Introduction
Gestational diabetes mellitus (GDM), defined as
glucose intolerance that develops or is diagnosed during pregnancy,
is one of the most common complications of pregnancy, leading to
considerable maternal-fetal risks (1). There has been an increase in the
prevalence of GDM worldwide, as a consequence of the increase in
the frequency of type 2 diabetes mellitus (T2DM) and maternal
obesity. Patients with GDM are 2-3 times more likely to develop
T2DM throughout their lifetime, a pathology that is the seventh
leading cause of death worldwide, and whose treatment requires
numerous resources (2).
The International Association of Diabetes and
Pregnancy Study Groups (IADPSG) report an average worldwide
incidence rate of 17.8% for GDM (between 9.3-25.5%) (3).
GDM is known to negatively influence pregnancy as
early as the first trimester; the frequency of malformations being
higher in patients with GDM than in the general population. The
most frequent anomalies are cardiovascular and neural tube defects
(4). Therefore, an early diagnosis
of GDM would help improve maternal-fetal prognosis (5).
Current trends in therapeutic management aim at the
discovery of biomarkers for the early detection of GDM or a better
selection of the patients at risk. Adipokines (AKs) have also been
studied in this regard, and adiponectin (AN) and leptin (L) have
been reported to play an important part in the early detection of
GDM. AKs are proteins secreted by adipocytes that interfere with
the glycoregulatory processes and are involved in numerous other
endocrine and metabolic processes such as insulin secretion,
insulin resistance regulation, as well as a number of inflammatory
processes, and body weight regulation (6). Studies have shown abnormal regulation
of AKs at the placental level in patients with GDM, due to a low
expression of AN (7).
Metabolomics, that can identify metabolites
resulting from biochemical reactions, is yet another promising
direction of research attempting to explain the pathophysiology of
GDM. These metabolites cause subtle metabolic changes in human
fluids and tissues and may be involved in the development of GDM.
3-Carboxy-4-methyl-5-propyl-2-furanpropanoic acid (CMPF) is a
furan-fatty acid whose level appears to be elevated in the blood of
patients with T2DM and GDM compared to patients without diabetes.
Furan-fatty acids are incorporated by phospholipids or cholesterol
esters and are metabolized into dibasic urofuranic acids that also
include CMPF, which are excreted in the urine (8,9).
In mice, elevated CMPF levels cause glucose
intolerance, inadequate insulin secretion, and decreased peripheral
glucose utilization (10). Some of
the mechanisms of action of CMPF are improper mitochondrial
function in pancreatic β-cells, low ATP glucose storage, increased
oxidative stress, dysfunction of cellular transcription mechanisms
and, finally, decreased insulin secretion. As antioxidant
treatments counteract the negative effects of CMPF on pancreatic
β-cells, we could envisage future treatment of GDM (10).
Starting from the assumption that the onset of GDM
occurs early in the very first weeks of pregnancy, the present
study aimed to verify whether the biological markers tested (AN, L,
AN/L and CMPF) can be associated with the diagnosis of GDM.
The main objective of this study was to propose a
method for the early, first trimester diagnosis of GDM, which would
include a panel of biomarkers associated with certain clinical and
demographic parameters in the Caucasian population, considered to
be at low risk of developing GDM. The secondary endpoint was to
analyze the maternal-fetal complications associated with GDM.
Patients and methods
Study population
We conducted a prospective longitudinal study at the
Obstetrics and Gynecology Clinic I, County Clinical Emergency
Hospital of Cluj-Napoca, Romania, in the period between January
2018 and March 2019. The study included a total of 111 first
trimester pregnant women.
The inclusion criteria were: Pregnant women aged
18-40 years, gestational age of 11-13 weeks +6 days singleton
pregnancy, performance of an oral glucose tolerance test (OGTT) at
24-28 weeks of gestation (WG), compliance with the follow-up
conditions, and delivery at the Obstetrics and Gynecology Clinic I
of Cluj-Napoca.
All the procedures performed were in accordance with
the ethical standards of the Institutional and National Research
Ethics Committee and with the Declaration of Helsinki (1964) and
its later amendments. Our report is based on the STROBE Statement
(Strengthening the Reporting of Observational studies in
Epidemiology) (11).
The exclusion criteria were: Patients known to be
suffering from type 1 and 2 diabetes, with acute or chronic
infectious pathology, multiple or intrauterine fetal death,
pregnancies with chromosomal abnormalities or fatal fetal
malformations, patients who did not comply with the follow-up
conditions and those who refused to participate in the study.
