Introduction
Globally, acute myocardial infarction (AMI) is
associated with high mortality and morbidity rates (1,2).
Reperfusion therapy is the most effective way to treat this disease
in a clinical setting (3). Though
the therapeutic aim is to restore blood flow to the affected area,
restoration of the blood supply often activates a cascade of
cellular damage mechanisms, which may further aggravate myocardial
cell pathology, namely myocardial ischemia/reperfusion injury
(MIRI) (4). Cellular ischemic
injury involves multiple injury mechanisms, such as abnormal
calcium regulation in cells (5,6), free
radical production (7),
mitochondrial damage (8) and the
activation of apoptotic pathways (9). The cytotoxic cascade leads to the
excessive production of reactive oxygen species (ROS), and is
considered to be the primary factor responsible for myocardial
contractile dysfunction, cell death and inflammation (10). These injuries may have serious
consequences, such as heart failure or death. Therefore, there is
an urgent requirement for further investigation of the pathogenesis
of AMI, and to develop new therapeutic compounds and
strategies.
The pathological mechanisms underlying
ischemia-reperfusion injury (IRI) are complex, and include
autophagy, cardiomyocyte apoptosis, cellular infiltration, calcium
overload, oxidative stress, energy metabolism disorder and vascular
endothelial dysfunction (11-13).
There are multiple signaling pathways involved in I/R injury, such
as the matrix metalloenzyme-associated, ATP-sensitive potassium
channel, angiotensin II and Fas signaling pathways. Klotho is a
protein that is involved in human aging and the length of the human
lifespan (14,15). A large number of previous studies
have revealed that klotho is involved in the occurrence and
development of various human diseases, including chronic kidney
disease (15,16), arteriosclerosis (17), myocardial hypertrophy (18,19),
diabetes and obesity (20-22),
as well as various types of cancer (23,24).
Klotho protein has been reported to exert anti- or
pro-apoptotic functions in different diseases (25,26).
In an acute pancreatitis model, klotho was found to alleviate
inflammation and apoptosis (27).
Sugiura et al (28) found
that klotho is involved in the pathophysiology of renal IRI, and
that it alleviated apoptosis in a renal IRI model via heat shock
protein (Hsp) 70. In an oxidative damage model, klotho was reported
to attenuate oxidant-induced alveolar epithelial cell apoptosis and
mitochondrial DNA damage (29).
Moreover, klotho was also found to suppress ROS-induced apoptosis
to improve cardiac function (30).
In a stress-induced cardiac injury model, klotho inhibited
cardiomyocyte apoptosis partly by suppressing the activation of the
p38 and JNK pathways (31).
Additionally, klotho inhibited the effects of dexamethasone via the
NF-κB signaling pathway in MC3T3-E1 osteoblasts (32). Moreover, in human umbilical vein
endothelial cells, klotho suppressed apoptosis by reducing the
activation of the PI3K/AKT pathway (33). Thus, klotho may be a potential
therapeutic target for acute inflammatory disease.
The present study aimed to identify whether klotho
exerts a protective effect on hypoxia/reoxygenation (H/R) injury in
H9c2(2-1) cells, as well as the potential molecular mechanisms
underlying this process. The results provide a useful reference for
clarifying the molecular mechanisms of klotho during H/R
progression, and suggest klotho as a potential therapeutic target
for acute MIRI.
Materials and methods
Regents and cell lines
The H9c2(2-1) rat heart myoblast cell line
(ATCC® CRL-1446™) was purchased from ATCC and cultured
in Dulbecco's modified Eagle's medium (DMEM) supplemented with 10%
fetal bovine serum. The pCMV3-C-HA negative control vector
(C-terminal HA-tagged) and klotho cDNA ORF clone were purchased
from Sino Biological, Inc. The klotho overexpression lentivirus and
control plasmid lentivirus were constructed by and purchased from
Kilton Biotechnology (Shanghai) Co., Ltd. Klotho shRNA (m) and
control shRNA lentiviral particles (containing a scrambled shRNA
sequence) were purchased from Santa Cruz Biotechnology, Inc.
H/R injury model
H9c2(2-1) cells were precultured in serum-free DMEM
overnight prior to H/R injury. Then, the cells were cultured and
exposed to hypoxic conditions for 4 h using an AnaeroPack pouch
(Mitsubishi Gas Chemical). The hypoxic conditions were 37˚C, 2%
O2, and 5% CO2. After 4 h, reoxygenation was
achieved by returning the cells to normal culture conditions, which
involved incubation at 37˚C in a humidified atmosphere containing
95% air and 5% CO2 for 4 h.
Transfection
H9c2(2-1) cells were plated into 6-well plate at the
cell density of 4x105 cells/well 10 h prior to
transfection. The transfection agent Lipofectamine® 2000
was obtained from Invitrogen; Thermo Fisher Scientific, Inc. For
transfection, 1 µg pCMV3-C-HA negative control vector or 1 µg
klotho cDNA ORF clone was diluted in 100 µl Opti-MEM (Thermo Fisher
Scientific, Inc.); 4 µl of Lipofectamine® 2000 was
diluted in 200 µl of Opti-MEM. The mixtures were incubated at room
temperature for 5 min. Then, 100 µl plasmid dilution liquid and 100
µl diluted Lipofectamine® 2000 liquid was mixed gently
and kept at room temperature for 20 min. The transfection mixture
was added into each 6-well plate at room temperature. After 6 h,
the transfection medium was discarded and 2 ml completed DMEM
medium was added. The transfected cells were cultured for 24 h and
the target proteins such as caspase-3, Bcl2 and Bax were detected
by western blotting after H/R treatment.
Additionally, for shRNA lentiviral particles
transduction, Klotho shRNA (m) and control shRNA lentiviral
particles were used in order to knockdown klotho, H9c2(2-1) cells.
Firstly, the cells were grown to ~50% confluency and a mixture of
complete DMEM medium with Polybrene® (cat. no.
sc-134220; Santa Cruz Biotechnology, Inc.) at a final concentration
of 5 µg/ml was prepared. 10 µl klotho shRNA lentivirus or 10 µl
control shRNA lentivirus was added and cultured at 37˚C for 24 h.
Subsequently, the culture medium was removed and the complete DMEM
medium (without Polybrene®) was replaced, and the cells
were cultured for another 24 h. The stable clones expressing the
shRNA were selected and the levels of klotho, p-Akt and total Akt
were detected by western blotting. Percentages of apoptotic
H9c2(2-1) cells transfected with klotho or control vector were
subsequently investigated.
