Introduction
Among all patients with type 2 diabetes mellitus
(T2DM), ~60% have peripheral neuropathy and one-third of these have
neuropathic pain (1). Chronic pain
is the most common health problem in the developed world (2,3).
Diabetic neuropathy (PDN) is one of the most common cause of
chronic pain (1). Furthermore,
approximately two-thirds of patients with PDN also present with
anxiety and depression (4), which
contribute to a poor quality of life (5). However, the curative efficacy of PDN
treatment remain unsatisfactory (1).
A previous study investigated the central
pathophysiological mechanisms of PDN (5). The thalamus is the hub of the
cortical-subcortical connections and is considered as the ‘central
core’. Furthermore, it is closely connected with regions involved
in different emotional and cognitive tasks (6). The thalamus is also regarded as a
relay station projecting to the cerebral cortex, which is
responsible for sensations, such as pain (7). Patients with PDN have been indicated
to exhibit a reduction in the volume of gray matter around the
somatosensory cortex, which may have important implications for the
long-term prognosis of DPN (8). A
previous study also demonstrated that PDN was associated with
thalamic and limbic dysfunctions, as well as impaired default and
attention networks (5). Thus, the
importance of the thalamus in PDN is being recognized.
Resting-state functional MRI (rs-fMRI) is a
neuroimaging method that enables researchers to measure the
activities of different brain regions (9). Furthermore, rs-fMRI has been used to
explore the intrinsic functional connectivity (FC) of the brain in
the resting state (10,11). A previous study by our group
indicated that patients with PDN had abnormal spontaneous
activities in several brain regions, including somatosensory,
cognitive and emotional activities, which were associated with
increased insulin resistance, depression and anxiety (12). Furthermore, the duration of
diabetes, glycated hemoglobin (HbA1c) levels, homeostasis model
assessment-insulin resistance and the estimated glomerular
filtration rate were significantly associated with abnormal
spontaneous activity in the brain.
The present study aimed to explore abnormalities of
the thalamo-cortical FC in patients with PDN using rs-fMRI. The
study may provide novel information on the underlying mechanisms of
PDN.
Materials and methods
Participants
A cross-sectional study was performed to explore the
FC of the thalamus in patients with PDN using rs-fMRI at the
Department of Endocrinology of Nanjing First Hospital (Nanjing,
China) between September 2016 and March 2017. Patients were
categorized into three groups: i) Patients with T2DM and PDN (Group
PDN), ii) patients with T2DM and non-painful neuropathy (Group NDN)
and iii) healthy subjects as a control (Group C). The protocol was
approved by the Institutional Review Board of Nanjing First
Hospital (Nanjing, China). All procedures were in accordance with
the Declaration of Helsinki from 1964 and its later amendments or
comparable ethical standards. Written informed consent was obtained
from all participants.
The inclusion criteria for the patients were as
follows: i) Voluntary participation and written informed consent;
ii) age between 18 and 60 years with junior high school education
or above; and iii) met the 1999 World Health Organization T2DM
diagnostic criteria (fasting plasma glucose ≥7.0 mmol/l or 2-h
postprandial glucose ≥11.1 mmol/l) (13).
The criteria for the diagnosis of PDN were as
follows: i) Neuropathy occurred after diagnosis of diabetes; ii)
patients had clinical symptoms, such as pain, numbness or abnormal
sensation; and iii) patients had abnormalities in one of the
following five examinations: Ankle reflex, vibratory sensation,
pressure sensation, temperature sensation and acupuncture pain
(12).
The inclusion criteria for the healthy controls were
as follows: i) Voluntary participation and written informed
consent; ii) age between 18 to 60 years with junior high school
education or above; iii) no history of diabetes and HbA1c levels of
4-6%; and iv) normal results of anxiety and depression scales.
The exclusion criteria for all subjects were as
follows: i) Left-hand writers; ii) neuropathy caused by other
causes, such as cervical spondylosis, cerebral infarction, Green
Barre syndrome, severe arteriovenous disease, drug neurotoxicity
and renal insufficiency; iii) patients with severe cerebral
vascular disease; iv) disorders such as depression, anxiety or
Alzheimer's disease; v) history of any serious medical, psychiatric
or neurologic disorders; vi) substance abuse; vii) head trauma or
loss of consciousness; and viii) any contraindications to MRI.
Laboratory assessments
HbA1c was measured using a high-performance liquid
chromatography assay (D-100 system; Bio-Rad Laboratories, Inc.).
C-peptide was measured using a chemiluminescent immunometric assay,
which employs the Modular Analytics E170 (Roche Diagnostics GmbH).
