Introduction
As one of most life-threatening fungal diseases,
cryptococcosis affects mostly immunocompromised individuals but
also several immunocompetent populations. Cryptococcus
(C.) neoformans and C. gattii are both
etiological causes of cryptococcosis. Cases of cryptococcosis
caused by C. neoformans infection occurred mostly in
immunocompromised populations, such as patients with acquired
immunodeficiency syndrome or organ transplantation, whereas
cryptococcosis caused by C. gattii occurs more frequently in
immunocompetent individuals (1).
C. neoformans is the most commonly isolated species from
clinical cases, accounting for a large portion of cases of
cryptococcosis.
C. neoformans and C. gattii exhibit
notable differences in phenotype, ecology, epidemiology and
resistance to drugs. First, the morphology of yeast cells is
different; C. neoformans cells are almost globose, while
C. gattii cells are both globose and oblong (2). Furthermore, soil and pigeon droppings
account for the majority of saprophytic sources of C.
neoformans, while decaying trees are identified as
environmental reservoirs of C. gattii (3,4). Cases
caused by C. neoformans are observed worldwide, but cases
caused by C. gattii are not frequently observed globally. In
addition, C. gattii exhibits relatively higher resistance to
antifungal drugs, while both C. gattii and C.
neoformans have been recognized as having different degrees of
resistance to certain antifungal drugs, such as azoles (5,6). The
differences in epidemiology and drug resistance exhibited by C.
gattii may be attributable to different pathogenic mechanisms.
Several studies have indicated that C. gattii exhibits
different virulence management mechanisms from C.
neoformans (7,8). C. gattii employs various
virulence factors to survive and disseminate in the host, such as
capsule production and melanin synthesis, growth at the host's body
temperature and degradation enzymes (9-12).
Under hostile conditions, Cryptococci may sense and adjust
themselves to severe stress stimuli, such as high osmotic pressure,
by activating multiple stress conduction pathways. Several studies
have identified abundant and distinctive roles of the different
signaling pathways by comparing the stress regulation mechanism
between C. gattii and C. neoformans (13-15).
Yeast is able to maintain osmotic homeostasis across the cell
membrane by adjusting the internal environment to a steady state.
As one of the most important stress regulatory systems, the
high-osmolarity glycerol (HOG) signaling pathway has a notable
effect on the osmotic stress reaction and is essential for
virulence regulation of C. neoformans (16); however, in C. gattii, this
pathway has remained to be investigated.
The HOG pathway is one of the important signaling
pathways in C. neoformans and is structurally similar to
those present in other fungi; this pathway regulates stress, sexual
differentiation and virulence (17). External stress stimuli are sensed
and transmitted by the HOG pathway, which governs protective
reactions against various deleterious stimuli, including osmotic
stress, oxidative response, high ion concentration, antifungal
drugs, high temperature, ultraviolet irradiation and toxic
metabolites (13,16,18,19).
HOG1 is one of the most important components of the MAPK cascade. A
large number of studies on the HOG pathway have focused on
C. neoformans. As reported for the North American
outbreak comprising numerous cryptococcosis cases, C. gattii
has emerged as a life-threatening primary pathogen infecting
immunocompetent patients (20). The
strains were observed to be significantly more virulent in
vivo than others. Furthermore, central nervous system infection
caused by C. gattii is usually associated with the
development of more cryptococcosis; more severe complications, such
as headaches; and a poor survival and recovery rate, and it usually
requires more frequent neurosurgical interventions and follow-ups
compared with cases caused by C. neoformans infection
(21). Previous studies suggested
that the ecology and pathogenesis of C. gattii were changing
significantly and merited further research (7). It was hypothesized that HOG1 may also
be involved in the pathogenic mechanism of C. gattii.
In the present study, the HOG1 gene in C.
gattii was characterized. To functionally characterize HOG1, a
mutant strain was obtained by deleting targeted genes for HOG1 by
using the clinical strain CZ2012 as a model, and a series of
phenotypic strains were compared with the wild-type (WT) and
reconstitution strains. In C. gattii, the present results
suggested that HOG1 has an essential role in regulating the stress
response, antifungal drug susceptibility and virulence factor
production, including processes such as capsule production and
melanin synthesis. Deletion mutation of the HOG1 gene in C.
gattii resulted in notable growth weakness, not only under
stressful conditions but also under normal conditions, and was
associated with attenuated virulence in infected mice. The C.
gattii hog1Δ mutant exhibited reduced capsule production and
only a small amount of melanin synthesis, contrary to results
obtained with C. neoformans, indicating that HOG1 has
developed a distinctive virulence regulatory mechanism in the two
Cryptococcus species. In summary, the present study
demonstrated certain convergent and divergent functions of HOG1 in
C. gattii compared with those in C. neoformans, which
provides a more detailed understanding of the pathogenic mechanisms
of C. gattii.
Materials and methods
Strains and media
Cryptococcus isolates exhibit various
mechanisms for enhancing virulence, such as growth at 39˚C,
adaption to stress and capsule production and marked amplification
of ergosterol (22). The strain
used in the present study was the clinical strain CZ2012 [C.
gattii (Cg), serotype B, mate-α; purchased from the
Cryptococcus Laboratory of China Medical Fungi preservation and
Management Center], which was isolated from a patient with
cryptococcal meningitis in China. Furthermore, the clinical isolate
strain of C. neoformans (Cn) from a Chinese patient with
cryptococcal meningitis was used (23). Yeast extract peptone dextrose (YPD)
agar media (1% yeast extract, 2% peptone, 2% dextrose and 2% agar;
Invitrogen; Thermo Fisher Scientific, Inc.) was utilized for
culture.
Complementary (c)DNA synthesis and
cloning of HOG1
Yeast cells were incubated overnight at 30˚C in
fresh YPD medium and stimulated with a high concentration of
glycerol, and total RNA was isolated with a yeast RNA extraction
kit and TRIzol (Invitrogen Inc.; Thermo Fisher Scientific, Inc.)
according to the manufacturer's protocol. The HOG1 gene was
amplified by Nested PCR. Regarding primers for the construction of
the HOG1 gene knockout fragment, H1 and H2 were outer primers,
located respectively at both ends of the target sequence, and H3
and H4 were inner nested primers, which were designed for the
encoding region. Restriction enzyme cutting sites (EcoRI and
NotI) were respectively added to the 5' end of H3 and H4.
The primers were as follows: Outer primers H1 (primer up),
5'-GTATACCAACCGGTCTCAAC-3' and H2 (primer down),
5'-CAGGCTCCTGAATACAACAC-3'; inner primers H3 (primer up),
5'-GAATTCATGGCCGATTTTGTCAAGCTC-3' (EcoRI) and H4 (primer
down), 5'-GCGGCCGCCTAGCTAGCAGGAGCAGCCGA-3' (NotI). The
underlined sites represent the restriction sites (EcoRI and
NotI).
The HOG1 cDNA sequence was 1,098 bp in length. The
complete HOG1 cDNA sequence was generated using reverse
transcription PCR using first-strand cDNA (24). The full-length HOG1 cDNA was cloned
into a plasmid vector by the pCR-Blunt Kit (Invitrogen; Thermo
Fisher Scientific, Inc.), creating the recombinant clone vector
pCR-Blunt-HOG1, which was transformed into competent DH5a cells
(Takara Bio, Inc.) for enveloping, the positive clones were then
subjected to screening using PCR analysis and DNA sequencing
(25).
