Introduction
Homocysteine (Hcy) is a thiol-containing amino acid,
and an intermediate product of methionine and cysteine amino acid
metabolism (1-3).
Hcy was first isolated from bladder stones in 1931 and was thought
to be related to the development of atherosclerosis as early as in
1969(4). When the metabolic pathway
is altered due to genetic or acquired factors, the Hcy level
increases, exceeding maximum normal levels, leading to a condition
termed hyperhomocysteinemia (HHcy) (5,6). HHcy
is used as an independent risk factor for predicting cardiovascular
disease, stroke and vitamin B12 deficiency (7-9).
Research to date has indicated that the two most important systems
affected by HHcy are the cardiovascular and nervous systems.
Studies have found that Hcy plays an important role
in the occurrence and development of carotid atherosclerosis
(10-12).
Elevated plasma levels of Hcy are associated with asymptomatic
carotid artery disease in patients with hypertension. Stroke, head
trauma and pressure cause the blood-brain barrier (BBB) to be
destroyed, exposing the brain to plasma components, including Hcy
(13-17).
Hcy also mediates cardiovascular conditions due to its adverse
effects on the cardiovascular endothelium and smooth muscle cells,
leading to changes in subclinical arterial structure and function
(13-15).
In addition, acute ischemic stroke seems to be associated with the
increase in inflammatory cytokine levels induced by Hcy and the
permeability of the BBB (16-17).
Through this mechanism, studies have found that multivitamin
therapy can protect the BBB against damage by reducing plasma Hcy
levels (18-20).
In addition, the activation of the inflammatory cascade following
brain lesions can trigger changes in the human immune system, and
different inflammatory cells play differential roles (21,22). T
helper cell 17 (Th17) and regulatory T cells (Tregs) play an
important role in maintaining the immune balance (23). Th17 cells secrete high levels of
IL-17A, which mainly mediates the inflammatory response and can
promote the maturation, proliferation and chemotaxis of neutrophils
(23-25).
Tregs secrete certain inhibitory cytokines, such as IL-10, IL-4 and
TGF-β, which mainly mediate immune tolerance and play an important
role in maintaining the body's immune balance (24,25).
Th17 and Tregs antagonize each other in function and
differentiation (24). When the
body is in a normal state, the two maintain a relative balance;
however, but when the body is in an abnormal state, an imbalance
occurs between Th17 and Tregs. On the other hand, CD4+ T
cells can differentiate into various Tregs, which can suppress
adaptive T cell responses and prevent autoimmunity (25). The control of the Th17/Treg balance
is also crucial to the development of inflammatory diseases and the
inflammatory response in brain lesions (24,25).
In addition, it has been indicated that transcription factors, such
as retinoic acid-related orphan receptor γt (RORγt) and forkhead
box P3 (FoxP3) play an important role in Th17 and Tregs cells
(26,27). This suggests that Th17 and Tregs
should not only be analyzed, but also the underlying mechanism and
the regulatory network involved should be considered. Th17 and
Tregs have been extensively investigated in the context of the
inflammatory response following brain injury; however, the role of
the two in the brain ischemic inflammatory response remains
controversial (28,29). This is not only due to the
interdependence between the nervous system and the immune system,
but also due to the different response of the two systems in the
process of brain ischemic inflammation.
In the present study, an animal model of HHcy was
established using Wistar-Kyoto (WKY) rats administered a high
methionine diet. The rats were then fed with a therapeutic diet
(including vitamins B6 and B12, and folic acid). Changes in body
weight, systolic blood pressure and plasma Hcy contents were
observed in the rats. In addition, the levels of inflammatory
cytokines (IL-6, IL-17A, IL-10 and TGF-β) associated with Th17 and
Tregs were detected, and changes in the plasma levels of Th17 and
Tregs, as well as key transcription factor (RORγt and FoxP3)
expression in brain tissue were determined to assess brain tissue
damage and the systemic immune response.
Materials and methods
Construction of animal models and
animal welfare
A total of 60 male WKYs (weight, 185.3-228.6 g; age,
8 weeks) were purchased from the Laboratory Animal Center of the
Chinese Academy of Military Medical Sciences. All animal
experiments were approved by the Animal Experiment Ethics Committee
of the Second Hospital of Tianjin Medical University (Tianjin,
China). All animals were allowed food and water ad libitum
and were housed under conditions of a 12-h light/dark cycle (lights
on at 7:00 a.m.) at a temperature of 22˚C with 40-60% humidity. The
experiments were carried out in accordance with the National
Institutes of Health Guide for the Care and use of Laboratory
Animals (30). For inhalant
anesthesia during the physical examination procedures, after 3%
anesthetic induction, anesthesia maintenance with 1.5% isoflurane
(Macklin Inc.) was used for each rat. At the end of the experiment,
all rats were euthanized by intraperitoneal injection of sodium
pentobarbital (80 mg/kg; cat. no. P-010; Cerilliant Corporation),
and exsanguination was used to euthanize them, and blood samples
were collected according to the AVMA Guidelines on the Euthanasia
of Animals (31). Brain tissues
were removed and immediately frozen in liquid nitrogen and stored
at -80˚C.
A total of 60 WKYs were randomly divided into three
groups: WKY control group (WKY-C group), WKY methionine group
(WKY-M group) and WKY treatment group (WKY-T group), with 20 rats
in each group. The experiment was intended to intervene in animal
feeding conditions for 16 weeks. Throughout the experiment, WKY-C
rats were given normal animal feed (without methionine), while
WKY-M and WKY-T rats received 2% methionine-supplemented (cat. no.
M9500; Merck KGaA) feed. Starting from the first day of week 9,
WKY-T rats were treated by gavage daily for 8 weeks, with a regimen
of 12 mg/kg vitamin B6 (cat. no. P5669), 0.09 mg/kg vitamin B12
(cat. no. V2876), and 4 mg/kg folic acid (cat. no. F7876; all from
Merck KGaA). At the same time, the WKY-M and WKY-C groups received
normal saline for 8 weeks. The dose was adjusted and calculated
according to the weight change of each rat.
Physical examination and plasma Hcy
analysis
The body weights of rats were measured and recorded
on the first day (week 0) and weeks 4, 8, 12 and 16. At the same
time, a non-invasive blood pressure measurement system was used to
monitor the systolic blood pressure (SBP) of the tail artery in the
state of consciousness (Taimen BP-100A automatic large-scale
non-invasive blood pressure measurement system; Chengdu Taimeng
Software Co., Ltd.). In order to ensure the accuracy of the SBP
measurement, the temperature of each rat was controlled at 37˚C,
the blood pressure of each rat was measured three times after
obtaining a stable baseline (Taimen BP-100A small animal heater;
Chengdu Taimeng Software Co., Ltd.). The plasma Hcy concentrations
in WKYs were determined by a 7500B automatic immunobiochemical
analyzer (Applied Biosystems; Thermo Fisher Scientific, Inc.)
following the manufacturer's instructions.
Cytokine release assay
Starting at the 9th week of gastric gavage treatment
for the WKY-T group, the tail blood of the rats in all groups was
collected by centrifugation every 4 weeks. The collected blood
samples were centrifuged at 4˚C at 1,500 x g for 10 min to collect
serum. The cytokine concentrations of IL-17A (cat. no. DY8410-05),
IL-6 (cat. no. DY506), IL-10 (cat. no. DY522) and TGF-β (cat. no.
PMB100B; all from R&D Systems, Inc.) in peripheral blood were
measured by ELISA. Each sample was tested in triplicate. The OD
value of each well was measured at a wavelength of 450 nm within 15
min. The concentration was calculated according to the
manufacturer's instructions.
Flow cytometric analysis
Th17/Treg lymphocytes were determined by flow
cytometry. The peripheral blood samples of WKY-C/M/T rats were
selected for analysis at weeks 0, 4, 8, 12 and 16. Briefly,
peripheral venous blood (1 ml) was collected in anticoagulation
tubes and mixed with 1 ml of PBS (cat. no. P1020; Beijing Solarbio
Science & Technology Co., Ltd.). The PBS liquid was then slowly
added to the wall of the tube and centrifuged at 4˚C, 500 x g for
20 min. Following centrifugation, the lymphocyte layer was
collected into a new tube. Subsequently, 1ml PBS solution was
added, and the mixture was centrifuged at 4˚C, 500 x g for 10 min.
