Introduction
Allergic rhinoconjunctivitis is an IgE
(immunoglobulin E)-mediated disorder that affects the nasal mucosa
and the conjunctiva. Its prevalence in adults varies within Europe,
ranging from 17% (in Italy) to 29% (Belgium) (1,2).
Inhaled allergens are a significant factor in morbidity and may
severely affect quality of life (3-5).
Symptoms of allergic rhinoconjunctivitis include sneezing, nasal
itching and obstruction, watery nasal discharge, eye itching and
congestion, and tearing, while coughing episodes, difficulty
breathing or wheezing are associated with asthma. Major causative
agents for allergic rhinoconjunctivitis are pollen grains, house
dust mites, pet danders and fungal spores (6,7).
Over the last few decades, a matter of particular
concern in this field has been Ambrosia (ragweed) pollen,
its presence in the air being caused by the plants dispersing it
and meteorological processes that alter pollen release,
dissemination, transport or deposition on surfaces (8).
Ragweed (family Asteraceae, genus Ambrosia)
is an invasive species of annual herbaceous flowering plant,
originally native to Northern America, which started to spread
through Europe in the 19th century (9). This weed can often be found on
roadsides, riverbanks, or abandoned lands and fields. Ragweed seeds
are able to survive for many decades in the soil and the plant
grows again under favorable conditions (10). Climate change (particularly rising
temperatures and carbon dioxide levels), urbanization and pollution
have led to the increase in ragweed biomass, length of the pollen
season and atmospheric pollen counts, while current forecasts show
an upward trend in the future (11). These factors play and will continue
to play a major role in the increasing burden generated by ragweed
sensitization for people living in affected areas (12).
The most prevalent species is Ambrosia
artemisiifolia (common or short ragweed), which is also
clinically the most relevant for its high potential to cause
allergic sensitization. Ragweed pollen can be transported over
great distances, while trade and traffic globalization have been
identified as the most important factors for ragweed distribution.
Ragweed pollen has been identified at great distances, of more than
600 km offshore and over 3 km up in the air (13). A single ragweed plant is estimated
to produce one billion pollen grains during one season (14,15).
The symptoms induced by ragweed pollen in sensitized individuals
correlate with and may last beyond the pollen season. In Romania,
only partial statistics regarding ragweed pollen spread and the
number of ragweed-allergic patients are available (16,17).
The highest atmospheric ragweed pollen concentration is encountered
in August and September, but the pollen is present in the air for a
much longer period, starting with mid-July and up to late October,
depending on weather conditions (18). There are currently 11 recognized
ragweed pollen allergens, numbered from 1 to 11 (Amb a 2 was
renamed as isoform Amb a 1.05), out of which Amb a 1 and Amb a 11
are considered major allergens, inducing sensitization in more than
50% of ragweed-allergic patients (19). Moreover, some ragweed pollen
allergens have homology to pollen allergens from Artemisia
vulgaris (mugwort), for example, Amb a 1 and Art v 6, Amb a 4
and Art v 1(20), raising the
question of whether some patients are truly co-sensitized to both
pollens, or whether cross-reactivity is at play (21). Mugwort belongs to the plant family
Asteraceae; its pollen season is longer than that of ragweed, with
which it partly overlaps. However, mugwort pollen concentrations in
the atmosphere do not reach such high values as ragweed, therefore
it does not affect as great a number of individuals.
The diagnosis of allergic rhinoconjunctivitis and
asthma relies on the precise medical history of the patient,
especially regarding potential sources of exposure, seasonal
symptoms of nasal inflammation and airway hyperresponsiveness. The
main clinical test used in practice is the skin prick test (SPT),
which can be accompanied by other diagnostic tools such as nasal
allergen challenge or spirometry. Paraclinical testing includes
molecular diagnosis and allows the identification of sensitizing
allergens and cross-reactions by measuring specific serum IgE to
relevant allergens. SPT is usually considered the first line of
diagnosis of IgE-mediated allergies, due to its advantages over
specific serum IgE, such as rapid results, flexibility, low cost
and good tolerability. The sensitivities and specificities of the
two methods have been compared in studies, with varying results,
some showing that SPT is more sensitive and less specific (22,23),
while others show the complete opposite (24,25).
Precise diagnosis is necessary for adequate management of allergic
rhinitis, which may involve allergen immunotherapy (AIT).
The aim of the present study was to determine the
symptom pattern of ragweed pollen-induced allergic disease on
sensitized patients from Western Romania, to investigate potential
cross-sensitivities or co-sensitivities with other allergens, and
to compare the molecular diagnosis of allergy by specific IgE
measurement with the SPT, with the goal of improving allergic
patient characterization and therapeutic guidance.
Patients and methods
Ethics approval and consent to
participate
All SPTs were performed and all peripheral blood
samples were obtained from the participants after the signing of
the informed consent elaborated under an approved protocol by the
Ethics in Scientific Research Commission of the ‘Pius Brinzeu’
County Clinical Emergency Hospital Timisoara, which complies with
Romanian laws (Law no. 95/2006, article 67, and article 28, chapter
VIII 904/2006) and with EU GCP Directive 2005/28/EC (26), International Conference of
Harmonisation of Technical Requirements for Registration of
Pharmaceuticals for Human Use (ICH) (27) and the Declaration of
Helsinki-Recommendations Guiding Medical Doctors in Biomedical
Research Involving Human Subjects (28).
Study design and patients
A total of 83 ragweed allergic patients and 14
heathy controls (97 subjects in total) were recruited and observed
prospectively in one ragweed pollen season (August, 2018 to
November, 2018), in a cross-sectional study. The clinical research
was performed in allergy clinics from Timisoara, the main town from
the Western part of Romania. The study included patients that
during ragweed pollen season presented rhinitis, with or without
conjunctivitis, diagnosed according to ARIA (Allergic Rhinitis and
its Impact on Asthma) criteria (29), with or without asthma, diagnosed
according to GINA (Global Initiative for Asthma) criteria (30), and that also had a positive result
on the SPT with ragweed pollen extract. Patients with chronic
pathologies such as cancer and autoimmune disease were excluded, as
well as those with histamine skin wheals on SPT of less than 2 mm.
Negative controls, without any allergic symptoms and with negative
SPT were also included. The healthy controls were recruited to
ensure that the SPT technique was correct and that the allergen
extracts were not providing false-positive results, in order to
consider the SPT as the gold standard; these subjects were not
included in the further analyses.
Symptom evaluation
A self-reported symptom evaluation score was also
applied (Appendix S1). Briefly,
the patients were asked to rate the intensity of their current, or,
in case they were already under antiallergic therapy, their maximal
symptoms, on a scale from 0 to 10. ‘0’ was defined as asymptomatic,
and ‘10’ as intense symptoms that greatly interfere with daily
activities, including sleep, thereby making them impossible. A
symptom score, calculated by summing up all the symptoms pertaining
to allergy that a patient presents, was also evaluated.