Disruption and reconstitution of
HOG1
Using restriction enzyme identification, PCR and
sequence analysis (25), the
recombinant plasmid pGAPza-HOG1 containing the intact HOG1 gene was
successfully constructed. The HOG1 gene knockout expression vector
pGAPza-dHOG1 was constructed by knocking out 400-bp fragment of
pGAPza-HOG1 using a single restriction, Sac I (Invitrogen, Thermo
Fisher Scientific, Inc.). The knockout product was transformed into
Cryptococcus gattii CZ2012 cells by electroporation (electric shock
condition: 1,500 V, 400 Ω, 25 µF, 5 mS; twice, internal: 5 min).
Stable transformants were obtained through screening on YPD medium
containing zeocin and subsequently confirmed by diagnostic PCR, DNA
sequencing and Southern blot analysis (25).
To construct the hog1Δ+HOG1 reconstituted
strain, pGAPza-HOG1 was linearized and transformed into the mutant
(deletion of HOG1) by electroporation (26). Stably transfected colonies were
selected on medium containing ampicillin and zeocin. The
reconstitution of the HOG1 gene was confirmed by diagnostic PCR and
Southern blotting (25).
Assay for capsule and melanin
production
For capsule production, three strains were incubated
for 24 h in YPD medium, spotted onto DMEM (agar plates; Gibco;
Thermo Fisher Scientific, Inc.) at a concentration of
5x106/ml and cultured for 48 h at 30 or 37˚C.
Subsequently, the cell capsule was stained with India ink at room
temperature for 10-15 min and images were acquired under the
microscope. The relative capsule size was determined by measuring
the diameter of the capsule and the cell by using rod tool within
the Photoshop software (Adobe Photoshop CS6; Adobe Systems Europe,
Ltd.). The relative capsule size was expressed as the mean ±
standard deviation. All tests above were repeated three times.
Three independent experiments with technical triplicates were
performed in parallel.
To assess melanin synthesis, cells were spotted onto
caffeic acid agar medium (Oxid Corporation.) separately for 30 and
37˚C and observed at 48 and 72 h. The depth of the color of the
spots was observed and images were acquired using a light
microscope (Canon, Inc.; ESO 200D; x10 magnification). A darker
color represented a higher level of melanin. All tests above were
repeated three times. Three independent experiments with technical
triplicates were performed in parallel.
Assay for urease activity
Cell suspension (5 µl at the same concentration as
above) was spotted onto Christensen urea agar medium (Oxid
corporation.), followed by culture for 2 days at 30 or 37˚C. Urease
turns the medium red and the color change was monitored daily and
images were captured. All tests above were repeated three times.
Three independent experiments with technical triplicates were
performed in parallel.
Sensitivity test for stress
Each strain was incubated overnight at 30˚C in solid
YPD medium and subcultured in fresh YPD medium to an optical
density at 600 nm (OD600 nm) of 0.7-0.9. The cells were
centrifuged, washed with PBS and serially diluted
(1-104). To test the osmotic stress response, cell
suspensions were spotted (5 µl per spot) onto solid YPD medium
containing 1 or 1.5 M KCl and 1 or 1.5 M NaCl. To test for
oxidation stress, media containing 2.5 or 3.0 mmol/ml hydrogen
peroxide were prepared. To test the sensitivity of strains to
antifungal drugs, cells were spotted on solid YPD medium containing
antifungal drugs at the indicated concentrations [16 µl/ml
fluconazole (FLC), 0.2 µl/ml itraconazole (ITCZ) or 1.0 µg/ml
amphotericin B (AMB)]. All plates were incubated for 3 days at 30˚C
and images were acquired. All tests above were repeated three time.
Three independent experiments with technical triplicates were
performed in parallel.
In addition, the three strains were analyzed to
determine their sensitivities to common antifungal drugs, such as
AMB, ITCZ, FLC and 5-flucytosine (5-FC). Tests were conducted
according to the National Committee for Clinical Laboratory
Standards protocol. M-27A (27) and
Candida parapsilosis ATCC22019 (Microbiologics, Inc.) was
employed as a quality control strain. The minimal inhibitory
concentration (MIC50) was determined to compare the
antifungal activity among different strains. All tests above were
repeated three times. Three independent experiments with technical
triplicates were performed in parallel.
Virulence assays
In total, 24 female C57BL/6 mice (Fudan University
Animal Laboratories; body weight, 20-24 g; age, 4-6 weeks) were
used for the present study. The mice were housed at 18-22˚C under
50-60% humidity in a quiet room with dim light, with free access to
food and water provided.
The three Crytococcus gattii yeast strains
(WT, hog1Δ and hog1Δ+HOG1) were grown in solid YPD
medium at 30˚C for 16 h and subsequently subcultured on fresh YPD
medium to an OD600 nm of 0.7-0.9. Cell suspensions were centrifuged
and washed three times with sterile PBS and the final concentration
was adjusted to 5x106 CFU/ml with sterile PBS. Female
C57BL mice in each test group (8 mice per group) were anesthetized
with an intraperitoneal injection of 1% pentobarbital sodium at a
dose of 50 mg/kg and were then inoculated with 2.5x105
CFU in a suspension of 50 µl via intravenous injection (28). Mice were sacrificed using
CO2 inhalation at a displacement rate equivalent to 20%
of the chamber volume per minute when they appeared to be in pain,
miserable, moribund and rapidly losing weight (>15%), the mice
were observed daily. Survival analysis for the different groups was
performed using Kaplan-Meier curves and for comparison between
groups, the log-rank test was employed with PRISM software version
7.0 (GraphPad Software, Inc.).
Statistical analysis
Statistical analysis of the relative capsule size
among the three groups was performed using one-way ANOVAs and post
hoc LSD tests using PRISM software version 7.0 (GraphPad Software,
Inc.). P<0.05 was considered to indicate a statistically
significant difference.
Results
Knockout of HOG1 gene
The complete HOG1 cDNA was generated by RT-PCR and
then cloned into pCR-Blunt vector, transformed. The positive clones
were screen using PCR analysis and DNA sequencing. The sequence was
consistent with GeneBank (Fig.
S1). To identify the recombinant clones of pCR-Blunt-HOG1 and
pGAPza-HOG1, enzyme digestion using NotI/EcoRI was performed, and
the vectors and fragments were obtained. A successful expressor
vector was constructed (Fig. S2).
Similarly, the pGAPza-dHOG1 was generated by knocking out the
400-bp fragment of pGAPza-HOG1 (Fig.
S3). The pGAPza-HOG1 was transformed into hog1Δ mutant
strain and the successful reconstitution strains were constructed
and confirmed using PCR (Fig.
S4)
HOG1 has an essential role in the
regulation of the stress response in C. gattii in vitro
First, to evaluate the role of HOG1 in the
regulation of the stress response in C. gattii, various
plate tests were performed. The density represents the tolerance to
stress with a higher density indicating a higher tolerance to
stress.
The C. gattii (Cg)-hog1Δ strain
exhibited increased susceptibility to various stress factors
(Fig. 1). The experimental data
indicated that the Cg-hog1Δ strain was more sensitive to
high osmotic stress induced by high concentrations of
Na+ and K+. In terms of oxidative stress, the
Cg-hog1Δ strain exhibited no enhanced sensitivity,
suggesting that HOG1 may not be directly involved in the oxidative
stress resistance of C. gattii. (29).