Flow cytometric analysis
H9c2(2-1) cells were infected with klotho lentivirus
or control plasmid lentivirus for 24 h prior to H/R treatment.
Apoptosis was assessed by Annexin V-FITC/propidium iodide (PI)
staining according to the kit protocols (Santa Cruz Biotechnology,
Inc.). For flow cytometric analysis, the cells were digested with
0.25% trypsin for 1 min and washed twice with precooled
phosphate-buffered saline (PBS). The cells were then resuspended in
500 µl binding buffer with Annexin V-FITC (0.1 µg/µl) and PI (0.05
µg/µl) in the dark for 15 min on ice. From each sample,
1x104 cells were collected for detection. The samples
were then run through a flow cytometer (BD LSRFortessa X-20; BD
Biosciences) and the data was analyzed using FlowJo v10 software
(FlowJo LLC).
MTT assay
Cell viability was determined with an MTT assay.
Briefly, H9c2(2-1) cells were transfected with klotho or negative
control lentivirus for 24 h. Then, 3x104 cells/well were
plated into 96-well plates for 6 h and treated by H/R injury as
aforementioned. Then, 10 µl MTT reagent was added to each well and
the plate was cultured for 4 h at 37˚C. The absorbance was measured
at 570 nm with a microplate reader, and viability was calculated as
follows: Cell viability = (OD570 nm of treatment
group-OD570 nm of blank wells)/(OD570 nm of
untreated group-OD570 nm of blank wells) x100%.
Lactate dehydrogenase (LDH) assay
Cellular injury was evaluated using the LDH test.
LDH is a stable and abundant cytoplasmic enzyme that is unable to
pass through the cell membrane. When a cell is damaged or dead, LDH
is quickly released into the cell culture medium. Thus, LDH
activity in the supernatant is proportional to the number of dead
cells. Briefly, H9c2(2-1) cells were infected with klotho
lentivirus or control plasmid lentivirus for 24 h prior to H/R
treatment. Cell supernatants were obtained from each group and LDH
activity was determined using an LDH release assay according to the
manufacturer's protocol (Nanjing KeyGen Biotech Co., Ltd.).
Scanning confocal microscopy
H9c2(2-1) cells were infected with klotho lentivirus
or control plasmid lentivirus for 24 h prior to H/R treatment.
Then, the cells were washed twice with PBS and fixed with 100%
methanol for 5 min at room temperature. Next, the cells were
permeabilized with 0.1% Triton X-100 for 5 min and treated with 2%
BSA in 0.1% PBS-Tween for 1 h to block non-specific protein-protein
interactions at room temperature. Primary Hsp70 antibody was added,
and the cells were incubated overnight at 4˚C. The Anti-Hsp70
antibody [EPR16892 (Alexa Fluor® 647; cat. no.
ab204691)] was purchased from Abcam. The cell nuclei were stained
with DAPI at a concentration of 1.43 µM, and were observed by
fluorescence confocal microscopy at x200 magnification. In an
alternative assay, H9c2(2-1) cells were infected, permeabilized and
blocked as aforementioned, and then stained with Hoechst 33258
(Qcbio Science & Technologies Co. Ltd.) to assess the effect of
klotho overexpression on the morphology of H/R-induced apoptotic
cells.
Western blot analysis
The levels of caspase-3, Bax, Bcl-2, klotho, Hsp70,
phosphorylated (p-)Akt, Akt, p-Bad and Bad were detected by western
blotting. Cell lysis buffer was purchased from Beyotime Institute
of Biotechnology (cat. no. P0013). Briefly, cell lysates were
prepared and total protein concentration was determined using a BCA
Protein Assay kit (Thermo Fisher Scientific, Inc.). A 20-µg sample
of total protein was loaded into each lane and separated by 10%
SDS-PAGE. The proteins were then transferred onto PVDF membranes at
300 mA for 2 h. The membranes were blocked with 5% non-fat milk at
room temperature for 40 min, and then incubated with primary
antibodies at a ratio of 1:2,000 overnight at 4˚C. The following
primary antibodies were used: Anti-caspase-3 (cat. no. 3138-100;
BioVision, Inc.), anti-Bcl-2 (cat. no. AP1303a-ev; Abgent, Inc.),
anti-Bax (cat. no. AP1302a-ev; Abgent, Inc.), recombinant
anti-Hsp70 (EPR16892; cat. no. ab181606; Abcam); anti-Klotho
(EPR6856; cat. no. ab181373; Santa Cruz Biotechnology, Inc.);
anti-p-Akt (B-5; cat. no. sc-271966; Santa Cruz Biotechnology,
Inc.), anti-Akt (EPR16798; cat. no. ab179463; Abcam), anti-Bad
[phospho S112 (EPR1891(2)); cat.
no. ab129192; Abcam]; anti-Bad (cat. no. ab90435; Abcam) and
β-actin (cat. no. AM1021B; Abgent, Inc.). The membrane was then
incubated with horseradish peroxidase-conjugated goat anti-rabbit
secondary antibody (1:10,000, cat. no. sc-2004; Santa Cruz
Biotechnology, Inc.) for 1 h at room temperature. The protein bands
were detected in a dark room using an ECL detection kit
(Cytiva).
Statistical analysis
All results were analyzed with SPSS 20.0 software
(IBM Corp) using one-way analysis of variance followed by Tukey's
post hoc test. The independent samples were analyzed using an
unpaired t-test, including the comparison between klotho protein
expression in H/R-injured cells and control H9c2(2-1) cells.
P<0.05 was considered to indicate a statistically significant
difference.
