Introduction
In recent years, sevoflurane has become one of the
most commonly used anesthetics for medical procedures during
pregnancy (1). A previous
investigation has determined the efficacy and safety of sevoflurane
in different organ systems and patient populations (2). A wide range of studies have suggested
that during brain development, a long-term risk of neurobehavioral
disorders and neurodegeneration is apparent following exposure to
anesthetics (3,4), and that the concentration of
sevoflurane plays an important role in the process of
neurodevelopmental damage (5).
Consequently, the administration of anesthetic drugs during early
brain development may result in neural dysfunction and damage
(6).
The common consensus is that exposure to anesthetics
during pregnancy is likely to affect fetal brain development, thus
the methods used to assess the safety of surgery and anesthesia are
primarily based on animal models (7). As an inhaled anesthetic with a rapid
recovery profile, sevoflurane is prevalently used worldwide. Human
neural development is similar to that of rats, and studies of model
animals are helpful in research and the discovery of novel
therapeutic options (8,9). Although previous studies have
described the efficacy and safety of sevoflurane, the present study
primarily focused on the effects of exposure duration on
neurodevelopment (10,11). Due to the differences in drug
responses, doses and time-scales of embryological development, as
well as a number of other confounding variables, the conclusions
drawn by retrospective human studies can be difficult to
interpret.
A previous study has reported that
sevoflurane-induced neurotoxicity promotes changes in the
development of the hippocampus (12). The first trimester is a stage at
which there are high levels of neurogenesis throughout the cortex,
and the development of the hippocampus, which is responsible for
high-level cognitive functions, plays important roles in the onset
and development of various neurodevelopmental defects (13).
In the present study, the duration of sevoflurane
general anesthesia in the hippocampus and parietal cortex was
assessed in early pregnancy. The expression of marker genes such as
interferon-inducible protein AIM2 (AIM2) and CD45 was detected to
reveal neuronal cell changes. The protein levels of glial
fibrillary acidic protein (GFAP), AIM2, CD45, IL-1β, pro-caspase-1
and cleaved-caspase-1 were used to further verify these
results.
Materials and methods
Animals
A total of 18 Sprague-Dawley (SD) rats were
purchased from Beijing Weitong Lihua Experimental Animal Technology
Company Co., Ltd. [license number SCXK (Beijing) 20160006]. The
rats were of specific-pathogen free grade and at the gestational
age of 5-7 days (weight, 250±30 g). The animals were housed at
23±2˚C, 55±5% humidity with a 12-h light/dark cycle, and with free
access to food and water. All animal experiments were conducted
following the National Institutes of Health (NIH) guidelines
(14) and were reviewed and
approved by the Yantaishan Hospital Animal Protection and Use
Committee (approval no. 2018-10087). Animal health and behavior
were monitored every day, including assessment of diet, weight,
mental states and mortality.
Animal grouping
Pregnant SD rats were randomly divided into 3 groups
(n=6/group): The control group (control), sevoflurane general
anesthesia group 1 (S1) and sevoflurane general anesthesia group 2
(S2).
Sevoflurane exposure
Rats in the control group were untreated, and those
in the two sevoflurane exposure groups (S1 and S2) were exposed to
2% sevoflurane (15-18).
All rats were placed in separate cages and treated using 100%
oxygen as a carrier gas, with a 4 l/min total gas flow. Rats in the
S1 and S2 group were anesthetized with 5% sevoflurane for 1-2 min
until they became unconscious. The 2% sevoflurane was used for
maintenance, and anesthesia was performed for 2 and 4 h in S1 and
S2 groups, respectively. During sevoflurane anesthesia, the
concentrations of oxygen, carbon dioxide and sevoflurane in the
anesthesia chamber were monitored using a gas detector (Drägerwerk
AG & Co. KGaA). Sevoflurane exposure was stopped at the
indicated time points and the pregnant rats were returned to their
cages until giving birth. In the control group, there were 35
female offspring rats and 28 male offspring rats. In the S1 group,
there were 32 female offspring rats and 33 male offspring rats. In
the S2 group, there were 29 female offspring rats and 34 male
offspring rats. The animals were housed at 23±2˚C, 55±5% humidity
with a 12-h light/dark cycle, and with free access to food and
water. A total of 30 days post-birth, 12 offspring (male:female
ratio 1:1) rats (weight, 110±20 g) were randomly selected from each
group, anesthetized by an intraperitoneal injection of 3% sodium
pentobarbital (50 mg/kg) and sacrificed by decapitation. Five
minutes after cardiac arrest, death was confirmed. Brain specimens
were then collected. The brain samples of six rats/group were fixed
in 4% paraformaldehyde for 24 h at 4˚C, and those from the other
six rats/group were snap frozen and stored in liquid nitrogen for
western blot analysis. In each group, 18 offspring rats were
euthanized. No animals died during the experiment.
Morris water maze test
A total of 6 offspring rats were randomly selected
from each group and maintained for 30 days prior to Morris water
maze testing. A round swimming pool (diameter, 180 cm; depth, 60
cm) was prepared with a water temperature of 23±1˚C. An underwater
platform (diameter, 10 cm; 2 cm below the water surface) was placed
in the first quadrant of the pool. Testing was initiated at 9 a.m.
on the 30th day after birth, and lasted for 5 days, with 4
tests/day. The rats were placed into the water at the designated
release point and allowed 90 sec to locate the platform. After
remaining on the platform for 15 sec, the rats were removed from
the pool and the experiment was terminated. If the rats failed to
locate the platform within 90 sec, they were manually placed on the
platform for 15 sec. Swimming time and speed were recorded and the
platform was removed to conduct the probe experiment, as previously
described (19).