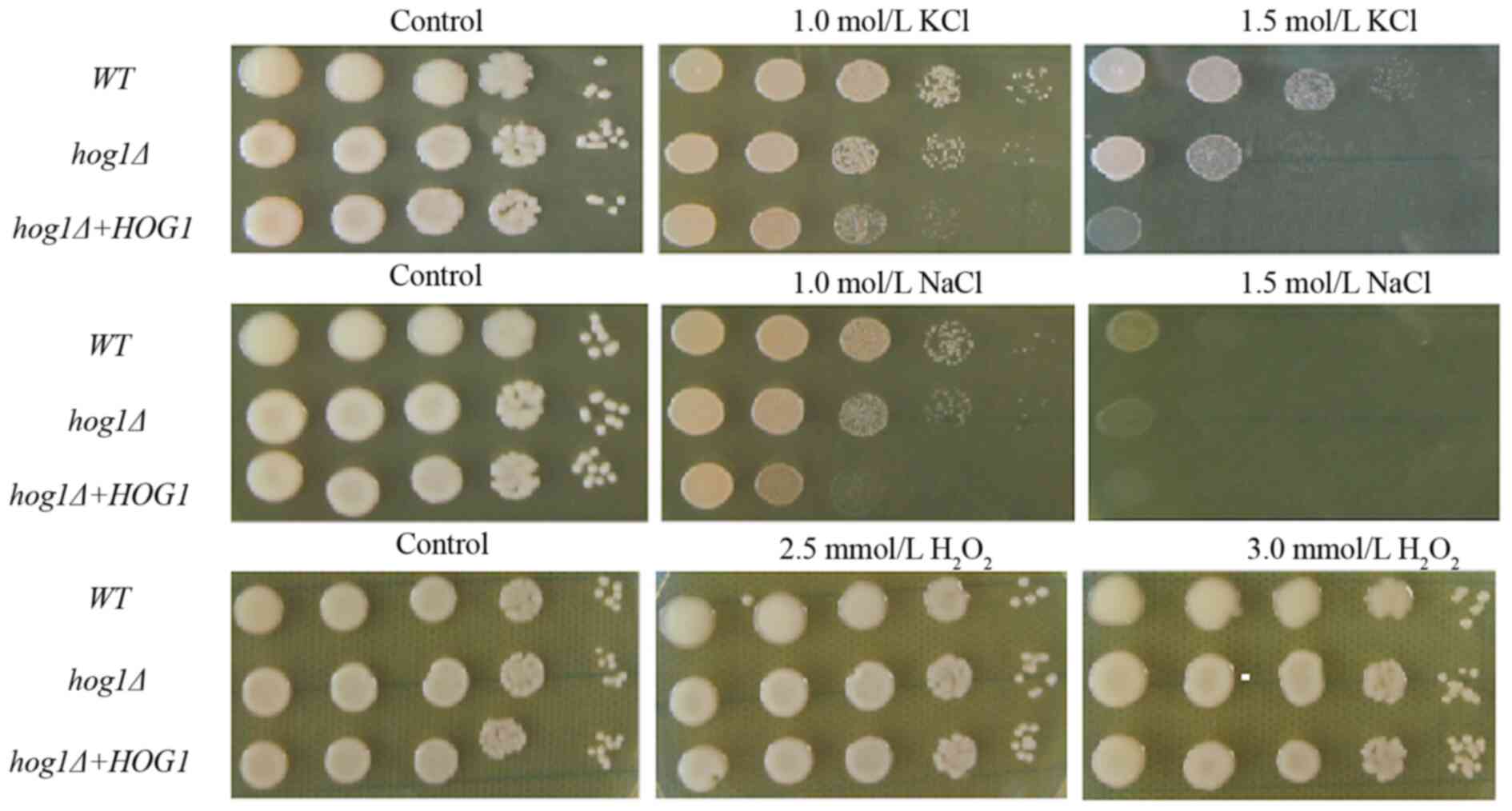 | Figure 1HOG1 is required for growth under
certain stress conditions. Each strain (WT strain, hog1Δ
strain or hog1Δ+HOG1 reconstitution strain) was grown to the
midlogarithmic phase in YPD medium, 10-fold serially diluted
(1-104 dilutions) and 2 µl of each diluted cell
suspension was spotted on YPD medium containing 1 or 1.5 mol/l
NaCl, 1 or 1.5 mol/l KCl for hyperosmotic stress and 2.5 or 3 mM
H2O2 for oxidative stress. Following
incubation for 3 days, images were acquired. All tests are for
C.gattii, the top panel is shows the KCl stress, the middle shows
the NaCl stress, the bottom shows the H2O2
stress. All tests were repeated three times, with representative
images being shown. WT, wild-type; HOG, high-osmolarity glycerol;
YPD, yeast extract peptone dextrose. |
In addition, the HOG1 gene was disrupted to identify
its effect on the susceptibility of C. gattii to several
antifungal drugs, including AMB, FLC and ITCZ. Compared to that of
the WT strain, the Cg-hog1Δ mutant strain displayed a
twofold decrease in the MIC of FLC and AMB, a more than fourfold
decrease in the MIC of ITCZ and a twofold increase in the MIC of
FC-5 (Table I). Of note,
reconstitution of the C. gattii HOG1 gene did not restore
the growth of C. gattii at high concentrations of
K+ and in the presence of ITCZ, although tolerance to
the other stressors (FLC) was observed in vitro. This
difference may be attributable to damage caused by repeated
biolistic transformations and/or ectopic integration. Also, this
could be due to transfection with a relatively large amount of
restoration. Taken together, these results demonstrated that HOG1
positively controls the stress response in vitro, although
with slight differences, in both C. neoformans and C.
gattii.
 | Table IHOG1 has a positive effect on
antifungal susceptibility of C. gattii. |
Table I
HOG1 has a positive effect on
antifungal susceptibility of C. gattii.