The control group included 47 singleton pregnancy
patients with physiological pregnancies who accepted to provide a
blood sample at 11-13 weeks+6 days in order to determine L, AN and
CMPF levels, with a normal OGTT result, and who gave birth at the
Obstetrics and Gynecology Clinic I of Cluj-Napoca.
Structured questionnaires and hospital medical
records provided information regarding maternal age, ethnicity,
height, pre-pregnancy weight, smoking before pregnancy,
reproductive, obstetrical and medical history during pregnancy and
after delivery, and the APGAR score.
Blood collection and biochemical
assays
Maternal blood samples were collected between 11 and
13 +6 weeks of gestation (WG) at the time of the combined
first-trimester screening for aneuploidy, according to standardized
surgical procedures. Venous blood samples were collected from
peripheral vessels into commercially available Vacutainer CAT 6-ml
PET tubes with clot activator to determine L, AN, and CMPF levels.
The blood samples were centrifuged at 4,000 x g for 5 min after
allowing the blood to clot for 30 min at room temperature, while
maintained in a vertical position. Serum and plasma samples were
stored at -80˚C until analysis. The diagnosis of
gestational diabetes was set between 24-28 WG based on OGTTs and in
compliance with the international recommendations of the
International Association of the Diabetes and Pregnancy Study
Groups criteria (IADPSG) (12). The
OGTT was performed with 75 mg of glucose intake (after 8 h of
fasting). Gestational diabetes was diagnosed when one of the 3
values determined was altered: Fasting blood glucose >92 mg/dl
(5.1 mmol/l), 1-h glycemia >180 mg/dl (10 mmol/l), 2-h glycemia
>153 mg/dl (8.5 mmol/l).
Serum CMPF, AN and L levels were determined using
commercially available enzyme-linked immunosorbent assay (ELISA)
kits. The concentrations of CMPF and of AN and L were determined
according to the manufacturers' instructions included in the
commercially available kits (MyBioSource, Inc. and
Invitrogen/ThermoFisher Scientific, Inc., respectively).
Statistical analysis
Statistical analysis was performed using MedCalc
Statistical Software version 19.1.5 (MedCalc Software by, Ostend,
Belgium; https://www.medcalc.org; 2020). The
continuous variable data were tested for normal distribution
(Shapiro Wilk test) and were described by median and 25-75
percentiles. All quantitative variables had a non-normal
distribution. The nominal data were characterized by frequency and
percentage. Comparisons between groups were performed using the
Man-Whitney or Chi-square test, whenever appropriate. Correlations
between quantitative variables were verified using the Spearman's
rank correlation coefficient. The cut-off value for L was
calculated in order to differentiate between GDM and normal
patients, using a receiver operating characteristic (ROC) curve.
The independent association between variables and the presence of
GDM were assessed by multivariate logistic regression. The model
included the variables that achieved a P-value <0.05 in the
univariate analysis. A P-value <0.05 was considered
statistically significant.
Results
Out of the selected 111 patients, 68 patients
underwent OGTT and were able to be monitored until delivery. The
rest of the patients included in the study did not meet the
follow-up criteria (43 were excluded from the study: 25 patients
gave birth in others hospitals and 18 patients were lost during
follow-up). Twenty-one patients were diagnosed with GDM based on
the test results and 47 patients were non-GDM and were selected as
a control group. The prevalence of GDM in the study group was
18.91% (21 patients confirmed with GDM/111 cases). The demographic
data of the groups are summarized in Table I. The age of the patients with GDM
was significantly more advanced than that of the patients who did
not develop diabetes, and they also had a higher pre-pregnancy BMI
(P=0.030 and P=0.003, respectively). Smokers were more likely to
develop GDM (P=0.030). The group of patients with GDM included more
multiparous women (61.9% compared to 34% in the control group),
especially women who had previously developed GDM in a previous
pregnancy. This group also included a higher percentage of patients
with fetus macrosomia in previous pregnancies (53.8% in GDM group
vs. 18.7% in control group) (Table
I).
 | Table IDemographic characteristics of the
study groups. |
Table I
Demographic characteristics of the
study groups.
