Introduction
Osteoarthritis (OA) is a degenerative joint disease
caused by changes in joint cartilage, subchondral bone, bursa,
synovium, ligament and other joint structure, which can cause pain
and joint stiffness. Its main pathological features include
cartilage degeneration, synovial inflammation and subchondral bone
changes (1). The incidence rate of
OA accounts for 4 to 13% of the world's population, which seriously
affects patients' quality of life, and is one of the main causes of
disability (2). Since OA has a
higher incidence rate in the elderly population, its impact will
grow exponentially in the coming decades (3). Medications currently recommended by
the guidelines include oral painkillers, non-steroidal
anti-inflammatory drugs and intra-articular corticosteroids.
However, these treatments only temporarily relieve symptoms and
have side effects such as irritating the gastrointestinal tract and
increasing the risk of cardiovascular disease (4). Therefore, exploring better OA
treatment methods has been a research focus in the field of
orthopedics. Although the pathogenesis of OA is unclear, it is
generally believed that OA is associated with increased
proinflammatory cytokines, activation of inflammation-associated
signaling pathways, and the degradation of the extracellular matrix
(ECM) (5-7).
Quercetin, a flavonoid compound widely found in
vegetables and fruits, is an excellent free radical scavenging
compound, which has many effects such as anti-oxidative stress and
anti-inflammatory, and can lower the risk of OA, rheumatoid
arthritis and other chronic diseases associated with oxidative
stress (8). Kanzaki et al
(9) tested the intake of oral
quercetin (45 mg/d) combined with glucosamine and chondroitin as
the experimental group, and OA pain symptoms were significantly
relieved compared with the control group. Quercetin can
significantly decrease the formation of inflammatory mediators in
macrophages in vivo and in vitro, significantly
decrease the expression and secretion of interleukin-1β (IL-1β),
tumor necrosis factor α (TNF-α) and interleukin-6 (IL-6) (10), and downregulate the expression of
matrix metalloproteinase 13 (MMP-13) (11), which has potential medicinal value
in the treatment of OA. The pathogenesis of OA and the apoptosis of
chondrocytes are regulated by a variety of signaling pathways,
including signaling pathway of p38 MAPK, Wnt-β catenin, nuclear
factor (NF)-κβ, OPG, PANK, PANKL and Hedgehog (12-15).
Among them, p38 MAPK signaling pathway is relatively clear, which
is involved in the degradation of the ECM of cartilage and
mediating inflammatory response, and involves a variety of kinases
and substrates, laying a solid foundation for further study on the
pathogenesis of OA. Therefore, the mechanism that quercetin
protecting articular chondrocytes may be mediated by targeting and
blocking the p38 MAPK signaling pathway was tested. Based on
bioinformatics, the present study used network pharmacology to
explore the targets of quercetin and analyze the mechanism of
action of the drug, which is of great significance for the
application and promotion of quercetin in the field of OA.
Experimental studies in cells in vitro were performed to
detect the expression of factors of the p38 MAPK signaling pathway
in articular chondrocytes and to observe the effect of quercetin on
articular chondrocytes. This will help to further explore the
pathogenesis of OA and the target of quercetin, in order to provide
theoretical and experimental basis for the prevention and treatment
of OA.
Materials and methods
Materials
Dulbecco's modified Eagle's medium (DMEM; cat. no.
CM15019) and fetal bovine serum (FBS; cat. no. 10100147) were
obtained from Gibco (Thermo Fisher Scientific, Inc.). Quercetin
(cat. no. HY-18085; purity >98.0%) and p38 MAPK signal pathway
blocker (SB203580; cat. no. HY-10256) were purchased from
MedChemExpress. Toluidine Blue (cat. no. G3660) was purchased from
Beijing Solarbio Science & Technology Co., Ltd. Cell Counting
Kit-8 (CCK-8; cat. no. CCK805) and Annexin V-FITC/PI Apoptosis kit
(cat. no. 70-AP101-100) were purchased from MULTI SCIENCES (LIANKE)
BIOTECH CO., LTD. Anti-MMP13 antibody (cat. no. ab51072), anti-p38
antibody (cat. no. ab47363), anti-ADAMTS-4 antibody (cat. no.
ab185722) and anti-collagen II antibody (cat. no. ab34712) were
purchased from Abcam. Anti-phosphorylated (P)-p38 antibody (cat.
no. AF4001) was obtained from Affinity Biosciences. Horseradish
peroxidase (HRP)-conjugated goat anti-rabbit IgG antibodies (cat.
no. GB23303), anti-β-actin antibody (cat. no. TA-09) and first
Strand cDNA Synthesis kit were purchased from Wuhan Servicebio
Technology Co., Ltd. Enzyme-linked immunosorbent assay (ELISA) kit
for rat 1L-1β (cat. no. EK301B/3-96) and ELISA kit for rat TNF-α
(cat. no. EK382/3-96) were supplied by Wuhan Servicebio Technology
Co., Ltd. Specific primers were designed and synthesized by Wuhan
Servicebio Technology Co., Ltd., as displayed in Table I.
 | Table IList of primer sequences of rat for
reverse transcription-quantitative PCR. |
Table I
List of primer sequences of rat for
reverse transcription-quantitative PCR.
| Genes | Primer sequences
(forward and reverse) |
|---|
| p38 | Forward,
5'-GTGCCCGAACGATACCAGAAC-3' |
| | Reverse,
5'-TGAATTCCTCCAGTGACCTTGC-3' |
| MMP-13 | Forward,
5'-CTATCCCTTGATGCCATTACCAG-3' |
| | Reverse,
5'-TAAGGTCACGGGATGGATGTTC-3' |
| ADAMTS-4 | Forward,
5'-ACCGTCAAGGCTCCTTCTGG-3' |
| | Reverse,
5'-ACCAAGTTGACAGGGTTTCGG-3' |
| Collagen Ⅱ | Forward,
5'-ACGCTACACTCAAGTCACTGAACAAC-3' |
| | Reverse,
5'-TCAATCCAGTAGTCTCCGCTCTTC-3' |
| β-actin | Forward,
5'-GTGACGTTGACATCCGTAAAGA-3' |
| | Reverse,
5'-GTAACAGTCCGCCTAGAAGCAC-3' |
Network pharmacology of quercetin
against OA
The Traditional Chinese Medicine Systems
Pharmacology Database and Analysis Platform database (TCMSP,
https://tcmspw.com/index.php) search was
used to find protein targets of quercetin, and the Uniprot database
(https://www.uniprot.org/) was used to find the
gene name of the associated protein. Quercetin and its
corresponding target genes were introduced into Cytoscape 3.6.1
(https://apps.cytoscape.org/apps/bisogenet) to
construct the information network of quercetin and gene targets.
Online Mendelian Inheritance in Man (OMIM, https://omim.org/), Therapeutic Target Database (TTD,
http://db.idrblab.net/ttd/) and PharmGKB
databases (https://www.pharmgkb.org/) were
searched for the targets associated with OA, and then the screening
results were introduced into Cytoscape. Bisogenet bioinformatics
plug-in (https://apps.cytoscape.org/apps/bisogenet) was used to
construct a network of osteoarthritis target genes. Through
Cytoscape, the aforementioned chemical components, OA and
associated target gene networks were combined to screen common
target genes, which were analyzed by GO (Gene Ontology, https://david.ncifcrf.gov/), KEGG (Kyoto Encyclopedia
of Genes and Genomes, https://david.ncifcrf.gov/) and molecular docking
technology (Autodock Vina software, http://vina.scripps.edu/download.html), respectively
to further discover and elucidate the application mechanism of
quercetin in the protection of articular cartilage in OA.
Primary cell extraction
All rats were used according to the national
guidelines of the care and use of laboratory animals with the
approval of the Animal Ethics Committee of Affiliated Hospital of
Shandong University of Traditional Chinese Medicine (AWE-2019-043).
A total of 2 Sprague-Dawley (S-D) male rats (age, 1 week) were
purchased from Experimental Animal Center of Shandong University
(Shandong, China). According to the method recommended by American
Veterinary Medical Association (AVMA), S-D male rats were
euthanized with pentobarbital sodium (100-150 mg/kg,
intraperitoneally), with their hair cleaned, and disinfected with
75% alcohol. The articular cartilage of the femoral condyle and
tibial plateau was extracted under aseptic conditions, washed with
1% dual-antibody PBS solution, and the tissue was cut into
fragments (1 mm3 in size), digested in 0.25% trypsin,
incubated at 37˚C for 30 min and digested with 0.2% collagen Ⅱ
solution for 4 h. Chondrocytes were seeded at a density of
2x105/ml in a 25-cm2 culture flask and
cultured in an incubator at 37˚C, in a humidified atmosphere
containing 5% CO2. Then, the primary cells were
isolated. When the bottom of the culture flask was covered with
chondrocyte (80% confluence), the cell passage was carried out,
with a ratio of 1:3. The experiment was carried out when the cells
were transferred to the second generation and entered the
logarithmic growth stage.
