Introduction
Despite the recent decline in the incidence and
death rate, lung cancer still remains the leading cause of death
from cancer in the US, accounting for ~27% of all cancer-related
deaths (1). Only 17.7% of all
patients with lung cancer are alive ≥5 years after diagnosis
(2). Non-small cell lung cancer
(NSCLC) is the most common subtype of lung cancer, with a higher
incidence in developed countries (3). NSCLC accounts for >80% of all lung
cancer cases (4). Thus, there is an
urgent requirement for more accurate detection methods and more
effective treatment options to address the high mortality rate of
NSCLC. Ultrasound-targeted microbubble destruction (UTMD) was
recently developed for the local release of drugs and genes
(5). During UTMD, the gene is
integrated into a microvesicle to induce its release upon reaching
the target region, and the microbubble can promote the appearance
of irreversible pores on the target cell membrane contributing to
gene transfer to the nucleus, thereby enhancing the expression and
transfection of the target gene (5,6). After
the microvesicle enters the target tissues, ultrasound breaks the
microbubbles, and then the drugs or genes that are carried by the
microbubbles are directionally released for therapeutic purposes,
leading to a therapeutic effect in the target tissues (7). Therefore, UTMD has been considered a
promising mediator for target therapy in human malignancies.
MicroRNAs (miRNAs/miRs) are non-coding RNAs with a
length of 22-26 nucleotides (8).
Accumulating evidence has indicated that altered expression of
miRNAs is crucial for carcinogenesis and miRNAs may either have
tumor suppressor or oncogenic functions (9). In recent decades, the functional roles
of miRNAs in tumor progression have been determined in different
types of human cancer, which has increased the attention of
researchers to the aberrant expression of miRNAs in tumor samples
(10). miR-4284 is differentially
expressed in several diseases, including cancer (11,12).
Gene chip analysis has indicated that miR-4284 was associated with
disease recurrence in lung adenocarcinoma (13). However, the biological roles of
miRNA-4284 in NSCLC have rarely been investigated.
The present study aimed to evaluate the expression
of miR-4284 in NSCLC tissues and cell lines and further explored
the biological functions of miR-4284 in NSCLC progression. Several
studies have reported on the enhancing effects of UTMD on the
functional role of miRNAs in disease progression, UTMD-mediated
miR-767 inhibition has been indicated to result in a notable
suppressive effect of tumor cell proliferation, migration and
invasion, and UTMD has also been revealed to assist the exosome
delivery of miR-21, serving a protective role in the heart
(14,15). Therefore, the present study further
compared the differences in biological functional changes between
UTMD-mediated and conventional transfection of miR-4284 in NSCLC
cells. The results may provide a theoretical basis for
UTMD-mediated targeted therapy of miRNAs in NSCLC.
Materials and methods
NSCLC tissue collection
Between May 2017 and May 2019, a total of 65
patients with NSCLC underwent surgical tumor resection at Zibo
Central Hospital (Shandong, China) and were diagnosed by
histopathological examination. Cancer tissues and matched
non-cancerous tissues were collected from each patient and all
tissues were frozen in liquid nitrogen and stored at -80˚C for
further use. Patients who received preoperative treatment were
excluded from the study. The protocols for tissue collection and
analysis were approved by the Ethics Committee of Zibo Central
Hospital (Shandong, China; approval no. 170239) and written
informed consent was provided by the patients prior to
sampling.
Cell culture and conventional cell
transfection
NSCLC cell lines, including SK-MES-1, A549, NCI-H460
(RRID: CVCL_0459) and H522, and the normal lung cell line NHBE were
obtained from the Cell Bank of the Chinese Academy of Sciences. All
cells were maintained in Dulbecco's modified Eagle's medium
(Invitrogen; Thermo Fisher Scientific, Inc.) supplemented with 10%
fetal bovine serum (FBS; Invitrogen; Thermo Fisher Scientific,
Inc.) in a humidified atmosphere with 5% CO2 at
37˚C.
In this study, cell transfection was used to achieve
the regulation of miR-4284 in vitro. The cell lines A549 and
H460 were selected to perform the transfection experiments due to
significantly higher expression of miR-4284 in these two cell lines
compared with that in the normal cells. Inhibitor negative control
(NC) and miR-4284 inhibitor were synthesized from Gene
Pharmaceuticals and transfected into A549 and H460 cells using
Lipofectamine 3000 (Invitrogen; Thermo Fisher Scientific, Inc.).
Cells treated with transfection reagents alone were set as a mock
group. After 48 h of transfection, the cells were used for further
analysis.
Preparation of microbubbles and cell
transfection
The method of obtaining microbubbles was according
to the protocol of a previous study (16). It was performed by sonication of 0.4
mg/ml 1,2-distearoyl-3-trimethylammoniumpropane (Avanti Polar
Lipids, Inc.) with 1 mg/ml polyethyleneglycol-2000 stearate (Avanti
Polar Lipids, Inc.), 2 mg/ml distearoylphosphatidylcholine (Avanti
Polar Lipids, Inc.) and perfluoropropane gas. miR-4284 inhibitor
(5'-AUGGGGUAUGUGAGCCC-3') or non-targeting inhibitor-NC
(5'-CAGUACUUUUGUGUAGUACAA-3') was incubated with the microbubbles
for 30 min at 37˚C. According to the manufacturer's protocol, the
mixtures were added to A549 and H460 cells and transfected with
Lipofectamine 3000 (Invitrogen; Thermo Fisher Scientific,
Inc.).