After discarding the supernatant, the cells were washed twice with
PBS, and prepared to perform flow cytometric analysis. CD3-APC
(cat. no. 17-0030-82; clone, eBioG4.18), CD4-PC5 (cat. no.
15-0041-82; clone, GK1.5), CD25-FITC (cat. no. MA1-35144; clone,
CD25-3G10), FoxP3-PE (cat. no. 12-5773-82; clone, FJK-16s) and
IL-17A-PC7 (cat. no. 25-7177-82; clone, eBio17B7) antibodies were
purchased from eBioscience; Thermo Fisher Scientific, Inc. The
isotype controls were used as follows: Rat IgG2b κ isotype control
PE-Cyanine5 (clone, eB149/10H5; cat. no. 15-4031-82; eBioscience);
Mouse IgG2b κ isotype control FITC (clone, eBMG2b; cat. no.
11-4732-81; eBioscience); Rat IgG2a κ isotype control PE (clone,
eBR2a; cat. no. 12-4321-80; eBioscience); Rat IgG2a κ isotype
control PE-Cyanine7 PE (clone, eBR2a; cat. no. 25-4321-82;
eBioscience). The cells were incubated with indicated antibodies at
4˚C for 20 min and then washed once with PBS and resuspended in
PBS. For intracellular staining of FoxP3, 1.0 ml 1X
fixation/permeabilization solution (Foxp3 Transcription Factor
Fixation/Permeabilization Concentrate and Diluent; cat. no.
00-5521-00; eBioscience) was added to each tube, which was vortexed
and incubated at 4˚C for 60 min in the dark. Following incubation,
1X permeabilization buffer solution (10X Permeabilization Buffer;
eBioscience, Inc.) was added to each tube, and the tubes were
vortexed and washed two times and centrifuged at 4˚C, 400 x g for
10 min. The supernatant was subsequently removed, and 5.0 µl
FoxP3-PE antibody (cat. no. 11-5773; eBioscience, Inc.) was added.
Cells were run on a CyAn ADP flow cytometer (Beckman Coulter, Inc.)
for flow cytometry. Treg cells were characterized as the percentage
of CD25+FoxP3+, and Th17 cells as the
percentage of CD4+IL17+ cells among the
CD3+CD4+ cell population. Data were analyzed
using Flow Jo software (version 10.4; FlowJo LLC). Positive and
negative cell populations for each marker were determined using
fluorescence controls and unstained cells were used as a negative
control. Instrument settings were verified and adjusted with the
mid-peak bead of the eight-peak calibration bead set (Spherotech
Inc.) before each acquisition session. Compensation beads (BD
Biosciences) were used to correct for spectral overlap between
channels, as previously described (32).
Reverse transcription-quantitative PCR
(RT-qPCR)
Total RNA was isolated from ~40 mg brain tissues
using TRIzol® reagent (Invitrogen; Thermo Fisher
Scientific, Inc.) according to the manufacturer's instructions.
Total RNA (~5 µg) of with oligo (dT) primers was reverse
transcribed in a 20-µl volume using the RevertAid First Strand cDNA
Synthesis kit (Thermo Fisher Scientific, Inc.) according to the
manufacturer's instructions. qPCR was performed using an ABI PRISM
7000 Sequence Detection System (Applied Biosystem; Thermo Fisher
Scientific, Inc.). The reactions were set up as follows: 10 µl
FastStart Universal SYBR Green Master (cat. no. 4913850001; Roche
Diagnostics), 0.4 µl forward primer, 0.4 µl reverse primer, 1 µl
cDNA template and 8.2 µl ddH2O, for a total reaction volume of 20
µl. The reaction system was preheated at 95˚C for 10 min, followed
by 40 cycles of 95˚C for 15 sec and 72˚C for 30 sec. After gene
amplification, the melting and amplification curves of the target
genes were recorded. Expression levels of target genes were
calculated using the 2-∆∆Cq method with β-actin control
(33). All experiments were
repeated three times. The IL-17A, RORγt, IL-10, FoxP3 and
β-actin-specific primers are listed in Table I. The PCR primers were selected from
different exons of the corresponding genes to discriminate PCR
products that might arise from possible chromosomal DNA
contaminants.
 | Table IThe mRNA primers of this study. |
Table I
The mRNA primers of this study.
| Target gene | Primer
direction | Primer sequence
(5'-3') |
|---|
| IL-17A | Forward |
GCAGCGGTACTCATCCCTCAA |
| | Reverse |
TCATTGCGGCTCAGAGTCCAG |
| RORγt | Forward |
GAACCAGAACAGGGCTCAGAC |
| | Reverse |
TAGAAGGTCCTCCAGGCGTAG |
| IL-10 | Forward |
ACGCTGTCATCGATTTCTCCC |
| | Reverse |
TCCCACACTCCAGGTTCGGTC |
| FoxP3 | Forward |
CCCTTTCACCTATGCCACCCT |
| | Reverse |
TTGTGGCGGATGGCATTCTTC |
| β-actin | Forward |
TCAGGTCATCACTATCGGCAA |
| | Reverse |
AGCACTGTGTTGGCATAGAGG |
Western blot analysis
At week 16, the total protein was extracted from
brain tissues, and the protein expression of RORγt and FoxP3 was
detected by western blotting. RIPA protein lysis buffer (cat. no.
R0020; Beijing Solarbio Science & Technology Co., Ltd.) and
protease inhibitor were used to extract tissue protein. The volume
ratio of RIPA and protease inhibitor was 100:1. Bradford assay
(Pierce Bradford Assay kit; Pierce; Thermo Fisher Scientific, Inc.)
was used to measure the protein concentration at 595 nm. Equal
amount (10 µg/lane) proteins from each group were separated by 10%
SDS-PAGE and then transferred onto a polyvinylidene fluoride
membrane. The membrane was blocked with 5% fat-free milk for 1 h at
room temperature. Membranes were then incubated with specific
primary antibodies against FoxP3 (cat. no. ab215206; 1:800
dilution; Abcam), RORγt (cat. no. ab207082; 1:1,000 dilution;
Abcam) and actin (cat. no. ab179467; 1:5,000 dilution; Abcam)
overnight at 4˚C. Then membranes were incubated with a secondary
antibody (goat anti-rabbit IgG HRP-linked; cat. no. ab6721;
1:10,000 dilution; Abcam) for 1 h at room temperature. Enhanced
chemiluminescence reagent (cat. no. 32106; Thermo Fisher
Scientific, Inc.) was used to visualize bands with a Tanon WB
camera (Tanon Science and Technology Co., Ltd.). ImageJ software
(version, 1.53d_19; National Institutes of Health) was used to
perform densitometry analysis to quantify the protein expression
(34). All experiments were
repeated three times.
MRI
A 7.0T MRI small animal magnetic resonance scanner
(Bruker Corporation) was used for MRI analysis. The selected scan
sequences included fast spin echo T1WI sequence and T2WI sequence,
both with the coronal scan, as commonly used in rats (35). A total of 12 rats in the WKY-C,
WKY-M and WKY-T groups at 24 weeks of age were selected, and 3 rats
in each group were subjected to routine head MRI examination.
Continuous respiratory anesthesia was performed using 2% isoflurane
mixed with 98% oxygen, followed by MRI scan under anesthesia. The
following parameters were used: Scan range, full head; tuning,
<200 MHz prior to scanning; shimming, 100 MHz. The scan
parameters were as follows: T2WI sequence imaging parameters:
Repetition time (TR), 3000 msec; echo time (TE), 75 msec; echo
train length (ETL), 8; layer thickness, 1.5 mm; layer spacing, 0.2
mm; number of the excitation (NEX), 4; matrix, 256x256; field of
view (FOV), 35x35 mm; imaging time, ~6 min and 30 sec. T1WI
sequence imaging parameters were as follows: TR, 500 msec; TE, 17
msec; layer thickness, 1.5 mm, layer spacing, 0.2 mm; NEX, 3;
matrix, 256x256; FOV, 35x35 mm; imaging time, ~5 min and 15
sec.
