1. Introduction
In 2012, 143,000 deaths caused by kidney cancer were
reported worldwide, with an increased incidence in the Czech
Republic, followed by Eastern and Northern Europe, North America
and Australia. Africa and South-East Asia had the lowest mortality
rates from kidney cancer. RCC accounts for over 90% of all renal
malignancies and is characterized histologically by the presence of
three cell types: 70% clear cells, between 10 and 15% papillary
cells, and only 5% chromophobe cells (1,2). The
incidence and mortality of renal cell carcinoma (RCC) in the Czech
Republic are among the highest in the world with 27.14 new cases
and 11.13 deaths per 100,000 persons per year, while in men, the
incidence is 63%, with 40% of cases diagnosed at the advanced or
metastatic stage (3).
According to Globocan, in 2018, 403,000 cases of RCC
were diagnosed, representing 2.2% of all cancer cases, with 254,000
cases among men and 148,000 among women. In developed countries,
the risk of kidney cancer is 0.69% in men and 0.35% in women. In
the USA, in 2019, according to published statistics, 74,000 new
cases of RCC were reported, representing 4.2% of all cancers
(4). Unfortunately, the RCC
incidence rate doubled in 2016, being 14.9/100,000 inhabitants,
compared to 1975, when the incidence was 7.1/100.00(4).
In Romania, in 2018, in accordance with results
published by Globocan, kidney cancer ranked 12th out of all
cancers, representing 2.4%, with a mortality risk of 1.8% and a
5-year survival rate of 2.878% (5).
Chronic kidney disease (CKD) and diabetic nephropathy represent
other renal pathologies among the adult population worldwide
(6-9).
The leading cause of death among patients with CKD are
cardiovascular diseases, which can be associated with vascular
calcification (10-12).
Uric acid is a biomarker for the cardiovascular risk, hyperuricemia
being associated with endothelial dysfunction, inflammation, and
the activation of the renin-angiotensin-aldosterone system
(13-15).
Smoking is a risk factor for the development of RCC,
the risk increasing by 50% for male smokers, while for female
smokers there is an increase of 20%. Obesity is another risk factor
for RCC, and a body mass index of 5 kg/m2 increases the
risk by 24% for men and 34% for women (1). Hypertension increases the risk of
kidney cancer, affecting the renal glomeruli and tubular apparatus
(4,7). In addition, a higher rate of
hypertension is reported for CKD patients (16,17).
Moreover, male sex, an increased BMI and smoking represent a few
risk factors for diabetic nephropathy (18). In the pathogenesis of RCC, in
addition to histological characterization, a molecular evaluation
is required, which can provide crucial information for future
therapeutic targets. The phosphoinositide 3-kinase (PI3K)/protein
kinase B (AKT)/mammalian target of rapamycin (mTOR) signalling
pathway is dysregulated in systemic cancers, including RCC.
Hyper-activation of this molecular pathway is correlated with
aggressive behaviour of RCC tumours and poor patient prognosis
(19,20).
2. PI3K/AKT signalling pathway: Roles,
activation, and isoforms
The PI3K/AKT/mTOR, signalling pathway plays a
pivotal role in cell survival and growth, being frequently
disrupted in malignant pathologies. A frequent enhanced activity of
the PI3K/AKT/mTOR pathway in malignant cells is observed, thus the
inhibition of mTOR is an attractive strategy to treat cancer
(21-29).
Phosphatidylinositol 3-kinases (PI3Ks) are a family
of lipid kinases. PI3Ks phosphorylate a component of the eukaryotic
cell membrane, namely phosphatidylinositol. Three classes of PI3K
(I, II and III) have been identified to date, based on differences
in sequence homology and lipid substrate preference (30). Growth factors, cytokines, and
hormones bind to receptor tyrosine kinases (RTKs) and
G-protein-coupled receptors (GPCRs) and activate PI3K. On the
intracellular membrane, class I PI3K phosphorylates the substrate
phosphatidylinositol 4,5-bisphosphate (PIP2) which
transforms into phosphatidylinositol 3,4,5-triphosphate (PIP3),
recruiting signalling proteins such as AKT (31,32).
All activated PI3K classes are involved in a diversity of cellular
processes such as proliferation, survival, metabolism, trafficking,
and immunity (33). Phosphatase and
tensin homologue (PTEN), the main negative regulator of PI3K,
dephosphorylates PIP3 into PIP2 (34). AKT is activated by two
phosphorylation processes. Phosphoinositide-dependent-protein
kinase 1 (PDK1) phosphorylates AKT1 at threonine 308, and the
second phosphorylation takes place at serine 473 by mTOR complex 2
(35,36). Based on differences in
serine/threonine residues, AKT is divided into three isoforms
(AKT1, AKT2 and AKT3). AKT1 is ubiquitously present in tissues,
being involved in cell growth and survival. AKT2 is present mainly
in muscle and adipocytes and contributes to glucose homeostasis,
while AKT3 is found in the brain and testes. All three isoforms
share more than 80% homology, contain pleckstrin homology (PH),
catalytic and regulatory domains and have common specific functions
(Fig. 1) (37-41).
