Introduction
Systemic lupus erythematosus (SLE) is an autoimmune
disease with a wide spectrum of clinical manifestations and a
prevalence of 20-50 persons per 100,000 population. The
pathogenesis of SLE is related to a disruption of immune
homeostasis, resulting from loss of self-tolerance, presence of
autoantibodies and formation of immune complexes, and dysregulation
of autoreactive lymphocytes, which are responsible for damage to
various systemic organs (1,2). Especially, the failure of regulatory T
cells (Treg)-mediated suppression is considered as a factor
implicated in the loss of immune homeostasis (3,4). The
elimination of Treg can lead to the development of lupus-like
manifestations, including glomerulonephritis and production of
autoantibodies, which indicates that failure of Treg-mediated
suppression is implicated in the pathogenesis of SLE, even though
the reports on the counts and function of Treg in patients with SLE
have shown conflicting results (5,6).
In recent years, several studies have revealed the
pathological role of endoplasmic reticulum (ER) stress in the
autoimmune and inflammatory diseases (7-10).
ER is the largest cellular organelle that is responsible for
protein synthesis and folding, transportation and storage of
calcium, and lipid synthesis (11-13).
In the process of ER proteostasis, the proteins are supposed to be
modified and folded correctly by the folding enzymes and chaperone.
However, these elaborate processes are prone to be disrupted during
diseased status, resulting in overwhelming protein folding demand
over protein folding capacity (14). Consequently, the misfolded proteins
are accumulated in the ER, which is known as ER stress, and it
activates unfolded protein response (UPR) to acquire opportunities
to correct misfolded proteins. However, if the UPR signaling
pathway is affected, the cell apoptosis is initiated (15). We also have shown that aberrant UPRs
were found in T lymphocytes of patients with SLE and that higher
levels of apoptosis response to ER stress may contribute to the
pathogenesis of SLE (16). Based on
previous findings, we investigated whether ER stress inhibition can
alleviate clinical manifestations in lupus and the effect of ER
stress inhibition on the Treg. To address this issue, we used
4-phenylbutyric acid (4-PBA) to suppress the ER stress signaling.
4-PBA, a low molecular weight chemical chaperone, increases protein
folding capacity of ER and consequently prevents accumulation of
misfolded protein. Because of the action to mitigate ER stress,
several researches have used 4-PBA for the purpose of amelioration
of ER stress-related inflammatory diseases (17,18).
In our experiments, we administrated 4-PBA intraperitoneally in an
induced SLE murine model generated by application of toll-like
receptor (TLR) 7 agonist. Herein, we report that ER stress
inhibition ameliorates systemic autoimmunity and consequently
improves symptoms in the murine lupus model by Treg modulation.
Materials and methods
Mice and in vivo treatment
BALB/C mice were purchased from Central Lab animal
Inc.. All mice were 7-8-week-old females. They were maintained in
the conventional cage with 12 h/12 h light/dark cycle and fed with
standard diet. The Resiquimod (R848)-induced model has been used
for our experiments. Several studies have demonstrated that
systemic autoimmune features developed following topical treatment
with Resiquimod. The induced mouse models of lupus have presented
phenotypic changes including marked splenomegaly, edematous and a
swollen appearance, elevated level of autoantibodies and multiple
organ involvement (19,20). The mice were divided into four
groups, namely controls, lupus model treated with vehicle, lupus
model treated with 4-PBA, and lupus model treated with steroid.
First, to induce the experimental lupus model, the skin on the back
of mice was treated with 100 µg of Resiquimod (R848), which is a
TLR7 agonist (Enzo) in 100 µg of acetone, 3 times weekly from week
0 to week 4. Second, the mice were treated with phosphate buffered
saline (PBS), 4-PBA dissolved in 100 µl of PBS intraperitoneally
three times weekly (500 mg/kg) (Calbiocam), and dexamethasone
dissolved in 100 µl of PBS intraperitoneally once a day (1 mg/kg)
(Daewon Pharm) for 4 weeks, from week 8 to week 12. At the age of
12 weeks, mice were sacrificed. All mice were anaesthetized by an
intraperitoneal injection of sodium pentobarbital (50 mg/kg). The
blood was collected from retro-orbital sinus after anaesthesia
procedure. Following collection of blood sample, the all mice were
immediately euthanized by cervical dislocation. Following the
completion of the euthanasia procedure, death was confirmed the
combination of signs including ascertaining cardiac arrest, lack of
breathing and loss of a corneal reflex.
All mouse experiments were performed according to
the protocols approved by the Institutional Animal Care and Use
Committee of the Chonbuk National University, Jeonju, Korea (CBNU
2017-0027).
Histopathologic assessment and
immunofluorescence
Kidneys were harvested after perfusion with buffered
saline, fixed with 4% formaldehyde (Biosesang) for 24 h, and
embedded in paraffin. For the determination of renal
histopathology, 10-µm sections of the kidney were stained with
hematoxylin and eosin and periodic acid-Schiff. Renal pathology was
evaluated based on a previously described scoring scale (21). Glomerular pathology and interstitial
pathology were assessed semiquantitatively on a scale of 0-3 in 10
randomly selected high-power fields for each mouse.
The 10-µm-thick acetone-fixed sections were stained
with rabbit anti-mouse IgG-heavy and light chain antibody
FITC-conjugated, goat anti-mouse IgM cross-absorbed antibody
FITC-conjugated (Bethyl Laboratories Inc.), and were then incubated
at room temperature for 1 h. For C3 staining, the kidney sections
were stained with rat anti-mouse C3 (Abcam) and then incubated at
room temperature overnight, followed by staining with goat anti-rat
IgG-heavy and light chain antibody FITC-conjugated and then
incubated for 1 h at room temperature. Sections were mounted with
glycerin and the images were acquired by a Nikon Eclipse E600
fluorescence microscopy (Nikon). In addition, fluorescence
intensity scores were assessed based on the methods described in a
previous study (21). The intensity
scores were evaluated for each section with at least 10
glomeruli.
For detection of antinuclear antibodies (ANAs),
serum was diluted 1:40 and placed on HEp-2 slides (Antibodies
Incorporated) with rabbit anti-mouse IgG-heavy and light chain
antibody FITC-conjugated. After mounting with glycerin, images were
analyzed by Nikon Eclipse E600 fluorescence microscopy.
Serologic analysis and urinalysis
Urine albumin and creatinine (Exocell Inc.), Serum
anti-double stranded DNA (anti-dsDNA) antibodies (Shibayagi Co.)
and serum cytokines, including TNF-α (Enzo), were quantified by
ELISA according to the manufacturers' instructions.
Cell isolation and flow cytometry
Spleens were harvested and weighed, and splenocytes
were isolated by passing tissues through a 70-µm cell strainer (BD
Biosciences). For fluorescence-activated cell sorting analysis of
splenocytes, the following antibodies from commercial sources were
used: anti-CD45 (B220), anti-CD69, anti-CD3, anti-CD4, anti-CD25,
and anti-Foxp3 (BD Biosciences). All antibodies were
fluorochrome-conjugated. The stained cells were analyzed using
FACSCalibur flow cytometer (BD Biosciences) and data analysis was
performed using FlowJo software (TreeStar Inc.).
