Introduction
Rheumatoid arthritis (RA), a common systemic
autoimmune disease, is primarily characterized by chronic
inflammation and cell infiltration in synovial tissues, which
contribute to the destruction or loss of cartilage and bone
(1-4).
Although several risk factors, including genetic factors, viral
infection and sex hormones, have been reported to be strongly
associated with the occurrence of RA, the etiology of RA is not
completely understood (5,6). During the progression of RA, various
inflammatory cells are activated by a series of cytokines and
chemokines or via cell-cell contact (7). Fibroblast-like synoviocytes (FLSs), a
type of inflammatory cell, are highly enriched in the synovial
membrane (8). Additionally, FLSs
have been widely reported to serve a critical role in the
initiation and development of RA (9). Moreover, FLSs secrete a number of
proinflammatory cytokines, such as TNF-α, IL-1β and IL-6, resulting
in aggravation of chronic inflammation (10). Therefore, further studies
investigating the molecular mechanism underlying inflammation in RA
are required.
MicroRNAs (miRNAs/miRs), a subgroup of non-coding
RNAs, are short and single-stranded RNAs (11), which have been reported to exert an
important impact on a broad spectrum of cellular processes,
including cell proliferation, differentiation, inflammation and
fibrosis in various diseases (12,13).
For example, hematopoietic miR-126 inhibits fibrosis in renal
ischemia/reperfusion injury via regulating C-X-C motif chemokine
receptor 4(14). In addition, it
has been reported that miR-21 genetic ablation promotes fibrosis
and inflammation to aggravate cardiac remodeling and dysfunction in
hypertension (15). Furthermore,
the methylation of miR-495 promoter activates NOD-like receptor
protein 3 inflammasome to facilitate acute lung injury progression
via promoting inflammation (16).
It has been reported that miR-23 overexpression induces trophoblast
cell apoptosis by binding to X-linked inhibitor of apoptosis
(17). In addition, miR-23 promotes
myelination in the central nervous system (18). Moreover, it has been demonstrated
that miR-23 is downregulated in patients with RA who are treated
with anti-TNF-α, indicating that miR-23 may serve as a potential
biomarker for RA therapy (19).
However, the biological functions and regulatory network of miR-23
in RA are not completely understood.
Furthermore, the regulation of inflammation in
diverse diseases via chemokine C-X-C motif ligand 12 (CXCL12) has
been widely reported (20,21). For example, CXCL12 promotes
α-synuclein-triggered neuroinflammation in Parkinson's disease
(22). Additionally,
tissue-infiltrating inflammatory cells highly express CXCL12,
serving a significant role in chronic periaortitis (23). A previous study demonstrated that
CXCL12 downregulation in synovial fibroblasts via DNA demethylation
inhibited RA development (24).
Nevertheless, the mechanism underlying the miR-23/CXCR12 axis in RA
is not completely understood.
The NF-κB signaling pathway is involved in the
regulation of inflammation (25-27).
However, the association between miR-23 or CXCL12 and NF-κB
signaling requires further investigation.
In conclusion, the present study investigated the
function and mechanism underlying miR-23 in RA, with the aim to
develop a novel diagnostic and therapeutic strategy for the
treatment of RA.
Materials and methods
Samples of synovial tissue and
serum
Synovial (n=22) and healthy (n=22) tissue samples
were obtained from patients with RA (10 males and 12 females;
average age, 51 years old) and healthy volunteers (9 males and 13
females; average age, 47 years old) at The Second Changzhou
People's Hospital Affiliated to Nanjing Medical University from
March 2016 to April 2018. Following surgery, samples were
immediately stored at -80˚C. In addition, blood samples were
collected from patients with RA (n=22) and healthy volunteers
(n=22). Written informed consent was obtained from patients and
volunteers prior to surgery. The present study was approved by the
Ethics Committee of The Second Changzhou People's Hospital
Affiliated to Nanjing Medical University.
FLSs
FLSs were isolated from synovial tissues. Briefly,
the synovial tissues were sectioned and digested with 0.25% trypsin
to isolate the synoviocytes at 37˚C for 30 min. Following culturing
overnight in DMEM supplemented with 10% FBS as well as penicillin
(100 IU/ml) and streptomycin (100 µg/ml) at 37˚C, the non-adherent
cells were removed and the adherent cells were cultured in DMEM
(Thermo Fisher Scientific, Inc.) containing 10% FBS and 1% mixture
of penicillin and streptomycin at 37˚C in a humidified atmosphere
containing 5% CO2. Cells were collected and used for
subsequent assays at passage 4-8. FLSs were stimulated with 10
µg/ml lipopolysaccaride (LPS; Sigma-Aldrich; Merck KGaA) for 24 h
at 37˚C to establish the RA model.
Cell transfection
miR-23 mimic (5'-AAACCGUUAGGGGUUC-3'), miR-23
inhibitor (5'-GCUGUCAUUCGUUAUC-3') and their corresponding negative
controls (NCs; NC mimic; 5'-AAGUGGCAACGAACCG-3'; NC inhibitor;
5'-AUGCACUUAGUAAUGA-3') were synthesized by Shanghai GenePharma
Co., Ltd. Small interfering (si)RNAs targeting CXCL12 (siCXCL12;
5'-CGAGGGCGAGCAUGCGUGUUGAUUG-3') and inhibitor κBα (IκBα; siIκBα;
5'-GCUAGGUAAAUCGGUUGGGUCGUGA-3') were obtained from Shanghai
GenePharma Co., Ltd., were used to knockdown CXCL12 and IκBα,
respectively, and siNC (5'-UCAAGUCCAGCACGACAUUG-3') was used as the
negative control. The full length CXCL12 gene was inserted into the
pcDNA3.1 vector (Shanghai GenePharma Co., Ltd.) to overexpress
CXCL12, whereas the empty pcDNA3.1 vector served as a control. FLSs
(5x104) were transfected with 50 nM vectors using
Lipofectamine® 3000 (Invitrogen; Thermo Fisher
Scientific, Inc.) for 48 h prior to subsequent assays.