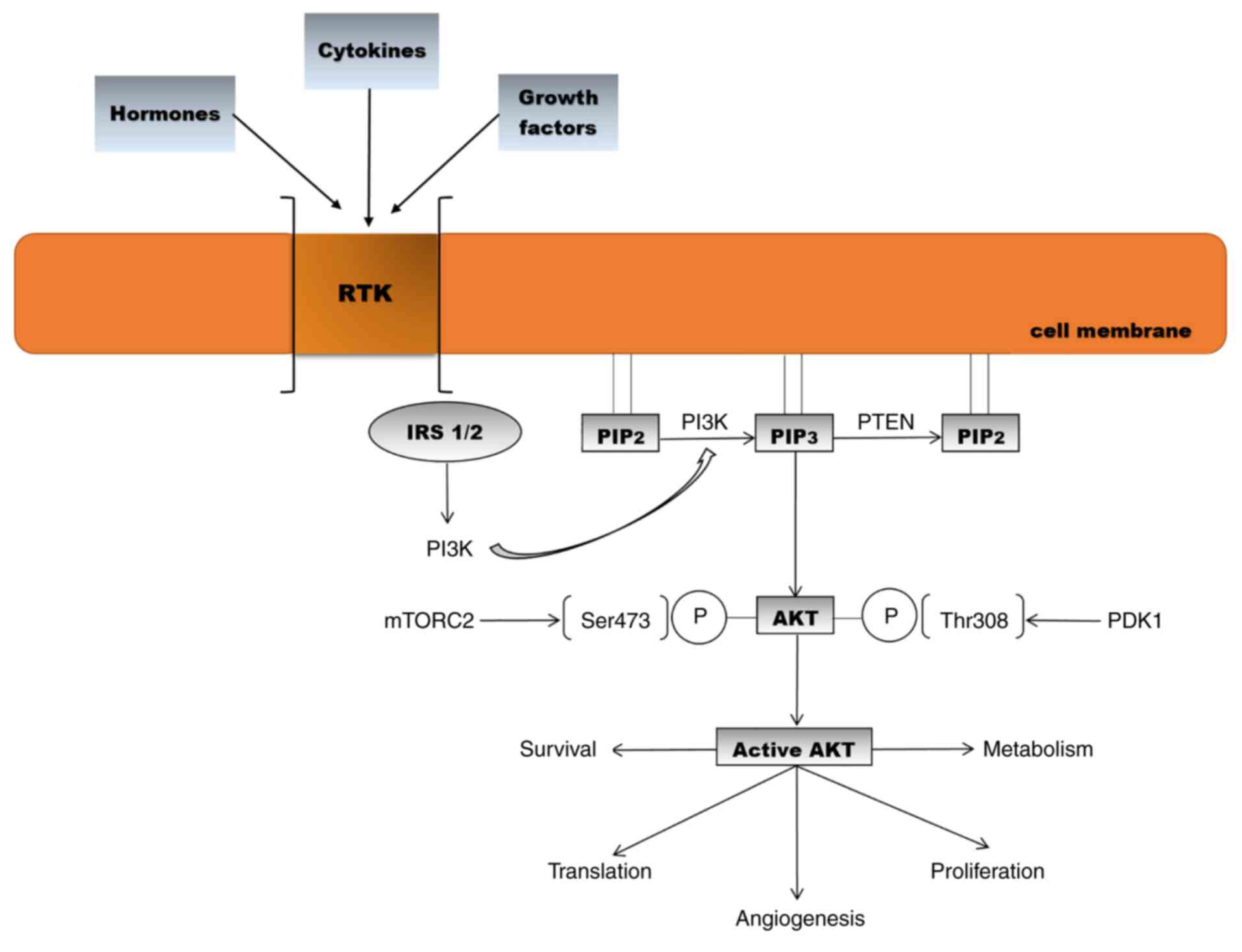 | Figure 1Activation of the AKT signalling
pathway and biological effects [adapted from Araki et al
(2003) and Meric-Bernstam and Gonzalez-Angulo (2009) (32,42)].
RTK, receptor tyrosine kinase; IRS, insulin receptor substrate;
PIP2, phosphatidylinositol 4,5-bisphosphate;
PIP3, phosphatidylinositol 3,4,5-triphosphate; PTEN,
phosphatase and tensin homologue; PI3K, phosphoinositide 3-kinase;
AKT, protein kinase B; PDK1, phosphoinositide-dependent-protein
kinase 1; mTORC2. mammalian target of rapamycin complex 2. |
3. Structure and function of the mTOR
pathway
mTOR is a component of the AKT signalling pathway,
which promotes cell growth and proliferation in eukaryotic cells
(42,43).
mTOR is a central regulator of cell metabolism,
proliferation, growth, and survival. This protein kinase is
activated in various pathological cellular processes such as tumour
formation, angiogenesis, adipogenesis, insulin resistance and
activation of T lymphocytes (42-45).
Overexpression of mTOR signalling pathway has been observed in
various systemic pathologies such as type 2 diabetes and multiple
neoplasms including RCC (42-45).
mTOR protein is a serine threonine kinase belonging to the PI3K
kinase family, which exists in two distinct multi-protein
complexes: mTOR1 and mTOR2(46).
Both complexes have certain common protein
components: mTOR (serine/threonine kinase), mLST8 (lethal mammalian
with sec-13 protein 8) and DEPTOR (DEP-domain containing
mTOR-interacting protein). The mTORC1 complex additionally contains
scaffold protein Raptor (regulatory-associated protein of TOR) and
AKT substrate protein PRAS40 (proline-rich AKT substrate 40-kDa)
(47,48). Scaffold protein Rictor (rapamycin
insensitive companion of mTOR), mSIN1 (stress-activated protein
kinase-interacting protein 1) and protein associated with Rictor 1
and 2, named PROTOR1, represent the core components of the mTORC2
complex (Fig. 2) (47,48).
