1. Introduction
Traditional Chinese medicine is attracting great
interest due to the high efficacy of its biological components for
the treatment of several diseases. For example, Astragalus
membranaceus is commonly used in various herbal formulations to
cure inflammatory diseases and cancers (1), and Forsythia suspense-based
treatments have provided notable protection against bacterial
infections, allergies, neurodegeneration and cancers (2). Triptolide, a diterpenoid triepoxide,
is one of the main active ingredients extracted from the
traditional Chinese herb, Tripterygium wilfordii Hook F.,
which possesses several pharmacological activities. In preclinical
in vitro and in vivo models, triptolide has exhibited
a broad spectrum of potent antitumor activity (3,4).
Triptolide can induce cell cycle arrest, interfere with tumor cell
proliferation, suppress cell migration, invasion and metastasis,
prevent angiogenesis, enhance caspase-dependent and -independent
cell death, and produce a synergistic effect in combination with
antitumor drugs (3,5). In addition, triptolide significantly
inhibits the expression of pro-inflammatory cytokines and
chemokines, and is considered a promising anti-inflammatory agent
for the treatment of diseases, including rheumatoid arthritis
(6). Triptolide exhibits profound
immunosuppressive activity by regulating the proportion of immune
cells and inhibiting the release of immune factors (7). Triptolide also alleviates renal and
myocardial pathological injuries, and exerts protective roles in
kidney and cardiovascular diseases (8,9).
However, despite its desirable clinical applications, treatment
with triptolide is restricted due to its potential multiorgan
toxicity, including hepatic, cardiac and reproductive toxicity
(10).
Currently, the molecular mechanisms underlying the
pharmacological activities of triptolide have been extensively
investigated (11,12), which have revealed various cellular
targets and the involvement of different signaling pathways,
including the heat shock protein 70 (Hsp70), vascular endothelial
growth factor (VEGF), c-Jun and protein kinase B (AKT) pathways
(13). MicroRNAs (miRNAs/miRs) are
highly conserved endogenous small non-coding RNA molecules that
negatively regulate gene expression. The phylogenetic conservation
of several miRNAs across mammals highlights the importance of the
miRNAs regulatory network (14).
Consistently, extensive studies of miRNA knockout and
overexpression models have suggested that miRNAs participate in
various essential cellular processes, including cell
differentiation, organ development, metabolism and apoptosis
(15,16). In addition, several diseases are
associated with aberrant expression of miRNAs, and miRNAs exhibit
potential as biomarkers and therapeutic targets for diseases, and
are highly implicated in drug treatment (17). Increasing evidence suggest that
miRNAs also serve as key regulatory elements for
triptolide-mediated activity (18-22).
Some studies assessing the effect of triptolide on miRNAs have
demonstrated that the expression levels of specific miRNAs change
several folds in different cell lines and tissues (23-25).
The present review summarizes miRNAs targeted by
triptolide and discusses the underlying molecular mechanisms
defining the association between the expression of miRNAs and
triptolide to outline the critical roles these miRNAs play in the
pharmacological activities of triptolide.
2. An overview of miRNAs
miRNAs are single-stranded RNA molecules that are 22
nucleotides in length, and their genes mostly reside in either
introns or exons of non-coding transcripts, while others are
located within introns of neighboring protein-coding pre-mRNAs and
dispersed across the genome (26).
miRNA genes are transcribed in the nucleus by RNA polymerase II or
III into long primary transcripts called pri-miRNA, which are
folded into double-stranded RNA hairpin. The pri-miRNA is cleaved
by a microprocessor to release the miRNA precursor named pre-miRNA,
containing a stem-loop of ~60 nucleotides, which is subsequently
exported to the cytoplasm by exportin 5 and Ran-GTP (15,27).
In the cytoplasm, the pre-miRNA is further processed by the
endonuclease, Dicer, to generate the small double-stranded miRNA
duplex (28). Once formed, the
miRNA duplex recruits argonaute protein (AGO) for unwinding
(29). A strand of the duplex,
namely mature miRNA, associates with the RNA induced silencing
complex and mediates mRNA degradation or translational repression
by binding to the partially complementary sequences of the
3'-untranslated region of specific mRNAs (30).
Similar to protein-coding genes, miRNAs are
subjected to stringent regulation, and transcriptional regulation
is a major contributor to the tissue- or development-specific gene
expression of miRNAs (31).
Furthermore, during the maturation steps, specific RNA binding
proteins intricately interact with the processing machineries of a
range of miRNAs through functional interactions, which subsequently
modulates the expression of their target mRNAs (32). In addition, single nucleotide
polymorphisms, RNA editing and methylation are considered important
mechanisms that control the expression levels and functions of
miRNAs (33).
3. Triptolide modulates the expression of
miRNAs
Recent studies have demonstrated that miRNAs are
involved in antitumor, anti-inflammatory and immunosuppressive
activities of triptolide (18,20,21,34-37).
In addition, triptolide exerts renal protective, cardioprotective
and antiangiogenesis functions by regulating the expression of
miRNAs (38-42).
Furthermore, miRNAs are closely associated with the multiorgan
toxicity of triptolide (23,43).
Tables I and II list evidence supporting miRNAs as
pivotal mediators in the pharmacological properties of
triptolide.
 | Table ITriptolide modulates the expression
of miRNAs in vitro. |
Table I
Triptolide modulates the expression
of miRNAs in vitro.
| miRNAs | Triptolide dosage
and treatment time | Cell lines | miRNA status | Downstream
targets | Related biological
effects | Refs. |
|---|
| miR-16-1 | 80 nmol/l, 3, 6 or
12 h; 20, 40 or 80 nmol/l, 8 h | Human T-cell
lymphocytic leukemia cell line, Molt-4 | ↓ | Not mentioned | Apoptosis↑ | (46) |
| miR-16-1 | 20, 40 or 80
nmol/l, 8 h | Human T-cell
lymphocytic leukemia cell line, Jurkat | ↓ | Not mentioned | Not mentioned | (46) |
| miR-138-2 | 80 nmol/l, 3, 6 or
12 h; 20, 40 or 80 nmol/l, 8 h | Human T-cell
lymphocytic leukemia cell line, Molt-4 | ↑ | Not mentioned | Not mentioned | (46) |
| miR-138-2 | 20, 40 or 80
nmol/l, 8 h | Human T-cell
lymphocytic leukemia cell line, Jurkat | ↑ | Not mentioned | Not mentioned | (46) |
| miR-17-92,
miR-106b-25 clusters | 200 nmol/l, 24
h | Human liver cancer
cell line, HepG2 | ↓ | PTEN and BIM | BIM and PTEN↑;
apoptosis↑ | (24) |
| miR-204 | 100 nmol/l, 24
h | Human pancreatic
cancer cell line, MIA PaCa-2 | ↑ | Mcl-1 | Mcl-1↓;
apoptosis↑ | (48) |
| miR-204 | 100 nmol/l, 24
h | Human pancreatic
cancer cell line, S2-VP10 | ↑ | Mcl-1 | Mcl-1↓; autophagic
cell death↑ | (48) |
| miR-142-3p | 100 nmol/l, 24
h | Human pancreatic
cancer cells lines, MIA PaCa-2, Capan-1 and S2-013 | ↑ | Hsp70 | Hsp70↓; cell
proliferation↓ | (50) |
| miR-191 | 50 or 100 nmol/l,
24 h | Human colorectal
cancer cell lines, HT-29 and SW480 | ↓ | Not mentioned | EMT↓; NF-κB and
Wnt/β-catenin pathways↓; cell proliferation↓; migration↓;
apoptosis↑ | (34) |
| miR-146a | 15 ng/ml, 24 h | Human breast cancer
cell line, MDA-MB-231 | ↑ | Rac1; RhoA | RhoA and Rac1↓;
cell invasion and metastasis↓ | (18) |
| miR-144 | 40 nmol/l, 36
h | Human
nasopharyngeal carcinoma cell lines, NPC-TW039 and NPC-TW076 | ↓ | PTEN | PTEN↑; p85α–PTEN
complex↑; p-CDK2↓; S phase arrest | (35) |
| miR-193b-3p | 25 nmol/l, 72
h | Human malignant
rhabdoid kidney tumor cell line, G-401 | ↑ | KLF4 | KLF4↓; PI3K/AKT and
ERK signaling pathways↓; cell viability↓; cell migration↓;
apoptosis↑ | (51) |
| miR-193b-3p | 10, 25 or 50
nmol/l, 72 h | Human malignant
rhabdoid kidney tumor cell line, WiT49 | ↑ | Not mentioned | Not mentioned | (51) |
| miR-138 | 100 nmol/l, 24
h | Human
medulloblastoma cell line, Daoy | ↑ | CDK6 | CDK6↓; PI3K/AKT and
Notch signaling pathways↓; cell proliferation↓; cell migration↓;
apoptosis↑ | (52) |
| miR-218 | 100 nmol/l, 24
h | Human benign
prostatic hypertrophy epithelial cell line, BPH-1 | ↑ | Survivin | Survivin↓; mTOR
signaling pathway↓; apoptosis↑ | (53) |
| miR-215, miR-146a,
miR-199b, miR-449a, miR-190b | 10 nmol/l, 48
h | Human non-small
cell lung cancer cell line, H460 | ↑ | Not mentioned | FAK↓; p-FAK, p-Src,
p-p130Cas↓; p-ERK1/2, MMP14↑; cell migration, invasion and
metastasis↓ | (25) |
| miR-92a, miR-222,
miR-23b, miR-27a, miR-25, miR-296 | 10 nmol/l, 48
h | Human non-small
