Introduction
Parkinson's disease (PD) is the second most
prevalent neurodegenerative disorder after Alzheimer's disease
(1). PD is characterized by
progressive loss of nigral dopamine neurons and decreased dopamine
levels in the striatum of the basal ganglia. Patients with PD
present with symptoms such as tremor at rest, rigidity,
bradykinesia, postural abnormalities and the freezing phenomenon
(1). Studies have reported a
prevalence of PD of 0.5-1% among individuals aged 65-69 years and
1-3% among those aged 80 years and above (2). Despite nearly 50 years of research, no
effective treatment has been developed for PD (3). L-DOPA has been the most widely used PD
treatment, however, its therapeutic effects decrease with long-term
therapy. Furthermore, numerous alternative therapies produce severe
side effects during therapy (4).
The current pharmacological treatments for PD only treat symptoms
and cannot stop the progressive loss of dopaminergic neurons in
patients with PD (5). Therefore, it
is essential to discover other potential therapeutic agents with
better efficacy for PD. Furthermore, reports of the failures of
candidate drugs for PD suggest the need for strategies to enhance
the probability of effective translation into animal research,
therefore providing improved clinical benefits (6). Numerous preclinical systematic reviews
have been proposed to promote candidate drug development and
discovery as well as clinical drug development. For centuries,
ginseng has been used in Traditional Chinese Medicine as a tonic
for vitality and stamina. The major active components of ginseng
are ginsenosides (7), which exert
beneficial effects in humans, including alleviating learning and
memory impairment as well as reversing pathological and
physiological changes induced by stress and aging. Ginsenoside-Rg1
(G-Rg1) is the most significant bioactive component responsible for
the pharmaceutical actions of ginseng (8). It has a wide range of neurotrophic and
neuroprotective effects and low toxicity (9). In vivo studies have reported
that G-Rg1 protects dopaminergic neurons against glutamate,
1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) and rotenone
toxicities (10-12).
Chen et al (13)
demonstrated the protective effect of Rg1 against MPTP-induced
nigral neuronal loss. Heng et al (14) reported that Rg1 improved animal
survival rates, dopamine loss, motor neuron deficits and abnormal
induced ultrastructural changes.
However, to date, only a small number of systematic
reviews have established the effects of G-Rg1 in animal models of
PD. Song et al (15)
published a systematic review using tyrosine hydroxylase
(TH)-positive cells in the nigra as the outcome, which is
insufficient to judge dopamine neuron loss (16). Therefore, in the present study,
further outcomes were included in a meta-analysis, including the
number of Nissl stain-positive cells. The majority of the published
experimental studies have small sample sizes. Systematically
reviewing and meta-analyzing all of these studies in an objective
manner is likely to offer reliable and credible evidence on whether
a G-Rg1 therapy is effective in experimental PD, allowing for the
selection of optimal drug administration requirements for clinical
trials. Therefore, a systematic review and meta-analysis was
performed to provide evidence supporting the role of G-Rg1 as a
neuroprotectant in experimental PD. TH-positive cells, pole test
times, Nissl-positive cell counts and DA levels were integrated to
perform the meta-analysis.
Materials and methods
Search strategy
The search strategy was designed according to the
criteria of the Preferred Reporting Items for Systematic reviews
and Meta-Analyses statement and with no language restrictions
(17). An independent search of
studies on the effects of G-Rg1 therapy on PD was performed in the
following databases from their inception to 2019: PubMed, EMBASE,
Web of Science, VIP, Chinese National Knowledge Infrastructure and
Wanfang databases. References of articles and reviews of interest
were also scanned for additional relevant studies.
The literature search for the meta-analysis was
restricted to published animal studies. In addition, references of
relevant original papers and review articles were screened. Using
the grouped terms, the PubMed search strategy was as follows and
was altered to suit other databases: i) ‘Paralysis agitans’; ii)
‘idiopathic Parkinson's disease’; iii) ‘Parkinsons disease’; iv)
‘Parkinson’s disease’; v) ‘Parkinson disease’; vi) ‘Parkinsonism’;
vii) or/i-v; viii) ‘ginseng ginsenoside’; ix) ‘ginsenoside-Rg1’; x)
‘G-Rg1’; xi) ‘Rg1’; xii) or/vii-xi, vi and xii.
Selection criteria
The included studies assessed the effects of G-Rg1
in animal models of PD, with the outcomes measured being
TH-positive cells in the nigra, Nissl-positive cells in the nigra,
and pole test time and/or dopamine (DA) level in the striatum. The
following inclusion criteria were established: i) Studies testing
the effect of G-Rg1 in animal models of PD; ii) in the treatment
group, the TH-positive cells, pole test times, and/or DA levels
were compared with vehicle-treated or untreated model animals; and
iii) in the treatment group, G-Rg1 was not tested in combination
with other neuroprotective agents. The pre-specified exclusion
criteria were as follows: i) Reviews, case reports, abstracts,
letters or comments, as well as clinical trials; ii) studies not
measuring TH-positive cells and/or pole test times and/or
Nissl-positive cell counts and/or DA levels as the outcome; and
iii) studies not reporting the effect of G-Rg1 in PD. TH-positive
cell counts and DA contents are commonly used to measure
dopaminergic neurons in the nigra and the striatum, respectively,
in animal models of PD (18,19).
The pole test is an effective method of estimating bradykinesia and
motor coordination in animal models (20). The PD models used have yet to
predict the efficacy of a single effective treatment, although they
have been useful in selecting certain symptomatically beneficial
drugs (21).
Data extraction
The following information was extracted from the
studies: i) Name of first author and year of publication, and the
method of generating the animal model; ii) sample size, sex,
species and body weight of the animals; iii) timing and dosage of
treatment as well as the treatment procedure; iv) outcome measures.
If the outcome was evaluated at several time-points, the time-point
of the last sacrifice was also extracted. The authors were
requested to provide additional information if the data required
for the review were incomplete or only presented graphically. When
no response was received, digital ruler software was used to
measure the data from the graphs. Data on the mean value and
standard deviation were extracted for each treatment and control
group. The time-point of lesion and drug administration were both
set at zero.
Definitions of subgroups
It was expected that the numbers of TH-positive
cells in the nigra would vary based on different animal strains and
PD models. In the present review, the animals were classified into
two groups. In one group, C57BL mice were injected with MPTP, while
in the second group, Wistar rats were injected with
6-hydroxydopamine (6-OHDA).
Quality assessment
Methodological quality was assessed based on an
eight-item modified scale from the STAIR list (21). The modified scale included the
following items: i) Peer-reviewed publication; ii) sample size
calculation; iii) randomization; iv) allocation concealment; v)
report of animals excluded from analysis; vi) blinded assessment of
the outcome; vii) compliance with animal welfare regulations; viii)
report on potential conflicts of interest and funding sources. For
calculation of the quality assessment aggregate score, each item on
the eight-item scale was equal to one point.
Statistical analysis
Statistical analysis was performed using RevMan
v.5.3 software (https://training.cochrane.org/online-learning/core-software-cochrane-reviews/revman).
Publication bias was analyzed using STATA/SE 12.0 software
(StataCorp). P<0.05 was considered to indicate statistical
significance. Data on TH-positive cells, pole test time and DA
levels were considered continuous data. These indicators were used
to estimate the combined effect size using the standardized mean
difference (SMD). The SMD is utilized as a summary statistic in a
meta-analysis when all studies assess the same outcome but measure
it in different ways (22).
Publication bias was assessed using a funnel plot and Egger's test
(23). The I2 statistic
was used to assess heterogeneity. The fixed-effects model
(Mantel-Haenszel method) was used if heterogeneity was negligible
and the random-effects model (DerSimonian and Laird method) was
used if heterogeneity was significant. To examine the robustness of
the results, a sensitivity analysis was performed by omitting each
study in turn from the total and reanalyzing the quality of the
remaining studies. Furthermore, the impact of factors influencing
the outcome was evaluated using a pre-specified subgroup analysis
based on the following features: Quality score, G-Rg1 dosage and
animal weight. The difference between groups was measured by
partitioning heterogeneity and using the χ2 distribution
with n-1 degrees of freedom, where n equals the number of groups.
One-way ANOVA followed by Tukey's test was used to determine
significance between groups by using GraphPad Prism 7.0 (GraphPad
Software, Inc.).