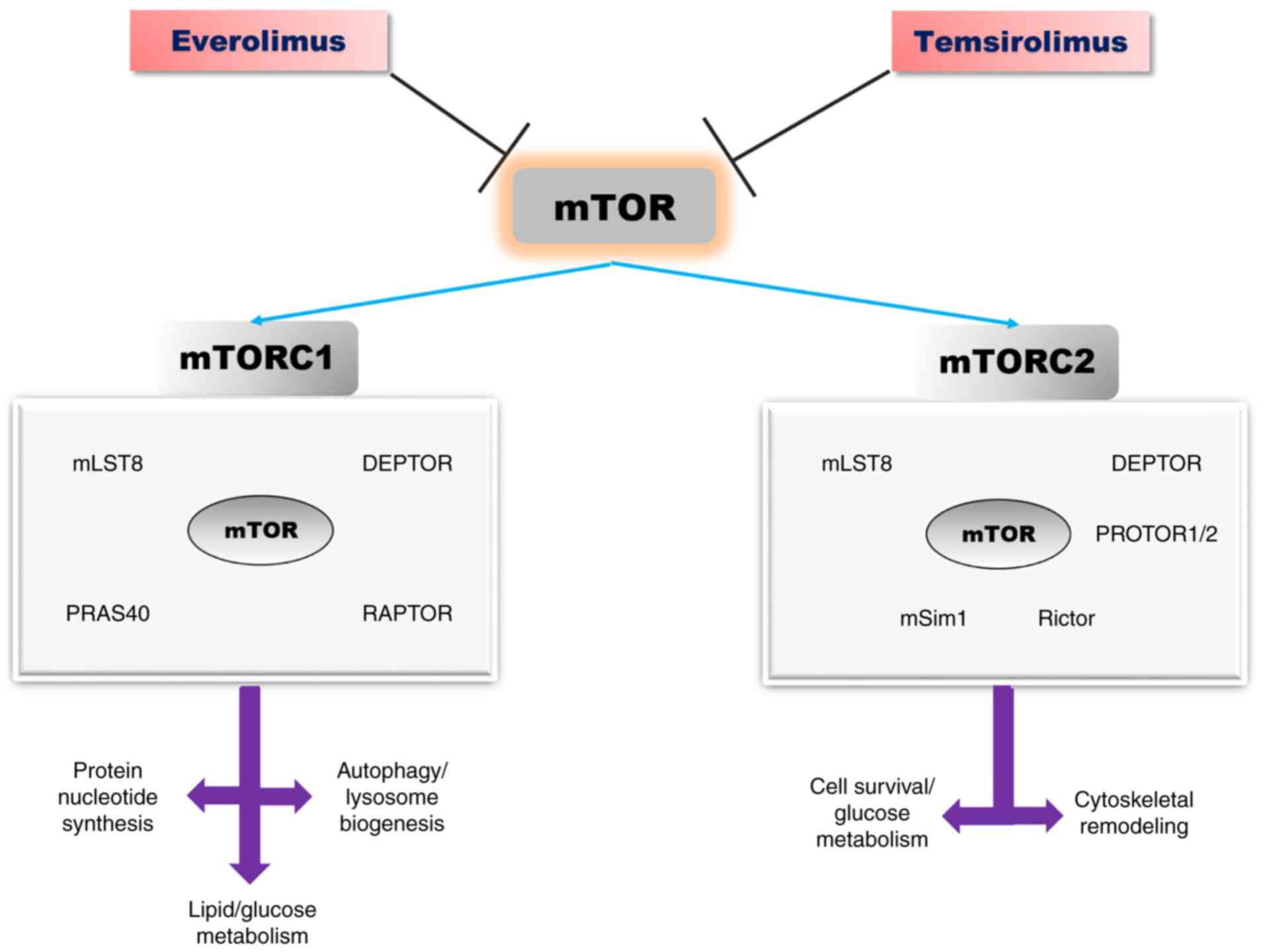 | Figure 2The mTOR signalling pathway:
Structure, biological effects, and inhibitors [adapted from Saxton
and Sabatini (2017) and Laplante and Sabatin (2012) (47,48)].
mTOR, mammalian target of rapamycin; DEPTOR, DEP-domain containing
mTOR-interacting protein; PRAS40, proline-rich AKT substrate
40-kDa; mLST8, lethal mammalian with sec-13 protein 8; Raptor,
regulatory-associated protein of TOR; Rictor, rapamycin insensitive
companion of mTOR; mSIN1, stress-activated protein
kinase-interacting protein 1. |
In response to environmental factors such as amino
acids, stress and growth factors, mTORC1 maintains a cellular
balance between catabolism and anabolism. Several growth factors
such as insulin, insulin-like growth factor (IGF) and amino acids
activate mTORC1 through signalling PI3K/AKT-Tuberous sclerosis
complex 1/2 (TSC1/2)-RHEB. PI3K-AKT pathway phosphorylates and
inhibits TSC1, which causes RHEB (the small GTPase Ras homologue
enrich in brain) activation, which represents the GTPase-activating
protein (47,48). Hypoxia and DNA damage, are also
signals for mTORC1 activation through TSC1/2 (48,49).
mTORC1 activation promotes protein synthesis by
ribosome biogenesis and mRNA translation. Ribosomal S6 kinase (S6K)
and the inhibitory Eif4e-binding proteins (4E-BPs) are the major
downstream effectors of mTORC1, activated by phosphorylation, which
will further phosphorylate other substrata, such as ribosomal
protein S6, and protein synthesis initiation factor 4B (eIF4B)
(47,50,51).
mTORC1 regulates cell growth and proliferation, through its
downstream effectors, S6K and 4E-BP, in response to IGF, amino
acids, hypoxia and DNA damage (50,52).
Overexpression of eIF4E is implicated in malignant transformation
of some specific cells (53,54).
mTORC1 promotes synthesis of purine nucleotides, de novo
lipogenesis. Moreover, mTORC1 stimulates glycolysis and glucose
uptake through transcription factor hypoxia-inducible factor α
(HIFα) (55,56).
mTORC1 is implicated in autophagy-lysosome and
ubiquitin-proteasome pathways, involved in protein and organelle
turnover. In the nutrient state, ULK1, the mammalian
autophagy-initiating kinase is phosphorylated by mTORC1, leading to
its inhibition, which further blocks autophagy. Adenosine
5'-monophosphate (AMP)-activated protein kinase (AMPK) activates
ULK1 (57,58). mTORC1 activation suppresses the
transcription factor EB (TFEB), the most important regulator of the
lysosome pathway. mTORC1 inactivation and nutrient deprivation
activate TBEB, then its nuclear translocation takes place, leading
further to lysosomal and autophagic genes expression (59). Zhao et al and Rousseau and
Bertolotti report that the ubiquitin proteasome system (UPS) is
increased when mTORC1 is inactivated in mammalian cells (60,61).
Moreover, mTORC1 controls the translation of cyclin D, c-Myc, and
other key proteins involved in cell proliferation (62). Insulin and IGF activate the mTORC2
signalling pathway. The downstream substrata for this kinase
protein are less known. Once activated, mTORC2 phosphorylates
members of the AGC kinase family, AKT, SGK and PKCα, which are
involved in cellular survival, metabolism, and cytoskeletal
remodelling. AKT is the most well characterized substratum for
mTORC2, being phosphorylated at serine 473 (41,63).
Furthermore, AKT phosphorylates TSC2, which is the upstream
inhibitor for mTORC1 (41,63). mTOR may be activated by
cAMP-dependent protein kinase (AMP kinase), and TSC1/2. In nutrient
and energy depletion states, cAMP and TSC 1/2 suppress mTOR
(64).
4. PI3K/AKT/mTOR in RCC
In endothelial cells, there are RTKs to which
vascular endothelial growth factor (VEGF) binds (VEGFR1/R2),
promoting cell proliferation and migration, by activating the
mitogen-activated protein kinases (MAPK) and PI3K/AKT/mTOR
signalling pathways (65).