| | MIC (µg/ml) |
|---|
| Strain | AMB | FLC | ITCZ | 5-FC |
|---|
| WT | 0.50 | 2.00 | 0.13 | 1.00 |
| hog1Δ | 0.25 | 1.00 | 0.03 | 2.00 |
|
hog1Δ+HOG1 | 0.25 | 4.00 | 0.25 | 1.00 |
| ATCC22019 | 2.00 | 2.00 | 0.25 | 0.25 |
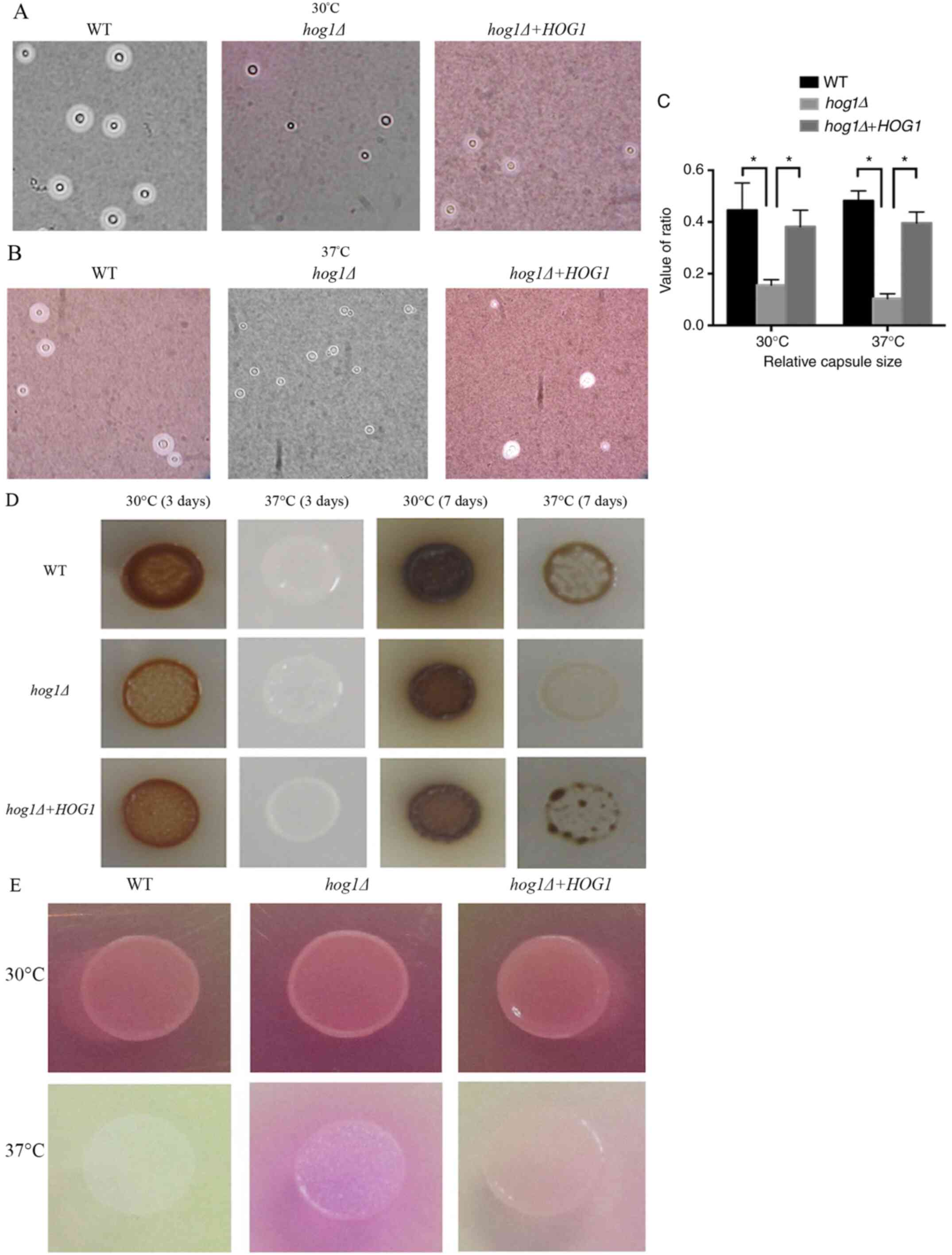
Deletion of HOG1 attenuates capsule
production and melanin synthesis but enhances urease excretion in
C. gattii
The effect of HOG1 on capsule production and melanin
synthesis, two important virulence factors that are essential for
virulence regulation, were observed in C. gattii. The
Cg-hog1Δ-mutant strain displayed a smaller capsule size in
DMEM than the WT strains and the hog1Δ+HOG1 reconstituted
strains (Fig. 2A and B). A total of 30 cells from different
strains were collected and images were captured that were subjected
to measurements, and finally, the relative capsule size was
calculated. In contrast to the WT strain and the reconstituted
strain, the Cg-hog1Δ mutant strain exhibited smaller
relative capsule sizes at 30 and 37˚C, whereas the WT strain had
capsule sizes similar to those of the reconstituted strain
(Fig. 2C). The
Cg-hog1Δ-mutant strain displayed hypomelanization in the
colonies compared with the WT strain and reconstituted strain when
incubated on caffeic acid medium at 30 and 37˚C for 3 days.
Furthermore, all of the strains produced less melanin at 30˚C than
at 37˚C, which demonstrated that temperature may negatively
regulate melanin production (Fig.
2D). Taken together, these results indicated that HOG1 has an
essential role in capsule production and melanin synthesis, two
important virulence factors in C. gattii.
As urease is an important virulence factor, it is
involved in the dissemination of C. neoformans in the host,
which promotes the accumulation of immature dendritic cells within
lung-associated lymph nodes and may enhance nonprotective T2 immune
responses during lung infection and promote invasion of the central
nervous system (9,30). In the assay, the color represents
the activity of urease, with a darker color indicating a higher
activity of urease. When the HOG1 mutant strain was incubated on
Christianson's urea agar medium, it exhibited an obvious color
development, changing the medium from yellow to a bright color,
indicating higher urease activity than that of the WT and the
hog1Δ+HOG1 reconstituted strains (Fig. 2E). In the present study, HOG1 was
observed to negatively regulate the production of urease.
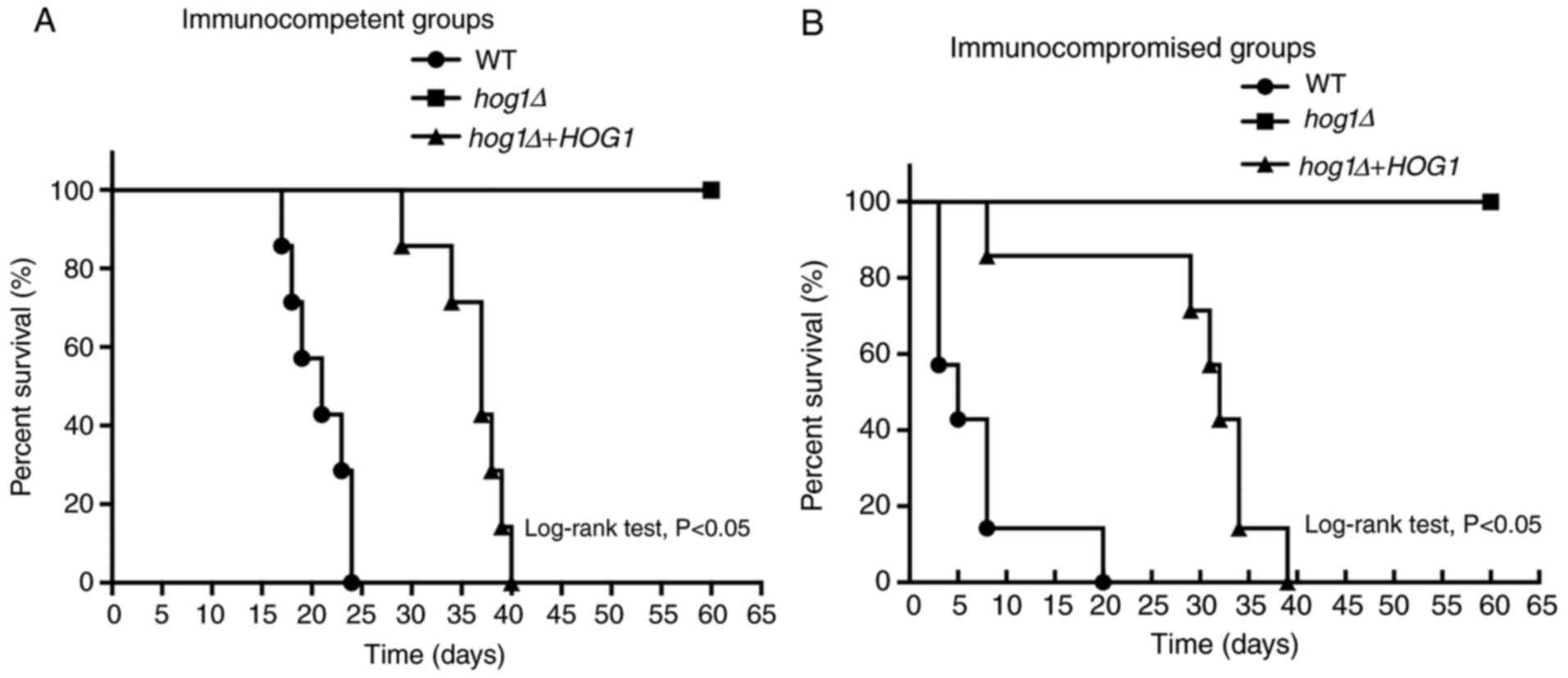
HOG1 is important for virulence in C.