Western blot analysis
Proteins were extracted from the spleen tissue
samples using a lysis buffer. Protein levels were determined using
Bio-Rad DC Protein assay (Bio-Rad Laboratories, Inc.). Proteins
were separated on 10% SDS-PAGE gels and were then transferred to
nitrocellulose membranes. Membranes were blocked in 5% fat-free
milk in Tris-buffered saline for 60 min at room temperature with
shaking and then probed with primary antibodies (1:1,000) against
GRP78 (Bioworld Technology), PERK (Santa Cruz Biotechnology, Inc.),
IRE1α (Cell Signaling Technology, Inc.), eIF2α (Cell Signaling
Technology, Inc.), ATF-6α (Bioworld Technology), CHOP (Bioworld
Technology), cleaved ATF4 (Cell Signaling Technology, Inc.), p-PERK
(Santa Cruz Biotechnology, Inc.), p-IRE1α (Thermo Fisher
Scientific, Inc.), p-eIF2α (Cell Signaling Technology, Inc.), and
Actin (Bethyl Laboratories) overnight at 4˚C. After three washes,
the membranes were incubated with secondary horseradish
peroxidase-conjugated antibodies (1:3,000) for 2 h at room
temperature. The reactive proteins were detected using ECL
(Amersham Life Sciences/GE Healthcare) and the intensity of bands
was quantified densitometrically using the Vilber Lourmat Fusion
fx7 system (Vilber Lourmat).
Inhibition assays
CD4+CD25- target cells that
were labelled with carboxyfluorescein diacetate succinimidyl ester
(CFSE; Molecular Probes) were co-cultured for 5 days with
regulatory CD4+CD25+ T cells at target cell:
Treg ratio of 1:1. The cells were incubated in the presence of
anti-CD3 and anti-CD28 antibodies coated beads (cells to beads
ratio 10:1) (Life Technologies; Thermo Fisher Scientific, Inc.) as
previously described (22). The
stained cells were analyzed using FACSCalibur flow cytometer (BD
Biosciences) and data analysis was performed using FlowJo software
(TreeStar Inc.). We calculated inhibitory index (%) of cell
proliferation in a same manner as described previously (22).
Statistical analysis
SPSS 22.0 software (SPSS Inc.) was used for
statistical analysis. Kruskal-Wallis tests for group comparisons
and Mann-Whitney test with Bonferroni's correction for comparisons
between pairs of groups were applied to the analysis of pathology
and IF scoring. All other data, which were parametically
distributed, were analyzed by one-way ANOVA with a Tukey post hoc
test for significance when comparing groups including proteinuria,
autoantibody, cytokine, flow cytometry assay. P values <0.05
were considered statistically significant. The results were
expressed as mean ± EM.
Results
4-PBA decreased lupus manifestations
and disease activity in murine lupus model
We investigated the effect of ER stress inhibition
on the development of clinical manifestations in murine lupus. The
murine lupus model which is induced with TLR7 agonist exhibits
severe SLE-phenotypes, such as splenomegaly, edema, presence of
autoantibodies to nuclear antigen, and glomerulonephritis. To
determine whether ER stress suppression prevents lupus
immunopathology, 4-PBA was administrated in induced lupus group at
8 weeks of age, when mice did not present overt lupus
manifestations. Additionally, steroid treatment was initiated in
another group with induced lupus to compare the inhibitory effects
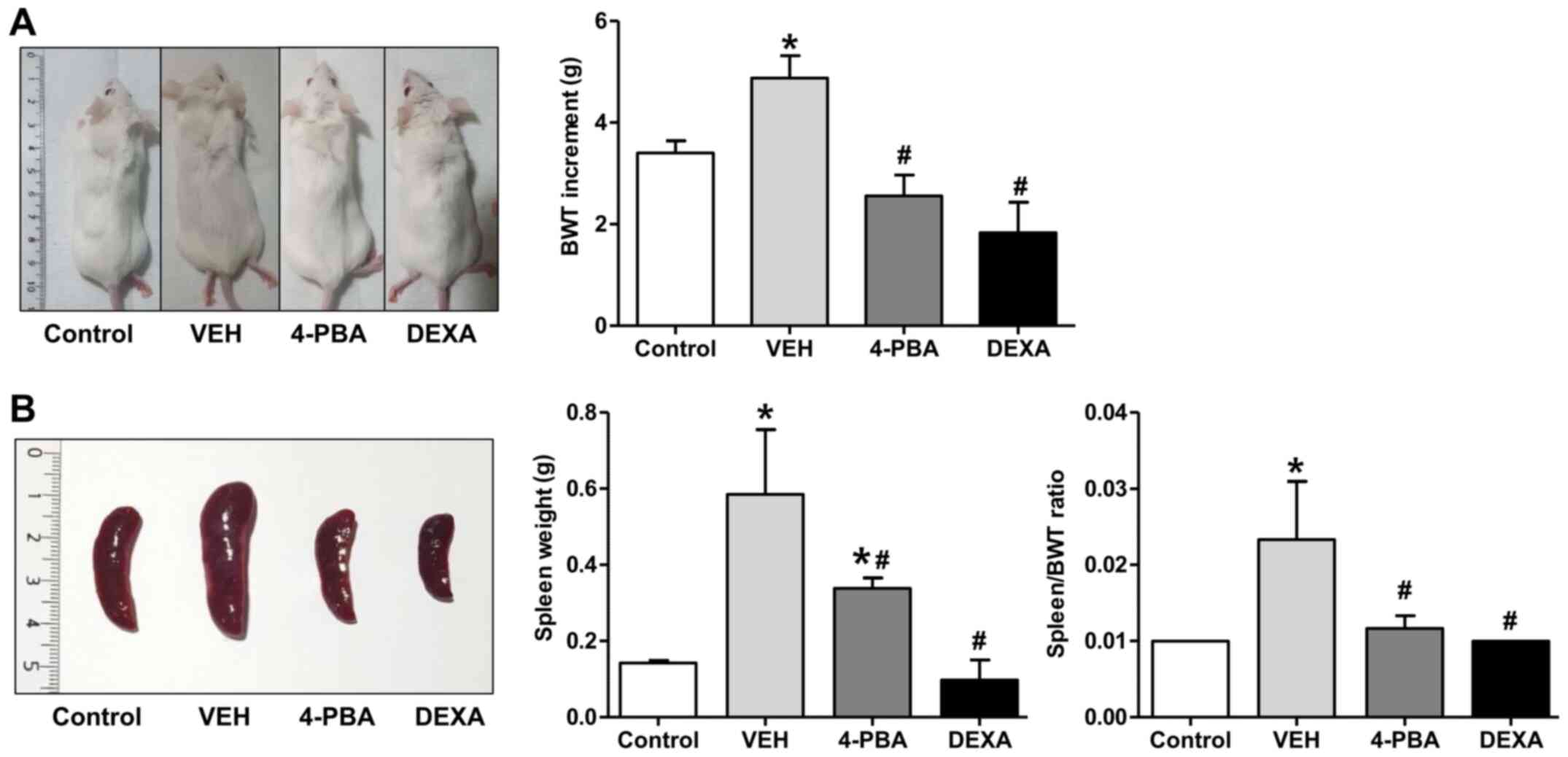
of 4-PBA with those of steroids. At 12 weeks, vehicle-treated mice
appeared edematous and swollen compared to 4-PBA or steroid-treated
mice (Fig. 1A). Regarding body
weight change, The TLR7 agonist-stimulated group showed an increase
in weight compared to the control mice. The TLR7 agonist-stimulated
group also presented marked splenomegaly from the expansion of
lymphocytes due to plasma dendritic cell activation following TLR7
stimulation and displayed an edematous and swollen appearance
because of systemic inflammation as treatment continued. Body
weight likely increased in these mice due to splenomegaly, which
was increased over three times compared to the control mice by the
12th week. The increment of body weight from baseline to 12th week
was significantly higher in vehicle-treated lupus mice (4.875±1.246
g) compared to 4-PBA-treated (2.556±1.236 g) and steroid-treated
group (1.833±1.472 g) (Fig. 1A).