cell lung cancer cell line, H460 | ↓ | Not mentioned | FAK↓; p-FAK, p-Src,
p-p130Cas↓; p-ERK1/2, MMP14↑; cell migration, invasion and
metastasis↓ | (25) |
| miR-204-5p | 50 or 100 nmol/l,
20 h | Human non-small
cell lung cancer cell line, A549 | ↑ | Sirt-1 | Sirt-1↓, Cav-1↓;
apoptosis↑ | (58) |
| miR-21 | 25 or 50 nmol/l, 48
h | Human non-small
cell lung cancer cell line, PC-9 | ↓ | PTEN | PTEN↑; cell
viability↓ | (62) |
| miR-21 | 5 nmol/l, 72 h | Human
multidrug-resistant chronic myeloid leukemia cell line,
K562/A02 | ↓ | PTEN | PTEN↑; adriamycin
resistance↓ | (63) |
| miR-6751 | 100 nmol/l, 24
h | Cisplatin-resistant
human ovarian cancer cell line, A2780/CP70 | ↑ | HK2 | HK2↓; apoptosis↑;
cisplatin resistance↓ | (64) |
| miR-142-5p and
miR-181a | 10 ng/ml, 24 h |
Dexamethasone-treated human multiple
myeloma cell line, MM.1S | ↓ | GR | GR↑; dexamethasone
resistance↓ | (65) |
| miR-181a | 150 nmol/l, 24
h | Human osteosarcoma
cell lines, SAOS2 and U2OS | ↓ | PTEN | PTEN↑; cell
proliferation↓; apoptosis↑; cell invasion↓ | (66) |
| miR-181a | 20 nmol/l, 24
h | Human neuroblastoma
cell line, SH-SY5Y | ↑ | Not mentioned | MAPK and NF-kB
signaling pathways↑; cell proliferation↓; apoptosis↑; cell
migration↓ | (67) |
| miR-155 | 0.05, 0.1 or 0.5
µmol/l, 0.5 h | LPS-stimulated
murine macrophage cell line, RAW264.7 | ↓ | Not mentioned | Proinflammatory
cytokines (TNF-α, IL1β, and IL-6) ↓ | (70) |
| miR-155 | 15 nmol/l, 24
h | LPS-stimulated
monocytes of patients with rheumatoid arthritis | ↓ | SHIP-1 | SHIP-1↑;
proinflammatory cytokines (TNF-α, IL-6)↓ | (19) |
| miR-155 | 40 nmol/l, 12
h | Human wild-type
αSyn preformed fibrils-treated mouse primary microglia | ↓ | SHIP-1 | SHIP-1↑; PI3K/AKT
signaling pathway↓; NF-κB activity↓; proinflammatory cytokines (TNFα
and IL-1β)↓ | (20) |
| miR-16-1 | 20 ng/ml, 24 h | Human primary
intestinal fibroblasts from strictured anastomosis tissue | ↓ | Hsp70 | Hsp70↑; cell
migration↓; cell proliferation↓; extracellular matrix-associated
proteins (Col-I, Col-III and α-SMA)↓ | (36) |
| miR-20b | 20 ng/ml, 1 h | LPS- and
ATP-treated human monocytic cell line, THP-1 | ↓ | NLRP3 | NLRP3↑; cleaved
caspase-1↓; proinflammatory cytokines (IL-1β and TNF-α)↓ | (37) |
| miR-96 | 12.5 nmol/l, 24
h | LPS-treated murine
microglial cell line, BV2 | ↑ | IKKβ | IKKβ↓; Iba-1↓;
proinflammatory cytokines (TNF-α and IL-1β)↓ | (100) |
| miR-96 | 10 nmol/l, 0.5
h | LPS-treated rat
primary microglia | ↑ | Not mentioned | Iba-1↓; NF-κB
signaling pathway↓; proinflammatory cytokines (TNF-α and
IL-1β)↓ | (100) |
| miR-125a-5p | 10 nmol/l, 3
days | Splenocytes of B6
mice | ↑ | Not mentioned | Foxp3↑; Treg
proportion↑ | (21) |
| miR-125a-5p | 0.2 mg/kg/day, 91
days | Splenocytes of
MRL/lpr mice | ↑ | Not mentioned | Foxp3↑; Treg
proportion↑ | (21) |
| miR-30 | 10 ng/ml, 24 h | TGF-β-treated
immortalized human podocyte cell line | ↑ | Not mentioned | Cell injury
mediators (MAPK, NF-κB and NFATC3)↓ | (22) |
| miR-188-5p | 5 ng/ml, 48 h | High
glucose-treated human proximal tubular epithelial cell line,
HK-2 | ↓ | PTEN | PTEN↑; PI3K/AKT
signaling pathway↓; renal EMT↓ | (38) |
| miR-141-3p | 10 µmol/l, 48
h | High
glucose-treated human mesangial cell | ↓ | PTEN | PTEN↓; autophagy↑;
AKT/mTOR signaling pathway↓; diabetic renal fibrosis↓ | (75) |
| miR-137 | 10 µg/l, 48 h | High
glucose-treated human renal mesangial cell | ↑ | Notch1 | Notch1 signaling
pathway↓; extracellular matrix proteins (Col IV and FN)↓ | (39) |
| miR-21 | 10 ng/ml, 24 h | Rat myocardial cell
line, H9C2 | ↓ | TLR4 | TLR4↓; MAPK/NF-κB
signaling pathway↓; proinflammatory cytokines (TNF-α, IL-6, and
IL-17)↓ | (40) |
| miR-92a | 3 µmol/l, 12 h | Human dermal
microvascular endothelial cell line, HMEC-1 | ↑ | Integrin subunit
alpha 5 (ITGA5) | Angiogenic
mediators (eNOS, VEGFR2 and VEGF)↓; ITGA5↓; ERK and PI3K/AKT
signaling pathways↓ | (42) |
| miR-26a | 120 nmol/l, 24
h | Mouse Leydig cell
line MLTC-1 | ↑ | GSK3β | GSK3β↓;
apoptosis↑ | (43) |
 | Table IITriptolide modulates the expression
of miRNAs in vivo. |
Table II
Triptolide modulates the expression
of miRNAs in vivo.
| miRNAs | Triptolide dosage
and treatment time | Tissue type | miRNA status | Downstream
targets | Related biological
effects | Refs. |
|---|
| miR-17-92;
miR-106b-25 clusters | 0.2 mg/kg/day, 14
days | Xenografted
hepatocellular carcinoma from BALB/c nude mice | ↓ | Not mentioned | Tumor volume↓;
apoptosis↑ | (24) |
| miR-204 | 0.42 mg/kg/day, 7
days | Xenografted human
pancreatic ductal adenocarcinoma from SCID mice | ↑ | Mcl-1 | Mcl-1↓; tumor
volume↓ | (48) |
| miR-142-3p | 0.42 mg/kg/day, 7
days | Xenografted human
pancreatic ductal adenocarcinoma from SCID mice | ↑ | Hsp70 | Hsp70↓ | (50) |
| miR-191 | 0.1, 0.3 or 1
mg/kg/day, 28 days | Xenografted human
colon carcinoma from BALB/c nude mice | ↓ | Not mentioned | Tumor volume and
weight↓ | (34) |
| miR-155 | 0.07 mg/kg every 2
days, 56 days | Small intestinal of
IL-10 deficient mice performed ileocecal resection | ↓ | SHIP-1 | SHIP-1↑;
anastomosis inflammation score↓; MPO and calprotectin↓;
inflammatory cytokines TGF-β ↑, IFN-γ and IL-4, IL-17 ↓ | (72) |
| miR-16-1 | 0.07 mg/kg every 2
days, 56 days | Small intestinal of
IL-10 deficient mice performed ileocecal resection | ↓ | Hsp70 | Hsp70↑; anastomosis
inflammation score↓; CD4+ cell infiltration area↓; fibrosis score↓;
extracellular matrix-associated proteins (collagen, procollagen I
and III)↓; inflammatory cytokines (TGF-β1, IL-6 and TNF-α)↓ | (73) |
| miR-96 | 0.1 mg/kg/day, 10
days | Spinal cord of
spinal cord injury rat | ↑ | Not mentioned | Iba-1↓; NF-κB
pathway↓; inflammatory cytokines (TNF-α and IL-1β)↓ | (100) |
| miR-344b-3p;
miR-30b-3p | 200 µg/kg/day, 56
days | Rat renal cortex
with adriamycin- induced nephropathy | ↓ | Not mentioned | Nephrin↑;
proteinuria↓; renal pathological lesions↓ | (76) |
| miR-30a | 10 ng/ml, 24 h | TGF-β1-treated
isolated glomeruli of mouse or rats | ↑ | Not mentioned | Not mentioned | (22) |
| miR-188-5p | 200 µg/kg/day, 84
days | Diabetic rat
kidney | ↓ | PTEN | PTEN↑; renal EMT↓;
PI3K/AKT signaling pathway↓ | (38) |
| miR-137 | 100 µg/kg/day, 84
days | Diabetic rat
kidney | ↑ | Not mentioned | Extracellular
matrix proteins (Col IV and FN)↓; Notch1 signaling pathway↓ | (39) |
| miR-21 | 0.4 mg/kg every 7
days, 28 days | Adjuvant arthritis
rat cardiac tissue | ↓ | TLR4 | TLR4↓; apoptosis↓;
proinflammatory cytokines (TNF-a, IL-6, and IL-17)↓; MAPK/NF-κB
signaling pathway↓ | (41) |
| miR-546, miR-343,
108 miRNAs | 0.1 mg/kg, single
oral dose, 14 days | Left ventricular
tissue from rats | ↑ | Not mentioned | Regulation of cell
adhesion, cell cycling, action potential, cell-cell communication,
and DNA binding | (23) |
| miR-384-3p,
miR-384-5p, 8 miRNAs | 0.1 mg/kg, single
oral dose, 14 days | Left ventricular
tissue from rats | ↓ | Not mentioned | Regulation of
calmodulin activity, heterodimerization activity, and signal
transduction | (23) |
| miR-483-3p | 0.1 mg/kg, single
oral dose, 14 days | Left ventricular
tissue from rats | ↑ | AhR | AhR↓; CYP1A1↓ | (23) |
| miR-15a-3p,
miR-615, miR-4833p, miR-127-5p | 0.1 mg/kg, single
oral dose, 14 days | Plasma from
rats | ↑ | Not mentioned | Not mentioned | (23) |
| miR-122 | 0.2, 0.4 or 0.8
µmol/l, 48 h | Zebrafish
larvae | ↑ | Not mentioned | Histology score of
hepatocyte vacuolation, hepatocyte disarray and oncotic necrosis↑;
liver volume↓ | (83) |
miRNAs involved in the antitumor
activity of triptolide
Several miRNAs act as tumor-suppressor genes or
oncogenes, and increasing evidence suggest that the suppressive
function of triptolide on multiple tumors is mediated by regulating
miRNAs (44,45). In human T-cell lymphocytic leukemia
cells, triptolide significantly increases miR-16-1 expression and
decreases miR-138-2 expression, and downregulation of miR-16-1
expression contributes to triptolide-induced apoptosis (46). Triptolide-induced hepatocellular
carcinoma cell death is associated with the suppression of two
oncogenic miRNA clusters, miR-17-92 and miR-106b-25(24). In pancreatic cancer cells, treatment
with triptolide increases miR-204 expression, which directly binds
to myeloid cell leukemia-1 (Mcl-1), an antiapoptotic gene essential
for cell survival (47), thereby
inhibiting Mcl-1 protein expression and inducing cell-type
dependent apoptosis or autophagic cell death (48). In addition, triptolide decreases
miR-142-3p expression, which negatively regulates Hsp70 expression,
a stress protein recognized as an apoptosis inhibitor (49), and suppresses pancreatic cancer cell
proliferation (50). In vivo
studies have confirmed that triptolide abrogates human pancreatic
tumors xenografts by concurrent upregulation of miR-204 and
miR-142-3p expression and downregulation of Mcl-1 or Hsp70
expression, respectively (48,50).