Results
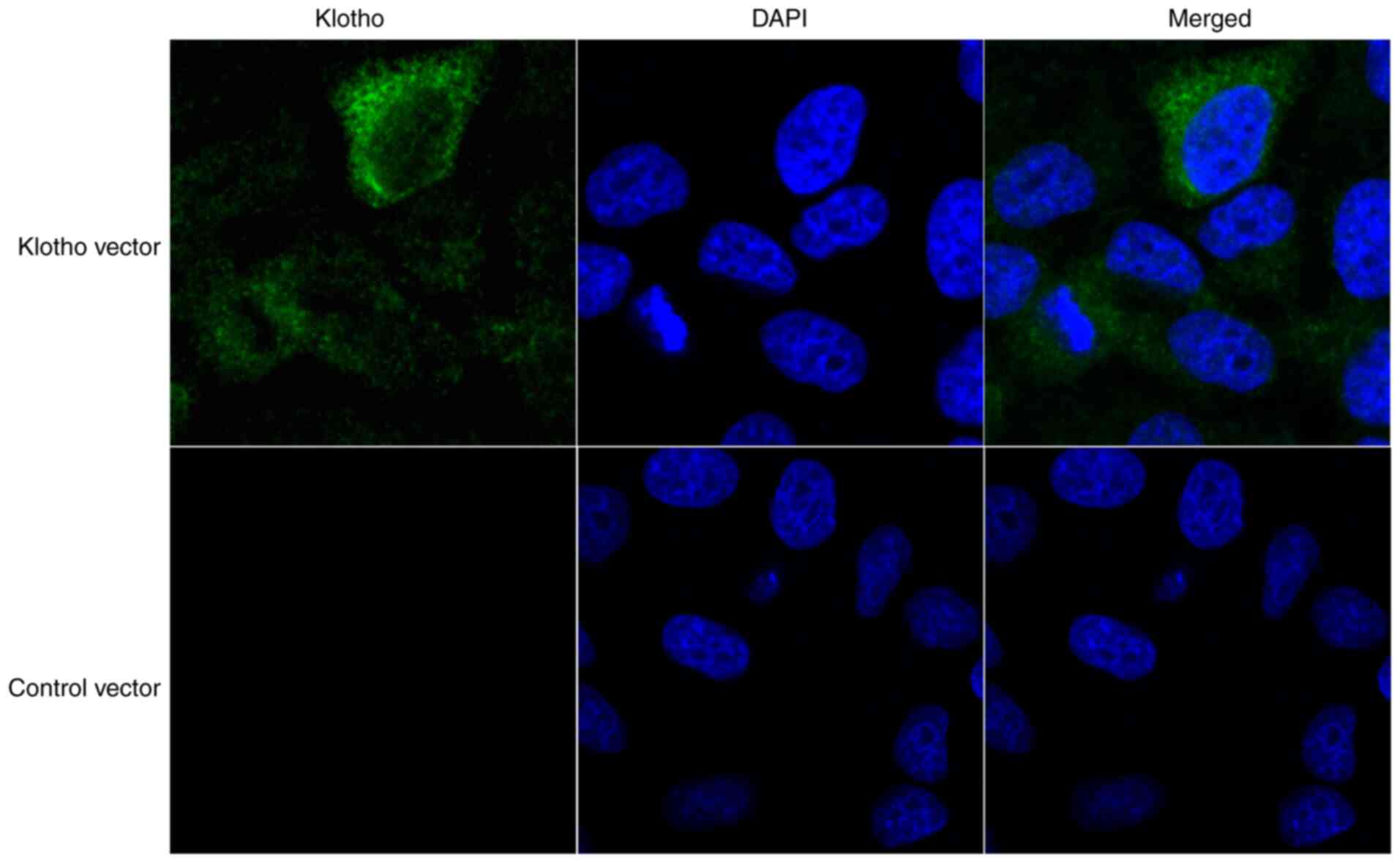
Klotho is primarily located in the
cytoplasm of H9c2(2-1) cells
In order to determine the role of klotho in the H/R
cardiomyocyte model, H9c2(2-1) cells were transfected the klotho
cDNA ORF clone and its negative control pCMV3-C-GFPSpark vector
(with C-terminal GFPSpark-tag). After 24 h, the distribution of
klotho in H9c2(2-1) cells was determined by confocal microscopy. As
shown in Fig. 1, klotho was
primarily distributed in the cytoplasm of H9c2(2-1) cells.
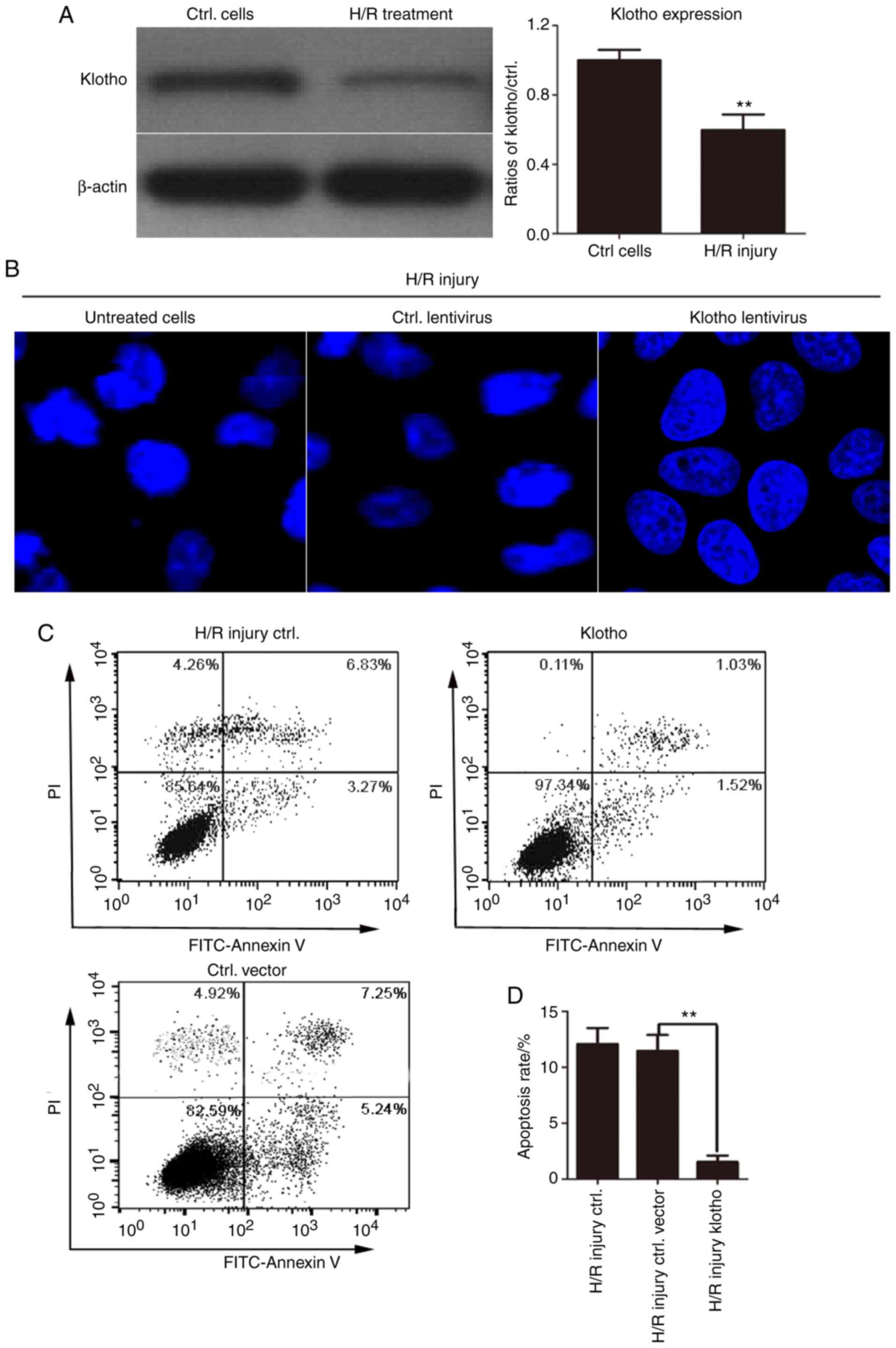
Overexpression of klotho markedly
inhibits the H/R-induced apoptosis of H9c2(2-1) cells
Klotho levels were first detected in H/R-treated
H9c2(2-1) cells by western blotting. As shown in Fig. 2A, klotho protein expression was
reduced by H/R injury. Additionally, to further confirm the role of
klotho in apoptosis, Hoechst 33258 was used to stain the nuclei and
to detect the effects of klotho overexpression on the morphology of
H/R-induced H9c2(2-1) cells. The results revealed that the nuclear
membrane of some cells shrank, the nuclear chromatin appeared dense
with enhanced fluorescence staining, and nuclear fragmentation and
apoptotic body formation were apparent in a number of cells.