Allergen extracts
Patient evaluation was performed by SPT to a panel
of 19 extracts of inhaled allergens (HAL, Düsseldorf, Germany),
containing standardized cutaneous extracts for prick tests: Hazel
(Corylus avellana), alder (Alnus incana), birch
(Betula alba), plane (Platanus vulgaris), oak
(Quercus robur), grass mix (Poa pratensis,
Dactilis glomerata, Lolium perenne, Phleum
pratense, Festuca pratensis, Helictotrichon
pretense), cereal mix (Triticum aestivum, Hordeum
vulgare, Secale cereale), mugwort (Artemisia
vulgaris), ragweed (Ambrosia artemisiifolia), fungi
(Alternaria alternata, Cladosporium herbarum,
Aspergillus fumigatus tested separately), yeasts (Candida
albicans, Saccharomyces mellis tested separately), dog
(Canis familiaris), cat (Felis catus), house dust
mites (Dermatophagoides pteronyssinus, Dermatophagoides
farinae tested separately), and cockroach (Blatella
germanica). Histamine dihydrochloride (10 mg/ml, equivalent to
6 mg histamine) was used as the positive control, and a phenolated
glycero-saline solution as the negative control. The panel of
allergen extracts was selected according to the recommendation of
the Global Allergy and Asthma European Network (GA2LEN)
for a common panel of allergens for Europe (31); however, it did not include cypress
and olive tree pollens, nor Parietaria pollen, as these
plant species are not commonly encountered in Romania.
Allergy skin prick tests
Allergy SPT was performed according to the
GA2LEN recommendation for harmonization of skin prick
testing (32). Briefly, drops of
allergen extract were applied on the volar aspect of the forearm
with a distance of at least 2 cm between them and then the skin was
pricked with lancets. Mean wheal diameter was recorded after 15 min
and a wheal diameter of at least 3 mm was considered a positive
SPT. The tests were performed by trained specialists on healthy
skin, and after appropriate withdrawal of treatments that may
interfere with the skin reaction, such as antihistamines, long-term
or high-dose glucocorticoids, antidepressants, or omalizumab. The
following notations were made, according to wheal mean diameter:
>3 to <4 mm, ‘+’ (very mildly reactive), ≥4 to <10 mm,
‘++’ (mildly reactive), ≥10 to <15, ‘+++’ (moderately reactive)
and ≥15, ‘++++’ (very reactive), and the presence of pseudopodia
was also recorded. This classification of the skin response was
used in order to better correlate it with the IgE class.
ImmunoCAP ISAC assay
For the evaluation of allergen-specific IgE
antibodies in blood, the ImmunoCAP ISAC (Immuno-Solid phase Allergy
Chip) assay was used, which is a semi-quantitative molecular
diagnostic test that reports results in ISAC standard units (ISU),
indicating allergen-specific IgE levels; the operating range is
0.3-100 ISU. The specific ISAC microarray chip that was used was
developed through the European Union-funded project Mechanisms for
the Development of Allergies; (MeDALL) (33), which can measure IgE antibodies to
176 allergen components (34),
using only 20 µl of serum. A sample consisting of 5 ml of venous
blood was collected from each patient in a red-top blood collection
tube (without anticoagulant or preservative). After collection of
the whole blood, it was allowed to clot by leaving it undisturbed
at room temperature for 30 min, and then the clot was removed by
centrifugation at 1,500 x g for 10 min at room temperature. The
resulting supernatant was immediately apportioned into 0.5 ml
aliquots and stored at -70˚C until further processing.
Allergen-specific IgE was measured in sera from ragweed-allergic
patients using a fluoroenzyme immunoassay auto-analyser (Thermo
Fisher Scientific Inc., Phadia AB), according to the manufacturer's
guidelines. Briefly, 20 µl of serum was added to each microarray
and incubated at room temperature for 120 min. The samples were
washed and then incubated for 30 min with 20 µl of
fluorescence-labeled antihuman IgE antibodies. Unbound antibodies
were removed by washing and the fluorescence of the processed ISAC
slides was measured in a GenePix4000B microarray scanner (Molecular
Devices). Image analysis was performed using a microarray image
analyzer software (MIA, Thermo Fisher Scientific Inc., Phadia AB).
Fluorescence measurements were compared with a calibration curve
and expressed as ISU. Established cut-off values were used to
interpret the results: Values <0.3 ISU were considered negative
(undetectable or very low) with regards to sensitization to a
specific allergen, and values of ≥0.3 ISU were distributed into
three classes, as follows: For ISU values ≥0.3 to <1, class 1
(low); for ISU values ≥1 to <15, class 2 (moderate to high); and
for ISU values ≥15, class 3 (very high). IgE class according to ISU
was also correlated with the intensity of the response to ragweed
pollen extract in the SPT. The chip included only the established
major allergen from ragweed pollen, Amb a 1, and not other ragweed
pollen allergens, nor ragweed pollen extract. The sensitivity and
specificity of ImmunoCAP ISAC were compared against the SPT, which
was considered the standard method for allergy diagnosis, as it was
the method used for patient selection.
Statistical analysis
Statistical analysis and data collection were
performed using Microsoft Office Excel 2013, two-tailed tests and
non-parametric tests such as Spearman's rho to measure the strength
of association between two variables. A statistical significance
threshold of 0.05 was used (P<0.05).
Results
Demographical data, clinical symptoms,
and skin prick test results
A total of 83 ragweed-allergic patients were
recruited in the study, according to their symptoms and the results
of the SPT to ragweed pollen extract. Mean patient age was 31.2±8.9
years, with a mean allergic disease age (duration since first
appearance of symptoms) of 3.62±3.15 years. A total of 40% of the
patients were male, and 60% female. There were no significant
differences between male and female patients regarding skin
sensitivity. Most patients (73%) were diagnosed with
moderate-severe intermittent allergic rhinoconjunctivitis, and 25%
of the patients also had allergic asthma. Mean maximum intensity of
symptoms was 8.15 out of 10 on the self-evaluation scale. The most
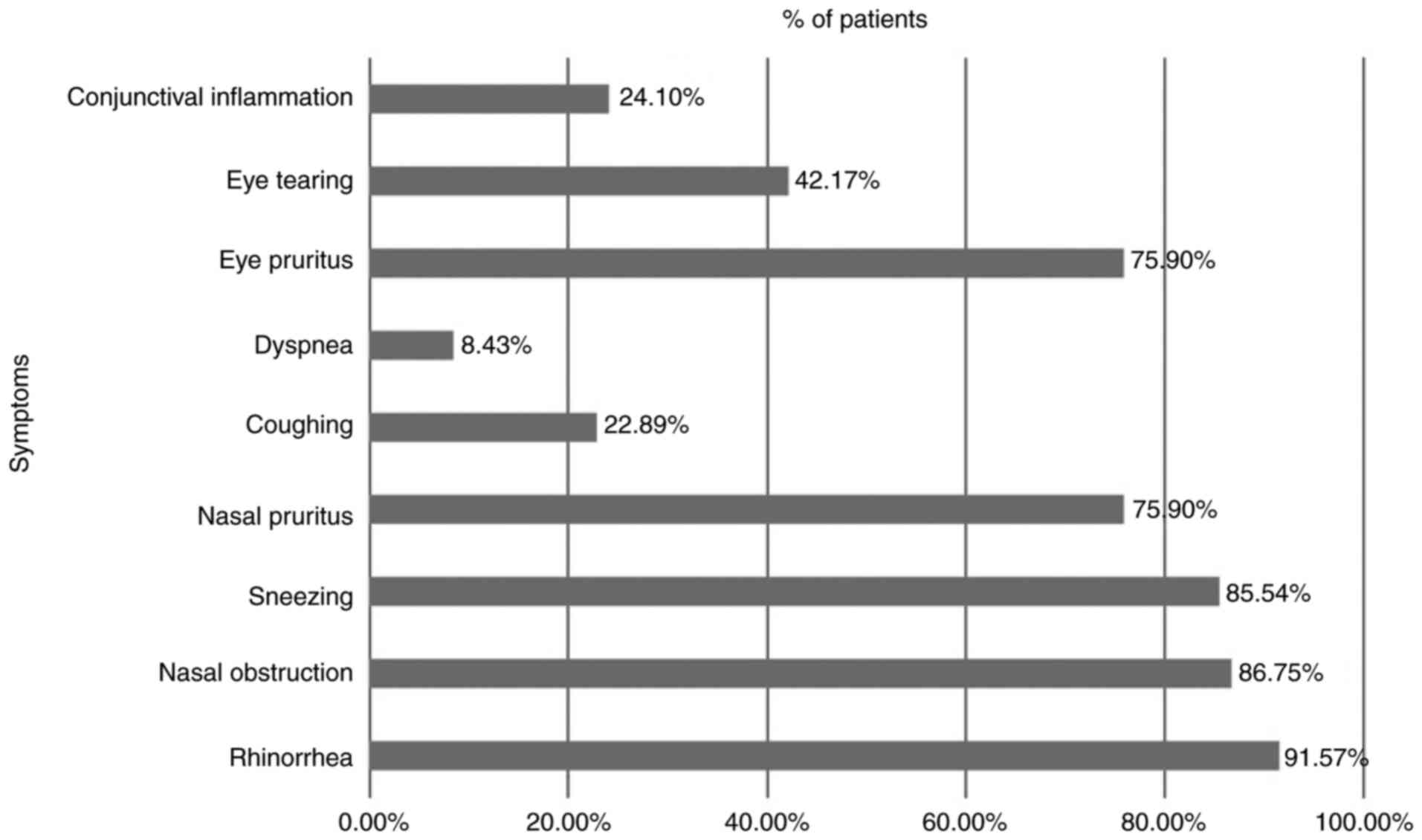
common symptoms were: Watery rhinorrhea (in 91.57% of the
patients), nasal obstruction (in 86.75% of the patients), sneezing
(in 85.54% of the patients), nasal pruritus (in 75.90% of the
patients), and eye pruritus (in 75.90% of the patients) (Fig. 1).