In colon carcinoma cells, treatment with triptolide downregulates
miR-191 expression, which in turn suppresses cell proliferation and
migration, induces apoptosis and activates the nuclear factor
kappa-B (NF-κB) and Wnt/β-catenin signaling pathways (34). Notably, these effects were reversed
following transfection with miR-191 mimics, suggesting that the
anti-colorectal cancer activities of triptolide are associated with
the downregulation of miR-191 expression (34). Through upregulation of miR-146a
expression, triptolide markedly decreases the expression levels of
the RhoA and Rac1 genes in breast cancer cells, which
are both key members of the Rho GTPase family involved in tumor
invasion and metastasis, and thus act as metastasis suppressors
(18). Triptolide also induces S
phase cell cycle arrest of human nasopharyngeal carcinoma cells by
enhancing p85α-phosphatase and tensin homolog (PTEN) complex
formation and inactivating AKT-mediated cyclin-dependent kinase 2
phosphorylation, which requires downregulation of miR-144
expression (35). Triptolide also
inhibits the proliferation of nephroblastoma and medulloblastoma
cells, and benign prostatic epithelial cells. In addition,
triptolide inhibits the migratory ability of these cells but
promotes apoptosis by upregulating the expression levels of
miR-193b-3p, miR-138 and miR-218 (51-53).
The latter activities involving miRNAs corresponded to inactivation
of the phosphatidylinositol 3 kinase (PI3K)/AKT and extracellular
regulated protein kinase (ERK) signaling pathways by downregulating
Kruppel-like factor, inactivating the Notch signaling pathway, as
well as suppressing CDK6 expression, or negatively regulating
survivin and inactivating the mammalian target of rapamycin (mTOR)
signaling pathway, respectively (51-53).
Sequencing data indicate that triptolide markedly
alters the expression profiles of miRNAs in human non-small cell
lung cancer cells. miRNAs associated with cell motility, such as
miR-146a, miR-23b and miR-199b (54-56),
are significantly upregulated or downregulated, and subsequent
studies have validated that triptolide notably decreases lung
cancer cell migration, invasion and metastasis by suppressing focal
adhesion kinase expression and disrupting its downstream signaling
pathways (25). Furthermore,
treatment with triptolide causes a dose-dependent upregulation of
miR-204-5p expression, along with decreased expression of its
target, SIRT-1, a member of the class III histone deacetylase
family required for caveolin-1 (Cav-1) expression (57), thereby coupling Cav-1 downregulation
with activation of classical AKT/Bax-mediated apoptosis in
non-small cell lung cancer cells (58).
Drug resistance is one of the main reasons for
therapy failure in cancer treatment (59). miRNAs are critical regulators of
molecular pathways implicated in cancer drug resistance (60). miR-21 is highly expressed and
associated with disease progression and multidrug-resistance in
different types of cancer (61).
Triptolide notably decreases miR-21 expression in non-small cell
lung cancer cells, and increases PTEN protein expression, which
acts as a tumor suppressor and participates in tumor occurrence and
development, and decreases cell proliferation and enhances
apoptosis (62). However, ectopic
miR-21 expression rescues the effect of triptolide on PTEN protein
expression and cell viability, suggesting that triptolide mediates
the decrease in miR-21 expression, which in turn promotes cell
death by upregulating PTEN expression (62). Furthermore, triptolide significantly
enhances adriamycin-induced cytotoxicity by decreasing miR-21
expression and increasing PTEN expression, and reverses drug
resistance to human chronic myeloid leukemia cells (63).
In cisplatin-resistant human ovarian cancer cells,
upregulation of miR-6751 expression via triptolide decreases
hexokinase 2 protein expression, which confers resistance to
cisplatin by enhancing autophagic activity, and sensitizes cells to
the proapoptotic effect of cisplatin (64). A similar synergistic proapoptotic
effect was detected following combined treatment with triptolide
and dexamethasone in human multiple myeloma cells, which has the
ability to overcome the glucocorticoid resistance of these cells by
enhancing glucocorticoid receptor expression by inhibiting
miR-142-5p and miR-181a expression (65). Furthermore, miR-181a expression is
notably attenuated in osteosarcoma cells following treatment with
triptolide, which directly upregulates PTEN expression, whereas
transfection with miR-181a mimics restores PTEN expression, and
decreases the inhibitory effect of triptolide on osteosarcoma cell
proliferation and invasion, suggesting the anti-osteosarcoma
properties of triptolide depended on the regulation of miR-181a and
its targeting of the PTEN gene (66). Conversely, upregulated miR-181a
expression participates in the suppressive effect of triptolide
against human neuroblastoma cell proliferation and migration, and
via activation of the mitogen-activated protein kinase (MAPK) and
NF-κB signaling pathways (67).
Thus, triptolide can upregulate or downregulate the expression of
specific miRNAs, influence downstream targeting signaling pathways,
and thus exert antitumor activities (Fig. 1).