gattii
To determine the role that the HOG1 gene has in the
virulence of C. gattii, a survival analysis was performed
through intravenous injection of a murine animal model. The
Cg-hog1Δ strain and the reconstituted strain
Cg-hog1Δ+HOG1 were collected and a suspension was prepared
for each strain. Finally, groups of immunocompromised and
immunocompetent C57BL/6 mice were inoculated intravenously with one
of each of the strains (105 cells per animal).
In the immunocompetent groups, mice infected by the
WT strain had a survival time of up to 24 days and the median
survival time was 21 days. The survival pattern of mice inoculated
with the reconstituted strain was similar, with the longest
survival time among all the mice was 40 days and the median
survival time was 37 days, but there was no significant difference.
By contrast, mice infected with the hog1Δ mutant strain
survived until the endpoint at 60 days after infection, suggesting
that disruption of the HOG1 gene significantly attenuated the
virulence of C. gattii (P<0.05; Fig. 3A).
In the immunocompromised group, similar results were
obtained. Mice infected with the Cg-hog1Δ strain survived
significantly comparatively longer and had not died at 60 days
after infection, whereas the median survival times of the mice
infected with the WT strain and WT reconstituted strain were 5 and
32 days, respectively (Fig. 3B).
There was a significant difference among the groups.
Taken together, the results stated above
demonstrated that HOG1 has a significant role in the virulence
regulation of C. gattii.
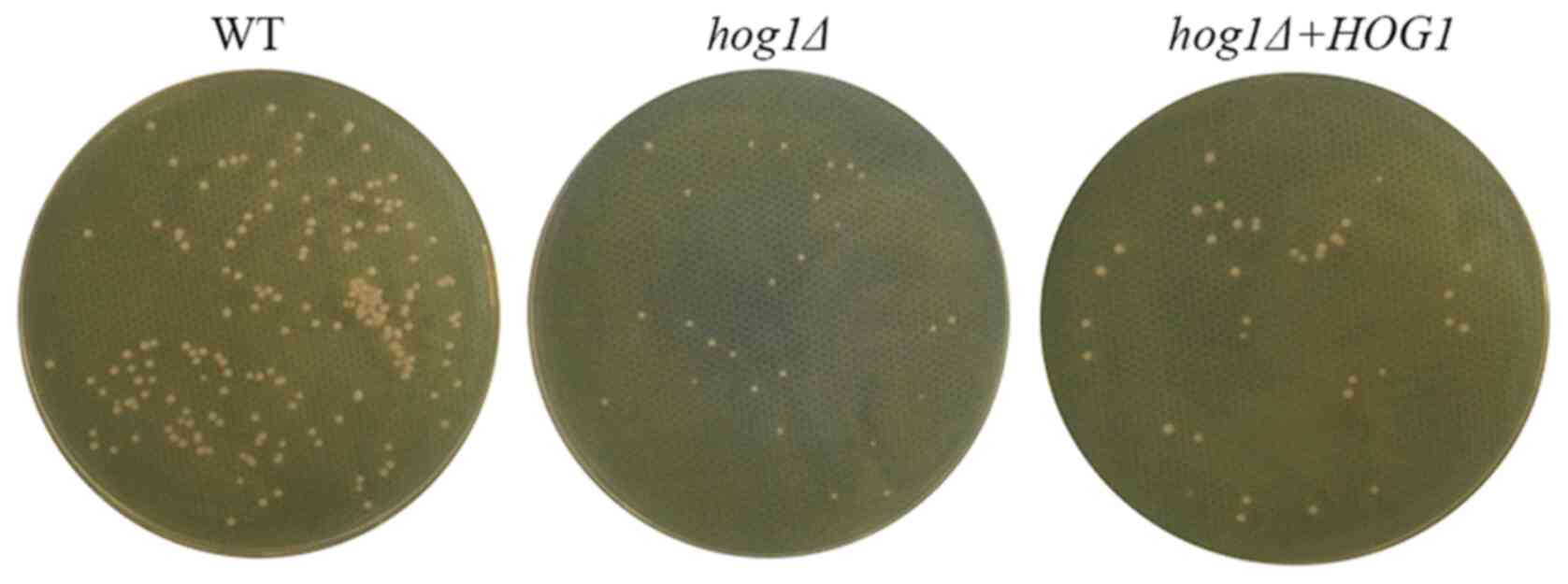
C. gattii requires HOG1 to propagate
in the mammal host
Finally, the roles of HOG1 in survival and
dissemination in the mammalian host were evaluated. The WT strain,
the hog1Δ strain and the reconstituted strain
hog1Δ+HOG1 were isolated from the lung tissues of the
infected mice and cultured on YPM medium for 3 days at 30˚C. As
presented in Fig. 4, colonies of
the hog1∆ strain were fewer and their sizes were smaller
than those of the WT strain and the reconstituted strain
hog1Δ+HOG1. These differences in the colonies further
verified that the hog1Δ strain had poorer growth in the host
environment, suggesting that HOG1 is able to prompt the propagation
of C. gattii and promote the dissemination in the mammalian
host.
Discussion
C. gattii is a major pathogenic fungus that
is most commonly detected among immunocompetent patients and occurs
not only in China but also in other parts of the world (31). However, C. gattii is only
scarcely distributed in temperate zones of the world. A previous
outbreak of C. gattii infection in the Pacific Northwest
United States and Vancouver Island in Canada indicates that this
species is spreading to other geographical areas (32), which has received increased global
scientific attention (33). To
date, only a small number of experimental studies on the pathogenic
mechanisms of C. gattii have been performed. In the present
study, the effect of HOG1 on virulence regulation of C.
gattii was functionally determined using the clinical strain
CZ2012 and certain distinctive and shared properties with C.
neoformans were defined.
HOG1 is one of the most important components of the
HOG-MAPK signaling pathway and was first characterized in C.
albicans (34). In C.
neoformans, HOG1 is also responsible for various cellular
processes. Knockout of the HOG1 gene results in numerous phenotypic
changes, such as sensitivity to hyperosmotic stimuli and oxidative
stress, resistance to azole, UV irradiation, growth at body
temperature and increased production of capsules and melanin
(29). However, HOG1 exhibits
different regulatory mechanisms in different environments and
clinical strains (35). A small
portion of C. neoformans isolates, such as JEC21, the
HOG1 gene is mostly dephosphorylated under normal circumstances and
is phosphorylated under stress shock, similar to HOG1 homologs in
other fungi, such as Saccharomyces cerevisiae. Conversely,
HOG1 is mostly phosphorylated under normal conditions and is
rapidly dephosphorylated under an external stress response, which
is common in almost all C. neoformans strains,
including H99. This type of pattern of HOG1 phosphorylation may
contribute to differences in the development of virulence
attributes. These differences have all been observed in the C.
neoformans strains, while the role that the signaling pathway
has in C. gattii has remained to be elucidated to date.