Statistical analysis
All animals were randomized into three groups. The
results are presented as the mean ± standard error of the mean. The
experiments (including physical examination, ELISA, RT-qPCR and
flow cytometric analysis) were repeated three times. Statistical
analysis was performed using GraphPad Prism 6.0 software (GraphPad
Software, Inc.). Differences between three groups were measured
using one-way ANOVA with Tukey's post hoc test. P<0.05 was
considered to indicate a statistically significant difference.
Results
Detection of body weight and plasma
Hcy content
In order to explore the preliminary effects of a
high methionine diet, a statistical analysis of the body weights of
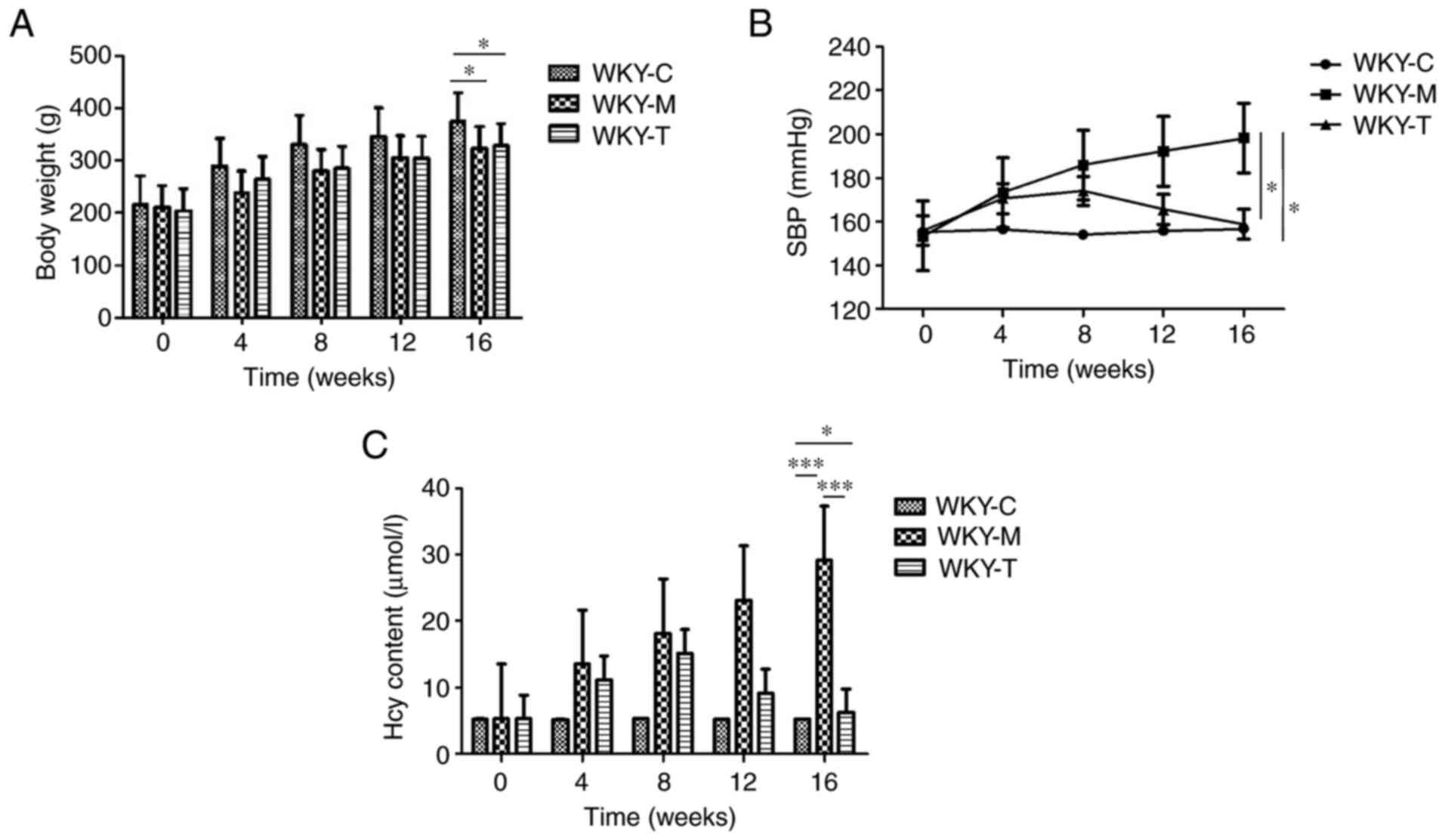
WKY rats was conducted (Fig. 1A).
The overall observation was that the weights of the rats in the
three groups during the 16-week period exhibited a gradual upward
trend. The results revealed that from 0 to 16 weeks, the rats in
the WKY-C group exhibited the heaviest weight and the most rapid
increase in weight. The amplitudes of body weight of the rats in
the WKY-M group (n=20) and WKY-T (n=20) group were lower than those
in the WKY-C (n=20) group. The WKY-M group exhibited the highest
SBP level, which was higher than the baseline of the WKY-C group
(Fig. 1B). Following treatment, it
was observed that the SBP value began to gradually decrease in the
WKY-T group and was significantly lower than that in the WKY-M
group at week 16. To further investigate the effects of a high
methionine diet and to confirm the successful establishment of the
HHcy model, plasma Hcy concentration was examined in each group
(Fig. 1C). Among the groups, the
WKY-M group exhibited the highest plasma Hcy concentration (≤6-fold
higher than the other groups). Compared with the WKY-M group, the
plasma Hcy concentration in the WKY-T group was significantly
reduced to levels comparable with those in the WKY-C group at week
16 (Fig. 1C). In summary, these
results indicated that a high methionine diet exerted a significant
effect on body weight and SBP. These data also indicated that the
HHcy model was successfully established.
Determination of plasma cytokine
secretion and expression of related genes in brain tissue
In order to examine the effects of a high methionine
diet on the main secreted factors of Th17 and Tregs in rat veins,
ELISA was performed to detect the expression levels of various
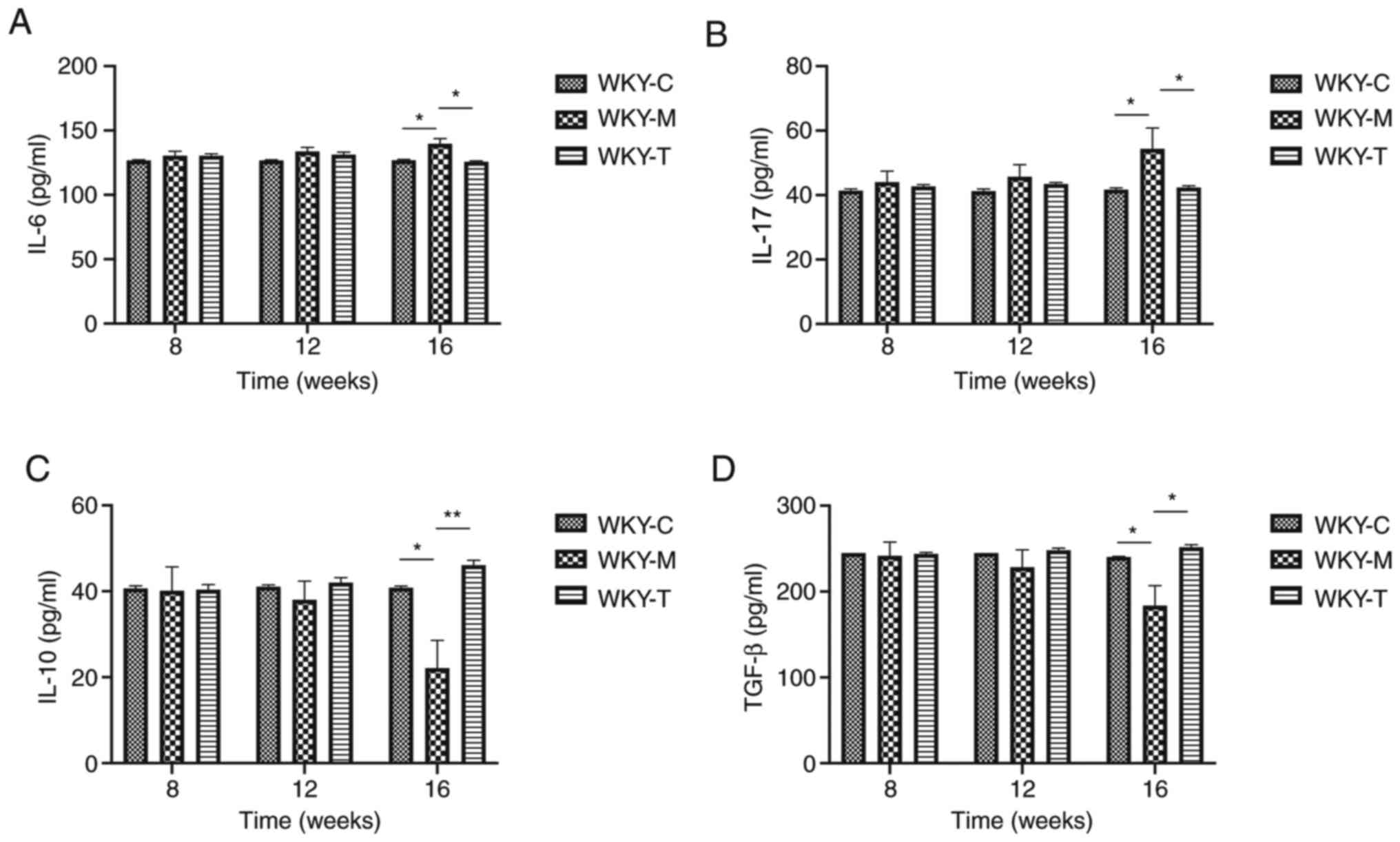
cytokines (IL-6, IL-17A, IL-10 and TGF-β; Fig. 2). Compared with the WKY-C group,
IL-17A secretion was increased in the WKY-M group, while the IL-10
and TGF-β levels were decreased in the WKY-M group at week 16.