Blood glucose, serum creatinine and lipid profiles (total
cholesterol and triglycerides) were measured by enzymatic assays
(Olympus AU5400 autoanalyzer; Beckman Coulter).
Nerve assessment
Three independently-trained doctors assessed the
nerve and mental conditions of the patients in accordance with the
pain symptoms and neurological signs: i) The visual analogue scale
(VAS) (12,14); ii) the Toronto Clinical Scoring
System (TCSS) (12,14); and iii) the Leeds Assessment of
Neuropathic Symptoms and Signs (12,15).
MRI acquisition
The whole-blood oxygen level-dependent (BOLD)
signals were collected using an Ingenia 3.0T MRI machine (Philips
Medical Systems B.V.). The MRI scanning technique was performed as
described previously (12). The
parameters of T1-weighted imaging were as follows: i) Repetition
time (TR)/echo time (TE), 8.2/3.8 msec; ii) field of view (FOV),
240x240 mm; iii) matrix, 240x222; iv) slice thickness, 1 mm; and v)
scanning time, 5 min and 29 sec. For subjects with no structural
brain abnormalities, a resting-state functional imaging scan was
performed. Subjects were required to close their eyes during the
scan, and stay awake and quiet without any further movements. The
parameters were as follows: i) TR/TE, 2,000/30 msec; ii) FOV,
220x220 mm; iii) matrix, 72x70, slice thickness, 4 mm; and iv)
scanning time, 12 min and 45 sec.
MRI processing
The standard pre-processing steps were performed
using the Statistical Parametric Mapping (SPM) version 8
(http://www.fil.ion.ucl.ac.uk/spm/)
and the Data Processing Assistant for re-fMRI on the MATLAB R2012b
platform (MathWorks) (16).
The initial 10 time-points were removed to eliminate
early detection interference. Subsequently, slice timing and
head-motion correction were performed. Head movements were
calibrated with 2-mm translations and were angled at 2 degrees to
eliminate inconsistencies. Subsequently, the image space was
normalized in accordance with the Montreal standard head anatomic
template and was resampled to a 3 mm x 3 mm x 3 mm size using a
unified segmentation algorithm on the T1 image (12). Nuisance signals, including white
matter signals and cerebral spinal fluid, were regressed.
Detrending and temporal band-pass filtering (0.01.0.08 Hz) were
performed. A Gauss kernel function of 4 mm with full width and half
height were used for spatial smoothing.
FC analysis
The thalamus was divided into two subregions
(Thalamus_L and Thalamus_R) for selecting the regions of interest
(ROIs). Masks of ROIs were obtained using the WFU Pick Atlas 3.0.5
(http://fmri.wfubmc.edu/software/PickAtlas) from the
Montreal Neurological Institute, which automatically generated
segmented atlas ROI templates.
The mean time course for calculating thalamic ROI
was determined by averaging the time course of the voxels within
the thalamus (17). Subsequently,
the thalamo-cortical FC was calculated. The z scores were obtained
from the correlation coefficients by Fisher's transformation
(18,19).
Statistical analysis
To examine inter-group differences in the clinical
characteristics, one-way analysis of variance (ANOVA), an
independent-samples t-test and the χ2 test were applied
using SPSS 22.0 software (IBM Corp.).
To compare differences in rs-FC among three groups,
ANOVA was performed using the resting-state fMRI data analysis
toolkit (REST) software (20). The
t-test was conducted to explore differences in rs-FC between of PDN
and NDN groups by treating age, sex and years of education as
covariates. Multiple comparative corrections were performed using
Monte Carlo simulation in conjunction with the REST AlphaSim
program. Voxels with cluster sizes ≥4 (108 mm3) and
P<0.01 were regarded as significant brain areas, corresponding
to the corrected P<0.05.
Results
Demographic and clinical
characteristics
All available cases were collected. Finally, a total
of 52 right-handed subjects were recruited for the present study.