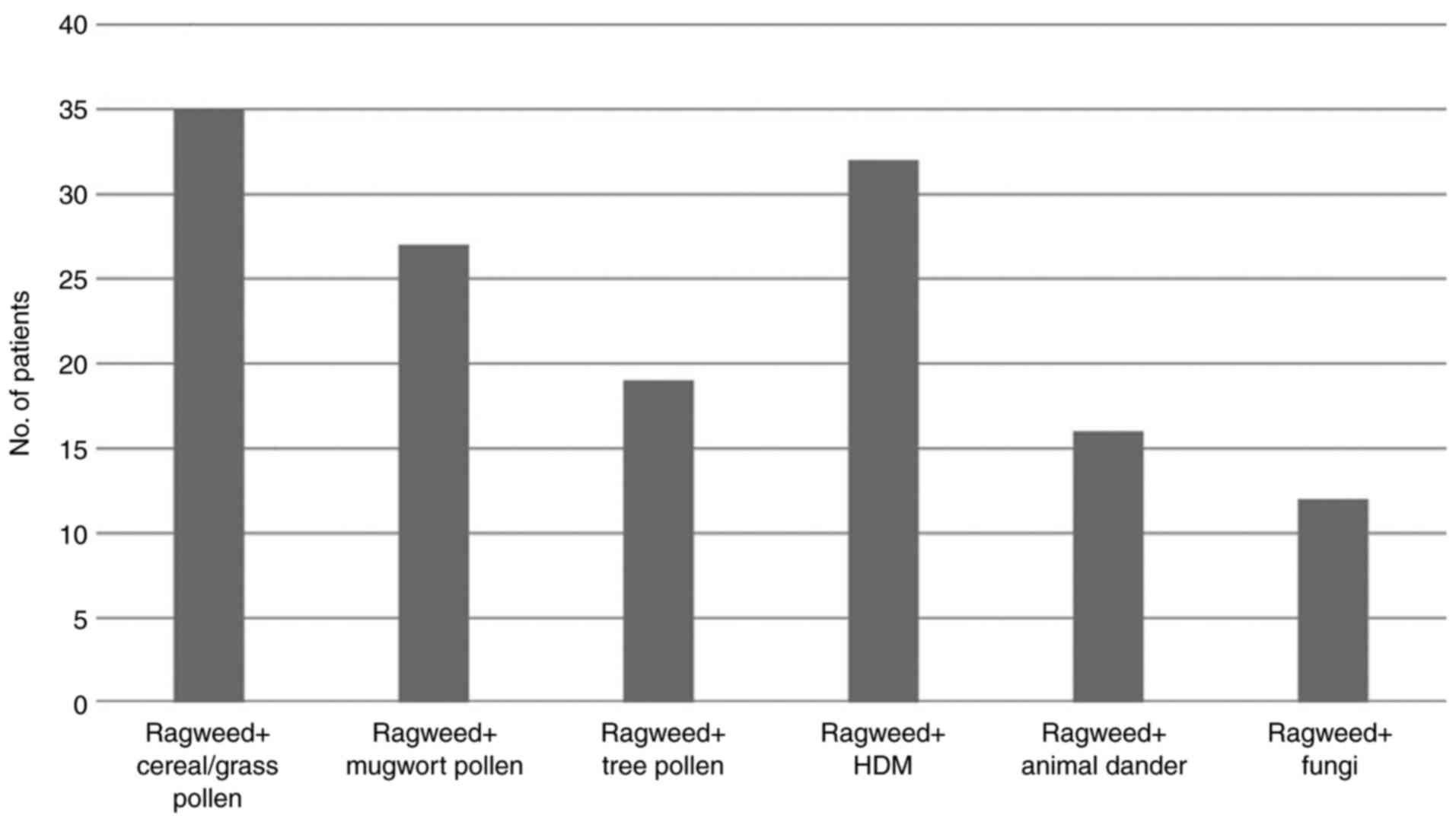
The majority of the patients were polysensitized
(62.65%) according to the SPT results. Most of the polysensitized
patients were sensitized to other pollens (cereals, grasses,
Artemisia, birch, oak), but a large percentage was also sensitized
to house dust mites (HDM), the main perennial allergen in this
geographical area (Fig. 2).