RNA extraction and reverse
transcription-quantitative PCR
Total RNA was isolated from fresh tissue samples and
cells using TRIzol reagent (Invitrogen; Thermo Fisher Scientific,
Inc.). RNA was reversed transcribed into single-stranded
complementary DNA with the PrimeScript reverse transcriptase kit
(Takara Bio, Inc.), according to the manufacturer's protocol. The
expression levels of miR-4284 were determined by quantitative PCR
with the SYBR-Green I Master Mix kit (Invitrogen; Thermo Fisher
Scientific, Inc.) on a 7500 real-time PCR system (Applied
Biosystems; Thermo Fisher Scientific, Inc.). The following
thermocycling conditions were used for the qPCR: Initial
denaturation at 95˚C for 10 min; followed by 40 cycles at 95˚C for
20 sec, 60˚C for 15 sec and 72˚C for 20 sec. U6 was used as an
endogenous control for miR-4284. The final expression value was
calculated using the 2-ΔΔCq method (17). The sequences of primers used were as
follows: miR-4284 forward, 5'-GCCGAGGGGCTCACATCACCC CAT-3' and
reverse, 5'-CTCAACTGGTGTCGTGGA-3'; U6 forward,
5'-CTCGCTTCGGCAGCACA-3' and reverse,
5'-AACGCTTCACGAATTTGCGT-3'.
Cell Counting Kit 8 (CCK-8) assay
After cell transfection, the effect of miR-4284 on
the proliferation of A549 and H460 cells was detected using a CCK-8
assay. Cells were seeded into a 96-well plate (3x103
cells/well) and incubated for 0, 24, 48 or 72 h. Subsequently, 10
µl of CCK-8 reagent (Beyotime Institute of Biotechnology) was added
to each well and the plates were further incubated for 2 h. Cell