Toluidine blue staining
The chondrocytes of the knee joint (the second
generation) were taken and inoculated into a 6-well plate at
5x104 cells/well. When the number of cells reached more
than half of the area, 4% paraformaldehyde was used to fix them at
room temperature for 30 min. After rinsing for 5 min, toluidine
blue dye solution was added evenly, and after staining at room
temperature for 15 min, distilled water and PBS buffer solution
were rinsed until dark blue disappeared. Finally, the cells were
rinsed with anhydrous ethanol until colorless and observed (10x
magnification, Axiovert 40 inverted phase-contrast microscope,
Zeiss AG).
Optimal intervention concentration of
quercetin screened by CCK-8
Inoculation density of cells was 2,000/well, for
each group of five wells. The normal complete culture medium was
used in the apoptosis group and the blank group, and 10 ng/ml IL-1β
was used in the drug group. After 24 h culture in the incubator,
the medium was discarded. Normal medium (100 µl) was added to the
apoptosis group and the blank group, and quercetin medium at
different concentrations (0, 50, 100, 150, 200, 400 and 600 µmol/l)
was added to the drug group, respectively. After intervention for
24 h, 10 µl CCK-8 solution was added to each well and incubated at
37˚C for 2 h. OD values of each well were detected at 450 nm
wavelength.
Experimental grouping and
intervention
The cells were divided into 6 groups. All groups
except W1 were treated with 10 ng/ml IL-1β and cultured for 24 h at
37˚C in a humidified atmosphere containing 5% CO2. After
successful intervention, normal complete medium was added to group
W1 and group W2, complete medium containing 0.1% DMSO was added to
group W3, complete medium containing 100 mM quercetin was added to
W4 group, and complete medium containing 10 µM SB 203580 was added
to W5 group. A complete culture medium containing 100 µmol/l
quercetin and 10 µM SB 203580 was added to group W6 and all cells
were cultured for 24 h. Supernatant was collected and stored at
-80˚C.
ELISA for detecting IL-1β and
TNF-α
Standard samples with 2X diluted standard was added
to the standard wells in turn, cell culture medium was added to the
blank wells, and buffer (1X) and samples were added to the sample
wells, with three samples in each group. The treated detection
antibody was added to each well, the plate was sealed, and the
samples were incubated whilst shaking at room temperature for 2 h.
After all the samples were washed, horseradish peroxidase-labeled
streptomycin was added to each well, and the samples were incubated
at room temperature for 45 min. The plate was washed. Then, after
the color-rendering substrate, TMB was added to 96 orifice plate,
the samples were cultured at room temperature for 20 min, avoiding
light. OD value at 450 nm wavelength was measured within 30 min by
an enzyme marker.
Apoptosis detected by flow
cytometry
After adjusting the instrument parameters, 500 µl 1X
binding buffer was added to each group of cells at room
temperature. 5 µl annexin V-FITC and 10 µl PI were added to each
tube. After gentle vortexing, the tubes were incubated for 5 min in
dark at room temperature prior to detection with the flow cytometer
used (Cyto FLEX, Beckman Coulter, Inc.).
Reverse transcription-quantitative
(RT-q)PCR for detecting inflammatory factors
After the completion of cell intervention in each
group, the medium was removed and the cells were washed with the
PBS buffer three times. TRIzol® was added to the cells,
and total RNA was extracted. After extraction, RNA concentration in
each group was detected by Nanodrop 2000. cDNA was synthesized from
mRNA by Revert Aid First Strand cDNA Synthesis kit at 42˚C for 60
min and at 80˚C for 5 min. The Light Cycler 480 fluorescence
quantitative PCR instrument (Roche Diagnostics) and SYBR Premix Ex
Taq II (Takara Biotechnology Co., Ltd.) were used for amplification
and detection, respectively. A 25-µl PCR reaction mixture contained
2 µl cDNA, 12.5 µl SYBR Premix Ex Taq II, 2 µl each primer and 8 µl
diethyl pyrocarbonate water. Following an initial denaturation step
at 95˚C for 10 min, p38, MMP-13, ADAMTS-4, Collagen Ⅱ and ß-actin
were amplified with 40 cycles at 95˚C for 15 sec, 60˚C for 30 sec
and 68˚C for 30 sec. The levels of β-actin were used as an internal
control. Each reaction was repeated three times. Quantitative data
were calculated with 2-ΔΔCq method (16).
Western blot analysis for detecting
the expression levels of proteins associated with the p38 MAPK
signaling pathway
Following treatment with quercetin as described for
the immunofluorescent staining assay, protein was extracted from
the cartilage cells using radioimmunoprecipitation assay buffer
(Beyotime Institute of Biotechnology) containing phenylmethane
sulfonyl fluoride. Protein samples (30 µg/lane) were separated by
SDS-PAGE (8%) and transferred to polyvinylidene difluoride
membranes. All primary antibodies were prepared to the same
dilution (dilution, 1:1,000), the membranes were incubated
overnight at 4˚C with primary anti-p38 antibody (cat. no. ab47363,
Abcam), anti-phosphorylated (P)-p38 antibody (cat. no. AF4001,
Affinity Biosciences), anti-MMP13 antibody (cat. no. ab51072,
Abcam), anti-ADAMTS-4 antibody (cat. no. ab185722, Abcam) and
anti-collagen II antibody (cat. no. ab34712, Abcam) followed by the
HRP-conjugated goat anti-rabbit IgG secondary antibody (dilution,
1:3,000) at room temperature for 30 min. The HRP-conjugated goat
anti-rabbit IgG antibodies (cat. no. GB23303) were purchased from
Wuhan Servicebio Technology Co., Ltd.. The blots were then
visualized using a chemiluminescent detection kit (Amersham; GE
Healthcare Life Sciences). A Typhoon Phosphor Imager with Image
Quant TL software version 7.0 (both GE Healthcare Life Sciences)
was used to quantify the protein. The antibody against β-actin
served as an internal reference. The expression level of Gray scale
integration of target protein in each sample was determined as
follows: Expression level of target protein-densitometric value of
target protein/densitometric value of β-actin.
Statistical analysis
All data were expressed as mean ± standard deviation
(X±S), and SPSS 20.0 statistical software (IBM Corp.) was used to
perform a one-way analysis of variance (ANOVA) among multiple
groups, Dunnett's multiple comparisons test was used as the
post-hoc test. P<0.05 was considered to indicate a statistically
significant difference.
