Introduction
In recent years, with ongoing lifestyle and diet
changes, population aging, and the rise in rates of obesity, the
incidence of diabetes mellitus (DM) is increasing at an alarming
rate (1). One of the common
complications of DM is diabetic cystopathy (DCP) and, even in
patients with stable blood glucose control, the incidence of DCP is
as high as 25% (2). DCP is
insidious and has an asymptomatic progressive development, which is
easily ignored by clinicians and diabetic patients before
ultimately causing chronic urinary retention and urinary
incontinence. DCP mainly manifests in affected patients as reduced
bladder contractility and decreased urination. Upon progressing to
the end stage, the disease is irreversible and is accompanied by
urinary tract infections and reflux hydronephrosis, which seriously
affect the quality of life of patients and may even be
life-threatening (3).
The exact pathogenesis of DCP remains inadequately
understood to date and the condition is mainly attributed to
factors, including peripheral autonomic neuropathy and impaired
detrusor function of the bladder caused by DM. An increasing number
of studies have suggested that certain signaling pathways
affiliated with neurotransmitter-receptor binding serve an
important role in elements of DCP, including the levels of peptide
energy, nitrogen energy, sputum energy, serotonin, endothelin,
gamma-aminobutyric acid and prostaglandins. Changes in the levels
or activity of corresponding neurotransmitters and receptors may
trigger bladder urethral dysfunction.
At present, although different conventional medical
treatments are available for DCP, the problem of the high
recurrence rate among patients cannot be satisfactorily solved.
Cystostomy is considered an effective treatment for cystopathy but
it seriously affects the quality of life of patients (4). It is generally believed that medical
nutrition therapy is an important part of DM management (5). Previous guidelines and research have
focused on the type and amount of carbohydrates consumed. However,
how other food ingredients, including caffeine, affect DM remains
unknown. Caffeine is the main ingredient in coffee and tea, which
are consumed daily by numerous people. The association between
caffeine and human health has long been of concern to scientists. A
recent study suggested that caffeine may improve bladder function
by promoting increased expression levels of nerve growth factor
(NGF) and c-fos, improving bladder contractility, and repairing
damaged bladder nerves (6), while
our previous study reported that caffeine may improve bladder
dysfunction in rats with DM (7).
However, the underlying mechanism of this remains unclear. Another
previous study has also indicated that calcitonin gene-related
peptides (CGRP) are the main transmitters affiliated with bladder
sensory nerves; specifically, the content of CGRP in the bladder
wall of diabetic rats, particularly in the submucosal plexus and
the CGRP nerve distribution, were significantly decreased,
suggesting that CGRP serves a key role in the development of DCP
(8).
Therefore, we hypothesized in the present study that
caffeine may improve bladder function in rats with DM by protecting
bladder sensory neurons and promoting the release of related
neurotransmitters.
Materials and methods
Animal models
The present study was approved by the Ethical
Committee on Animal Experiment Committee of Nanjing Medical
University (Nanjing, China). The animal models were prepared using
a protocol described previously by WenBo et al (9). Female Sprague Dawley (SD) rats (n=64;
weight, 200-220 g) were purchased from Nanjing Medical University's
Animal Experiment Center. All rats were housed under similar
conditions (temperature, 22±2˚C; relative humidity, 50±5%;
light/dark cycle, 12-h) with free access for food and water. A
total of 40 rats were intraperitoneally injected with 60 mg/kg
streptozotocin (STZ) (Sigma-Aldrich; Merck KGaA), while the other
24 rats were intraperitoneally injected with the same volume of
citric acid buffer. A level of fasting blood glucose (FBG) measured
three days after STZ injection of >16.7 mmol/l was considered to
indicate that the DM model had been successfully induced. In total,
31 DM rats were successfully induced, constituting 77.5% of the
total study population. Next, 24 diabetic rats were stratified as
the DM and DM plus caffeine treatment (DM + caffeine) groups (n=12
each), while 24 STZ-untreated rats were divided into the control
and caffeine groups (n=12 each). The rats in the DM + caffeine and
caffeine groups were treated with caffeine by oral gavage (10
mg/kg/day) for 16 weeks. The urination time, micturition interval,
bladder wet weight and maximum voiding pressure of rats were used
to measure bladder function as reported in our previous study
(10). Next, the rats were
sacrificed and the lumbosacral-segment dorsal root ganglion (DRG)
and bladder were prepared for analysis.
Hematoxylin and eosin (H&E)
staining for the DRG tissues
After the rats were sacrificed by being
intraperitoneally injected with 2% pentobarbital sodium (180
mg/kg), the spinal canal was opened posteriorly to expose the
spinal cord and the posterior roots of the spinal nerves, the white
and bright nodule on the posterior root of the spinal nerve near
the intervertebral foramen was the DRG. DRG specimens were fixed
with 10% neutral formalin at room temperature for 4 h, routinely
dehydrated, embedded, and cut into 4-µm-thick sections for H&E
staining at room temperature for 8 min. The result is shown in
Fig. S1.
