Song KJ, Suh GJ and Shin
S: Prognosis of patients with out-of-hospital cardiac arrest and
early biochemical markers: Ischemia modified albumin,
procalictonin, and S-100 protein. J Korean Soc Emerg Med.
17:281–290. 2006.
|
|
19
|
Sokhanvar S, Mellati AO, Mousavinasab SN,
Taran L, Vahdani B and Golmmohamadi Z: Ischemia-modified albumin
(IMA) in differential diagnosis of transient myocardial ischemia
from non ischemic chest pain. Bratisl Lek Listy. 113:612–615.
2012.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Zhong Y, Wang N and Xu H: The value of
ischaemia modified albumin in the diagnosis on coronary
atherosclerotic heart disease. Heart. 97 (Suppl
3)(A134)2011.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Zhang CL, Jiang YM, Gao X and Wang X:
Clinical value of h-FABP, hs-CRP, cTnT examination to diagnose
acute myocardial infarction. J Dalian Med Univ. 30:170–172.
2008.
|
|
22
|
Devaki RN, Suma MN, Gowdappa HB, et al:
HS-CRP levels in myocardial infarction patients in relation to
cardiac markers, 2011.
|
|
23
|
Keller T, Zeller T, Peetz D, Tzikas S,
Roth A, Czyz E, Bickel C, Baldus S, Warnholtz A, Fröhlich M, et al:
Sensitive troponin I assay in early diagnosis of acute myocardial
infarction. N Engl J Med. 361:868–877. 2009.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Chan D and Ng LL: Biomarkers in acute
myocardial infarction. BMC Med. 8(34)2010.PubMed/NCBI View Article : Google Scholar
|