Results
Network pharmacology study of
quercetin in the treatment of osteoarthritis
In total, there were 89 corresponding targets of
quercetin. Protein names were found through Uniprot online
database. Meanwhile, the target network of quercetin was
constructed and analyzed by using the Cytoscape3.6.1 software. Each
node represents the target gene or compound, each edge represents
the association between nodes, and the degree represents the
corresponding number of nodes associated with other nodes. The
greater the degree is, the more likely it is to be the ‘center’ in
the construction network diagram. The network has 90 nodes (1
chemical component, 89 target genes) and 89 edges, which reflects
the complex network of multi-target associations of one drug and
further validates the synergistic prevention and treatment effect
of quercetin on multi-targets and multi-paths. Three databases
(OMIM, TTD and PharmGKB) were screened to obtain the target genes
associated with osteoarthritis. The duplicated genes were
eliminated, and 2976 genes associated with OA remained. The
Bisogenet plug-in was used to construct a network of
osteoarthritis-target genes, in which the constructed network has a
total of 436451 edges and 14141 nodes. Through the analysis, the
complex association between the pathogenic factors and pathogenesis
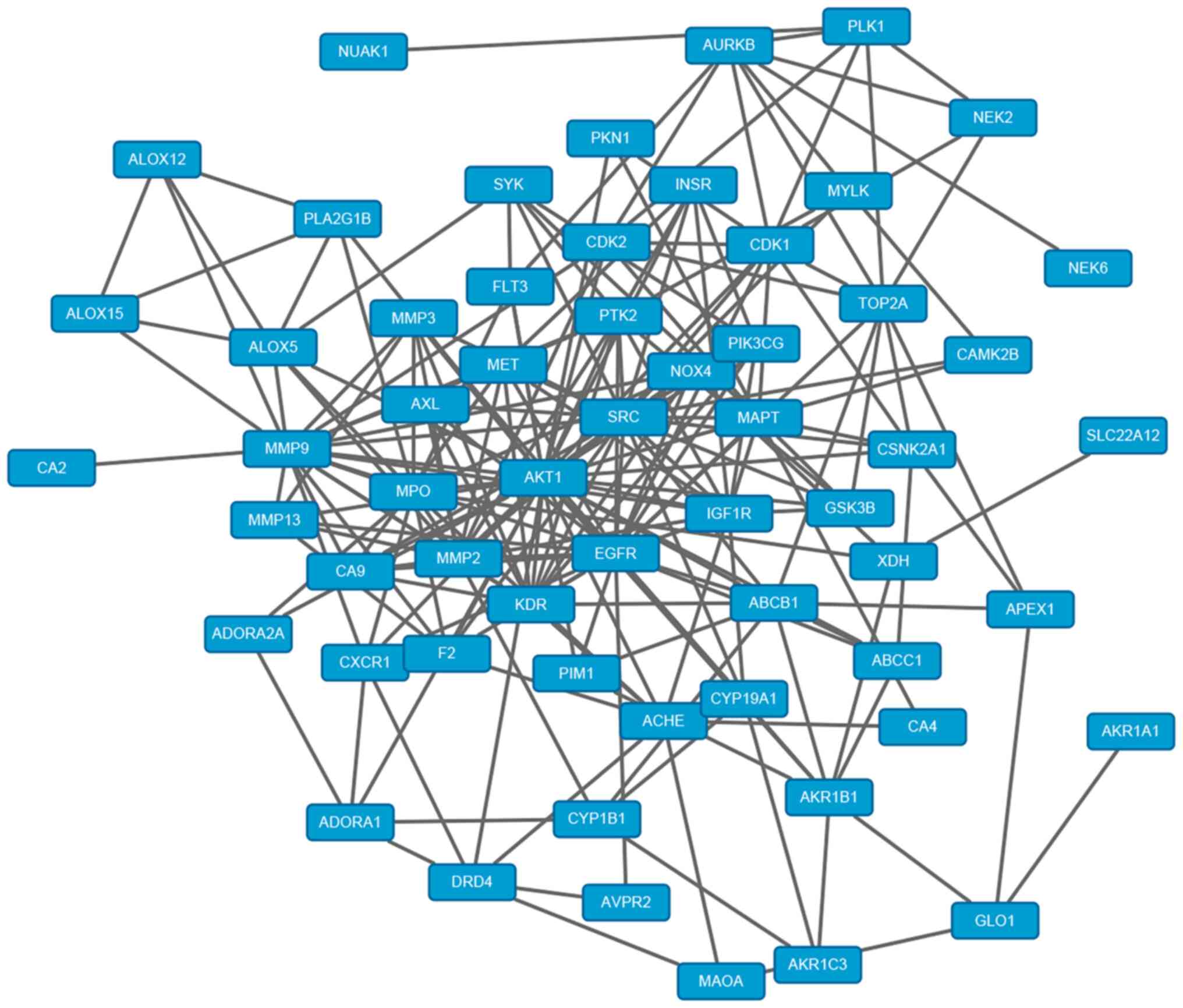
of osteoarthritis was demonstrated. A total of 66 co-acting target
genes were screened out by combining the network of chemical
component-target gene and that of osteoarthritis-target gene
(Fig. 1). Autodock Vina software
was used to dock quercetin with OA inflammatory factor target
proteins (TNF-α, 1L-1β, MMP-13, ADAMTS-4, ADAMTS-5 and MAPKK6)
under the p38 MAPK signaling pathway. Quercetin had the strongest
binding energy with MMP-13 (Table
II).
 | Table IITable of molecular docking between
quercetin and compounds. |
Table II
Table of molecular docking between
quercetin and compounds.
| Chemical
compound | Binding energy,
kcal/mol |
|---|
| TNF-α | -6.1 |
| IL-1β | -8.3 |
| MMP-13 | -9.7 |
| ADAMTS-4 | -9.3 |
| ADAMTS-5 | -8.2 |
| MAP2K6 | -9 |
Extraction and identification of
articular chondrocytes in rats
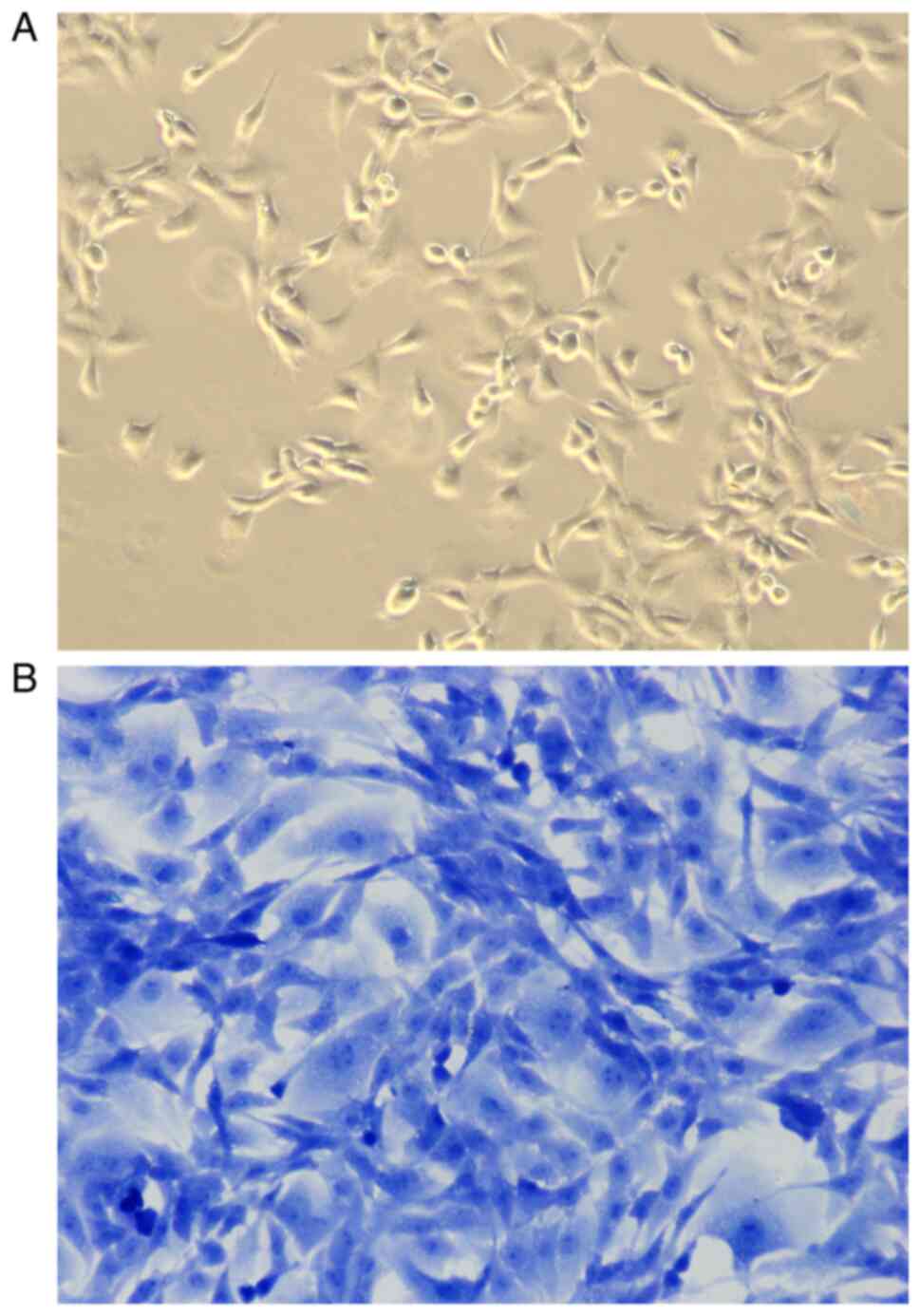
In the present study, toluidine blue staining was
used to identify articular chondrocytes isolated from articular
cartilage tissues in SD rats. As shown in Fig. 2, chondrocytes grew adherent to the
wall, in the shape of polygonal or fusiform, with basically the
same shape and size, abundant cytoplasm, clear nuclei, and good
growth status (Fig. 2A). After
staining with toluidine blue, the nuclei were stained dark blue and
the cytoplasm and stroma were stained light blue (Fig. 2B).