proliferation was quantified by determining the optical density at
450 nm using a microplate reader.
Transwell assay
Transwell chambers (Corning, Inc.) were applied in
the present study for the measurement of cell migration and
invasion of NSCLC cells. Transwell chambers precoated with Matrigel
(Corning, Inc.) were used for the invasion assay, while chambers
without Matrigel coating were used for migration assay. The
transfected cells were seeded into the upper chambers with
serum-free medium at a density of 3x105 cells/chamber,
while the lower chambers were filled with culture medium
supplemented with 10% FBS as a chemoattractant. The cells that had
transgressed through the filter/membrane to the lower chambers were
stained after 48 h of incubation and were counted under an inverted
microscope (Olympus Corp.).
Statistical analysis
Values are expressed as the mean ± standard
deviation and were analyzed in SPSS 21.0 (IBM Corp.) and GraphPad
Prism 7.0 (GraphPad Software, Inc.). A paired Student's t-test was
used to compare the differences between two groups and one-way
ANOVA followed by Tukey's test was used for differences between
multiple groups. P<0.05 was considered to indicate a
statistically significant difference.
Results
Expression of miR-4284 in NSCLC
tissues and cell lines
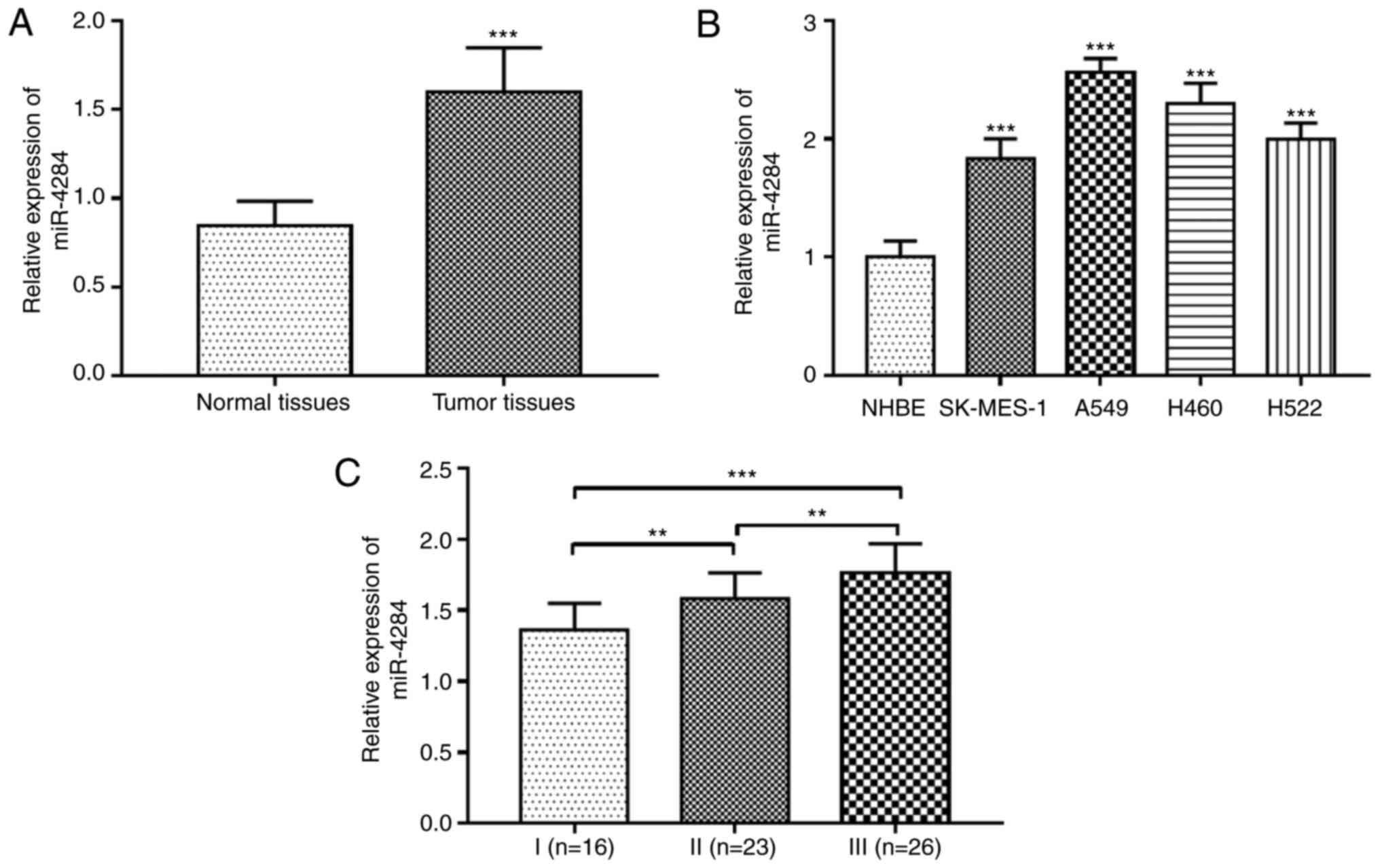
The expression level of miR-4284 was evaluated in 65
patients with NSCLC, including 41 males and 24 females with an
average age of 59.8±13.9 years (Table
I). The results indicated that the expression levels of