Following treatment, compared with the WKY-M group, IL-17A
secretion in the WKY-T group was decreased, while the IL-10 and
TGF-β levels were increased at week 16. In order to further verify
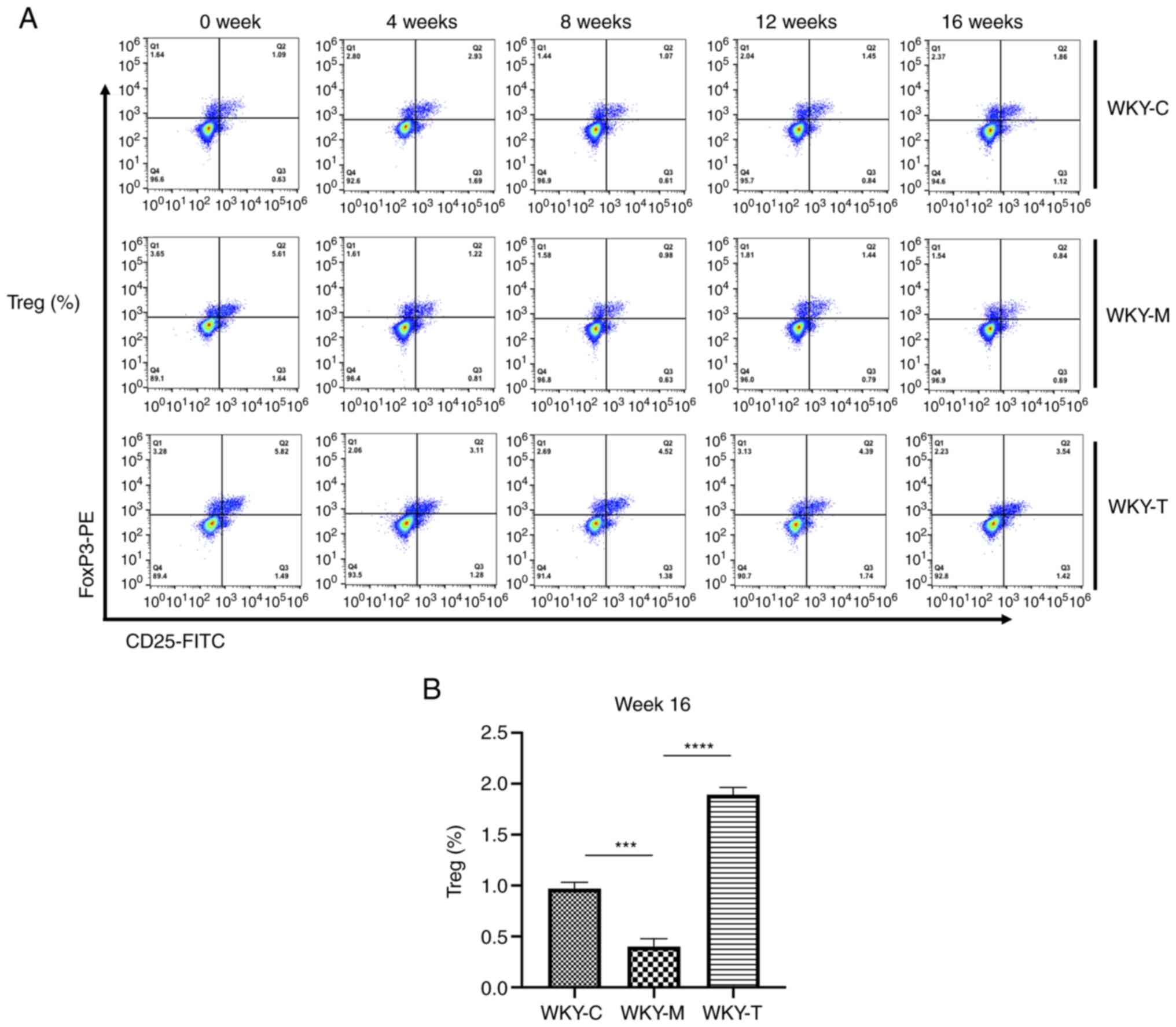
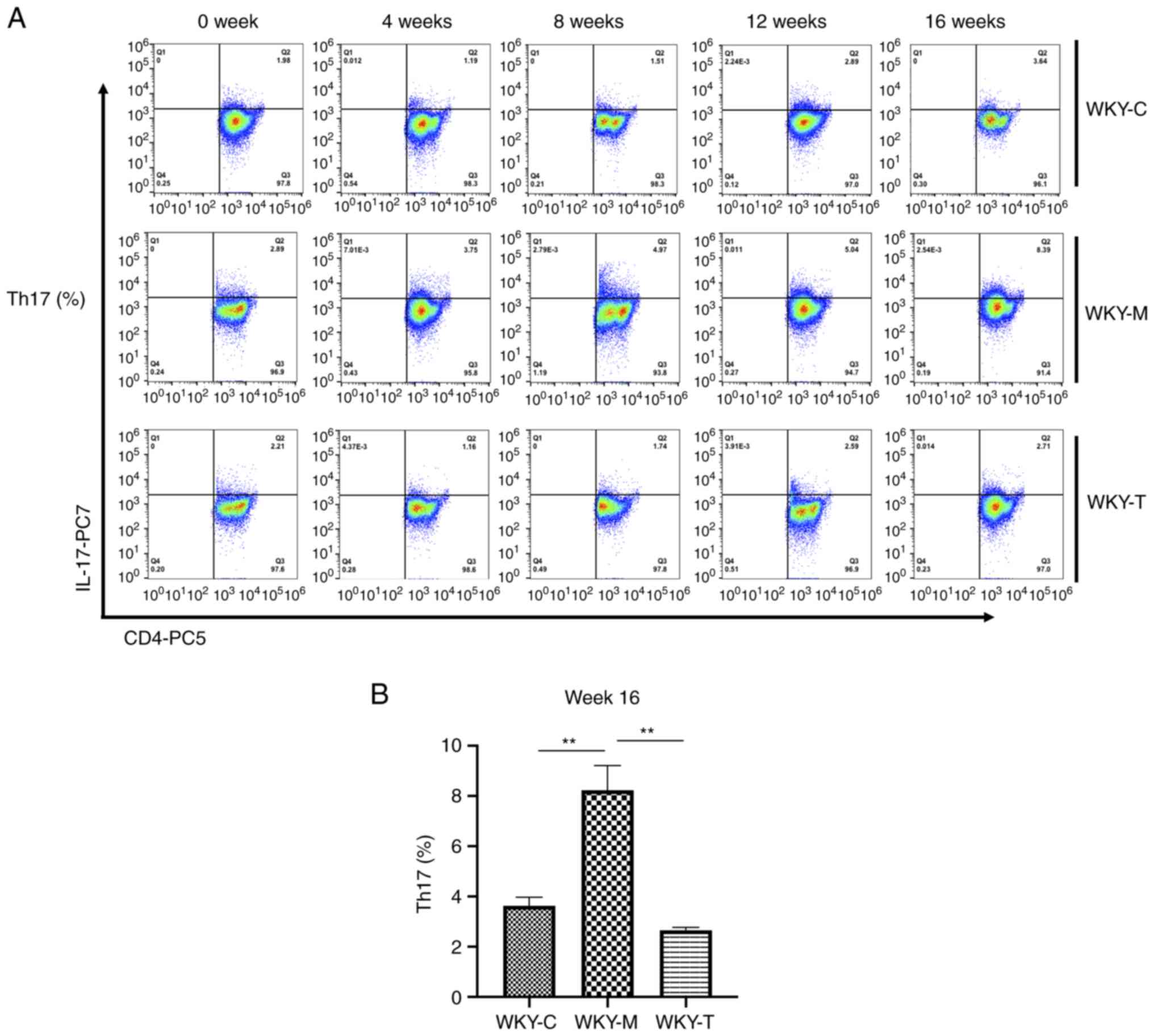
the role of Th17 and Tregs, the levels of Tregs and Th17 cells were
detected in peripheral blood at weeks 0, 4, 8, 12 and 16 using flow
cytometry (Figs. 3A and 4A). Under the joint action of HHcy and
hypertension, the levels of Treg were significantly inhibited,
while those of Th17 cells were upregulated (Fig. 3B and 4B). Following folic acid, VitB6, and
VitB12 intervention, these effects were reversed. The levels of
Tregs were significantly enhanced, while those of Th17 cells were
downregulated.