Optimal concentration of quercetin to
prevent IL-1β-induced inflammatory cytokines expression in
chondrocytes
The optimal concentration of quercetin in
intervening with articular chondrocytes of rats was screened by
CCK-8 (Fig. 3A). The cell viability
of the 0 µmol/l quercetin intervention group was significantly
lower compared with that of the non-treated control after culturing
in medium containing 1L-1β for 24 h, and the difference was
statistically significant. Thus, IL-1β at the concentration of 10
ng/ml could induce the degeneration of rat chondrocytes, and the
model was successfully established. At the same time, compared with
the cell viability of degenerative articular chondrocytes of the 0
µmol/l quercetin intervention group cultured in normal complete
medium, the increase in cell viability of the 100 µmol/l quercetin
intervention group was significant, which shows that the optimal
concentration of quercetin is 100 µmol/l. In the present study, 100
µmol/l quercetin was used for subsequent interventions.
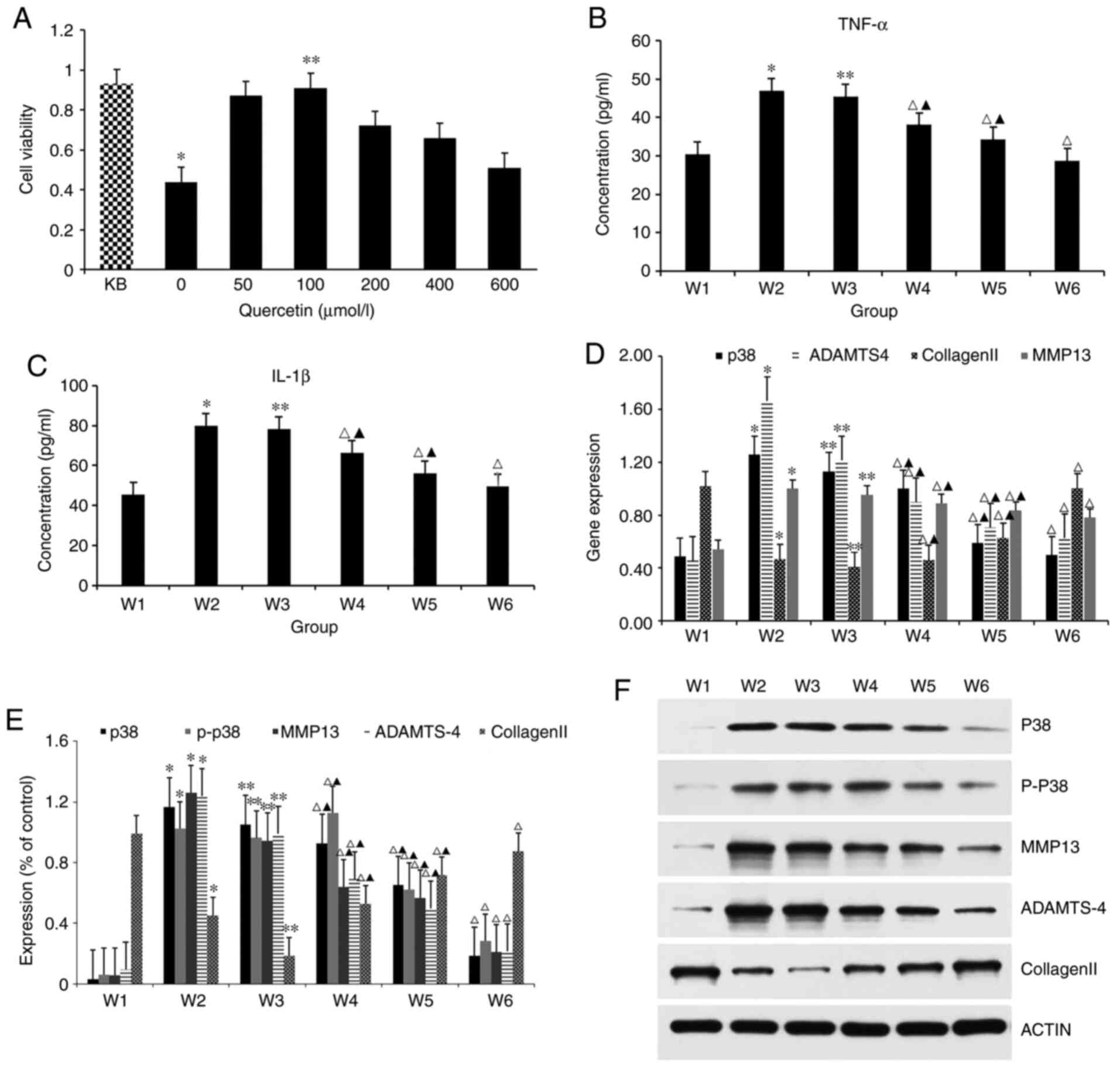 | Figure 3Various studies in IL-1β-induced
chondrocytes treated with quercetin. (A) Cell viability was
evaluated in IL-1β-induced chondrocytes treated with quercetin by
Cell Cycle Kit-8. KB represents group without 10 ng/ml IL-1 β
intervention. *P<0.05 vs. KB. **P<0.05
vs. 0 µmol/l. (B and C) Detection of the expression of IL-1β and
TNF-α in p38 MAPK signaling pathway were measured by enzyme-linked
immunosorbent assay kit. *P<0.05 vs. W1;
**P>0.05 vs. W2; ΔP<0.05;
▲P<0.05 vs. W1. (D) Reverse
transcription-quantitative PCR was performed to determine the
expression levels of p38, ADAMTS-4, collagen Ⅱ and MMP-13 in
chondrocytes under IL-1β stimulation. *P<0.05 vs. W1;
**P>0.05 vs. W2; ΔP<0.05;
▲P<0.05 vs. W1. (E and F) The associated proteins
(p38, P-p38, MMP-13, ADAMTS-4 and collagen Ⅱ) were evaluated by
western blotting. *P<0.05 vs.
W1;**P>0.05 vs. W2; ΔP1,0.05;
▲P<0.05 vs. W1. IL, interleukin; TNF, tumor necrosis
factor; MMP, matrix metalloprotease; P-, phosphorylated. |
Effects of quercetin on the expression
levels of inflammatory factors upstream of the p38 MAPK
pathway
The cell culture supernatant was collected as a
specimen, and the expression levels of inflammatory factors, IL-1β
and TNF-α in the p38 MAPK signaling pathway were detected by ELISA
(Fig. 3B and C). The ELISA results showed that the
expression levels of IL-1β and TNF-α were the lowest in the W1
group and the highest in the W2 group, and there was a significant
difference between them, indicating that IL-1β at 10 ng/ml could
cause chondrogenic degeneration. Compared with the inflammatory
factor expression level of the W2 group, the expression level in
the W3 group (DMSO) was approximately the same, and no obvious
difference was found between the two groups, showing that 0.1% DMSO
had no effect on the expression of inflammatory factors in
cartilage cells. DMSO can be applied as a solvent to dissolve the
blocker, SB2035804, when its concentration is <0.1%. For the
expression levels of inflammatory factors, IL-1β and TNF-α in the
p38 MAPK signaling pathway, quercetin intervention in W4 group, W5
group and W6 group can effectively decrease the expression of
inflammatory factors in degenerative chondrocytes, compared with
that in the W2 group inflammatory factor expression level. However,
when quercetin is used in combination with the blocker, the
expression level of inflammatory factors is the lowest, with a
significant statistical difference, indicating that both quercetin
and SB203580 can lower the expression of inflammatory factors in
chondrocytes, with similar effects, but the effect of quercetin is
weaker than SB203580.