Receptors for growth factors, such as VEGF, IGF, epidermal growth
factor (EGF), have been identified at the level of clear cell RCC
(65). Overexpression of EGF,
transforming growth factor (TGF)-β, and IGF leads to RTK
activation, which further activates various signalling pathways,
such as RAS/mitogen-activated protein (MEK)/extracellular
signal-regulated kinases (ERK) or PI3K/AKT/mTOR, leading to the
production of hypoxia-inducible factor (HIF)-α, which promotes
tumour progression (65). Genetic
alterations may activate mTOR, reduce the function of PTEN, which
cause abnormal activation of AKT, by increasing the function of the
catalytic subunit of PI3K (41,65-67).
Sato et al report mutations in the AKT/mTOR signalling
pathway in clear cell RCC (68). In
RCC tumours, PI3K/AKT/mTOR signalling pathway activation is
correlated with aggressive development and poor survival rate
(69,70).
Hyperactivity of mTOR may occur through several
mechanisms: Over-activation of growth factors, mutations of the
PI3K/AKT signalling pathway, decreased expression of TSC1/2,
epigenetic suppression of PTEN and Von Hippel-Lindau (VHL)
gene inactivation (65,71). Activation of mTOR leads to increased
angiogenesis in neighbouring endothelial cells (65,71).
In cancer cells, mTOR regulates mRNA translation for HIF1-α, HIF-2α
and p70S6 kinase. In the pathogenesis of RCC, overexpression of
HIF-1α and HIF-2α, appears to play an important role, together with
overexpression of p70S6K (65,72-75).
mTOR is involved in upregulation of HIF-α subunits, while VEGF and
other molecules increase angiogenesis (65,76).
RCC is characterized by alterations of the VHL gene. The
loss of VHL function leads to the deregulation of cyclin D1,
a cyclin-dependent kinase cofactor important for cell cycle
progression (65,77,78).
In clear cell RCC tumorigenesis, the accumulation of HIF-1α and
HIF-2α represents a critical step, as a result of bi-allelic
alteration of the VHL gene. In the presence of phospholipase
D, mTOR enhances the expression of HIF-1α and HIF-2α, at the
translational level rather than transcriptional (65,79).
Chen et al identified the activation of the PI3K/AKT/mTOR
signalling pathway in the tissues and cells of patients with RCC.
The study reports that inhibition of this pathway may reduce
epithelial to mesenchymal cell transition in RCC (21). In 2013, Sato and colleagues
published the results of integrated molecular analyses in over 100
cases of clear cell RCC, analysing whole-genome and/or whole-exome
and RNA sequencing. VHL gene defect, HIF accumulation,
mutations in the PI3K/AKT/mTOR signalling pathway, p53 and DNA
methylation were identified in patients with clear cell RCC genetic
lesions (68).
5. mTOR inhibitors in RCC
To date, three methods have been proposed for
developing therapeutic agents for RCC: VEGF, RTK and mTOR
inhibitors. The mechanism of action of rapamycin drug analogs
includes both angiogenesis and tumour cell proliferation. Drug
resistance may reduce the utility of mTOR inhibitors (80). Temsirolimus and everolimus are two
mTOR inhibitors, which are currently utilized for the treatment of
certain RCC subtypes (19,81-84).
In May 2007, FDA approved temsirolimus as treatment in a phase III
trial, which included 626 patients with poor-prognostic metastatic
RCC. The patients received temsirolimus (25 mg intravenous weekly),
interferon (IFN)-γ (3x106 U subcutaneously three-times
weekly) or a combination of both (temsirolimus 15 mg weekly and
63x106 U of IFN-γ three-times weekly). The survival rate
of patients with metastasis RCC treated with temsirolimus was
statistically prolonged when compared with patients who received
only IFN-γ (P=0.0069; hazard ratio, HR=0.73). The median overall
survival time reported in the temsirolimus group was 10.9 months,
7.3 months in the interferon group and 8.4 months in the
combination group. Anaemia, nausea, peripheral oedema, rash,
asthenia, hyperlipidemia and hyperglycemia were the common adverse
effects observed in patients who received temsirolimus treatment.
Based on the results, temsirolimus is the best therapeutic option
for metastatic RCC patients. However, the survival rate was not
improved in the interferon group by temsirolimus addition (85).
In 2018, Park and colleagues published the results
of a phase II clinical trial that included 40 non-clear-cell
recurrent or metastatic RCC patients, who received axitinib or
temsirolimus. The results of the 3-year study reported promising
effects for axitinib in terms of progression-free survival and
objective response rate, but not for temsirolimus (86).
In 2017, Bedke et al published the results of
a study which included patients with metastatic RCC, who received
inhibitors for RTK, mTOR and VEGF administered in 3 stages.
Axitinib, sorafenib, sunitinib and pozapanib were the RTK
inhibitors used in the study, while temsirolimus and everolimus
were the inhibitors for mTOR. Sunitinib is the first novel drug,
which doubles progression-free survival in patients with metastatic
RCC. In the first line of treatment, 626 patients with RCC, divided
into three groups-namely, 80% with clear RCC cells, 20% with
non-clear RCC and 72% with metastatic RCC, received temsirolimus,
INF-α or a combination of the two. Survival rates were higher in
patients receiving temsirolimus vs. INF-α (10.9 months compared
with 7.4 months). RTK and mTOR inhibitors were administered in the
second line therapy, the results being unclear as to the best
treatment option. In the third line of treatment, VEGF and mTOR
inhibitors (everolimus) were administered, with everolimus showing
beneficial effects. Everolimus and temsirolimus reduced S6K1 and
4EBP1 activities and increased the synthesis of HIF1-α. These
rapamycin analogues inhibit cell proliferation, growth, and
survival, blocking the cell cycle in the G1-phase (84). Until 2006, a high dose of
interleukin (IL)-2 remained the best therapeutic target for
metastatic clear-cell RCC, but the therapy has evolved and
currently three therapeutic targets are proposed. They are
inhibitors for RTK-VEGF and mTOR, used in phase III trials, which
were found to increase the survival rate (87). In RCC, GNE-477 may be a novel and
efficacious dual inhibitor for PI3K-mTOR. Ye et al observed,
in primary cultured human RCC cells, that GNE-477 inhibited cell
growth, viability, proliferation, cell cycle progression,
migration, and invasion. GNE-477 inhibited the PI3K-AKT-mTOR
signalling pathway in primary RCC cells, by blocking AKT1, p70S6K1,
p85 and S6. In vivo studies made on nude mice demonstrated
that intraperitoneal injection of GNE-477 suppressed tumour growth.