RORγt and FoxP3 are important transcription factors
associated with the differentiation of Th17 and Tregs,
respectively. In addition, at weeks 8, 12 and 16, fluorescent qPCR
was used to detect the mRNA levels of RORγt, IL-17A, FoxP3 and
IL-10 in the brain tissues of the three groups of rats to determine
the possible regulatory changes. The results revealed that at week
16, the mRNA levels of IL-17A and RORγt in the WKY-M group were
significantly upregulated, while the mRNA levels of FoxP3 in the
WKY-M group were significantly downregulated. The mRNA levels of
IL-17A and RORγt were downregulated in the WKY-T group. Conversely,
the mRNA levels of FoxP3 in the WKY-T group increased significantly
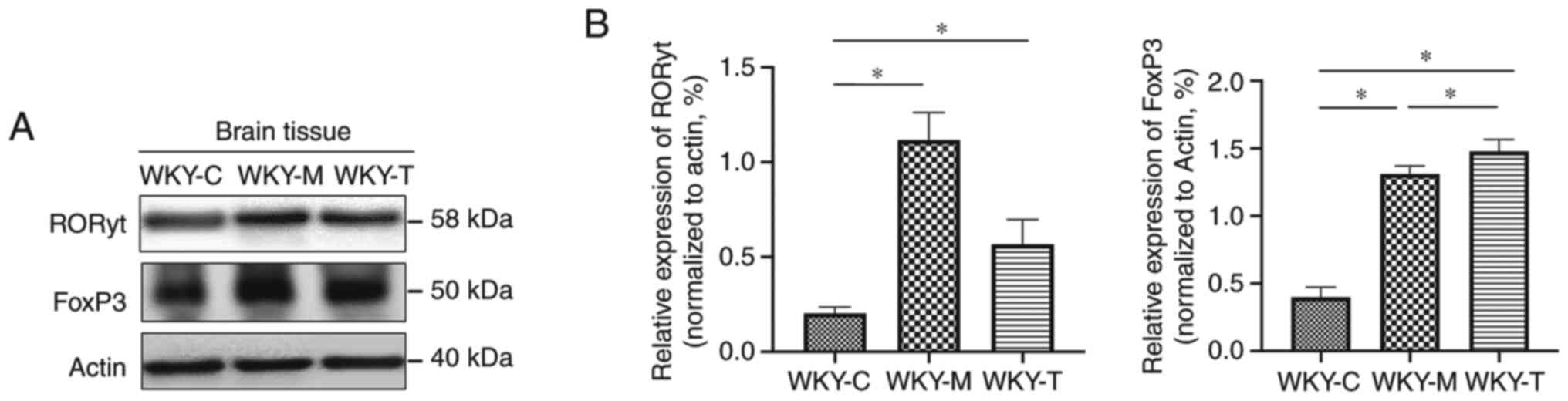
(Fig. 5). Furthermore, the protein
levels of RORγt and FoxP3 in the brain tissues were further
detected by western blot analysis (Fig.
6A and B). The relative protein
expression levels of RORγt and FoxP3 in the WKY-M and WKY-T group
were significantly higher than those in the WKY-C group. The
expression of RORγt in the WKY-M group was higher than that in the
WKY-T group. The expression of FoxP3 in the WKY-M group was
significantly lower than that in the WKY-T group.
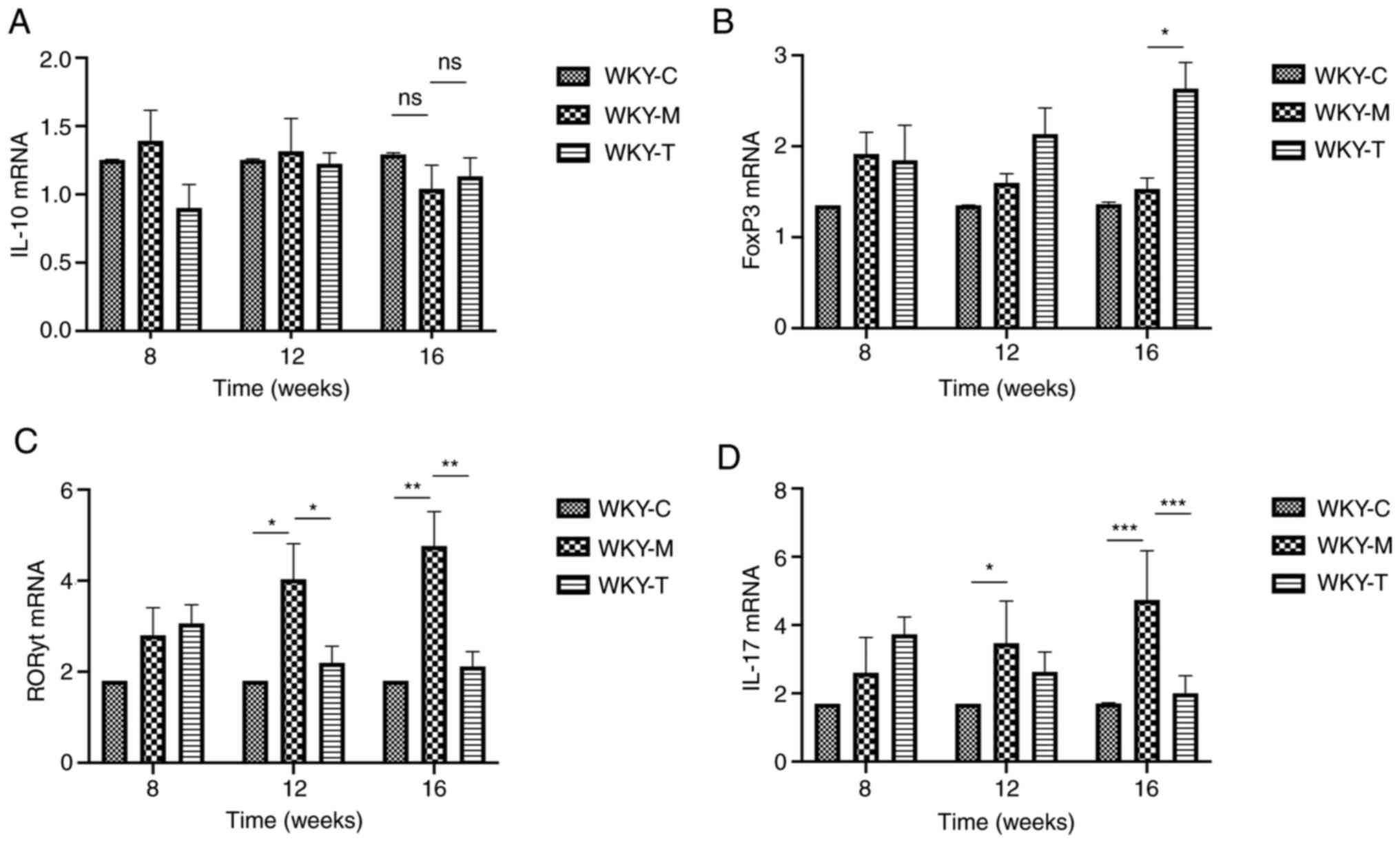 | Figure 5Expression of IL-10, FoxP3, RORγt and
IL-17A mRNA in brain tissues. mRNA expression levels of (A) IL-10,
(B) FoxP3, (C) RORγt and (D) IL-17A were determined by reverse
transcription-quantitative PCR and normalized to β-actin gene. Data
are presented as the mean ± standard error of the mean. n=3/group.