| Variables | GDM group (N=21) | Control group
(N=47) | P-value |
|---|
| Maternal age, years
[mean (range)] | 32 (30-35.5) | 30 (27-33) | 0.030 |
| Smoking, n (%) | 7 (33.3%) | 5 (10.6%) | 0.030 |
| BMI
(kg/m2) | 24.3 (21.3;
28.4) | 20.9 (19.8;
24.1) | 0.003 |
| Family history of
diabetes, n (%) | 8 (38.1%) | 8 (17%) | 0.007 |
| Parity, n (%) | | | |
|
Nulliparous | 8 (38.8%) | 31 (66%) | 0.060 |
|
Multiparous | | | 0.070 |
|
Previous
GDM | 3 (14.28%) | - | |
|
Non-previous
GDM | 10 (47.61%) | 16 (34%) | |
| Previous macrosomia,
% | 7 (53.84%) | 3 (18.7%) | NS |
Patients who developed GDM had significantly higher
levels of L compared with those who did not develop diabetes
(P<0.001). AN levels did not differ significantly between the
patient groups. The AN/L ratio was significantly lower in the
patients with GDM (P=0.030). We did not find significant values of
CMPF in patients with GDM compared to those in the control group
(Table II).
 | Table IIComparison of biochemical markers
according to GDM status. |
Table II
Comparison of biochemical markers
according to GDM status.
| Biochemical
markers | GDM groupmedian (25;
75 percentiles) | Control group median
(25; 75 percentiles) | P-value |
|---|
| Adiponectin (AN) | 26.8 (17.6;
70.1) | 28.4 (18.2;
50.79) | 0.800 |
| Leptin (L) | 32.7 (25; 48.1) | 16.8 (9.5; 32) |
<0.001 |
| AN/L | 0.88 (0.58;
2.9) | 1.42 (0.89;
7.4) | 0.030 |
| CMPF | 180.6 (154.4;
201.9) | 179.2 (153.1;
213.1) | 0.900 |
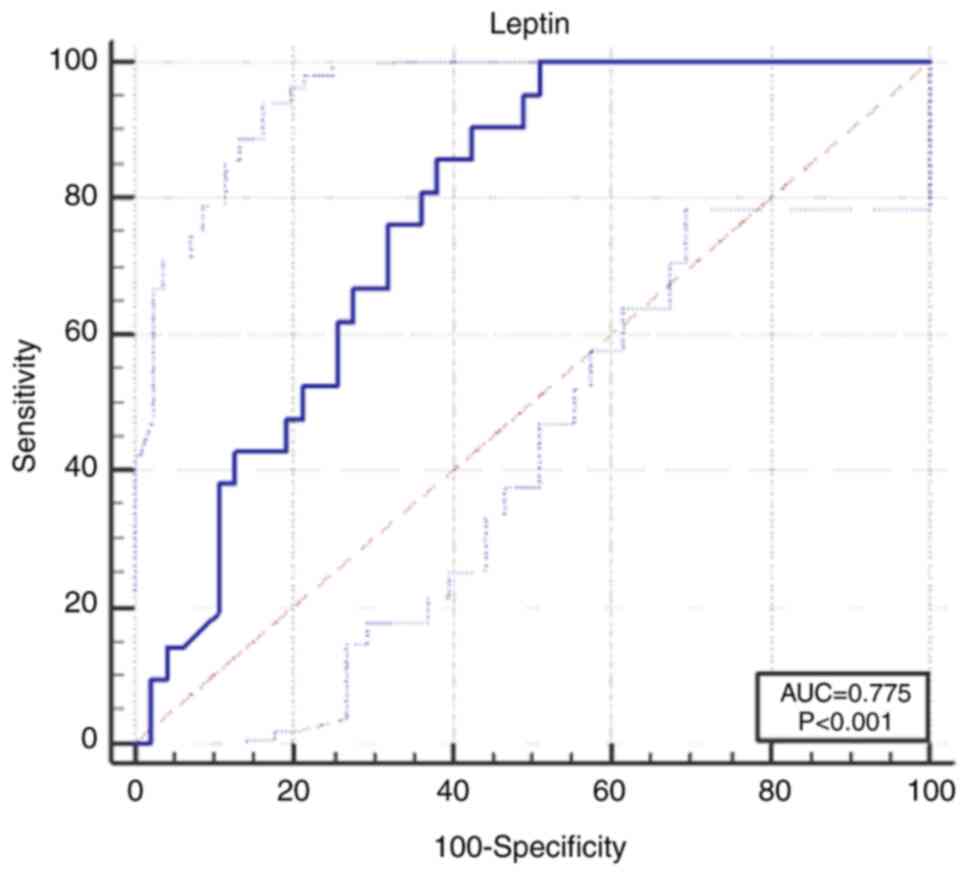
The L cut-off value we calculated was 16 ng/ml. The
probability of developing GDM was higher in the case of patients
with levels above this cut-off value [area under the curve
(AUC)=0.775, 95% confidence interval (CI), 0.658-0.867],
sensitivity 100% (95% CI, 83.9-100), specificity 48.9% (95% CI,
34.1-63.9) (P<0.001) (Fig.