Compared with those of the control cells, the nuclei of the klotho
lentivirus-infected H9c2(2-1) cells were smooth, intact and uniform
in density, and the chromatin in the nucleus was evenly stained.
These results revealed that klotho overexpression alleviated
apoptosis induced by H/R injury (Fig.
2B).
To determine the role of klotho in H/R-induced
apoptosis, H9c2(2-1) cells were infected with klotho lentivirus or
the control vector lentivirus for 24 h followed by H/R treatment.
The apoptotic rate in each group was determined by Annexin
V-FITC/PI dual staining. As shown in Fig. 2C, under H/R conditions, the
apoptotic rate was significantly higher in control vector
lentivirus-infected cells. However, infection with klotho plasmid
lentivirus significantly inhibited the apoptosis with H/R injury.
These results revealed that H/R treatment markedly increased the
number of apoptotic cells, while klotho overexpression inhibited
the H/R-induced apoptosis of H9c2(2-1) cells.
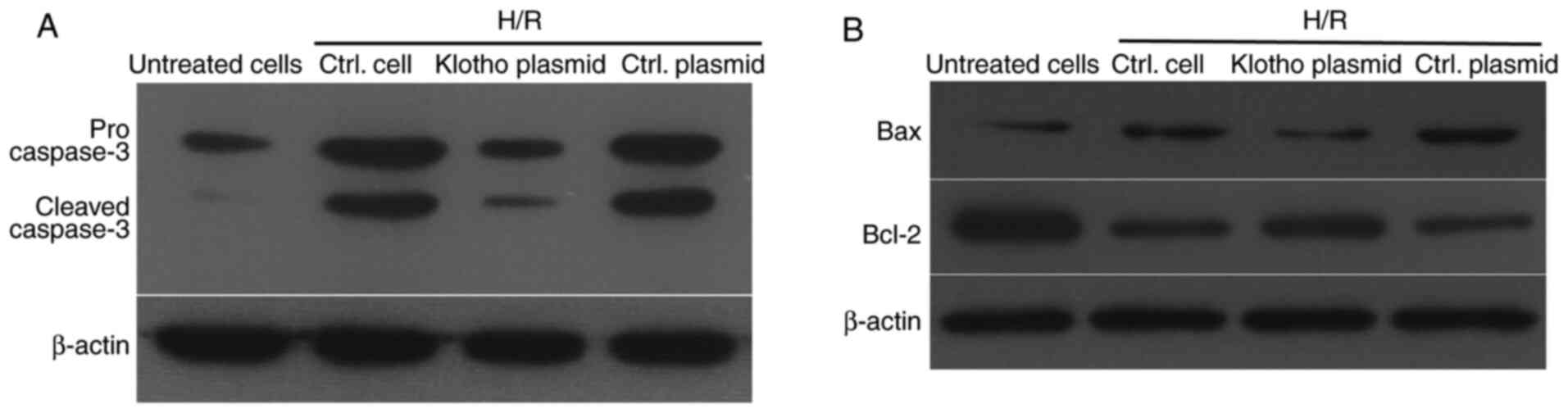
Klotho suppresses the activation of
caspase-3, upregulates Bcl2 and downregulates Bax expression
following H/R treatment
In order to investigate the inhibitory effects of
klotho on H9c2(2-1) cell apoptosis after H/R treatment, the levels
of pro- and cleaved caspase-3 were detected by western blotting. As
shown in Fig. 3A, H/R treatment
increased the levels of pro- and cleaved caspase-2 in H9c2(2-1)
cells, while infection with the klotho lentivirus inhibited the
increase in these proteins, compared with those in the control
plasmid-infected group. Moreover, the ratio of Bcl-2 to Bax is a
key factor in reflecting apoptosis induced by various stimuli.
Thus, the levels of Bcl-2 and Bax were also detected in H/R-treated
H9c2(2-1) cells after infection with klotho lentivirus or control
vector lentivirus. As shown in Fig.
3B, klotho induced a higher level of Bcl-2 and decreased the
level of Bax in H9c2(2-1) cells compared with the control vector
lentivirus after H/R injury. These results demonstrate that H/R
treatment promoted the activation of pro-caspase-3, and that klotho
overexpression inhibited H/R injury-induced apoptosis by inhibiting
caspase-3 activation and increasing the ratio of Bcl-2 to Bax.
Taken together, these data reveal that klotho has an anti-apoptotic
effect on H/R-induced apoptosis in H9c2(2-1) cells.