*P<0.05, **P<0.01 and
***P<0.001 as indicated. C, control; M, methionine;
T, treatment; WKY, Wistar-Kyoto; ns, no statistical significance;
FoxP3, forkhead box P3; RORγt, retinoic acid-related orphan
receptor γ t. |
Preliminary analysis of changes
associated with ischemic stroke
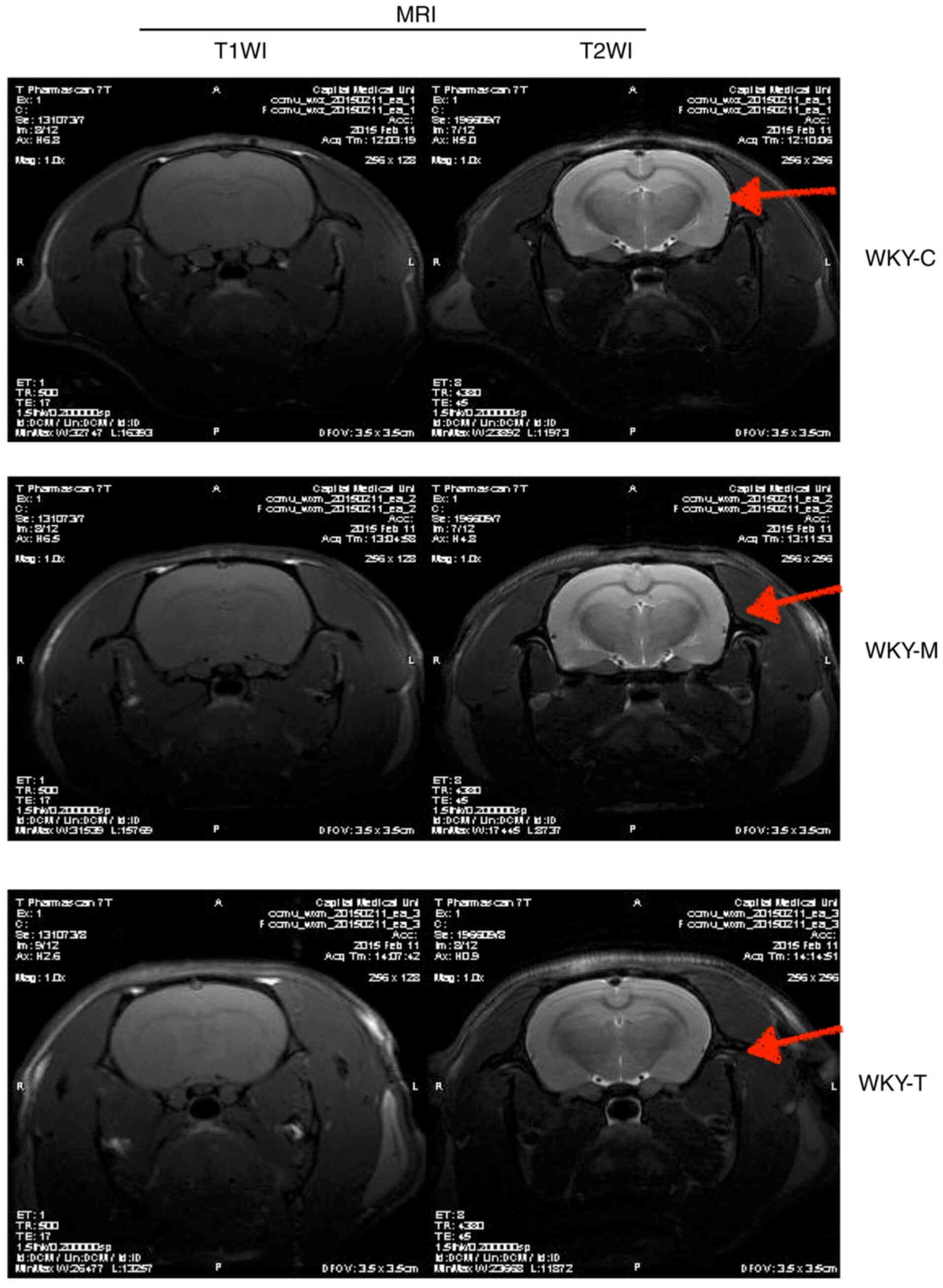
In order to determine whether ischemic lesions
appeared in rat brain tissue, MRI imaging was performed (Fig. 7). Different from the classic thread
embolization method to induce the middle cerebral artery occlusion
of rats to establish a rat model of ischemic stroke, the rats were
directly selected after the feeding intervention test for routine
head MRI detection. The experiment did not yield a definitive
positive result, indicating that no obvious damage was observed in
the brain structure of each group. Further analysis revealed that
the animal species, age and modeling method differed compared to
several other classic models (36,37).
Although the expected results were not obtained, the experiment did
provide some experience for future use; namely that the selection
of species, rat age and modeling methods need to be carefully
selected.
Discussion
The blood vessels that comprise the central nervous
system (CNS) vasculature have unique characteristics, termed the
blood-brain barrier (BBB) (38).
The precise control of CNS homeostasis by the barrier effect of the
BBB allows neurons to perform appropriate functions and also
protects nerve tissue from toxins and pathogens (39). At the same time, the change in
barrier characteristics of the BBB has also become an important
link in the pathology and development of various diseases (40-42).
As regards Hcy, researchers have observed that a diet high in Hcy
can lead to damage to capillaries in the hippocampal CA1 region,
local and irregular thickening of the basement membrane, swelling
of the mitochondria and cytoplasm, and the appearance of fibrosis
in the hippocampal CA1 region (43). Another study also demonstrated that
elevated Hcy levels exerted a significant toxic effect on cerebral
microvessels in the brain, indicating that Hcy was closely related
to the disruption of the BBB (16).
However, the specific mechanisms behind the changes caused by Hcy
remain to be fully determined.
On the one hand, HHcy alters the structure and
function of the BBB to increase its permeability; on the other
hand, HHcy alters the function of neurons by affecting astrocytes
(13,20,44).
Hcy becomes the direct cause of brain tissue damage, and the
inflammatory response and the permeability changes of the BBB
mutually promote each other to accelerate the damage to brain
tissue caused by high Hcy levels (45-49).
For hypertensive patients with HHcy, current research focuses on
the immune function of Th17 and Tregs (50-52).