GNE-477 inhibited RCC cell growth in vitro and in
vivo (88).
6. Conclusions
Obesity, smoking, and hypertension are risk factors
for RCC, representing, in 2018, 2.2% of all cancers worldwide, with
a higher incidence in men compared with women. The PI3K/AKT/mTOR
signalling pathway is involved in protein and nucleotide synthesis,
lipid/glucose metabolism, autophagy, translation, angiogenesis,
cell survival and proliferation. Over-activation of the
PI3K/AKT/mTOR signalling pathway is crucial for RCC cell survival,
proliferation, migration, and metastasis.
Drugs or pharmacological inhibitors of this
signalling cascade are promising and important targets for RCC.
Temsirolimus and everolimus are two mTOR inhibitors that are used
for RCC therapy at present, which have been found to increase the
patient survival rate.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
All information provided in this review is
documented by relevant references.
Authors' contributions
DM, DGB, AT, OS, IAV, DAM, CCP, RIP, ME, NAS, GV,
DEG, AEN and CS designed the review, performed the literature
search, selected the included studies and wrote the manuscript and.
DM, DGB, AT, OS, IDV, DAM, CCP, RIP, ME, NAS, GV, DEG, AEN and CS
critically revised the manuscript. All authors read and approved
the final manuscript. The contributions of all the authors on this
review are greatly valued and appreciated.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Znaor A, Lortet-Tieulent J, Laversanne MA,
Jemal A and Bray F: International variations and trends in renal
cell carcinoma incidence and mortality. Eur Urol. 67:519–530.
2015.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Saad AM, Gad MM, Al-Husseini MJ, Ruhban
IA, Sonbol MB and Ho TH: Trends in renal-cell carcinoma incidence
and mortality in the United States in the last 2 decades: A
SEER-based study. Clin Genitourin Cancer. 17:46–57.e5.
2019.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Poprach A, Bortlíček Z, Büchler T,
Melichar B, Lakomý R, Vyzula R, Brabec P, Svoboda M, Dušek L and
Gregor J: Patients with advanced and metastatic renal cell
carcinoma treated with targeted therapy in the Czech Republic:
Twenty cancer centres, six agents, one database. Med Oncol.
29:3314–3320. 2012.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Padala SA and Barsouk A, Thandra KC,
Saginala K, Mohammed A, Vakiti A, Rawla P and Barsouk A:
Epidemiology of renal cell carcinoma. World J Oncol. 11:79–87.
2020.PubMed/NCBI View Article : Google Scholar
|
|
5
|
World Health Organization: Globocan 2020.
Cancer Today. Data visualization tools for exploring the global
cancer burden in 2020. https://gco.iarc.fr/today/data/factsheets/populations/642-romania-fact-sheets.
Accessed August, 2020.
|
|
6
|
Bălan DG, Balcangiu-Stroescu AE, Tănăsescu
MD, Diaconescu AC, Răducu L, Mihai A, Tănase M, Stănescu II and
Ionescu D: Nutritional intervention in patients with diabetic renal
disease-a brief presentation. Rev Chim Buchar. 69:4078–4082.
2018.
|
|
7
|
Măndiță A, Timofte D, Balcangiu-Stroescu
AE, Bălan DG, Răducu L, Tănăsescu MD, Diaconescu AC, Dragoș D,
Coșconel I and Ionescu D: Treatment of high blood pressure in
patients with chronic renal disease. Rev Chim Buchar. 70:993–995.
2019.
|
|
8
|
Totan A, Balcangiu-Stroescu AE, Melescanu
Imre M, Miricescu D, Balan DG, Stanescu II, Ionescu D, Timofte D,
Tanasescu MD and Greabu M: XOR-possible correlations with oxidative
stress and inflammation markers in the context of diabetic kidney
disease. Rev Chim Buchar. 70:1396–1398. 2019.
|
|
9
|
Alicic RC, Rooney MT and Tuttle KR:
Diabetic kidney disease: Challenges, progress, and possibilities.
Clin J Am Soc Nephrol. 12:2032–2045. 2017.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Disthabanchong S: Vascular calcification
in chronic kidney disease: Pathogenesis and clinical implication.
World J Nephrol. 6:43–53. 2012.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Timofte D, Ionescu D, Medrihan L, Măndiță
A, Rășină A and Damian L: Vascular calcification and bone disease
in hemodialysis patients assessment, association and risk factors.
Nephrology Dialysis Transplantation; Oxford Univ Press. 22:325–326.
2007.
|
|
12
|
Timofte D, Dragoș D, Balcangiu-Stroescu A,
Tănăsescu M, Bălan DG, Răducu L, Tulin A, Stiru O and Ionescu D:
Abdominal aortic calcification in predialysis patients:
Contribution of traditional and uremia-related risk factors. Exp
Ther Med. 20:97–102. 2020.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Timofte D, Măndiță A, Balcangiu-Stroescu
AE, Bălan DG, Răducu L, Tănăsescu MD, Diaconescu AC, Dorin D,
Coșconel CI and Ionescu D: Hyperuricemia and cardiovascular
diseases-clinical and paraclinical correlations. Rev Chim Buchar.
70:1045–1046. 2019.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Epingeac ME, Gaman MA, Diaconu C, Gad M
and Gaman AM: The evaluation of oxidative stress in obesity. Rev
Chim Buchar. 70:2241–2244. 2019.
|
|
15
|
Diaconu C: Midaortic syndrome in a young
man. Cor et Vasa. 59:e171–e173. 2017.
|
|
16
|
Balcangiu-Stroescu AE, Tănăsescu MD,
Diaconescu A, Răducu L, Constantin AM, Bălan DG, Țărmure V and
Ionescu D: Cardiovascular comorbidities, inflammation and serum
albumin levels in a group of hemodialysis patients. Rev Chim
Buchar. 69:926–929. 2019.
|
|
17
|
Diaconu C: Treatment of Diabetes in
Patients with Heart Failure. The 3rd International Conference on
Interdisciplinary Management of Diabetes Mellitus and its
Complications-Diabetes Mellitus in Internal Medicine, INTERDIAB
2017 Proceedings. Serafinceanu C, Negoita O and Elian V (eds).
Niculescu, Bucharest, pp170-177, 2017.
|
|
18
|
Balcangiu-Stroescu AE, Tănăsescu MD,
Diaconescu AC, Răducu L, Bălan DG, Mihai A, Tănase M, Stănescu II
and Ionescu D: Diabetic nephropathy: A concise assessment of the
causes, risk factors and implications in diabetic patients. Rev
Chim Buchar. 69:3118–3121. 2018.
|
|
19
|
Husseinzadeh HD and Garcia JA: Therapeutic
rationale for mTOR inhibition in advanced renal cell carcinoma.