miR-4284 in NSCLC tissues were significantly upregulated compared
with those in matched normal tissues (P<0.001; Fig. 1A). Furthermore, compared with that
in the normal NHBE cell line, miR-4284 expression levels were also
observed to be increased in four NSCLC cell lines (SK-MES-1, A549,
H460 and H522) (all P<0.001; Fig.
1B). In addition, the expression levels of miRNA-4284 were
different among patients with different stages of TNM: The level in
patients with stage II was higher than that in patients with stage
I, it was higher in patients with stage III than that in patients
with stage II and thus, it was the highest in patients with stage
III (all P<0.01; Fig. 1C).
 | Table IDemographics and clinical
characteristics of patients with NSCLC. |
Table I
Demographics and clinical
characteristics of patients with NSCLC.
| Characteristics | Patients with NSCLC
(n=65) |
|---|
| Age, years | 59.8±13.9 |
| Tumor size, cm | 3.9±1.7 |
| Sex | |
|
Male | 41 |
|
Female | 24 |
| Smoking status | |
|
Never | 22 |
|
Current/ever | 43 |
| Differentiation | |
|
Well/moderate | 39 |
|
Poor | 26 |
| TNM stage | |
|
I-II | 37 |
|
III-IV | 28 |
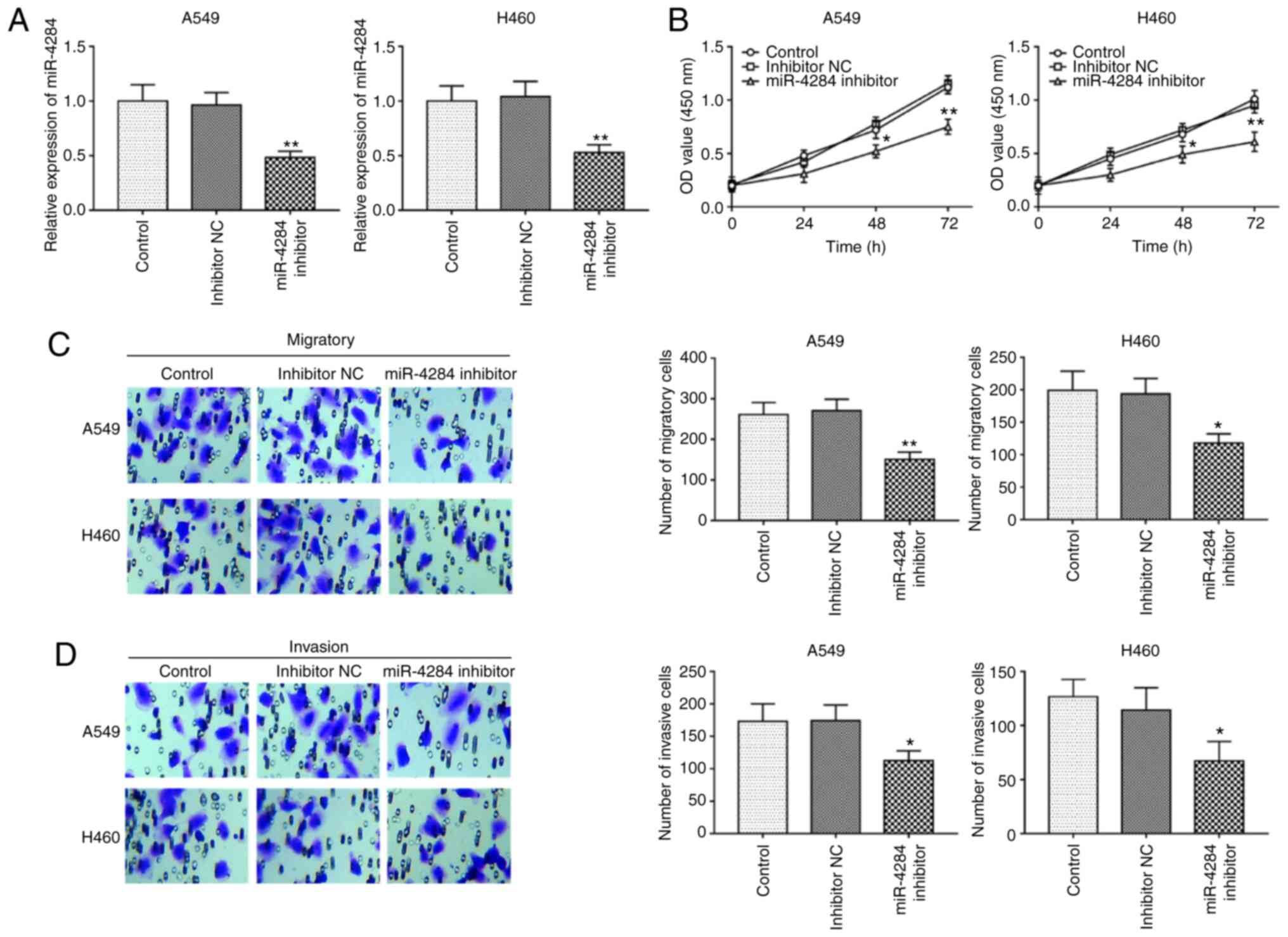
miR-4284 inhibition suppresses cell
proliferation, migration and invasion of NSCLC cells
Next, cell experiments were performed to determine
the role of miR-4284 in NSCLC. The cell lines A549 and H460 were
subjected to cell transfection, as they had significantly higher
miR-4284 expression compared with that in the normal cell line