Results
Characteristics of the included
studies
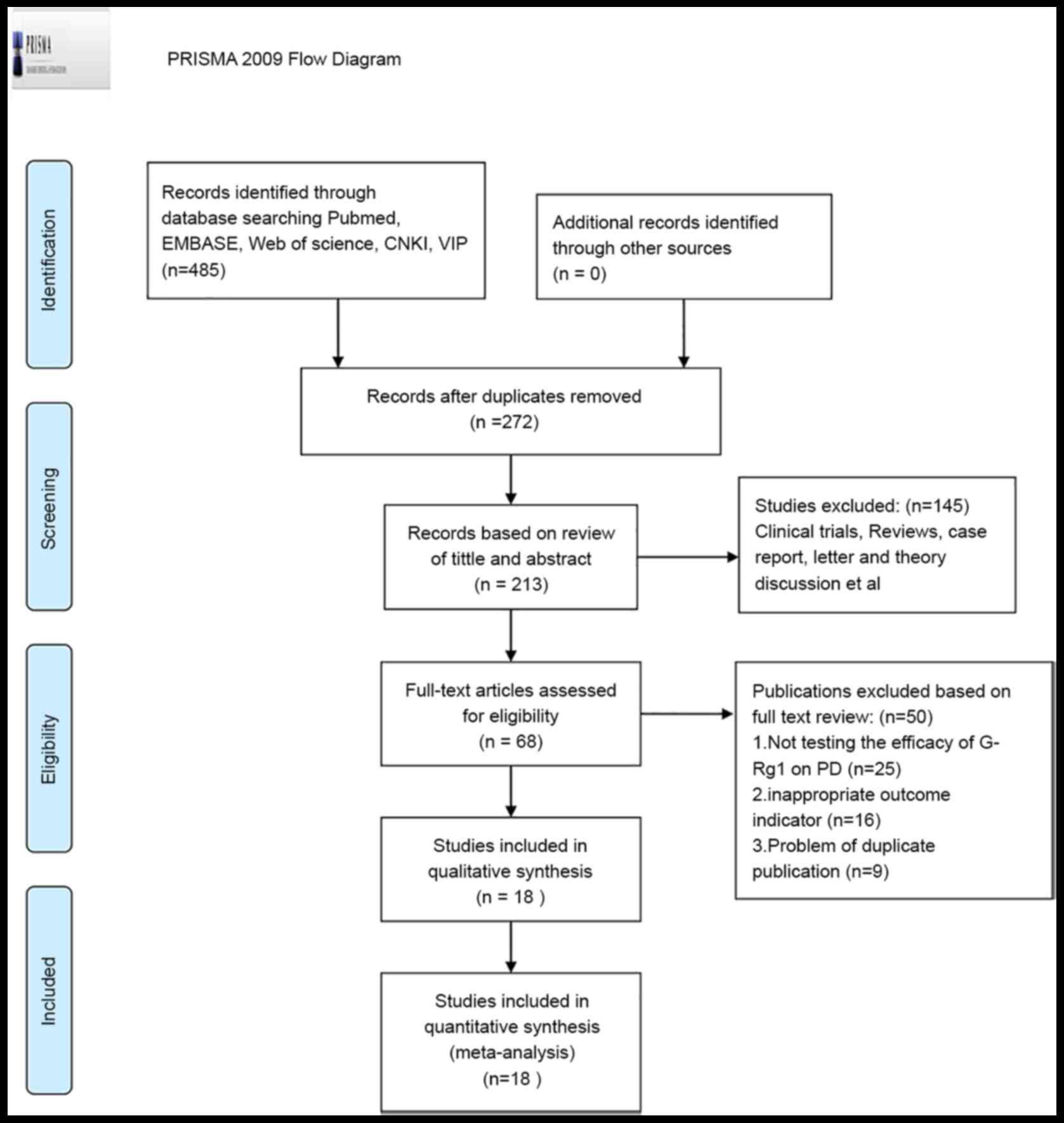
following an independent review, 485 papers were
identified. After removing duplicates, 213 unique articles were
identified and 145 papers were excluded after reviewing the titles
and abstracts due to at least one of the following reasons: i)
Clinical trial and/or ii) review, case report, letter or theory
discussion. After reading the remaining 68 papers, which reported
the effect of G-Rg1 on PD models, 18 articles (13,14,24-39)
were identified as meeting the eligibility criteria (Fig. 1).
The studies involved a total of 343 animals (G-Rg1
group, n=167; control group, n=176) and they all belonged to two
species: Wistar rats (n=18) (34,35)
and C57BL/6 mice (n=325). Furthermore, 16 out of the 18 studies
used 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP), -induced
models, whereas the remaining two studies used 6-hydroxydopamine
(6-OHDA)-induced models (34,35).
The sex of the animals used was male in 14 studies and female in 3
studies (34-36).
One study did not report animal sex (25). All-female animals were
ovariectomized and chloral hydrate and Euthanal were used in 4
studies and 1 study, respectively. The remaining 13 studies did not
report the anesthetic drug used. The publication year of the
studies ranged from 2002 to 2019. The sample size used in the
studies varied from 10 to 47 animals. The mice used weighed 16-30
g, while the rats weighed 220-250 g. However, only the mean or
range of the data in each study was used for this meta-analysis,
rather than individual data. The schedule of the MPTP injection
differed, as 30 mg/kg/day (d) intraperitoneally (i.p.) for 5 d was
used in 13 studies (13,24,33,37,38),
25 mg/kg/d i.p. every 4 d on a 40-d schedule was used in 1 study
(14) and 60 mg/kg/d i.p. for 1 d
was used in 2 studies (36,39). Treatment regimens included 2.5 and 3
µl 6-OHDA (3.6 mg/µl in 0.9% saline containing 0.02% w/v ascorbic
acid) unilaterally injected into the medial forebrain bundle per
treatment in two studies (34,35). A
total of five studies used a dose gradient of G-Rg1. Among them,
four studies used 5-, 10- and 20 mg/kg doses (24,36-38)
and one study utilized 10-, 20- and 40-mg/kg doses (14), while the remaining studies applied
2.5-, 5- and 10-mg/kg doses (13).
A total of 13 studies included a single dose of G-Rg1, 10 mg/kg in
12 studies (25-27,29,36,39)
and 5 mg/kg in 1 study (28). The
number of TH-positive cells in the nigra was the outcome measure in
13 studies (13,24,26,27,29-35,37,38),
Nissl-positive cells were the outcome measure in 3 studies
(13,23,37),
the pole test time was the outcome measure in 4 studies (14,25,37,39)
and DA content in the striatum was the outcome measure in 3 studies
(28,34,36).
In the 13 studies assessing the TH-positive nigra cells, the
TH-positive cell count was appraised using diaminobenzidine
staining before incubation with a TH polyclonal antibody and
biotinylated IgG as the secondary antibody. The plasma and positive
cell processes were stained brown and measured using analytical
software. In the 3 studies with Nissl-positive cells as the outcome
measure, the staining method was as follows: Paraffin sections were
deparaffinized and hydrated, stained with methylene blue buffer for
10 min and immersed into an acetic acid buffer for 2 min. The four
studies in which the outcome was the pole test time employed a
previously reported protocol (40).
The total time required to climb down the pole was measured. The 3
studies in which the DA content in the striatum was the outcome
measure, DA contents were measured by high-performance liquid
chromatography with electrochemical detection and the results were
expressed as ng/mg wet weight of brain tissue (Table I).
 | Table IBasic characteristics of the included
studies. |
Table I
Basic characteristics of the included
studies.
| First author
(year) | Species (sex,
n) | Body weight
(g) | Model, agent (dose,
route) and duration | Anesthetic | Intervention
method | Outcomes | P-value | (Refs.) |
|---|
| Chen, 2002 | C57BL mice (male,
6/6) | 20±2 | MPTP (30 mg/kg/d,
i.p.) for 5d | NR | Rg1 (2.5, 5 and 10
mg/kg/d, i.p.) for 3d; D4, P-T 2 h before MPTP injection for
5d | 1. TH+
cells | 1. <0.01 | (13) |
| | | | | | | 2. Nissl/Tunel
neuron | 2. <0.01 | |
| | | | | | | 3.
iNOS/caspase-3/NOS | 3. <0.05 | |
| Chen, 2005 | C57BL mice (male,
8/8) | 20±2 | MPTP (30 mg/kg/d,
i.p.) for 5d | Euthanal | Rg1 (5, 10 and 20
mg/kg/d, i.p.) for 3d before MPTP injection | 1. TH+
cells | 1. <0.01 | (24) |
| | | | | | | 2. Nissl
cells/Tunel neuron | 2. <0.01 | |
| | | | | | | 3.