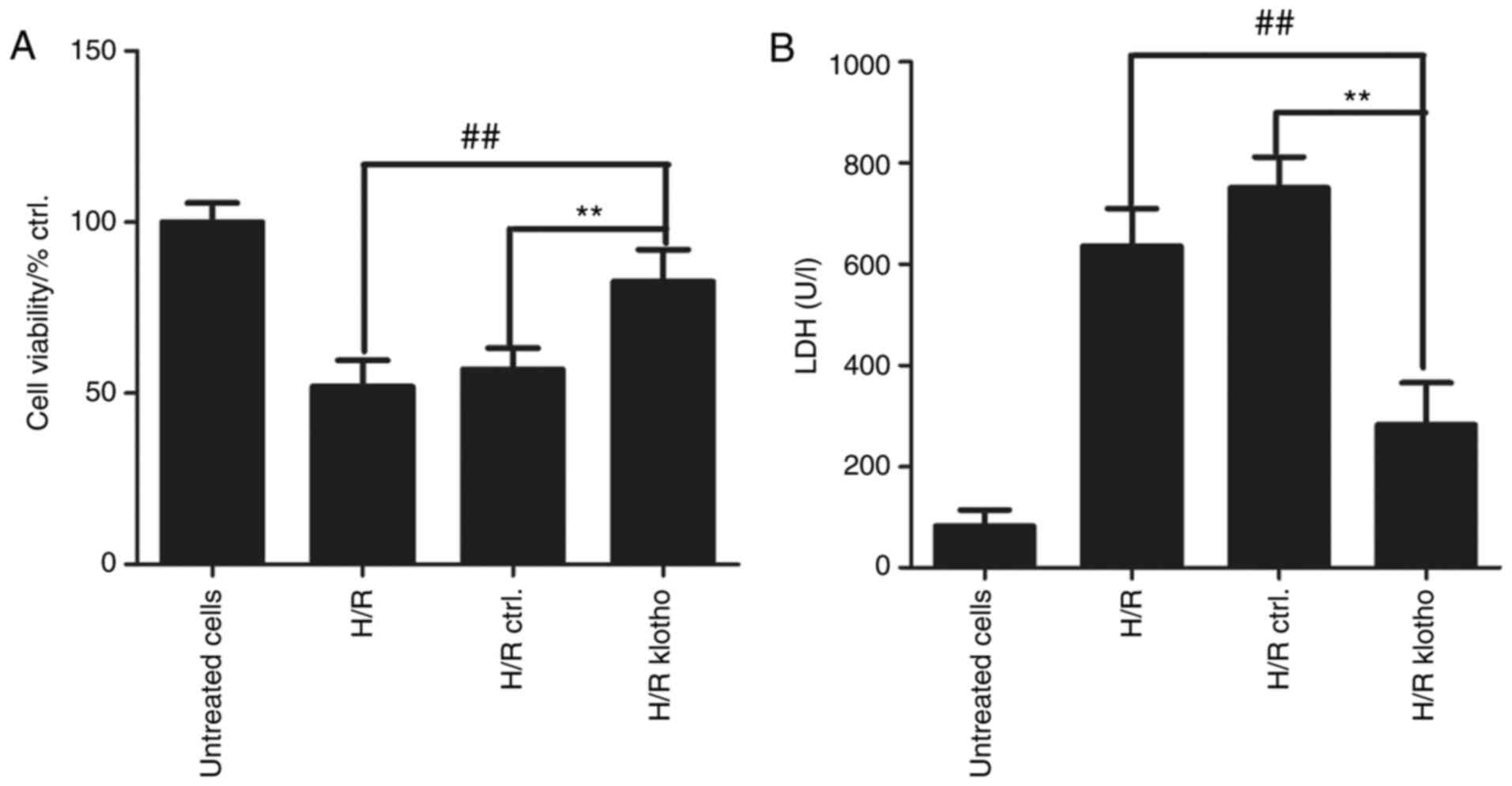
Klotho retains cell viability and
decreases H9c2(2-1) cell injury
MTT and LDH assays were used to evaluate the effects
of klotho on myocardial cell viability and damage after H/R
treatment. Cardiomyocytes were treated with H/R for 4 h, and the
cells were then transfected with klotho or control plasmid for 24
h. As shown in Fig. 4A, cell
viability decreased after H/R treatment for 4 h, and klotho
retained H9c2(2-1) cell viability following H/R treatment
(P<0.01 compared with the control group). The level of LDH can
represent the degree of cardiomyocyte damage, and cardiomyocyte
injury results in increased LDH levels. As shown in Fig. 4B, LDH levels were significantly
higher in H/R-treated cells than in normal cells, suggesting that
H/R results in cellular injury. The level of LDH in the
cardiomyocyte culture medium was significantly lower in the
klotho-transfected group than in the control group (P<0.01).
These results demonstrate that klotho reduces myocardial cell
damage.
Overexpression of klotho in
H/R-treated H9c2(2-1) cells increases the levels of Hsp70, p-Akt
and p-Bad
It has been reported that klotho mitigates apoptosis
in cells involved in ischemic acute kidney injury by regulating the
level of Hsp70(28). The present
study investigated whether Hsp70 was involved in IRI in
klotho-overexpressing H9c2(2-1) cells. Firstly, cells were infected
with klotho lentivirus for 24 h prior to H/R treatment, and Hsp70
expression was detected by confocal laser scanning microscopy and
western blotting. As shown in Fig.
5A, Hsp70 and klotho were colocalized in the cytoplasm of
H/R-treated cells. Moreover, western blotting revealed that klotho
overexpression in H9c2(2-1) cells with H/R injury was accompanied
by increased levels of Hsp70, p-Akt and p-Bad; the level of total
Akt was not markedly altered, and total Bad was markedly decreased
in klotho lentivirus-infected cells (Fig. 5B). Conversely, klotho-knockdown with
H/R injury resulted in decreased expression of Hsp70 and p-Akt,
though total Akt levels were unchanged in klotho shRNA
lentivirus-infected cells (Fig.
5C). These results show that klotho overexpression alleviated
H/R injury in H9c2(2-1) cells by upregulating the levels of Hsp70,
p-Akt and p-Bad.