1).
In order to ascertain which variables independently
predict the onset of GDM, we constructed a model using logistic
multivariate regression. We introduced the statistically
significant variables that were associated with GDM in the
univariate analysis. Patients of advanced maternal age and higher L
levels had a 1.16 and 1.06 time higher risk, respectively, of
developing GDM (Table III).
 | Table IIIMultivariate logistic regression for
the presence of GDM. |
Table III
Multivariate logistic regression for
the presence of GDM.
| | 95% CI for OR |
|---|
| | B | P-value | OR | Min | Max |
|---|
| Maternal age | 0.15 | 0.050 | 1.16 | 1.00 | 1.36 |
| Pre-pregnancy
BMI | 0.05 | 0.500 | 1.05 | 0.88 | 1.26 |
| Smoking | 0.39 | 0.600 | 1.48 | 0.31 | 7.07 |
| Leptin | 0.06 | 0.020 | 1.06 | 1.00 | 1.11 |
There was a weak negative correlation between AN
values and newborn weight and a weak positive correlation with the
initial weight of the mother. AN/L coefficient was moderately
correlated with newborn weight (Table
IV).
 | Table IVCorrelations for newborn weight and
APGAR score. |
Table IV
Correlations for newborn weight and
APGAR score.
| | Newborn weight | APGAR score |
|---|
| | R | P-value | R | P-value |
|---|
| Maternal age | 0.024 | 0.800 | 0.088 | 0.400 |
| Pre-pregnancy
BMI | 0.280 | 0.020 | 0.030 | 0.800 |
| Adiponectin | -0.289 | 0.020 | -0.014 | 0.900 |
| Leptin | 0.175 | 0.100 | -0.048 | 0.600 |
| AN/L | -0.332 | 0.007 | -0.049 | 0.700 |
| CMPF | 0.102 | 0.400 | -0.215 | 0.070 |
In patients diagnosed with GDM, the rate of
obstetric complications, such as preterm birth, premature rupture
of membranes (PROM), hydramnios, dystocia of the shoulders,
cervical or perineal lacerations, was higher compared to the
control group, but only polyhydramnios presented with a significant
difference. In addition, the rate of Cesarean section (P<0.001),
the number of cases of fetal macrosomia (P=0.01) and the frequency
of hypoglycemia in newborns (P=0.030) was higher in the GDM group
compared to the control group. Newborns born to mothers in the GDM
group, who required neonatal intensive care unit (NICU) admission
were more numerous than those of the non-diabetic mothers (P=0.040)
(Table V).
 | Table VPeripartum parameters in the GDM and
control groups. |
Table V
Peripartum parameters in the GDM and
control groups.
| | GDM group (N=21)
(%) | Control group
(N=47) (%) | P-value |
|---|
| Maternal
complications, n (%) | | | |
|
PROM | 2 (9.52) | 2 (4.25) | |
|
Preterm
labor | 2 (9.52) | 1 (2.12) | |
|
Polyhydramnios | 7 (33.3) | - |
<0.001 |
|
Postterm
pregnancy | - | 2 (4.25) | |
|
Shoulder
dystocia | 2 (9.52) | | NS |
|
Lacerations | 2 (9.52) | 1 (2.12) | NS |
|
Fetal
dystocia | 4 (19.1) | 5 (10.63) | NS |
| Fetal
complications, n (%) | | | |
|
IUGR | 1 (4.76) | - | NS |
|
Perinatal
asphyxia | 1 (4.76) | 1 (2.12) | NS |
| Mode of delivery, n
(%) | | | |
|
Vaginal
birth | 7 (33.3) | 32(68) |
<0.001 |
|
Cesarean
section | 14 (66.6) | 15(32) | |
| Newborn gender, n
(%) | | | |
|
Male | 10 (47.6) | 22 (46.8) | NS |
|
Female | 11 (52.3) | 23 (53.2) | |
| Birth weight
(g) | | | |
|
≥2,500 | 2 (9.52) | 1 (2.12) | 0.040 |
|
2,500-3,900 | 11 (52.38) | 41 (87.23) | 0.010 |
|
≤3,900 | 8 (38.1) | 5 (10.6) | |
| APGAR score, n
(%) | | | |
|
≥7 | 1 (4.76) | 1 (2.12) | NS |
|
<7 | 20 (95.23) | 46 (97.87) | |
| Neonatal morbidity,
n (%) | | | |
|
Monitoring
in NICU | 7 (33.3) | 6 (12.76) | 0.040 |
|
Hypoglycemia | 6 (28.57) | 1 (2.12) | 0.030 |
Discussion
The present study demonstrated that L levels and
AN/L ratio can predict GDM development as early as the first
trimester of pregnancy, alone or in combination with demographic
parameters such as age, smoking, or pre-pregnancy body mass index
(BMI), while AN and 3-carboxy-4-methyl-5-propyl-2-furanpropanoic
acid (CMPF) levels do not. Elevated L levels proved to be a good
predictor of GDM. In addition, the AN/L ratio was found to be
significantly correlated with the development of GDM. However, in
our research, AN and CMPF levels were not associated with GDM. The
results were independent of age, BMI and smoking habits. Elevated L
levels were found in all patients with GDM, regardless of age. The
more advanced the age of the patients, the higher the frequency of
GDM when several risk factors were accumulated. Nonetheless, the
changes in L levels and the AN/L ratio occurred independently of
age. The logistic regression (Table
III) showed that the independent nature of the variables that
were introduced in the model, was preserved.