GSH/T-SOD/p-Jnk/p-c-Jun/ | 3. <0.01 | |
| Heng 2016 | C57BL/6 mice (male,
19/28) | 23±2 | MPTP (25 mg/kg/d,
i.p.), probenecid (250 mg/kg/d, i.p.) every 4d on a 40-d
schedule | 10% Chloral hydrate
(400 mg/kg, i.p.) | Rg1 (10, 20 and 40
mg/kg/d, i.p.) from D(-3) to day 49 | 1. MB (rotarod/pole
tests) | 1. <0.01 | (14) |
| | | | | | | 2. TH+
protein | 2. <0.01 | |
| | | | | | | 3. GFAP/IBA-1” | 3. <0.01 | |
| Jiang, 2015 | C57BL/6 mice (NR,
10/10) | 25-30 | MPTP (30 mg/kg/d,
i.p.) for 5d | NR | Rg1 (10 mg/kg/d,
i.p.) from the first day of MPTP injection until 10 days after the
last injection of MPTP | 1. MB (rotarod/pole
tests) | 1. <0.05 | (25) |
| | | | | | | 2. TH/α-synuclein
proteins | 2. <0.05 | |
| | | | | | | 3. TH fibers | 3. <0.01 | |
| Liu, 2008 | C57BL/6N mice
(male, 15/15) | 18-23 | MPTP (30 mg/kg/d,
i.p.) for 5d | NR | Rg1 (10 mg/kg/d,
i.p.) for 3d; D4, P-T 2-3 h before MPTP injection for 5d | 1. TH+
cells | 1. <0.01 | (26) |
| | | | | | | 2.
p-c-Jun/Tunel+ cells | 2. <0.05 | |
| | | | | | | 3. p-c-Jun
protein | 3. <0.01 | |
| Shi, 2009 | C57BL/6N mice
(male, 5/5) | 25-30 | MPTP (30 mg/kg/d,
i.p.) for 5d | NR | Rg1 (10 mg/kg/d,
i.p.) for 3d; D4, P-T 2 h before MPTP injection for 5d | 1. TH+
cells | 1. <0.01 | (27) |
| | | | | | | 2. iNOS/p-erk
cells | 2. <0.01 | |
| | | | | | | 3. iNOS/p-erk
protein” | 3. <0.05 | |
| Wang, 2008 | C57BL/6N mice
(male, 10/10) | 25-30 | MPTP (30 mg/kg/d,
i.p.) for 5d | NR | Rg1 (10 mg/kg/d,
i.p.) for 3d; D4, P-T 2 h before MPTP injection for 5d | 1. TH+
cells | 1. <0.01 | (32) |
| | | | | | | 2. TH/COX-2/PGE/p38
protein | 2. <0.01 | |
| Wang, 2009 | C57BL/6N mice
(male, 9/9) | 25-30 | MPTP (30 mg/kg/d,
i.p.) for 5d | NR | Rg1 (10 mg/kg/d,
i.p.) for 3 d; D4, P-T 2-3 h before MPTP injection for 5d | 1. TH+
cells | 1. <0.01 | (33) |
| | | | | | | 2.
COX-2+ cells | 2. <0.01 | |
| | | | | | | 3. TH, COX-2,
p-c-Jun protein | 3. <0.01 | |
| Wang, 2012 | C57BL/6N mice
(male, 6/6) | 25-30 | MPTP (30 mg/kg/d,
i.p.) for 5d | NR | Rg1 (10 mg/kg/d,
i.p.) for 3d; D4, P-T 2-3 h before MPTP injection for 5d | 1. TH+
cells | 1. <0.01 | (31) |
| | | | | | | 2.
p-P38/NF-κB/COX-2/ TH protein | 2. <0.01 | |
| Wang, 2013 | C57BL/6N mice
(male, 9/9) | 25-30 | MPTP (30 mg/kg/d,
i.p.) for 5d | NR | Rg1 (10 mg/kg/d,
i.p.) for 3d; D4, P-T 2-3 h before MPTP injection for 5d | 1. TH+
cells | 1. <0.01 | (29) |
| | | | | | | 2. NF-κB/iNOS
cells | 2. <0.01 | |
| | | | | | | 3. NF-κB/iNOS/TH
protein expression | 3. <0.05 | |
| Wang, 2014 | C57BL/6N mice
(male, 9/9) | 25-30 | MPTP (30 mg/kg/d,
i.p.) for 5d | NR | Rg1 (10 mg/kg/d,
i.p.) for 3d; D4, P-T 2 h before MPTP injection for 5d. | 1. TH+
cells | 1. <0.01 | (30) |
| | | | | | | 2.
NF-κB/iNOS+ cells | 2. <0.01 | |
| Wang, 2009 | C57BL/6 mice (male,
6/6) | 20–22 | MPTP (30 mg/kg/d,
i.p.) for 5d | 10% Chloral
hydrate | Rg1 (5 mg/kg/d,
i.p.) for 3d; D4, P-T 2 h before MPTP injection for (400 mg/kg,
i.p.) 5d. | 1. DA/DOPAC/HVA
iron+ cells | 1. <0.01 | (28) |
| | | | | | | 2. TH
protein/mRNA/ | 2. <0.01 | |
| | | | | | | 3. DMT1 ± IRE
positive cell numbers” | 3. <0.01 | |
| Yan, 2014 | Ovariectomized
C57BL/6 mice (female, 10/10) | 20±2 | MPTP (60 mg/kg/d,
i.p.) for 1d | NR | Rg1 (10 mg/kg/d,
i.p.) for 3d; D4, P-T 2 h before MPTP injection for 5d. | 1. DA | 1. <0.01 | (36) |
| | | | | | | 2. TH-IR cells | 2. <0.01 | |
| Xu, 2008 | Ovariectomized
Wistar rats | 220-250 | 2.5 and 3 µl 6-OHDA
injected into MFB per time | 10% Chloral
hydrate | Rg1 (10 mg/kg i.p.
q.d.) for | 1. TH+
cells | 1. <0.01 | (35) |
| | | | | | | 2. TH mRNA
expression | 2. <0.01 | |
| | (female,6/6) | | | (400 mg/kg,
i.p.) | 14 days | | | |
| Xu, 2009 | Ovariectomized
Wistar rats | 220-250 | 2.5 and 3 µl 6-OHDA
injected into MFB per time | 10% Chloral
hydrate | Rg1 (10 mg/kg i.p.
q.d.) for | 1. MB (pole test,
rotarod test) | 1. <0.01 | (34) |
| | | | | | | 2. TH+
cells | 2. <0.01 | |
| | (female,
12/12) | | | (400 mg/kg,
i.p.) | 14 days | 3. DA/DAT | 3. <0.01 | |
| | | | | | | 4. TH/DAT/Bcl-2
mRNA expression” | 4. <0.01 | |
| Zhou, 2003 | C57BL mice (male,
8/8) | 20±2 | MPTP (30 mg/kg/d,
i.p.) for 5d | NR | Rg1 (5, 10, 20
mg/kg/d, i.p.) for 3d; D4, P-T 2 h before MPTP injection for
5d | 1.TH+
cells | 1. <0.01 | (38) |
| | | | | | | 2.