ELISA
NGF, brain-derived neurotrophic factor (BDNF), and
CGRP levels in bladder tissue were measured using an ELISA kit for
rats; specifically, the NGF ELISA kit (cat. no. ab193736; Abcam),
BDNF ELISA kit (cat. no. ab213899; Abcam) or CGRP ELISA kit (cat.
no. 589001; Cayman Chemical Company), according to the
manufacturer's protocols. In brief, total proteins were extracted
from the bladder using lysis buffer (50 mM Tris-HCl, pH 7.5, 150 mM
NaCl, 1 mM EDTA, 1% NP-40, 0.5% deoxycholic acid, 0.1% SDS, 10
µl/ml protease inhibitor cocktail and 1 mM PMSF) and the
supernatants were collected by centrifuging at 4˚C at 12,000 x g
for 15 min. Subsequently, 96-well microplates were coated with 100
µl biotinylated primary antibodies mixed with 100 µl EIA buffer
provided in the kit, plus 100 µl standard and sample aliquots.
Plates were incubated for 2 h at 30˚C, followed by aspiration of
the samples and subsequent washing of them three times with wash
buffer. Next, 100 µl solution of streptavidin-horseradish
peroxidase conjugate was added to each well and incubated for 30
min at 30˚C, prior to washed again. Thereafter, 100 µl substrate
solution provided in the kits was added to each well and the plates
were incubated for 30 min at 30˚C. The optical density values were
read at 450 nm using a Biotek Synergy 2 plate reader (BioTek
Instruments, Inc.).
Terminal deoxynucleotidyl
transferase-mediated dUTP nick end labeling (TUNEL) assay
The apoptosis in DRG was measured using a TUNEL kit
for rats (cat. no. C1086; Beyotime Institute of Biotechnology,)
according to the manufacturer's protocols. Briefly, DRG specimens
were fixed with 10% neutral formalin at room temperature for 4 h,
routinely dehydrated with an ascending ethanol gradient and xylene,
embedded with paraffin, and cut into 4-µm-thick sections for TUNEL
assay. The section was incubated with 100 µl TUNEL reagent (5 µl
TdT enzyme, 45 µl fluorescent labeling solution, 50 µl TUNEL test
solution) in the dark at 37˚C for 60 min. Cell nuclei were
counter-stained with Hoechst 33342 (1:2,000, Thermo Fisher
Scientific, Inc.) for 30 min at room temperature. The sections were
observed and photographed with a fluorescent microscope (Leica
Microsystems GmbH) at x400 magnification. Under the microscope, six
fields were randomly selected to count the number of apoptotic
cells for each section.
Western blot analysis
Western blot analysis was applied to determine the
expression levels of B-cell lymphoma-2 (Bcl-2), Bcl-2-associated X
protein (Bax), caspase-3, cleaved caspase-3, caspase-9 and cleaved
caspase-9 proteins in DRG tissues. Tissues were homogenized in RIPA
lysis buffer (Thermo Fisher Scientific, Inc.) with protease
inhibitor cocktail (Roche Diagnostics), before the supernatants
were collected by centrifuging at 4˚C at 12,000 x g for 15 min. The
total protein was quantified by UV spectrophotometry and 50 µg
protein was separated by 12% SDS-PAGE. Next, they were transferred
onto the nitrocellulose membrane (EMD Millipore). The transferred
membrane was washed with rinse buffer, prior to being incubated
with blocking buffer (5% skimmed milk in rinse buffer) for 30 min
at room temperature. Subsequently, the membrane was incubated with
the following primary antibodies for 4 h at room temperature:
rabbit anti-Bcl-2 (cat. no. ab194583; dilution, 1:800; Abcam),
rabbit anti-Bax (cat. no. ab32503; dilution, 1:800; Abcam), rabbit
anti-cleaved caspase-3 (cat. no. ab214430; dilution, 1:1,000;
Abcam), rabbit anti-caspase-3 (cat. no. ab184787; dilution,
1:1,000; Abcam), rabbit anti-cleaved caspase-9 (cat. no. 20750;
dilution, 1:1,000; Cell Signaling Technology, Inc.), rabbit
anti-caspase-9 (cat. no. ab184786; dilution, 1:1,000; Abcam) and
mouse anti-β-actin (cat. no. A1978; dilution, 1:5,000;
Sigma-Aldrich; Merck KGaA). Next, each membrane was washed with
rinse buffer and incubated with the following secondary antibodies
for 1.5 h at room temperature: IRDye 680-conjugated
affinity-purified goat anti-mouse immunoglobulin G (cat. no.
610-144-121; dilution, 1:5,000; Rockland Immunochemicals) and IRDye
800-conjugated affinity-purified goat anti-rabbit immunoglobulin G
(cat. no. 611-145-122; dilution, 1:5,000; Rockland
Immunochemicals). The results were visualized using the Odyssey
laser scanning system (LI-COR Biosciences). The relative quantity
of the protein bands was determined using the Odyssey version 3.0
software program (LI-COR Biosciences).
Statistical analysis
The data obtained in the present study were
processed and analyzed using the SPSS version 21.0 software program
(IBM Corp.). The measurement data are expressed as the mean ±
standard deviation. Differences between groups were estimated by
one-way analysis of variance and compared using the Tukey's post
hoc test. P<0.05 was considered to indicate a statistically
significant difference.