In the present study, the hog1Δ strains had a
poor growth performance under stress conditions. Similar to the
role exerted by HOG1 in C. neoformans, this protein
positively regulated the responses of C. gattii to various
stresses, including high K+/Na+. There are
numerous possible explanations for the effect of HOG1 in stress
control. Intracellular glycerol appeared to be increased after
Cryptococcus was exposed to various stresses to adapt to
environmental changes, which are controlled by the HOG-MAPK
signaling pathway. The HOG-MAPK signaling pathway also has an
essential role in stress responses in other fungi, such as
Candida albicans, Schizosaccharomyces pombe and
Saccharomyces cerevisiae (36-38).
As a core component of the HOG-MAPK pathway, HOG1 has an essential
role in maintaining the cellular balance and responding to various
stresses. HOG1 knockout results in reduced accumulation of
intracellular glycerol, thereby affecting normal cell growth and
eventually causing cell swelling and bursting (39). Therefore, a growth defect of the
hog1Δ strains was observed in the present experiment.
However, deletion of HOG1 resulted in a difference
between C. gattii and C. neoformans regarding
susceptibility to oxidation stress. The C. gattii hog1Δ
strain and C. neoformans had a similar sensitivity to
H2O2. Multiple signaling pathways and
regulatory systems control the response to various stresses.
However, HOG1 may act on different substrates to differentially
regulate the reaction to oxidation stress.
HOG1 regulates numerous key downstream proteins and
governs several virulence traits in C. neoformans, such as
ergosterol biosynthesis, which is a target antifungal drug to which
it binds. After knockout of the HOG1 gene, the expression levels of
545 genes changed significantly, more than two times the normal
level, which was confirmed by transcriptomics analysis (40). In the hog1Δ mutant, the
expression levels of genes involved in the synthesis and content of
ergosterol were significantly increased, indicating that HOG1
inhibits the synthesis of ergosterol under normal conditions. There
is another finding that supports this possibility. The hog1Δ
mutant strain had a greater sensitivity to AMB but was resistant to
azole antifungal drugs, probably because ergosterol is the binding
site of AMB, and the expression level was clearly increased. The
effect of the HOG signaling pathway on the synthesis of ergosterol
varies significantly among different strains (38). Deletion of HOG1 results MICs of FLC
and ITCZ increased (40). However,
in the present study, the hog1Δ strains exhibited distinct
antifungal susceptibility, such as a twofold decrease in the MIC
for AMB and FLC, at least a 4-fold decrease in the MICs of ITCZ,
but a 2-fold increase in the MIC of 5-FC, which are divergent
compared with C. neoformans. These results suggested that
there may be another pathway that negatively coordinates with
HOG-MAPK, affecting ergosterol biosynthesis. Another explanation
may be that during evolution, C. gattii lost either precise
upstream or downstream feedback control of HOG1, resulting in
phosphorylation changes in the MAPK pathway and contributing to
antifungal drug sensitivity. This phenomenon may provide certain
benefits to C. gattii regarding host infection, such as high
stress resistance.
To further determine the effect of HOG1 on virulence
factors, capsule production and melanin synthesis were evaluated,
which are essential for protection from oxidants, phagocytosis and
dissemination (41). Both capsule
production and melanin synthesis are controlled by the cyclic
adenosine monophosphate (cAMP)/protein kinase A (PKA) signaling
pathway (13,42-44).
HOG-MAPK is involved in crosstalk between these processes and the
cAMP/PKA signaling pathway (15).
In C. neoformans, HOG1 controls capsule production and
melanin synthesis differentially in different serotype strains; for
instance, HOG1 decreases capsule production and melanin synthesis
in the H99 mutant strain but not in other strains. In the present
study, HOG1 was evidenced to participate in crosstalk with the
cAMP/PKA signaling pathway and knockout of the HOG1 gene resulted
in a significant reduction in capsule production and melanin
synthesis in C. gattii. These results indicated that HOG1
may positively modulate a component of the cAMP/PKA signaling
pathway controlling capsule and melanin synthesis in C.
gattii. Taken together, these findings indicate that in
contrast to C. neoformans, HOG1 also has an important role
in governing virulence factors in C. gattii. This result
suggests that the phosphorelay system diverges functionally and
structurally, which, in turn, differentially regulates the HOG1
cascade in both Cryptococcus species. Because of the
complexity of the interactions between cAMP/PKA and the HOG
pathway, further study is required to understand the interplay
between the two pathways.
Urease activity is essential for the spread of
Cryptococcus in lung infections, as the enzyme induces a
nonprotective T2 immune response by promoting the accumulation of
immature dendritic cells (9).
Urease may facilitate cell body transmigration to the blood-brain
barrier by enhancing sequestration within microvascular beds
(45) and promoting central nervous
system (CNS) invasion (46). The
function of urease activation has been extensively studied in such
organisms as plants and bacteria but rarely in fungi, particularly
in C. gattii. However, prior research demonstrated that
after translocation of the pathogen into the CNS, the virulence of
urease is attenuated, which results in scarcity or absence of
inflammation in brain tissues (30). In the present study, unexpectedly,
the hog1Δ strain exhibited more viable urease activities
than the WT strain and the reconstituted strain at 37˚C, but the
difference in urease activities was not as obvious at 37˚C. These
results suggest that HOG1 may regulate urease activities in C.
gattii strains within the host environment.
The Cryptococcus species may utilize multiple
tools to persist in the host environment and cause damage to the
host (47). Numerous virulence
factors are considered to contribute to virulence, such as adoption
to stress, secreted enzymes and capsule and melanin synthesis
(30,48,49).
In C. gattii, knockout of the HOG1 gene led to a significant
reduction in the pathogen's virulence in the mouse model, despite
the higher level of urease excretion in the hog1Δ strain.
The fact that the hog1Δ strain isolated from infected mice
had a growth defect compared with the WT strain and the
reconstituted strain further indicated the role of HOG1 in
virulence. All of these results suggested that HOG1 has a
significant role in virulence regulation in both C.
neoformans and C. gattii, with several shared and
distinctive mechanisms.
In conclusion, the present study demonstrated the
role of HOG1 in the regulation of various virulence factors of the
C. gattii strain CZ2012. HOG1 is essential for propagation
in the lung, resistance to stress, capsule production and melanin
synthesis, as well as the pathogenicity of C. gattii in a
mouse model. Furthermore, the commonalities and differences of HOG1
in both Cryptococcus species demonstrated that C.
gattii may have developed certain specific mechanisms to adapt
to changes in the environment in vivo and in vitro.
However, the mechanism by which HOG1 affects the immune function of
the host during the infection process has remained to be elucidated
and warrants further investigation.
Supplementary Material
PCR screening of HOG1 gene cloning
into pCR-Blunt. The HOG1 gene is 1,098 bp in length. Lane no. 6 is
positive, which is consistent with the GenBank sequence. M, marker;
HOG1, high osmolarity glycerol 1.
pGAPza-HOG1 expression vector
construction (PCR and enzyme restriction analysis). The pGAPza-HOG1
expression vector was enzyme-digested by NotI/EcoRI and vector
(left panel) and fragment (right panel) were obtained, which were
then cloned, transformed and screened. M, marker; HOG1, high
osmolarity glycerol 1.