Hematoxylin and eosin (H&E) and
Nissl staining
After dehydration, the brain specimens were fixed in
4% paraformaldehyde solution, as aforementioned, and embedded in
paraffin. Paraffin blocks of brain tissue included sections of the
hippocampus and parietal cortex (5-µm thick). The sections were
routinely dewaxed with xylene at 60˚C and hydrated using a graded
ethanol series. The tissues were stained with hematoxylin
(Sigma-Aldrich; Merck KGaA) for 5 min at room temperature, and then
rinsed with tap water. The tissues were then differentiated for 30
sec using hydrochloric acid and ethanol, immersed in tap water for
15 min at room temperature, and then placed in eosin staining
solution (Sigma-Aldrich; Merck KGaA) for 2 min at room temperature.
All samples were routinely dehydrated and sealed. For Nissel
staining, the sections were incubated at 37˚C overnight, rehydrated
and then subjected to Nissl staining for 10 min at room
temperature. These samples were also dehydrated, cleared and
sealed. The morphology of neurons in the hippocampus and parietal
cortex was observed using a light microscope at x400 magnification
(Olympus BX51; Olympus Corporation).
Immunohistochemistry
After conventional sectioning of the hippocampal and
parietal cortex tissues (5 µm thick), the specimens were dewaxed
with xylene at 60˚C and hydrated with a graded series of ethanol
solutions. The sections were inactivated using 3%
H2O2 for 20 min at room temperature, fixed in
citrate buffer (pH 6.0) with high-temperature heating to boiling
for 10 min, and then treated with 5% BSA (cat. no. SW3015: Beijing
Solarbio Science & Technology Co., Ltd.) for 20 min at room
temperature. Rabbit anti-rat GFAP (1:800; cat. no. orb10706), AIM2
(1:200; cat. no. orb45726), CD45 (1:300; cat. no. orb10328) and
IL-1β (1:300; cat. no. orb499934) (all Biorbyt Ltd.) were added and
the tissues were incubated at 4˚C overnight. After rewarming, the
specimens were incubated with goat anti-rabbit horseradish
peroxidase IgG (1:1,000; cat. no. ABIN101988; Antibodies Online
GmbH). The sections were developed with 3,3'-diaminobenzidine,
followed by counterstaining with hematoxylin for 10 min at room
temperature, dehydration, clearing and sealing. The relevant brain
regions (5 randomly selected fields per sample) were observed under
a light optical microscope (Olympus Corporation) at x400
magnification, and cells were counted using Aperio ImageScope 11.1
software (Leica Microsystems, Inc.).
Western blotting
Sections of the hippocampus and parietal cortex were
ground and homogenized using a total protein extraction kit (cat.
no. BC3710; Beijing Solarbio Science & Technology Co., Ltd.)
according to the manufacturer's instructions. After centrifugation
at 12,000 x g for 10 min at 4˚C, the supernatant was removed and a
BCA kit was used to determine protein concentration (Beijing
Solarbio Science & Technology Co., Ltd.). A total of 40 µg
protein/lane was separated by SDS-PAGE (10% gel) in a 1:1 dilution
with 5X protein loading buffer (cat. no. P1040; Beijing Solarbio
Science & Technology Co., Ltd.) and heated at 95˚C for 5 min to
denature the protein. The samples were then transferred to PVDF
membranes (Merck KGaA) at a voltage of 80 V for 30 min and blocked
with a TBS with 0.1% Tween-20 (TBST) solution containing 5% skimmed
milk powder for 1 h at 4˚C. Rabbit anti-rat CD45 (1:1,000; cat. no.
orb10328; Biorbyt Ltd.), IL-1β (1:1,000; cat. no. orb499934;
Biorbyt Ltd.), caspase-1 (1:800; cat. no. PA5-86936; Thermo Fisher
Scientific, Inc.), caspase-1 p10 (1:1,000; cat. no. PA5-39882;
Thermo Fisher Scientific, Inc.) and β-actin (1:2,000; cat. no.
orb178392; Biorbyt Ltd.) polyclonal antibodies were diluted with a
TBST solution containing 3% bovine serum protein (cat. no. ab64009;
Abcam) and used to probe the membranes at 4˚C overnight. After
rewarming, the membranes were incubated with goat anti-rabbit
horseradish peroxidase-labeled IgG (1:1,000; cat. no. ABIN101988;
Antibodies Online GmbH) for 1 h at room temperature and washed with
ECL (cat. no. PE0010; Beijing Solarbio Science & Technology
Co., Ltd.) for 3-5 min. The protein levels were normalized to those
of β-actin and grayscale scanning and semi-quantification were
performed using ImageJ 1.8 software (NIH).
Statistical analysis
The data were processed using SPSS 19.0 software
(IBM Corp.) and are presented as the mean ± SEM. The number of
repeats was 6. Statistical significance was determined by ANOVA
followed by Tukey's test for multiple comparisons. The values of
swimming speed and escape latency were analyzed using one-way
repeated-measures ANOVA followed by Bonferroni post hoc analysis.
P<0.05 was considered to indicate a statistically significant
difference.