In our study, L levels were elevated as early as the
first trimester in pregnant women who developed GDM, independently
of age, pre-pregnancy BMI and smoking habits (OR=1.16). Moreover,
there was a direct correlation between elevated L levels and the
risk of developing GDM [AUC 0.775 (95% CI, 0.658-0.867),
sensitivity 100% (95% CI, 83.9-100), specificity 48.9% (95 % CI
34.1-63.9), P<0.001].
The strong point of this study consists in the way
in which the patients were selected. OGTT was performed in all
patients included in the study, unlike in other studies, in which
OGTT was performed only in patients with risk factors. Thus, we
avoided overdiagnosis in the monitored groups and implicitly the
highlighting of some biased AN, L, AN/L and CMPF values (13).
It is also important to note that this study was
conducted on a Caucasian population, with a very low rate of
diabetes compared to the black population (14,15).
These results are consistent with those of a meta-analysis
published by Bao et al which concluded that L levels in the
first trimester or in the early second trimester were 7.25 ng/ml
higher (95% CI 3.27-11.22), among women who later developed GDM
than women who did not (16).
The results obtained in the present study are
confirmed by other studies. In a study performed on 47 pregnant
women Qiu et al obtained a GDM frequency rate of 5.7% (18.9%
in our study). The increase in L levels in the first trimester were
found to be highly significant for the development of GDM
(P<0.001). An increase in L levels above 31.0 ng/ml resulted in
a 4.7 times increase in the risk of developing GDM (95% CI,
1.2-18.0). At the same time, other authors found a close linear
increased association between L levels and the risk of developing
GDM. Thus, a 10 ng/m increase in leptin levels was followed by a
20% increase in the risk of developing GDM (17). In our study, L values that were
double compared to those in the control group were associated with
a 16% increased risk in GDM.
While conducting a study in which only pregnant
women at risk for GDM underwent OGTT, Thagaard et al
reported an increase in L levels only in obese patients with GDM
and not in normal weight patients (13). In the present research, the low AN/L
ratio showed a statistically significant correlation with the
development of GDM and fetal macrosomia. Thagaard et al also
reported that alterations of this ratio in the first trimester of
pregnancy have a good predictive value for GDM in patients with
normal weight or moderate obesity (BMI <35 kg/m2)
(13). Skvarca et al showed
that the AN/L ratio is the best marker for assessing insulin
resistance in normal weight pregnant women, being correlated with
the HOMA-IR index (6).
Various studies have reported maternal obesity as
the leading cause of increased L levels in pregnant women with GDM.
In this case, elevated L levels would be the result of changes due
to obesity combined with pregnancy-related physiological changes,
and not the result of independent changes due to GDM. In
normal-weight pregnant women GDM is caused by inadequate insulin
synthesis, while in overweight/obese pregnant women, GDM is the
result of inadequate insulin synthesis and increased peripheral
insulin resistance (15-22).
Therefore, increased L levels are the result of the
above mentioned changes and obese patients may develop peripheral
resistance to L, similar to insulin resistance. However, the
mechanisms involved in the correlation between L levels, peripheral
insulin resistance, insulin levels and obesity during pregnancy,
are still incompletely elucidated and represent a current study
issue of international interest on family health (23).