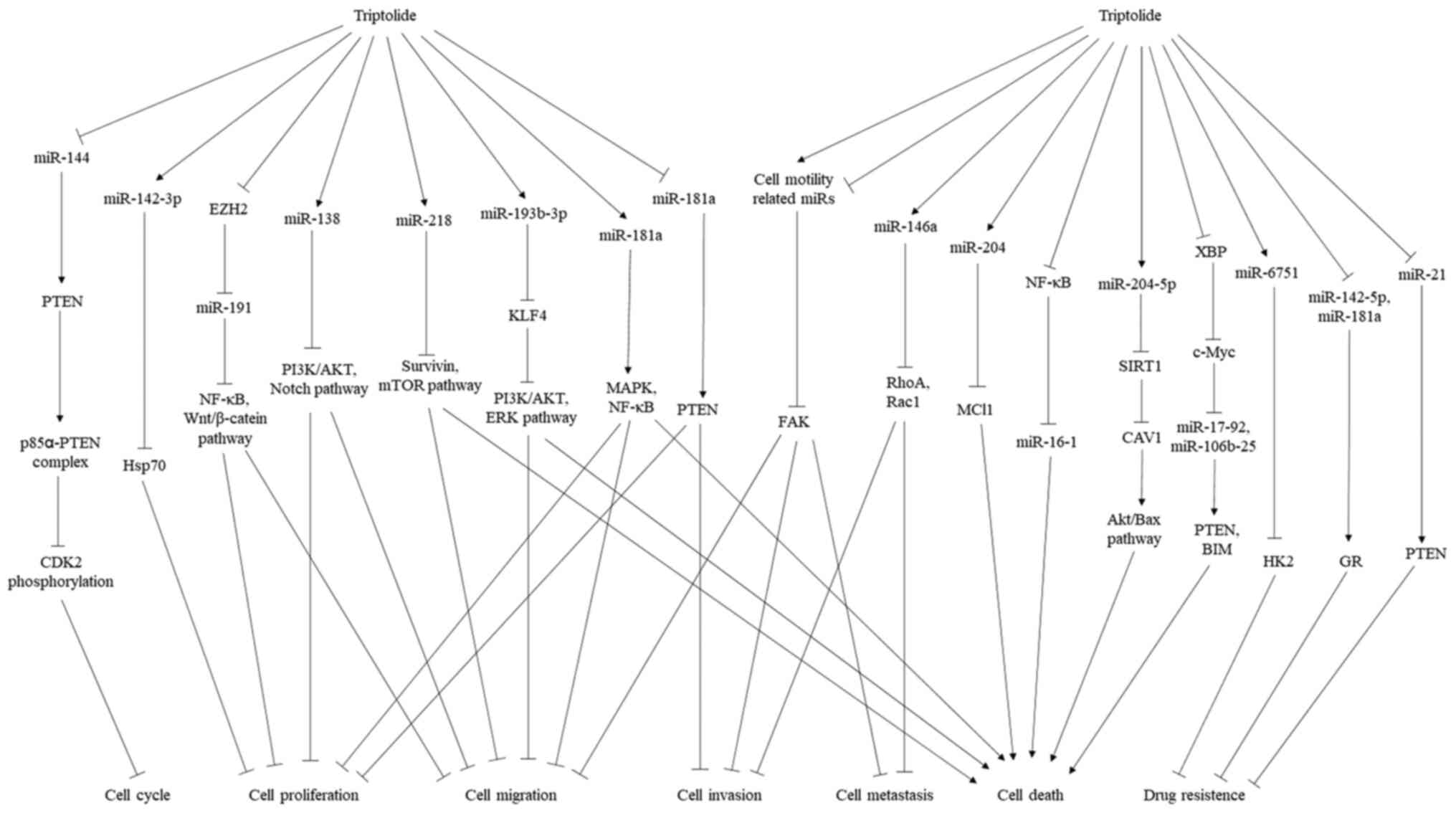 | Figure 1miRNAs involved in the antitumor
activity of triptolide. Through upregulation and downregulation of
specific miRNAs, triptolide affects downstream signaling pathways,
which induces tumor cell cycle arrest, interferes with cell
proliferation, suppresses cell migration, invasion and metastasis,
enhances cell death and reverses drug resistance. miRNA, microRNA;
PTEN, phosphatase and tensin homolog; CDK, cyclin-dependent kinase;
Hsp, heat shock protein; EZH2, enhancer of zeste homolog 2; NF-κB,
nuclear factor kappa-B; PI3K, phosphatidylinositol 3 kinase; AKT,
protein kinase B; mTOR, mammalian target of rapamycin; KLF4,
Kruppel-like factor; ERK, extracellular regulated protein kinase;
Cav-1, caveolin-1; HK2, hexokinase 2; GR, glucocorticoid
receptor. |
miRNAs involved in the
anti-inflammatory and immunosuppressive activities of
triptolide
Several miRNAs are substantially activated by
inflammatory stimuli, such as the Toll-like receptor (TLR), ligand
lipopolysaccharide (LPS) and components of the inflammatory
processes by post-transcriptional regulation of either signal
transduction proteins of inflammatory pathways or specific
inflammatory cytokines (68). For
example, miR-155, which is implicated in the pathogenesis of
inflammatory diseases (69), has
prompted investigation on the association between triptolide and
miR-155 in these inflammatory diseases. cDNA array and northern
blot analysis have demonstrated that the expression of inflammatory
cytokine, as well as miR-155 are markedly upregulated in
macrophages following LPS stimulation, whereas triptolide
attenuates the induction of these genes in a dose-dependent manner
(70).
Similar observations were obtained in monocytes from
patients with rheumatoid arthritis (19), whereby overexpression of miR-155
reverses the inhibitory effect of triptolide on LPS-induced
interleukin (IL)-6 and tumor necrosis factor-α production, and
antagonizes the effect of triptolide on Src homology 2-containing
inositol phosphatase-1 (SHIP-1), which is a target of miR-155 and
functions as a potent inhibitor of several inflammatory pathways
(71). Thus, it has been proposed
that triptolide inhibits miR-155 expression, which negatively
affects its downstream target, SHIP-1, and suppresses the
inflammatory response stimulated by LPS.
The miR-155/SHIP-1 axis plays a critical role in
triptolide-induced improvement on the symptoms of other
inflammatory diseases. Inhibiting the miR-155/SHIP-1 axis via
triptolide suppresses NF-κB activity via the PI3K/AKT pathway,
which significantly inhibits microglial activation and stimulates
inflammatory cytokines stimulated by prion-like preformed fibril
(20).
Triptolide has also been reported to suppress
miR-155 expression and simultaneously promote SHIP-1 expression in
the small intestine, particularly in the anastomosis of IL-10
deficient mice subjected to ileocecal resection (72). These effects are also associated
with decreased levels of inflammatory cytokines, and eventually
exert therapeutic effects on Crohn's disease, an inflammatory bowel
disease (72). Studies on Crohn's
disease have reported that triptolide effectively reverses
upregulated miR-16-1 expression and downregulated Hsp70 expression
at the anastomosis sites in IL-10 deficient mice, as well as in
patients with Crohn's disease, which represents a protective
mechanism against postsurgical inflammation and anastomotic
fibrosis (37,73).
With regards to the effects on osteoarthritis,
downregulation of miR-20b expression following treatment with
triptolide notably upregulates its target inflammasome-related
gene, nucleotide-binding oligomerization domain-like receptor
family pyrin domain-containing protein 3, which limits the
activation of caspase-1 and subsequently inhibits the maturation of
inflammatory cytokines, thus preventing the development of
osteoarthritis (41). In the
treatment of spinal cord injury (SCI), the anti-inflammatory
activity of triptolide is mediated by upregulated miR-96
expression, which inactivates NF-κB by negatively regulating the
inhibitor of NF-κB kinase subunit β expression, and decreases
inflammatory cytokine levels in LPS-induced primary microglia and
spinal cord tissues of SCI rats (42).
Triptolide is frequently used as an
immunosuppressive compound in the treatment of chronic autoimmune
diseases, including systemic lupus erythematosus (SLE) (12). Recently, a novel miRNA target was
identified following treatment of SLE by triptolide. Triptolide was
demonstrated to markedly increase miR-125a-5p expression, as well
as the percentage of regulatory T cells (Treg), which are important
in modulating self-tolerance and autoimmunity (74). Conversely, miR-125a-5p inhibitor
significantly abrogates the effects of triptolide on Treg,
suggesting that triptolide stimulates Treg activation via
miR-125a-5p, and thereby alleviates the clinical and histological
symptoms observed in the SLE mouse model (21). Taken together, these findings
suggest that changes in the expression levels of specific miRNAs
contribute to the anti-inflammatory and immunosuppressive
properties of triptolide.
miRNAs involved in the renal
protective and cardioprotective activities of triptolide
Increasing evidence suggest that triptolide exhibits
renal protective effects by regulating the expression of miRNAs
(22,38,39,75,76).
In adriamycin-induced nephropathy rat models, elevated expression
levels of miR-344b-3p and miR-30b-3p were observed, the effects of
which were reversed following treatment with triptolide. Triptolide
also increases the levels of nephrin protein, which is involved in
the maintenance of the glomerular filtration barrier structure in
the kidney cortex, thereby attenuating podocyte damage and
partially improving renal function (76). Thus, it has been speculated that
miR-344b-3p and miR-30b-3p are involved in the protective effect of
triptolide towards podocytes from adriamycin-induced nephropathy
(76). In the transforming growth
factor-β (TGF-β)-induced podocyte injury model, the presence of
triptolide completely reversed TGF-β-induced miR-30 downregulation,
suppressed TGF-β-stimulated activation of downstream damaging
pathways, including MAPK, NF-κB and calcineurin/NFATC3, and
alleviated podocyte injury in vitro and ex vivo
(22).
During the development of diabetic kidney disease
(DKD), miRNAs are closely associated with multiple pathological
modifications (77). Triptolide
notably mitigates the impaired renal function by exerting a
therapeutic effect on DKD through the regulation of miRNA-mediated
signaling pathways (38,75). For example, triptolide significantly
reverses the increase in miR-188-5p expression induced by high
glucose in human proximal tubular epithelial cells and diabetic
kidneys, which enhances the expression of action target PTEN, and
inactivates the downstream PI3K/AKT signaling pathway and inhibits
renal epithelial-to-mesenchymal transition in DKD (38). Similarly, downregulation of
miR-141-3p expression via triptolide affects the target PTEN
protein in human renal mesangial cells, under high glucose, with an
opposite tendency. In addition, triptolide-induced autophagic
activation and fibrosis alleviation in association with the
activation of the PTEN/AKT/mTOR pathway is blocked following
overexpression of miR-141-3p (75).
The miR-137/Notch1 pathway prevents
glomerulosclerosis under diabetic conditions (39). Unlike miR-188-5p and miR-141-3p,
miR-137 expression significantly decreases in high
glucose-incubated renal mesangial cells and in diabetic rat kidney
tissues, but returns to normal levels following treatment with
triptolide (39). Triptolide
inactivates the Notch1 pathway reliance on miR-137, which
suppresses extracellular matrix protein accumulation of collagen IV
and fibronectin, thus improving DKD by protecting against
glomerulosclerosis (39).