Results
Effects of sevoflurane general
anesthesia in early pregnancy on the learning ability of offspring
rats
In order to study the effect of sevoflurane general
anesthesia in early pregnancy on the long-term learning and memory
of offspring rats, Morris water maze tests were performed on each
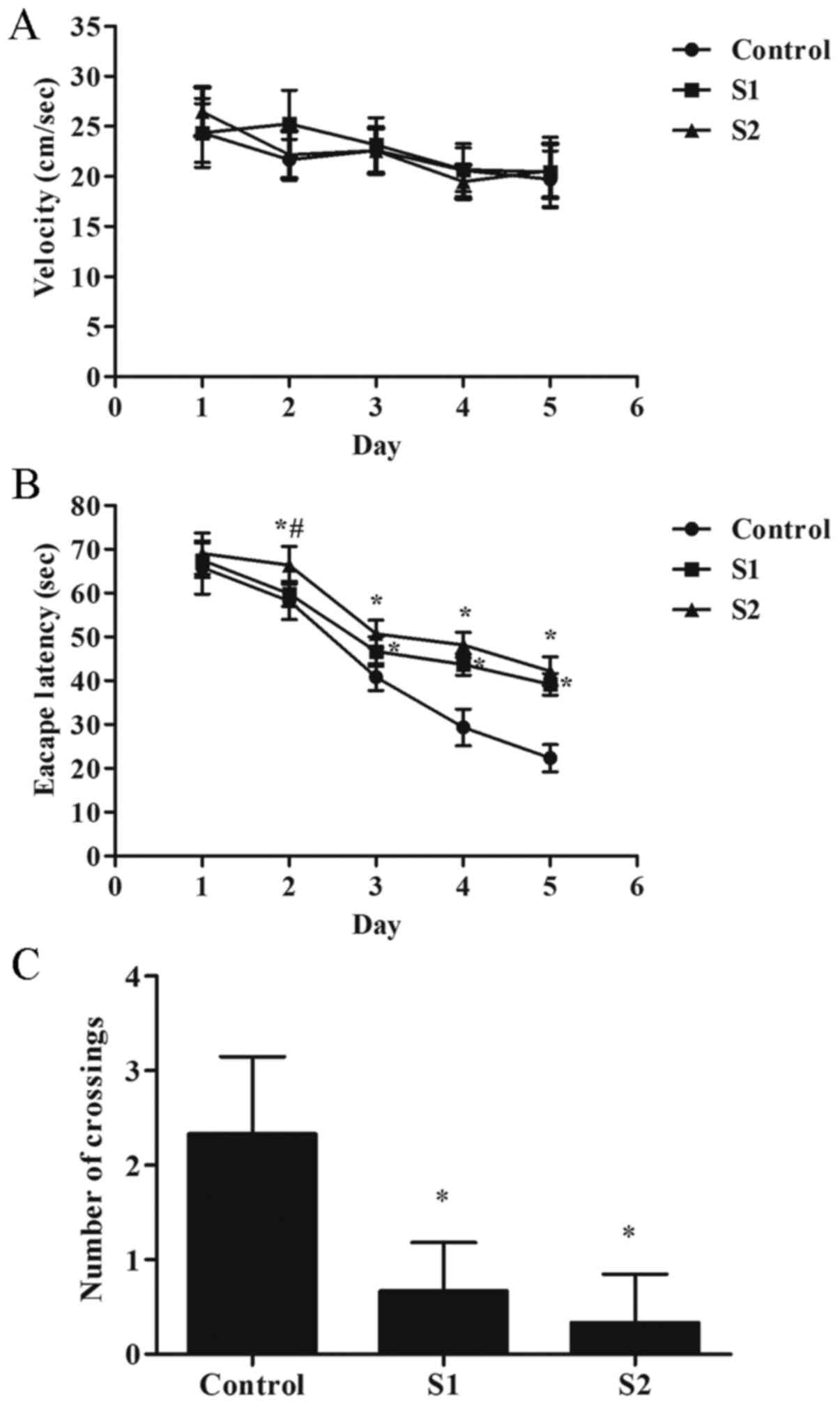
group of rats. The results showed no significant differences in the
activity speed of each group of rats (Fig. 1A). Compared with the control group,
the escape latency of the rats in the S1 and S2 group increased on
days 3-5 of training (P<0.05), and the number of crossings of
the original platform decreased significantly (Fig. 1B and C; P<0.05). Compared with the S1 group,
the S2 group exhibited poorer learning ability, the escape latency
of the rats in the S2 group increased on day 2 of training
(Fig. 1B; P<0.05).