Our study did not show statistically significant
associations between AN levels and GDM development; the values
being similar between the GDM and the non-GDM group (26.8 vs. 28.4
g/ml). Therefore, AN does not predict GDM development. Instead, our
research showed an inversely proportional association between AN
levels and the weight of newborns at birth (P=0.020). This result
was also confirmed by Nanda et al, who showed the role of
hypoadiponectinemia in predicting fetal macrosomia (24).
Studies on the connection between the decrease in AN
levels in pregnancy and the development of GDM are controversial.
Many of these studies could not find a correlation between changes
in AN levels in the first trimester and the development of GDM,
similarly to our findings (6,25-27).
Another study reported a positive correlation
between AN levels and fetal weight at birth, as well as a negative
correlation between AN levels and head circumference (28). Paradisi et al reported a 5%
physiological decrease in AN levels during pregnancy in the second
trimester of pregnancy compared to the first trimester, as well as
a 20% decrease in AN levels in the third trimester, compared to the
first trimester, which was not due to GDM. However, given that AN
level variations in the first trimester in pregnant women with GDM
were not significantly altered compared to non-GDM patients, this
biomarker did not prove to be effective as a predictive factor for
GDM (29).
On the other hand, some studies have shown that AN
levels are decreased in GDM, highlighting that this decrease in AN
levels is independent of maternal adiposity and could be predictive
of GDM development (17,30,31).
Another biomarker of interest when trying to explain
the changes leading to the development of GDM is CMPF, a metabolite
of furan whose level is elevated in the plasma of patients with
T2DM and in pregnant women with GDM. Our research did not reveal
significant differences between the two groups (GDM and non-GDM
group) in as far as this metabolite is concerned [mean (25; 75
percentiles): 180.6 (154.4; 201.9) vs. 179.2 (153.1; 213.1)].
Lankinen et al showed that elevated CMPF levels may be the
result of a diet rich in fish, and that elevated levels are not
associated with the development of GDM (32). However, further studies on a large
number of cases are needed to demonstrate the relationship between
the increase in CMPF levels and the development of GDM.
The limitations of this study consist, first of all,
in the small number of patients, the short time allotted to the
selection of cases, and the number of samples collected during
pregnancy.
The markers (L, AN and CMPF) were determined at the
same time with the genetic screening, and there may be variations
depending on various factors (fasting prior to sample collection or
not). Multiple harvests during pregnancy would be needed in order
to clarify how these markers change with the evolution of the
pregnancy.
A second issue includes the determination of the way
in which BMI can influence L and AN levels or, in other words, to
what extent L and AN levels are altered by the presence of GDM
and/or by obesity in patients with a high BMI.
Continuing this research on large groups of patients
could help physicians select patients at risk to develop GDM, open
new horizons in as far as the therapeutic conduct in the case of
these patients is concerned, and provide a better understanding of
the pathophysiological mechanisms of this disease.
The use of biomarkers for the early diagnosis of GDM
can have a beneficial impact on maternal health decreasing the
mortality rate and maternal-fetal morbidity by changing lifestyle,
diet, and early treatment.
In conclusion, our results concerning L values are
encouraging and predictive of the development of GDM as early as
the first trimester of pregnancy. Most importantly, this parameter
is independent of the patient BMI, contrary to what many other
studies report. A low AN/L ratio value is predictive of GDM
development and is associated with fetal macrosomia. Research on
far larger study groups is needed to demonstrate the predictive
role of CMPF and AN.
Acknowledgements
Not applicable.
Funding
This study was partially funded by the ‘Iuliu Hatieganu’
University of Medicine and Pharmacy of Cluj-Napoca, Romania for
Doctoral Research Projects (no. 1300/48/13.01.2017).
Availability of data and materials
The datasets used and/or analyzed in the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
ARF was the main coordinator of the project and was
responsible for the study design. GC and ARF drafted the manuscript
of the present paper. RMP was involved in the supervising of the
data collection and stratification. AS contributed to data assembly
and analysis. SF and MD contributed to the study design and
manuscript revision. All authors contributed intellectually to this
manuscript and have approved this final version.
Ethics approval and consent to
participate
The study was approved by the Bioethics Commission
of the ‘Iuliu Hatieganu’ University of Medicine and Pharmacy in
Cluj-Napoca, Romania (Nr.247/08.06.2017). Patients who participated
in this research had complete clinical data. Signed informed
consents were obtained from the patients or the guardians.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Soheilykhah S, Mojibian M, Rahimi-Saghand
S, Rashidi M and Hadinedoushan H: Maternal serum leptin
concentration in gestational diabetes. Taiwan J Obstet Gynecol.