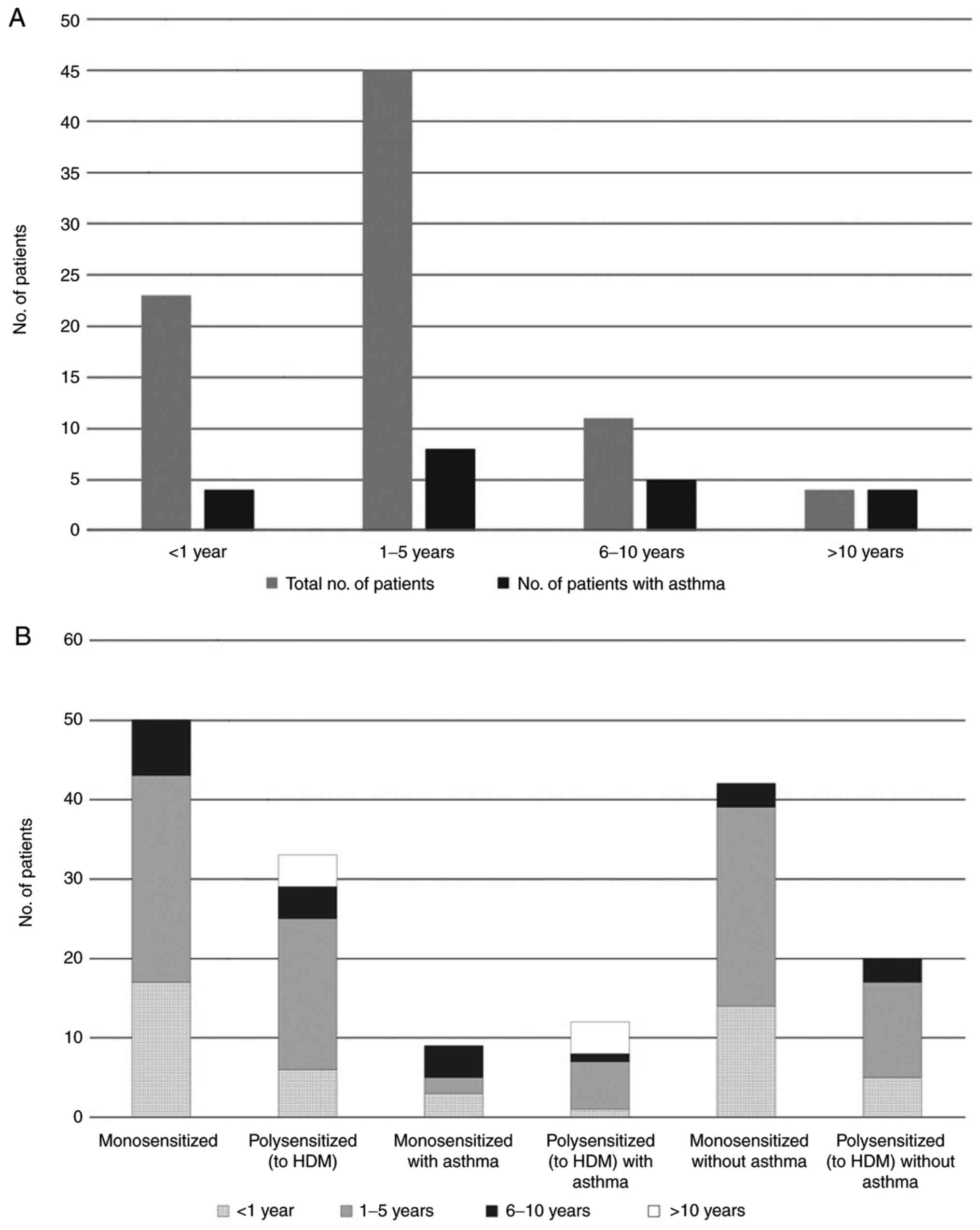
All patients with a disease history of more than 10
years had developed allergic asthma, with exacerbations during the
ragweed pollen season. (Fig. 3A).
However, when looking separately at patients only sensitized to
ragweed pollen vs. patients also sensitized to house dust mites
(HDM), only the HDM-sensitized patient group had a disease history
of >10 years (Fig. 3B).
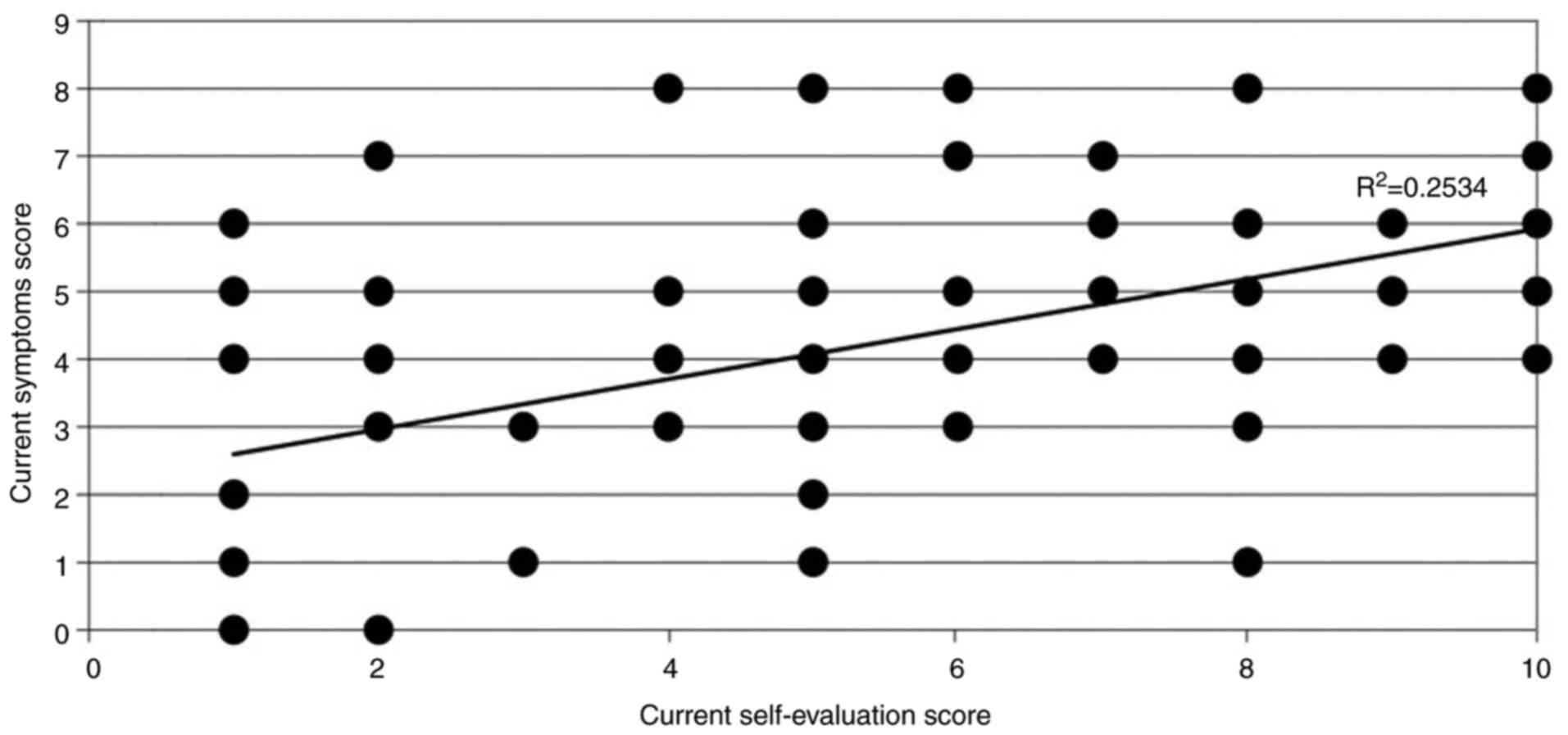
Correlation between self-reported
evaluation score and a computed symptoms score
A moderate correlation was found between the
self-reported evaluation score (on a scale 0-10) and a computed
symptoms score (by adding up all the symptoms a patient presents),
by using Spearman's rho (0.584), which was extremely significant
(P<0.0001), indicating an accurate correlation between the
patients' estimate of disease severity and the number of symptoms
(Fig. 4). However, the
R2 obtained was 0.25, which only partially explains the
variability of the response data around its mean. This may occur
because only the number of symptoms present in a patient was taken
into account, not also the severity of each symptom. Therefore,
there could be patients with only one, very severe symptom,
significantly affecting their quality of life, or patients with
several mild symptoms that do not significantly interfere with
their daily activities.