Effects of sevoflurane general
anesthesia during early pregnancy on the morphology of hippocampal
and parietal cortex neurons in offspring rats
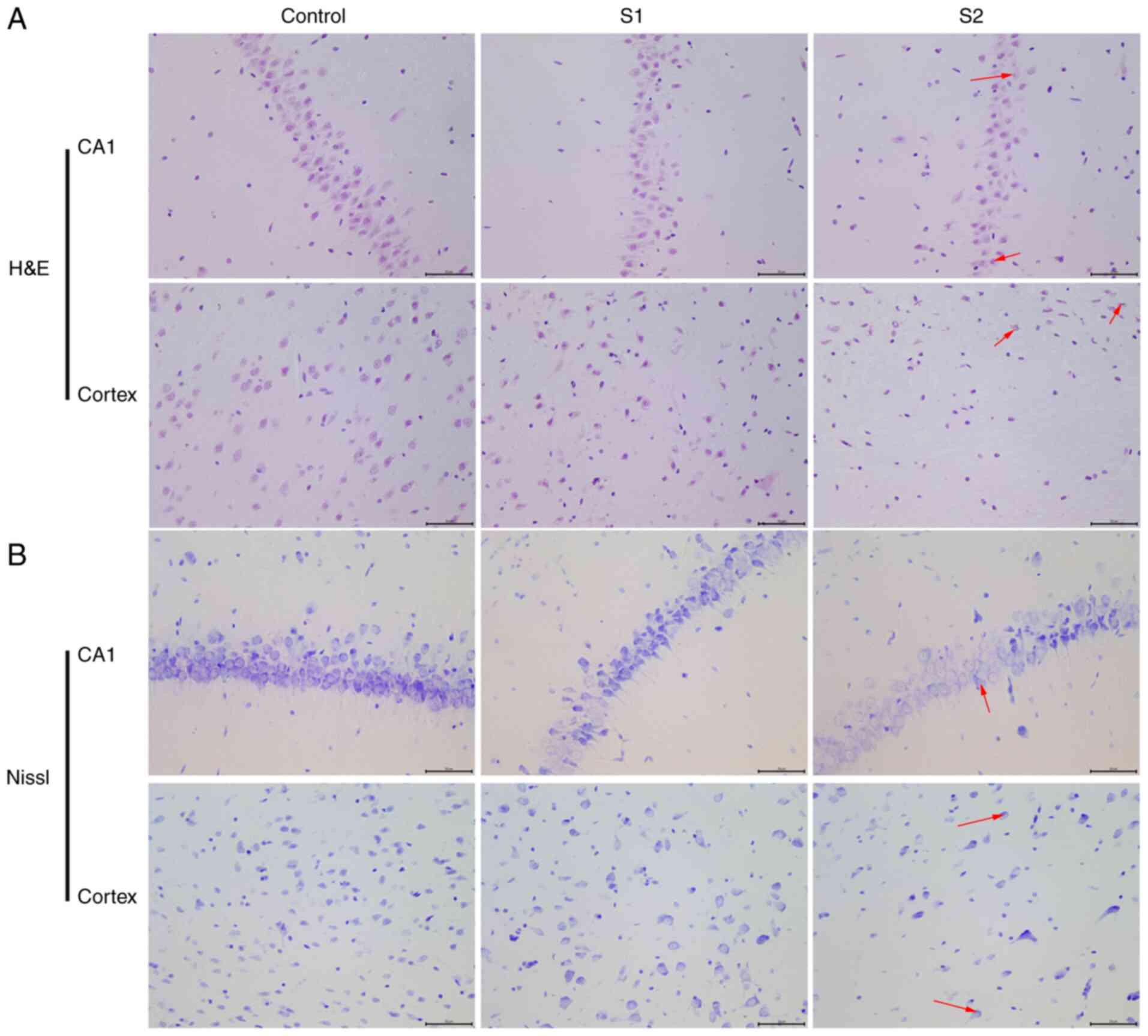
The objective of the present study was to
characterize the effects of sevoflurane general anesthesia in early
pregnancy on offspring rats. The morphological changes of neurons
in the hippocampus and parietal cortex were observed by H&E and
Nissl staining. The H&E staining results showed that the
hippocampus and parietal cortex neurons of offspring rats in the
control group were uniform in size, arranged neatly and exhibited a
clear outline (Fig. 2A). When
compared with the control group, the neuronal cells of the S1 group
were arranged relatively neatly, and a small number showed swelling
and neuronal degeneration. However, cells from the S2 group were
arranged in a disordered manner, the cell bodies were shrunken and
the nuclei were condensed into triangles or polygons. The Nissl
staining results illustrated that the hippocampus and parietal
cortex of offspring rats in the control group had normal, clear and
complete Nissl bodies (Fig. 2B).
When compared with the control group, the Nissl bodies appeared
diffuse in the S1 and S2 group. In the S2 group, the Nissl bodies
appeared more diffuse than in the S1 group.
Effects of sevoflurane general
anesthesia during early pregnancy on the expression of GFAP and