Results
General condition of the rats and
urinary function of the bladder
As mentioned previously, 40 rats were prepared for
the DM model with STZ and, of these, 31 rats were successfully
induced for a success rate of 77.50%. There was no significant
difference in body weight between the control and caffeine groups
(P>0.05). However, compared with that in the control and
caffeine groups, the body weights of the rats in the DM and
DM+caffeine groups were significantly decreased, with the decrease
being more notable in the DM group, and the variations among the
groups were statistically significant (P<0.05). There was no
significant difference in FBG between the control and caffeine
groups (P>0.05) and no significant difference in FBG
between the DM and DM+caffeine groups (P>0.05). Compared
with that in the control and caffeine groups, the bladder wet
weight of the rats in the DM and DM+caffeine groups was
significantly increased, with the bladder wet weight in the
DM+caffeine group being lower than that in the DM group
(P<0.05). By contrast, there was no significant
difference in the urinary function between the control and caffeine
groups (P>0.05). Compared with that in the control and
caffeine groups, urinary function in the DM and DM+caffeine groups
was significantly decreased, but the urinary function of the
DM+caffeine group was significantly better than that of DM group
(P<0.05). The results are presented in Table I.
 | Table IParameters in the animals after 16
weeks. |
Table I
Parameters in the animals after 16
weeks.
| Parameters | Control | Caffeine | DM | DM + caffeine |