Nissl/caspase-3/Tunel/p-Jnk | 2. <0.01 | |
| Zhou, 2016 | C57B/6J mice (male,
10/10) | 16-25 | MPTP (30 mg/kg/d,
i.p.) for 5d | NR | Rg1 (5, 10, 20
mg/kg/d, i.p.) for 3d; D4, P-T 2 h before MPTP injection for
5d | 1. TH+
cells | 1. <0.01 | (37) |
| | | | | | | 2. MB (pole
test) | 2. <0.01 | |
| | | | | | | 3. Wnt1/GSK-3b/
p-GSK-3b | 3. <0.01 | |
| Zhu, 2014 | C57BL/6J mice
(male, 9/9) | 22-30 | MPTP (60 mg/kg/d,
i.p.) for 1d | 60 g/l Chloral
hydrate (30 mg/kg, i.p.) | Rg1 (10 mg/kg/d,
i.p.) for 3d before MPTP injection | 1. MB (pole
tests) | 1. <0.01 | (39) |
| | | | | | | 2. Ephrin
B2/p-c-Jun protein | 2. <0.01 | |
| | | | | | | 3. TH mRNA | | |
Methodological quality
The quality scores of the 18 studies ranged from 3
to 8, out of which three studies had a score of 3, six studies had
a score of 4, four studies had a score of 5, four studies had a
score of 6 and one study had a score of 8 (Table II). Only one study mentioned the
sample size calculation. All studies presented detailed methods for
the random allocation to the treatment group. Allocation
concealment was performed in 9 studies. In addition, two studies
reported conditions under which the animals were excluded from
analysis, while 13 studies included a description of the blinded
assessment of the outcome. Furthermore, five studies provided a
statement of potential conflict of interest and funding sources
(Table II).
 | Table IIQuality assessment of included
studies. |
Table II
Quality assessment of included
studies.
| | Criterion no. | |
|---|
| Author (year) | i | ii | iii | iv | v | vi | vii | viii | Total criteria
fulfilled (n) | (Refs.) |
|---|
| Chen, 2002 | √ | | √ | | | √ | √ | | 4 | (13) |
| Chen, 2005 | √ | | √ | √ | | √ | √ | | 5 | (24) |
| Heng, 2016 | √ | √ | √ | √ | √ | √ | √ | √ | 8 | (14) |
| Jiang, 2015 | √ | | √ | √ | | √ | √ | √ | 6 | (25) |
| Liu, 2008 | √ | | √ | √ | | √ | √ | | 5 | (26) |
| Shi, 2009 | √ | | √ | | | | √ | | 3 | (27) |
| Wang, 2008 | √ | | √ | | | √ | √ | | 4 | (32) |
| Wang, 2009 | √ | | √ | | | √ | √ | | 4 | (33) |
| Wang, 2012 | √ | | √ | | | | √ | | 3 | (31) |
| Wang, 2013 | √ | | √ | √ | | √ | √ | | 5 | (29) |
| Wang, 2014 | √ | | √ | √ | √ | | √ | | 5 | (30) |
| Wang, 2009 | √ | | √ | √ | | √ | √ | √ | 6 | (28) |
| Yan, 2014 | √ | | √ | | | √ | √ | | 4 | (36) |
| Xu, 2008 | √ | | √ | √ | | √ | √ | √ | 6 | (35) |
| Xu, 2009 | √ | | √ | | | √ | √ | | 4 | (34) |
| Zhou, 2003 | √ | | √ | | | | √ | | 3 | (38) |
| Zhou, 2016 | √ | | √ | √ | | √ | √ | √ | 6 | (37) |
| Zhu, 2014 | √ | | √ | | | √ | √ | | 4 | (39) |
Effectiveness
The analysis of TH-positive cells in the nigra
included 204 animals in 13 studies, out of which 180 animals in 11
studies were included in the subgroup analysis of MPTP-induced mice
and the remaining 24 animals in two studies were included in the
subgroup analysis of 6-OHDA-induced rats. However, one study
(25) was excluded from the pooled
analysis because the data were presented in the form of percentages
(TH-positive cells/control %), and the means and standard
deviations generated from the raw data were inaccessible. The whole
data for analysis were pooled and a significant difference in the
G-Rg1 treatment group compared to the control group was determined
(n=204, SMD: 4.23, 95% CI: 3.22 to 5.24, P<0.00001; Fig. 2). The 6-OHDA-induced mice exhibited
a larger effect size than the MPTP-induced mice (n=24, SMD: 10.38,
95% CI: 6.71 to 14.05 vs. n=180, SMD: 3.76, 95% CI: 2.88 to 4.63,
P<0.00001; Fig. 2). Furthermore,
there was obvious heterogeneity between studies for the analysis of
TH-positive cells in the MPTP-induced group (Tau2=1.19,
Chi2=24.61, P=0.006, I2=59%; Fig. 2). The results on TH-positive cells
and heterogeneity were inconsistent after sequentially excluding
each of the studies. The outlier studies (27) were excluded to produce more
homogeneous results (Tau2=0.13, Chi2=10.29,
P=0.33, I2=13%) and a larger effect size (n=170, SMD:
3.89, 95% CI: 3.26 to 4.51, P<0.00001) in the subgroup analysis
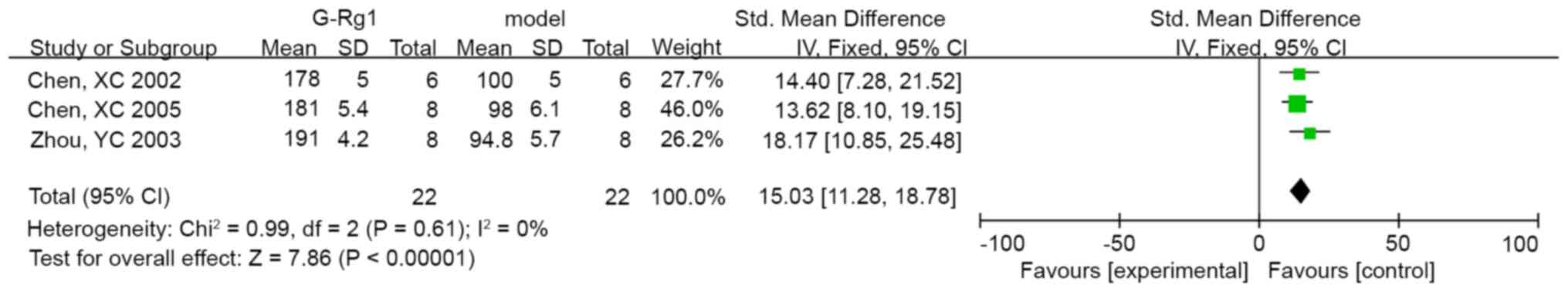
of MPTP-induced mice. In addition, 3 studies revealed significant
effects of G-Rg1 on Nissl-positive cells compared with the control
group (n=44, SMD: 15.03, 95% CI: 11.28 to 18.78, P<0.00001;
heterogeneity: Chi2=0.99, P=0.61, I2=0%;
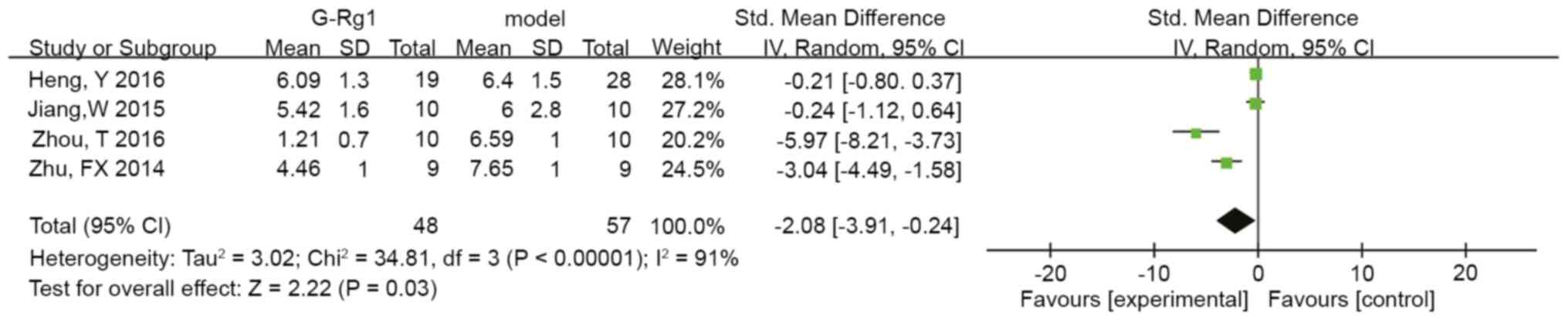
Fig. 3). Furthermore, four studies
reported that G-Rg1 decreased the pole test time compared to the
control group (n=105, SMD: -2.08, 95% CI: -3.91 to -0.24, P=0.03;
heterogeneity: Tau2=3.02, Chi2=34.81,
P<0.00001, I2=91%; Fig.