AIM2 in hippocampal and parietal cortex neurons in offspring
rats
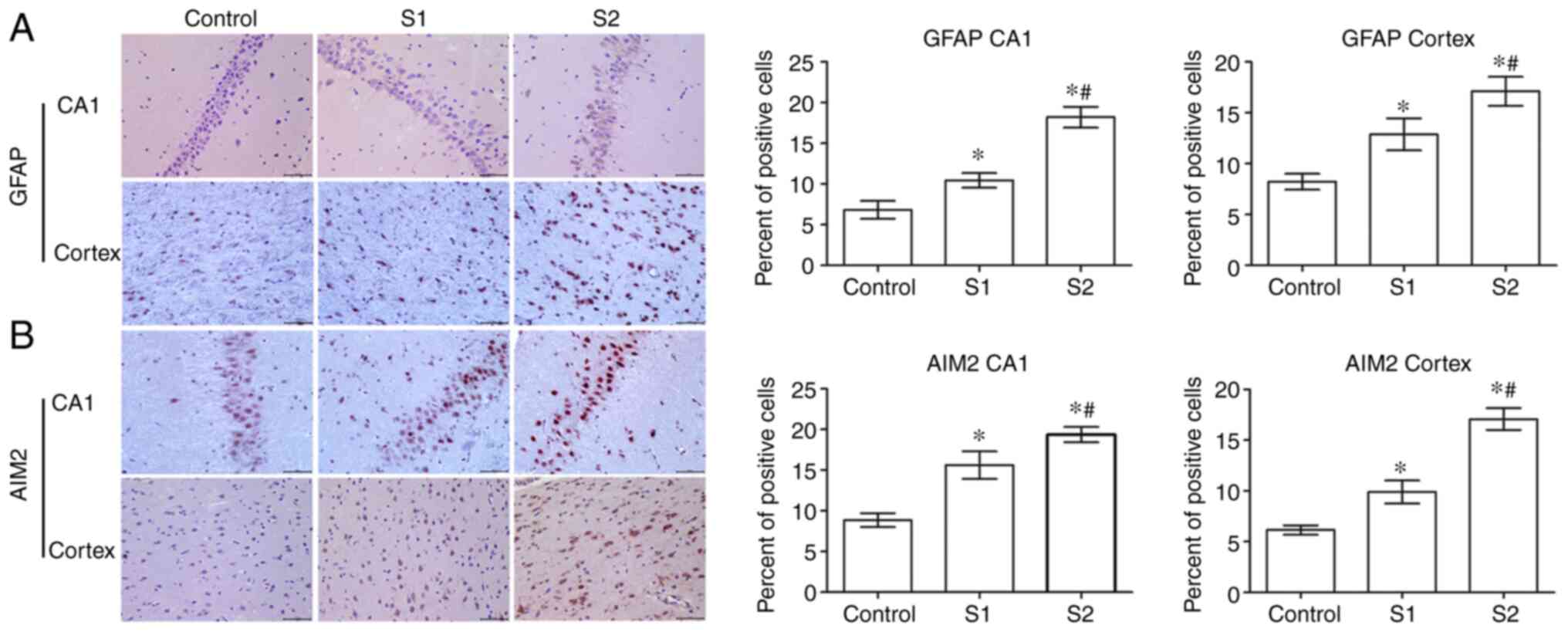
The expression of GFAP in offspring rats was
evaluated to determine the effects of sevoflurane on the
hippocampus and parietal cortex. Compared with the control group,
the expression of GFAP in the S1 and S2 groups was significantly
increased (P<0.05) in the hippocampus and parietal cortex, and
GFAP expression was higher in the S2 group than in the S1 group
(P<0.05; Fig. 3A). Further
experiments demonstrated that the expression of AIM2 in the S1 and
S2 groups was significantly increased compared with the control
group (P<0.05). Furthermore, the S2 group exhibited a higher
expression level of AIM2 than the S1 group (P<0.05; Fig. 3B).
Effects of sevoflurane general
anesthesia during early pregnancy on the expression of CD45 and
IL-1β in the hippocampus and parietal cortex of offspring rats
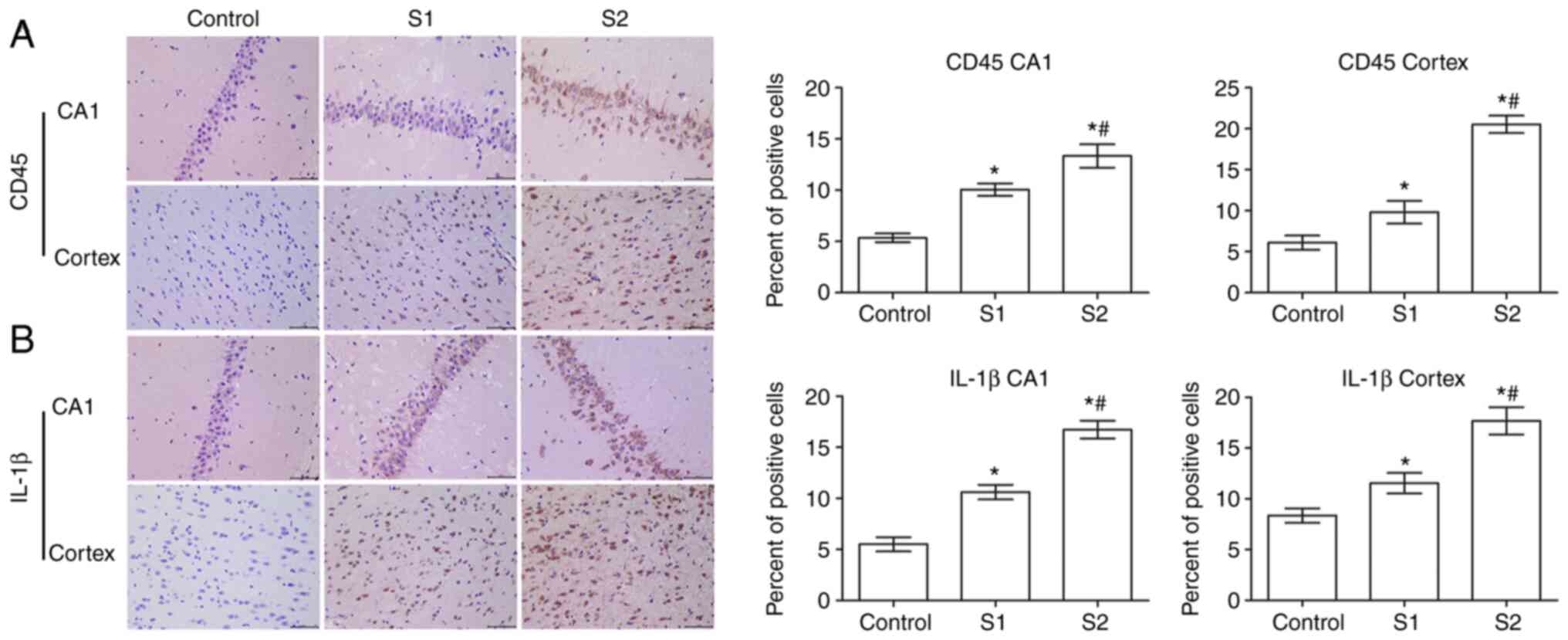
In order to study the effect of sevoflurane general
anesthesia during early pregnancy on inflammatory factors in
offspring rats, the expression of CD45 and IL-1β in the hippocampus
and parietal cortex of offspring rats was observed. As shown in
Fig. 4A, the expression of CD45 in
the hippocampus and parietal cortex of the S1 group and the S2
group was significantly higher than that in the control group
(P<0.05), and the expression of CD45 was higher in the S2 group
than that in the S1 group (P<0.05).