Reverse transcription-quantitative PCR
(RT-qPCR)
Total RNA was isolated from cultured FLSs, serum or
synovial tissues using an Eastep Super Total RNA Extraction kit
(Promega Corporation). Total RNA was reverse transcribed into cDNA
using the Perfect Real Time RT Reagent kit (Takara Bio, Inc.) at
37˚C for 15 min for the measurement of CXCL12 and IκBα expression
levels according to the manufacturer's instructions. Additionally,
total RNA was reverse transcribed into cDNA using the TransScript
Green miRNA RT SuperMix (Beijing Transgen Biotech Co., Ltd.) for
the measurement of miR-23 expression levels. Subsequently, qPCR was
performed using the SYBR-Green PCR Master mix kit or the TaqMan
miRNA assay kit on a 7900HT Fast Real-Time system (all from Applied
Biosystems; Thermo Fisher Scientific, Inc.). The thermocycling
conditions were as follows: Initial denaturation at 95˚C for 3 min,
followed by 40 cycles of denaturation at 95˚C for 30 sec, annealing
at 60˚C for 30 sec and extension at 72˚C for 20 sec, followed by a
final extension at 72˚C for 5 min. miRNA and mRNA expression levels
were quantified using the 2-ΔΔCq method (28) and normalized to the internal
reference genes U6 and GAPDH, respectively. Primer sequences used
for RT-qPCR analysis were as follows: miR-23 forward,
5'-CCTACTGTCGTCCCAAGACCT-3' and reverse, 5'-GGGGCTCGTGCAGAAGAAT-3';
and CXCL12 forward, 5'-TCCAAACTGTGCCCTTCA-3' and reverse,
5'-CTCTTCTTCTGTCGCTTCTT-3'; and IκBα forward,
5'-TGGCCAGTGTAGCAGTCTTG-3' and reverse, 5'-GACATCAGCACCCAAAGTCA-3';
and GAPDH forward, 5'-GCTGGCGCTGAGTACGTCGT-3' and reverse,
5'-ACGTTGGCAGTGGGGACACG-3'; and U6 forward, 5'-CTCGCTTCGGCAGCACA-3'
and reverse 5'-AACGCTTCACGAATTTGCGT-3'.
Western blotting
Total protein was extracted from FLSs using RIPA
buffer (Beyotime Institute of Biotechnology) and protein
concentrations were measured using the BCA protein assay kit
(Beyotime Institute of Biotechnology). Equal quantities (15
µg/lane) of protein were separated via 10% SDS-PAGE and transferred
onto PVDF membranes (EMD Millipore). Following blocking with 5%
skimmed milk for 2 h at room temperature, membranes were incubated
overnight at 4˚C with the following primary antibodies:
Anti-phosphorylated (p)-p65 (1:1,000; cat. no. 3033; Cell Signaling
Technology, Inc.), anti-IκBα (1:1,000; cat. no. 4812; Cell
Signaling Technology, Inc.) and anti-GAPDH (1:1,000; cat. no. 5174;
Cell Signaling Technology, Inc.). Subsequently, the membranes were
incubated with horseradish peroxidase-conjugated goat anti-rabbit
(1:3,000; cat. no. GB23303; Wuhan Servicebio Technology Co., Ltd.)
secondary antibody in the dark at room temperature for 1 h. Protein
bands were visualized using an ECL reagent (EMD Millipore). GAPDH
was used as the loading control. Protein expression was quantified
using Image-Pro Plus software (version 6.0; Media Cybernetics,
Inc.).
Luciferase reporter assay
StarBase V2.0 (http://starbase.sysu.edu.cn/index.php) was used to
validate the putative bindings between miR-23 and CXCL12
3'-untranslated region (3'-UTR). The wild-type (WT) or mutant (Mut)
binding sequences of CXCL12 3'-UTR were cloned into the pmirGLO
vector (Promega Corporation). 293T cells (ATCC; 5x104)
were co-transfected with 50 nM miR-23 mimic, miR-23 inhibitor, NC
mimic or NC inhibitor and CXCL12-WT or CXCL12-Mut using
Lipofectamine® 2000 (Thermo Fisher Scientific, Inc.)
according to manufacturer's instructions. At 48 h
post-transfection, luciferase activities were detected using the
Dual-Luciferase Reporter assay system (Promega Corporation)
according to the manufacturer's instructions. Firefly luciferase
activity was normalized to Renilla (Promega
Corporation).
ELISA
Cell culture media from cultured FLSs was harvested
and stored at -80˚C. Subsequently, The supernatants were collected
by centrifugation at 1,000 x g for 5 min at 4˚C, and the secretory
levels of TNF-α, IL-1β and IL-8 in the supernatants of cultured
FLSs were measured using a Duoset ELISA kit (cat. no. dy206;
R&D Systems, Inc.) according to the manufacturer's
instructions.
Statistical analysis
Data are presented as the mean ± standard deviation
of three independent experimental repeats. Statistical analyses
were performed using SPSS 17.0 (SPSS, Inc.) and GraphPad Prism
(version 5; GraphPad Software, Inc.) software. Comparisons between
two groups were analyzed using the paired or unpaired Student's
t-test. Comparisons among multiple groups were analyzed using
one-way ANOVA followed by Tukey's post hoc test. P<0.05 was
considered to indicate a statistically significant difference.