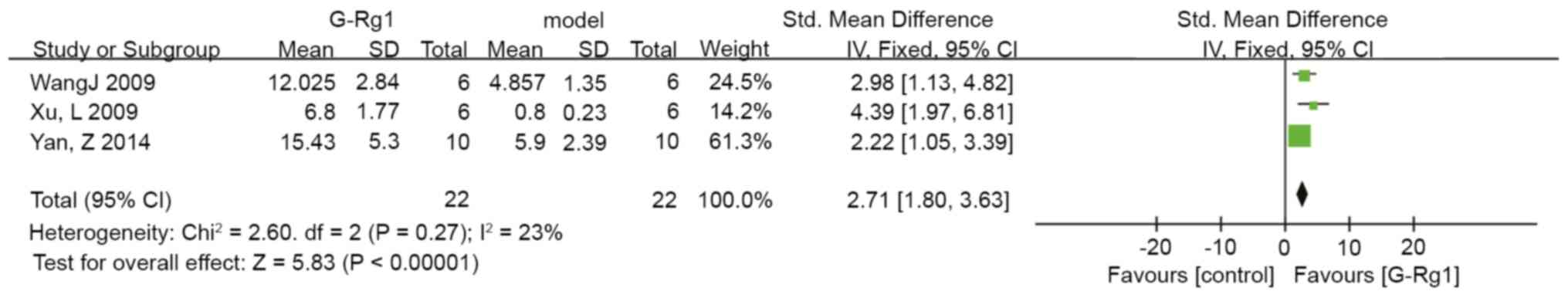
4), and the data were stable based on sensitivity analysis. In
three studies, the DA levels in the striatum were indicated to be
significantly improved in the G-Rg1 group compared with those in
the control group (n=44, SMD: 2.71, 95% CI: 1.80 to 3.63,
P<0.00001; heterogeneity: Chi2=2.60, P=0.27,
I2=23%; Fig. 5). The
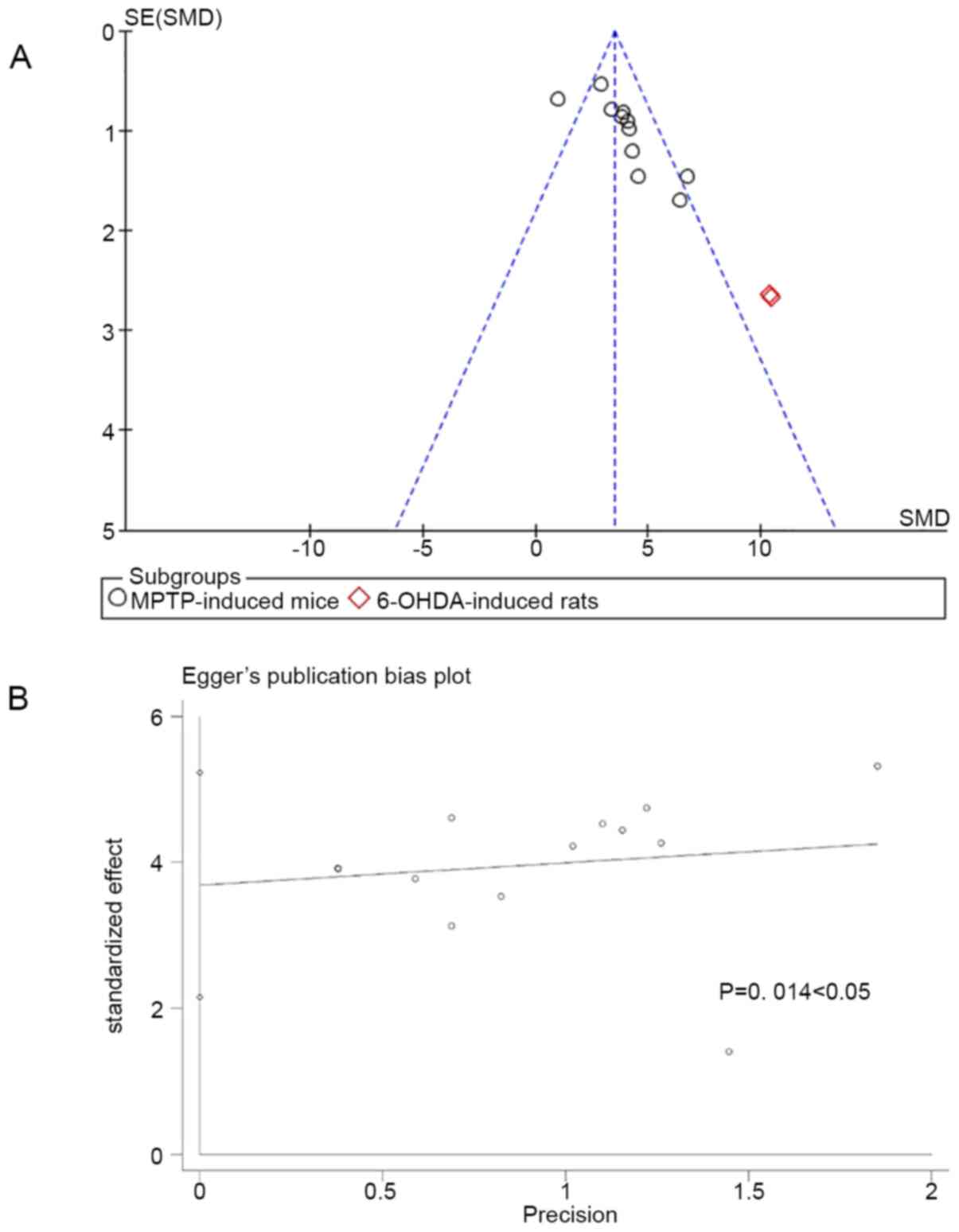
funnel plot indicated mild publication bias concerning the outcome
of TH-positive cells upon visual inspection (Fig. 6A). In addition, Egger's test
revealed moderate publication bias for the studies with TH-positive
cells as the outcome (P=0.014; Fig.
6B).
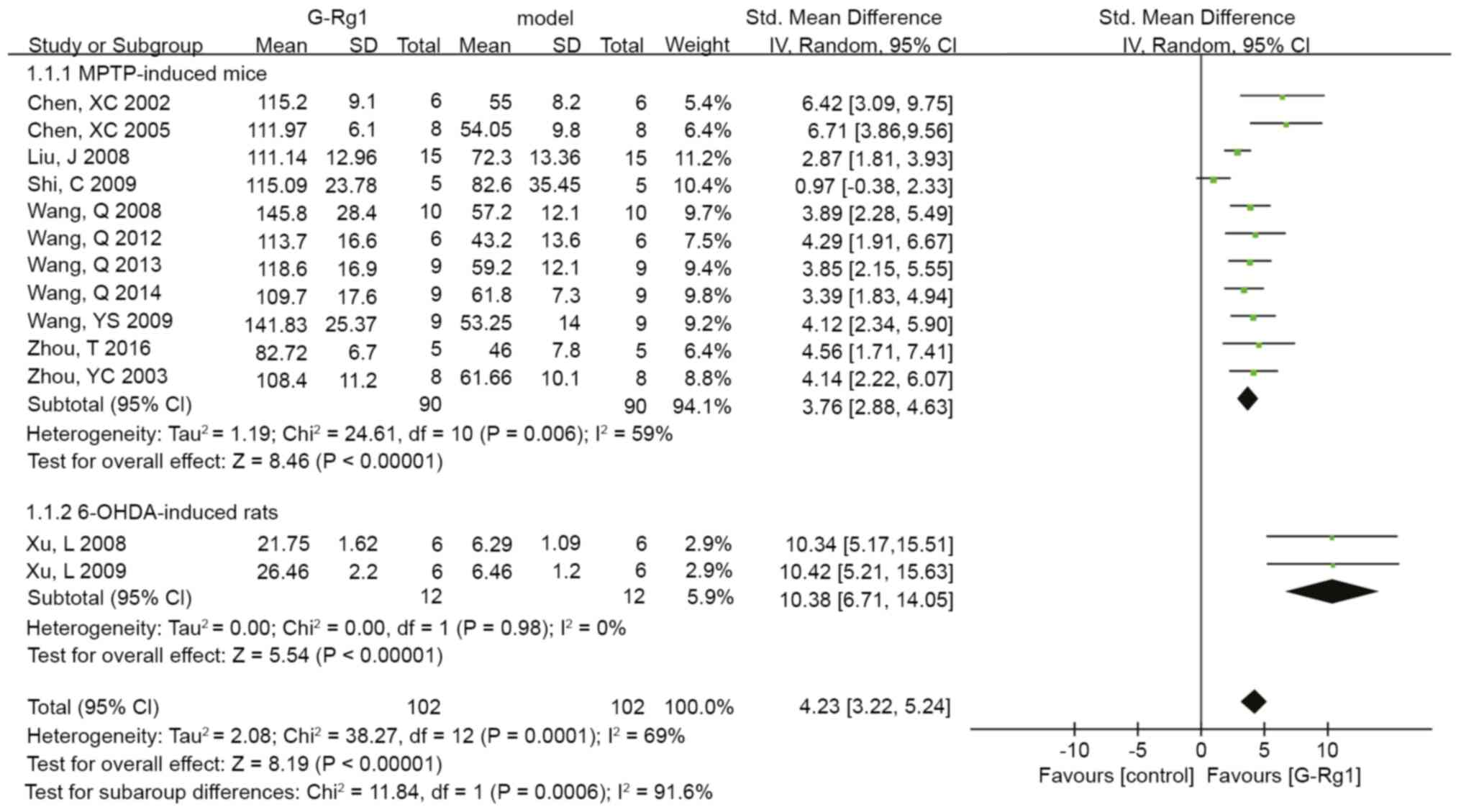 | Figure 2Pooled estimate of improvement in
tyrosine hydroxylase-positive cells in the nigra. CI, confidence
interval; df, degrees of freedom; SD, Std. deviation; Std.,
standard; IV, inverse variance; G-Rg1, ginsenoside Rg1; MPTP,
1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine; OHDA,
6-hydroxydopamine. |
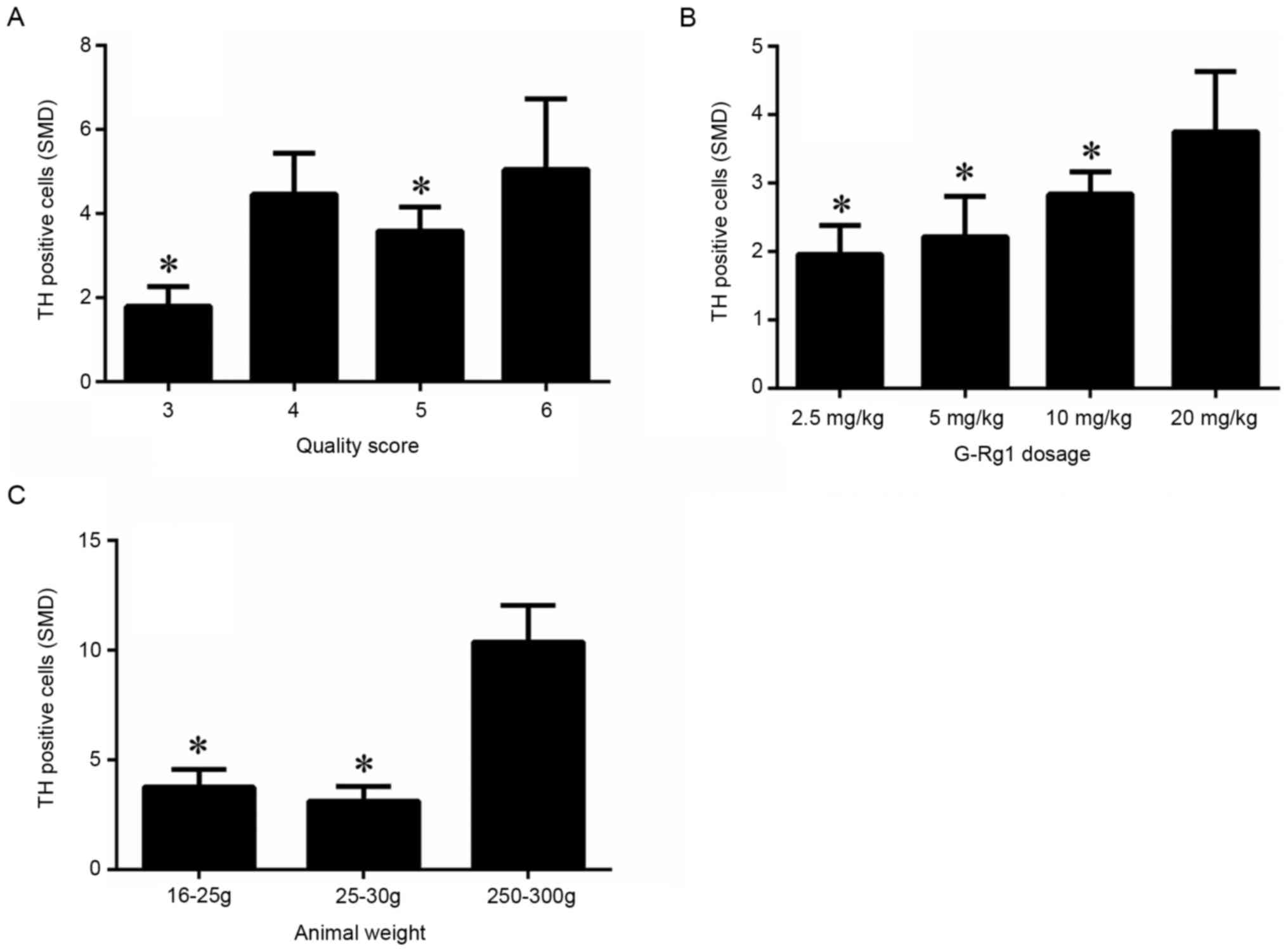
Pre-specified subgroup analysis
In the subgroup analysis for the outcome measure of
TH-positive cells, the effect size of G-Rg1 in low-quality studies
(quality score=3) was much smaller than that in higher-quality
studies (Fig. 7A) (P<0.05). The
G-Rg1 dose effect on TH-positive cells was then investigated. A
high dose of G-Rg1 (20 mg/kg; n=26, 3 studies) was more effective
at increasing dopaminergic neurons than a moderate dose (10 mg/kg;
n=180, 11 studies) (P<0.05), low dose (5 mg/kg; n=32, 4 studies)
or very low dose (2.5 mg/kg; n=6, 1 study; Fig. 7B (P<0.05). Of note, the effect
size was observed to be higher in younger mice (body weight, 16-25
g) than in older mice (body weight, 25-30 g) (P<0.05). Based on
effect size, rats (body weight, 220-250 g) were preferable to mice
(body weight, 16-30 g; Fig. 7C)
(P<0.05).
Discussion
The present study provided a systematic review and
meta-analysis to explore the effect of G-Rg1 in animal models of
PD. A total of 18 studies with the outcomes of TH-positive cells in
the nigra, total pole test time and DA contents in the striatum
indicated significant improvement in animal models of PD after
G-Rg1 treatment. The present meta-analysis revealed that
pretreatment with specific doses of G-Rg1 is able to minimize the
loss of dopaminergic neurons in both the nigra and the striatum and
improve motor function in animal models of PD.
Methodological quality was assessed based on an
eight-item modified scale from the STAIR list. The quality scores
of the 18 studies ranged from 3 to 8. High-quality papers (quality
scores ≥6) (14,25,28,35,37)
are more rigorous in their research design and they generally