Marked splenomegaly also was detected in vehicle-treated mice
(weight, 0.585±0.416 g; ratio 0.023±0.018), while 4-PBA (weight
0.338±0.066 g; ratio 0.011±0.004) and steroid-treated mice (weight
0.097±0.105 g; ratio 0.010±0) showed a decrease in spleen size and
spleen to body weight ratio (Fig.
1B).
4-PBA reduced tumor necrosis factor-α
level and production of auto-antibodies
To assess the effects of ER stress suppression on
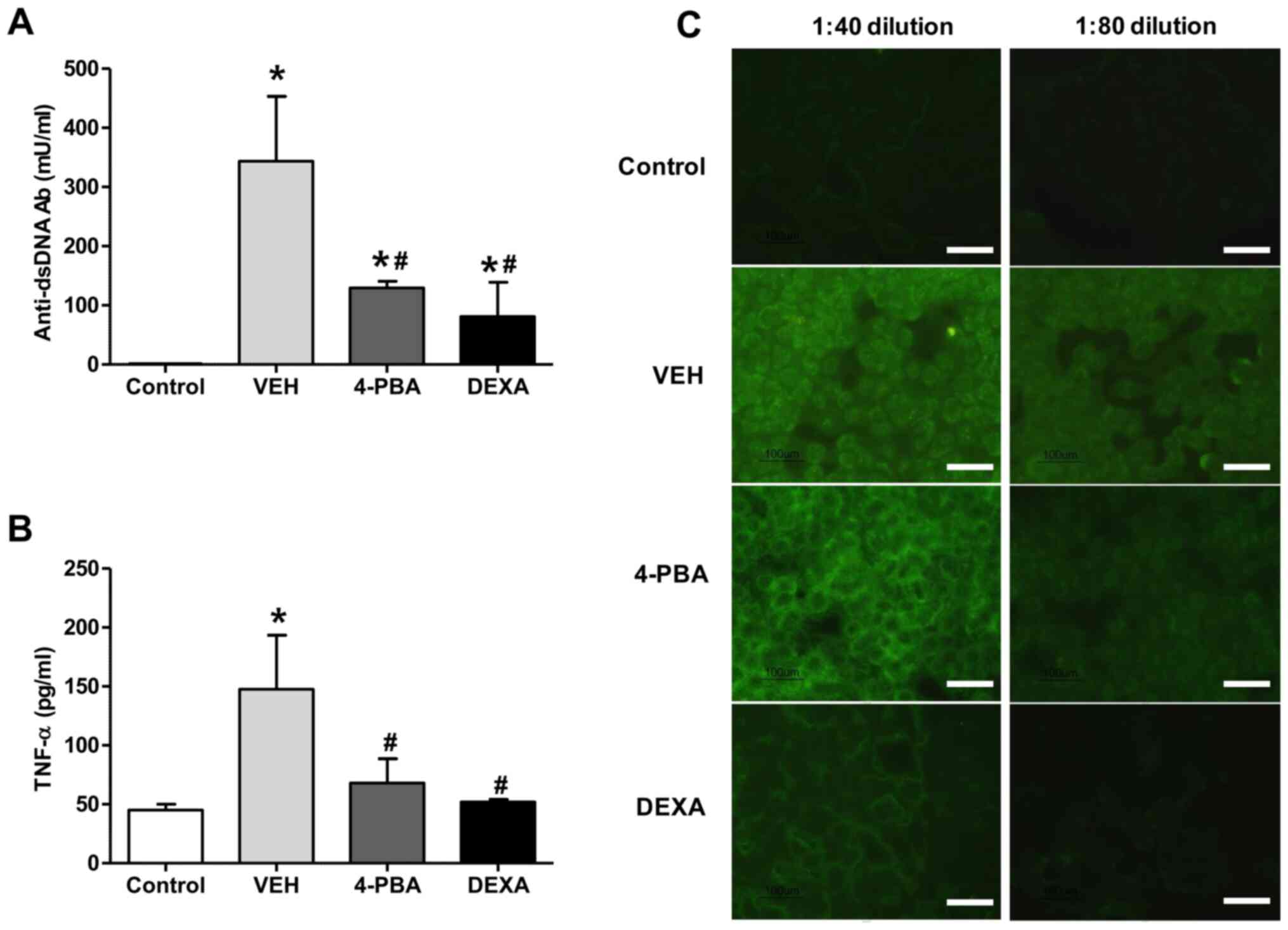
autoantibody production, the serum level of anti-dsDNA antibody was
measured. The sera from vehicle-treated mice showed higher level of
anti-dsDNA (343.5±219.6 mU/ml), whereas the mice treated with 4-PBA
showed decreased level of anti-dsDNA (129.5±21.9 mU/ml) at 12th
week and the level was similar to that of the steroid-treated group
(81.0±82.0 mU/ml) (Fig. 2A). The
presence of ANAs in sera of murine lupus model was assayed by
indirect immunofluorescence using HEp-2 slides. The serum was
diluted at the concentration of 1:40 and 1:80 to determine the
difference of fluorescence intensity between the groups. Enhanced
expression of ANA with speckled pattern was detected in the serum
of vehicle group with 1:40 dilution and the fluorescent activity
was also detected at 1:80 dilution, even though the activity was
decreased. In the serum of 4-PBA-treated mice, the fluorescent
activity against ANA was detected at 1:40 concentration; however,
it was rarely detected in the serum diluted at the concentration of
1:80 (Fig. 2C). These results
indicate that 4-PBA treatment blocked the production of
autoantibodies.
Our experiments revealed an increased concentration
of TNF-α (147.7±79.4 pg/ml) in vehicle-treated group, in agreement
with the results of previous reports. However, treatment with 4-PBA
reduced the levels of TNF-α (68.0±35.6 pg/ml) in murine lupus model
(Fig. 2B). The steroid-treated
group showed similar level of TNF-α (52.0±2.8 pg/ml). Taken
together, these data suggest that 4-PBA administration decreases
autoantibodies production and pro-inflammatory cytokine production,
consequently ameliorating the disease activity of murine lupus
model.