NHBE, and among the four cells lines (SK-MES-1, A549, H460 and
H522) the expression level of miR-4284 was higher in A549 and H460
cell lines. After transfection with inhibitor, the expression
levels of miR-4284 in the A549 and H460 cell lines decreased
compared with those in the corresponding negative controls (all
P<0.01; Fig. 2A). A cell
proliferation assay indicated that decreased expression of miR4284
inhibited cell proliferation (all P<0.05; Fig. 2B). In addition to cell
proliferation, the regulatory effects of miR-4284 on the migration
and invasion of the A549 and H460 cell lines were further analyzed,
revealing that knockdown of miR-4284 inhibited cell migration and
invasion (all P<0.05; Fig. 2C
and D)
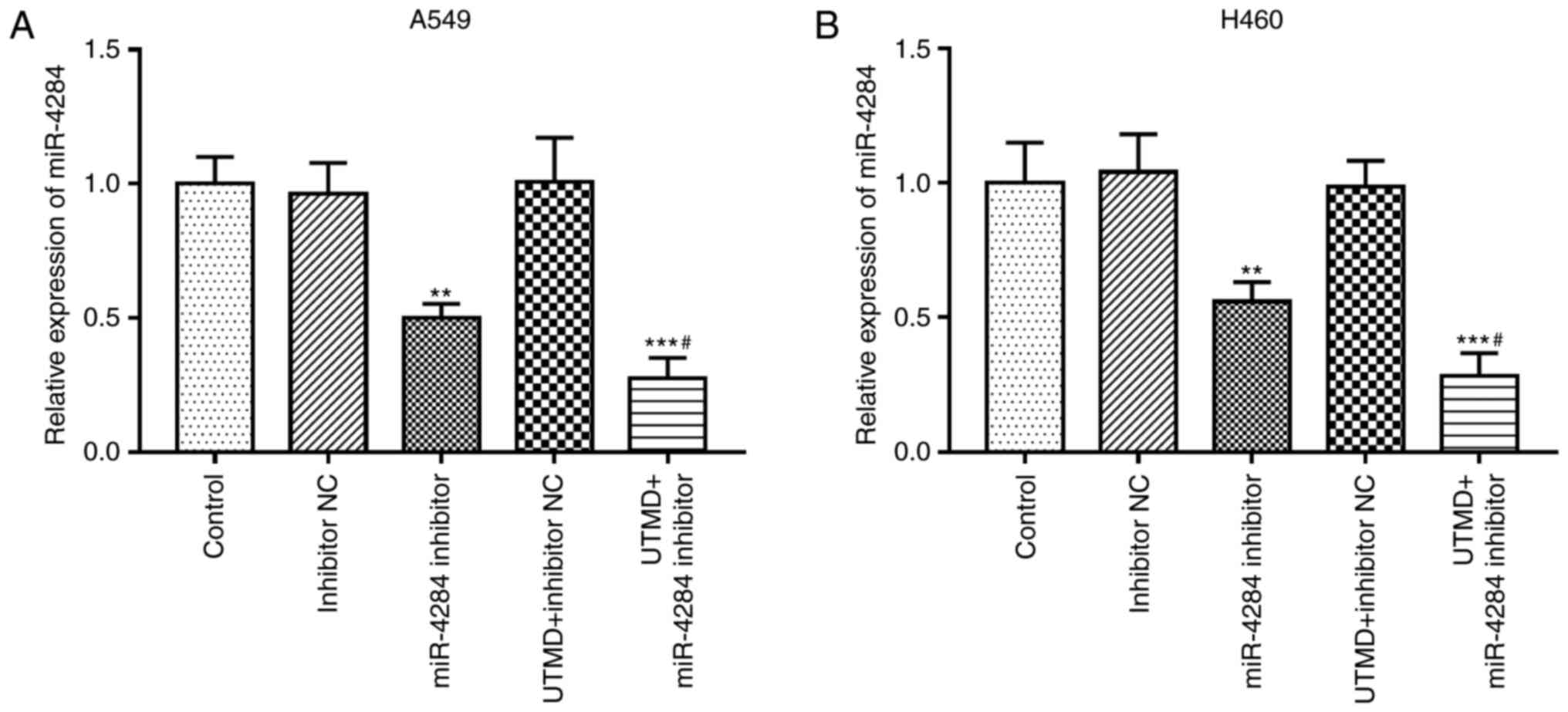
UTMD enhances the cell transfection
efficiency of miR-4284
UTMD is able to improve the transfection efficiency
of foreign genes into target tissues and organs (6). The effect of UTMD on the transfection
efficiency of miR-4284 was thus investigated. In A549 and H460
cells, UTMD significantly increased the inhibitory effects of
miR-4284 inhibitor on miR-4284 in both A549 and H460 cells, which
manifested as markedly decreased miR-4284 expression levels induced
by UTMD-mediated miR-4284 inhibitor compared with the inhibition
achieved by miR-4284 inhibitor alone (all P<0.05; Fig. 3).
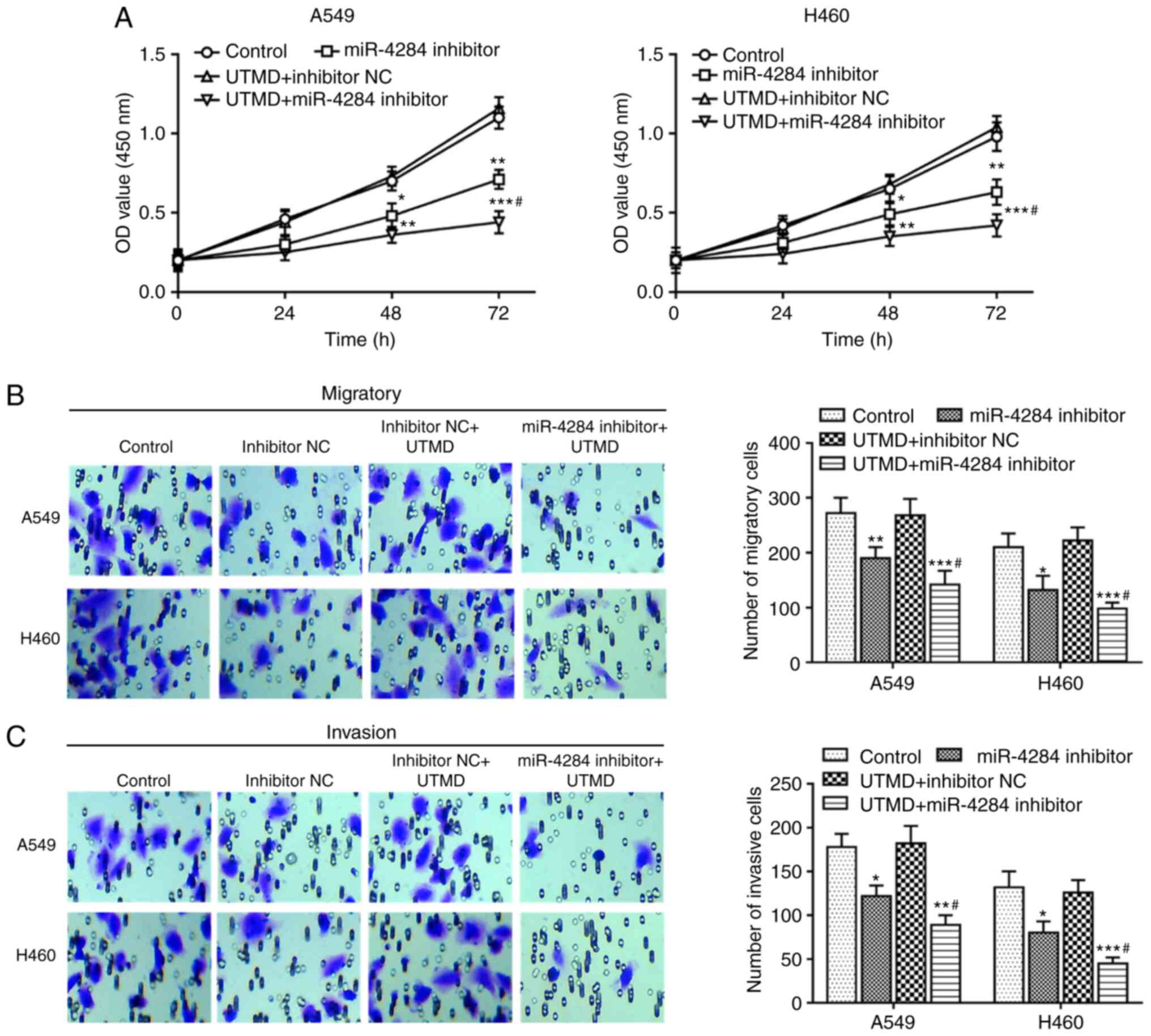
Effects of UTMD-mediated miR-4284
transfection on NSCLC cell proliferation, migration and
invasion
As presented in Fig.