Deletion of HOG1 gene and analysis of
dHOG1. The HOG1 gene knockout expression vector pGAPza-dHOG1 was
constructed by knocking out 400-bp fragments of pGAPza-HOG,
followed by reconnection and transformation. M, marker; HOG1, high
osmolarity glycerol 1.
PCR analysis of knockout strain and
reconstitution strain. Knockout of dHOG1 gene and reconstitution of
HOG1 gene were confirmed by reverse transcription PCR. Lanes no. 1,
2 and 5 are PCR-amplified products of the target HOG1 gene fragment
from reconstitution strains. Lanes a-f are PCR-amplified products
of the target dHOG1 gene from knockout strains. M, marker; HOG1,
high osmolarity glycerol 1.
Acknowledgements
Not applicable.
Funding
This study was supported by the Zhejiang Provincial Natural
Science Foundation of China (grant no. LY20H110002), the General
Project Funds from the Health Department of Zhejiang Province
(grant nos. 2020KY446 and 2021KY069) and the Outstanding Young
People's Fund of Zhejiang Provincial People's Hospital (grant no.
ZRY2018C004).
Availability of data and materials
The datasets used and/or analyzed during the
current study are available from the corresponding author on
reasonable request.
Authors' contributions
YMH and YΒF confirmed the authenticity of all the
raw data. YMH, WQX and YΒF designed the present study. YMH and XHT
collected clinical samples and performed analysis of data. YMH and
YT performed statistical analysis. DFX and YY performed the
experiments. YMH wrote the manuscript. All authors read and
approved the final manuscript.
Ethics approval and consent to
participate
The study was approved by the Medical Ethics
Committee of Zhejiang Provincial People's Hospital (Hangzhou,
China; reference no. 2019-180).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Heitman J: American Society for
Microbiology. (2011). ‘Cryptococcus: From human pathogen to model
yeast.’ Washington, DC, ASM Press.
|
|
2
|
Kamari A, Sepahvand A and Mohammadi R:
‘Isolation and molecular characterization of Cryptococcus
species isolated from pigeon nests and Eucalyptus trees’.
Curr Med Mycol. 3:20–25. 2017.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Lin KH, Lin YP and Chung WH: Two-step
method for isolating Cryptococcus species complex from
environmental material using a new selective medium. Environ
Microbiol Rep. 11:651–658. 2019.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Pakshir K, Fakhim H, Vaezi A, Meis JF,
Mahmoodi M, Zomorodian K, Javidnia J, Ansari S, Hagen F and Badali
H: Molecular epidemiology of environmental Cryptococcus
species isolates based on amplified fragment length polymorphism. J
Mycol Med. 28:599–605. 2018.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Bandalizadeh Z, Shokohi T, Badali H,
Abastabar M, Babamahmoudi F, Davoodi L, Mardani M, Javanian M,
Cheraghmakani H, Sepidgar AA, et al: Molecular epidemiology and
antifungal susceptibility profiles of clinical Cryptococcus
neoformans/Cryptococcus gattii species complex. J Med
Microbiol. 69:72–81. 2020.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Herkert PF, Hagen F, Pinheiro RL, Muro MD,
Meis JF and Queiroz-Telles F: Ecoepidemiology of Cryptococcus
gattii in developing countries. J Fungi (Basel).
3(62)2017.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Kwon-Chung KJ, Fraser JA, Doering TL, Wang
Z, Janbon G, Idnurm A and Bahn YS: Cryptococcus neoformans
and Cryptococcus gattii, the etiologic agents of
cryptococcosis. Cold Spring Harb Perspect Med.
4(a019760)2014.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Olave MC, Vargas-Zambrano JC, Celis AM,
Castañeda E and González JM: Infective capacity of Cryptococcus
neoformans and Cryptococcus gattii in a human
astrocytoma cell line. Mycoses. 60:447–453. 2017.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Pini G, Faggi E and Campisi E: Enzymatic
characterization of clinical and environmental Cryptococcus
neoformans strains isolated in Italy. Rev Iberoam Micol.
34:77–82. 2017.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Casadevall A, Coelho C, Cordero RJ,
Dragotakes Q, Jung E, Vij R and Wear MP: The capsule of
Cryptococcus neoformans. Virulence. 10:822–831.
2019.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Oliveira DL, Freire-de-Lima CG, Nosanchuk
JD, Casadevall A, Rodrigues ML and Nimrichter L: Extracellular
vesicles from Cryptococcus neoformans modulate macrophage
functions. Infect Immun. 8:1601–1609. 2010.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Lee D, Jang EH, Lee M, Kim SW, Lee Y, Lee
KT and Bahn YS: Unraveling melanin biosynthesis and signaling
networks in Cryptococcus neoformans. mBio. 10:e02267–19.
2019.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Caza M and Kronstad JW: The cAMP/protein
kinase a pathway regulates virulence and adaptation to host
conditions in Cryptococcus neoformans. Front Cell Infect
Microbiol. 9(212)2019.PubMed/NCBI View Article : Google Scholar
|
|
14
|
So YS, Lee DG, Idnurm A, Ianiri G and Bahn
YS: The TOR pathway plays pleiotropic roles in growth and stress
responses of the fungal pathogen Cryptococcus neoformans.
Genetics. 212:1241–1258. 2019.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Fu C, Donadio N, Cardenas ME and Heitman
J: Dissecting the roles of the calcineurin pathway in unisexual
reproduction, stress responses, and virulence in Cryptococcus
deneoformans. Genetics. 208:639–653. 2018.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Jung KW, Strain AK, Nielsen K, Jung KH and
Bahn YS: Two cation transporters Ena1 and Nha1 cooperatively
modulate ion homeostasis, antifungal drug resistance, and virulence
of Cryptococcus neoformans via the HOG pathway. Fungal Genet
Biol. 49:332–345. 2012.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Meyers GL, Jung KW, Bang S, Kim J, Kim S,
Hong J, Cheong E, Kim KH and Bahn YS: The water channel protein
aquaporin 1 regulates cellular metabolism and competitive fitness
in a global fungal pathogen Cryptococcus neoformans. Environ
Microbiol Rep. 9:268–278. 2017.PubMed/NCBI View Article : Google Scholar
|
|
18
|
So YS, Jang J, Park G, Xu J, Olszewski MA
and Bahn YS: Sho1 and Msb2 play complementary but distinct roles in
stress responses, sexual differentiation, and pathogenicity of
Cryptococcus neoformans. Front Microbiol.
9(2958)2018.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Bahn YS: Master and commander in fungal
pathogens: The two-component system and the HOG signaling pathway.
Eukaryot Cell. 7:2017–2036. 2008.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Hoang LMN, Maguire JA, Doyle P, Fyfe M and
Roscoe DL: Cryptococcus neoformans infections at vancouver
hospital and health sciences centre (1997-2002): Epidemiology,
microbiology and histopathology. J Med Microbiol. 53:935–940.
2004.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Nascimento E, Vitali LH, Kress MR and
Martinez R: Cryptococcus neoformans and C. gattii
isolates from both HIV-infected and uninfected patients: Antifungal
susceptibility and outcome of cryptococcal disease. Rev Inst Med
Trop Sao Paulo. 59(e49)2017.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Chen Y, Farrer RA, Giamberardino C,
Sakthikumar S, Jones A, Yang T, Tenor JL, Wagih O, Van Wyk M,
Govender NP, et al: Microevolution of serial clinical isolates of
Cryptococcus neoformansvar grubii and C. gattii.
mBio. 8:e00166–17. 2017.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Sang J, Yang Y, Fan Y, Wang G, Yi J, Fang
W, Pan W, Xu J and Liao W: Isolated iliac cryptococcosis in an
immunocompetent patient. PLoS Negl Trop Dis.