4-PBA ameliorated lupus nephritis
SLE exhibits a broad range of clinical
manifestations, of which lupus nephritis is the most serious form
of major organ involvement and the leading cause of high morbidity
and mortality (23). Thus, it is
essential to assess the effects of 4-PBA on renal pathology in
murine lupus model. The BALB/c mice treated with TLR7 agonist
developed spontaneously a disease with characteristic features of
human SLE, including nephritis (19). In the present experiment,
progressive albuminuria was observed over time in vehicle-treated
group with a urine albumin-to-creatinine ratio (UACR) of up to
59±44 mg/g. On the other hand, 4-PBA group exhibited decreased
albuminuria compared to the vehicle group at 12th week, with an
UACR of 41±27 mg/g, but the difference was not statistically
significance. The steroid-treated group showed the lower range of
UACR, 10±5 mg/g, that was significantly lower compared to that of
vehicle group (Fig. 3A).
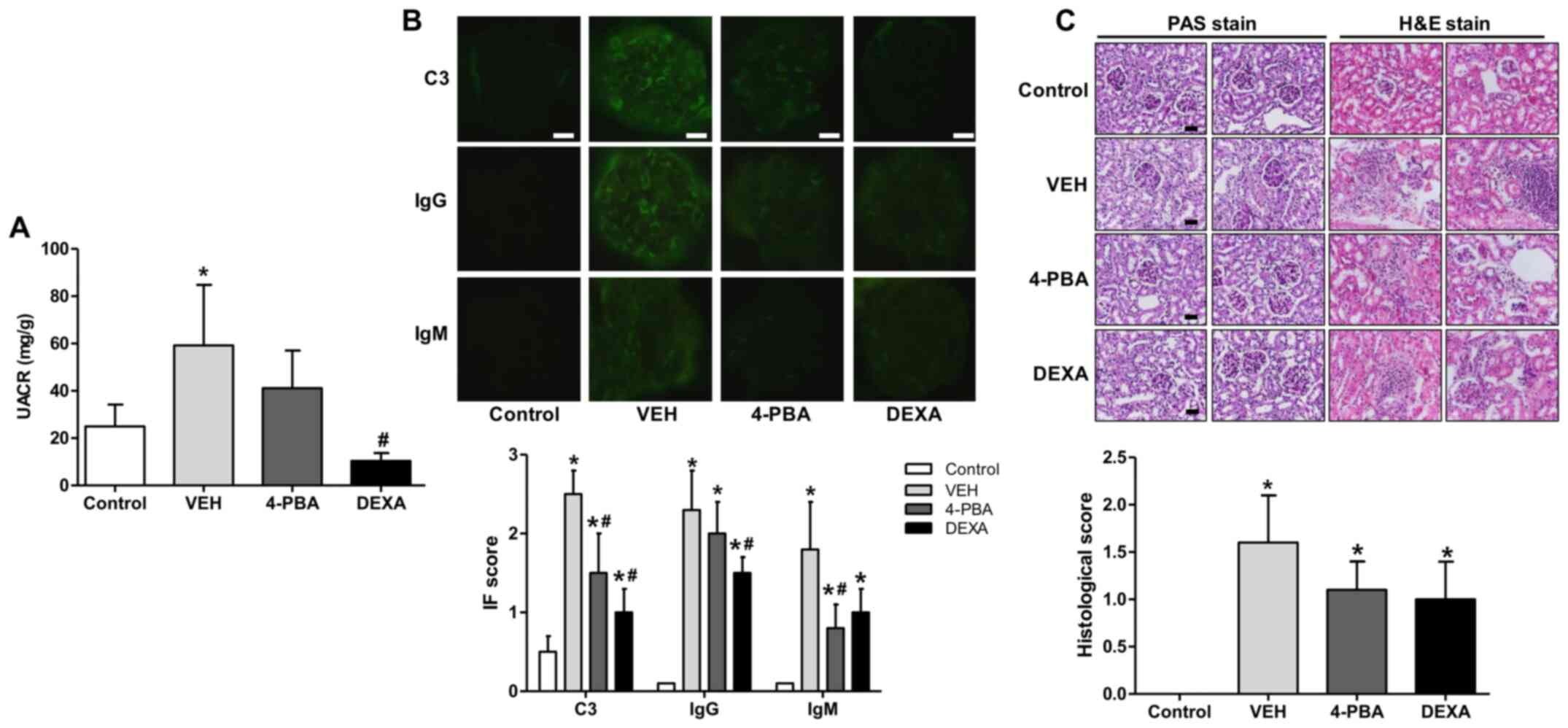 | Figure 3Decreased albuminuria and renal
deposition of immune complex and C3 and ameliorated pathologic
severity of kidney injury induced by 4-PBA treatment. (A) UACR in
BALB/c and lupus mice that were treated with VEH, 4-PBA and DEXA.
The mouse urine was collected for 24 h at the age of 12 week using
a metabolic cage, and urinary albumin and creatinine levels were
quantified using ELISA. (B) Representative renal sections stained
for C3, IgG and IgM (original magnification, x400) and IF staining
scores are shown. Scale bar, 10 µm. (C) Representative kidney
sections stained with PAS stain and hematoxylin and eosin stain
(original magnification, x400) and histologic scores are shown.
Scale bar, 25 µm. *P<0.05 vs. BALB/c mice;
#P<0.05 vs. vehicle-treated mice. Results are
presented as the mean ± EM of three independent experiments per
group. 4-PBA, 4-phenylbutyric acid; DEXA, dexamethasone; IF,
immunofluorescence; PAS, periodic acid-Schiff; UACR, urine
albumin-to-creatinine ratio; VEH, vehicle. |
To determine whether 4-PBA attenuates
immunopathology, we performed indirect fluorescent assay of kidney
to detect IgG, IgM, and C3 deposition. In the vehicle group, IgG,
IgM, and C3 were heavily deposited within glomeruli; however, 4-PBA
treatment significantly reduced deposition of C3 and IgM (Fig. 3B). We also compared the
histopathology of kidneys between the groups. The assessment
revealed obvious mesangial hypercellularity and thickening of
glomerular basement membrane in the vehicle-treated lupus mice
compared to BALB/c mice. Moreover, the interstitium also showed
advanced fibrosis and mononuclear inflammatory cell infiltration.
However, compared to vehicle group, the glomeruli of 4-PBA-treated
or dexamethasone-treated groups showed relatively mild mesangial
hypercellularity and decreased interstitial fibrosis and
inflammatory cell infiltration (Fig.
3C), even though there was not a statistical significance.
Elevated expression of ER stress
markers in murine lupus and attenuation of ER stress by 4-PBA
treatment
Our next goal was to demonstrate whether ER stress
inhibition is involved in the amelioration of lupus phenotypes,
including nephritis, in murine model. The immunoblot analysis of
the spleen, a largest lymphatic organ and the site of innate and
adaptive immune processes, was performed for measuring ER stress
markers. Immunoblot analysis revealed that ER stress markers,
including GRP78, CHOP, p-PERK, and p-IRE1α, were elevated in the
vehicle-treated murine lupus group compared to wild-type mice.
However, GRP78, p-IRE1α, and CHOP were greatly suppressed by
treatment of the mice with 4-PBA (Fig.
4A-C). Accordingly, these results indicate that: i) 4-PBA
suppressed ER stress signaling that was upregulated in the murine
lupus model; and ii) 4-PBA treatment induced a marked improvement
in lupus manifestation, including nephritis; thus, these results
suggest the beneficial effects of ER stress inhibition on murine
lupus.