4A, UTMD-mediated knockdown of miR-4284 significantly inhibited
the proliferation of A549 and H460 cells compared with that in the
mock group. As expected, further inhibition of cell proliferation
was observed in cells co-transfected with UTMD and miR-4284
inhibitor compared with that in cells transfected with miR-4284
inhibitor alone (all P<0.05). Similarly, further experiments
suggested that cell migration and invasion were significantly
reduced after in vitro transfection with UTMD-mediated
miR-4284 inhibitor compared with those in the mock group and the
miR-4284 inhibitor group (all P<0.05; Fig. 4B and C).
Discussion
Lung cancer is the most common cause of
cancer-related death worldwide (18). The number of patients with NSCLC
accounts for 85% of all patients with lung cancer and the 5-year
overall survival rate is only 15% (19). NSCLC is the leading cause of
malignancy-associated mortality worldwide (20). Lung cancer is a molecularly
heterogeneous disease with differences in the growth rate, invasive
ability, sensitivity to drugs and prognosis of the tumor during its
progression (21). Thus, the
heterogeneity provides ample opportunity for multiple treatment
approaches and target pathways. MiRNAs are small non-coding RNAs
that regulate gene expression at the post-transcriptional level,
and increasing evidence suggests that miRNAs are involved in
carcinogenesis and the development of human cancers (22,23).
For instance, in colorectal cancer (CRC), studies have indicated
the tumor suppressor function of miR-421; therefore, the miR-421/
mating-type locus (MAT1) axis is expected to be one of the targets
for CRC targeted therapy (24).
miR-4319 is able to inhibit the proliferation of CRC by targeting
ankyrin repeat and BTB/POZ domain containing protein 1 and miR-4319
may become a meaningful treatment for CRC (25). Yang et al (26) confirmed that miR-497 may be used as
a biomarker for cancer diagnosis and prognosis and is a promising
therapeutic target for future clinical applications. Ji et
al (27) studied the effect of
UTMD of miR-133a on breast cancer treatment and determined that it
inhibited tumor growth and improved the survival rate in a breast
cancer model of mice, which may indicate the safety and
effectiveness of the UTMD method for miRNA delivery in the
regulation of tumorigenesis. However, the role of miR-4284 in NSCLC
has remained elusive.
In the present study, it was determined that
miR-4284 was aberrantly expressed in NSCLC. Previous studies have
indicated that the expression of miR-4284 was altered in various
diseases, suggesting that miR-4284 may be involved in their
pathogenesis (12,28). For instance, Liu et al
(29) reported that bone
marrow-derived mesenchymal stem cells (BMSCs) had a stronger
ability to inhibit osteoclastogenesis through the miR-4284/C-X-C
motif chemokine ligand 5 axis, which provided a novel perspective
on pathological osteogenesis mechanisms of BMSCs. miR-4284 may
serve as a tissue and prognostic biomarker for diffuse large B-cell
lymphoma (30). In gastric cancer,
the expression level of miR-4284 was significantly upregulated,
which indicated that it may represent a novel diagnostic and
prognostic biomarker for this tumor type (11). In the present study, the expression
levels of miR-4284 was revealed to be upregulated in NSCLC tumors
and in the A549 and H460 cell lines as compared with those in the
corresponding normal control group. In addition, the expression
level of miR-4284 exhibited a gradual enhancement with the increase
of the TNM stage and was the highest in tumors from stage III
patients. Furthermore, cell proliferation assays suggested that the
proliferation ability of NSCLC cells decreased after miR-4284
silencing. Cell migration and invasion experiments were further
performed, further demonstrating inhibitory effects of miR-4284:
When the expression level of miR-4284 decreased, the cell migration
and invasion ability declined. Therefore, miR-4284 may have an
oncogenic role in NSCLC.
UTMD is a promising targeted gene delivery method
that has been successfully applied in the treatment of numerous
diseases over the past decade (31). As a potential drug/gene delivery
system, UTMD may be used to improve the permeability of biological
barriers and enhance the therapeutic effect of tumors (32). For instance, UTMD technology is able
to significantly facilitate the co-transmission of gemcitabine and
miR-21i and thus provides a promising strategy for the effective
treatment of pancreatic cancer (33). In the study of Yang et al
(34), miR-let-7b was transfected
into ovarian cancer stem cells by flow cytometry, and the results
indicated that miR-let-7b transfection efficiency using UTMD was
significantly higher. In NSCLC, the expression level of miR-767
could be successfully downregulated by miR-767 inhibitor and UTMD
further enhanced the transfection efficacy of miR-767 inhibitor,
while downregulation of miR-767 was indicated to inhibit the
proliferation, migration and invasion of tumor cells (15). In the present study, UTMD improved
the transfection efficiency of a miR-4284 inhibitor in NSCLC cells.