included sample size calculation, allocation concealment, reporting
of animals excluded from analysis, potential conflicts of interest
and funding in their study. A total of three studies (27,31,38)
had poor quality (quality scores=3) and they were peer-reviewed
publications featuring randomization and compliance with animal
welfare regulations, but did not describe the sample size
calculation, allocation concealment, reporting of animals excluded
from analysis, blinded assessment of outcomes, reporting potential
conflicts of interest and study funding, which may decrease the
reliability of the results.
Several limitations of the present study should be
considered. First, seven papers were included in English-language
databases (PubMed, EMBASE and Web of Science) and the remaining 11
papers were published in the Chinese language, which may lead to
selection bias. Furthermore, the present analysis focused on animal
studies, as no published studies were reporting on clinical trials
of G-Rg1 treatment for PD and data from clinical studies are more
valuable than those from animal studies. In addition, the included
studies in the present meta-analysis did not report any negative
results on TH-positive cells in the nigra, DA levels in the
striatum or pole test time. There may have been an overestimation
of the results because only available data were included in the
analysis, and articles with negative results are more difficult to
publish. In addition, the treatment regimens of Rg1 in the included
studies varied widely (e.g. in terms of pre- and post-treatment to
MTPT or 6-OHDA, frequency and duration), which was also a
limitation of the present study. As another limitation, only the
association, rather than causation, was evaluated, since the
present meta-analysis was an observational research study rather
than being experimental. None of the studies reported the effect of
G-Rg1 in PD in other species, such as primates. Furthermore, there
is no standard way to produce and most importantly assess
dopaminergic lesions following toxin-induced lesion in rodents and
the complexity includes the dose, the method used to assess
denervation and the timing of the assessment after intoxication. In
the present study, the SMD rather than the normalized mean
difference (NMD) was used. However, SMD is more conservative than
the NMD (22). Finally, among the
studies included in the present meta-analysis, mild publication
bias was suggested by the funnel plot and Egger's test. Studies
with non-significant results may remain unpublished because the
authors do not submit their manuscripts to journals for publishing,
resulting in potential publication bias. Selective publishing and
reporting also contribute to this bias, which must be considered.