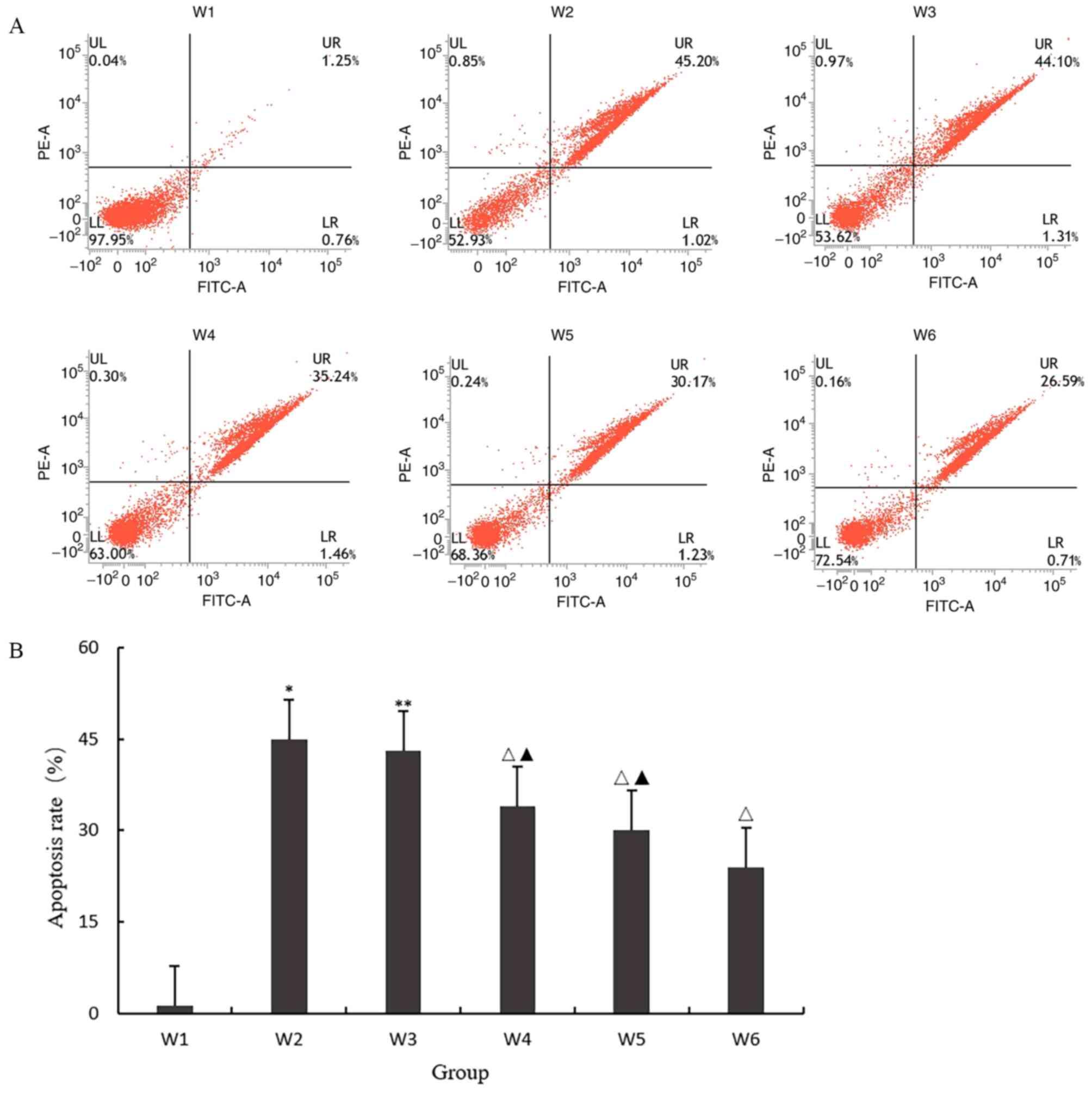
Effect of quercetin on apoptosis
As shown in Fig. 4A
and B, the apoptosis rate of the W2
group was significantly higher compared with that of the W1 group
(P<0.05), indicating that 10 ng/ml IL-1β could induce
chondrocyte degeneration and apoptosis; the apoptosis rate of W3
group was approximately the same as that of the W2 group
(P>0.05), indicating that 0.1% DMSO had no effect on the
expression of inflammatory factors in chondrocytes. When the final
concentration was <0.1%, DMSO solution could be used as solvent
to dissolve SB203580; W4, W5 and W6 had the same effect, and the
apoptosis rate of the three groups was significantly decreased. The
results showed that quercetin and SB203580, a p38 MAPK signal
pathway blocker, could inhibit the apoptosis of degenerative
chondrocytes, but the effect of quercetin was weaker than that of
the pathway blocker.
Expression levels of associated
proteins by targeting p38 MAPK signaling pathway via quercetin
Western blot analysis was used to detect the
expression levels of associated proteins p38, P-p38, MMP-13,
collagen Ⅱ and ADAMTS-4 in the p38 MAPK signal pathway of cells in
each group (Fig. 3E and F). Results showed that the expression
level of collagen II was highest in the W1 group and lowest in the
W2 group. The expression of MMP-13, p38, P-p38, and ADAMTS-4 in the
W1 group was lowest but highest in the W2 group. There was
significant difference between them, showing that IL-1β at 10 ng/ml
can stimulate the expression of MMP-13, p38, P-p38 and ADAMTS-4 in
chondrocytes, and inhibit the expression of type II collagen,
causing chondrocyte degeneration. Compared with protein expression
level in the W2 group, the expression level in the W3 group was
about the same, and there was no significant difference between
them, indicating that 0.1% DMSO had no effect on chondrocyte
protein expression, and 0.1% DMSO solution can be used as a solvent
to dissolve SB2035803. For expression levels of associated
proteins, quercetin intervention in the W4, W5 and W6 groups can
effectively lower the expression levels of MMP-13, p38, P-p38, and
ADAMTS-4, compared with that in the W2 group, while increasing the
expression of type II collagen. When combined, the protein
expression level changed most significantly, and the difference was
statistically significant, showing that quercetin and the blocking
agent SB203580 can change the expression level of proteins
associated with p38 MAPK signaling pathway of chondrocytes;
however, the effect of quercetin is weaker than that of SB 2035803
blocking agent.
Quercetin decreases the expression of
IL-1β-induced inflammatory cytokines in chondrocytes with blocking
the p38 MAPK pathway
For mRNA levels, RT-qPCR results showed that the
expression level of collagen II mRNA was highest in the W1 group
and lowest in the W2 group. The expression of the MMP13 mRNA and
p38 mRNA in the W1 group was lowest and highest in the W2 group,
respectively. The significant difference shows that IL-1β at 10
ng/ml can stimulate the expression of MMP-13, p38 and ADAMTS-4 in
chondrocytes, and inhibit the expression of type II collagen,
causing chondrocyte degeneration. Meanwhile, compared with mRNA
expression level in the W2 group, W3 expression level in the DMSO
group was approximately the same, indicating that 0.1% DMSO had no
effect on the expression of chondrocyte genes. Quercetin
intervention in the W4, W5 and the W6 groups could effectively
lower the expression levels of MMP-13 mRNA, p38 mRNA and ADAMTS-4
mRNA, compared with that in the W2 group, but it increased the
expression of type II collagen in each group. However, when used in
combination, the gene expression level changed more significantly
and the difference was statistically significant, indicating that
quercetin and the blocking agent SB203580 both have the same
effect, and both can change the expression levels of genes
associated with the p38 MAPK signaling pathway of chondrocytes;
however, the effect of quercetin is weaker than that of the blocker
(Fig. 3D).
Discussion
Network pharmacology is a new research method based
on bioinformatics system and drug information system, which can use
official databases to explore data on drugs or their ingredients
and make scientific predictions of disease-associated target
factors and pathways. Cytokines are a type of high-activity
proteins secreted by cells, and are widely involved in the
physiological functions and pathological changes in the body,
playing an important role in the regulation of chondrocyte
proliferation, apoptosis and ECM metabolism. Specific signal
pathways are important for the development of diseases. Quercetin,
widely found in fruits, vegetables, and Chinese herbal medicines,
has anti-inflammatory and anti-oxidative stress effects. According
to the existing research, quercetin may have a protective effect on
the articular cartilage of osteoarthritis. Therefore, the network
pharmacology technology was used in the present study to explore
the targets and pathway of quercetin and analyze the mechanism of
the drug in the field of OA. Cell experiments were conducted to
verify that quercetin blocks the p38 signaling pathway to protect
articular cartilage, which is of great significance for the
development of quercetin as a therapeutic in this field.
By using network pharmacology to predict disease
targets, 66 target genes were identified, including MMP family,
ALOX family, CA family, and PI3K, which are most likely potential
targets for quercetin to protect articular cartilage. GO was used
to analyze and process relevant data, and it was found that the
predicted targets were involved in a number of body regulation,
such as active oxygen metabolism, injury response regulation,
vitamin metabolism, NO metabolism process, body aging, cell
movement regulation, cadmium response and other processes. The
aging of the body decreases the tolerance of the articular
cartilage, causing wear and tear, which leads to osteoarthritis
pain. Studies have shown that reactive oxygen are associated with
various diseases, including cancer, inflammation of various parts
of the body and cardiovascular diseases (17,18).
Nitric oxide (NO) plays an important role in osteochondral repair.