|---|
| Body weight, g | 303.92±10.52 | 302.92±10.40 |
190.33±8.02a,b |
215.00±22.54a-c |
| FBG, mmol/l | 4.60±1.27 | 4.59±1.11 |
25.33±3.07a,b |
23.13±5.96a,b |
| Bladder wet weight,
g | 0.20±0.02 | 0.20±0.03 |
0.40±0.07a,b |
0.28±0.04a-c |
| Urination time,
sec | 18.33±2.42 | 18.17±2.29 |
25.92±3.73a,b |
22.08±3.12a-c |
| Micturition interval,
sec | 40.67±3.87 | 41.50±3.66 |
236.33±63.94a,b |
73.67±13.18c |
| Maximum voiding
pressure, mmHg | 41.00±7.71 | 45.67±6.54 |
15.58±4.50a,b |
29.42±6.26a-c |
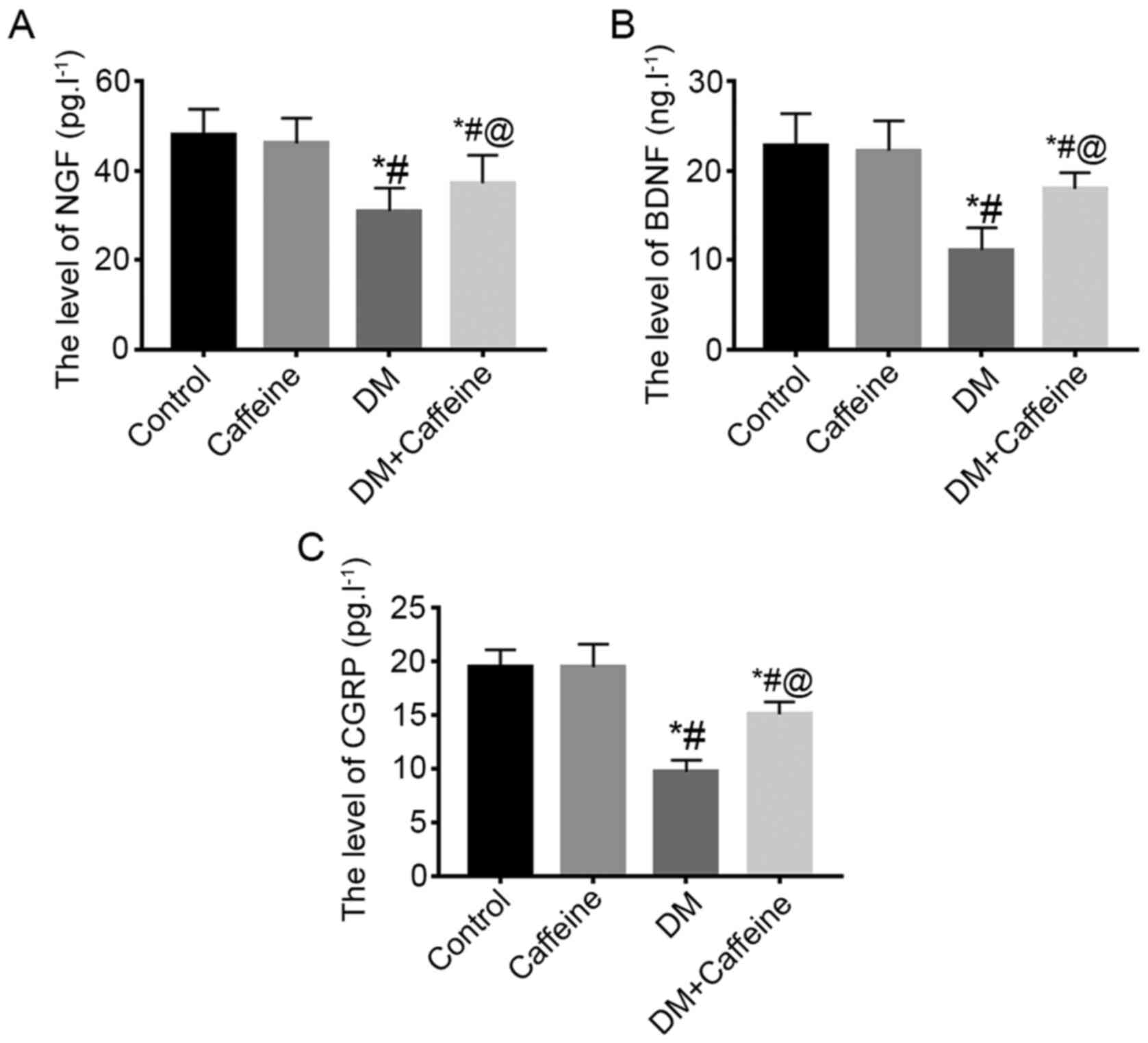
NGF, BDNF and CGRP levels in the
bladder tissue
ELISA results demonstrated that there were no
significant differences in the levels of NGF, BDNF and CGRP in the
bladder tissues between the control and caffeine groups
(P>0.05). Compared with that in the control and caffeine
groups, the levels of NGF, BDNF and CGRP in the bladder tissues of
the DM and DM+caffeine groups were significantly decreased, with
the levels of NGF, BDNF and CGRP in the bladder tissue of the
DM+caffeine group being higher than those in the DM group and the
differences being statistically significant (P<0.05;
Fig. 1).
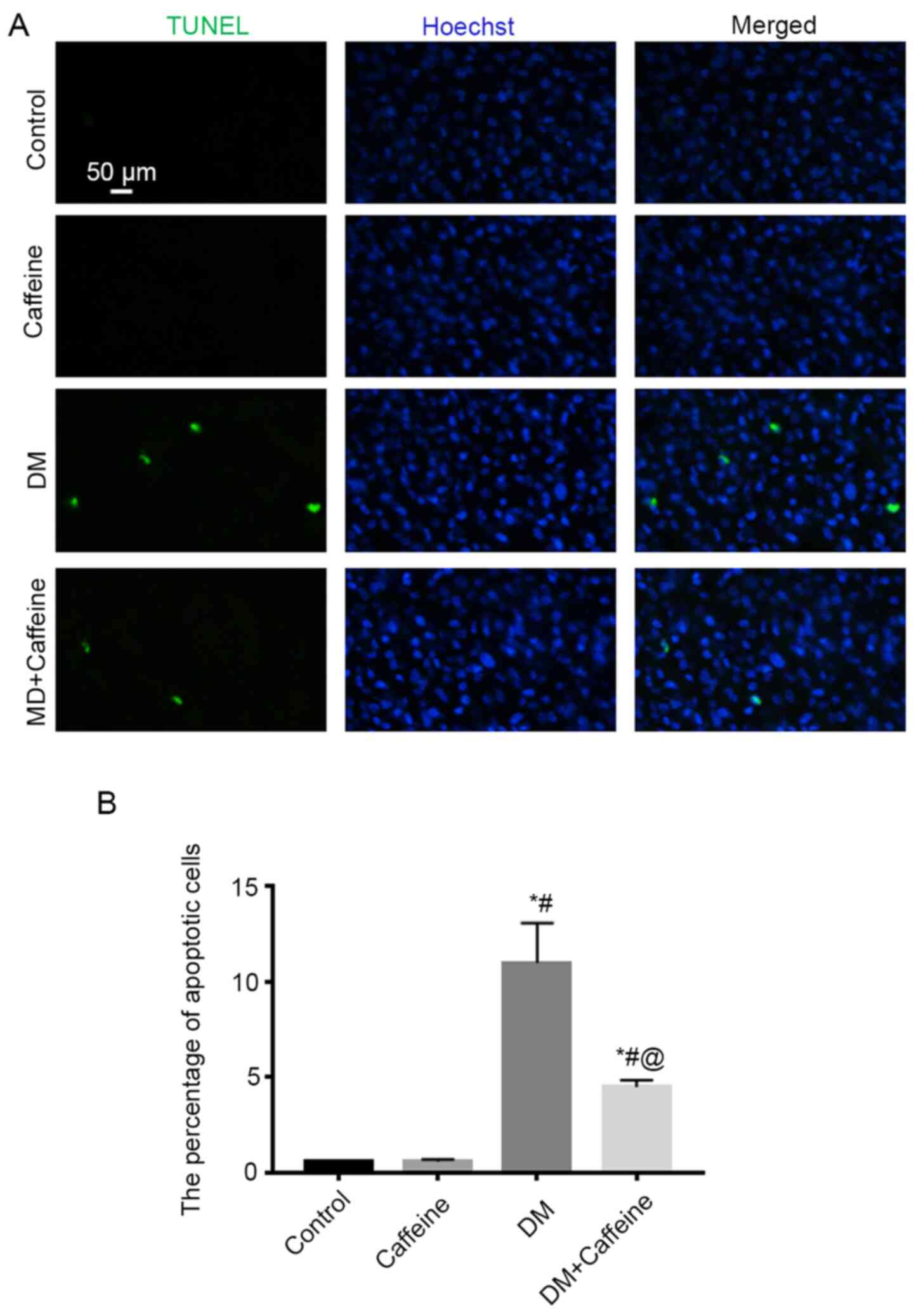
Cellular apoptosis in the DRG
TUNEL assay demonstrated that there were almost no
apoptotic cells present in the DRG in the control and caffeine
groups, while more apoptotic cells were observed in the DRG in the
DM and DM+caffeine groups. Furthermore, the apoptotic cell count in
the DRG in the DM+caffeine group was less than that in the DM group
and all differences were statistically significant
(P<0.05; Fig. 2).