Results
miR-23 is downregulated and CXCL12 is
upregulated in RA
To determine the expression levels of miR-23 and
CXCL12 in RA, serum and synovial tissues were collected from
patients with RA and healthy volunteers, and RT-qPCR was performed.
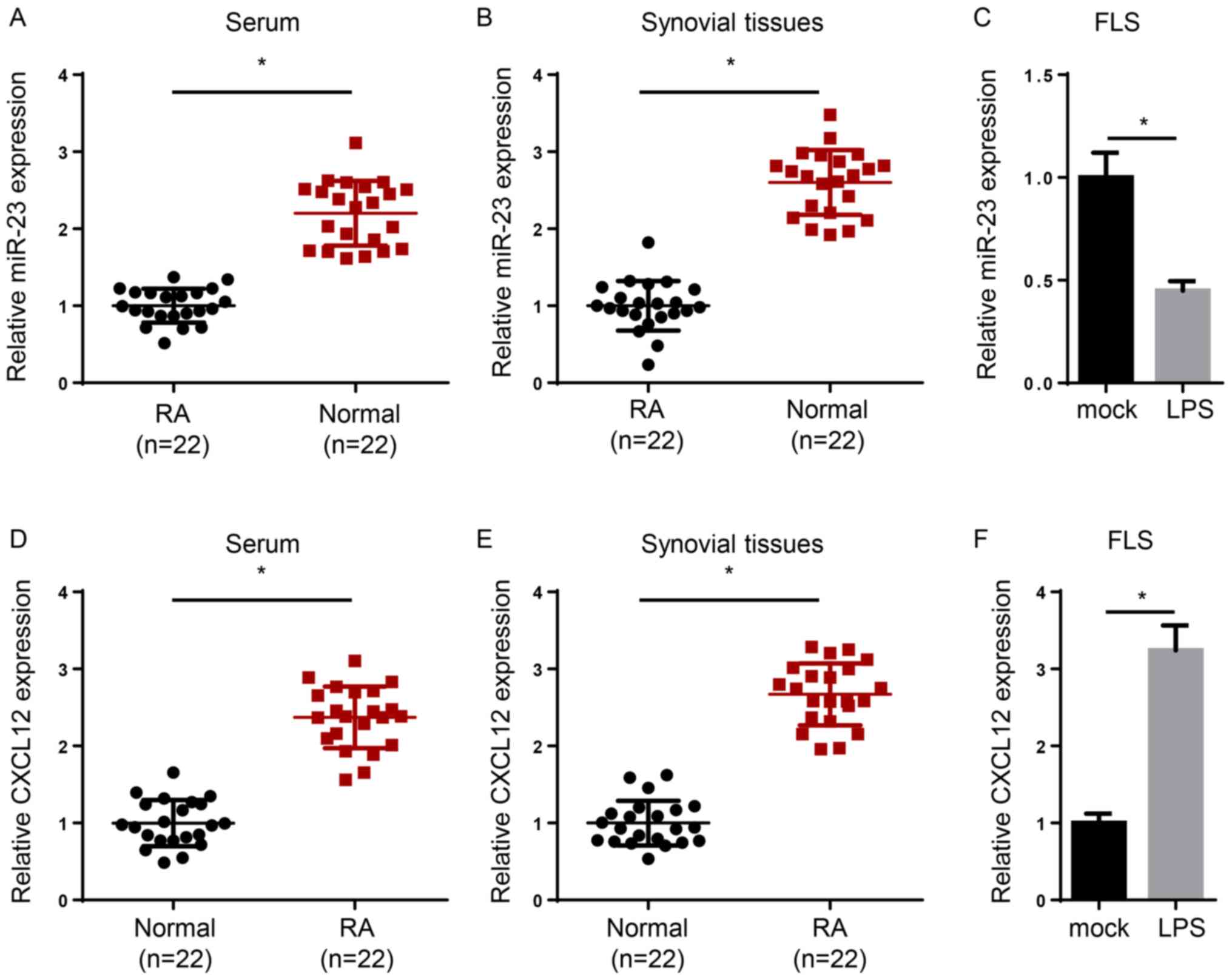
The expression levels of miR-23 were significantly reduced in the
serum and synovial tissues of patients with RA compared with
healthy samples (Fig. 1A and
B). In addition, induction of FLSs
with lipopolysaccharide (LPS) significantly decreased miR-23
expression levels compared with the mock group (Fig. 1C). By contrast, CXCL12 expression
levels were significantly increased in the serum or synovial
tissues of patients with RA compared with healthy controls
(Fig. 1D and E). Moreover, CXCL12 expression levels were
significantly increased in LPS-treated FLSs compared with the mock
group (Fig. 1F). Overall, the
results indicated that miR-23 was downregulated and CXCL12 was
upregulated in RA.
miR-23 suppresses inflammation and
binds to the 3'-UTR of CXCL12
Several miRNAs have been associated with the
inhibition of RA progression (29).
In addition, miR-23 is upregulated following
anti-TNF-α/disease-modifying antirheumatic drugs combination
therapy, thus suggesting that miR-23 may serve as a potential
biomarker for RA treatment (19).