Studies have found that nitric oxide synthase (NOS) inhibitors have
a significant effect on inhibiting the release of NO from
regenerating cartilage. When NOS activity is decreased, NOS
inhibitor has obvious effect in improving the quality of cartilage
regeneration (19,20). Vitamin metabolism has an inseparable
association with bone diseases. Vitamin D can regulate the cell
activity of osteoblasts and osteoclasts, Vitamin A can inhibit
osteoblast function, and Vitamin K can inhibit bone resorption
activation factors (IL-1 and IL-6), and lower osteoclast activity,
bone loss, and the risk of osteoarthritis (21). Furthermore, low concentrations of
vitamin B may also be a trigger for bone mass reduction (22,23).
The reaction process of cadmium can enhance the activity of
osteoclasts, and bones are in the list of important target organs
damaged by cadmium. Therefore, osteoclasts may be the target cells
for bone diseases caused by changes in cadmium (24-26).
Cadmium interferes bone metabolism, and its mechanism is associated
with the effect on bone formation and absorption.
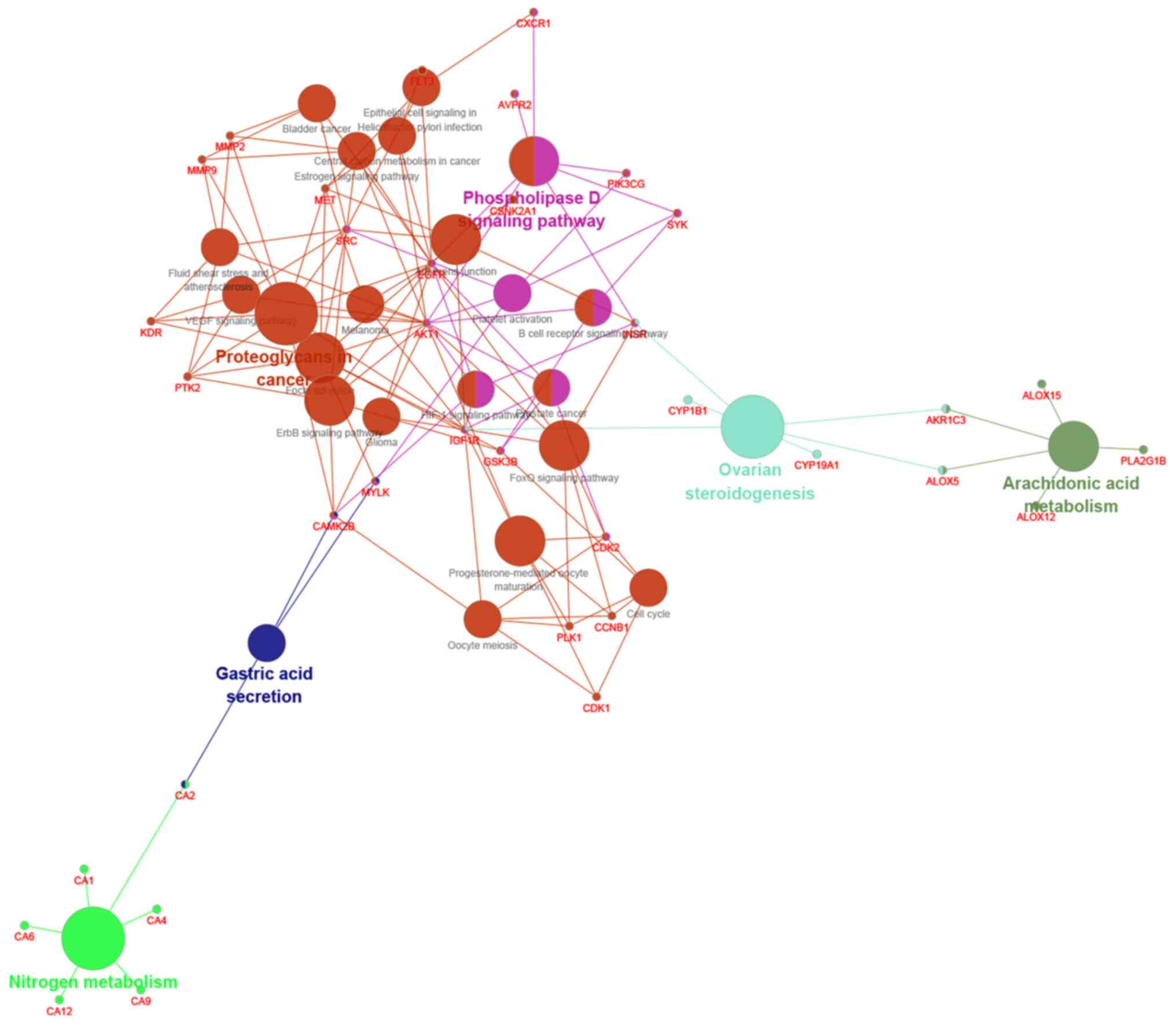
KEGG analysis for signaling pathways was used,
showing that quercetin is associated with multiple signaling
pathways, many of which can participate in the regulation of OA, as
shown in Fig. 5. According to
prediction results, there are certain associations between
osteoarthritis and MAPK signal pathway, Wnt/β-catenin signal
pathway, NF-κβ signal pathway, hypoxia-inducible factor (HIF)-1
signal pathway, Hedgehog signal pathway, Notch signal pathway and
other associated pathways. The Wnt/β-catenin signaling pathway,
which has been studied for a long time, has the function of
differentiating osteoblasts and osteoclasts (27). MAPK signaling pathway is the signal
transduction system that mediates and regulates degenerative
changes fin cartilage in OA, and the activation of MAPK-associated
signaling pathway can change the expression of matrix
metalloproteinases and regulate a series of reactions, such as
chondrocyte apoptosis and bone destruction (28). Pathological activation of the NF-κB
signaling pathway induces a variety of inflammatory reactions in
the body and affect the occurrence and development of arthritis
diseases, whose role is to regulate the inflammatory response of
cells (29). The Notch signal
pathway is to ensure the phenotype of chondrocytes under different
environments, regulate the differentiation of chondrocytes, and
affects the metabolism of cartilage matrix, whose activation can
affect the expression of MMP-13 and VEGFA, eventually leads to the
destruction of articular cartilage (30,31).
The p38 MAPK signaling pathway verified in the
present study experiment is highly activated in OA articular
chondrocytes, which is an important signaling pathway that mediates
the pathological progress of OA. This signal pathway belongs to the
MAPK signal pathway that was predicted through network pharmacology
technology. Fan et al (32)
found that the expression of phosphorylated p38 in the articular
cartilage of patients with OA was significantly higher compared
with that in normal articular cartilage tissue, which was
consistent with the expression trend of ERK. Rasheed et al
(33) detected the expression of
p38α, p38γ and p38δ in OA chondrocytes, but did not detect the
expression of p38β using RT-PCR, western blotting and other
methods. Meanwhile, IL-1β could significantly increase p38α and
p38γ phosphorylation, but did not have a significant effect on p38δ
phosphorylation. Xu et al (34) clarified that the p38 MAPK signaling
pathway plays a very important role in OA chondrocyte apoptosis
using western blotting and other methods. At the same time, in the
inflammatory cartilage matrix, activation of the p38 MAPK signaling
pathway can induce the secretion of inflammatory cytokines (TNF-α,
IL-1β, NO, SOD) and the expression of matrix metalloproteinases
(MMP-3, MMP-9, MMP-13), depolymerization with platelet
thrombin-sensitive protein domain and metal proteins (ADAMTS-4,
ADAMTS-5), leading to the degradation of type II collagen, which in
turn triggers chondrocyte apoptosis. Map2k3 and map2k6 were the
main targets of p38 MAPK signaling pathway. The activation reaction
showed that the three intracellular protein kinases were activated
in turn, and MAPK was finally activated. Therefore, it is believed
that the intervention of quercetin on the highly activated p38MAPK
signaling pathway of OA articular cartilage can achieve the
prevention and treatment of articular cartilage, based on
predictions and molecular docking technology.