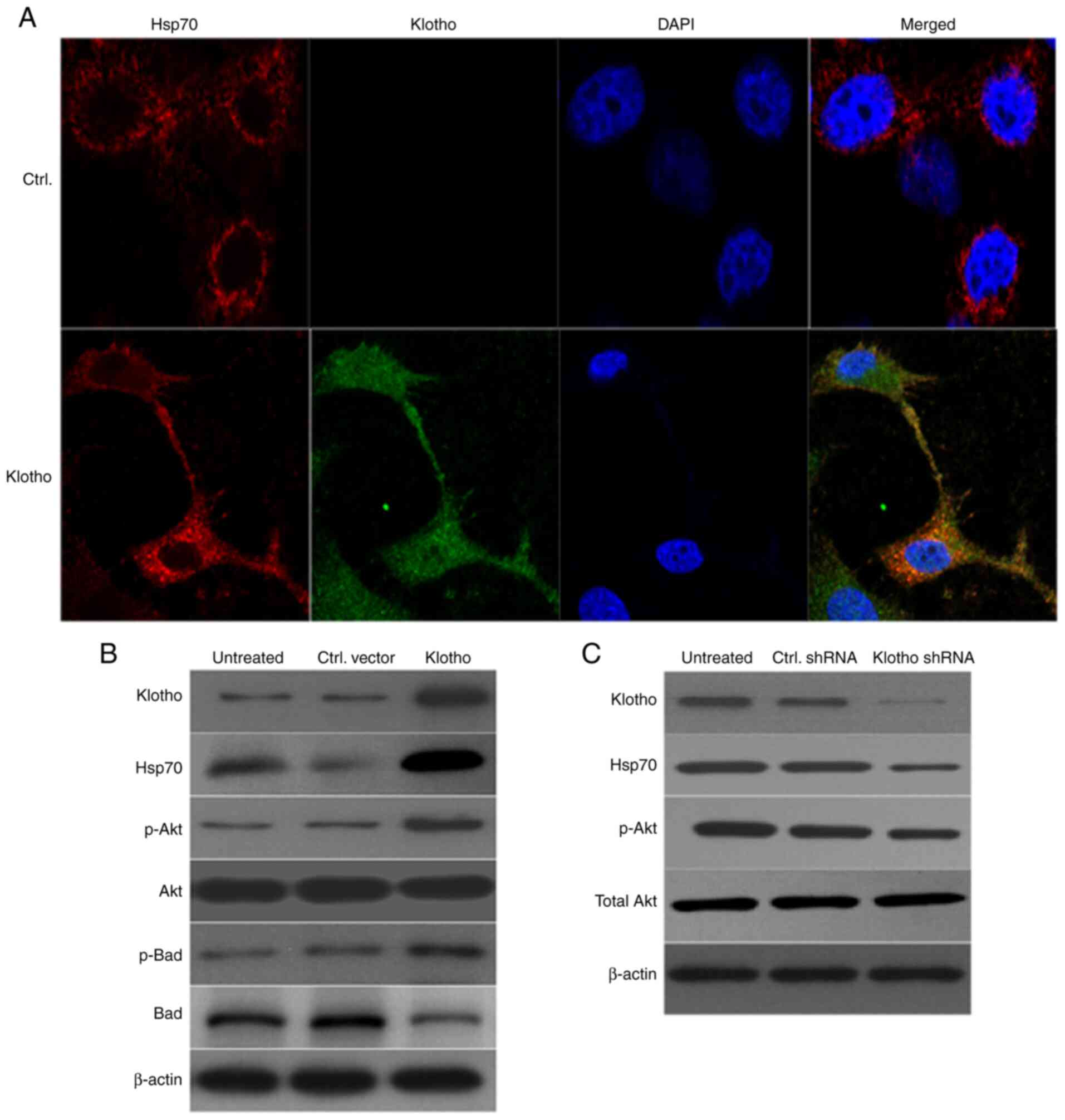 | Figure 5Klotho mitigates H/R injury by
regulating the Hsp70/Akt/Bad pathway. (A) H9c2(2-1) cells were
transfected with klotho plasmid for 24 h prior to H/R injury.
Expression levels of klotho and Hsp70 were detected by
immunofluorescence microscopy (magnification, x200). DAPI was used
to stain the nuclei. (B) Expression of klotho, p-Akt, p-Bad, total
Akt and total Bad was detected by western blotting. β-actin was
used as the internal reference gene. (C) H9c2(2-1) cells were
infected with klotho shRNA lentivirus or control shRNA lentivirus
for 48 h. The levels of klotho, p-Akt and total Akt were detected
by western blotting. H/R, hypoxia/reoxygenation; Ctrl, control;
Hsp70, heat shock protein 70; p-, phosphorylated; sh, short
hairpin. |
Discussion
AMI is a disease with high global incidence and
mortality rates (34). MIRI, which
refers to the interruption of blood supply to the myocardium during
pathological injury, is the most common cause of AMI (35). After the blood supply is restored,
the original ischemic myocardial injury does not improve, but
instead shows more serious damage than that prior to blood supply
recovery. In the present study, a H/R injury model was constructed
using H9c2(2-1) cells to mimic acute MIRI. The protective role of
klotho on myocardial injury was subsequently identified, and klotho
was found to suppress apoptosis by upregulating the levels of
Hsp70, p-Akt and p-Bad.
H/R injury significantly increased the apoptotic
rate of H9c2(2-1) cells and also resulted in decreased klotho
expression. Apoptosis was flow cytometrically evaluated by Annexin
V-FITC/PI dual staining, and the results showed that the apoptotic
rate of H9c2(2-1) cells was markedly increased in the H/R injury
group compared with that of the control group. Furthermore, klotho
overexpression prior to H/R treatment significantly inhibited
H/R-induced apoptosis. The effect of klotho on H9c2(2-1) cell
viability was subsequently assessed using MTT. The results
indicated that H/R treatment reduced the survival rate of
cardiomyocytes and that klotho overexpression significantly
inhibited cell injury and death. Additionally, when myocardial
cells are damaged, LDH level increases, thus LDH level represents
the degree of myocardial cell damage. An LDH assay was performed to
assess H9c2(2-1) cell injury following H/R; the results showed that
H/R treatment increased LDH release, suggesting that H/R results in
cardiomyocyte injury. However, the LDH level in the cardiomyocyte
culture medium of the klotho overexpression group was significantly
lower than that in the control group (P<0.01), suggesting that
klotho overexpression inhibited H9c2(2-1) cell damage.
Next, the molecular mechanism by which klotho
inhibits apoptosis was investigated by western blotting. The
results showed that klotho overexpression inhibited the activation
of caspase-3, increased the Bcl-2/Bax ratio, as well as the level
of p-Bad in H9c2(2-1) cells, compared with those in control vector
lentivirus-infected cells. Furthermore, colocalization of Hsp70 and
klotho was noted in the cytoplasm of H/R-treated cells via confocal
laser scanning microscopy and western blotting. These results
suggest that klotho and Hsp70 were co-localized in the cytoplasm
and inhibited the apoptosis of H/R-treated cells via hsp70. This
was consistent with the findings of Sugiura et al (28), which showed that in an ischemic
acute kidney injury model, klotho inhibited apoptosis via Hsp70.