The cardioprotective activity of triptolide has been
attributed to the changes in miR-21 expression. Treatment with
triptolide markedly decreases miR-21 expression, inactivates the
TLR4/MAPK/NF-κB signaling pathway and prevents cell apoptosis in
LPS-treated myocardial cells, as well as in cardiac tissues from
adjuvant arthritis rat models (40,41).
Collectively, these findings confirm the regulatory role of miRNAs
in the renal protective and cardioprotective activities of
triptolide.
miRNAs involved in the
antiangiogenesis activity of triptolide
A key feature of atherosclerosis is the dysregulated
progression of endothelial cell angiogenesis within the plaques, a
process that is regulated by several miRNAs, particularly miR-92a
(78). miR-92a blocks angiogenesis
both in vitro and in vivo (79), and participates in the inhibitory
effect of triptolide in atherosclerosis. In human dermal
microvascular endothelial cells, triptolide effectively induces
miR-92a expression in a dose-dependent manner, and impedes the
production of integrin subunit α 5 (ITGA5), which is a direct
target of miR-92a (42).
Overexpression of ITGA5 inactivates the ERK and PI3K/AKT signaling
pathways, as well as the accumulation of a number of
angiogenesis-related factors, such as endothelial nitric oxide
synthase, VEGF receptor-2 and VEGF, which inhibits angiogenesis
(42). These effects are reversed
following ITGA5 knockdown (42).
Thus, miR-92a acts as a crucial mediator in the antiangiogenesis
activity of triptolide by inactivating the ERK and PI3K/AKT
signaling pathways following downregulation of ITGA5
expression.
miRNAs involved in the multiorgan
toxicity of triptolide
Several miRNAs, such as miR-122 and miR-26a, are
involved in the multiorgan toxicity observed following treatment
with triptolide, and are considered sensitive early warning
indicators (80). Oral
administration of triptolide in male rats led to cardiac
dysfunction and myocardial cell death. These effects were
associated with at least a 2-fold increase in the expression levels
of 108 miRNAs, as well as a 2-fold decrease in the expression
levels of eight miRNAs in heart tissue samples. Furthermore,
changes in plasma miRNAs were also detected (81). Among these, 28 miRNAs were predicted
to simultaneously regulate the expression of aryl hydrocarbon
receptor (AhR), which is a transcription factor that is closely
associated with cardiac pathophysiology (81). Another study confirmed that
triptolide significantly decreases myocardial and plasma AhR
levels, as well as its downstream gene, CYP1A1 (23).
miR-122 expression is significantly upregulated in
adult zebrafish or mice livers following treatment with liver
toxicants, including acetaminophen or tamoxifen (82). A similar trend has been observed in
zebrafish larvae following treatment with triptolide, which causes
hepatic injury (83), which is
considered to be a crucial event resulting from deregulated hepatic
function caused by altered miR-122 expression (84). Taken together, these findings
suggest that miR-122 may be used as a diagnostic predictor and an
attractive therapeutic target for the hepatotoxicity activity of
triptolide.
miR-26a expression is upregulated in
triptolide-induced reproductive toxicity in mouse Leydig cells
(43). This results in cytotoxicity
via inhibition of its downstream target, glycogen synthase
kinase-3β, which possesses antiapoptotic effects (43). Collectively, these findings indicate
the association between miRNAs and the multiorgan toxicity of
triptolide.
4. Molecular mechanisms underlying
regulation of miRNAs by triptolide
The regulation of the expression of miRNAs by
triptolide is partly mediated by specific transcription factors.
Triptolide has been reported to interfere with TGF-β-induced
Smad2/3 phosphorylation and activation, and prevents phosphorylated
protein binding to Smad4 and their subsequent translocation to
nuclei where they are known to regulate gene expression as
transcription factors, and thereby completely restore miR-30
downregulation in podocytes (22).
As a multifunctional transcription factor, NF-κB positively and
negatively regulates the expression of miRNAs (85). Treatment with triptolide decreases
the expression and nuclear accumulation of NF-κB in a
dose-dependent manner, accompanied by the differential expression
of 23 miRNA genes in lymphocytic leukemic cell lines, suggesting
that triptolide-induced cytotoxic effects may occur by inhibiting
NF-κB transcriptional activity, and consequently influencing the
expression of miRNAs (46). c-Myc
also plays a critical role in repressing two oncogenic miRNA
clusters by triptolide in hepatocellular carcinoma cells (24). Through direct binding to the E-box
element in the promoter region of MCM7, c-Myc transactivates both
miR-7-92 and miR-106b-25, which function as oncogenes in cancer
initiation and progression (86).
Triptolide significantly antagonizes the transcription of these two
miRNA clusters by targeting c-Myc, and XPB (also known as ERCC3) is
involved in this process (24). XPB
is a subunit of general transcription factor TFIIH, which is
essential for RNA polymerase II-dependent transcription initiation
and nucleotide excision repair (87). It has been reported that triptolide
covalently binds to the Cys342 residue of XPB, the largest subunit
of the general transcription factor TFIIH (88), and impedes its ATPase activity,
inhibiting RNA polymerase II mediated transcription (89). Triptolide also acts as an inhibitor
of RNA polymerase II by selectively activating CDK7, and
subsequently by triggering proteasome-dependent degradation of
hyperphosphorylated Rpb1, which is the largest RNA polymerase II
subunit (90). On the other hand,
triptolide directly suppresses the recruitment of B-related factor
1 to TFIIIB complex, an essential transcription initiation factor
of RNA polymerase III (91), and
significantly inhibits RNA polymerase III transcription (92). Based on these observations and the
fact that miRNA genes are transcribed by RNA polymerase II or III,
it is reasonable to speculate that the inhibition of RNA polymerase
II and III mediated general transcription underlies miRNAs
regulation by triptolide.
Triptolide may also regulate the expression of
miRNAs via an enhancer of zeste homolog 2 (EZH2)-involved
mechanism. EZH2, a well-known histone methyltransferase (93), is capable of binding to the promoter
regions of miRNAs genes and catalyzing histone H3 trimethylation,
resulting in the transcriptional silencing of miRNAs genes
(94). It has been proven that EZH2
is a target for triptolide in prostate cancer and multiple myeloma
cells (95), and a recent study
demonstrated that treatment with triptolide significantly
downregulates miR-191 expression in colon carcinoma cells in an
EZH2-dependent manner, suggesting a modulatory activity of
triptolide on the expression of miRNAs at the epigenetic level
(34).
The regulation of miRNAs by triptolide may be
ascribed to the autophagy pathway. Autophagy can be modulated by
triptolide through multiple machineries and signaling pathways,
such as oxidative stress, cytoplasmic calcium and the
AKT/mTOR/p70S6K pathway (13).
miRNAs also play vital roles in autophagy as they can target
autophagy-related genes or other related regulators, and
participate in regulating the dynamic process of autophagy
(96). Triptolide has been reported
to significantly decrease miR-141-3p expression, which directly
acts on PTEN, and thereby reverses the induced autophagy in high
glucose treated human mesangial cells and in DKD rats (75). Conversely, autophagy selectively
regulates miRNA homeostasis, which is activated by promoting
autophagy receptor-mediated degradation of miRNA pathway
components, including DICER and AGO2, further proving an
association between autophagy and miRNAs (97). Although the molecular mechanisms
involved in the role of autophagy in triptolide regulation of
miRNAs remain unclear, it is possible that autophagy may play a
functional role, and the precise molecular targets and regulatory
networks remain to be elucidated. Collectively, these findings
suggest that the expression of triptolide-regulated miRNAs is
mediated by multiple intracellular components and molecular
mechanisms.
5. Conclusions and future perspective
Traditional Chinese medicines have been effectively
used to cure various diseases. Thus, investigating the molecular
mechanisms underlying their activities is highly warranted. As a
biologically active ingredient derived from the Chinese herb,
Tripterygium wilfordii Hook F., recent studies have
demonstrated that triptolide exerts effects on the expression of a
series of endogenous miRNAs, which in turn employ several
downstream cellular factors to achieve its multiple pharmacological
activities, highlighting miRNAs as critical mediators for
triptolide-induced effects (18,20,21,23,34-43).
However, determining the exact role of these miRNAs is limited by
the in vitro models offered by these studies. Haploid cells
are effective tools to study gene function due to having one set of
chromosomes (98,99). Thus, prospective studies will aim to
use haploid cells to screen novel miRNAs for specific functions
following treatment with triptolide, and additional evidence from
in vivo experiments will validate the conclusions. In
addition, regulation of miRNA expression is usually cell- or
tissue-specific, and as precise base pairing between miRNA and
their corresponding mRNA targets is not required. A single miRNA
may simultaneously target several mRNAs, whereas one mRNA can be
regulated by different miRNAs (28,30,33).
Triptolide may affect PTEN expression by regulating the expression
of several miRNAs, including miR-21 (62,63),
miR-181a (66), miR-188-5p
(38), miR-144(35), miR-141-3p (75), miR-17-92 and miR-106b-25 clusters
(24). miR-21 is a common target
when triptolide controls the expression of both TLR4 and PTEN
(40,41,62,63).
Thus, the intricate complexity and integration of the activity of
miRNAs present challenges in identifying their function and
regulatory pathways. Supplementary bioinformatics tools may provide
integrated analyses of these complicated networks that participate
in the pharmacological activities of triptolide. Prospective
studies are required to investigate whether miRNAs play roles in
other pharmacological activities of triptolide.