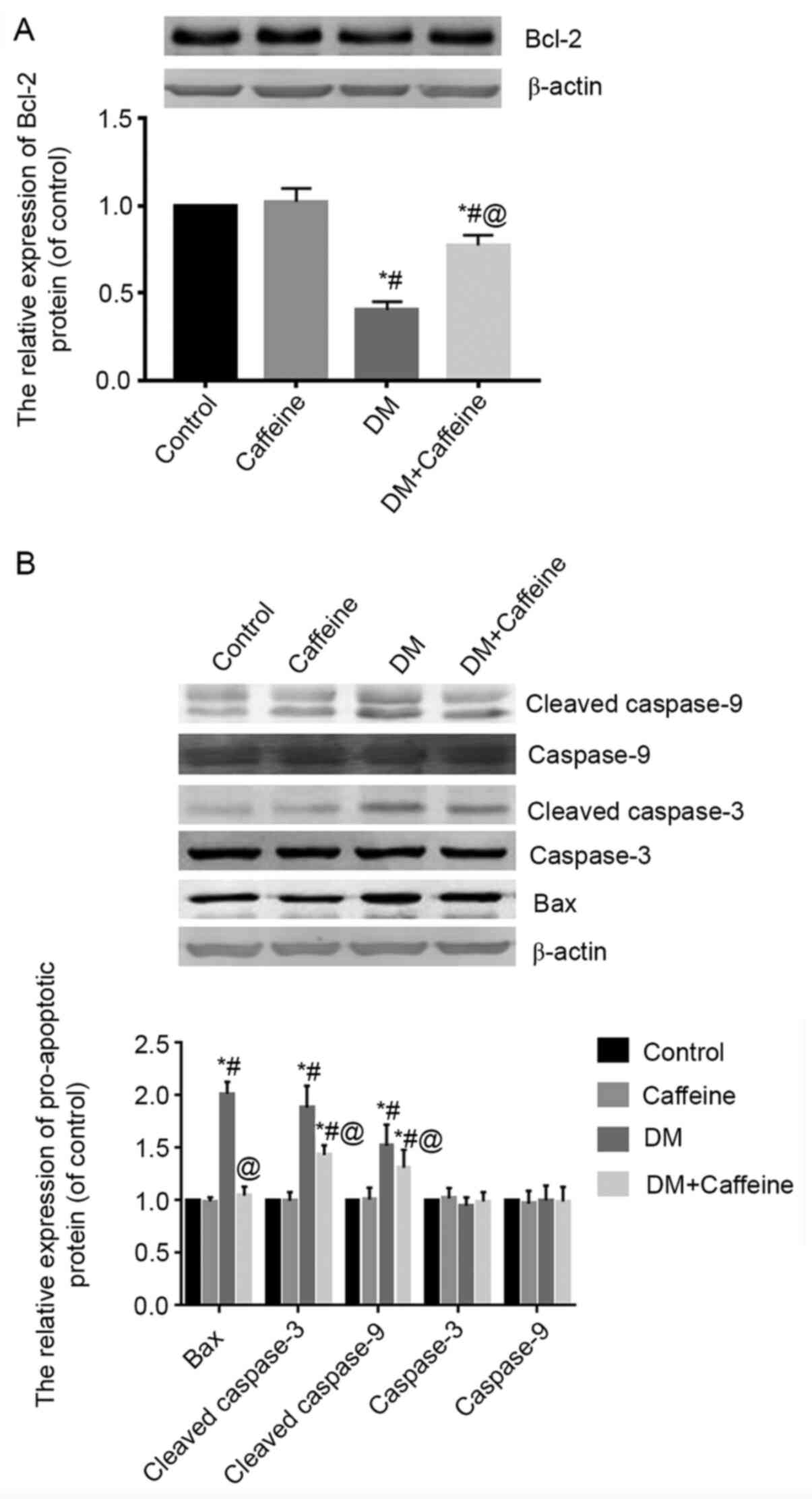
Expression of Bcl-2, Bax, caspase-3,
cleaved caspase-3, caspase-9 and cleaved caspase-9 proteins in the
DRG
Western blot analysis demonstrated that there was no
significant difference in the expression of Bcl-2 protein in the
DRG between the control and caffeine groups (P>0.05).