ImmunoCAP ISAC assay results
The results obtained in vivo, by SPT, were
compared against the immunoarray chip, which measures
semi-quantitatively specific serum IgE against 176 molecular
allergens. It includes several molecular allergens from sources
such as HDM (Dermatophagoides pteronyssinus and
farinae), timothy grass (Phleum pratense), or dog
(Canis familiaris), but only one allergen from ragweed. It
does not include any allergens from cultivated grasses or cereal
pollens, such as rye, wheat, or barley, nor from oak tree, which
induced sensitizations in some of the patients included in the
study, as measured by SPT. Significantly, it includes several mold
allergens, but none of the patients sensitized to molds, as
determined by SPT, had a positive result for specific serum IgE
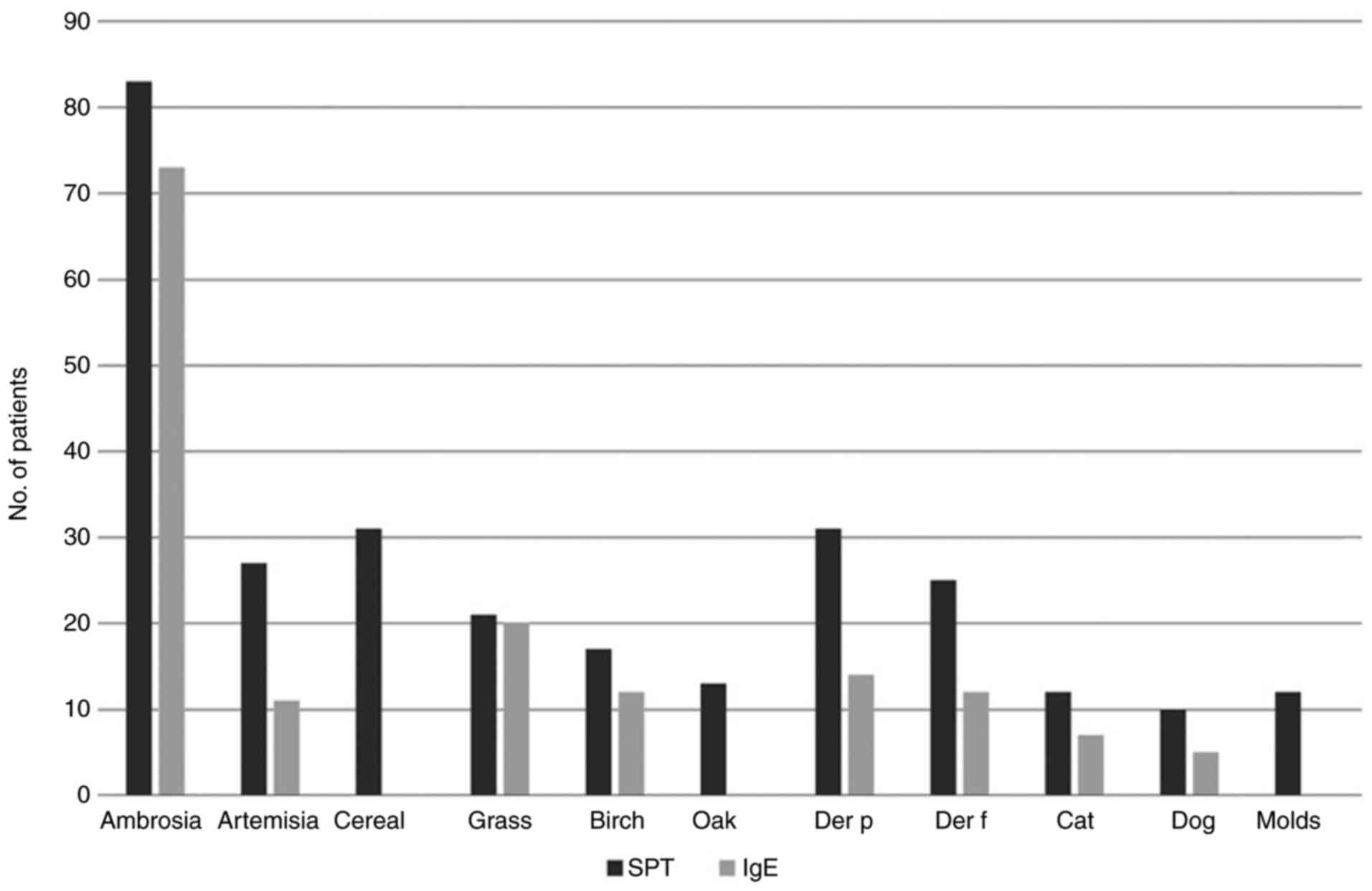
against molds (Fig. 5, Table I).
 | Table IComparison between SPT and IgE
results. |
Table I
Comparison between SPT and IgE
results.
| | Ambrosia | Artemisia | Cereal | Grass | Birch | Oak | Der p | Der f | Cat | Dog | Molds |
|---|
| % positive IgE out
of positive SPT | 87.95 | 40.74 | 0 | 95.23 | 70.58 | 0 | 45.16 | 48 | 58.33 | 50 | 0 |
Out of the 83 patients with positive SPT to ragweed
pollen extract, 90% also had increased specific serum IgE levels to
the major ragweed allergen, Amb a 1 (5% class 1, 35% class 2, and
45% class 3). Sensitivity and specificity of the ImmunoCAP ISAC
microarray (Amb a 1) vs. SPT with ragweed pollen extract was 85.88%
(95% CI: 76.63-92.48%), and 90.91% (95% CI: 58.67-98.49%),
respectively. However, IgE class did not correlate with the mean
diameter of the wheal in SPT.
A particular situation was noted regarding the 11
patients that had a positive SPT to ragweed pollen extract and
negative specific IgE to Amb a 1. The possibility of
cross-reactivity with allergens from mugwort pollen was examined in
more detail, due to the high homology shared by several allergens
from these two plants from the Asteraceae family, and a possible
interpretation is provided in Table
II.
 | Table IIInterpretation of SPT to ragweed
pollen extract and mugwort pollen extract, and specific IgE to Amb
a 1 and Art v 1. |
Table II
Interpretation of SPT to ragweed
pollen extract and mugwort pollen extract, and specific IgE to Amb
a 1 and Art v 1.
| Results | No. of
patients | Interpretation |
|---|
| SPT to mugwort neg
and Art v 1 neg | 7 | Probably sensitized
to other major and/or minor allergens from ragweed pollen, but
which do not share cross-reactivity with allergens from mugwort
(e.g., Amb a 11) |
| SPT to mugwort neg
and Art v 1 pos | 2 | Probably due to
cross-sensitization between Art v 1 and Amb a 4 |
| SPT to mugwort pos
and Art v 1 neg | 1 | Probably sensitized
to minor allergens from ragweed pollen which have cross-reactivity
with minor allergens from mugwort pollen (e.g., profilins-Amb a 8
with Art v 4, or polcalcins-Amb a 9 with Art v 5) |
| SPT to mugwort pos
and Art v 1 pos | 1 | Probably sensitized
to mugwort pollen |
Potential cross-reactivity between Amb a 1 and other
allergens from same pectate lyase family, but from species not
present in the local flora, such as Cry j 1 from Japanese cedar and
Cup a 1 from Arizona cypress was also investigated. Cry j 1
specific IgE were identified in ~24% of the Amb a 1 positive
patients, as opposed to none of the Amb a 1 negative patients,
which was a statistically significant difference (P<0.05). Cup a
1 specific IgE was identified in ~8% of the Amb a 1 positive
patients, as opposed to none of the Amb a 1 negative patients,
which was statistically insignificant (P>0.05) (Table III).