Related literatures demonstrated that there are many
commonly used methods for establishing in vitro degenerative
joint chondrocyte models. Qin et al (35) applied 10 ng/ml lipopolysaccharide
(LPS) to interfere with normal chondrocytes for 8 h, and
successfully obtained degenerative joint chondrocytes. LPS is
composed of lipid A, specific polysaccharides and non-specific core
polysaccharides, which can induce the expression of inflammatory
factors such as IL-1β and TNF-α, but this method acts on the NF-κB
pathway (36). Therefore, this
method does not meet the requirements because the signal pathway
target of the present study is the p38 MAPK pathway. There have
been many studies using 10 ng ml IL-1β for the induction of normal
chondrocytes for 24 h to successfully obtain degenerated
chondrocytes (37,38). This method uses inflammatory factors
to directly stimulate the cells to undergo inflammatory changes,
which is similar to the pathogenesis of OA with high modeling
success rate, and it is also one of the most widely used methods in
research. In addition, there are also modeling methods using 10 µM
trans-retinoic acid for 24 h or using NO to induce normal
chondrocytes, but the use of these methods is still in the
preliminary exploration stage, and they are not commonly used and
have not been widely promoted (39). Thus, the most recommended methods
are 10 ng ml IL-1β induction of normal chondrocytes for 24 h, 1 mM
sodium nitroprusside intervention for 24 h, and 10 ng/ml LPS
intervention for 8 h (40). The
purpose of the study was to verify that quercetin protects
articular cartilage through targeted regulation of the p38 MAPK
signaling pathway, and IL-1β induction is more direct and rapid,
which is closest to the pathogenesis of OA and has been used most
frequently in the literature with higher rate of success.
Experiments on IL-1β-induced degeneration of
cartilage cells showed that the expression level of TNF-α and IL-1β
was significantly increased. The expression quantity of p38 was
sharply increased and phosphorylated, generating P-p38. Then, the
expression of type II collagen declined, while the expression of
MMP-13 increased. It is postulated that TNF-α and IL-1β, as start
factors in the p38 MAPK inflammatory signaling pathways, are
increased in the degeneration of articular cartilage cells, and
then p38 MAPK signaling pathways is activated, leading to its
phosphorylation. As a result, phosphorylation levels increase after
p38 MAPK signaling pathways are activated, which in turn will
increase the secretion of TNF-α and IL-1β, inhibit the expression
of type II collagen, and activate the expression of MMP-13, leading
to further degeneration of articular cartilage cells. The
degradation of type II collagen fibers plays a major role in the
degeneration of cartilage. MMP-13 is 10 to 30 times as capable of
degrading type II collagen fibers as other MMPs, and MMP-13 is also
required for the degradation of other collagen fiber enzymes. MMPs
are a family of zinc-dependent proteinases that are characterized
by promoting the turnover of various ECM proteins, including
collagens and proteoglycans. MMP13 is the major enzyme involved in
OA cartilage erosion, due to its potent proteolytic effects on type
II collagen (41). Thus, the
inhibition of MMP-13 expression may impede type II collagen
degradation. The target gene of quercetin protecting articular
cartilage predicted by GO analysis is involved in the metabolism of
NO, which also indicates that quercetin could protect articular
cartilage. The ADAMTS protein family is involved in pathogenic
cartilage degradation. The most efficient aggrecanases relevant to
joint disease are ADAMTS-4 and ADAMTS-5(42). As for factors associated with the
signal pathway, the expression level of TNF-α and IL-1β was
significantly decreased with the intervention of quercetin, MAPK
signaling pathway blocker SB 203580, or the two mixed; the
expression of type II collagen rises, and that of p38, MMP-13, or
ADAMTS-4 drops. Thus, it can be seen that p38 MAPK signaling
pathway plays an important role in regulating the pathological
progress of OA and is one of the important targets for the
prevention and control of OA. Quercetin and blockers SB203580 both
can inhibit the expression of inflammatory factor, MMPs, TNF,
IL-1β, improve collagen Ⅱ expression to accelerate the degeneration
of cartilage cell repair, and protect the articular cartilage
cells. However, according to the experimental results, it is found
that its effect is weaker compared with that of the blocker
SB203580, which blocks the p38 MAPK signaling pathway but not
completely, and its specificity needs to be further explored.
Based on the data from network pharmacology and
in vitro experiments, it was demonstrated that quercetin
could lower the expression of inflammatory factors of cartilage for
the prevention and treatment of OA. By blocking the p38 MAPK
signaling pathway, the expression level of associated factors can
be improved. Quercetin can promote the repair of degenerative
chondrocytes and protect articular chondrocytes.
Acknowledgements
Not applicable.
Funding
Funding was provided by Jinan Science and Technology plan
project (grant no. 201805044).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
XPW and RXB contributed to the conception and design
of the study, and the acquisition, analysis and interpretation of
the data of the study. They also contributed to the drafting of the
work and its critical revision for important intellectual content.
WPX and SLW contributed to the conception and design of the study,
the acquisition, analysis and interpretation of the data of the
study, contributed to the drafting of the work and its critical
revision for important intellectual content. BAW, YFB and HBS
contributed to the acquisition, analysis and interpretation of data
of the study, contributed to the drafting of the work and its
critical revision for important intellectual content. XPW and RXB
confirm the authenticity of all the raw data. All authors read and
approved the final version of the manuscript and agreed to be
accountable for all aspects of the study in ensuring that questions
related to the accuracy or integrity of any part of the work are
appropriately investigated and resolved.
Ethics approval and consent to
participate
All rats were used according to the national
guidelines of the care and use of laboratory animals with the
approval of the Animal Ethics Committee of Affiliated Hospital of
Shandong University of Traditional Chinese Medicine (approval no.
AWE-2019-043).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Lane NE, Shidara K and Wise BL:
Osteoarthritis year in review 2016: Clinical. Osteoarthritis
Cartilage. 25:209–215. 2017.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Frank M, Bwemero J, Kalunga D, Sangu W,
Semeni S, Hamisi M and Julius M: OA60 Public health and palliative
care mix; a ccpmedicine approach to reverse the overgrowing burden
of non-communicable diseases in tanzania. BMJ Support Palliat Care.
5 (Suppl 1)(A19)2015.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Kim H, Kang D, Cho Y and Kim JH:
Epigenetic regulation of chondrocyte catabolism and anabolism in
osteoarthritis. Mol Cells. 38:677–684. 2015.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Mc Alindon TE, Bannuru RR, Sullivan MC,
Arden NK, Berenbaum F, Bierma-Zeinstra SM, Hawker GA, Henrotin Y,
Hunter DJ, Kawaguchi H, et al: OARSI guidelines for the
non-surgical management of knee osteoarthritis. Osteoarthritis
Cartilage. 22:363–388. 2014.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Hu G, Zhao X, Wang C, Geng Y, Zhao J, Xu
J, Zuo B, Zhao C, Wang C and Zhang X: MicroRNA-145 attenuates
TNF-α-driven cartilage matrix degradation in osteoarthritis via
direct suppression of MKK4. Cell Death Dis. 8(e3140)2017.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Xiang X, Zhou Y, Sun H, Tan S, Lu Z, Huang
L and Wang W: Ivabradine abrogates TNF-α-induced degradation of
articular cartilage matrix. Int Immunopharmacol. 66:347–353.
2019.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Favero M, Belluzzi E, Trisolino G,
Goldring MB, Goldring SR, Cigolotti A, Pozzuoli A, Ruggieri P,
Ramonda R, Grigolo B, et al: Inflammatory molecules produced by
meniscus and synovium in early and end-stage osteoarthritis: A
coculture study. J Cell Physiol. 234:11176–11187. 2019.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Basu A, Schell J and Scofield RH: Dietary
fruits and arthritis. Food Funct. 9:70–77. 2018.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Kanzaki N, Saito K, Maeda A, Kitagawa Y,
Kiso Y, Watanabe K, Tomonaga A, Nagaoka I and Yamaguchi H: Effect
of a dietary supplement containing glucosamine hydrochloride,
chondroitin sulfate and quercetin glycosides on symptomatic knee
osteoarthritis: A randomized, double-blind, placebo-controlled
study. J Sci Food Agric. 92:862–869. 2012.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Leyva-López N, Gutierrez-Grijalva EP,
Ambriz-Perez DL and Heredia JB: Flavonoids as cytokine modulators:
A possible therapy for inflammation-related diseases. Int J Mol
Sci. 17(921)2016.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Ahmad R, Sylvester J, Ahmad M and
Zafarullah M: Involvement of H-Ras and reactive oxygen species in
proinflammatory cytokine-induced matrix metalloproteinase-13
expression in human articular chondrocytes. Arch Biochem Biophys.