The patients with DN were categorized into two groups (PDN and
NDN). Group PDN was comprised of 12 males and 8 females, whereas
group NDN was comprised of 13 males and 7 females. A total of 13
age-, sex- and years of education-matched healthy volunteers (7
males and 6 females) were recruited. One patient was excluded from
Group-PDN due to head motion >2 mm during MRI. The patient
characteristics are presented in Table
I.
 | Table IDemographic and clinical
characteristics of the participants. |
Table I
Demographic and clinical
characteristics of the participants.
| Item | Group-PDN
(n=19) | Group-NDN
(n=20) | Group-C (n=13) | P-value |
|---|
| Sex
(male/female) | 12/7 | 13/7 | 7/6 | 0.800a |
| Age (year) | 53.8±8.1 | 54.1±6.4 | 53.9±5.3 | 0.994b |
| Education
(years) | 9.6±3.4 | 10.5±3.7 | 10.4±2.9 | 0.724b |
| Duration of disease
(months) | 109.4±65.5 | 100.4±66.9 | - | 0.671c |
| Fasting blood
glucose (mmol/l) | 9.2±2.9 | 7.4±3.5 | - | 0.083c |
| HbA1c (%) | 8.5±1.9 | 8.7±1.7 | - | 0.633c |
| C-peptide
(ng/ml) | 1.6±0.9 | 1.2±0.7 | - | 0.100c |
| Cholesterol
(mmol/l) | 4.9±1.2 | 5.1±1.2 | - | 0.650c |
| Triacylglycerol
(mmol/l) | 2.4±2.7 | 2.4±2.9 | - | 0.970c |
| Creatinine
(µmol/l) | 70.0±19.2 | 67.9±30.6 | - | 0.990c |
| VAS (score) | 6.8±1.9 | 0.0±0.0 | - |
<0.0001c |
| TCSS (score) | 9.7±3.4 | 6.2±1.6 | - | 0.0002c |
No significant difference in age, sex and years of
educationwas present among the three groups. Furthermore, there was
no significant difference in the course of disease, fasting blood