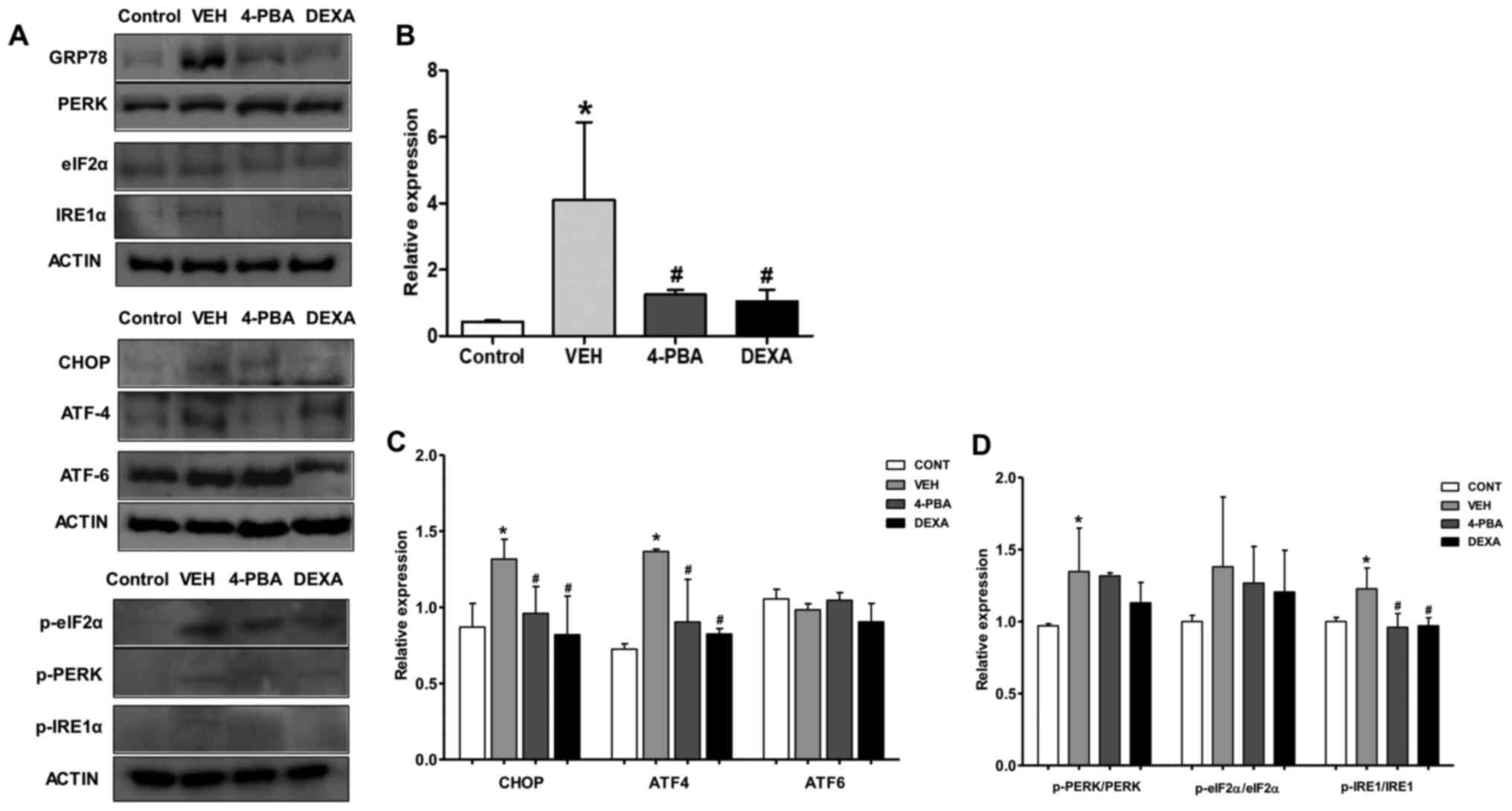 | Figure 4Upregulated ER stress markers in
murine lupus models and their attenuation by 4-PBA treatment. (A)
Representative western blotting images of the ER stress markers
from spleen tissue lysates. (B) The pan form or phosphorylated form
of GRP78 and (C) CHOP, ATF4, ATF6, (D) PERK, eIF2α and IRE1 were
semiquantified by densitometric analysis and normalized to ACTIN
expression or their unphosphorylated proteins.
*P<0.05 vs. BALB/c mice; #P<0.05 vs.
vehicle-treated mice. Values are presented as the mean ± EM of
three independent experiments per group. 4-PBA, 4-phenylbutyric
acid; CONT, control; DEXA, dexamethasone; ER, endoplasmic
reticulum; p-, phosphorylated-; TNF-α, tumor necrosis factor-α;
VEH, vehicle. |
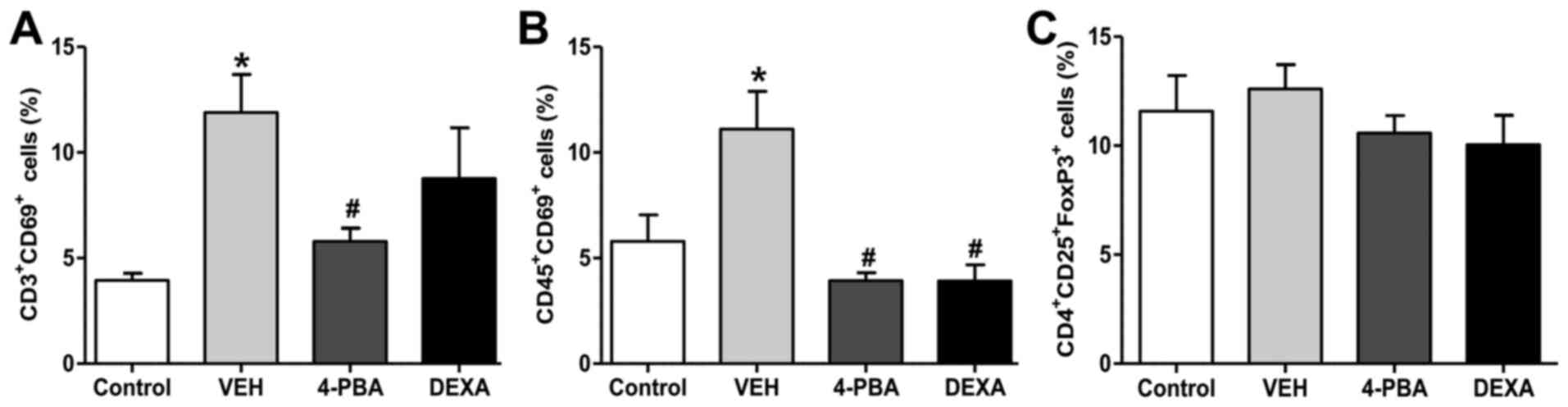
The proportion of activated T and B
lymphocytes was decreased by 4-PBA treatment
Next, we analyzed the immune cell populations,
including activated T and B lymphocytes, and Treg from the spleen
to determine the roles of these immune cells on the improvement of
lupus phenotypes by 4-PBA treatment. The frequency of activated T
and B lymphocyte by analysis of CD69+ expression was
higher in the vehicle group compared to that in the wild-type mice.