507:350–355. 2011.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Zhang HJ, Wei QF, Wang SJ, Zhang HJ, Zhang
XY, Geng Q, Cui YH and Wang XH: lncRNA HOTAIR alleviates rheumatoid
arthritis by targeting miR-138 and inactivating NF-κB pathway. Int
Immunopharmacol. 50:283–290. 2017.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Yuan X, Liu H, Huang H, Liu H, Li L, Yang
J, Shi W, Liu W and Wu L: The key role of canonical Wnt/β-catenin
signaling in cartilage chondrocytes. Curr Drug Targets. 17:475–484.
2016.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Saito T and Tanaka S: Molecular mechanisms
underlying osteoarthritis development: Notch and NF-κB. Arthritis
Res Ther. 19(94)2017.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Tat SK, Pelletier JP, Velasco CR, Padrines
M and Martel-Pelletier J: New perspective in osteoarthritis: The
OPG and RANKL system as a potential therapeutic target? Keio J Med.
58:29–40. 2009.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408.
2001.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Daina A, Michielin O and Zoete V:
SwissTargetPrediction: Updated data and new features for efficient
prediction of protein targets of small molecules. Nucleic Acids
Res. 47 (W1):W357–W364. 2019.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Di Meo S, Reed TT, Venditti P and Victor
VM: Role of ROS and RNS sources in physiological and pathological
conditions. Oxid MedCell Longev. 2016(1245049)2016.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Kanzaki H, Shinohara F, Kajiya M and
Kodama T: The Keap1/Nrf2 protein axis plays a role in osteoclast
differentiation by regulating intracellular reactive oxygen species
signaling. J Biol Chem. 288:23009–23020. 2013.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Lee SW, Song YS, Shin SH, Kim KT, Park YC,
Park BS, Yun I, Kim K, Lee SY, Chung WT, et al: Cilostazol protects
rat chondrocytes against nitric oxide-induced apoptosis in vitro
and prevents cartilage destruction in a rat model of
osteoarthritis. Arthritis Rheum. 58:790–800. 2008.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Yasuhara R, Miyamoto Y, Akaike T, Akuta T,
Nakamura M, Takami M, Morimura N, Yasu K and Kamijo R:
Interleukin-1beta induces death in chondrocyte-like ATDC5 cells
through mitochondrial dysfunction and energy depletion in a
reactive nitrogen and oxygen species-dependent manner. Biochem J.
389:315–323. 2005.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Booth SL, Broe KE, Peterson JW, Cheng DM,
Dawson-Hughes B, Gundberg CM, Cupples LA, Wilson PW and Kiel DP:
Associations between vitamin K biochemical measures and bone
mineral density in men and women. J Clin Endocrinol Metab.
89:4904–4909. 2004.PubMed/NCBI View Article : Google Scholar
|
|
23
|
McLean RR, Jacques PF, Selhub J, Fredman
L, Tucker KL, Samelson EJ, Kiel DP, Cupples LA and Hannan MT:
Plasma B vitamins, homocysteine, and their relation with bone loss
and hip fracture in elderly men and women. J Clin Endocrinol Metab.
93:2206–2212. 2008.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Faour WH, Mancini A, He QW and Di Battista
JA: T-cell-derived interleukin-17 regulates the level and stability
of cyclooxygenase-2 (COX-2) mRNA through restricted activation of
the p38 mitogen-activated protein kinase cascade: Role of distal
sequences in the 3'-untranslated region of COX-2 mRNA. J Biol Chem.
278:26897–26907. 2003.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Theoleyre S, Wittrant Y, Tat SK, Fortun Y,
Redini F and Heymann D: The molecular triad OPG/RANK/RANKL:
Involvement in the orchestration of pathophysiological bone
remodeling. Cytokine Growth Factor Rev. 15:457–475. 2004.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Brzoska MM and Moniuszko-Jakoniuk J:
Low-level exposure to cadmium during the lifetime increases the
risk of osteoporosis and fractures of the lumbar spine in the
elderly: Studies on a rat model of human environmental exposure.
Toxicol Sci. 82:468–477. 2004.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Brzóska MM, Majewska K and
Moniuszko-Jakoniuk J: Mineral status and mechanical properties of
lumbar spine of female rats chronically exposed to various levels
of cadmium. Bone. 34:517–526. 2004.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Albers J, Keller J, Baranowsky A, Beil FT,
Catala-Lehnen P, Schulze J, Amling M and Schinke T: Canonical Wnt
signaling inhibits osteoclastogenesis independent of
osteoprotegerin. J Cell Biol. 200:537–549. 2013.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Biniecka M, Connolly M, Gao W, Ng CT,
Balogh E, Gogarty M, Santos L, Murphy E, Brayden D, Veale DJ and
Fearon U: Redox-mediated angiogenesis in the hypoxic joint of
inflammatory arthritis. Arthritis Rheumatol. 66:3300–3310.
2014.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Kim SJ and Chun JS: Protein kinase C alpha
and zeta regulate nitric oxide-induced NF-kappa B activation that
mediates cyclooxygenase-2 expression and apoptosis but not
dedifferentiation in articular chondrocytes. Biochem Biophys Res
Commun. 303:206–211. 2003.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Hosaka Y, Saito T, Sugita S, Hikata T,
Kobayashi H, Fukai A, Taniguchi Y, Hirata M, Akiyama H, Chung UI
and Kawaguchi H: Notch signaling in chondrocytes modulates
endochondral ossification and osteoarthritis development. Proc Natl
Acad Sci USA. 110:1875–1880. 2013.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Fan Z, Söder S, Oehler S, Fundel K and
Aigner T: Activation of interleukin-1 signaling cascades in normal
and osteoarthritic articular cartilage. Am J Pathol. 171:938–946.
2007.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Rasheed Z, Akhtar N and Haqqi T:
Pomegranate extract inhibits the interleukin-1β-induced activation
of MKK-3, p38α-MAPK and transcription factor RUNX-2 in human
osteoarthritis chondrocytes. Arthritis Res Ther.
12(R195)2010.PubMed/NCBI View
Article : Google Scholar
|
|
34
|
Xu L, Zhai L, Ge Q, Liu Z and Tao R:
Vacuolar protein sorting 4B (VPS4B) regulates apoptosis of
chondrocytes via p38 mitogen-activated protein kinases (MAPK) in
osteoarthritis. Inflammation. 40:1924–1932. 2017.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Qin Y, Chen Y, Wang W, Wang Z, Tang G,
Zhang P, He Z, Liu Y, Dai SM and Shen Q: HMGB1-LPS complex promotes
transformation of osteoarthritis synovial fibroblasts to a
rheumatoid arthritis synovial fibroblast-like phenotype. Cell Death
Dis. 5(e1077)2014.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Lai JL, Liu YH, Liu C, Qi MP, Liu RN, Zhu
XF, Zhou QG, Chen YY, Guo AZ and Hu CM: Indirubin inhibits
LPS-induced inflammation via TLR4 abrogation mediated by the NF-κB
and MAPK signaling pathways. Inflammation. 40:1–12. 2017.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Lu Z, Liu Q, Liu L, Wu H, Zheng L and Zhao
JM: A novel synthesized sulfonamido-based gallate-JEZTC blocks
cartilage degradation on rabbit model of osteoarthritis: An in
vitro and in vivo study. Cell Physiol Biochem. 49:2304–2319.
2018.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Chen C, Zhu Z, Hu N, Liang X and Huang W:
Leonurine hydrochloride suppresses inflammatory responses and
ameliorates cartilage degradation in osteoarthritis via NF-κB
signaling pathway. Inflammation. 43:146–154. 2020.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Tchetina EV, Squires G and Poole AR:
Increased type II collagen degradation and very early focal
cartilage degeneration is associated with upregulation of
chondrocyte differentiation related genes in early human articular
cartilage lesions. J Rheumatol. 32:876–886. 2005.PubMed/NCBI
|
|
40
|
Wu TJ, Lin CY, Tsai CH, Huang YL and Tang
CH: Glucose suppresses IL-1β-induced MMP-1 expression through the
FAK, MEK, ERK, and AP-1 signaling pathways. Environ Toxicol.
33:1061–1068. 2018.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Liu J, Cao L, Gao X, Chen Z, Guo S, He Z,
Qian Y, Yu Y and Wang G: Ghrelin prevents articular cartilage
matrix destruction in human chondrocytes. Biomed Pharmacother.
98:651–655. 2018.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Yang Y, Gu Y, Zhao H and Zhang S: Loganin
attenuates osteoarthritis in rats by inhibiting IL-1β-induced
catabolism and apoptosis in chondrocytes via regulation of
phosphatidylinositol 3-kinases (PI3K)/akt. Med Sci Monit.
25:4159–4168. 2019.PubMed/NCBI View Article : Google Scholar
|