Further examination showed that compared with the
control group, the expression of IL-1β in the hippocampus and
parietal cortex of the S1 and S2 groups was significantly increased
(P<0.05), and that this expression was significantly higher in
the S2 group than that in the S1 group (P<0.05; Fig. 4B).
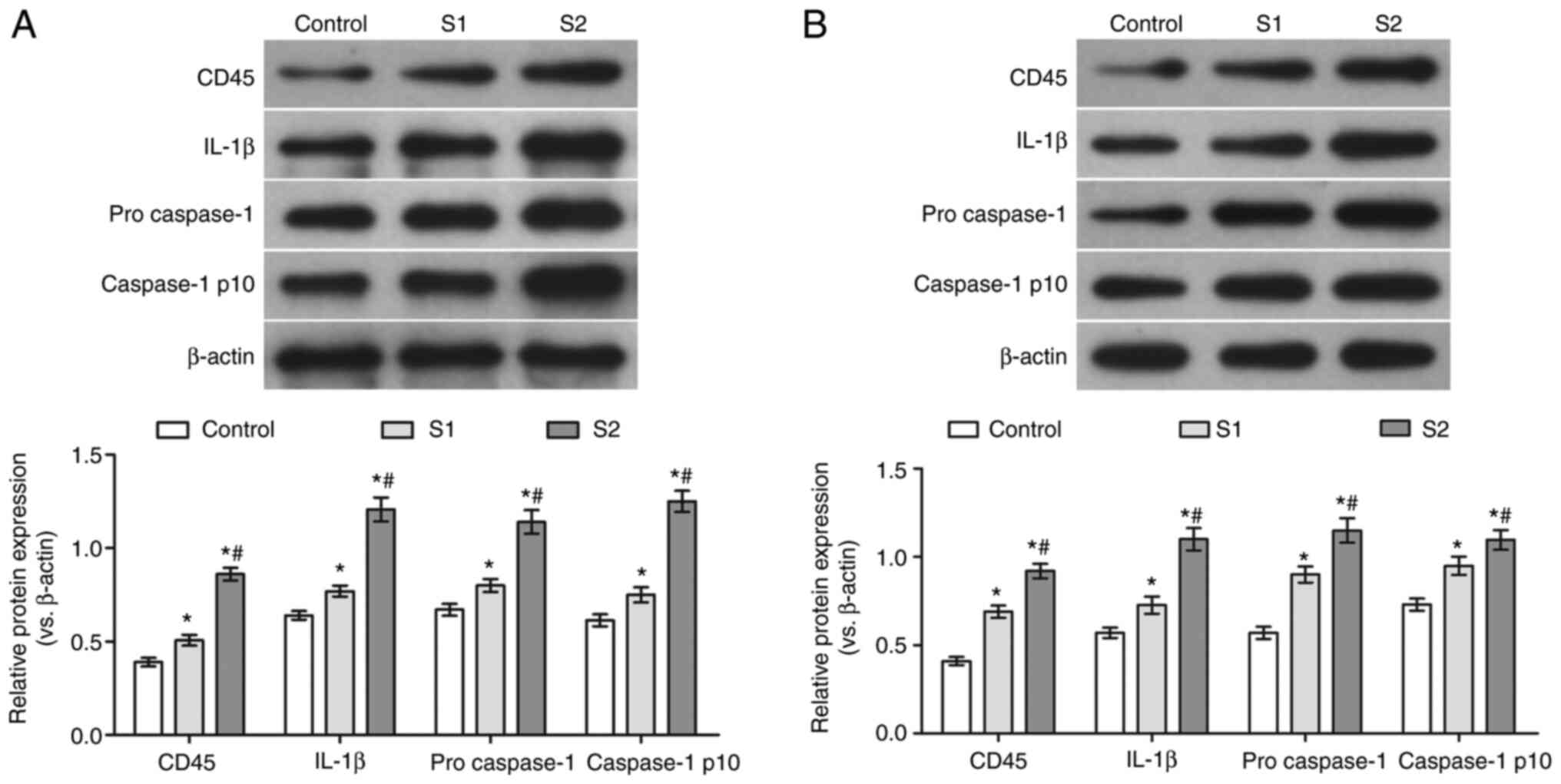
Effects of sevoflurane general
anesthesia during early pregnancy on the protein levels of CD45,
IL-1β, pro-caspase-1 and caspase-1 p10 in the hippocampus and
parietal cortex of offspring rats
To further verify the effects of sevoflurane general
anesthesia during early pregnancy on the hippocampus and parietal
cortex of offspring rats, the protein levels of CD45, IL-1β,
pro-caspase-1 and caspase-1 p10 were determined. Compared with the
control group, the protein levels of CD45, IL-1β, pro-caspase-1 and
caspase-1 p10 in the hippocampus and parietal cortex of the S1 and
S2 groups were significantly increased (P<0.05). Moreover, the
expression of these proteins was found to be higher in the S2 than
that in the S1 group (P<0.05; Fig.
5).
Discussion
To investigate the effects of sevoflurane exposure
on the hippocampus and parietal cortex, a comparison study was
designed to examine the expression of inflammation-associated
proteins in rats. Previous studies on the morphological changes in
neuronal cells indicated that exposure to sevoflurane during
gestation was associated with offspring brain development (13,20,21).
Previous research has revealed that anesthetics
cause significant damage to the developing brain (22). The degree of damage is primarily
dependent on the concentration of the anesthetic and the duration
of exposure (23). According to
previous hypotheses, in the present study, a concentration of 2%
sevoflurane was administered to rats during early pregnancy
(15-18).
Repeated sevoflurane exposure has also been reported to suppress
the proliferation of neural progenitors in offspring (24). Therefore, to investigate its effects
on brain development, sevoflurane exposure times of 2 and 4 h were
selected with reference to previous studies (18,25).