 | Table IIICross-reactivity between Amb a 1 and
Cry j 1, and Cup a 1 respectively. |
Table III
Cross-reactivity between Amb a 1 and
Cry j 1, and Cup a 1 respectively.
| | SPT ragweed
pos |
|---|
| | Amb a 1 neg
(%) | Amb a 1 pos
(%) |
|---|
| Cry j 1 neg | 100.00 | 76.06 |
| Cry j 1 pos | 0.00 | 23.94 |
| Cup a 1 neg | 100.00 | 91.55 |
| Cup a 1 pos | 0.00 | 8.45 |
Discussion
The principle of the skin prick test (SPT) is that
the introduction of relevant allergens in the skin induces a wheal
and flare response, which can be measured in a standardized
fashion. This reaction is due to the cross-linkage of specific IgE
bound on mast cells, which leads to their degranulation and
subsequent release of inflammatory mediators, such as histamine
(35). However, in vitro
methods have become an important complementary tool for allergy
diagnosis, and they are even starting to replace the in vivo
diagnosis, due to its limitations. SPT has several
contraindications, for example it cannot be used in patients with
extensive eczema, dermographism, urticaria with or without
angioedema (36), or in patients
under medication that may interfere with the test results, such as
antihistamines, glucocorticoids, and some classes of
antidepressants (31). Another
limitation is the fact that allergen extracts are biological
mixtures, containing different concentrations of allergens,
depending on the extraction method; therefore, results obtained
with allergen extracts from different manufacturers can vary
greatly (37,38). These variations are even more
relevant when the biological extracts are used for allergen
immunotherapy (39). Currently, the
numbers of available extracts for in vivo testing and for
immunotherapy has diminished greatly, due to the increasingly
stringent rules for market approval in Europe (40). Another point that has not been
addressed is the potential of SPT to induce de novo
sensitizations, as the skin has been known to be an effective route
for sensitization (41,42).
Despite all these limitations, the advantages of the
SPT such as its cost efficiency, extensive support by national
health insurance houses, and lack of expensive equipment
requirements make it an indispensable tool for the immediate
diagnosis of allergic patients by physicians of different
specialties, and enhance patient compliance by providing a
convincing visual image of the extent of their sensitivities.
Unlike allergen extracts and natural allergens,
recombinant allergens produced by techniques of molecular
engineering are pure, and produced under standardized, reproducible
conditions. They can be further used for singleplex (ImmunoCAP) or
multiplex (ISAC microarray chip, ALEX2) molecular
diagnosis of respiratory and food allergies, as well as for
allergen immunotherapy. Molecular allergy diagnosis can
differentiate between genuine sensitization and cross-reactivity,
as well as guide allergen immunotherapy to a more targeted and
personalized approach (43).
ImmunoCAP ISAC, based on 112 different molecular components, is the
most studied and most frequently used molecular diagnostic tool
based on a microarray. Yet, in the present study we used an
experimental microarray chip with 176 allergens, and technically,
chips with many more allergens can be blotted as well as
personalized chips with different allergen patterns, according to
patient symptoms and medical history. Currently microarray chips
are not used as the primary diagnostic tool, due to their high
costs; moreover, they are also not covered by national health
insurance. Unfortunately, socioeconomic status influences
healthcare.
Any result, either obtained by in vivo or
in vitro methods, should be interpreted in the context of
clinical symptoms and medical history, and, as using either method
by itself may lead to inappropriate diagnosis of some patients, it
is therefore recommended to use the in vivo and in
vitro testing methods in complementary fashion whenever
possible. When the test results and the medical history are
inconclusive, challenge tests may help in determining the clinical
relevance of sensitization.
The two major allergens from ragweed pollen are
considered to be Amb a 1, a pectate lyase, and Amb a 11, a cysteine
protease (44). SPT extracts may
contain variable amounts of these proteins and also of the other
allergenic proteins from ragweed pollen, depending on the
extraction methods as well as on the local characteristics of the
plants (45). However, to date,
only methods of testing for specific IgE against ragweed pollen
extract, Amb a 1 and Amb a 4 exist. When using the ImmunoCAP ISAC
microarray chip, which only includes Amb a 1, not all sera from
patients that tested positive to ragweed pollen extract by SPT
showed a positive reaction. This suggests that some
ragweed-allergic patients would be undiagnosed or misdiagnosed by
using current in vitro diagnosis methods, which rely on
component-resolved diagnosis, but do not have the full array of
allergens from ragweed pollen. As shown in Table II, these patients may fall under
several different scenarios-either sensitized primarily to other
major and/or minor allergens from ragweed pollen, some of which may
be shared with mugwort or with other plants, or they may be
sensitized primarily to other plants, which contain pan-allergens
such as profilins or polcalcins sharing homology with ragweed
allergens. Therefore, they may also not respond to allergen
immunotherapy (AIT) using recombinant Amb a 1, as they are likely
sensitized to Amb a 11 or minor allergens from ragweed pollen.
The identification of Cry j 1-specific IgE in
patients sensitized to Amb a 1 is interesting, as the sequence
identity between the molecules is not too high, 46-49% (46), even though both, as well as Cup a 1,
are pectate lyases. In addition, exposure to Japanese cedar was
minimal in this sample population, as it is not a local plant.
The clinical utility of a precise molecular
diagnosis in allergic diseases was demonstrated in a previous study
by Chen et al (47), who
performed a post hoc analysis of sera from house dust mite
(HDM)-allergic patients who had been treated by subcutaneous HDM
AIT in a double-blind, placebo-controlled clinical study, regarding
their IgE and IgG reactivity against a comprehensive panel of HDM
allergens by ImmunoCAP ISAC technology. The clinical effects of AIT
had been evaluated in the patients by controlled allergen exposure
in the Vienna Challenge Chamber. HDM extracts used for allergy
diagnosis with SPT and AIT vary regarding allergen concentration,
as they are only standardized for Der p 1 and Der p 2, even though
there are several other clinically relevant allergens, such as Der
p 5, Der p 7, Der p 21, and Der p 23. The study showed that only
anti-Der p 1 and anti-Der p 2 IgG levels increased in patients that
had undergone AIT with HDM extract. Patients that had also been
sensitized to other HDM allergens beside Der p 1 and Der p 2 showed
no signs of clinical improvement after AIT. Similar outcomes are
expected regarding ragweed-allergic patients undergoing AIT;
therefore, a precise diagnosis regarding the sensitization pattern
is required. Molecular diagnosis using all clinically relevant
ragweed pollen allergens could offer support to allergists, for
identification of several categories of patients: i) Those who may
benefit from AIT with extracts currently available from companies,
ii) those who may be treated with Amb a 1-only AIT, and iii) those
who may need a more personalized approach with a specific pattern
of clinically relevant allergens.
In conclusion, the present study showed that a small
fraction of ragweed-allergic patients, which are sensitized only to
minor ragweed pollen allergens and/or to Amb a 11, cannot be
identified with standard in vitro diagnostic procedures.
Therefore, the development of an improved component-resolved
diagnosis, using several ragweed pollen allergens, is required for
a better patient characterization and subsequent selection of an
appropriate AIT product. The improvement of component-resolved
diagnosis will allow for a more personalized approach to the
management of the ragweed-allergic patient, consisting in an AIT
product that contains only the allergens relevant to a particular
patient, thereby ensuring better patient outcomes.