Previously, Hsp90 was found to exert a profound ischemic
postconditioning cardioprotective effect and to alleviate
I/R-induced myocardial injury and apoptosis in vivo
(36). Notably, the western
blotting results of the present study showed that klotho
overexpression in H9c2(2-1) cells prior to H/R treatment
upregulated the levels of p-Akt and p-Bad. The total Akt level was
not notably altered, and the total Bad level was markedly decreased
in klotho-overexpressing cells. Together, these data suggest that
klotho inhibited apoptosis by interacting with Hsp70 and decreasing
the levels of Bad in H9c2(2-1) cells.
As mature cardiac myocytes are unable to
proliferate, their survival is important in maintaining cardiac
health and function. The proliferative abilities and apoptosis of
cardiomyocytes after MIRI have been widely studied. Although the
antiaging protein klotho reportedly possesses a protective role in
cardiac diseases, the precise mechanisms underlying this effect
remain unknown. A previous study indicated that klotho inhibited
angiotensin II-induced cardiomyocyte hypertrophy by suppressing the
angiotensin II type I receptor/β-catenin pathway (1). Another group found that klotho
suppressed cardiomyocyte apoptosis in mice with stress-induced
cardiac injury by downregulating endoplasmic reticulum stress
(2). In the present study, klotho
and HSP70 were found to co-localize in the cell cytoplasm, and
klotho overexpression was accompanied by HSP70 upregulation. Thus,
how klotho exerts its protective role in the H/R cardiomyocytes by
regulating the expression of Hsp70 was further investigated.
However, as the present study was comprised solely of in
vitro experiments, in vivo functions should be
investigated in the future.
In the present study, the role of klotho in H/R
injury-induced proliferation and apoptosis after MIRI was
investigated, and the associated molecular mechanism was further
clarified. Collectively, the results indicate that klotho reduces
apoptosis by upregulating Hsp70 and p-Akt after IRI, suggesting
that klotho may serve as a potential therapeutic target for I/R
injury and repair.
Acknowledgements
Not applicable.
Funding
The present study was supported by the National Key R&D Plan
(grant no. 2017YFC1307602), the Tianjin Science and Technology
Commission Support Plan (grant no. 15ZXLCSY00040).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Author's contributions
JH checked the references and performed several
western blotting assays. BS performed the flow cytometry and
several western blotting experiments. XL and JZ performed the MTT
assay, acquired the data and prepared the manuscript. YL designed
the experiments and wrote the manuscript. All authors read and
approved the final manuscript. JH and YL were responsible for the
authenticity of the raw data.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests
References
|
1
|
Alabas OA, Jernberg T, Pujades-Rodriguez
M, Rutherford MJ, West RM, Hall M, Timmis A, Lindahl B, Fox KA,
Hemingway H, et al: Statistics on mortality following acute
myocardial infarction in 842 897 Europeans. Cardiovasc Res.
116:149–157. 2020.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Ioacara S, Popescu AC, Tenenbaum J,
Dimulescu DR, Popescu MR, Sirbu A and Fica S: Acute Myocardial
Infarction Mortality Rates and Trends in Romania between 1994 and
2017. Int J Environ Res Public Health. 17(285)2019.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Neri M, Riezzo I, Pascale N, Pomara C and
Turillazzi E: Ischemia/reperfusion injury following acute
myocardial infarction: A critical issue for clinicians and forensic
pathologists. Mediators Inflamm. 2017(7018393)2017.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Wang M and Wan J: Soluble regulators of
Interleukin-1 signaling: Novel biomarkers for early acute
myocardial infarction diagnosis and to predict ischemia/reperfusion
injury? Int J Cardiol. 274(357)2019.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Feng M, Wang Q, Wang H and Guan W: Tumor
necrosis factor-alpha preconditioning attenuates liver
ischemia/reperfusion injury through preserving sarco/endoplasmic
reticulum calcium-ATPase function. J Surg Res. 184:1109–1113.
2013.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Li X, Dai Y, Yan S, Shi Y, Han B, Li J,
Cha L and Mu J: Down-regulation of lncRNA KCNQ1OT1 protects against
myocardial ischemia/reperfusion injury following acute myocardial
infarction. Biochem Biophys Res Commun. 491:1026–1033.
2017.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Tu CC, Wan BY and Zeng Y: STIM2 knockdown
protects against ischemia/reperfusion injury through reducing
mitochondrial calcium overload and preserving mitochondrial
function. Life Sci. 247(116560)2020.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Yue R, Xia X, Jiang J, Yang D, Han Y, Chen
X, Cai Y, Li L, Wang WE and Zeng C: Mitochondrial DNA oxidative
damage contributes to cardiomyocyte ischemia/reperfusion-injury in
rats: Cardioprotective role of lycopene. J Cell Physiol.
230:2128–2141. 2015.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Wang Z, Sun R, Wang G, Chen Z, Li Y, Zhao
Y, Liu D, Zhao H, Zhang F, Yao J, et al: SIRT3-mediated
deacetylation of PRDX3 alleviates mitochondrial oxidative damage
and apoptosis induced by intestinal ischemia/reperfusion injury.
Redox Biol. 28(101343)2020.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Wang X, He F, Liao Y, Song X, Zhang M, Qu
L, Luo T, Zhou S, Ling Y, Guo J, et al: Baicalin pretreatment
protects against myocardial ischemia/reperfusion injury by
inhibiting mitochondrial damage-mediated apoptosis. Int J Cardiol.
168:4343–4345. 2013.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Yang Y, Duan W, Jin Z, Yi W, Yan J, Zhang
S, Wang N, Liang Z, Li Y, Chen W, et al: JAK2/STAT3 activation by
melatonin attenuates the mitochondrial oxidative damage induced by
myocardial ischemia/reperfusion injury. J Pineal Res. 55:275–286.
2013.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Xie Y, Jiang D, Xiao J, Fu C, Zhang Z, Ye
Z and Zhang X: Ischemic preconditioning attenuates
ischemia/reperfusion-induced kidney injury by activating autophagy
via the SGK1 signaling pathway. Cell Death Dis.
9(338)2018.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Zhou LY, Zhai M, Huang Y, Xu S, An T, Wang
YH, Zhang RC, Liu CY, Dong YH, Wang M, et al: The circular RNA ACR
attenuates myocardial ischemia/reperfusion injury by suppressing
autophagy via modulation of the Pink1/ FAM65B pathway. Cell Death
Differ. 26:1299–1315. 2019.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Di Bona D, Accardi G, Virruso C, Candore G
and Caruso C: Association of Klotho polymorphisms with healthy
aging: A systematic review and meta-analysis. Rejuvenation Res.