glucose, HbA1c, C-peptide, total cholesterol, triacylglycerol and
creatinine between the PDN and NDN groups (Table I); however, significant differences
in nerve conditions as measured by the VAS score and TCSS were
detected between the two groups (Table
I).
FC differences between groups
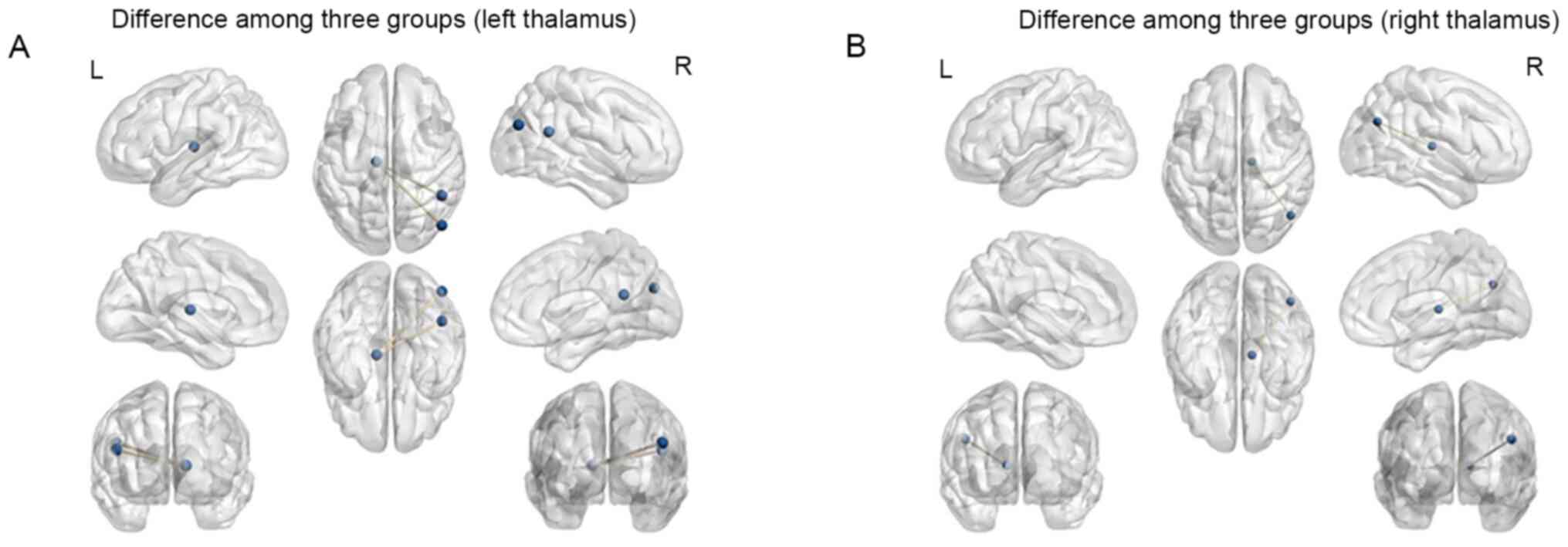
Considering the left thalamus as the ROI, the
results of the one-way ANOVA revealed significant differences in FC
values among the three groups in the vermis, right parahippocampal
gyrus automated anatomical labeling (aal) (21), right inferior temporal gyrus (aal),
right fusiform gyrus, right thalamus (aal), right middle temporal
gyrus (aal), left rolandic operculum (aal), right middle occipital
gyrus (aal), left median cingulate and paracingulate gyri (aal),
right angular gyrus (aal) and right middle occipital gyrus (aal)
(P<0.05, Alphasim correction; Table
II and Fig. 1). Compared with
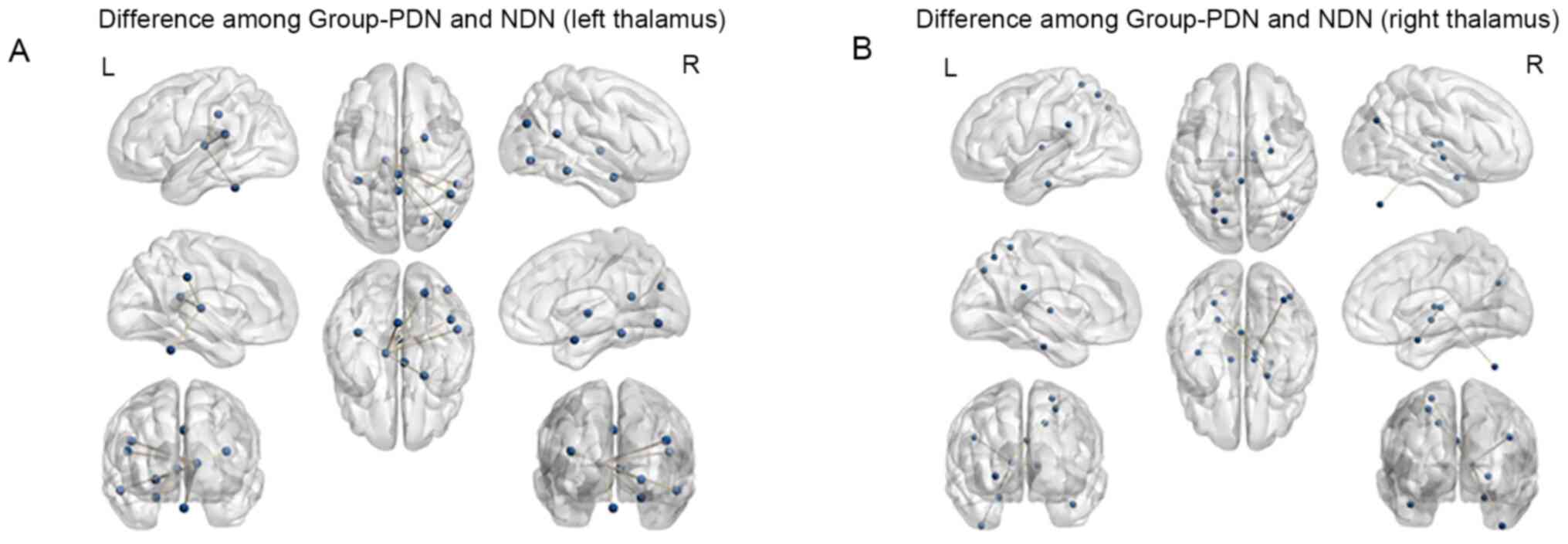
Group NDN, Group PDN exhibited significantly increased FC between
the left thalamus and the right angular gyrus, as well as the right
middle occipital lobe (P<0.05, AlphaSim correction; Table III and Fig. 2).
 | Table IIANOVA of differences in
thalamic-whole brain functional connectivity among three groups
when taking the bilateral thalamus as the region of interest. |
Table II
ANOVA of differences in
thalamic-whole brain functional connectivity among three groups
when taking the bilateral thalamus as the region of interest.