In conclusion, specific miRNAs have been identified
as primary targets when triptolide displays multiple
pharmacological effects. These findings also suggest that
triptolide can serve as a novel molecular probe for studying
miRNAs. A comprehensive understanding of the regulatory pathways
and specific functions of these miRNAs will help determine the
underlying molecular mechanisms of triptolide and provide more
effective strategies for drug development and disease
treatment.
Acknowledgements
Not applicable.
Funding
The present review was supported by the Applied Basic Research
Program of Science and Technology Department of Shanxi Province
(grant no. 201801D121357), the Research Project of Shanxi
Provincial Health and Family Planning Commission (grant no.
201601114) and the Research Fund from Shanxi Key Laboratory of
Innovative Drug for Treatment of Serious Diseases Basing on the
Chronic Inflammation (grant no. SXIDL-2018-08).
Availability of data and materials
Not applicable.
Authors' contributions
YW conceived and supervised the present study. KZ
drafted the initial manuscript. YC, BH, RL and YW reviewed the
manuscript for important intellectual content. All authors have
read and approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Auyeung KK, Han QB and Ko JK:
Astragalus membranaceus: A review of its protection against
inflammation and gastrointestinal cancers. Am J Chin Med. 44:1–22.
2016.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Wang Z, Xia Q, Liu X, Liu W, Huang W, Mei
X, Luo J, Shan M, Lin R, Zou D and Ma Z: Phytochemistry,
pharmacology, quality control and future research of Forsythia
suspensa (Thunb.) Vahl: A review. J Ethnopharmacol. 210:318–339.
2018.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Shi JF, Luo YY, Li JX, Luo RF, Chen L, Li
J, Zhang JM and Fu CM: Research progress on anti-tumor effects and
mechanisms of triptolide and its combined application. China J Chin
Mater Med. 44:3391–3398. 2019.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Noel P, Von Hoff DD, Saluja AK, Velagapudi
M, Borazanci E and Han M: Triptolide and its derivatives as cancer
therapies. Trends Pharmacol Sci. 40:327–341. 2019.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Yan P and Sun X: Triptolide: A new star
for treating human malignancies. J Cancer Res Ther. 14
(Suppl):S271–S275. 2018.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Huang G, Yuan K, Zhu Q, Zhang S, Lu Q, Zhu
M, Sheng H, Yu R, Luo G and Xu A: Triptolide inhibits the
inflammatory activities of neutrophils to ameliorate chronic
arthritis. Mol Immunol. 101:210–220. 2018.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Huang SH, Lin GJ, Chu CH, Yu JC, Chen TW,
Chen YW, Chien MW, Chu CC and Sytwu HK: Triptolide ameliorates
autoimmune diabetes and prolongs islet graft survival in nonobese
diabetic mice. Pancreas. 42:442–451. 2013.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Zheng CX, Chen ZH, Zeng CH, Qin WS, Li LS
and Liu ZH: Triptolide protects podocytes from puromycin
aminonucleoside induced injury in vivo and in vitro. Kidney Int.
74:596–612. 2008.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Yu H, Shi L, Zhao S, Sun Y, Gao Y, Sun Y
and Qi G: Triptolide attenuates myocardial ischemia/reperfusion
injuries in rats by inducing the activation of Nrf2/HO-1 defense
pathway. Cardiovasc Toxicol. 16:325–335. 2016.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Xi C, Peng S, Wu Z, Zhou Q and Zhou J:
Toxicity of triptolide and the molecular mechanisms involved.
Biomed Pharmacother. 90:531–541. 2017.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Chen SR, Dai Y, Zhao J, Lin L and Wang Y
and Wang Y: A mechanistic overview of triptolide and celestrol,
natural products from Tripterygium wilfordii Hook F. Front
Pharmacol. 9(104)2018.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Yuan K, Li X, Lu Q, Zhu Q, Jiang H, Wang
T, Huang G and Xu A: Application and mechanisms of triptolide in
the treatment of inflammatory diseases-a review. Front Pharmacol.
10(1469)2019.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Wei YM, Wang YH, Xue HQ, Luan ZH, Liu BW
and Ren JH: Triptolide, A potential autophagy modulator. Chin J
Integr Med. 25:233–240. 2019.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Jin W, Wang J, Liu CP, Wang HW and Xu RM:
Structural basis for pri-miRNA recognition by Drosha. Mol Cell.
78:423–433. 2020.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Olejniczak M, Kotowska-Zimmer A and
Krzyzosiak W: Stress-induced changes in miRNA biogenesis and
functioning. Cell Mol Life Sci. 75:177–191. 2018.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Dexheimer PJ and Cochella L: MicroRNAs:
From mechanism to organism. Front Cell Dev Biol.
8(409)2020.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Rupaimoole R and Slack FJ: MicroRNA
therapeutics: Towards a new era for the management of cancer and
other diseases. Nat Rev Drug Discov. 16:203–222. 2017.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Liu Q, Wang W, Li F, Yu D, Xu C and Hu H:
Triptolide inhibits breast cancer cell metastasis through inducing
the expression of miR-146a, a negative regulator of Rho GTPase.
Oncol Res. 27:1043–1050. 2019.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Peng A, Huang X, Liu R, Wang X and Zhuang
J: Triptolide inhibits the inflammatory response of monocytes from
rheumatoid arthritis patients by regulating miR-155. Chin J Cell
Mol Immunol. 30:635–638. 2014.PubMed/NCBI
|
|
20
|
Feng Y, Zheng C, Zhang Y, Xing C, Cai W,
Li R, Chen J and Duan Y: Triptolide inhibits preformed
fibril-induced microglial activation by targeting the
microRNA155-5p/SHIP1 pathway. Oxid Med Cell Longev.
2019(6527638)2019.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Zhao X, Tang X, Yan Q, Song H, Li Z, Wang
D, Chen H and Sun L: Triptolide ameliorates lupus via the induction
of miR-125a-5p mediating Treg upregulation. Int Immunopharmacol.
71:14–21. 2019.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Yang Q, Sun M, Chen Y, Lu Y, Ye Y, Song H,
Xu X, Shi S and Wang J: Triptolide protects podocytes from
TGF-β-induced injury by preventing miR-30 downregulation. Am J
Transl Res. 9:5150–5159. 2017.PubMed/NCBI
|
|
23
|
Wang SR, Chen X, Ling S, Ni RZ, Guo H and
Xu JW: MicroRNA expression, targeting, release dynamics and
early-warning biomarkers in acute cardiotoxicity induced by
triptolide in rats. Biomed Pharmacother. 111:1467–1477.
2019.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Li SG, Shi QW, Yuan LY, Qin LP, Wang Y,
Miao YQ, Chen Z, Ling CQ and Qin WX: C-Myc-dependent repression of
two oncogenic miRNA clusters contributes to triptolide-induced cell
death in hepatocellular carcinoma cells. J Exp Clin Cancer Res.
37(51)2018.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Reno TA, Kim JY and Raz DJ: Triptolide
inhibits lung cancer cell migration, invasion, and metastasis. Ann
Thorac Surg. 100:1817–1825. 2015.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Kalla R, Ventham NT, Kennedy NA, Quintana
JF, Nimmo ER, Buck AH and Satsangi J: MicroRNAs: New players in
IBD. Gut. 64:504–517. 2015.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Michlewski G and Caceres JF:
Post-transcriptional control of miRNA biogenesis. RNA. 25:1–16.
2019.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Saliminejad K, Khorram Khorshid HR,
Soleymani Fard S and Ghaffari SH: An overview of microRNAs:
Biology, functions, therapeutics, and analysis methods. J Cell
Physiol. 234:5451–5465. 2019.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Vishnoi A and Rani S: miRNA biogenesis and
regulation of diseases: An overview. Methods Mol Biol. 1509:1–10.
2017.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Yates LA, Norbury CJ and Gilbert RJ: The
long and short of microRNA. Cell. 153:516–519. 2013.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Turner MJ and Slack FJ: Transcriptional
control of microRNA expression in C elegans: Promoting
better understanding. RNA Biol. 6:49–53. 2009.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Kedde M, Strasser MJ, Boldajipour B, Oude
Vrielink JA, Slanchev K, le Sage C, Nagel R, Voorhoeve PM, van
Duijse J, Orom UA, et al: RNA-binding protein Dnd1 inhibits
microRNA access to target mRNA. Cell. 131:1273–1286.
2007.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Krol J, Loedige I and Filipowicz W: The
widespread regulation of microRNA biogenesis, function and decay.
Nat Rev Genet. 11:597–610. 2010.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Qi Y and Li J: Triptolide inhibits the
growth and migration of colon carcinoma cells by down-regulation of
miR-191. Exp Mol Pathol. 107:23–31. 2019.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Wu CW, Wang SG, Lin ML and Chen SS:
Downregulation of miR-144 by triptolide enhanced p85α-PTEN complex
formation causing S phase arrest of human nasopharyngeal carcinoma
cells. Eur J Pharmacol. 855:137–148. 2019.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Chen M, Wang JM, Wang D, Wu R and Hou HW:
Triptolide inhibits migration and proliferation of fibroblasts from
ileocolonic anastomosis of patients with Crohn's disease via
regulating the miR161/HSP70 pathway. Mol Med Rep. 19:4841–4851.
2019.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Qian K, Zhang L and Shi K: Triptolide
prevents osteoarthritis via inhibiting hsa-miR-20b.