However, the biological functions and molecular mechanism
underlying miR-23 in RA are not completely understood. In the
present study, a series of experiments were designed to investigate
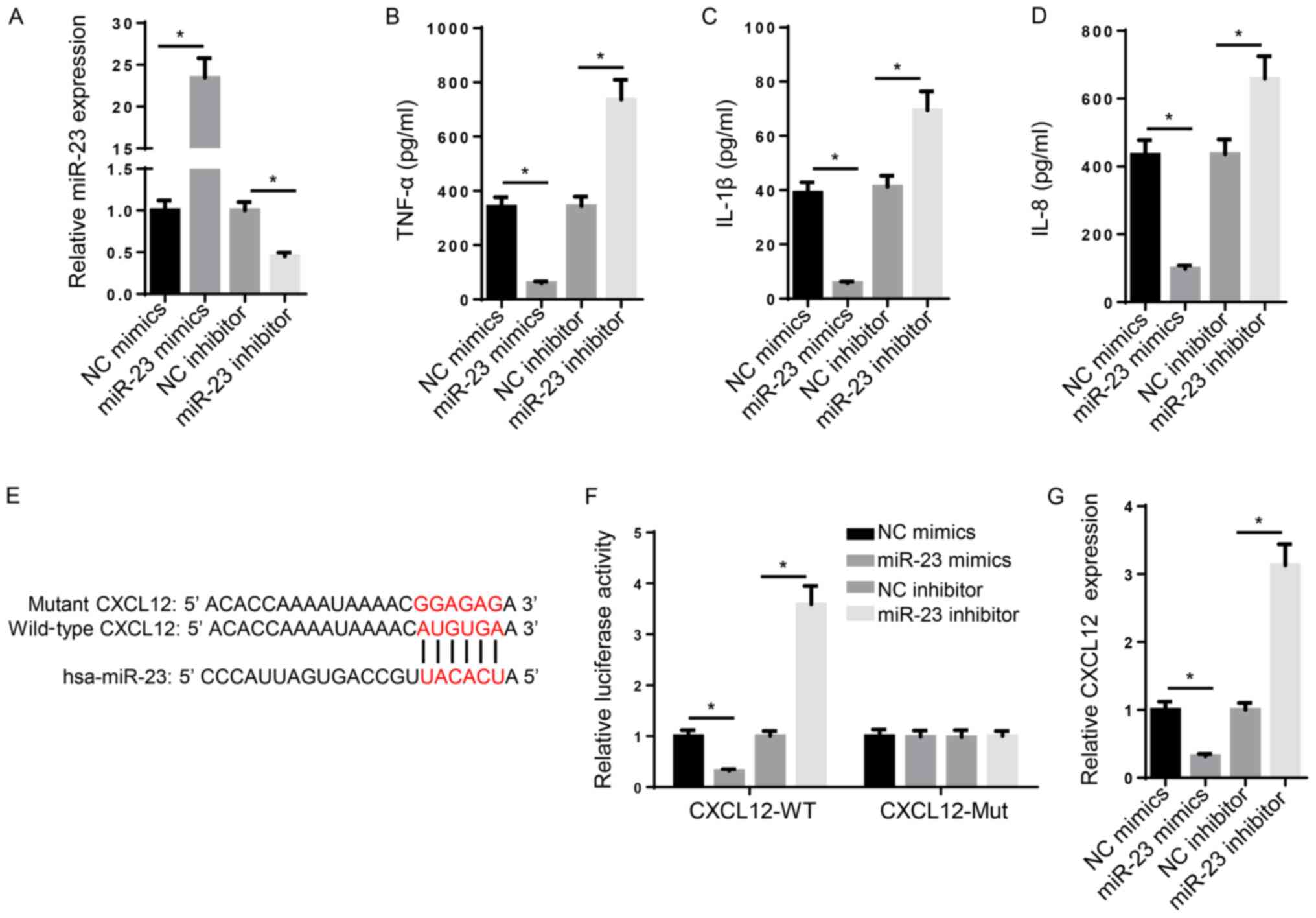
the role of miR-23 in RA. Compared with NC mimic and NC inhibitor,
miR-23 mimic and miR-23 inhibitor significantly increased and
decreased the expression levels of miR-23, respectively (Fig. 2A). Subsequently, the effect of
miR-23 on inflammation was investigated. TNF-α, IL-1β and IL-8 are
typical markers of inflammation (30). The ELISA results suggested that the
levels of TNF-α, IL-1β and IL-8 were significantly increased in
miR-23 inhibitor-transfected FLSs compared with the NC inhibitor
group, whereas miR-23 mimic significantly decreased the secretory
levels of inflammatory markers compared with the NC mimic group
(Fig. 2B-D). Furthermore, the
mechanism underlying the effects of miR-23 on RA progression was
investigated. Increasing evidence has suggested that miRNAs
regulate mRNA expression at the post-transcriptional level via
interacting with the 3'-UTR of the target mRNA (31). The binding sequence between miR-23
and CXCL12 3'-UTR (3280-3287 bp) was predicted using StarBase
(http://starbase.sysu.edu.cn/) (Fig. 2E). Furthermore, the luciferase
reporter assay indicated that miR-23 mimic significantly decreased
the luciferase activity of CXCL12-WT compared with NC mimic,
whereas miR-23 inhibitor displayed the opposite effect compared
with NC inhibitor. By contrast, the luciferase activity of
CXCL12-Mut was not significantly altered by miR-23 mimic or
inhibitor compared with NC mimic and inhibitor, respectively
(Fig. 2F). Similarly, the RT-qPCR
results suggested that miR-23 mimic and miR-23 inhibitor
significantly decreased and increased CXCL12 mRNA expression levels
compared with NC mimic and NC inhibitor, respectively (Fig. 2G). Collectively, the aforementioned
results suggested that miR-23 suppressed inflammation and bound to
the 3'-UTR of CXCL12.