12(e0006206)2018.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Ho EC, Donaldson ME and Saville BJ:
Detection of antisense RNA transcripts by strand-specific RT-PCR.
Methods Mol Biol. 630:125–138. 2010.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Meng Y, Zhang C, Yi J, Zhou Z, Fa Z, Zhao
J, Yang Y, Fang W, Wang Y and Liao WQ: Deubiquitinase Ubp5 is
required for the growth and pathogenicity of Cryptococcus
gattii. PLoS One. 11(e0153219)2016.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Lin X, Chacko N, Wang L and Pavuluri Y:
Generation of stable mutants and targeted gene deletion strains in
Cryptococcus neoformans through electroporation. Med Mycol.
53:225–234. 2015.PubMed/NCBI View Article : Google Scholar
|
|
27
|
National Committee for Clinical Laboratory
Standards: Reference method for broth dilution antifungal
susceptibility testing of yeasts. Approved standard NCCLS document
M27-A. National Committee for Clinical Laboratory Standards, Wayne,
PA, 1997.
|
|
28
|
Liu TB and Xue C: Fbp1-mediated
ubiquitin-proteasome pathway controls Cryptococcus
neoformans virulence by regulating fungal intracellular growth
in macrophages. Infect Immun. 82:557–568. 2014.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Upadhya R, Kim H, Jung KW, Park G, Lam W,
Lodge JK and Bahn YS: Sulphiredoxin plays peroxiredoxin-dependent
and -independent roles via the HOG signalling pathway in
Cryptococcus neoformans and contributes to fungal virulence.
Mol Microbiol. 90:630–648. 2013.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Toplis B, Bosch C, Schwartz IS, Kenyon C,
Boekhout T, Perfect JR and Botha A: The virulence factor urease and
its unexplored role in the metabolism of Cryptococcus
neoformans. FEMS Yeast Res. 20(foaa031)2020.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Fang W, Fa Z and Liao W: Epidemiology of
Cryptococcus and Cryptococcosis in China. Fungal
Genet Biol. 78:7–15. 2015.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Acheson ES, Galanis E, Bartlett K, Mak S
and Klinkenberg B: Searching for clues for eighteen years:
Deciphering the ecological determinants of Cryptococcus
gattii on Vancouver Island, British Columbia. Med Mycol.
6:129–144. 2018.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Firacative C, Torres G, Meyer W and
Escandón P: Clonal dispersal of Cryptococcus gattii VGII in
an endemic region of Cryptococcosis in Colombia. J Fungi
(Basel). 5(32)2019.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Day AM, McNiff MM, da Silva Dantas A, Gow
NA and Quinn J: Hog1 regulates stress tolerance and virulence in
the emerging fungal pathogen Candida auris. mSphere. 3:e00506–18.
2018.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Kojima K, Bahn YS and Heitman J:
Calcineurin, Mpk1 and Hog1 MAPK pathways independently control
fludioxonil antifungal sensitivity in Cryptococcus
neoformans. Microbiology (Reading). 152:591–604.
2006.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Guirao-Abad JP, Sánchez-Fresneda R, Román
E, Pla J, Argüelles JC and Alonso-Monge R: The MAPK Hog1 mediates
the response to amphotericin B in Candida albicans. Fungal
Genet Biol. 136(103302)2020.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Shiraishi K, Hioki T, Habata A, Yurimoto H
and Sakai Y: Yeast Hog1 proteins are sequestered in stress granules
during high-temperature stress. J Cell Sci.
131(jcs209114)2018.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Sharmeen N, Sulea T, Whiteway M and Wu C:
The adaptor protein Ste50 directly modulates yeast MAPK signaling
specificity through differential connections of its RA domain. Mol
Biol Cell. 30:794–807. 2019.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Petelenz-Kurdziel E, Kuehn C, Nordlander
B, Klein D, Hong KK, Jacobson T, Dahl P, Schaber J, Nielsen J,
Hohmann S and Klipp E: Quantitative analysis of glycerol
accumulation, glycolysis and growth under hyper osmotic stress.
PLoS Comput Biol. 9(e1003084)2013.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Ko YJ, Yu YM, Kim GB, Lee GW, Maeng PJ,
Kim S, Floyd A, Heitman J and Bahn YS: Remodeling of global
transcription patterns of Cryptococcus neoformans genes
mediated by the stress-activated HOG signaling pathways. Eukaryot
Cell. 8:1197–1217. 2009.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Geddes JM, Caza M, Croll D, Stoynov N,
Foster LJ and Kronstad JW: Analysis of the protein kinase
a-regulated proteome of Cryptococcus neoformans identifies a
role for the ubiquitin-proteasome pathway in capsule formation.
mBio. 7:e01862–15. 2016.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Huston SM, Ngamskulrungroj P, Xiang RF,
Ogbomo H, Stack D, Li SS, Timm-McCann M, Kyei SK, Oykhman P,
Kwon-Chung KJ and Mody CH: Cryptococcus gattii capsule
blocks surface recognition required for dendritic cell maturation
independent of internalization and antigen processing. J Immunol.
196:1259–1271. 2016.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Maybruck BT, Lam WC, Specht CA, Ilagan MX,
Donlin MJ and Lodge JK: The aminoalkylindole BML-190 negatively
regulates chitosan synthesis via the Cyclic AMP/protein kinase A1
pathway in Cryptococcus neoformans. mBio. 10:e02264–19.
2019.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Bruni GO, Battle B, Kelly B, Zhang Z and
Wang P: Comparative proteomic analysis of Gib2 validating its
adaptor function in Cryptococcus neoformans. PLoS One.
12(e0180243)2017.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Fu MS, Coelho C, De Leon-Rodriguez CM,
Rossi DC, Camacho E, Jung EH, Kulkarni M and Casadevall A:
Cryptococcus neoformans urease affects the outcome of
intracellular pathogenesis by modulating phagolysosomal pH. PLoS
Pathog. 14(e1007144)2018.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Squizani ED, Oliveira NK, Reuwsaat JC,
Marques BM, Lopes W, Gerber AL, de Vasconcelos AT, Lev S,
Djordjevic JT, Schrank A, et al: Cryptococcal dissemination to the
central nervous system requires the vacuolar calcium transporter
Pmc1. Cell Microbiol: Feb 20, 2018 (Epub ahead of print).
|
|
47
|
Kronstad J, Saikia S, Nielson ED,
Kretschmer M, Jung W, Hu G, Geddes JM, Griffiths EJ, Choi J,
Cadieux B, et al: Adaptation of Cryptococcus neoformans to
mammalian hosts: Integrated regulation of metabolism and virulence.
Eukaryot Cell. 11:109–118. 2012.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Do E, Park M, Hu G, Caza M, Kronstad JW
and Jung WH: The lysine biosynthetic enzyme Lys4 influences iron
metabolism, mitochondrial function and virulence in Cryptococcus
neoformans. Biochem Biophys Res Commun. 77:706–711.
2016.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Chang AL, Kang Y and Doering TL: Cdk8 and
Ssn801 regulate oxidative stress resistance and virulence in
Cryptococcus neoformans. mBio. 10:e02818–18. 2019.PubMed/NCBI View Article : Google Scholar
|