Inflammopharmacology. 27:109–119. 2019.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Xue M, Cheng Y, Han F, Chang Y, Yang Y, Li
X and Chen L, Lu Y, Sun B and Chen L: Triptolide attenuates renal
tubular epithelial-mesenchymal transition via the
miR-188-5p-mediated PI3K/AKT pathway in diabetic kidney disease.
Int J Biol Sci. 14:1545–1557. 2018.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Han F, Wang S, Chang Y, Li C, Yang J, Han
Z, Chang B, Sun B and Chen L: Triptolide prevents extracellular
matrix accumulation in experimental diabetic kidney disease by
targeting microRNA-137/Notch1 pathway. J Cell Physiol.
233:2225–2237. 2018.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Cao Y, Guo Y, Wang Y, Cao Y, Zong R, Huang
C and Liu J: Drug-containing serum of Xinfeng capsules protect
against H9C2 from death by enhancing miRNA-21 and inhibiting
toll-like receptor 4/phosphorylated p-38 (p-p38)/p-p65 signaling
pathway and proinflammatory cytokines expression. J Tradit Chin
Med. 38:359–365. 2018.PubMed/NCBI
|
|
41
|
Cao YX, Huang D, Liu J, Zong RK, Wan L,
Huang CB, Zhang WD and Wang Y: A novel chinese medicine, xinfeng
capsule, modulates proinflammatory cytokines via regulating the
toll-like receptor 4 (TLR4)/Mitogen-activated protein kinase
(MAPK)/Nuclear Kappa B (NF-κB) signaling pathway in an adjuvant
arthritis rat model. Med Sci Monit. 25:6767–6774. 2019.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Xu X, Tian L and Zhang Z: Triptolide
inhibits angiogenesis in microvascular endothelial cells through
regulation of miR-92a. J Physiol Biochem. 75:573–583.
2019.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Liang H, Zhang S and Li Z: Ginsenoside Rg3
protects mouse leydig cells against triptolide by downregulation of
miR-26a. Drug Des Devel Ther. 13:2057–2066. 2019.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Mamoori A, Gopalan V and Lam AK: Role of
miR-193a in cancer: Complexity and factors control the patterns of
its expression. Curr Cancer Drug Targets. 18:618–628.
2018.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Svoronos AA, Engelman DM and Slack FJ:
OncomiR or tumor suppressor? The duplicity of microRNAs in cancer.
Cancer Res. 76:3666–3670. 2016.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Meng HT, Zhu L, Ni WM, You LS, Jin J and
Qian WB: Triptolide inhibits the proliferation of cells from
lymphocytic leukemic cell lines in association with downregulation
of NF-kappaB activity and miR-16-1*. Acta Pharmacol Sin.
32:503–511. 2011.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Xiang W, Yang CY and Bai L: Mcl-1
inhibiton in cancer treatment. Onco Targets Ther. 11:7301–7314.
2018.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Chen Z, Sangwan V, Banerjee S, Mackenzie
T, Dudeja V, Li X, Wang H, Vickers SM and Saluja AK: miR-204
mediated loss of Myeloid cell leukemia-1 results in pancreatic
cancer cell death. Mol Cancer. 12(105)2013.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Roufayel R and Kadry S: Molecular
chaperone HSP70 and key regulators of apoptosis - a review. Curr
Mol Med. 19:315–325. 2019.PubMed/NCBI View Article : Google Scholar
|
|
50
|
MacKenzie TN, Mujumdar N, Banerjee S,
Sangwan V, Sarver A, Vickers S, Subramanian S and Saluja AK:
Triptolide induces the expression of miR-142-3p: A negative
regulator of heat shock protein 70 and pancreatic cancer cell
proliferation. Mol Cancer Ther. 12:1266–1275. 2013.PubMed/NCBI View Article : Google Scholar
|
|
51
|
Hang S, Wang X and Li H: Triptolide
inhibits viability and migration while promotes apoptosis in
nephroblastoma cells by regulation of miR-193b-3p. Exp Mol Pathol.
108:80–88. 2019.PubMed/NCBI View Article : Google Scholar
|
|
52
|
Zhang H, Li H, Liu Z, Ge A, Guo E, Liu S
and Chen Z: Triptolide inhibits the proliferation and migration of
medulloblastoma Daoy cells by upregulation of microRNA-138. J Cell
Biochem. 119:9866–9877. 2018.PubMed/NCBI View Article : Google Scholar
|
|
53
|
Yao C, Li H and Zhang W: Triptolide
inhibits benign prostatic epithelium viability and migration and
induces apoptosis via upregulation of microRNA-218. Int J
Immunopathol Pharmacol. 32(2058738418812349)2018.PubMed/NCBI View Article : Google Scholar
|
|
54
|
Liu Q, Wang W, Yang X, Zhao D, Li F and
Wang H: miRNA-146a inhibits cell migration and invasion by
targeting RhoA in breast cancer. Oncol Rep. 36:189–196.
2016.PubMed/NCBI View Article : Google Scholar
|
|
55
|
Hu H, Tang J, Liu C and Cen Y: miR-23b
promotes the migration of keratinocytes through downregulating
TIMP3. J Surg Res. 254:102–109. 2020.PubMed/NCBI View Article : Google Scholar
|
|
56
|
Wang J, Zhou F, Yin L, Zhao L, Zhang Y and
Wang J: MicroRNA-199b targets the regulation of ZEB1 expression to
inhibit cell proliferation, migration and invasion in non-small
cell lung cancer. Mol Med Rep. 16:5007–5014. 2017.PubMed/NCBI View Article : Google Scholar
|
|
57
|
Charles S, Raj V, Arokiaraj J and Mala K:
Caveolin1/protein arginine methyltransferase1/sirtuin1 axis as a
potential target against endothelial dysfunction. Pharmacol Res.
119:1–11. 2017.PubMed/NCBI View Article : Google Scholar
|
|
58
|
Philips BJ, Kumar A, Burki S, Ryan JP,
Noda K and D'Cunha J: Triptolide-induced apoptosis in non-small
cell lung cancer via a novel miR204-5p/Caveolin-1/Akt-mediated
pathway. Oncotarget. 11:2793–2806. 2020.PubMed/NCBI View Article : Google Scholar
|
|
59
|
Cao Y: Adipocyte and lipid metabolism in
cancer drug resistance. J Clin Invest. 129:3006–3017.
2019.PubMed/NCBI View Article : Google Scholar
|
|
60
|
Gomes BC, Rueff J and Rodrigues AS:
MicroRNAs and cancer drug resistance. Methods Mol Biol.
1395:137–162. 2016.PubMed/NCBI View Article : Google Scholar
|
|
61
|
Pfeffer SR, Yang CH and Preffer LM: The
role of miR-21 in cancer. Drug Dev Res. 76:270–277. 2015.PubMed/NCBI View Article : Google Scholar
|
|
62
|
Li X, Zang A, Jia Y, Zhang J, Fan W, Feng
J, Duan M, Zhang L, Huo R, Jiao J and Zhu X: Triptolide reduces
proliferation and enhances apoptosis of human non-small cell lung
cancer cells through PTEN by targeting miR-21. Mol Med Rep.
13:2763–2768. 2016.PubMed/NCBI View Article : Google Scholar
|
|
63
|
Li H, Hui L, Xu W, Shen H, Chen Q, Long L
and Zhu X: Triptolide modulates the sensitivity of K562/A02 cells
to adriamycin by regulating miR-21 expression. Pharm Biol.
50:1233–1240. 2012.PubMed/NCBI View Article : Google Scholar
|
|
64
|
Wang R, Ma X, Su S and Liu Y: Triptolide
antagonized the cisplatin resistance in human ovarian cancer cell
line A2780/CP70 via hsa-mir-6751. Future Med Chem. 10:1947–1955.
2018.PubMed/NCBI View Article : Google Scholar
|
|
65
|
Huang X, Yang M and Jin J: Triptolide
enhances the sensitivity of multiple myeloma cells to dexamethasone
via microRNAs. Leuk Lymphoma. 53:1188–1195. 2012.PubMed/NCBI View Article : Google Scholar
|
|
66
|
Jiang C, Fang X, Zhang H, Wang X, Li M,
Jiang W, Tian F, Zhu L and Bian Z: Triptolide inhibits the growth
of osteosarcoma by regulating microRNA-181a via targeting PTEN gene
in vivo and vitro. Tumour Biol. 39(1010428317697556)2017.PubMed/NCBI View Article : Google Scholar
|
|
67
|
Jiang J, Song X, Yang J, Lei K, Ni Y, Zhou
F and Sun L: Triptolide inhibits proliferation and migration of
human neuroblastoma SH-SY5Y cells by upregulating MicroRNA-181a.
Oncol Res. 26:1235–1243. 2018.PubMed/NCBI View Article : Google Scholar
|
|
68
|
O'Connell RM, Rao DS and Baltimore D:
microRNA regulation of inflammatory responses. Annu Rev Immunol.
30:295–312. 2012.PubMed/NCBI View Article : Google Scholar
|
|
69
|
Mahesh G and Biswas R: miRNA-155: A master
regulator of inflammation. J Interferon Cytokine Res. 39:321–330.
2019.PubMed/NCBI View Article : Google Scholar
|
|
70
|
Matta R, Wang X, Ge H, Ray W, Nelin LD and
Liu Y: Triptolide induces anti-inflammatory cellular responses. Am
J Transl Res. 1:267–282. 2009.PubMed/NCBI
|
|
71
|
Tang H, Mao J, Ye X, Zhang F, Kerr WG,
Zheng T and Zhu Z: SHIP-1, a target of miR-155, regulates
endothelial cell responses in lung fibrosis. FASEB J. 34:2011–2023.