CXCL12 overexpression reverses the
inhibitory effect of miR-23 on inflammatory cytokine
expression
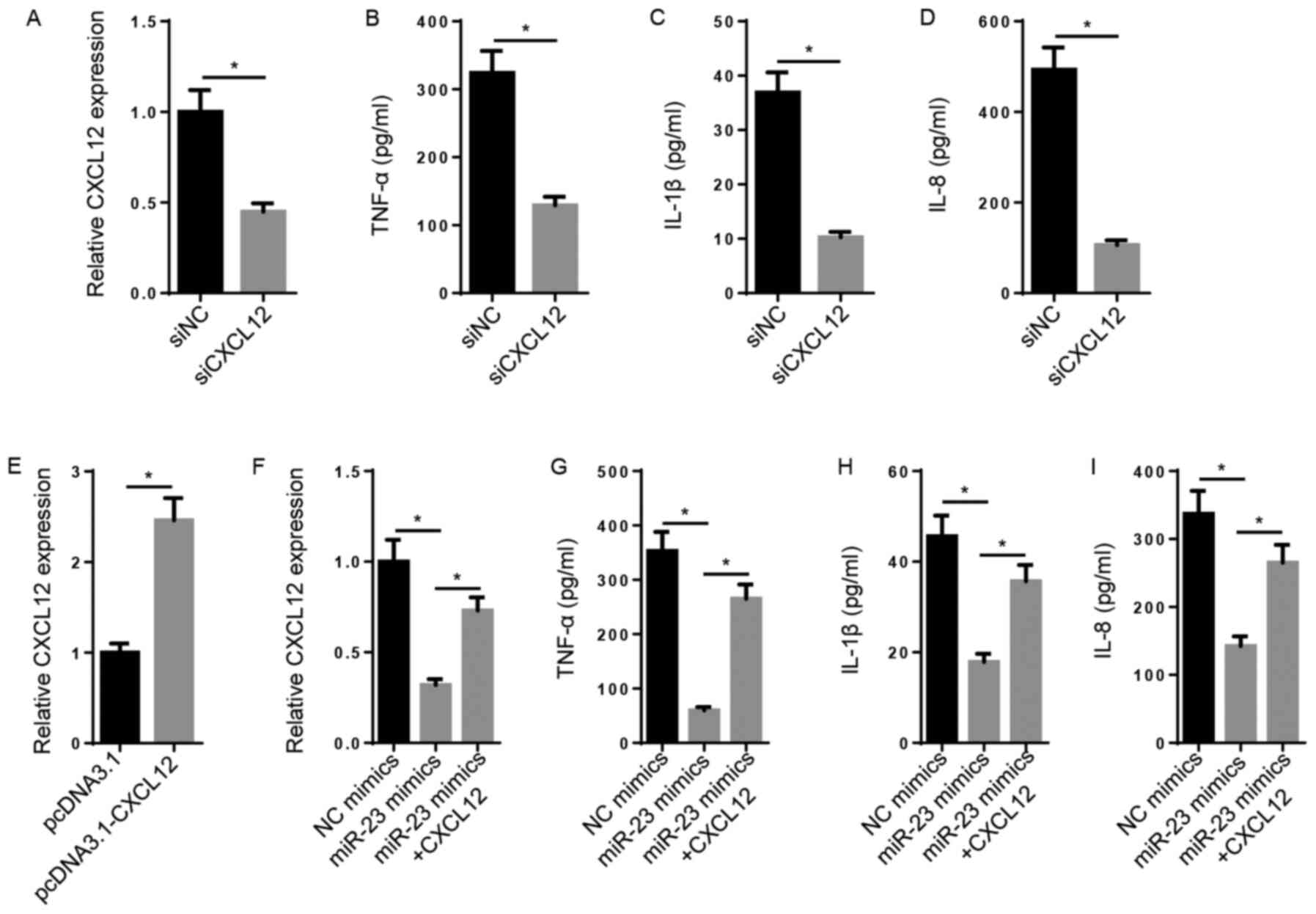
Subsequently, the biological function of CXCL12 in
RA was explored. The results indicated that the levels of CXCL12,
TNF-α, IL-1β and IL-8 were significantly decreased in the siCXCL12
group compared with the siNC group (Fig. 3A-D). CXCL12 expression levels were
significantly increased in pcDNA3.1-CXCL12-transfected FLSs
compared with pcDNA3.1-transfected FLSs (Fig. 3E). Furthermore, miR-23
mimic-mediated decreased levels of CXCL12 mRNA were significantly
reversed following CXCL12 overexpression (Fig. 3F). Likewise, the inhibitory effect
of miR-23 overexpression on TNF-α, IL-1β and IL-8 levels was
significantly reversed by CXCL12 overexpression (Fig. 3G-I). The results demonstrated that
miR-23 inhibited inflammation via regulating CXCL12 expression.
NF-κB signaling is crucial for
miR-23-mediated regulation of inflammatory cytokine expression
It has been reported that NF-κB signaling, an
inflammation-associated signaling pathway, may be activated by
CXCL12 via the CXCL12/CXCR7 axis (32). CXCL12 knockdown decreased the
protein expression levels of p-p65 and increased the protein
expression levels of IκBα compared with the siNC group (Fig. 4A). The transfection efficacy assay
indicated that the expression of IκBα was significantly decreased
in siIκBα-transfected FLSs compared with siNC-transfected FLSs
(Fig. 4B). The RT-qPCR and western
blotting results indicated that the mRNA and protein expression
levels of IκBα were markedly increased by miR-23 mimic compared
with NC mimic, but decreased following co-transfection with siIκBα
(Fig. 4C and D). Furthermore, the suppressive effects of
miR-23 mimic on TNF-α, IL-1β and IL-8 secretory levels were
significantly reversed by co-transfection with siIκBα (Fig. 4E-G). In conclusion, the results
suggested that CXCL12 promoted inflammation by activating NF-κB
signaling.