| A, FC with the left
thalamus |
|---|
| | MNI coordinate | |
|---|
| Area | Side | x | y | z | K | F-value |
|---|
| Vermis_10 | L | 0 | -45 | -30 | 45 | 11.64 |
|
Parahippocampal | R | 24 | 3 | -21 | 7 | 9.44 |
| Temporal_Inf | R | 54 | -39 | -15 | 9 | 13.26 |
| Fusiform | R | 24 | -72 | -6 | 15 | 12.52 |
| Thalamus | R | 6 | -9 | 3 | 26 | 10.8 |
| Temporal_Mid | R | 48 | -48 | 18 | 6 | 11.34 |
| Rolandic_Oper | L | -36 | -36 | 18 | 10 | 9.76 |
| Occipital_Mid | R | 45 | -75 | 27 | 10 | 9.49 |
| Cingulum_Mid | L | 0 | -30 | 36 | 12 | 10.19 |
| B, FC with the
right thalamus |
| | MNI coordinate | |
| Area | Side | x | y | z | K | F-value |
|
Cerebelum_Crus2 | R | 39 | -66 | -45 | 7 | 11.7 |
| Temporal_Inf | L | -39 | -18 | -27 | 6 | 14.55 |
|
Parahippocampal | R | 24 | 3 | -21 | 11 | 9.97 |
| Pallidum | R | 27 | -9 | -3 | 19 | 11.16 |
| Thalamus | R | 12 | -12 | 9 | 40 | 15.44 |
| Thalamus | L | -9 | -12 | 6 | 6 | 9.89 |
| Angular | R | 45 | -69 | 30 | 19 | 11.58 |
| Cingulum_Post | L | 0 | -36 | 27 | 8 | 9.57 |
| Parietal_Sup | L | -15 | -72 | 42 | 6 | 12.39 |
| Parietal_Sup | L | -24 | -63 | 54 | 12 | 13.05 |
| Parietal_Sup | L | -21 | -48 | 63 | 7 | 10.53 |
 | Table IIIAnalysis of the differences in
thalamic-whole brain functional connectivity between the Group-PDN
and Group-NDN when taking the bilateral thalamus as the region of
interest. |
Table III
Analysis of the differences in
thalamic-whole brain functional connectivity between the Group-PDN
and Group-NDN when taking the bilateral thalamus as the region of
interest.
| A, FC with the left
thalamus |
|---|
| | MNI coordinate | |
|---|
| Area | Side | x | y | z | K | t-value |
|---|
| Angular gyrus | R | 48 | -48 | 21 | 6 | 4.83 |
| Middle occipital
gyrus | R | 48 | -75 | 27 | 5 | 4.28 |
| B, FC with the
right thalamus |
| | MNI coordinate | |
| Area | Side | x | y | z | K | t-value |
| Angular gyrus | R | 48 | -66 | 3 | 19 | 4.47 |
Considering the right thalamus as the ROI, the
results of the one-way ANOVA indicated that significant differences
in FC values among three groups were present in the right
cerebellum, left inferior temporal gyrus, right parahippocampal
gyrus (aal), right lenticular nucleus, pallidum (aal), right
thalamus, left thalamus, right angular gyrus (aal), left posterior
cingulate gyrus and left superior parietal gyrus (aal) (P<0.05,
Alphasim correction; Table II and
Fig. 1). Compared with Group NDN,
Group PDN exhibited significantly increased FC between the right
thalamus and the right angular gyrus (P<0.05 AlphaSim
correction; Table III and
Fig. 2).
Discussion
Functional MRI has been widely used to investigate
brain dysfunction in patients with psychiatric disorders (22-25).
To the best of our knowledge, the present study was the first to
indicate enhanced thalamic-whole brain FC in Chinese patients with
PDN using thalamic seed-based analysis. The results indicated that
Group PDN had increased FC between the bilateral thalami and the
right angular gyrus and between the left thalamus and the right
occipital lobe, when compared with Group NDN. Furthermore, it was
determined that Group PDN had increased FC between the left
thalamus and the right angular gyrus, as well as the right
occipital gyrus. Group PDN also had an increased FC between the
right thalamus and the right angular gyrus.
The thalamus is the central cortical-subcortical
connectivity hub and serves as the gateway to the cerebral cortex.
The thalamus is considered to have a significant role in the
process of pain sensation (26).
The thalamus was indicated to be associated with spontaneous and
evoked pain in chronic conditions (7). In 2011, it was reported that PDN was
associated with increased thalamic vascularity in T1DM (27). To date, there has been a scarcity of
research focusing particularly on the thalamus and the role of PDN
in T2DM using rs-fMRI. Therefore, the present study may expand the
current understanding of the underlying neurobiological mechanisms
of these conditions.
The angular gyrus is one of the components within
the default mode network (DMN), which was reported to be involved
in the process of episodic memory retrieval (28). The angular gyrus is involved in
several cognitive domains (i.e., language processing and attention)
and serves as an important node of the DMN (29,30).
The DMN was indicated to be altered in chronic pain diseases such
as fibromyalgia, which is considered to be the prototypical central
chronic pain syndrome (31).