50:149–153. 2011.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Moini J: Chapter 6: The Health Impact of
Diabetes. In: Epidemiology of Diabetes. Elsevier, pp115-145,
2019.
|
|
3
|
Sacks DA, Hadden DR, Maresh M,
Deerochanawong C, Dyer AR, Metzger BE, Lowe LP, Coustan DR, Hod M,
Oats JJ, et al: Frequency of gestational diabetes mellitus at
collaborating centers based on IADPSG consensus panel-recommended
criteria: The hyperglycemia and adverse pregnancy outcome (HAPO)
study. Diabetes Care. 35:526–528. 2012.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Ornoy A, Reece EA, Pavlinkova G, Kappen C
and Miller RK: Effect of maternal diabetes on the embryo, fetus,
and children: Congenital anomalies, genetic and epigenetic changes
and developmental outcomes. Birth Defects Res C Embryo Today.
105:53–72. 2015.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Standards of Medical Care in
Diabetes-2016: Summary of revisions. Diabetes Care. 39 (Suppl
1):S4–S5. 2016.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Skvarca A, Tomazic M, Blagus R, Krhin B
and Janez A: Adiponectin/leptin ratio and insulin resistance in
pregnancy. J Int Med Res. 41:123–128. 2013.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Li H, Dong A and Lv X: Advanced glycation
end products and adipocytokines and oxidative stress in placental
tissues of pregnant women with gestational diabetes mellitus. Exp
Ther Med. 18:685–691. 2019.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Prentice KJ, Luu L, Allister EM, Liu Y,
Jun LS, Sloop KW, Hardy AB, Wei L, Jia W, Fantus IG, et al: The
furan fatty acid metabolite CMPF is elevated in diabetes and
induces β cell dysfunction. Cell Metab. 19:653–666. 2014.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Diaz SO, Pinto J, Graça G, Duarte IF,
Barros AS, Galhano E, Pita C, Almeida Mdo C, Goodfellow BJ,
Carreira IM and Gil AM: Metabolic biomarkers of prenatal disorders:
An exploratory NMR metabonomics study of second trimester maternal
urine and blood plasma. J Proteome Res. 10:3732–3742.
2011.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Nolan CJ: Lipotoxicity, β cell
dysfunction, and gestational diabetes. Cell Metab. 19:553–554.
2014.PubMed/NCBI View Article : Google Scholar
|
|
11
|
von Elm E, Altman DG, Egger M, Pocock SJ,
Gøtzsche PC and Vandenbroucke JP: STROBE Initiative. The
strengthening the reporting of observational studies in
epidemiology (STROBE) Statement: Guidelines for reporting
observational studies. PLoS Med. 16(e296)2007.PubMed/NCBI View Article : Google Scholar
|
|
12
|
International Association of Diabetes and
Pregnancy Study Groups Consensus Panel. Metzger BE, Gabbe SG,
Persson B, Buchanan TA, Catalano PA, Damm P, Dyer AR, Leiva Ad, Hod
M, et al: International Association of Diabetes and Pregnancy Study
Groups recommendations on the diagnosis and classification of
hyperglycemia in pregnancy. Diabetes Care. 33:676–682.
2010.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Thagaard IN, Krebs L, Holm JC, Lange T,
Larsen T and Christiansen M: Adiponectin and leptin as first
trimester markers for gestational diabetes mellitus: A cohort
study. Clin Chem Lab Med. 55:1805–1812. 2017.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Savvidou M, Nelson SM, Makgoba M, Messow
CM, Sattar N and Nicolaides K: First-trimester prediction of
gestational diabetes mellitus: Examining the potential of combining
maternal characteristics and laboratory measures. Diabetes.
59:3017–3022. 2010.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Hedderson MM, Xu F, Darbinian JA,
Quesenberry CP, Sridhar S, Kim C, Gunderson EP and Ferrara A:
Prepregnancy SHBG concentrations and risk for subsequently
developing gestational diabetes mellitus. Diabetes Care.
37:1296–1303. 2014.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Bao W, Baecker A, Song Y, Kiely M, Liu S
and Zhang C: Adipokine levels during the first or early second
trimester of pregnancy and subsequent risk of gestational diabetes
mellitus: A systematic review. Metabolism. 64:756–764.