Supplementary Material
Self-reported allergic symptom
score.
Acknowledgements
Not applicable.
Funding
This work was supported through the project ‘INnovative
Strategies for Prevention, diagnosis and therapy of ragweed pollen
Induced REspiratory Diseases’ (INSPIRED), MySMIS 103663, COP
2014-2020 92/09.09.2016, funded by the National Authority for
Scientific Research and Innovation (ANCSI), under the Operational
Program Competitiveness 2014-2020.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
LH conceptualized and visualized the experiments,
developed the methodology, and prepared the original draft of the
manuscript. LH and TPT performed the formal analysis, validated and
interpreted the data. LH and CP supervised the study. LH, TPT and
CP performed the investigations. LH, FS, RFPP, KWC and CP reviewed
the data, revised the manuscript and edited the final manuscript.
All authors have read and agreed to the published version of the
manuscript.
Ethics approval and consent to
participate
All skin prick tests were performed and all
peripheral blood samples were obtained after signing the informed
consent elaborated under an approved protocol by the Ethics in
Scientific Research Commission of the ‘Pius Brinzeu’ Clinical
Emergency County Hospital Timisoara, which complies with Romanian
laws (Law no. 95/2006, article 67, and article 28, chapter VIII
904/2006) and with EU GCP Directive 2005/28/EC, International
Conference of Harmonisation of Technical Requirements for
Registration of Pharmaceuticals for Human Use (ICH) and the
Declaration of Helsinki-Recommendations Guiding Medical Doctors in
Biomedical Research Involving Human Subjects.
Patient consent for publication
Not applicable.
Competing interests
The authors declare no competing interests. The
funders had no role in the design of the study; in the collection,
analyses, or interpretation of data; in the writing of the
manuscript, or in the decision to publish the results.
Authors' information
ORCiD: Laura Haidar: https://orcid.org/0000-0002-5703-2578; Carmen
Panaitescu: https://orcid.org/0000-0001-8749-9972.
References
|
1
|
Bauchau V and Durham SR: Epidemiological
characterization of the intermittent and persistent types of
allergic rhinitis. Allergy. 60:350–353. 2005.PubMed/NCBI View Article : Google Scholar
|
|
2
|
D'Amato G, Cecchi L, Bonini S, Nunes C,
Annesi-Maesano I, Behrendt H, Liccardi G, Popov T and Van
Cauwenberge P: Allergenic pollen and pollen allergy in Europe.
Allergy. 62:976–990. 2007.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Pawankar R, Canonica GW, Holgate ST,
Lockey RF and Blaiss M: The World Allergy Organization (WAO) White
Book on Allergy: Update 2013. World Allergy Organization (WAO),
Milwaukee, WI, pp87-92, 2013.
|
|
4
|
Bumbacea RS, Corcea SL, Ali S, Dinica LC,
Fanfaret IS and Boda D: Mite allergy and atopic dermatitis: Is
there a clear link? (Review). Exp Ther Med. 20:3554–3560.
2020.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Solomon I, Ilie MA, Draghici C, Voiculescu
VM, Caruntu C, Boda D and Zurac S: The impact of lifestyle factors
on evolution of atopic dermatitis: An alternative approach. Exp
Ther Med. 17:1078–1084. 2019.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Singh AB and Mathur C: An aerobiological
perspective in allergy and asthma. Asia Pac Allergy. 2:210–222.
2012.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Bumbacea R, Berghea E and Giurcaneanu C:
Frequency of contact sensitisation in children with atopic
dermatitis. Allergy. 62 (Suppl 83)(S319)2007.
|
|
8
|
Šaulienė I and Veriankaitė L: Analysis of
high allergenicity airborne pollen dispersion: Common ragweed study
case in Lithuania. Ann Agric Environ Med. 19:451–455.
2012.PubMed/NCBI
|
|
9
|
Smith M, Cecchi L, Skjøth CA, Karrer G and
Šikoparija B: Common ragweed: A threat to environmental health in
Europe. Environ Int. 61:115–126. 2013.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Buters J, Alberternst B, Nawrath S, Wimmer
M, Traidl-Hoffmann C, Starfinger U, Behrendt H, Schmidt-Weber C and
Bergmann KC: Ambrosia artemisiifolia (ragweed) in
Germany-current presence, allergological relevance and containment
procedures. Allergo J Int. 24:108–120. 2015.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Ziska LH, Gebhard DE, Frenz DA, Faulkner
S, Singer BD and Straka JG: Cities as harbingers of climate change:
Common ragweed, urbanization, and public health. J Allergy Clin
Immunol. 111:290–295. 2003.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Oswalt ML and Marshall GD: Ragweed as an
example of worldwide allergen expansion. Allergy Asthma Clin
Immunol. 4:130–135. 2008.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Smith M, Jäger S, Berger U, Šikoparija B,
Hallsdottir M, Saulienė I, Bergmann KC, Pashley CL, de Weger L,
Majkowska-Wojciechowska B, et al: Geographic and temporal
variations in pollen exposure across Europe. Allergy. 69:913–923.
2004.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Fumanal B, Chauvel B and Bretagnolle F:
Estimation of pollen and seed production of common ragweed in
France. Ann Agric Environ Med. 14:233–236. 2007.PubMed/NCBI
|
|
15
|
Bullock JM, Chapman D, Schafer S, Roy D,
Girardello M, Haynes T, Beal S, Wheeler B, Dickie I, Phang Z, et
al: Assessing and controlling the spread and the effects of common
ragweed in Europe. Final Report to the European Commission, DG
Environment. 2012. Available online: https://ec.europa.eu/environment/nature/invasivealien/docs/Final_Final_Report.pdf.
Accessed June 18, 2020).
|
|
16
|
Leru PM, Matei D and Ianovici N: Health
impact of Ambrosia artemisiifolia reflected by allergists
practice in Romania. A questionnaire-based survey. Ann West Univ
Timisoara Series Biol. 18:43–54. 2015.
|
|
17
|
Popescu FD and Tudose AM: Ambrosia
pollen sensitization in allergic rhinitis patients from the central
part of the Romanian Plain. Romanian J Rhinol. 1:26–30. 2011.
|
|
18
|
Ianovici N, Panaitescu CB and Brudiu I:
Analysis of airborne allergenic pollen spectrum for 2009 in
Timişoara, Romania. Aerobiologia (Bologna). 29:95–111. 2013.
|
|
19
|
Chen KW, Marusciac L, Tamas PT, Valenta R
and Panaitescu C: Ragweed pollen allergy: Burden, characteristics,
and management of an imported allergen source in Europe. Int Arch
Allergy Immunol. 176:163–180. 2018.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Pichler U, Hauser M, Wolf M, Bernardi ML,
Gadermaier G, Weiss R, Ebner C, Yokoi H, Takai T, Didierlaurent A,
et al: Pectate lyase pollen allergens: Sensitization profiles and
cross-reactivity pattern. PLoS One. 10(e0120038)2015.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Asero R, Bellotto E, Ghiani A, Aina R,
Villalta D and Citterio S: Concomitant sensitization to ragweed and
mugwort pollen: Who is who in clinical allergy? Ann Allergy Asthma
Immunol. 113:307–313. 2014.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Kianifar HR, Pourreza A, Azad FJ,
Yousefzadeh H and Masomi F: Sensitivity comparison of the skin
prick test and serum and fecal radio allergosorbent test (RAST) in
diagnosis of food allergy in children. Rep Biochem Mol Biol.