Curr Clin Pharmacol. 6:214–221. 2011.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Chen H, Zhu D, Zheng Z, Cai Y, Chen Z and
Xie W: CEP55 promotes epithelial-mesenchymal transition in renal
cell carcinoma through PI3K/AKT/mTOR pathway. Clin Transl Oncol.
21:939–949. 2019.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Xiang RF, Wang Y, Zhang N, Xu WB, Cao Y,
Tong J, Li JM, Wu YL and Yan H: MK2206 enhances the cytocidal
effects of bufalin in multiple myeloma by inhibiting the AKT/mTOR
pathway. Cell Death Dis. 8(e2776)2017.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Martini M, de Santis MC, Braccini L,
Gulluni F and Hirsch E: PI3K/AKT signalling pathway and cancer: An
updated review. Ann Med. 46:372–383. 2014.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Costa RLB, Han HS and Gradishar WJ:
Targeting the PI3K/AKT/mTOR pathway in triple-negative breast
cancer: A review. Breast Cancer Res Treat. 69:397–406.
2018.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Sathe A and Nawroth R: Targeting the
PI3K/AKT/mTOR pathway in bladder cancer. Methods Mol Biol.
1655:335–350. 2018.PubMed/NCBI View Article : Google Scholar
|
|
25
|
O'Donnell JS, Massi D, Teng MW and Mandala
M: PI3K-AKT-mTOR inhibition in cancer immunotherapy, redux. Semin
Cancer Biol. 48:91–103. 2018.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Chamcheu JC, Roy T, Uddin MB,
Banang-Mbeumi S, Chamcheu RN, Walker AL, Liu YY and Huang S: Role
and therapeutic targeting of the PI3K/AKT/mTOR signaling pathway in
skin cancer: A review of current status and future trends on
natural and synthetic Agents therapy. Cells. 8(803)2019.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Bertacchini J, Heidari N, Mediani L,
Capitani S, Shahjahani M, Ahmadzadeh A and Saki N: Targeting
PI3K/AKT/mTOR network for treatment of leukemia. Cell Mol Life Sci.
72:2337–2347. 2015.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Aggarwal S, John S, Sapra L, Sharma SC and
Das SN: Targeted disruption of PI3K/AKT/mTOR signaling pathway, via
PI3K inhibitors, promotes growth inhibitory effects in oral cancer
cells. Cancer Chemother Pharmacol. 83:451–461. 2019.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Li Z, Liu J, Que L and Tang X: The
immunoregulatory protein B7-H3 promotes aerobic glycolysis in oral
squamous carcinoma via PI3K/AKT/mTOR pathway. J Cancer.
10:5770–5784. 2019.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Graupera M and Potente M: Regulation of
angiogenesis by PI3K signaling networks. Exp Cell Res.
319:1348–1355. 2013.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Carnero A, Blanco-Aparicio C, Renner O,
Link W and Leal JF: The PTEN/PI3K/AKT signalling pathway in cancer,
therapeutic implications. Curr Cancer Drug Targets. 8:187–198.
2008.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Fresno Vara JA, Casado E, de Castro J,
Cejas P, Belda-Iniesta C and González-Barón M: PI3K/AKT signalling
pathway and cancer. Cancer Treat Rev. 30:193–204. 2004.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Araki N, Hatae T, Furukawa A and Swanson
JA: Phosphoinositide-3-kinase independent contractile activities
associated with Fcgamma-receptor-mediated phagocytosis and
macropinocytosis in macrophages. J Cell Sci. 116:247–257.
2003.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Zhang J, Yu XH, Yan YG, Wang C and Wang
WJ: PI3K/AKT signaling in osteosarcoma. Clin Chim Acta.
444:182–192. 2015.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Regan MM and Phillip AD: AKT-dependent and
independent mechanisms of mTOR regulation in cancer. Cell Signal.
21:656–664. 2009.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Sarbassov DD, Guertin DA, Ali SM and
Sabatini DM: Phosphorylation and regulation of AKT/PKB by the
rictor-mTOR complex. Science. 307:1098–1101. 2005.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Krycer JR, Sharpe LJ, Luu W and Brown AJ:
The AKT-SREBP nexus: Cell signaling meets lipid metabolism. Trends
Endocrinol Metab. 21:268–276. 2010.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Abeyrathna P and Su Y: The critical role
of AKT in cardiovascular function. Vascul Pharmacol. 74:38–48.
2015.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Nicholson KM and Anderson NG: The protein
kinase B/AKT signalling pathway in human malignancy. Cell Signal.
14:381–395. 2002.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Brazil DP, Yang ZZ and Hemmings BA:
Advances in protein kinase B signalling: AKTion on multiple fronts.
Trends Biochem Sci. 29:233–242. 2004.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Hers I, Vincent EE and Tavaré JM: AKT
signalling in health and disease. Cell Signal. 23:1515–1527.
2011.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Meric-Bernstam F and Gonzalez-Angulo AM:
Targeting the mTOR signaling network for cancer therapy. J Clin
Oncol. 27:2278–2287. 2009.PubMed/NCBI View Article : Google Scholar
|
|
43
|
JIlha J, Espírito-Santo CC and de Freitas
GR: mTOR signaling pathway and protein synthesis: From training to
aging and muscle autophagy. Adv Exp Med Bio. 1088:139–151.
2018.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Ayuk SM and Abrahamse H: mTOR signaling
pathway in cancer targets photodynamic therapy in vitro. Cells.
8(431)2019.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Battelli C and Cho DC: mTOR inhibitors in
renal cell carcinoma. Therapy. 8:359–367. 2011.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Guertin DA and Sabatini DM: Defining the
role of mTOR in cancer. Cancer Cell. 12:9–22. 2007.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Saxton RA and Sabatini DM: mTOR signaling
in growth, metabolism, and disease. Cell. 168:960–976.