AIM2 is an inflammasome cytokine that plays an
essential role in the defense against bacterial and viral elements
and is a reliable indicator for assessing brain changes (26). In the present study, a significant
increase in AIM2 expression was observed in the offspring of the
sevoflurane-treated groups. AIM2 has been reported to engage the
caspse-1-activating adaptor protein to form a caspase-1-activating
inflammasome (27). CD45, the
lymphocyte common antigen, is a receptor-linked protein tyrosine
phosphatase that plays a crucial role in leucocyte function
(28). In the current study, the
expression of CD45 was higher in the sevoflurane groups than that
in the control group. These findings are consistent with previous
studies indicating that the use of sevoflurane anesthesia in
pregnant animals may affect the development of the fetal brain
(29).
Abnormalities in the hippocampus and parietal cortex
usually result in mental disorders such as autism spectrum disorder
and neurodegeneration (30).
Previous studies have shown that sevoflurane reduces the protein
expression levels of caspase-3 in the fetal brain (31). IL-1β is a key pro-inflammatory
cytokine that is essential for host defense responses to injury and
infection. Caspase-1 is a functional enzyme that proteolytically
cleaves other proteins, such as the precursors of the inflammatory
cytokine IL-1, and thus plays a pivotal role in cellular immunity
as an inflammatory response initiator. Once activated, caspase-1
usually initiates a proinflammatory response through the cleavage
and thus activation of two inflammatory cytokines, IL-1β and
IL-18(32). In the present study,
significant changes in the protein levels of CD45, IL-1β,
pro-caspase-1 and caspase-1 p10 were observed in the hippocampus
and parietal cortex. These results suggest that sevoflurane
exposure had an impact on different regions of the fetal brain.
The results of the present study indicated that
damage to different regions of the brain, including the hippocampus
and parietal cortex, following sevoflurane exposure may ultimately
lead to functional and neurological impairments in adult offspring,
which provides insights into the potential mechanisms of
postoperative neurological impairment following prenatal
sevoflurane exposure.
However, the present study had certain limitations.
In addition to the hippocampus and parietal cortex, whether other
regions of brain are affected remains to be elucidated.
In conclusion, sevoflurane general anesthesia during
early pregnancy promoted the expression of AIM2 in the hippocampus
and parietal cortex of offspring SD rats, and also promoted the
inflammatory response in these tissues.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The dataset used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
YZ and CL carried out experimental work, as well as
data collection and interpretation, revised the manuscript. YZ and
JL participated in the design and coordination of the experimental
work, in addition to data acquisition. SS and WJ contributed to the
study design, experimental data collection and analysis, and
preparation of the manuscript. CL and JL assessed the data to
ensure its legitimacy. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
All animal experiments were conducted following the
National Institutes of Health guidelines and were reviewed and
approved by the Yantaishan Hospital Animal Protection and Use
Committee (approval no. 2018-10087; Yantai, China).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Brioni JD, Varughese S, Ahmed R and Bein
B: A clinical review of inhalation anesthesia with sevoflurane:
From early research to emerging topics. J Anesth. 31:764–778.
2017.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Choi ES, Shin JY, Oh AY, Park HP, Hwang
JW, Lim YJ and Jeon YT: Sevoflurane versus propofol for
interventional neuroradiology: A comparison of the maintenance and
recovery profiles at comparable depths of anesthesia. Korean J
Anesthesiol. 66:290–294. 2014.PubMed/NCBI View Article : Google Scholar
|
|
3
|
O'Farrell RA, Foley AG, Buggy DJ and
Gallagher HC: Neurotoxicity of inhalation anesthetics in the
neonatal rat brain: Effects on behavior and neurodegeneration in
the piriform cortex. Anesthesiol Res Pract.
2018(6376090)2018.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Ji MH, Wang XM, Sun XR, Zhang H, Ju LS,
Qiu LL, Yng JJ, Jia M, Wu J and Yang J: Environmental ehrichment
ameliorates neonatal sevoflurane exposure-induced cognitive and
synaptic plasticity impairments. J Mol Neurosci. 57:358–365.
2015.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Wu J, Yu J, Xie P, Maimaitili Y, Wang J,
Yang L, Ma H, Zhang X, Yang Y and Zheng H: Sevoflurane
postconditioning protects the myocardium against
ischemia/reperfusion injury via activation of the JAK2-STAT3
pathway. PeerJ. 5(e3196)2017.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Juhasz-Boss I, Solomayer E, Strik M and
Raspe C: Abdominal surgery in pregnancy-an interdisciplinary
challenge. Dtsch Arztebl Int. 111:465–472. 2014.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Olutoye OA, Baker BW, Belfort MA and
Olutoye OO: Food and Drug Administration warning on anesthesia and
brain development: Implications for obstetric and fetal surgery. Am
J Obstet Gynecol. 218:98–102. 2018.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Malhotra A, Yosh E and Xiong M: Propofol's
effects on the fetal brain for non-obstetric surgery. Brain Sci.