Another previous study indicated that music was able to reduce pain
and increase the amplitude of rs-fMRI BOLD signals in the left
angular gyrus in patients with fibromyalgia. The angular gyrus is
involved in the top-down regulation of the pain modulatory network
by the DMN (32). Furthermore,
increased activity in the left angular gyrus following
verbally-induced placebo analgesia was observed in patients with
chronic pain (33). These results
suggested that the angular gyrus may have interactions with brain
areas or pathways involved in pain modulation (33).
A previous multivariate analysis of resting FC
demonstrated that the angular gyrus had similar connectivity to
that in the default mode network (DMN) (28). Another study determined that the
angular gyrus were involved in numerous tasks and processes
(29). In the present study, it was
indicated that in PDN group the bilateral thalamus had increased FC
with the right angular gyrus, which was related to the pain
modulatory network. Therefore, it was speculated that impaired FC
between the thalamus and angular gyrus in PDN may be related to the
pain modulatory network within the DMN.
The present study also indicated that the thalamus
had increased FC with the right middle occipital gyrus. The
thalamus is responsible for interpreting visual information and
forming conscious perception (34).
Changes in the thickness of the middle occipital gyrus associated
were determined in blind patients (35). Furthermore, local synchronicity and
abnormal occipital lobe function were reported in patients with
hemifacial spasm (36). Patients
with classical trigeminal neuralgia (CTN) had symptoms
characterized by orbital pain, decreased corneal reflex and
decreased vision (34). Individuals
with chronic post-hysterectomy pain had decreased FC between the
left sensorimotor insula and the right angular and middle occipital
gyri (MOG), as well as between the left chemosensory insula, the
bilateral angular gyri and the MOG following pain stimulation
(37).
Furthermore, increased regional homogeneity (ReHo)
values in the right middle occipital gyrus were reported in
patients with CTN (34). Patients
with drug-induced headaches exhibited an increased volume of the
bilateral middle occipital gyrus (38). Furthermore, patients with
pain-related conditions had altered neuronal activity or structural
functions in the middle occipital gyrus (38,39).
These results suggested that the middle occipital gyrus may
participate in the processes leading to the perception of pain in
patients with diabetic polyneuropathy. The abnormal FC between the
thalamus and other brain regions were indicated to be associated
with PDN.
The present study has several limitations. First,
the sample size was small. Furthermore, no associations between the
altered brain regions and clinical characteristics were explored in
the present study. Further studies with a larger sample size are
required to understand the mechanisms of T2DM with PDN. Finally, it
was not possible to exclude the potential influences of medication,
such as oral antidiabetic drugs, in the present study.
In conclusion, the present study revealed enhanced
FC between the bilateral thalamic-angular gyrus and the left
thalamic-right MOG in patients with T2DM and PDN. The increased
thalamic-whole brain FC may be involved in PDN. Furthermore, the
abnormal thalamic-angular gyrus FC may be related to the DMN. These
results may be helpful for understanding the central
pathophysiological mechanisms of PDN in patients with T2DM.
Acknowledgements
Not applicable.
Funding
The present study was supported by grants from the Nanjing
Science and Technology Development Project (grant no. 201605027)
and the Natural Science Foundation of Jiangsu Province (grant no.
BK20170136).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
XL, XX and CM acquired and interpreted the patient
data, constructed the figures and wrote the manuscript. PZ, QZ, LJ,
YY, JM, LY and KL acquired and analyzed the patient data, and
revised the manuscript. JW and ZY designed the study and revised
the manuscript. JW and ZY confirm the authenticity of all the raw
data. All authors have read and approved the final manuscript.
Ethics approval and consent to
participate
The protocol was approved by the Institutional
Review Board of Nanjing First Hospital (Nanjing, China). All
procedures were in accordance with the 1964 Helsinki Declaration
and its later amendments or comparable ethical standards. Written
informed consent was obtained from all participants.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Feldman EL, Nave KA, Jensen TS and Bennett
DLH: New horizons in diabetic neuropathy: Mechanisms,
bioenergetics, and pain. Neuron. 93:1296–1313. 2017.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Gaskin DJ and Richard P: The economic
costs of pain in the United States. J Pain. 13:715–724.
2012.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Fayaz A, Croft P, Langford RM, Donaldson
LJ and Jones GT: Prevalence of chronic pain in the UK: A systematic
review and meta-analysis of population studies. BMJ Open.