17:212–216. 2014.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Wang Q, Su W, Shen Z and Wang R:
Correlation between soluble alpha-Klotho and renal function in
patients with chronic kidney disease: A Review and meta-analysis.
Biomed Res Int. 2018(9481475)2018.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Mosa O, Skitek M and Jerin A: Validity of
Klotho, CYR61 and YKL-40 as ideal predictive biomarkers for acute
kidney injury: Review study. Sao Paulo Med J. 135:57–65.
2017.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Kuwahara N, Sasaki S, Kobara M, Nakata T,
Tatsumi T, Irie H, Narumiya H, Hatta T, Takeda K, Matsubara H, et
al: HMG-CoA reductase inhibition improves anti-aging klotho protein
expression and arteriosclerosis in rats with chronic inhibition of
nitric oxide synthesis. Int J Cardiol. 123:84–90. 2008.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Yang K, Wang C, Nie L, Zhao X, Gu J, Guan
X, Wang S, Xiao T, Xu X, He T, et al: Klotho protects against
indoxyl sulphate-induced myocardial hypertrophy. J Am Soc Nephrol.
26:2434–2446. 2015.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Ramez M, Rajabi H, Ramezani F, Naderi N,
Darbandi-Azar A and Nasirinezhad F: The greater effect of
high-intensity interval training versus moderate-intensity
continuous training on cardioprotection against
ischemia-reperfusion injury through Klotho levels and attenuate of
myocardial TRPC6 expression. BMC Cardiovasc Disord.
19(118)2019.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Takenaka T, Kobori H, Miyazaki T, Suzuki
H, Nishiyama A, Ishii N, Yamashita M and Hayashi M: Klotho protein
supplementation reduces blood pressure and renal hypertrophy in
db/db mice, a model of type 2 diabetes. Acta Physiol (Oxf).
225(e13190)2019.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Donate-Correa J, Martín-Núñez E, Delgado
NP, de Fuentes MM, Arduan AO, Mora-Fernández C and Navarro González
JF: Implications of Fibroblast growth factor/Klotho system in
glucose metabolism and diabetes. Cytokine Growth Factor Rev.
28:71–77. 2016.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Fountoulakis N, Maltese G, Gnudi L and
Karalliedde J: Reduced levels of anti-ageing hormone Klotho predict
renal function decline in type 2 diabetes. J Clin Endocrinol Metab.
103:2026–2032. 2018.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Zhou X and Wang X: Klotho: A novel
biomarker for cancer. J Cancer Res Clin Oncol. 141:961–969.
2015.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Fakhar M, Najumuddin Gul M and Rashid S:
Antagonistic role of Klotho-derived peptides dynamics in the
pancreatic cancer treatment through obstructing WNT-1 and Frizzled
binding. Biophys Chem. 240:107–117. 2018.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Li Q, Li Y, Liang L, Li J, Luo D, Liu Q,
Cai S and Li X: Klotho negatively regulated aerobic glycolysis in
colorectal cancer via ERK/HIF1alpha axis. Cell Commun Signal.
16(26)2018.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Pako J, Bikov A, Barta I, Matsueda H,
Puskas R, Galffy G, Kerpel-Fronius A, Antus B and Horvath I:
Assessment of the circulating klotho protein in lung cancer
patients. Pathol Oncol Res. 26:233–238. 2020.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Wang N, Ma J, Ren Y, Xiang S and Jia R:
Secreted klotho from exosomes alleviates inflammation and apoptosis
in acute pancreatitis. Am J Transl Res. 11:3375–3383.
2019.PubMed/NCBI
|
|
28
|
Sugiura H, Yoshida T, Mitobe M, Yoshida S,
Shiohira S, Nitta K and Tsuchiya K: Klotho reduces apoptosis in
experimental ischaemic acute kidney injury via HSP-70. Nephrol Dial
Transplant. 25:60–68. 2010.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Kim SJ, Cheresh P, Eren M, Jablonski RP,
Yeldandi A, Ridge KM, Budinger GR, Kim DH, Wolf M, Vaughan DE, et
al: Klotho, an antiaging molecule, attenuates oxidant-induced
alveolar epithelial cell mtDNA damage and apoptosis. Am J Physiol
Lung Cell Mol Physiol. 313:L16–L26. 2017.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Zhu H, Gao Y, Zhu S, Cui Q and Du J:
Klotho Improves cardiac function by suppressing reactive oxygen
species (ROS) mediated apoptosis by modulating Mapks/Nrf2 signaling
in doxorubicin-induced cardiotoxicity. Med Sci Monit. 23:5283–5293.
2017.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Song S, Gao P, Xiao H, Xu Y and Si LY:
Klotho suppresses cardiomyocyte apoptosis in mice with
stress-induced cardiac injury via downregulation of endoplasmic
reticulum stress. PLoS One. 8(e82968)2013.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Liang X, Li B, Huang Q, Liu D and Ma H:
Klotho prevents DEX-induced apoptosis in MC3T3-E1 osteoblasts
through the NF-κB signaling pathway. Biochem Biophys Res Commun.
507:355–361. 2018.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Cui W, Leng B, Liu W and Wang G:
Suppression of apoptosis in human umbilical vein endothelial cells
(HUVECs) by Klotho protein is associated with reduced endoplasmic
reticulum oxidative stress and activation of the PI3K/AKT pathway.
Med Sci Monit. 24:8489–8499. 2018.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Aijaz S, Ahmed N, Akhter Z, Sattar S,
Lakhani S, Malik R and Pathan A: Clinical characteristics and
in-hospital outcome in percutaneous coronary interventions with ST
elevation myocardial infarction patients developing acute kidney
injury. J Pak Med Assoc. 69:1827–1833. 2019.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Wang C, Pei YY, Ma YH, Ma XL, Liu ZW and
Zhu JH: Risk factors for acute kidney injury in patients with acute
myocardial infarction. Chin Med J (Engl). 132:1660–1665.
2019.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Wang DX, Huang Z, Li QJ, Zhong GQ, He Y,
Huang WQ, Cao XL, Tu RH and Meng JJ: Involvement of HSP90 in
ischemic postconditioning-induced cardioprotection by inhibition of
the complement system, JNK and inflammation. Acta Cir Bras.
35(e202000105)2020.PubMed/NCBI View Article : Google Scholar
|