Th17 and Tregs maintain a relative balance when the body is in a
normal state; however, when the body malfunctions, the balance
between Th17 and Tregs is altered (21). This immune imbalance is related to
the occurrence and development of a variety of diseases, such as
inflammation, infection, tumors and autoimmune diseases (53).
In the present study, the systemic effects (body
weight, SBP and plasma Hcy levels) of the Hcy diet were first
explored. Following folic acid and vitamin B diet therapy
intervention, further analysis revealed that the expression levels
of the main secretory factor, IL-17A, and the transcriptional
regulator, RORγt, of Th17 cells in the brain tissue of the WKY-T
group decreased, and returned to the corresponding levels of the
normal diet group. It was demonstrated that folic acid and vitamin
B treatment attenuated the effects of HHcy-induced hypertension by
suppressing the role of Th17 cells and weakening the inflammatory
response process. This was consistent with the findings that
patients with cardiovascular disease have high blood pressure, and
plasma Hcy levels are higher than in normal subjects, while folic
acid, vitamin B6, vitamin B12 levels are lower than normal
(54). In the present study, flow
cytometry revealed that the proportion of Tregs in the WKY-M group
was significantly lower than that of the normal diet group, and
following folic acid and vitamin B intervention, the proportion of
Tregs and the expression level of their regulatory factor, FoxP3,
were increased in the WKY-T group. The results of RT-qPCR and
western blot analysis revealed that the numbers of Th17 cells were
decreased in the brain tissues of the WKY-T group, while those of
Tregs were increased. The numbers of Th17 cells in the WKY-M group
were increased gradually at 16 weeks and the protein expression of
RORγt and FoxP3 in the brain tissues was also consistently altered.
This indicated that HHcy not only enhanced the Th17 response, but
also reduced the body's immune suppression mechanism by reducing
the level of Tregs. Based on the current observation, dietary
interventions of folic acid and vitamin B may suppress the
inflammatory response process by rectifying the Th17/Treg balance,
and may attenuate the adverse effects induced by hypertension. A
limitation of the present study was that no differences in brain
structure were observed by MRI, and the imbalance of the Treg/Th17
immune response by HHcy was detected in a preliminary perspective
and not fully explained by relevant molecular mechanisms. Further
studies are required to investigate the molecular mechanisms
responsible for brain injury caused by HHcy. Future studies should
focus on investigating the molecular mechanisms of brain injury
caused by HHcy. Although any damage to the rat brain structure in
the experiment was not observed, the experiment did provide certain
experience for future use; namely that the selection of species,
rat age and modeling methods need to be carefully selected.
In conclusion, the present study examined the
effects of a high methionine diet on the main secretory factors
(IL-6, IL-17A, IL-10 and TGF-β) and transcriptional regulators
(RORγt and FoxP3) of rat Th17 and Tregs. Imaging changes of rat
brain tissue and changes following folic acid and vitamin B
intervention were combined, and it was found that HHcy could cause
a Th17/Treg immune imbalance. This immune imbalance state was
closely associated with the inflammatory response. The diet
intervention (supplements of folic acid, vitamin B6 and vitamin
B12) not only reduced the damage to brain tissue induced by Hcy by
reducing the level of Hcy in the blood, but also reduced the
inflammatory response and rectified the Treg/Th17 immune imbalance
to attenuate brain tissue damage.
Acknowledgements
Not applicable.
Funding
This study was supported by Tianjin Municipal Science and
Technology Commission (grant no. 16ZCZDSY03100).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
YZ and JG carried out the experiments and drafted
the manuscript. YZ, LW, XL and JG performed the statistical
analysis and participated in the study design. LW and XL helped to
collect data and performed the statistical analysis. YZ and JG
confirm the authenticity of all the raw data. All authors read and
approved the final manuscript.
Ethics approval and consent to
participate
All animal experiments were approved by the Animal
Experiment Ethics Committee of the Second Hospital of Tianjin
Medical University (Tianjin, China), and were conducted according
to the American Association for Accreditation of Laboratory Animal
Care and the IACUC guidelines.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Djuric D, Jakovljevic V, Zivkovic V and
Srejovic I: Homocysteine and homocysteine-related compounds: An
overview of the roles in the pathology of the cardiovascular and
nervous systems. Can J Physiol Pharmacol. 96:991–1003.
2018.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Ganguly P and Alam SF: Role of
homocysteine in the development of cardiovascular disease. Nutr J.
14(6)2015.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Spence JD: Homocysteine lowering for
stroke prevention: Unravelling the complexity of the evidence. Int
J Stroke. 11:744–747. 2016.PubMed/NCBI View Article : Google Scholar
|
|
4
|
McCully KS: Vascular pathology of
homocysteinemia: Implications for the pathogenesis of
arteriosclerosis. Am J Pathol. 56:111–128. 1969.PubMed/NCBI
|
|
5
|
Kim J, Kim H, Roh H and Kwon Y: Causes of
hyperhomocysteinemia and its pathological significance. Arch Pharm
Res. 41:372–383. 2018.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Azad MAK, Huang P, Liu G, Ren W, Teklebrh
T, Yan W, Zhou X and Yin Y: Hyperhomocysteinemia and cardiovascular
disease in animal model. Amino Acids. 50:3–9. 2018.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Capelli I, Cianciolo G, Gasperoni L,
Zappulo F, Tondolo F, Cappuccilli M and La Manna G: Folic acid and
vitamin B12 administration in CKD, why not? Nutrients.
11(383)2019.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Dardiotis E, Arseniou S, Sokratous M,
Tsouris Z, Siokas V, Mentis AA, Michalopoulou A, Andravizou A,
Dastamani M, Paterakis K, et al: Vitamin B12, folate, and
homocysteine levels and multiple sclerosis: A meta-analysis. Mult
Scler Relat Disord. 17:190–197. 2017.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Pawlak R: Is vitamin B12 deficiency a risk
factor for cardiovascular disease in vegetarians? Am J Prev Med.
48:e11–e26. 2015.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Dinavahi R and Falkner B: Relationship of
homocysteine with cardiovascular disease and blood pressure. J Clin
Hypertens (Greenwich). 6:494–498; quiz 499-500. 2004.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Stehouwer CD and van Guldener C: Does
homocysteine cause hypertension? Clin Chem Lab Med. 41:1408–1411.
2003.PubMed/NCBI View Article : Google Scholar
|
|
12
|
van Guldener C, Nanayakkara PW and
Stehouwer CD: Homocysteine and blood pressure. Curr Hypertens Rep.
5:26–31. 2003.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Beard RS Jr, Reynolds JJ and Bearden SE:
Hyperhomocysteinemia increases permeability of the blood-brain
barrier by NMDA receptor-dependent regulation of adherens and tight
junctions. Blood. 118:2007–2014. 2011.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Ehrlich D and Humpel C: Chronic vascular
risk factors (cholesterol, homocysteine, ethanol) impair spatial
memory, decline cholinergic neurons and induce blood-brain barrier
leakage in rats in vivo. J Neurol Sci. 322:92–95. 2012.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Kalani A, Kamat PK, Familtseva A,
Chaturvedi P, Muradashvili N, Narayanan N, Tyagi SC and Tyagi N:
Role of microRNA29b in blood-brain barrier dysfunction during
hyperhomocysteinemia: An epigenetic mechanism. J Cereb Blood Flow
Metab. 34:1212–1222. 2014.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Kamath AF, Chauhan AK, Kisucka J, Dole VS,
Loscalzo J, Handy DE and Wagner DD: Elevated levels of homocysteine
compromise blood-brain barrier integrity in mice. Blood.
107:591–593. 2006.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Le Stunff H, Véret J, Kassis N, Denom J,
Meneyrol K, Paul JL, Cruciani-Guglielmacci C, Magnan C and Janel N:
Deciphering the link between hyperhomocysteinemia and ceramide
metabolism in Alzheimer-type neurodegeneration. Front Neurol.
10(807)2019.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Lee H, Kim HJ, Kim JM and Chang N: Effects
of dietary folic acid supplementation on cerebrovascular
endothelial dysfunction in rats with induced hyperhomocysteinemia.