The murine lupus with 4-PBA treatment showed a significantly
reduced proportion of activated T and B lymphocyte and the same
results were observed in steroid-treated group (Figs. 5 and S1). Thus, these findings led us to
speculate that the reduction of frequencies of activated T and B
lymphocytes by 4-PBA may contribute to ameliorate clinical
manifestation, organ damage, and decrease autoantibodies
production. However, Treg, which play an important role in the
negative regulation of dysregulated lymphocytes, did not show
significant differences in the population of the four groups
(Fig. 5). The decreased proportion
of activated T and B lymphocytes by 4-PBA and steroid treatment was
in accordance with previous results; however, a paradoxical finding
was that no changes were observed in the frequency of Treg among
the groups. Thus, qualitative evaluation in the suppressive
function of Treg in vehicle-, 4-PBA-, and steroid-treated group was
performed to define the exact effect of ER stress inhibitor on
Treg.
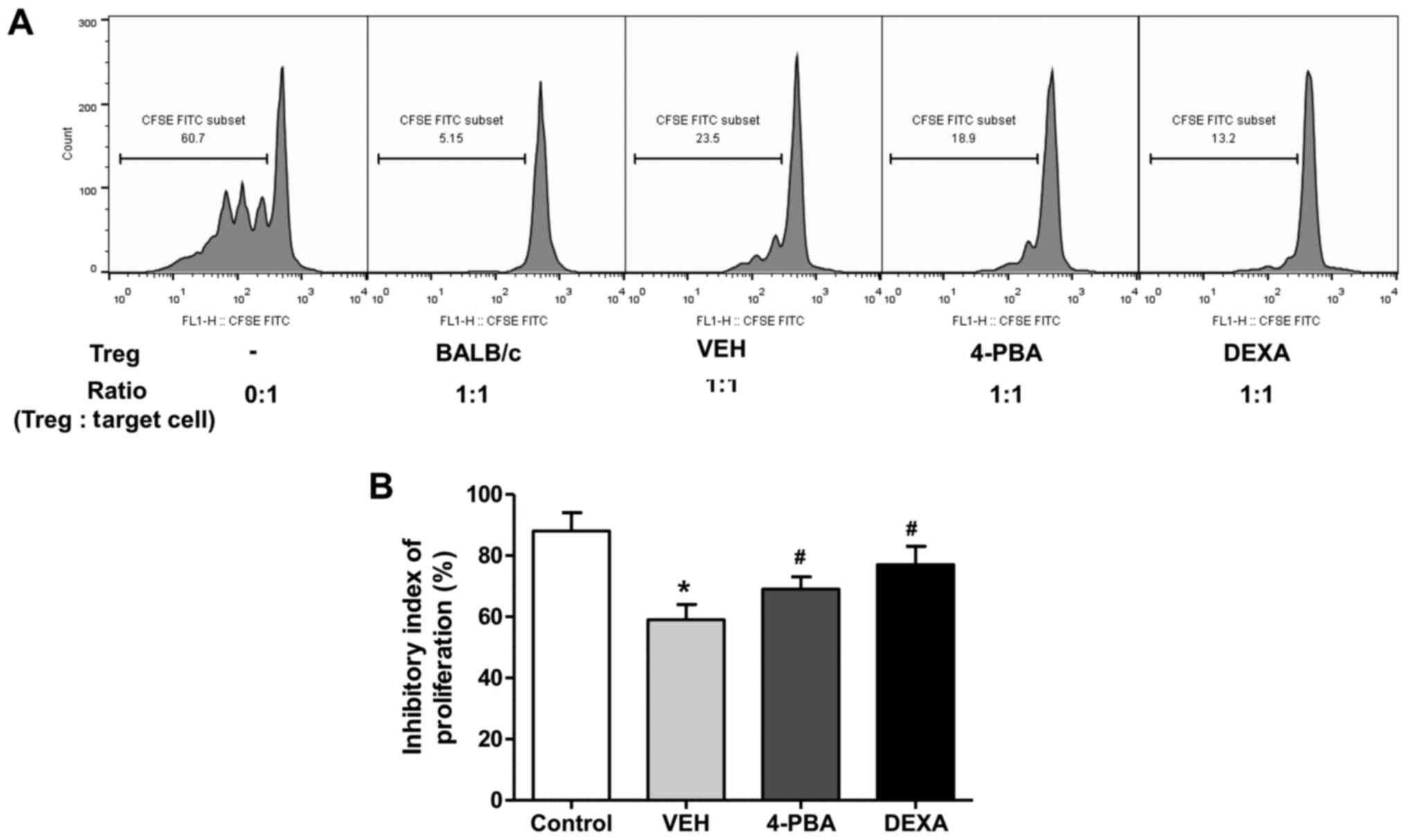
4-PBA improved the suppressive
capacity of Treg in murine lupus
We evaluated the suppressive function of
CD4+CD25+ Treg in vehicle-, 4-PBA-, and
dexamethasone-treated groups by measuring the proliferation of
CD4+CD25- T cells, as target cells, in
vitro. Freshly isolated CD4+CD25+ T cells
were co-cultured with CFSE-stained target cells at a ratio of 1:1
for 5 days and the inhibitory index of proliferation was acquired
using the same equation as described above. The ability of Treg to
suppress target cell proliferation was significantly lower in
vehicle-treated group than in wild-type mice. The Treg of 4-PBA
group and steroid group exhibited markedly improved suppressive
activity compared to that of vehicle-treated group (vehicle,
59.2±6.8%; 4-PBA 68.8±6.2%, steroid, 77.2±7.1%; P=0.03 and P=0.01)
(Fig. 6A and B). Thus, these results suggested that ER
stress inhibition improves the function of Treg in murine lupus,
even if it did not affect the frequency of Treg.
Discussion
By administration of 4-PBA, clinical manifestations
of SLE, such as splenomegaly or generalized edema, were improved
and serum levels of anti-dsDNA antibodies and inflammatory
cytokines were also decreased. In particular, with respect to the
renal involvement, 4-PBA treatment showed a trend of attenuating
the albuminuria and histological damage, including inflammatory
cell infiltration and IgM and C3 deposition. The deposition of IgG
was not affected by 4-PBA treatment in our experiment, but there
are two possible explanations for this result. First, the
characteristics of immunoglobulin can influence this finding.
Contrary to IgG, IgM is the first antibody to appear in the
response to the initial exposure to an antigen. Therefore, it is
possible that the antigen presentation of dendritic cells was
suppressed by the suppression of regulatory T cells by 4-PBA.
Second, this result could arise due to variation between the lupus
mice. The level of IgG in the 4-PBA group was lower than that in
the vehicle group, although the difference was not statistically
significant. It is possible that variations between mice have
suppressed the derivation of statistically significant results,
even though a reduction trend is seen. Further studies will be
needed to identify the precise mechanism behind this reduction.
To evaluate the effects of ER stress on the immune
cells, we investigated the population of activated T and B cells
and function of Treg. The cell population showed different
percentages among the treatment groups and a higher proportion of
activated T and B cells was observed in the vehicle group compared
to the 4-PBA group; however, no significant statistical difference
in Treg population was detected between the two groups. However,
enhanced suppressive capacity of Treg in 4-PBA treated group was
observed. These results suggest that the ER stress suppression
through 4-PBA may improve the function of Treg, resulting in more
efficacious regulation of over-activated T and B cells.