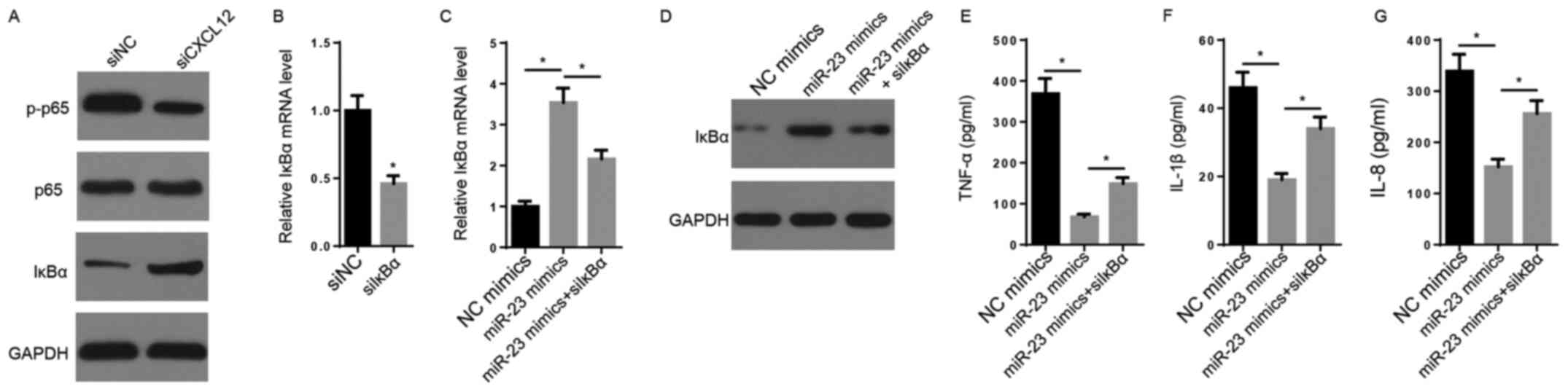 | Figure 4NF-κB signaling is crucial for
miR-23-mediated regulation of inflammatory cytokine expression. (A)
The effects of CXCL12 knockdown on the protein expression levels of
p65, p-p65 and IκBα were measured via western blotting. (B) IκBα
expression levels following transfection with siIκBα and siNC were
measured via RT-qPCR. IκBα (C) mRNA and (D) protein expression
levels were measured via RT-qPCR and western blotting,
respectively. ELISAs were performed to measure the secretory levels
of (E) TNF-α, (F) IL-1β and (G) IL-8. *P<0.05. miR,
microRNA; CXCL12, chemokine C-X-C motif ligand 12; p,
phosphorylated; IκBα, inhibitor κBα; si, small interfering RNA; NC,
negative control; RT-qPCR, reverse transcription-quantitative
PCR. |
Discussion
RA impacts the quality of life of patients (33). Modern advanced therapies, including
surgical resection, medical treatment and other complementary
therapies, have been applied for the treatment of RA (34). However, the treatment of RA is
challenging due to inflammatory cell infiltration and bone
destruction (35).
Emerging evidence has demonstrated that miRNA
expression is dysregulated in FLSs or synovial tissues of patients
with RA, which is potentially associated with the pathology of RA
(36). Although miRNAs do not
encode protein products, they may modulate gene expression at the
post-transcriptional level via interacting with the 3'-UTR of
target mRNAs (29,37). For instance, miR-338-5p promotes
cell viability, proliferation and migration of RA-FLSs via
targeting nuclear factor of activated T cells 5(38). In addition, miR-20a regulates the
secretion of IL-6, IL-1β and TNF-α in FLSs via targeting apoptosis
signal-regulating kinase 1(39).
Furthermore, it has been previously demonstrated that miR-23 is
downregulated in patients with RA who are treated with anti-TNF-α
(19). Therefore, the present study
further explored the function and mechanism underlying miR-23 in
RA. The results suggested that miR-23 negatively regulated the
expression of IL-8, IL-1β and TNF-α in FLSs, indicating that miR-23
served as an anti-inflammatory factor in FLSs. On the basis of the
underlying mechanism, it was predicted that miR-23 directly
targeted CXCL12 3'-UTR.
CXCL12 has been widely reported to regulate
inflammatory cell infiltration. For example, high mobility group
box 1 induces tissue damage by recruiting inflammatory cells via
forming a complex with CXCL12(40).
Furthermore, it has been demonstrated that CXCL12 accumulation
results in an increase in matrix metalloproteinases, resulting in
the perpetuation of RA (41).
However, the present study focused on the effect of CXCL12 on
inflammation. The results suggested that CXCL12 knockdown reduced
inflammation, whereas CXCL12 overexpression reversed the
suppressive effect of miR-23 mimic on inflammation.
NF-κB signaling is closely associated with
inflammatory cell infiltration (42-44).
On the basis of the underlying mechanism, phosphorylation of NF-κB
(p65) and downregulation of IκBα may activate NF-κB signaling
(45). Therefore, it was
hypothesized that CXCL12 induces inflammation via NF-κB signaling.
The present study indicated that CXCL12 knockdown increased IκBα
and decreased p-p65 expression levels compared with siNC, resulting
in inactivation of the NF-κB signaling pathway. Furthermore, rescue
assays in FLSs indicated that IκBα knockdown significantly reversed
miR-23 mimic-mediated downregulation of the inflammatory process.
Overall, the results suggested that miR-23 was downregulated and
CXCL12 was upregulated in RA.
Collectively, the present study suggested that
miR-23 inhibited inflammation to alleviate RA by regulating CXCL12
via the NF-κB signaling pathway. The novel mechanism identified in
the present study may provide a potential target for RA treatment.
However, the present study had a number of limitations. Firstly, an
animal model was not established, therefore the role of miR-23
in vivo requires further investigation. Secondly, although
miR-23 may serve a role in cell proliferation, migration and
apoptosis in RA, the present study only focused on inflammation.