6(e010364)2016.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Dermanovic Dobrota V, Hrabac P, Skegro D,
Smiljanic R, Dobrota S, Prkacin I, Brkljacic N, Peros K, Tomic M,
Lukinovic-Skudar V and Basic Kes V: The impact of neuropathic pain
and other comorbidities on the quality of life in patients with
diabetes. Health Qual Life Outcomes. 12(171)2014.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Tesfaye S, Selvarajah D, Gandhi R, Greig
M, Shillo P, Fang F and Wilkinson ID: Diabetic peripheral
neuropathy may not be as its name suggests: Evidence from magnetic
resonance imaging. Pain. 157 (Suppl 1):S72–S80. 2016.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Modha DS and Singh R: Network architecture
of the long-distance pathways in the macaque brain. Proc Natl Acad
Sci USA. 107:13485–13490. 2010.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Yen CT and Lu PL: Thalamus and pain. Acta
Anaesthesiol Taiwan. 51:73–80. 2013.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Selvarajah D, Wilkinson ID, Maxwell M,
Davies J, Sankar A, Boland E, Gandhi R, Tracey I and Tesfaye S:
Magnetic resonance neuroimaging study of brain structural
differences in diabetic peripheral neuropathy. Diabetes Care.
37:1681–1688. 2014.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Biswal B, Yetkin FZ, Haughton VM and Hyde
JS: Functional connectivity in the motor cortex of resting human
brain using echo-planar MRI. Magn Reson Med. 34:537–541.
1995.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Zuo XN, Kelly C, Di Martino A, Mennes M,
Margulies DS, Bangaru S, Grzadzinski R, Evans AC, Zang YF,
Castellanos FX and Milham MP: Growing together and growing apart:
Regional and sex differences in the lifespan developmental
trajectories of functional homotopy. J Neurosci. 30:15034–15043.
2010.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Gorges M, Muller HP, Lule D, Ludolph AC,
Pinkhardt EH and Kassubek J: Functional connectivity within the
default mode network is associated with saccadic accuracy in
Parkinson's disease: A resting-state FMRI and videooculographic
study. Brain Connect. 3:265–272. 2013.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Zhang Q, Zhang P, Yan R, Xu X, Mao C, Liu
X, Li F, Ma J, Ye L, Yao Z and Wu J: A single-blinded trial using
resting-state functional magnetic resonance imaging of brain
activity in patients with type 2 diabetes and painful neuropathy.
Diabetes Ther. 10:135–147. 2019.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Gabir MM, Hanson RL, Dabelea D, Imperatore
G, Roumain J, Bennett PH and Knowler WC: The 1997 American Diabetes
Association and 1999 World Health Organization criteria for
hyperglycemia in the diagnosis and prediction of diabetes. Diabetes
Care. 23:1108–1112. 2000.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Essmat A and Hussein MS: Green tea extract
for mild-to-moderate diabetic peripheral neuropathy A randomized
controlled trial. Complement Ther Clin Pract.
43(101317)2021.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Garoushi S, Johnson MI and Tashani OA:
Translation and cultural adaptation of the Leeds Assessment of
Neuropathic Symptoms and Signs (LANSS) pain scale into Arabic for
use with patients with diabetes in Libya. Libyan J Med.
12(1384288)2017.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Chao-Gan Y and Yu-Feng Z: DPARSF: A MATLAB
toolbox for ‘Pipeline’ data analysis of resting-state fMRI. Front
Syst Neurosci. 4(13)2010.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Salvador R, Suckling J, Schwarzbauer C and
Bullmore E: Undirected graphs of frequency-dependent functional
connectivity in whole brain networks. Philos Trans R Soc Lond B
Biol Sci. 360:937–946. 2005.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Liu Y, Yu C, Liang M, Li J, Tian L, Zhou
Y, Qin W, Li K and Jiang T: Whole brain functional connectivity in
the early blind. Brain. 130:2085–2096. 2007.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Hale JR, White TP, Mayhew SD, Wilson RS,
Rollings DT, Khalsa S, Arvanitis TN and Bagshaw AP: Altered
thalamocortical and intra-thalamic functional connectivity during
light sleep compared with wake. Neuroimage. 125:657–667.
2016.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Song XW, Dong ZY, Long XY, Li SF, Zuo XN,
Zhu CZ, He Y, Yan CG and Zang YF: REST: A toolkit for resting-state
functional magnetic resonance imaging data processing. PLoS One.
6(e25031)2011.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Tzourio-Mazoyer N, Landeau B,
Papathanassiou D, Crivello F, Etard O, Delcroix N, Mazoyer B and
Joliot M: Automated anatomical labeling of activations in SPM using
a macroscopic anatomical parcellation of the MNI MRI single-subject
brain. Neuroimage. 15:273–289. 2002.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Zhu X, He Z, Luo C, Qiu X, He S, Peng A,
Zhang L and Chen L: Altered spontaneous brain activity in
MRI-negative refractory temporal lobe epilepsy patients with major
depressive disorder: A resting-state fMRI study. J Neurol Sci.