Consequently, 4-PBA may limit the exaggerated immune response,
leading to improvement of overall SLE manifestations, including
lupus nephritis. Numerous attempts have been made to prove the role
of Treg by its modulation, such as adoptive transfer or depletion
of Treg, and many animal studies have shown different effects of
Treg on prevention or development of autoimmune diseases (24,25).
Especially, adoptive transfer of Treg from ‘young’ F1 mice or from
the mice that transduced to express Ccr2 to recipients showed
ameliorated immune-mediated manifestations, including pneumonitis
or autoimmune sialoadenitis, in (NZB x NZW)F1 mice or MRL-Faslpr
mice (26,27). Taken together these reports, even
though we did not modulate Treg directly, our experiments indicate
that enhanced suppressive function of Treg in 4-PBA-treated mice
substantially exert therapeutic effects on the disease.
Recently, a number of researches have reported that
ER stress suppression blocks disease progression and ameliorates
clinical manifestations (7-9).
Our experiments are the first to show the ameliorative effect of
4-PBA in murine lupus nephritis. Based on our current results, we
can speculate that increasing ER folding capacity and facilitating
misfolded proteins translocation by chemical ER stress inhibitor
may result in improvement of suppressive capacity of Treg, although
no statistical difference was detected in studied population.
Treg occupies only 5-10% of total CD4+ T
lymphocytes in the healthy controls; consequently, the transfer of
Tregs to the patients with lupus is limited. Thus, the efforts in
expansion of Treg in vivo or in vitro have been made
and their efficacy and safety profile has been evaluated in several
studies that studied the immune disorders (28-30).
Together with these approaches, improving the function of Treg by
ER stress inhibition might be an option to optimize immunological
homeostasis. Furthermore, we investigated the effects of steroids
in order to estimate and compare the efficacy of 4-PBA with
clinical improvements obtained by steroid treatment, and the
results showed that the ameliorating effects of 4-PBA in murine
lupus were comparable with steroid treatment. However, more
researches are needed to elucidate how ER stress inhibition is
involved in restoration of the Treg function. The dynamic
organelle, ER, is responsible for the calcium storage,
gluconeogenesis, cholesterol and lipid synthesis, as well as
proteostasis (31); therefore, the
impaired function of Treg under the condition of elevated ER stress
could be based on these disrupted metabolic processes; however, the
accurate mechanism remains unknown. The clarification of this link
between improved function of Treg and ER stress inhibition is
necessary.
Some limitations of this study should be
acknowledged. First, absence of several serum cytokines or serial
urine proteins of lupus murine, and unidentified identification of
various cell markers in flow cytometry analysis. However, we
quantified important and basic cytokines and autoantibodies that
should be identified in the lupus murine model and analyzed the
flow cytometry using a representative cell activation marker, and
the data obtained through these analyses are judged to support our
results meaningfully. Second, we focused only on the Treg and did
not evaluate the effects of 4-PBA on other innate immune cells,
including macrophage, dendritic cells or neutrophils, and the
subsets of B and T lymphocytes. Because immune responses occur in
synergy of diverse types of cells, clinical improvement induced by
4-PBA in murine lupus may be affected by the other altered function
of different subset of cells. However, it is noteworthy that 4-PBA
ameliorates disease severity and that the improved suppressive
function of Treg is associated with the phenomenon, at least in
part. Another limitation is that the expression of ER stress
markers was evaluated in the entire population of splenocytes, not
the Treg. Because the limited cell counts of Treg, assessment of
markers in specific subsets of cells was technically difficult.
Third, the murine lupus model we used was not a SLE-prone mouse
model, but a TLR7-agonist induced model. Differences in types of
manifestations, autoantibodies levels, and disease severity could
exist between the mice. However, because the enhanced sensing of
RNA-containing antigen, overproduction of autoantibodies, aberrant
activation of T and B lymphocytes are the mechanisms in the TLR7
agonist-induced mice model (19),
it was more apparent to evaluate the function of Treg in this
murine lupus model. Fourth, we did not show changes in regional
lymph nodes, which could clarify the effect of 4-PBA in the lupus
model. The spleen showed marked changes in the expansion of myeloid
and lymphoid cells between vehicle- and 4-PBA-treated mice and may
be representative of regional lymph nodes as the spleen is the
largest lymphatic organ in the body. However, further studies
analyzing immune cell expansion and activation using regional lymph
nodes are necessary to prove the suppressive capacity of 4-PBA.
In conclusion, the present study demonstrated for
the first time that ER stress inhibition can improve Treg function,
thereby inducing proper regulation of aberrant immune
hyper-activation, and consequently leading to phenotypical
improvements, especially in lupus nephritis, in lupus model. These
findings provide strong support for a potential therapeutic effect
of 4-PBA in patients with SLE.
Supplementary Material
Murine splenocytes were stained with
antibodies against CD3, CD45, CD69, CD4, CD25 and FoxP3, and then
analyzed by flow cytometry. The gating was performed for activated
T cells (CD3+CD69+), activated B cells
(CD45+CD69+) and regulatory T cells
(CD4+CD25+FoxP3+). 4-PBA,
4-phenylbutyric acid; DEXA, dexamethasone; VEH, vehicle.
Acknowledgements
This abstract was presented at the Annual European
Congress of Rheumatology June 12-15, 2019, Madrid, Spain and was
published as Abstract no. THU0209, at the ACR/ARHP Annual Meeting
November 8-13, 2019, Atlanta, USA, and was published as Abstract
no. 995, and at the Annual Meeting of the Korean College of
Rheumatology May 18-19, 2018, Seoul, South Korea, and was published
as Abstract no. 17S-0063.
Funding
This research was supported by Basic Science Research Program
through the National Research Foundation of Korea (NRF) funded by
the Ministry of Science, ICT and Future Planning (grant no.
2015006120) and Fund of Biomedical Research Institute, Chonbuk
National University Hospital (grant no. CUH2018-0006).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
YC and WHY designed the experiment. JHJ and EGL
performed the main experiment. KMK performed the histological
examination of the kidney. YC and WHY statistically analyzed the
data. YC, JHJ, EGL and KMK interpreted the results. YC prepared a
draft of the manuscript. WHY and KMK revised the manuscript. WHY
acquired funding and contributed to resources. YC and WHY
authenticate the raw data. All authors have read and approved the
final manuscript.
Ethics approval and consent to
participate
All animal experiments were approved by the
Institutional Animal Care and Use Committee of the Chonbuk National
University, Jeonju, Korea (CBNU 2017-0027).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests
References
|
1
|
Tsokos GC: Systemic lupus erythematosus. N
Engl J Med. 365:2110–2121. 2011.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Kiriakidou M, Cotton D, Taichman D and
Williams S: Systemic lupus erythematosus. Ann Intern Med.
159:ITC4–ITC1. 2013.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Sakaguchi S: Regulatory T cells: Key
controllers of immunologic self-tolerance. Cell. 101:455–458.
2000.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Shevach EM: CD4+
CD25+ suppressor T cells: More questions than answers.