However, in the present study, publication bias was a possible
explanation because all of the 18 included studies had positive
rather than negative results.
Significant differences were observed between high-
and low-quality papers based on the outcome measures. Specifically,
for TH-positive cell outcomes, low-quality studies indicated the
lowest effect of G-Rg1, suggesting that a poor-quality research
design may have influenced the outcomes of certain previous studies
(41). High-quality studies may
have a lower variance, which increases the effect size. On the
other hand, improving the quality of studies may help reduce bias
when such trials are included in meta-analyses. However, when the
data from lower-quality trials are used in meta-analyses, the
treatment efficacy may be statistically exaggerated by 30-50%
(42). This may explain why the
effect size of moderate-quality studies (quality score=4) is
slightly higher than that of higher-quality studies (quality
score=5). Certain scholars consider allocation concealment or
randomization to be the major factors that inflate estimates of
treatment efficacy (43).
Consequently, high-quality, well-designed studies are required to
determine the efficacy of G-Rg1 in PD. Based on the effect size, a
high dose of G-Rg1 (20 mg/kg) was indicated to have the highest
efficacy in PD models. However, only three studies used this dosage
and 13 studies used a dose of 10 mg/kg to examine the outcome of
TH-positive cells in the nigra. Therefore, these results should be
interpreted with caution in this subgroup analysis. The effects of
different dosages of G-Rg1, including their toxic effects, should
be explored in future studies. In the present meta-analysis,
according to the effect size, the efficacy of G-Rg1 to improve
dopaminergic neurons was better in the 6-OHDA-induced rats than in
the MPTP-induced mice. However, these results should be interpreted
with caution as, in the present meta-analysis, 16 studies used the
MPTP-induced model, whereas only 2 studies used 6-OHDA treatment of
rats to induce the model of PD. By now, there is sufficient
literature illustrating the neuroprotective effects of G-Rg1 in
animal models (15). Therefore, in
future research, more evidence should be gathered regarding the
efficacy of G-Rg1 in 6-OHDA-induced rats. Only 3 studies included
in the present meta-analysis measured Nissl stain-positive cells,
which may directly indicate the survival of neurons. Loss of TH
expression is not necessarily related to cells dying (16,44),
and following MPTP and 6-OHDA treatment, there is a temporal
association of tyrosine nitration or cysteine oxidation with
inactivation of TH in vitro, suggesting that this covalent
post-translational modification is responsible for the in
vivo loss of TH neurons (45-47).
Nissl staining may make the conclusions more stable in experimental
models of PD and future research should pay close attention to
this. Furthermore, no published papers are exploring the joint
action of G-Rg1 with other neuroprotectants on PD, which should be
investigated in future clinical trials. To the best of our
knowledge, there are still no clinical studies reporting the
effects of G-Rg1 on PD. However, the above results suggest a
potential treatment effect of G-Rg1 on PD suitable for a clinical
study.
The studies included in the present meta-analysis
that used the MPTP-induced model did not strictly follow the
protocol of Jackson-Lewis and Przedborski from 2007(48). Besides, the studies reported on
whether G-Rg1 interfered with MPTP toxicokinetics and pre-treatment
or if co-administration with G-Rg1 invalidated the interpretation
of the data. It is also uncertain whether G-Rg1 prevented the
uptake of MPTP by blocking DAT (DA transporter), preventing the
conversion of MPTP to MPP and detoxifying MPTP. Therefore, the
method of pretreatment with G-Rg1 may not be scientific (48). Further studies on the
pharmacological relationship between G-Rg1 and MPTP are required.
Furthermore, all studies in the present meta-analysis counted the
cell numbers immediately after the last injection of MPTP. This may
have led to the reporting of higher numbers as cells take time to
die and the best option is to determine the cell numbers after 15
days of toxin application (16,44).
No obvious toxicity of G-Rg1 toward the animals was
observed in the 18 studies analyzed in the present study. However,
one study that included toxicity testing reported that the
intravenous median lethal dose of the combination of salvianolic
acid B (SalB) and G-Rg1 was 1,747 mg/kg. This dose was 100-fold
greater than the effective dose (15 mg/mg), suggesting that
SalB-Rg1 and G-Rg1 is a safe combination that may be considered for
future clinical development (49).
However, the safety of intravenous ginsenoside-Rg1 calls for
extensive basic research and large-scale clinical trials.
Ongoing research is investigating the proposed
mechanisms of G-Rg1, including the stimulation of antioxidants
(50), anti-inflammatory (51), anti-apoptotic (52) and immune activities (53), the potentiation of nerve growth
factor activity (54), maintenance
of cellular ATP levels (55),
inhibition of excitotoxicity (56),
Ca2+ over-influx into neurons (57) and the preservation of the structural
integrity of neurons (58). The
possible mechanisms underlying the effects of G-Rg1 should be
further investigated in future studies.
In conclusion, G-Rg1 was able to attenuate the
damage caused by toxicants in the nigra and the striatum, as
evidenced by increased numbers of TH-positive cells and DA levels
in the striatum and of Nissl-positive cells, and improved motor
function associated with a reduction in the total pole test time in
animal models of PD. G-Rg1 has positive effects in attenuating
damage in models of PD, suggesting that it is a possible candidate
neuroprotective drug for treating human PD. However, there is
potential publication bias in most of the reported studies and the
limited quality of the experiments decreases the reliability of
these results. Further studies should confirm if the
neuroprotectant G-Rg1 is a promising drug candidate for PD.
Acknowledgements
Not applicable.
Funding
Funding: The current study was supported by the National TCM
Foundation of China (grant no. JDZX2015111), the Zhejiang Science
Foundation of China (grant nos. LY14H080004 and LY15H040011) and
the Projects of the Zhejiang Committee of Chinese medicine, China
(grant no. 2013ZB045).
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
YBH, YS and ZDY designed the current study, searched
databases, extracted and assessed the literature and drafted the
manuscript. YBH, JHL and YMG statistically analyzed the data. YBH
and YMC confirm the authenticity of all the raw data. YMC and YLL
conceived and designed the present study, provided general
supervision and finalized the manuscript. All authors read and
approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Barnett R: Parkinson's disease. Lancet.
387(217)2016.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Toulouse A and Sullivan AM: Progress in
Parkinson's disease-where do we stand? Prog Neurobiol. 85:376–392.
2008.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Schapira AH: Molecular and clinical
pathways to neuroprotection of dopaminergic drugs in Parkinson
disease. Neurology. 72 (Suppl 7):S44–S50. 2009.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Beal MF: Bioenergetic approaches for
neuroprotection in Parkinson's disease. Ann Neurol. 53 (Suppl
3):S39–S48. 2003.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Olanow CW and Schapira AH: Therapeutic
prospects for Parkinson disease. Ann Neurol. 74:337–347.
2013.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Schapira AH: Treatment options in the
modern management of Parkinson disease. Arch Neurol. 64:1083–1088.
2007.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Ellis JM and Reddy P: Effects of Panax
ginseng on quality of life. Ann Pharmacother. 36:375–379.
2002.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Rausch WD, Liu S, Gille G and Radad K:
Neuroprotective effects of ginsenosides. Acta Neurobiol Exp (Wars).
66:369–375. 2006.PubMed/NCBI
|
|
9
|
Ong WY, Farooqui T, Koh HL, Farooqui AA
and Ling EA: Protective effects of ginseng on neurological
disorders. Front Aging Neurosci. 7(129)2015.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Chen XC, Fang F, Zhu YG, Chen LM, Zhou YC
and Chen Y: Protective effect of ginsenoside Rg1 on
MPP+-induced apoptosis in SHSY5Y cells. J Neural Transm
(Vienna). 110:835–845. 2003.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Radad K, Gille G, Moldzio R, Saito H,
Ishige K and Rausch WD: Ginsenosides Rb1 and Rg1 effects on
survival and neurite growth of MPP+-affected
mesencephalic dopaminergic cells. J Neural Transm (Vienna).