386:29–35. 2018.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Tose K, Yoshihara Y and Takahashi H: FMRI
neurofeedback and its application to psychiatric disorders. Brain
Nerve. 70:1209–1216. 2018.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Tadayonnejad R, Deshpande R, Ajilore O,
Moody T, Morfini F, Ly R, O'Neill J and Feusner JD: Pregenual
anterior cingulate dysfunction associated with depression in OCD:
An integrated multimodal fMRI/(1)H MRS study.
Neuropsychopharmacology. 43:1146–1155. 2018.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Shen Z, Jiang L, Yang S, Ye J, Dai N, Liu
X, Li N, Lu J, Liu F, Lu Y, et al: Identify changes of brain
regional homogeneity in early and later adult onset patients with
first-episode depression using resting-state fMRI. PLoS One.
12(e0184712)2017.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Head H and Holmes G: Sensory disturbances
from cerebral lesions. Brain. 34:102–254. 1911.
|
|
27
|
Selvarajah D, Wilkinson ID, Gandhi R,
Griffiths PD and Tesfaye S: Microvascular perfusion abnormalities
of the Thalamus in painful but not painless diabetic
polyneuropathy: A clue to the pathogenesis of pain in type 1
diabetes. Diabetes Care. 34:718–720. 2011.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Bellana B, Liu Z, Anderson JAE, Moscovitch
M and Grady CL: Laterality effects in functional connectivity of
the angular gyrus during rest and episodic retrieval.
Neuropsychologia. 80:24–34. 2016.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Seghier ML: The angular gyrus: Multiple
functions and multiple subdivisions. Neuroscientist. 19:43–61.
2013.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Greicius MD, Supekar K, Menon V and
Dougherty RF: Resting-state functional connectivity reflects
structural connectivity in the default mode network. Cereb Cortex.
19:72–78. 2009.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Napadow V, LaCount L, Park K, As-Sanie S,
Clauw DJ and Harris RE: Intrinsic brain connectivity in
fibromyalgia is associated with chronic pain intensity. Arthritis
Rheum. 62:2545–2555. 2010.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Garza-Villarreal EA, Jiang Z, Vuust P,
Alcauter S, Vase L, Pasaye EH, Cavazos-Rodriguez R, Brattico E,
Jensen TS and Barrios FA: Music reduces pain and increases resting
state fMRI BOLD signal amplitude in the left angular gyrus in
fibromyalgia patients. Front Psychol. 6(1051)2015.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Craggs JG, Price DD and Robinson ME:
Enhancing the placebo response: Functional magnetic resonance
imaging evidence of memory and semantic processing in placebo
analgesia. J Pain. 15:435–446. 2014.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Xiang CQ, Liu WF, Xu QH, Su T, Yong-Qiang
S, Min YL, Yuan Q, Zhu PW, Liu KC, Jiang N, et al: Altered
spontaneous brain activity in classical trigeminal neuralgia as
determined by changes in regional homogeneity: A resting-state
functional MRI study. Pain Pract. 19:397–406. 2018.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Anurova I, Renier LA, De Volder AG,
Carlson S and Rauschecker JP: Relationship between cortical
thickness and functional activation in the early blind. Cereb
Cortex. 25:2035–2048. 2015.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Tu Y, Wei Y, Sun K, Zhao W and Yu B:
Altered spontaneous brain activity in patients with hemifacial
spasm: A resting-state functional MRI study. PLoS One.
10(e0116849)2015.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Ching YY, Wang C, Tay T, Loke YM, Tang PH,
Sng BL and Zhou J: Altered sensory insular connectivity in chronic
postsurgical pain patients. Front Hum Neurosci.
12(483)2018.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Chen X, Chen Z, Dong Z, Liu M and Yu S:
Morphometric changes over the whole brain in caffeine-containing
combination-analgesic-overuse headache. Mol Pain.
14(1744806918778641)2018.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Chen Y, Meng Z, Zhang Z, Zhu Y, Gao R, Cao
X, Tan L, Wang Z, Zhang H, Li Y and Fan Q: The right thalamic
glutamate level correlates with functional connectivity with right
dorsal anterior cingulate cortex/middle occipital gyrus in
unmedicated obsessive-compulsive disorder: A combined fMRI and
1H-MRS study. Aust N Z J Psychiatry. 53:207–218.
2019.PubMed/NCBI View Article : Google Scholar
|