Nat Rev Immunol. 2:389–400. 2002.PubMed/NCBI View
Article : Google Scholar
|
|
5
|
Sakaguchi S, Sakaguchi N, Asano M, Itoh M
and Toda M: Immunologic self-tolerance maintained by activated T
cells expressing IL-2 receptor alpha-chains (CD25). Breakdown of a
single mechanism of self-tolerance causes various autoimmune
diseases. J Immunol. 155:1151–1164. 1995.PubMed/NCBI
|
|
6
|
Buckner JH: Mechanisms of impaired
regulation by CD4(+)CD25(+)FOXP3(+) regulatory T cells in human
autoimmune diseases. Nat Rev Immunol. 10:849–859. 2010.PubMed/NCBI View
Article : Google Scholar
|
|
7
|
Mukai S, Ogawa Y, Urano F, Kudo-Saito C,
Kawakami Y and Tsubota K: Novel Treatment of Chronic
Graft-Versus-Host Disease in Mice Using the ER Stress Reducer
4-Phenylbutyric Acid. Sci Rep. 7(41939)2017.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Zeng M, Sang W, Chen S, Chen R, Zhang H,
Xue F, Li Z, Liu Y, Gong Y, Zhang H, et al: 4-PBA inhibits
LPS-induced inflammation through regulating ER stress and autophagy
in acute lung injury models. Toxicol Lett. 271:26–37.
2017.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Mohammed-Ali Z, Lu C, Marway MK, Carlisle
RE, Ask K, Lukic D, Krepinsky JC and Dickhout JG: Endoplasmic
reticulum stress inhibition attenuates hypertensive chronic kidney
disease through reduction in proteinuria. Sci Rep.
7(41572)2017.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Zheng P, Lin Y, Wang F, Luo R, Zhang T, Hu
S, Feng P, Liang X, Li C and Wang W: 4-PBA improves lithium-induced
nephrogenic diabetes insipidus by attenuating ER stress. Am J
Physiol Renal Physiol. 311:F763–F776. 2016.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Braakman I and Hebert DN: Protein folding
in the endoplasmic reticulum. Cold Spring Harb Perspect Biol.
5(a013201)2013.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Fagone P and Jackowski S: Membrane
phospholipid synthesis and endoplasmic reticulum function. J Lipid
Res. 50 (Suppl):S311–S316. 2009.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Clapham DE: Calcium signaling. Cell.
131:1047–1058. 2007.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Bhandary B, Marahatta A, Kim HR and Chae
HJ: An involvement of oxidative stress in endoplasmic reticulum
stress and its associated diseases. Int J Mol Sci. 14:434–456.
2012.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Lee AS: The ER chaperone and signaling
regulator GRP78/BiP as a monitor of endoplasmic reticulum stress.
Methods. 35:373–381. 2005.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Lee WS, Sung MS, Lee EG, Yoo HG, Cheon YH,
Chae HJ and Yoo WH: A pathogenic role for ER stress-induced
autophagy and ER chaperone GRP78/BiP in T lymphocyte systemic lupus
erythematosus. J Leukoc Biol. 97:425–433. 2015.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Ozcan U, Yilmaz E, Ozcan L, Furuhashi M,
Vaillancourt E, Smith RO, Görgün CZ and Hotamisligil GS: Chemical
chaperones reduce ER stress and restore glucose homeostasis in a
mouse model of type 2 diabetes. Science. 313:1137–1140.
2006.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Kawasaki N, Asada R, Saito A, Kanemoto S
and Imaizumi K: Obesity-induced endoplasmic reticulum stress causes
chronic inflammation in adipose tissue. Sci Rep.
2(799)2012.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Yokogawa M, Takaishi M, Nakajima K,
Kamijima R, Fujimoto C, Kataoka S, Terada Y and Sano S:
Epicutaneous application of toll-like receptor 7 agonists leads to
systemic autoimmunity in wild-type mice: A new model of systemic
lupus erythematosus. Arthritis Rheumatol. 66:694–706.
2014.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Hasham MG, Baxan N, Stuckey DJ, Branca J,
Perkins B, Dent O, Duffy T, Hameed TS, Stella SE, Bellahcene M, et
al: Systemic autoimmunity induced by the TLR7/8 agonist Resiquimod
causes myocarditis and dilated cardiomyopathy in a new mouse model
of autoimmune heart disease. Dis Model Mech. 10:259–270.
2017.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Yan JJ, Lee JG, Jang JY, Koo TY, Ahn C and
Yang J: IL-2/anti-IL-2 complexes ameliorate lupus nephritis by
expansion of CD4+CD25+Foxp3+
regulatory T cells. Kidney Int. 91:603–615. 2017.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Lee YA, Kim HR, Lee JS, Jung HW, Kim HY,
Lee GM, Lee J, Sim JH, Oh SJ, Chung DH, et al: CD4+
FOXP3+ Regulatory T Cells Exhibit Impaired Ability to
Suppress Effector T Cell Proliferation in Patients with Turner
Syndrome. PLoS One. 10(e0144549)2015.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Yu F, Haas M, Glassock R and Zhao MH:
Redefining lupus nephritis: Clinical implications of
pathophysiologic subtypes. Nat Rev Nephrol. 13:483–495.
2017.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Sakaguchi S: Naturally arising
CD4+ regulatory t cells for immunologic self-tolerance
and negative control of immune responses. Annu Rev Immunol.
22:531–562. 2004.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Masteller EL, Tang Q and Bluestone JA:
Antigen-specific regulatory T cells - ex vivo expansion and
therapeutic potential. Semin Immunol. 18:103–110. 2006.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Hasegawa H, Inoue A, Muraoka M, Yamanouchi
J, Miyazaki T and Yasukawa M: Therapy for pneumonitis and
sialadenitis by accumulation of CCR2-expressing
CD4+CD25+ regulatory T cells in MRL/lpr mice.
Arthritis Res Ther. 9(R15)2007.PubMed/NCBI View
Article : Google Scholar
|
|
27
|
Weigert O, von Spee C, Undeutsch R, Kloke
L, Humrich JY and Riemekasten G: CD4+Foxp3+
regulatory T cells prolong drug-induced disease remission in
(NZBxNZW) F1 lupus mice. Arthritis Res Ther. 15(R35)2013.PubMed/NCBI View
Article : Google Scholar
|
|
28
|
Lamarche C and Levings MK: Guiding
regulatory T cells to the allograft. Curr Opin Organ Transplant.
23:106–113. 2018.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Ukena SN, Höpting M, Velaga S, Ivanyi P,
Grosse J, Baron U, Ganser A and Franzke A: Isolation strategies of
regulatory T cells for clinical trials: Phenotype, function,
stability, and expansion capacity. Exp Hematol. 39:1152–1160.
2011.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Donnelly C, Dykstra B, Mondal N, Huang J,
Kaskow BJ, Griffin R, Sackstein R and Baecher-Allan C: Optimizing
human Treg immunotherapy by Treg subset selection and E-selectin
ligand expression. Sci Rep. 8(420)2018.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Kim I, Xu W and Reed JC: Cell death and
endoplasmic reticulum stress: Disease relevance and therapeutic
opportunities. Nat Rev Drug Discov. 7:1013–1030. 2008.PubMed/NCBI View
Article : Google Scholar
|