2020.PubMed/NCBI View Article : Google Scholar
|
|
72
|
Wu R, Li Y, Guo Z, Gong J, Zhu W, Li N and
Li J: Triptolide ameliorates ileocolonic anastomosis inflammation
in IL-10 deficient mice by mechanism involving suppression of
miR-155/SHIP-1 signaling pathway. Mol Immunol. 56:340–346.
2013.PubMed/NCBI View Article : Google Scholar
|
|
73
|
Hou HW, Wang JM, Wang D, Wu R and Ji ZL:
Triptolide exerts protective effects against fibrosis following
ileocolonic anastomosis by mechanisms involving the miR-16-1/HSP70
pathway in IL-10-deficient mice. Int J Mol Med. 40:337–346.
2017.PubMed/NCBI View Article : Google Scholar
|
|
74
|
Lee GR: The balance of Th17 versus Treg
cells in autoimmunity. Int J Mol Sci. 19(730)2018.PubMed/NCBI View Article : Google Scholar
|
|
75
|
Li XY, Wang SS, Han Z, Han F, Chang YP,
Yang Y, Xue M, Sun B and Chen LM: Triptolide restores autophagy to
alleviate diabetic renal fibrosis through the
miR-141-3p/PTEN/Akt/mTOR pathway. Mol Ther Nucleic Acids. 9:48–56.
2017.PubMed/NCBI View Article : Google Scholar
|
|
76
|
Jiang CB, Wei MG, Tu Y, Zhu H, Li CQ, Jing
WM and Sun W: Triptolide attenuates podocyte injury by regulating
expression of miRNA-344b-3p and miRNA-30b-3p in rats with
adriamycin-induced nephropathy. Evid Based Complement Alternat Med.
2015(107814)2015.PubMed/NCBI View Article : Google Scholar
|
|
77
|
Ignarski M, Islam R and Muller RU: Long
non-coding RNAs in kidney disease. Int J Mol Sci.
20(3276)2019.PubMed/NCBI View Article : Google Scholar
|
|
78
|
Bonauer A, Carmona G, Iwasaki M, Mione M,
Koyanagi M, Fischer A, Burchfield J, Fox H, Doebele C, Ohtani K, et
al: MicroRNA-92a controls angiogenesis and functional recovery of
ischemic tissues in mice. Science. 324:1710–1713. 2009.PubMed/NCBI View Article : Google Scholar
|
|
79
|
Zhang L, Zhou M, Qin G, Weintraub NL and
Tang Y: miR-92a regulates viability and angiogenesis of endothelial
cells under oxidative stress. Biochem Biophys Res Commun.
446:952–958. 2014.PubMed/NCBI View Article : Google Scholar
|
|
80
|
Marrone AK, Beland FA and Pogribny IP: The
role for microRNAs in drug toxicity and in safety assessment.
Expert Opin Drug Metab Toxicol. 11:601–611. 2015.PubMed/NCBI View Article : Google Scholar
|
|
81
|
Ichihara S, Li P, Mise N, Suzuki Y, Izuoka
K, Nakajima T, Gonzalez F and Ichihara G: Ablation of aryl
hydrocarbon receptor promotes angiotensin II-induced cardiac
fibrosis through enhanced c-Jun/HIF-1α signaling. Arch Toxicol.
93:1543–1553. 2019.PubMed/NCBI View Article : Google Scholar
|
|
82
|
Nam HS, Hwang KS, Jeong YM, Ryu JI, Choi
TY, Bae MA, Son WC, You KH, Son HY and Kim CH: Expression of
miRNA-122 induced by liver toxicants in Zebrafish. Biomed Res Int.
2016(1473578)2016.PubMed/NCBI View Article : Google Scholar
|
|
83
|
Vliegenthart ADB, Wei C, Buckley C,
Berends C, de Potter CMJ, Schneemann S, Del Pozo J, Tucker C,
Mullins JJ, Webb DJ and Dear JW: Characterization of
triptolide-induced hepatotoxicity by imaging and transcriptomics in
a novel Zebrafish model. Toxicol Sci. 159:380–391. 2017.PubMed/NCBI View Article : Google Scholar
|
|
84
|
Cheng B, Zhu Q, Lin W and Wang L:
MicroRNA-122 inhibits epithelial-mesenchymal transition of hepatic
stellate cells induced by the TGF-β1/Smad signaling pathway. Exp
Ther Med. 17:284–290. 2019.PubMed/NCBI View Article : Google Scholar
|
|
85
|
Yuan Y, Tong L and Wu S: microRNA and
NF-kappa B. Adv Exp Med Biol. 887:157–170. 2015.PubMed/NCBI View Article : Google Scholar
|
|
86
|
Ichikawa D, Komatsu S, Konishi H and
Otsuji E: Circulating microRNA in digestive tract cancers.
Gastroenterology. 1421074–1078. (e1071)2012.PubMed/NCBI View Article : Google Scholar
|
|
87
|
Titov DV, Gilman B, He QL, Bhat S, Low WK,
Dang Y, Smeaton M, Demain AL, Miller PS, Kugel JF, et al: XPB, a
subunit of TFIIH, is a target of the natural product triptolide.
Nat Chem Biol. 7:182–188. 2011.PubMed/NCBI View Article : Google Scholar
|
|
88
|
Chauhan AK, Li P, Sun Y, Wani G, Zhu Q and
Wani AA: Spironolactone-induced XPB degradtion requires TFIIH
integrity and ubiquitin-selective segregase VCP/p97. Cell Cycle.
20:81–95. 2021.PubMed/NCBI View Article : Google Scholar
|
|
89
|
He QL, Titov DV, Li J, Tan M, Ye Z, Zhao
Y, Romo D and Liu JO: Covalent modification of a cysteine residue
in the XPB subunit of the general transcription factor TFIIH
through single epoxide cleavage of the transcription inhibitor
triptolide. Angew Chem Int Ed Engl. 54:1859–1863. 2015.PubMed/NCBI View Article : Google Scholar
|
|
90
|
Manzo SG, Zhou ZL, Wang YQ, Marinello J,
He JX, Li YC, Ding J, Capranico G and Miao ZH: Natural product
triptolide mediates cancer cell death by triggering CDK7-dependent
degradation of RNA polymerase II. Cancer Res. 72:5363–5373.
2012.PubMed/NCBI View Article : Google Scholar
|
|
91
|
Abascal-Palacios G, Ramsay EP, Beuron F,
Morris E and Vannini A: Structural basis of RNA polymerase III
transcription initiation. Nature. 553:301–306. 2018.PubMed/NCBI View Article : Google Scholar
|
|
92
|
Liang X, Xie R, Su J, Ye B, Wei S, Liang
Z, Bai R, Chen Z, Li Z and Gao X: Inhibition of RNA polymerase III
transcription by Triptolide attenuates colorectal tumorigenesis. J
Exp Clin Cancer Res. 38(217)2019.PubMed/NCBI View Article : Google Scholar
|
|
93
|
Yamagishi M and Uchimaru K: Targeting EZH2
in cancer therapy. Curr Opin Oncol. 29:375–381. 2017.PubMed/NCBI View Article : Google Scholar
|
|
94
|
Ihira K, Dong P, Xiong Y, Watari H, Konno
Y, Hanley SJ, Noguchi M, Hirata N, Suizu F, Yamada T, et al: EZH2
inhibition suppresses endometrial cancer progression via
miR-361/Twist axis. Oncotarget. 8:13509–13520. 2017.PubMed/NCBI View Article : Google Scholar
|
|
95
|
Tamgue O, Chai CS, Hao L, Zambe JC, Huang
WW, Zhang B, Lei M and Wei YM: Triptolide inhibits histone
methyltransferase EZH2 and modulates the expression of its target
genes in prostate cancer cells. Asian Pac J Cancer Prev.
14:5663–5669. 2013.PubMed/NCBI View Article : Google Scholar
|
|
96
|
Akkoc Y and Gozuacik D: MicroRNAs as major
regulators of the autophagy pathway. Biochim Biophys Acta Mol Cell
Res 1867: Kappa B, 2020.
|
|
97
|
Gibbings D, Mostowy S, Jay F, Schwab Y,
Cossart P and Voinnet O: Selective autophagy degrades DICER and
AGO2 and regulates miRNA activity. Nat Cell Biol. 14:1314–1321.
2012.PubMed/NCBI View Article : Google Scholar
|
|
98
|
Peng K, Li X, Wu C, Wang Y, Yu J, Zhang J,
Gao Q, Zhang W, Zhang Q, Fan Y, et al: Derivation of haploid
trophoblast stem cells via conversion in vitro. iScience.
11:508–518. 2019.PubMed/NCBI View Article : Google Scholar
|
|
99
|
Wang H, Zhang W, Yu J, Wu C, Gao Q, Li X,
Li Y, Zhang J, Tian Y, Tan T, et al: Genetic screening and
multipotency in rhesus monkey haploid neural progenitor cells.
Development. 145(dev160531)2018.PubMed/NCBI View Article : Google Scholar
|
|
100
|
Huang Y, Zhu N, Chen T, Chen W, Kong J,
Zheng W and Ruan J: Triptolide suppressed the microglia activation
to improve spinal cord injury through miR-96/IKKβ/NF-κB pathway.
Spine (Phila Pa 1976). 44:E707–E714. 2019.PubMed/NCBI View Article : Google Scholar
|