Finally, as RA pathology is complex, future studies should
investigate further miR-23-mediated mechanisms.
Acknowledgements
Not applicable.
Funding
This study was supported by Changzhou Sci&Tech Program
(grant no. CJ20200101).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
BG and XC designed the present study. GS, YW, YG and
LZ performed all the experiments, analyzed the data and prepared
the figures. BG drafted the initial manuscript. XC reviewed and
revised the manuscript. All authors read and approved the final
version of the manuscript.
Ethics approval and consent to
participate
Written informed consent was obtained from patients
and volunteers prior to surgery. The present study was approved by
the Ethics Committee of The Second Changzhou People's Hospital
Affiliated to Nanjing Medical University.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Šenolt L: Rheumatoid arthritis. Vnitr Lek.
64:98–106. 2018.PubMed/NCBI(In Czech).
|
|
2
|
Wasserman A: Rheumatoid arthritis: Common
questions about diagnosis and management. Am Fam Physician.
97:455–462. 2018.PubMed/NCBI
|
|
3
|
Katz P: Causes and consequences of fatigue
in rheumatoid arthritis. Curr Opin Rheumatol. 29:269–276.
2017.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Baum R and Gravallese EM: Bone as a target
organ in rheumatic disease: Impact on osteoclasts and osteoblasts.
Clin Rev Allergy Immunol. 51:1–15. 2016.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Burmester GR and Pope JE: Novel treatment
strategies in rheumatoid arthritis. Lancet. 389:2338–2348.
2017.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Mellado M, Martínez-Muñoz L, Cascio G,
Lucas P, Pablos JL and Rodríguez-Frade JG: T cell migration in
rheumatoid arthritis. Front Immunol. 6(384)2015.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Hoxha M: A systematic review on the role
of eicosanoid pathways in rheumatoid arthritis. Adv Med Sci.
63:22–29. 2018.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Bustamante MF, Garcia-Carbonell R,
Whisenant KD and Guma M: Fibroblast-like synoviocyte metabolism in
the pathogenesis of rheumatoid arthritis. Arthritis Res Ther.
19(110)2017.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Doody KM, Bottini N and Firestein GS:
Epigenetic alterations in rheumatoid arthritis fibroblast-like
synoviocytes. Epigenomics. 9:479–492. 2017.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Li S, Zhang T, Xu W, Ding J, Yin F, Xu J,
Sun W, Wang H, Sun M, Cai Z and Hua Y: Sarcoma-targeting
peptide-decorated polypeptide nanogel intracellularly delivers
shikonin for upregulated osteosarcoma necroptosis and diminished
pulmonary metastasis. Theranostics. 8:1361–1375. 2018.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Lu TX and Rothenberg ME: MicroRNA. J
Allergy Clin Immunol. 141:1202–1207. 2018.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Vishnoi A and Rani S: MiRNA biogenesis and
regulation of diseases: An overview. Methods Mol Biol. 1509:1–10.
2017.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Rupaimoole R and Slack FJ: MicroRNA
therapeutics: Towards a new era for the management of cancer and
other diseases. Nat Rev Drug Discov. 16:203–222. 2017.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Bijkerk R, van Solingen C, de Boer HC, van
der Pol P, Khairoun M, de Bruin RG, van Oeveren-Rietdijk AM,
Lievers E, Schlagwein N, van Gijlswijk DJ, et al: Hematopoietic
microRNA-126 protects against renal ischemia/reperfusion injury by
promoting vascular integrity. J Am Soc Nephrol. 25:1710–1722.
2014.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Liu X, Zhang C, Wang C, Sun J, Wang D,
Zhao Y and Xu X: miR-210 promotes human osteosarcoma cell migration
and invasion by targeting FGFRL1. Oncol Lett. 16:2229–2236.
2018.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Ying Y, Mao Y and Yao M: NLRP3
inflammasome activation by microRNA-495 promoter methylation may
contribute to the progression of acute lung injury. Mol Ther
Nucleic Acids. 18:801–814. 2019.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Li L, Hou A, Gao X, Zhang J, Zhang L, Wang
J, Li H and Song Y: Lentivirus-mediated miR-23a overexpression
induces trophoblast cell apoptosis through inhibiting X-linked
inhibitor of apoptosis. Biomed Pharmacother. 94:412–417.
2017.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Lin ST, Huang Y, Zhang L, Heng MY, Ptácek
LJ and Fu YH: MicroRNA-23a promotes myelination in the central
nervous system. Proc Natl Acad Sci USA. 110:17468–17473.
2013.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Castro-Villegas C, Pérez-Sánchez C,
Escudero A, Filipescu I, Verdu M, Ruiz-Limón P, Aguirre MA,
Jiménez-Gomez Y, Font P, Rodriguez-Ariza A, et al: Circulating
miRNAs as potential biomarkers of therapy effectiveness in
rheumatoid arthritis patients treated with anti-TNFα. Arthritis Res
Ther. 17(49)2015.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Janssens R, Struyf S and Proost P: The
unique structural and functional features of CXCL12. Cell Mol
Immunol. 15:299–311. 2018.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Yang L, Wang M, Guo YY, Sun T, Li YJ, Yang
Q, Zhang K, Liu SB, Zhao MG and Wu YM: Systemic inflammation
induces anxiety disorder through CXCL12/CXCR4 pathway. Brain Behav
Immun. 56:352–362. 2016.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Xu J, Wang H, Hu Y, Zhang YS, Wen L, Yin
F, Wang Z, Zhang Y, Li S, Miao Y, et al: Inhibition of CaMKIIα
activity enhances antitumor effect of fullerene C60 nanocrystals by
suppression of autophagic degradation. Adv Sci (Weinh).