UTMD-mediated transfection further promoted the decrease in the
proliferation, migration and invasion ability of NSCLC cells
induced by miR-4284 inhibitor. This was due to UTMD enhancing the
transfection efficiency of the inhibitor of miR-4284 in
vitro, which thereby enhanced the efficacy of miR-4284
inhibition to impair the progression of NSCLC.
In conclusion, the present study indicated that the
expression of miR-4284 was increased in NSCLC tissues and cell
lines compared with that in the corresponding normal controls.
Decreased expression of miR-4284 was able to inhibit tumor cell
proliferation, migration and invasion. In addition, UTMD enhanced
the transfection efficiency of miR-4284 inhibitors, leading to more
significant inhibition of the biological function of NSCLC cells.
Therefore, miR-4284 may be a potential therapeutic target for NSCLC
and UTMD-mediated delivery of inhibitors of miR-4284 may be a
promising therapeutic strategy for NSCLC. There are still certain
limitations to the present study, such as the lack of elucidation
of the detailed mechanism of the role of miR-4284 in NSCLC
pathogenesis. In addition, the present study provided in
vitro results and it is esteemed that the effects of UTMD
introduction methods in vivo and their detailed molecular
mechanisms will be further addressed in depth in future
studies.
Acknowledgements
Not applicable.
Funding
Funding: The present study was supported by the Project of Zibo
Key Research and Development Plan (grant no. 2018kj010104).
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
PT and WD designed and conceived the study,
conducted the clinical studies, analyzed the clinical data and
wrote the manuscript. YW conducted the cell experiments and
analyzed the cell experimental data. All authors read and approved
the manuscript.
Ethics approval and consent to
participate
Written informed consent was obtained from each
patient and the experimental procedures were approved by the Ethics
Committee of Zibo Central Hospital (Shandong, China; approval no.
170239).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Smith RA, Andrews KS, Brooks D, Fedewa SA,
Manassaram-Baptiste D, Saslow D, Brawley OW and Wender RC: Cancer
screening in the United States, 2018: A review of current American
Cancer Society guidelines and current issues in cancer screening.
CA Cancer J Clin. 68:297–316. 2018.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Miller KD, Siegel RL, Lin CC, Mariotto AB,
Kramer JL, Rowland JH, Stein KD, Alteri R and Jemal A: Cancer
treatment and survivorship statistics, 2016. CA Cancer J Clin.
66:271–289. 2016.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Travis WD, Brambilla E, Nicholson AG,
Yatabe Y, Austin JHM, Beasley MB, Chirieac LR, Dacic S, Duhig E, et
al: The 2015 World Health Organization Classification of Lung
Tumors: Impact of Genetic, Clinical and Radiologic Advances Since
the 2004 Classification. J Thorac Oncol. 10:1243–1260.
2015.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Cai J, Fang L, Huang Y, Li R, Xu X, Hu Z,
Zhang L, Yang Y, Zhu X, Zhang H, et al: Simultaneous overactivation
of Wnt/β-catenin and TGFβ signalling by miR-128-3p confers
chemoresistance-associated metastasis in NSCLC. Nat Commun.
8(15870)2017.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Chen H and Hwang JH: Ultrasound-targeted
microbubble destruction for chemotherapeutic drug delivery to solid
tumors. J Ther Ultrasound. 1(10)2013.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Tinkov S, Bekeredjian R, Winter G and
Coester C: Microbubbles as ultrasound triggered drug carriers. J
Pharm Sci. 98:1935–1961. 2009.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Yang H, Sun Y, Wei J, Xu L, Tang Y, Yang
L, Zhang X and Lu Y: The effects of ultrasound targeted microbubble
destruction (UTMD) carrying IL 8 monoclonal antibody on the
inflammatory responses and stability of atherosclerotic plaques.
Biomed Pharmacother Oct. 118(109161)2019.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Lee Y, Jeon K, Lee JT, Kim S and Kim VN:
MicroRNA maturation: Stepwise processing and subcellular
localization. EMBO J. 21:4663–4670. 2002.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Barbato S, Solaini G and Fabbri M:
MicroRNAs in Oncogenesis and Tumor Suppression. Int Rev Cell Mol
Biol. 333:229–268. 2017.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Dan B, Luo J, Li K and Chen S: Prognostic
value of miR-375 for survival outcomes in various cancers: A
systematic review and meta-analysis. Oncol Res Treat. 41:47–50.
2018.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Li Y, Shen Z, Jiang H, Lai Z, Wang Z,
Jiang K, Ye Y and Wang S: MicroRNA 4284 promotes gastric cancer
tumorigenicity by targeting ten-eleven translocation 1. Mol Med
Rep. 17:6569–6575. 2018.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Yang F, Nam S, Brown CE, Zhao R, Starr R,
Ma Y, Xie J, Horne DA, Malkas LH, Jove R, et al: A novel berbamine
derivative inhibits cell viability and induces apoptosis in cancer
stem-like cells of human glioblastoma, via up-regulation of
miRNA-4284 and JNK/AP-1 signaling. PLoS One.