7(107)2017.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Walkden GJ, Pickering AE and Gill H:
Assessing long-term neurodevelopmental outcome following general
anesthesia in early childhood: Challenges and opportunities. Anesth
Analg. 128:681–694. 2019.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Vutskits L and Xie Z: Lasting impact of
general anaesthesia on the brain: Mechanisms and relevance. Nat Rev
Neurosci. 17:705–717. 2016.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Jevtovic-Todorovic V: Exposure of
developing brain to general anesthesia: What is the animal
evidence? Anesthesiology. 128:832–839. 2018.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Bi C, Cai Q, Shan Y, Yang F, Sun S, Wu X
and Liu H: Sevoflurane induces neurotoxicity in the developing rat
hippocampus by upregulating connexin 43 via the JNK/c-Jun/AP-1
pathway. Biomed Pharmacother. 108:1469–1476. 2018.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Song R, Ling X, Peng M, Xue Z, Cang J and
Fang F: Maternal sevoflurane exposure causes abnormal development
of fetal prefrontal cortex and induces cognitive dysfunction in
offspring. Stem Cells Int. 2017(6158468)2017.PubMed/NCBI View Article : Google Scholar
|
|
14
|
National Institutes of Health (NIH)
guidelines revised in 1996. https://nihrecord.nih.gov/.
|
|
15
|
Huang H, Liu CM, Sun J, Jin WJ, Wu YQ and
Chen J: Repeated 2% sevoflurane administration in 7-and 60-day-old
rats: Neurotoxicity and neurocognitive dysfunction. Aneasthesist.
66:850–857. 2017.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Guo Z, Zhao F, Wang Y, Wang Y, Geng M,
Zhang Y, Ma Q and Xu X: Sevoflurane Exerts an Anti-depressive
Action by Blocking the HMGB1/TLR4 Pathway in Unpredictable Chronic
Mild Stress Rats. J Mol Neurosci. 69:546–556. 2019.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Yu X, Zhang F and Shi J: Neonatal exposure
to sevoflurane caused cognitive deficits by dysregulating SK2
channels and GluA2-lacking AMPA receptors in juvenile rat
hippocampus. Neuropharmacology. 141:66–75. 2018.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Zheng SQ, An LX, Cheng X and Wang YJ:
Sevoflurane causes neuronal apoptosis and adaptability changes of
neonatal rats. Acta Anaesthesiol Scand. 57:1167–1174.
2013.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Zhang Y, Yang F, Gao Y, Shan Y, Dong Y and
Liu H: Neuroglobin protects offspring rats from neuronal damage
induced by sevoflurane exposure to pregnant rats by inhibiting
endogenous apoptosis. Int J Dev Neurosci. 76:17–24. 2019.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Wu Z, Li X, Zhang Y, Tong D, Wang L and
Zhao P: Effects of sevoflurane exposure during mid-pregnancy on
learning and memory in offspring rats: Beneficial effects of
maternal exercise. Front Cell Neurosci. 12(122)2018.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Fang F, Song R, Ling X, Peng M, Xue Z and
Cang J: Multiple sevoflurane anesthesia in pregnant mice inhibits
neurogenesis of fetal hippocampus via repressing transcription
factor Pax6. Life Sci. 175:16–22. 2017.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Sharma HS, Muresanu DF, Nozari A,
Castellani RJ, Dey PK, Wiklund L and Sharma A: Anesthetics
influence concussive head injury induced blood-brain barrier
breakdown, brain edema formation, cerebral blood flow, serotonin
levels, brain pathology and functional outcome. Int Rev Neurobiol.
146:45–81. 2019.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Rocha TL, Dias-Junior CA, Possomato-Vieira
JS, Goncalves-Rizzi VH, Nogueira FR, de Souza KM, Braz LG and Braz
MG: Sevoflurane induces DNA damage whereas isoflurane leads to
higher antioxidative status in anesthetized rats. Biomed Res Int.
2015(264971)2015.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Zhang MQ, Ji MH, Zhao QS, Jia M, Qiu LL,
Yang JJ, Peng YG, Yang JJ and Martynyuk AE: Neurobehavioural
abnormalities induced by repeated exposure of neonatal rats to
sevoflurane can be aggravated by social isolation and enrichment
deprivation initiated after exposure to the anaesthetic. Br J
Anaesth. 115:752–760. 2015.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Guo SB, Liu LD, Wang C, Jiang Q, Dong YX
and Tian Y: Repeated exposure to sevoflurane impairs the learning
and memory of older male rats. Life Sci. 192:75–83. 2018.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Ge X, Li W, Huang S, Yin Z, Xu X, Chen F,
Kong X, Wang H, Zhang J and Lei P: The pathological role of NLRs
and AIM2 inflammasome-mediated pyroptosis in damaged blood-brain
barrier after traumatic brain injury. Brain Res. 1697:10–20.
2018.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Lam HYP, Chen TT, Chen CC, Yang TH, Cheng
PC and Peng SY: Angiostrongylus cantonensis activates inflammasomes
in meningoencephalitic BALB/c mice. Parasitol Int.
77(102119)2020.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Rheinländer A, Schraven B and Bommhardt U:
CD45 in human physiology and clinical medicine. Immunol Lett.
196:22–32. 2018.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Hirotsu A, Iwata Y, Tatsumi K, Miyai Y,
Matsuyama T and Tanaka T: Maternal exposure to volatile anesthetics
induces IL-6 in fetal brains and affects neuronal development. Eur
J Pharmacol. 863(172682)2019.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Gilsoul M, Grisar T, Delgado-Escueta AV,
de Nijs L and Lakaye B: Subtle brain developmental abnormalities in
the pathogenesis of Juvenile Myoclonic epilepsy. Front Cell
Neurosci. 13(433)2019.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Perez-Zoghbi JF, Zhu W, Grafe MR and
Brambrink AM: Dexmedetomidine-mediated neuroprotection against
sevoflurane-induced neurotoxicity extends to several brain regions
in neonatal rats. Br J Anaesth. 119:506–516. 2017.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Malik A and Kanneganti TD: Inflammasome
activation and assembly at a glance. J Cell Sci. 30:3955–3963.
2017.PubMed/NCBI View Article : Google Scholar
|