6(1801233)2019.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Nicastro M, Vescovini R, Maritati F,
Palmisano A, Urban ML, Incerti M, Fenaroli P, Peyronel F, Benigno
GD, Mangieri D, et al: Fibrocytes in chronic periaortitis: A novel
mechanism linking inflammation and fibrosis. Arthritis Rheumatol.
71:1913–1922. 2019.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Karouzakis E, Rengel Y, Jüngel A, Kolling
C, Gay RE, Michel BA, Tak PP, Gay S, Neidhart M and Ospelt C: DNA
methylation regulates the expression of CXCL12 in rheumatoid
arthritis synovial fibroblasts. Genes Immun. 12:643–652.
2011.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Sun SC: The non-canonical NF-κB pathway in
immunity and inflammation. Nat Rev Immunol. 17:545–558.
2017.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Afonina IS, Zhong Z, Karin M and Beyaert
R: Limiting inflammation-the negative regulation of NF-κB and the
NLRP3 inflammasome. Nat Immunol. 18:861–869. 2017.PubMed/NCBI View
Article : Google Scholar
|
|
27
|
Novack DV: Role of NF-κB in the skeleton.
Cell Res. 21:169–182. 2011.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408.
2001.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Moran-Moguel MC, Petarra-Del Rio S,
Mayorquin-Galvan EE and Zavala-Cerna MG: Rheumatoid arthritis and
miRNAs: A critical review through a functional view. J Immunol Res.
2018(2474529)2018.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Hegemann N, Wondimu A, Ullrich K and
Schmidt MF: Synovial MMP-3 and TIMP-1 levels and their correlation
with cytokine expression in canine rheumatoid arthritis. Vet
Immunol Immunopathol. 91:199–204. 2003.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Huang Y: The novel regulatory role of
lncRNA-miRNA-mRNA axis in cardiovascular diseases. J Cell Mol Med.
22:5768–5775. 2018.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Liao YX, Zhou CH, Zeng H, Zuo DQ, Wang ZY,
Yin F, Hua YQ and Cai ZD: The role of the CXCL12-CXCR4/CXCR7 axis
in the progression and metastasis of bone sarcomas (review). Int J
Mol Med. 32:1239–1246. 2013.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Coskun Benlidayi I: Sleep impairment: An
obstacle to achieve optimal quality of life in rheumatoid
arthritis. Rheumatol Int. 38:2183–2192. 2018.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Wasserman AM: Diagnosis and management of
rheumatoid arthritis. Am Fam Physician. 84:1245–1252.
2011.PubMed/NCBI
|
|
35
|
Chinese Rheumatology Association. 2018
Chinese guideline for the diagnosis and treatment of rheumatoid
arthritis. Zhonghua Nei Ke Za Zhi. 57:242–251. 2018.PubMed/NCBI View Article : Google Scholar : (In Chinese).
|
|
36
|
Salehi E, Eftekhari R, Oraei M, Gharib A
and Bidad K: MicroRNAs in rheumatoid arthritis. Clin Rheumatol.
34:615–628. 2015.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Song YJ, Li G, He JH, Guo Y and Yang L:
Bioinformatics-based identification of microRNA-regulated and
rheumatoid arthritis-associated genes. PLoS One.
10(e0137551)2015.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Guo T, Ding H, Jiang H, Bao N, Zhou L and
Zhao J: miR-338-5p regulates the viability, proliferation,
apoptosis and migration of rheumatoid arthritis fibroblast-like
synoviocytes by targeting NFAT5. Cell Physiol Biochem. 49:899–910.
2018.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Philippe L, Alsaleh G, Pichot A, Ostermann
E, Zuber G, Frisch B, Sibilia J, Pfeffer S, Bahram S, Wachsmann D
and Georgel P: MiR-20a regulates ASK1 expression and TLR4-dependent
cytokine release in rheumatoid fibroblast-like synoviocytes. Ann
Rheum Dis. 72:1071–1079. 2013.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Cecchinato V, D'Agostino G, Raeli L,
Nerviani A, Schiraldi M, Danelon G, Manzo A, Thelen M, Ciurea A,
Bianchi ME, et al: Redox-mediated mechanisms fuel monocyte
responses to CXCL12/HMGB1 in active rheumatoid arthritis. Front
Immunol. 9(2118)2018.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Wu S, Zheng Q, Xing X, Dong Y, Wang Y, You
Y, Chen R, Hu C, Chen J, Gao D, et al: Matrix stiffness-upregulated
LOXL2 promotes fibronectin production, MMP9 and CXCL12 expression
and BMDCs recruitment to assist pre-metastatic niche formation. J
Exp Clin Cancer Res. 37(99)2018.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Castejón ML, Rosillo MA, Montoya T,
González-Benjumea A, Fernández-Bolaños JG and Alarcón-de-la-Lastra
C: Oleuropein down-regulated IL-1β-induced inflammation and
oxidative stress in human synovial fibroblast cell line SW982. Food
Funct. 8:1890–1898. 2017.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Zhang N, Liu Z, Luo H, Wu W, Nie K, Cai L,
Tan S, Chen X, Huang Y, Liu J, et al: FM0807 decelerates
experimental arthritis progression by inhibiting inflammatory
responses and joint destruction via modulating NF-κB and MAPK
pathways. Biosci Rep. 39(BSR20182263)2019.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Yao Z, Xing L and Boyce BF: NF-κB p100
limits TNF-induced bone resorption in mice by a TRAF3-dependent
mechanism. J Clin Invest. 119:3024–3034. 2009.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Noort AR, Tak PP and Tas SW: Non-canonical
NF-κB signaling in rheumatoid arthritis: Dr Jekyll and Mr Hyde?
Arthritis Res Ther. 17(15)2015.PubMed/NCBI View Article : Google Scholar
|