2015.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Qiu C, Williams MA, Vadachkoria S,
Frederick IO and Luthy DA: Increased maternal plasma leptin in
early pregnancy and risk of gestational diabetes mellitus. Obstet
Gynecol. 103:519–525. 2004.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Maple-Brown L, Ye C, Hanley AJ, Connelly
PW, Sermer M, Zinman B and Retnakaran R: Maternal pregravid weight
is the primary determinant of serum leptin and its metabolic
associations in pregnancy, irrespective of gestational glucose
tolerance status. J Clin Endocrinol Metab. 97:4148–4155.
2012.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Eriksson B, Löf M, Olausson H and Forsum
E: Body fat, insulin resistance, energy expenditure and serum
concentrations of leptin, adiponectin and resistin before, during
and after pregnancy in healthy Swedish women. Br J Nutr. 103:50–57.
2010.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Misra VK and Trudeau S: The influence of
overweight and obesity on longitudinal trends in maternal serum
leptin levels during pregnancy. Obesity (Silver Spring).
19:416–421. 2011.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Cruciat G, Nemeti G, Goidescu I, Anitan S
and Florian A: Hypertriglyceridemia triggered acute pancreatitis in
pregnancy-diagnostic approach, management and follow-up care.
Lipids Health Dis. 19(2)2020.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Park S, Kim MY, Baik SH, Woo JT, Kwon YJ,
Daily JW, Park YM, Yang JH and Kim SH: Gestational diabetes is
associated with high energy and saturated fat intakes and with low
plasma visfatin and adiponectin levels independent of prepregnancy
BMI. Eur J Clin Nutr. 67:196–201. 2013.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Froy O, Sherman H, Bhargava G, Chapnik N,
Cohen R, Gutman R, Kronfeld-Schor N and Miskin R: Spontaneous
caloric restriction associated with increased leptin levels in
obesity-resistant αmUPA mice. Int J Obes. 35:226–235.
2011.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Nanda S, Akolekar R, Sarquis R, Mosconi AP
and Nicolaides KH: Maternal serum adiponectin at 11 to 13 weeks of
gestationin the prediction of macrosomia. Prenat Diagn. 31:479–483.
2011.PubMed/NCBI View
Article : Google Scholar
|
|
25
|
Saucedo R, Zarate A, Basurto L, Hernandez
M, Puello E, Galvan R and Campos S: Relationship between
circulating adipokines and insulin resistance during pregnancy and
postpartum in women with gestational diabetes. Arch Med Res.
42:318–323. 2011.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Yamamoto Y, Hirose H, Saito I, Tomita M,
Taniyama M, Matsubara K, Okazaki Y, Ishii T, Nishikai K and Saruta
T: Correlation of the adipocyte-derived protein adiponectin with
insulin resistance index and serum high-density
lipoprotein-cholesterol, independent of body mass index, in the
Japanese population. Clin Sci. 103:137–142. 2002.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Mohammadi T and Paknahad Z: Adiponectin
concentration in gestational diabetic Women: A case-control study.
Clin Nutr Res. 6:267–276. 2017.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Vernini JM, Moreli JB, Antônio RA, Costa
RA, Negrato CA, Rudge MV and Calderon IM: Maternal adipokines and
insulin as biomarkers of pregnancies complicated by overweight and
obesity. Diabetol Metab Syndr. 8(68)2016.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Paradisi G, Ianniello F, Tomei C,
Bracaglia M, Carducci B, Gualano MR, La Torre G, Banci M and Caruso
A: Longitudinal changes of adiponectin, carbohydrate and lipid
metabolism in pregnant women at high risk for gestational diabetes.
Gynecol Endocrinol. 26:539–545. 2010.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Lain KY, Daftary AR, Ness RB and Roberts
JM: First trimester adipocytokine concentrations and risk of
developing gestational diabetes later in pregnancy. Clin Endocrinol
(Oxf). 69:407–411. 2008.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Lacroix M, Battista MC, Doyon M, Ménard J,
Ardilouze JL, Perron P and Hivert MF: Lower adiponectin levels at
first trimester of pregnancy are associated with increased insulin
resistance and higher risk of developing gestational diabetes
mellitus. Diabetes Care. 36:1577–1583. 2013.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Lankinen MA, Hanhineva K, Kolehmainen M,
Lehtonen M, Auriola S, Mykkänen H, Poutanen K, Schwab U and
Uusitupa M: CMPF does not associate with impaired glucose
metabolism in individuals with features of metabolic syndrome. PLoS
One. 10(e0124379)2015.PubMed/NCBI View Article : Google Scholar
|