Compared with that in the control and caffeine groups, the relative
expression levels of Bcl-2 protein in the DRGs of the DM and
DM+caffeine groups were significantly decreased, while the
expression level of Bcl-2 protein in the DM+caffeine group was
higher than that in the DM group and the difference was
statistically significant (P<0.05; Fig. 3A).
The results of western blotting demonstrated that
the expression levels of Bax, cleaved caspase-3 and cleaved
caspase-9 proteins in the DRGs of the DM group and DM+caffeine
group were higher than those in the control and caffeine groups and
all differences were statistically significant (P<0.05).
Furthermore, the levels of Bax, cleaved caspase-3 and cleaved
caspase-9 proteins in the DRGs of the DM+caffeine group were
significantly lower than those in the DM group (P<0.05).
The differences of caspase-3 and caspase-9 protein levels among
groups were not statistically significant (P>0.05;
Fig. 3B).
Discussion
Caffeine is a methylxanthine and acts as an agonist
of the sarcoplasmic reticulum RyR receptor in the cell, regulating
RyR receptor-mediated calcium release and mitochondria adenosine
triphosphate production, thereby exerting a biological function. In
the present study, a DM rat model was successfully prepared using
STZ, prior to the DM rats being treated with caffeine for 16 weeks.
The results demonstrated that bladder-function parameters,
including urination time, micturition interval and maximum voiding
pressure, were significantly improved in DM rats. Furthermore, the
wet weight of the bladder in treated DM rats was markedly
decreased. These findings suggested that caffeine may effectively
improve the bladder function of DM rats. Additionally, the results
of FBG analysis indicated that caffeine did not significantly
decrease the level of FBG in the DM rats, suggesting that caffeine
may have no impact on decreasing the blood glucose level and
supporting the hypothesis that its pharmacological effect may be
mainly associated with the target cells.
NGF is a member of the neurotrophic factor family
and is found primarily in sympathetic, partial sensory nerves and
distributed target organs. Previous studies have demonstrated that
NGF is associated with the pathogenesis of bladder dysfunction
caused by multiple etiologies (11-13).
NGF expression levels in tissues have been significantly decreased
in clinical animal models and the promotion of NGF expression may
improve the function of the bladder to a certain extent (11-13).
Additionally, BDNF is another important neurotrophic factor. Its
amino acid sequence is similar to that of NGF and is widely found
in the nervous system and corresponding target organs.
BDNF-overexpression is associated with an overactive bladder
(14). Certain treatments may
improve bladder function by promoting the recovery of the BDNF
expression level (15).
Finally, CGRP is produced by sensory neurons. Once
released from the cells, CGRP initiates a biological response by
binding to a specific CGRP receptor located on cell surfaces and
serves a key role in a variety of physiological and pathological
processes, including cell proliferation, differentiation,
apoptosis, and inflammatory and immune responses (16). It was reported that CGRP was the
main transmitter of bladder sensory nerves (8). Elsewhere, the content of CGRP in the
bladder walls of diabetic rats, particularly in the submucosal
plexus, was significantly decreased and CGRP nerve distribution was
also significantly decreased, suggesting that CGRP serves a key
role in the development of DCP (8).
The results of the present study indicated that the expression
levels of NGF, BDNF and CGRP were significantly decreased in the
bladder tissues of DM rats. After 16 weeks of caffeine treatment,
the expression levels of NGF, BDNF and CGRP were restored to a
certain extent in the bladder tissues of DM rats but remained lower
than those in the normal control rats. These results supported the
idea that caffeine may promote the synthesis and release of
neurotrophic factors in bladder tissues; however, the specific
mechanism behind this concept requires further investigation. NGF,
BDNF and CGRP have neurotrophic effects, which reduce the neuronal
damage caused by high glucose by exerting a protective effect on
DRG neurons, and may regulate certain biological signaling pathways
to improve bladder function by acting on corresponding receptors on
bladder smooth muscles (15,16).
DRG is the main source of bladder sensory nerve
fibers. Previous studies have demonstrated that, in the context of
chronic hyperglycemia, neuron apoptosis and the number of autonomic
nerves distributed in the bladder were decreased, further
aggravating the level of damage to the bladder smooth muscle
function (17,18). The results of the present study
revealed that apoptotic cells of the DRG in DM rats were
significantly decreased following caffeine treatment, suggesting
that caffeine has neuroprotective effects. This result may be
associated with the increased expression of NGF, BDNF and CGRP
triggered by caffeine. Several previous studies have reported that
NGF, BDNF and CGRP may bind to their specific receptors, activate
the related signaling pathways, and subsequently exert
anti-apoptotic cytoprotective actions (19-24).
There are numerous apoptotic proteins known to be involved in the
process of apoptosis, among which the anti-apoptotic protein
BCL2, the pro-apoptotic protein BAX, caspase-3 and
caspase-9 have been heavily studied (25-28).