2017.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Laplante M and Sabatini DM: mTOR signaling
in growth control and disease. Cell. 149:274–293. 2012.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Inoki K, Zhu T and Guan KL: TSC2 mediates
cellular energy response to control cell growth and survival. Cell.
115:577–590. 2003.PubMed/NCBI View Article : Google Scholar
|
|
50
|
Cornu M, Albert V and Hall MN: mTOR in
aging, metabolism, and cancer. Curr Opin Genet Dev. 23:53–62.
2013.PubMed/NCBI View Article : Google Scholar
|
|
51
|
Stallone G, Infante B, Prisciandaro C and
Grandaliano G: mTOR and aging: An old fashioned dress. Int J Mol
Sci. 20(2774)2019.PubMed/NCBI View Article : Google Scholar
|
|
52
|
Wullschleger K, Loewith R and Hall EB: TOR
signaling in growth and metabolism. Cell. 124:471–484.
2006.PubMed/NCBI View Article : Google Scholar
|
|
53
|
Fredrichkson RM, Mushynski WE and
Sonenberg N: Phosphorylation of translation initiation factor
eIf-4E is induced in a Ras-dependent manner during nerve growth
factor-mediated PC 12 cell differentiation. Mol Cell Biol.
12:1239–1247. 1992.PubMed/NCBI View Article : Google Scholar
|
|
54
|
Martin CK and Borden KL: The oncogene
eIF4E: Using biochemical insights to target cancer. J Interferon
Cytokine Res. 33:227–238. 2013.PubMed/NCBI View Article : Google Scholar
|
|
55
|
Ben-Sahra I, Hoxhaj G, Ricoult SJH, Asara
JM and Manning BD: mTORC1 induces purine synthesis through control
of the mitochondrial tetrahydrofolate cycle. Science. 351:728–733.
2016.PubMed/NCBI View Article : Google Scholar
|
|
56
|
Düvel K, Yecies JL, Menon S, Raman P,
Lipovsky AI, Souza AL, Triantafellow E, Ma Q, Gorski R, Cleaver S,
et al: Activation of a metabolic gene regulatory network downstream
of mTOR complex 1. Mol Cell. 39:171–183. 2010.PubMed/NCBI View Article : Google Scholar
|
|
57
|
Kim J, Kundu M, Viollet B and Guan KL:
AMPK and mTOR regulate autophagy through direct phosphorylation of
Ulk1. Nat Cell Biol. 13:132–141. 2011.PubMed/NCBI View Article : Google Scholar
|
|
58
|
Tripathi DN, Chowdhury R, Trudel LJ, Tee
AR, Slack RS, Walker CL and Wogan GN: Reactive nitrogen species
regulate autophagy through ATM-AMPK-TSC2-mediated suppression of
mTORC1. Proc Natl Acad Sci USA. 110:E2950–E2957. 2013.PubMed/NCBI View Article : Google Scholar
|
|
59
|
Settembre C, Zoncu R, Medina DL, Vetrini
F, Erdin S, Erdin S, Huynh T, Ferron M, Karsenty G, Vellard MC, et
al: A lysosome-to-nucleus signalling mechanism senses and regulates
the lysosome via mTOR and TFEB. EMBO J. 31:1095–1108.
2012.PubMed/NCBI View Article : Google Scholar
|
|
60
|
Zhao J, Zhai B, Gygi SP and Goldberg AL:
mTOR inhibition activates overall protein degradation by the
ubiquitin proteasome system as well as by autophagy. Proc Natl Acad
Sci USA. 112:15790–15797. 2015.PubMed/NCBI View Article : Google Scholar
|
|
61
|
Rousseau A and Bertolotti A: An
evolutionarily conserved pathway controls proteasome homeostasis.
Nature. 536:184–189. 2016.PubMed/NCBI View Article : Google Scholar
|
|
62
|
Fingar DC, Richardson CJ, Tee AR, Cheatham
L, Tsou C and Blenis J: mTOR controls cell cycle progression
through its cell growth effectors S6K1 and 4EBP1/eukaryotic
translation factor 4E. Mol Cell Biol. 24:200–216. 2004.PubMed/NCBI View Article : Google Scholar
|
|
63
|
Cai H, Dong LQ and Liu F: Recent advances
in adipose mTOR signaling and function: Therapeutic prospects.
Trends Pharmacol Sci. 37:303–317. 2016.PubMed/NCBI View Article : Google Scholar
|
|
64
|
Inoki K and Guan KL: Complexity of the TOR
signaling network. Trends Cell Biol. 16:206–212. 2006.PubMed/NCBI View Article : Google Scholar
|
|
65
|
Kumar A, Kumari N, Gupta V and Prasad R:
Renal cell carcinoma: Molecular aspects. Ind J Clin Biochem.
33:246–254. 2018.PubMed/NCBI View Article : Google Scholar
|
|
66
|
Neshat MS, Mellinghoff IK, Tran C, Stiles
B, Thomas G, Petersen R, Frost P, Gibbons JJ, Wu H and Sawyers CL:
Enhanced sensitivity of PTEN-deficient tumors to inhibition of
FRAP/mTOR. Proc Natl Acad Sci USA. 98:10314–10319. 2001.PubMed/NCBI View Article : Google Scholar
|
|
67
|
Jamaspishvili T, Berman DM, Ross AE, Scher
HI, De Marzo AM, Squire JA and Lotan T: Clinical implications of
PTEN loss in prostate cancer. Nat Rev Urol. 15:222–234.
2018.PubMed/NCBI View Article : Google Scholar
|
|
68
|
Sato Y, Yoshizato T, Shiraishi Y, Maekawa
S, Okuno Y, Kamura T, Shimamura T, Sato-Otsubo A, Nagae G, Suzuki
H, et al: Integrated molecular analysis of clear-cell renal cell
carcinoma. Nat Genet. 45:860–867. 2013.PubMed/NCBI View Article : Google Scholar
|
|
69
|
Pantuck AJ, Seligson DB, Klatte T, Yu H,
Leppert JT, Moore L, O'Toole T, Gibbons J, Belldegrun AS and Figlin
RA: Prognostic relevance of the mTOR pathway in renal cell
carcinoma: Implications for molecular patient selection for
targeted therapy. Cancer. 109:2257–2267. 2007.PubMed/NCBI View Article : Google Scholar
|
|
70
|
Damayanti NP, Budka JA, Khella HWZ, Ferris
MW, Ku SY, Kauffman E, Wood AC, Ahmed K, Chintala VN,
Adelaiye-Ogala R, et al: Therapeutic targeting of
TFE3/IRS-1/PI3K/mTOR axis in translocation renal cell carcinoma.