9(e94443)2014.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Sim J, Kim Y, Kim H, Shin SJ, Kim DH, Paik
SS and Jang K: Identification of recurrence-associated microRNAs in
stage I lung adenocarcinoma. Medicine (Baltimore).
97(e10996)2018.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Sun W, Zhao P, Zhou Y, Xing C, Zhao L, Li
Z and Yuan L: Ultrasound targeted microbubble destruction assisted
exosomal delivery of miR-21 protects the heart from chemotherapy
associated cardiotoxicity. Biochem Biophys Res Commun. 532:60–67.
2020.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Li X, Xu M, Lv W and Yang X:
Ultrasound-targeted microbubble destruction-mediated miR-767
inhibition suppresses tumor progression of non-small cell lung
cancer. Exp Ther Med. 19:3391–3397. 2020.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Leong-Poi H, Kuliszewski MA, Lekas M,
Sibbald M, Teichert-Kuliszewska K, Klibanov AL, Stewart DJ and
Lindner JR: Therapeutic arteriogenesis by ultrasound-mediated
VEGF165 plasmid gene delivery to chronically ischemic skeletal
muscle. Circ Res. 101:295–303. 2007.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) Method. Methods. 25:402–408.
2001.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Torre LA, Bray F, Siegel RL, Ferlay J,
Lortet-Tieulent J and Jemal A: Global cancer statistics, 2012. CA
Cancer J Clin. 65:87–108. 2015.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Akhurst T: Staging of non-small-cell lung
cancer. PET Clin. 13:1–10. 2018.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Soria JC, Massard C and Le Chevalier T:
Should progression-free survival be the primary measure of efficacy
for advanced NSCLC therapy? Ann Oncol. 21:2324–2332.
2010.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Schwartz AG and Cote ML: Epidemiology of
lung cancer. Adv Exp Med Biol. 893:21–41. 2016.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Connell LC, Harding JJ and Abou-Alfa GK:
Advanced Hepatocellular Cancer: The Current State of Future
Research. Curr Treat Options Oncol. 17(43)2016.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Wang Z, Sha HH and Li HJ: Functions and
mechanisms of miR 186 in human cancer. Biomed Pharmacother.
119(109428)2019.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Xue L and Yang D: miR 421 inhibited
proliferation and metastasis of colorectal cancer by targeting
MTA1. J BUON. 23:1633–1639. 2018.PubMed/NCBI
|
|
25
|
Huang L, Zhang Y, Li Z, Zhao X, Xi Z, Chen
H, Shi H, Xin T, Shen R and Wang T: miR-4319 suppresses colorectal
cancer progression by targeting ABTB1. United European
Gastroenterol J. 7:517–528. 2019.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Yang G, Xiong G, Cao Z, Zheng S, You L,
Zhang T and Zhao Y: miR-497 expression, function and clinical
application in cancer. Oncotarget. 7:55900–55911. 2016.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Ji Y, Han Z, Shao L and Zhao Y: Evaluation
of in vivo antitumor effects of low-frequency ultrasound-mediated
miRNA-133a microbubble delivery in breast cancer. Cancer Med.
5:2534–2543. 2016.PubMed/NCBI View
Article : Google Scholar
|
|
28
|
Munari E, Marchionni L, Chitre A, Hayashi
M, Martignoni G, Brunelli M, Gobbo S, Argani P, Allaf M, Hoque MO,
et al: Clear cell papillary renal cell carcinoma: micro-RNA
expression profiling and comparison with clear cell renal cell
carcinoma and papillary renal cell carcinoma. Hum Pathol.
45:1130–1138. 2014.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Liu W, Wang P, Xie Z, Wang S, Ma M, Li J,
Li M, Cen S, Tang S, Zheng G, et al: Abnormal inhibition of
osteoclastogenesis by mesenchymal stem cells through the
miR-4284/CXCL5 axis in ankylosing spondylitis. Cell Death Dis.
10(188)2019.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Tamaddon G, Geramizadeh B, Karimi MH,
Mowla SJ and Abroun S: miR-4284 and miR-4484 as putative biomarkers
for diffuse large B-cell lymphoma. Iran J Med Sci. 41:334–339.
2016.PubMed/NCBI
|
|
31
|
Wu J and Li RK: Ultrasound-targeted
microbubble destruction in gene therapy: A new tool to cure human
diseases. Genes Dis. 4:64–74. 2016.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Shi D, Guo L, Sun X, Shang M, Meng D, Zhou
X, Liu X, Zhao Y and Li J: UTMD inhibit EMT of breast cancer
through the ROS/miR-200c/ZEB1 axis. Sci Rep.
10(6657)2020.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Lin L, Fan Y, Gao F, Jin L, Li D, Sun W,
Li F, Qin P, Shi Q, Shi X, et al: UTMD-Promoted Co-Delivery of
gemcitabine and miR-21 inhibitor by dendrimer-entrapped gold
nanoparticles for pancreatic cancer therapy. Theranostics.
8:1923–1939. 2018.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Yang C, Li B, Yu J, Yang F, Cai K and Chen
Z: Ultrasound microbubbles mediated miR-let-7b delivery into
CD133+ ovarian cancer stem cells. Biosci Rep.
38(38)2018.PubMed/NCBI View Article : Google Scholar
|