The results of the present study demonstrated that the expression
levels of Bax, cleaved caspase-3 and cleaved caspase-9 proteins in
the DRG were increased in DM rats, while the expression level of
the Bcl-2 protein was decreased. Following caffeine treatment, the
expression levels of Bax, cleaved caspase-3, cleaved caspase-9 and
Bcl-2 proteins were partially restored. These results suggest that
the effect of caffeine on protecting DRG cells from apoptosis is
associated with the regulation of Bax, cleaved caspase-3, cleaved
caspase-9 and Bcl-2 expression levels.
There are certain limitations to the present study.
At present, the majority of previous studies have focused on
sensory neurons. However, the urinary bladder contractile function
is regulated by sensory neurons, the motor neurons innervating the
bladder, and the smooth muscle function. The bladder is controlled
by two types of motor neurons, innervated by sympathetic and
parasympathetic efferent nerves, which has highlighted certain
difficulties to the related research, which may also be the reason
for the lack of relevant literature. It may be necessary to design
more sophisticated experiments to solve this problem. In addition,
the mechanism by which caffeine attenuated the bladder hypertrophy
was not sufficiently addressed in the present study. We hypothesize
that CGRP may be associated with the mechanism by which caffeine
attenuated the bladder hypertrophy. Finally, considering the impact
on cells when digested into single cell suspension, flow cytometry
detection was not performed in the present study, and the level of
apoptosis was assessed using the percentage of the number of
apoptotic cells to the number of total cells and the detected of
related apoptotic proteins.
In summary, the results of the present study
suggested that caffeine promotes bladder function in rats with DM
through protective effects on the DRG, which may involve at least
the signaling pathways associated with Bax, caspase-3, caspase-9
and Bcl-2.
Supplementary Material
Hematoxylin an eosin staining of the
dorsal root ganglion tissue (magnification, x400).
Acknowledgements
Not applicable.
Funding
The present study was supported by National Natural Science
Foundation of China (grant no. 81400758), Six Top Talent Fund of
Jiangsu Province (grant no. 2014-wsw-12), and Nanjing Medical
Science and Technology Development Project (grant no.
YKK17210).
Availability of data and materials
The datasets generated and/or analyzed during the
current study are available in the Baidu Netdisk repository,
https://pan.baidu.com/s/1IkHrw9skcDaNP7lRtocS5A (code:
puem).
Authors' contributions
ZW designed the study. JX, YL and SZ performed the
experiment. LD, BS and YS performed the data analyses. JX and ZW
wrote the manuscript. JX and ZW authenticated the raw data in this
study. All authors reviewed and approved the final version of the
manuscript.
Ethics approval and consent to
participate
The present study was approved by the Ethical
Committee on Animal Experiment Committee of Nanjing Medical
University (Nanjing, China).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Xu Y, Wang L, He J, Bi Y, Li M, Wang T,
Wang L, Jiang Y, Dai M, Lu J, et al: Prevalence and control of
diabetes in Chinese adults. JAMA. 310:948–959. 2013.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Yuan Z, Tang Z, He C and Tang W: Diabetic
cystopathy: A review. J Diabetes. 7:442–447. 2015.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Arrellano-Valdez F, Urrutia-Osorio M,
Arroyo C and Soto-Vega E: A comprehensive review of urologic
complications in patients with diabetes. Springerplus.
3(549)2014.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Feifer A and Corcos J: Contemporary role
of suprapubic cystostomy in treatment of neuropathic bladder
dysfunction in spinal cord injured patients. Neurourol Urodyn.
27:475–479. 2008.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Franz MJ, Bantle JP, Beebe CA, Brunzell
JD, Chiasson JL, Garg A, Holzmeister LA, Hoogwerf B, Mayer-Davis E,
Mooradian AD, et al: Evidence-based nutrition principles and
recommendations for the treatment and prevention of diabetes and
related complications. Diabetes Care. 26 (Suppl 1):S51–S61.
2003.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Cho YS, Ko IG, Kim SE, Hwan L, Shin MS,
Kim CJ, Kim SH, Jin JJ, Chung JY and Kim KH: Caffeine enhances
micturition through neuronal activation in micturition centers. Mol
Med Rep. 10:2931–2936. 2014.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Yi CR, Wei ZQ, Deng XL, Sun ZY, Li XR and
Tian CG: Effects of coffee and caffeine on bladder dysfunction in
streptozotocin-induced diabetic rats. Acta Pharmacol Sin.
27:1037–1043. 2006.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Langdale CL, Thor KB, Marson L and Burgard
EC: Maintenance of bladder innervation in diabetes: A stereological
study of streptozotocin-treated female rats. Auton Neurosci.
185:59–66. 2014.PubMed/NCBI View Article : Google Scholar
|
|
9
|
WenBo W, Fei Z, YiHeng D, Wei W, TingMang
Y, WenHao Z, QianRu L and HaiTao L: Human umbilical cord
mesenchymal stem cells overexpressing nerve growth factor
ameliorate diabetic cystopathy in rats. Neurochem Res.
42:3537–3547. 2017.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Liu YD, Zhang SC, Xue J, Wei ZQ, Shen BX
and Ding LC: Caffeine improves bladder function in
streptozotocin-induced diabetic rats. Neurourol Urodyn. 38:81–86.