Brain Res. 996:139–147. 2004.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Ford TC, Downey LA, Simpson T, McPhee G,
Oliver C and Stough C: The effect of a high-dose vitamin B
multivitamin supplement on the relationship between brain
metabolism and blood biomarkers of oxidative stress: A randomized
control trial. Nutrients. 10(1860)2018.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Kennedy DO and Haskell CF: Vitamins and
cognition: What is the evidence? Drugs. 71:1957–1971.
2011.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Pirchl M, Ullrich C, Sperner-Unterweger B
and Humpel C: Homocysteine has anti-inflammatory properties in a
hypercholesterolemic rat model in vivo. Mol Cell Neurosci.
49:456–463. 2012.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Zhang Y, Wang L, Zhou X, Geng J and Li X:
The immunomodulatory mechanism of brain injury induced by
hyperhomocysteinemia in spontaneously hypertensive rats. J Cell
Biochem. 120:9421–9429. 2019.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Lee GR: The Balance of Th17 versus treg
cells in autoimmunity. Int J Mol Sci. 19(730)2018.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Crouser ED: Role of imbalance between Th17
and regulatory T-cells in sarcoidosis. Curr Opin Pulm Med.
24:521–526. 2018.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Melnik BC, John SM, Chen W and Plewig G: T
helper 17 cell/regulatory T-cell imbalance in hidradenitis
suppurativa/acne inversa: The link to hair follicle dissection,
obesity, smoking and autoimmune comorbidities. Br J Dermatol.
179:260–272. 2018.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Hang S, Paik D, Yao L, Kim E, Trinath J,
Lu J, Ha S, Nelson BN, Kelly SP, Wu L, et al: Bile acid metabolites
control TH17 and Treg cell differentiation.
Nature. 576:143–148. 2019.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Levine AG, Mendoza A, Hemmers S, Moltedo
B, Niec RE, Schizas M, Hoyos BE, Putintseva EV, Chaudhry A, Dikiy
S, et al: Stability and function of regulatory T cells expressing
the transcription factor T-bet. Nature. 546:421–425.
2017.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Coder B, Wang W, Wang L, Wu Z, Zhuge Q and
Su DM: Friend or foe: The dichotomous impact of T cells on
neuro-de/re-generation during aging. Oncotarget. 8:7116–7137.
2017.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Schmitt V, Rink L and Uciechowski P: The
Th17/Treg balance is disturbed during aging. Exp Gerontol.
48:1379–1386. 2013.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Health N: Guide for the care and use of
laboratory animals. NIH contract No. No1-RR-2-2135. 11–28.
1985.
|
|
31
|
Leary SL, Underwood W, Anthony R, et al:
AVMA guidelines for the euthanasia of animals: 2013 edition.
American Veterinary Medical Association Schaumburg, IL, 2013.
|
|
32
|
McGee HM, Daly ME, Azghadi S, Stewart SL,
Oesterich L, Schlom J, Donahue R, Schoenfeld JD, Chen Q, Rao S, et
al: Stereotactic ablative radiation therapy induces systemic
differences in peripheral blood immunophenotype dependent on
irradiated site. Int J Radiat Oncol Biol Phys. 101:1259–1270.
2018.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408.
2001.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Schindelin J, Rueden CT, Hiner MC and
Eliceiri KW: The ImageJ ecosystem: An open platform for biomedical
image analysis. Mol Reprod Dev. 82:518–529. 2015.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Wen M, Lian Z, Huang L, Zhu S, Hu B, Han
Y, Deng Y and Zeng H: Magnetic resonance spectroscopy for
assessment of brain injury in the rat model of sepsis. Exp Ther
Med. 14:4118–4124. 2017.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Kovalska M, Hnilicova P, Kalenska D,
Tothova B, Adamkov M and Lehotsky J: Effect of methionine diet on
metabolic and histopathological changes of rat hippocampus. Int J
Mol Sci. 20(6234)2019.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Jindal A, Rajagopal S, Winter L, Miller
JW, Jacobsen DW, Brigman J, Allan AM, Paul S and Poddar R:
Hyperhomocysteinemia leads to exacerbation of ischemic brain
damage: Role of GluN2A NMDA receptors. Neurobiol Dis. 127:287–302.
2019.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Daneman R and Prat A: The blood-brain
barrier. Cold Spring Harb Perspect Biol. 7(a020412)2015.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Keaney J and Campbell M: The dynamic
blood-brain barrier. FEBS J. 282:4067–4079. 2015.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Liebner S, Dijkhuizen RM, Reiss Y, Plate
KH, Agalliu D and Constantin G: Functional morphology of the
blood-brain barrier in health and disease. Acta Neuropathol.
135:311–336. 2018.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Sweeney MD, Zhao Z, Montagne A, Nelson AR
and Zlokovic BV: Blood-brain barrier: From physiology to disease
and back. Physiol Rev. 99:21–78. 2019.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Varatharaj A and Galea I: The blood-brain
barrier in systemic inflammation. Brain Behav Immun. 60:1–12.
2017.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Lee H, Kim JM, Kim HJ, Lee I and Chang N:
Folic acid supplementation can reduce the endothelial damage in rat
brain microvasculature due to hyperhomocysteinemia. J Nutr.
135:544–548. 2005.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Tchantchou F, Goodfellow M, Li F, Ramsue
L, Miller C, Puche A and Fiskum G: Hyperhomocysteinemia-induced
oxidative stress exacerbates cortical traumatic brain injury
outcomes in rats. Cell Mol Neurobiol. 41:487–503. 2021.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Faverzani JL, Hammerschmidt TG, Sitta A,
Deon M, Wajner M and Vargas CR: Oxidative stress in homocystinuria
due to cystathionine ß-synthase deficiency: Findings in patients
and in animal models. Cell Mol Neurobiol. 37:1477–1485.
2017.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Kamat PK, Kalani A, Givvimani S, Sathnur
PB, Tyagi SC and Tyagi N: Hydrogen sulfide attenuates
neurodegeneration and neurovascular dysfunction induced by
intracerebral-administered homocysteine in mice. Neuroscience.
252:302–319. 2013.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Lehotský J, Tothová B, Kovalská M, Dobrota
D, Beňová A, Kalenská D and Kaplán P: Role of Homocysteine in the
ischemic stroke and development of ischemic tolerance. Front
Neurosci. 10(538)2016.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Tóthová B, Kovalská M, Kalenská D,
Tomašcová A and Lehotský J: Histone hyperacetylation as a response
to global brain ischemia associated with hyperhomocysteinemia in
rats. Int J Mol Sci. 19(3147)2018.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Vacek JC, Behera J, George AK, Kamat PK,
Kalani A and Tyagi N: Tetrahydrocurcumin ameliorates
homocysteine-mediated mitochondrial remodeling in brain endothelial
cells. J Cell Physiol. 233:3080–3092. 2018.PubMed/NCBI View Article : Google Scholar
|
|
50
|
Gao X, Li J and Chen M: Effect of
homocysteine on the differentiation of CD4+T Cells into
Th17 cells. Dig Dis Sci. 63:3339–3347. 2018.PubMed/NCBI View Article : Google Scholar
|
|
51
|
Lin X, Meng X and Song Z: Homocysteine and
psoriasis. Biosci Rep. 39(BSR20190867)2019.PubMed/NCBI View Article : Google Scholar
|
|
52
|
Feng J, Zhang Z, Kong W, Liu B, Xu Q and
Wang X: Regulatory T cells ameliorate
hyperhomocysteinaemia-accelerated atherosclerosis in apoE-/-mice.
Cardiovasc Res. 84:155–163. 2009.PubMed/NCBI View Article : Google Scholar
|
|
53
|
Rodriguez-Iturbe B, Pons H and Johnson RJ:
Role of the immune system in hypertension. Physiol Rev.
97:1127–1164. 2017.PubMed/NCBI View Article : Google Scholar
|
|
54
|
Karolczak K, Kubalczyk P, Glowacki R,
Pietruszynski R and Watala C: Aldosterone modulates blood
homocysteine and cholesterol in coronary artery disease patients-a
possible impact on atherothrombosis? Physiol Res. 67:197–207.
2018.PubMed/NCBI View Article : Google Scholar
|