Clin Cancer Res. 24:5977–5989. 2018.PubMed/NCBI View Article : Google Scholar
|
|
71
|
Brugarolas J: Renal-cell
carcinoma-molecular pathways and therapies. N Engl J Med.
356:185–187. 2007.PubMed/NCBI View Article : Google Scholar
|
|
72
|
Robb VA, Karbowniczek M, Klein-Szanto AJ
and Henske EP: Activation of the mTOR signaling pathway in renal
clear cell carcinoma. J Urol1. 77:346–352. 2007.PubMed/NCBI View Article : Google Scholar
|
|
73
|
Hudson CC, Liu M, Chiang GG, Otterness DM,
Loomis DC, Kaper F, Giaccia AJ and Abraham RT: Regulation of
hypoxia-inducible factor 1a expression and function by the
mammalian target of rapamycin. Mol Cell Biol. 22:7004–7014.
2002.PubMed/NCBI View Article : Google Scholar
|
|
74
|
Miikkulainen P, Högel H, Seyednasrollah F,
Rantanen K, Elo LL and Jaakkola PM: Hypoxia-inducible factor
(HIF)-prolyl hydroxylase 3 (PHD3) maintains high HIF2A mRNA levels
in clear cell renal cell carcinoma. J Biol Chem. 294:3760–3771.
2019.PubMed/NCBI View Article : Google Scholar
|
|
75
|
Fiorini C, Massari F, Pedron S, Sanavio S,
Ciccarese C, Porcaro AB, Artibani W, Bertoldo F, Zampini C, Sava T,
et al: Methods to identify molecular expression of mTOR pathway: A
Rationale approach to stratify patients affected by clear cell
renal cell carcinoma for more likely response to mTOR inhibitors.
Am J Cancer Res. 4:907–915. 2014.PubMed/NCBI
|
|
76
|
Pantuck AJ, Zeng G, Belldegrun AS and
Figlin RA: Pathobiology, prognosis, and targeted therapy for renal
cell carcinoma: Exploiting the hypoxia-induced pathway. Clin Cancer
Res. 9:4641–4652. 2003.PubMed/NCBI
|
|
77
|
Baba M, Hirai S, Yamada-Okabe H, Hamada K,
Tabuchi H, Kobayashi K, Kondo K, Yoshida M, Yamashita A, Kishida T,
et al: Loss of von Hippel-Lindau protein causes cell density
dependent deregulation of cyclinD1 expression through
hypoxia-inducible factor. Oncogene. 22:2728–2738. 2003.PubMed/NCBI View Article : Google Scholar
|
|
78
|
Zatyka M, da Silva NF, Clifford SC, Morris
MR, Wiesener MS, Eckardt KU, Houlston RS, Richards FM, Latif F and
Maher ER: Identification of cyclin D1 and other novel targets for
the von Hippel-Lindau tumor suppressor gene by expression array
analysis and investigation of cyclin D1 genotype as a modifier in
von Hippel-Lindau disease. Cancer Res. 62:3803–3811.
2002.PubMed/NCBI
|
|
79
|
Toschini A, Edelstein J, Rockwell P, Ohh M
and Foster DA: HIF alpha expression in HVL-deficient renal cancer
cells is dependent on phospholipase D. Oncogene. 27:2746–2753.
2008.PubMed/NCBI View Article : Google Scholar
|
|
80
|
Carew JS, Kelly KR and Nawrocki ST:
Mechanisms of mTOR inhibitor resistance in cancer therapy. Target
Oncol. 6:17–22. 2011.PubMed/NCBI View Article : Google Scholar
|
|
81
|
Pal SK and Quinn DI: Differentiating mTOR
inhibitors in renal cell carcinoma. Cancer Treat Rev. 39:709–719.
2013.PubMed/NCBI View Article : Google Scholar
|
|
82
|
Konings IR, Verweij J, Wiemer EA and
Sleijfer S: The applicability of mTOR inhibition in solid tumors.
Curr Cancer Drug Targets. 9:439–450. 2009.PubMed/NCBI View Article : Google Scholar
|
|
83
|
Motzer RJ, Escudier B, Oudard S, Hutson
TE, Porta C, Bracarda S, Grünwald V, Thompson JA, Figlin RA,
Hollaender N, et al: Efficacy of everolimus in advanced renal cell
carcinoma: A double-blind, randomised, placebo-controlled phase III
trial. Lancet. 372:449–56. 2008.PubMed/NCBI View Article : Google Scholar
|
|
84
|
Bedke J, Gauler T, Grünwald V, Hegele A,
Herrmann E, Hinz S, Janssen J, Schmitz S, Schostak M, Tesch H, et
al: Systemic therapy in metastatic renal cell carcinoma. World J
Urol. 35:179–188. 2017.PubMed/NCBI View Article : Google Scholar
|
|
85
|
Hudes G, Carducci M, Tomczak P, Dutcher J,
Figlin R, Kapoor A, Staroslawska E, Sosman J, McDermott D, Bodrogi
I, et al: Temsirolimus, interferon alfa, or both for advanced
renal-cell carcinoma. N Engl J Med. 356:2271–2281. 2007.PubMed/NCBI View Article : Google Scholar
|
|
86
|
Park I, Lee SH and Lee JL: A Multicenter
phase II trial of axitinib in patients with recurrent or metastatic
non-clear-cell renal cell carcinoma who had failed prior treatment
with Temsirolimus. Clin Genitourin Cancer. 16:e997–e1002.
2018.PubMed/NCBI View Article : Google Scholar
|
|
87
|
Tegos T, Tegos K, Dimitriadou A and
Dimitriadis G: Current and emerging first-line systemic therapies
in metastatic clear-cell renal cell carcinoma. J BUON.
24:1340–1353. 2019.PubMed/NCBI
|
|
88
|
Ye X, Ruan JW, Huang H, Huang WP, Zhang Y
and Zhang F: PI3K-AKT-mTOR inhibition by GNE-477 inhibits renal
cell carcinoma cell growth in vitro and in vivo. Aging (Albany NY).
12:9489–9499. 2020.PubMed/NCBI View Article : Google Scholar
|
