2018.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Kashyap M, Pore S, Yoshimura N and Tyagi
P: Constitutive expression Of NGF, P75(NTR) affected by bladder
distension and NGF antisense treatment. Life Sci. 148:93–98.
2016.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Wada N, Shimizu T, Shimizu N, de Groat WC,
Kanai AJ, Tyagi P, Kakizaki H and Yoshimura N: The effect of
neutralization of nerve growth factor (NGF) on bladder and urethral
dysfunction in mice with spinal cord injury. Neurourol Urodyn.
37:1889–1896. 2018.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Tonyali S, Ates D, Akbiyik F, Kankaya D,
Baydar D and Ergen A: Urine nerve growth factor (NGF) level,
bladder nerve staining and symptom/problem scores in patients with
interstitial cystitis. Adv Clin Exp Med. 27:159–163.
2018.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Kashyap MP, Pore SK, de Groat WC,
Chermansky CJ, Yoshimura N and Tyagi P: BDNF overexpression in the
bladder induces neuronal changes to mediate bladder overactivity.
Am J Physiol Renal Physiol. 315:F45–F56. 2018.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Kashyap MP, Roberts C, Waseem M and Tyagi
P: Drug targets in neurotrophin signaling in the central and
peripheral nervous system. Mol Neurobiol. 55:6939–6955.
2018.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Parameswaran N, Disa J, Spielman WS,
Brooks DP, Nambi P and Aiyar N: Activation of multiple
mitogen-activated protein kinases by recombinant calcitonin
gene-related peptide receptor. Eur J Pharmacol. 389:125–130.
2000.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Nirmal J, Tyagi P, Chuang YC, Lee WC,
Yoshimura N, Huang CC, Rajaganapathy B and Chancellor MB:
Functional and molecular characterization of hyposensitive
underactive bladder tissue and urine in streptozotocin-induced
diabetic rat. PLoS One. 9(e102644)2014.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Daneshgari F, Liu G, Birder L,
Hanna-Mitchell AT and Chacko S: Diabetic bladder dysfunction:
Current translational knowledge. J Urol. 182 (Suppl 6):S18–S26.
2009.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Li R, Wu Y, Zou S, Wang X, Li Y, Xu K,
Gong F, Liu Y, Wang J, Liao Y, et al: NGF attenuates high
glucose-induced ER stress, preventing schwann cell apoptosis by
activating the PI3K/Akt/GSK3β and ERK1/2 pathways. Neurochem Res.
42:3005–3018. 2017.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Sun Z, Hu W, Yin S, Lu X, Zuo W, Ge S and
Xu Y: NGF protects against oxygen and glucose deprivation-induced
oxidative stress and apoptosis by up-regulation of HO-1 through
MEK/ERK pathway. Neurosci Lett. 641:8–14. 2017.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Cao J, Wu Y, Liu G and Li Z:
Over-expression of BDNF inhibits angiotensin II-induced apoptosis
of cardiomyocytes in SD rats. Xi Bao Yu Fen Zi Mian Yi Xue Za Zhi.
34:218–224. 2018.PubMed/NCBI(In Chinese).
|
|
22
|
Qi G, Mi Y, Wang Y, Li R, Huang S, Li X
and Liu X: Neuroprotective action of tea polyphenols on oxidative
stress-induced apoptosis through the activation of the
TrkB/CREB/BDNF pathway and Keap1/Nrf2 signaling pathway in SH-SY5Y
cells and mice brain. Food Funct. 8:4421–4432. 2017.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Ma YX, Guo Z and Sun T: CGRP inhibits
norepinephrine induced apoptosis with restoration of Bcl-2/Bax in
cultured cardiomyocytes of rat. Neurosci Lett. 549:130–134.
2013.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Yang JH, Zhang YQ and Guo Z: Endogenous
CGRP protects retinal cells against stress induced apoptosis in
rats. Neurosci Lett. 501:83–85. 2011.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Renault TT, Dejean LM and Manon S: A
brewing understanding of the regulation of Bax function by Bcl-xL
and Bcl-2. Mech Ageing Dev. 161:201–210. 2017.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Vogel MW: Cell death, Bcl-2, Bax, and the
cerebellum. Cerebellum. 1:277–287. 2002.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Song L, Gao LN, Wang J, Thapa S, Li Y,
Zhong XB, Zhao HW, Xiang XR, Zhang FG and Ji P: Stromal
cell-derived factor-1α alleviates calcium-sensing receptor
activation-mediated ischemia/reperfusion injury by inhibiting
caspase-3/caspase-9-induced cell apoptosis in rat free flaps.
Biomed Res Int. 2018(8945850)2018.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Lan T, Zhao H, Xiang B, Wang J and Liu Y:
Suture compression induced midpalatal suture chondrocyte apoptosis
with increased caspase-3, caspase-9, Bad, Bak, Bax and Bid
expression. Biochem Biophys Res Commun. 489:179–186.
2017.PubMed/NCBI View Article : Google Scholar
|