4:98–103. 2016.PubMed/NCBI
|
|
23
|
Bignardi D, Comite P, Mori I, Ferrero F,
Fontana V, Bruzzone M, Mussap M and Ciprandi G: Allergen-specific
IgE: Comparison between skin prick-test and serum assay in
real-life. Allergol Select. 40:16–22. 2017.
|
|
24
|
Asha'ari ZA, Suhaimi Y, Yusof RA, Rushdan
I and Maraina CH: Comparison of serum specific IgE with skin prick
test in the diagnosis of allergy in Malaysia. Med J Malaysia.
66:202–206. 2011.PubMed/NCBI
|
|
25
|
Kumar R, Gupta N, Kanuga J and Kanuga M: A
comparative study of skin prick test versus serum-specific IgE
measurement in Indian patients with bronchial asthma and allergic
rhinitis. Indian J Chest Dis Allied Sci. 57:81–85. 2015.PubMed/NCBI
|
|
26
|
Verheugen G: Commission directive
2005/28/EC laying down principles and guidelines for good clinical
practice as regards investigational medicinal products for human
use, as well as the requirements for authorization of the
manufacturing or importation of such products. Official J Eur
Union. 13(91)2005.
|
|
27
|
International Council on Harmonisation of
Technical Requirements for Registration of Pharmaceuticals for
Human Use (ICH). https://www.ema.europa.eu/en/partners-networks/international-activities/multilateral-organisations-initiatives/international-council-harmonisation-technical-requirements-registration-pharmaceuticals-human-use#ich-guidelines-and-technical-requirements-section.
Accessed June 18, 2020).
|
|
28
|
World Medical Association declaration of
Helsinki. Recommendations guiding physicians in biomedical research
involving human subjects. JAMA. 277:925–926. 1997.PubMed/NCBI
|
|
29
|
Brożek JL, Bousquet J, Agache I, Agarwal
A, Bachert C, Bosnic-Anticevich S, Brignardello-Petersen R,
Canonica GW, Casale T, Chavannes NH, et al: Allergic rhinitis and
its impact on Asthma (ARIA) guidelines-2016 revision. J Allergy
Clin Immunol. 140:950–958. 2017.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Global Initiative for Asthma. Global
Strategy for Asthma Management and Prevention, 2018. https://ginasthma.org/wp-content/uploads/2019/01/2018-GINA.pdf.
Accessed June 18, 2020).
|
|
31
|
Heinzerling L, Mari A, Bergmann KC,
Bresciani M, Burbach G, Darsow U, Durham S, Fokkens W, Gjomarkaj M,
Haahtela T, et al: The skin prick test-European standards. Clin
Transl Allergy. 3(3)2013.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Heinzerling LM, Burbach GJ, Edenharter G,
Bachert C, Bindslev-Jensen C, Bonini S, Bousquet J,
Bousquet-Rouanet L, Bousquet PJ, Bresciani M, et al: GA(2)LEN skin
test study I: GA(2)LEN harmonization of skin prick testing: Novel
sensitization patterns for inhalant allergens in Europe. Allergy.
64:1498–1506. 2009.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Mechanisms for the Development of
Allergies (MeDALL). https://cordis.europa.eu/project/id/261357. Accessed
June 18, 2020.
|
|
34
|
Lupinek C, Wollmann E, Baar A, Banerjee S,
Breiteneder H, Broecker BM, Bublin M, Curin M, Flicker S, Garmatiuk
T, et al: Advances in allergen-microarray technology for diagnosis
and monitoring of allergy: The MeDALL allergen-chip. Methods.
66:106–119. 2014.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Kowal K, DuBuske L and Wood RA: Overview
of skin testing for allergic disease. UpToDate, Waltham, MA.
https://www.uptodate.com/contents/overview-of-skin-testing-for-allergic-disease?search=Overview%20of%20skin%20testing%20for%20allergic%20disease&source=search_result&selectedTitle=1~150&usage_type=default&display_rank=1.
Accessed June 18, 2020.
|
|
36
|
Leru PM, Anton VF, Bocsan C, Muntean A and
Boda D: Acquired angioedema induced by angiotensin-converting
enzyme inhibitors-experience of a hospital-based allergy center.
Exp Ther Med. 20:68–72. 2020.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Focke M, Marth K and Valenta R: Molecular
composition and biological activity of commercial birch pollen
allergen extracts. Eur J Clin Invest. 39:429–436. 2009.PubMed/NCBI View Article : Google Scholar
|
|
38
|
González-Pérez R, Poza-Guedes P, del Pino
YB, Matheu V and Sánchez-Machín I: Evaluation of major mite
allergens from European standardized commercial extracts for in
vivo diagnosis: Addressing the need for precision medicine. Clin
Transl Allergy. 9:14–21. 2019.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Moreno Benítez F, Espinazo Romeu M, Letrán
Camacho A, Mas S, García-Cózar FJ and Tabar AI: Variation in
allergen content in sublingual allergen immunotherapy with house
dust mites. Allergy. 70:1413–1420. 2015.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Valenta R, Karaulov A, Niederberger V,
Zhernov Y, Elisyutina O, Campana R, Focke-Tejkl M, Curin M,
Namazova-Baranova L, Wang JY, et al: Allergen extracts for in vivo
diagnosis and treatment of allergy: Is there a future? J Allergy
Clin Immunol Pract. 6:1845–1855.e2. 2018.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Jensen-Jarolim E, Jensen AN and Canonica
GW: Debates in allergy medicine: Molecular allergy diagnosis with
ISAC will replace screenings by skin prick test in the future.
World Allergy Organ J. 10:33–38. 2017.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Agache I, Bilò M, Braunstahl GJ, Delgado
L, Demoly P, Eigenmann P, Gevaert P, Gomes E, Hellings P, Horak F,
et al: In vivo diagnosis of allergic diseases-allergen provocation
tests. Allergy. 70:355–365. 2015.PubMed/NCBI View Article : Google Scholar
|
|
43
|
van Hage M, Hamsten C and Valenta R:
ImmunoCAP assays: Pros and cons in allergology. J Allergy Clin
Immunol. 140:974–977. 2017.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Bouley J, Groeme R, Le Mignon M, Jain K,
Chabre H, Bordas-Le Floch V, Couret MN, Bussieres L, Lautrette A,
Naveau M, et al: Identification of the cysteine protease Amb a 11
as a novel major allergen from short ragweed. J Allergy Clin
Immunol. 136:1055–1064. 2015.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Pagani M, Antico A, Cilia M, Calabro D,
Poto S, Pecora S and Burastero SE: Comparison of different
diagnostic products for skin Prick testing. Eur Ann Allergy Clin
Immunol. 41:23–31. 2009.PubMed/NCBI
|
|
46
|
Mohapatra SS, Lockey RF and Polo F: Weed
Pollen Allergens. In: Allergens and Allergen Immunotherapy, 4th
edition. Lockey RF and Ledford DK (eds). Informa Healthcare, New
York, NY, pp127-140, 2008.
|
|
47
|
Chen KW, Zieglmayer P, Zieglmayer R,
Lemell P, Horak F, Bunu CP, Valenta R and Vrtala S: Selection of
house dust mite-allergic patients by molecular diagnosis may
enhance success of specific immunotherapy. J Allergy Clin Immunol.
143:1248–1252. 2019.PubMed/NCBI View Article : Google Scholar
|