111:37–45. 2004.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Radad K, Gille G, Moldzio R, Saito H and
Rausch WD: Ginsenosides Rb1 and Rg1 effects on mesencephalic
dopaminergic cells stressed with glutamate. Brain Res. 1021:41–53.
2004.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Chen XC, Chen Y, Zhu YG, Fang F and Chen
LM: Protective effect of ginsenoside Rg1 against MPTP-induced
apoptosis in mouse substantia nigra neurons. Acta Pharmacol Sin.
23:829–834. 2002.PubMed/NCBI
|
|
14
|
Heng Y, Zhang QS, Mu Z, Hu JF, Yuan YH and
Chen NH: Ginsenoside Rg1 attenuates motor impairment and
neuroinflammation in the MPTP-probenecid-induced parkinsonism mouse
model by targeting α-synuclein abnormalities in the substantia
nigra. Toxicol Lett. 243:7–21. 2016.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Song L, Xu MB, Zhou XL, Zhang DP, Zhang SL
and Zheng GQ: A preclinical systematic review of ginsenoside-Rg1 in
experimental Parkinson's disease. Oxid Med Cell Longev.
2017(2163053)2017.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Stanic D, Finkelstein DI, Bourke DW, Drago
J and Horne MK: Timecourse of striatal re-innervation following
lesions of dopaminergic SNpc neurons of the rat. Eur J Neurosci.
18:1175–1188. 2003.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Moher D, Liberati A, Tetzlaff J and Altman
DG: PRISMA Group. Preferred reporting items for systematic reviews
and meta-analyses: The PRISMA statement. Int J Surg. 8:336–341.
2010.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Fifel K and Cooper HM: Loss of dopamine
disrupts circadian rhythms in a mouse model of Parkinson's disease.
Neurobiol Dis. 71:359–369. 2014.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Morin N, Jourdain VA and Di Paolo T:
Modeling dyskinesia in animal models of Parkinson disease. Exp
Neurol. 256:105–116. 2014.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Matsumoto M: Dopamine signals and
physiological origin of cognitive dysfunction in Parkinson's
disease. Mov Disord. 30:472–483. 2015.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Jagmag SA, Tripathi N, Shukla SD, Maiti S
and Khurana S: Evaluation of models of Parkinson's disease. Front
Neurosci. 9(503)2016.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Vesterinen HM, Sena ES, Egan KJ, Hirst TC,
Churolov L, Currie GL, Antonic A, Howells DW and Macleod MR:
Meta-analysis of data from animal studies: A practical guide. J
Neurosci Methods. 221:92–102. 2014.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Egger M, Davey Smith G, Schneider M and
Minder C: Bias in meta-analysis detected by a simple, graphical
test. BMJ. 315:629–634. 1997.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Chen XC, Zhou YC, Chen Y, Zhu YG, Fang F
and Chen LM: Ginsenoside Rg1 reduces MPTP-induced substantia nigra
neuron loss by suppressing oxidative stress. Acta Pharmacol Sin.
26:56–62. 2005.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Jiang W, Wang Z, Jiang Y, Lu M and Li X:
Ginsenoside Rg1 ameliorates motor function in an animal model of
Parkinson's disease. Pharmacology. 96:25–31. 2015.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Liu J, Li R, Liu LN, Zhang XW, Zhang YX
and Zhang ZF: Protective effect of ginsenoside Rg1 on jnk signaling
pathway mediatethe loss of nigral nurons in the mice model of
Parkinson. Mod Prev Med. 35:1973–1975. 2008.(In Chinese).
|
|
27
|
Shi C, Zhang YX and Zhang ZF: Effect of
phosphorylated-ERK1/2 on inducible nitric oxide synthase expression
in the substantia nigra of mice with MPTP-induced Parkinson
disease. Nan Fang Yi Ke Da Xue Xue Bao. 29:60–63. 2009.PubMed/NCBI(In Chinese).
|
|
28
|
Wang J, Xu HM, Yang HD, Du XX, Jiang H and
Xie JX: Rg1 reduces nigral iron levels of MPTP-treated C57BL6 mice
by regulating certain iron transport proteins. Neurochem Int.
54:43–48. 2009.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Wang Q, Zhang H and Liu M: Influence of
NF-κB on i-NOS expression in substantia nigra of mouse models of
Parkinson's disease induced by MPTP. J Hebei United Univ.
15:297–299. 2013.(In Chinese).
|
|
30
|
Wang Q, Zhang H, Liu M, Li QJ, Geng LX,
Sun MH, Tian QY and Zhang YX: Influence of ginsenoside Rg1 in
expressions of FADD and FLIP in substantia nigra of Parkinson's
disease model mice. J Jilin Univ Med Ed. 40:962–966. 2014.
|
|
31
|
Wang Q, Zhang H, Zhang ZF, Wei ZF, Wang
YS, Zhou HX, et al: Role of P38 MAPK in regulating expression of
NF-κB and COX-2 in substantia-nigra of MPTP Parkinson's disease
mice model. China J Mod Med. 22:15–20. 2012.(In Chinese).
|
|
32
|
Wang Q, Zhang YX and Zhang ZF: Influence
of NF-κB on COX-2 expression insubstantia nigra of mouse models of
Parkinson's disease induced by MPTP. J Fourth Mil Med Univ.
29:1757–1760. 2008.
|
|
33
|
Wang YS, Li H, Zhang YX, Wei SP, Zhang ZF
and Tian QY: Influence of ginsenoside Rg1 on p-c-jun and cox-2
epression in substantia nigra of the MPTP mouse model of subacute
Parkinson's disease. Chin J Neuroanat. 25:432–436. 2009.
|
|
34
|
Xu L, Chen WF and Wong MS: Ginsenoside Rg1
protects dopaminergic neurons in a rat model of Parkinson's disease
through the IGF-I receptor signalling pathway. Br J Pharmacol.
158:738–748. 2009.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Xu L, Liu LX, Chen LX, Xie JX and Huang
WX: The protective effect of ginsenoside Rg1 on dopaminergic
neurons of substantia in the ovariectomized rat model of
Parkinson's disease. Zhongguo Ying Yong Sheng Li Xue Za Zhi.
24:1–5. 2008.PubMed/NCBI(In Chinese).
|
|
36
|
Yan Z, Wu L, Xue D, Gao XQ and Chen WF:
Effects of gesenoside Rg1 and insulin-like growth factor on
dopaminergic neurons in Parkinson. Acta Acad Med Qingdao Univ.
50:283–288. 2014.
|
|
37
|
Zhou T, Zu G, Zhang X, Wang X, Li S, Gong
X, Liang Z and Zhao J: Neuroprotective effects of ginsenoside Rg1
through the Wnt/β-catenin signaling pathway in both in vivo and in
vitro models of Parkinson's disease. Neuropharmacology.
101:480–489. 2016.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Zhou YC, Chen XC, Zhu YG, Fang F and Chen
LM: Down-regulation of oxidative stress is the possible mechanism
of ginsenoside Rg1 protecting the substantia nigra neurons in PD
mice. Chin J Clin Pharmacol Ther. 8:2003.(In Chinese).
|
|
39
|
Zhu FX, Chang HM, Duan Y, Li PY and Wang
SX: Effect of ginsenoside Rg1 on the expressions of tyrosine
hydroxylase,ephrin B2 and phosphorylated c-Jun in substantia nigra
of mice with Parkinson's disease. J Xinxiang Med Univ. 31:781–785.
2014.
|
|
40
|
Fleming SM, Salcedo J, Fernagut PO,
Rockenstein E, Masliah E, Levine MS and Chesselet MF: Early and
progressive sensorimotor anomalies in mice overexpressing wild-type
human alpha-synuclein. J Neurosci. 24:9434–9440. 2004.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Macleod MR, O'Collins T, Howells DW and
Donnan GA: Pooling of animal experimental data reveals influence of
study design and publication bias. Stroke. 35:1203–1208.
2004.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Moher D, Pham B, Jones A, Cook DJ, Jadad
AR, Moher M, Tugwell P and Klassen TP: Does quality of reports of
randomised trials affect estimates of intervention efficacy
reported in meta-analyses? Lancet. 352:609–613. 1998.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Bebarta V, Luyten D and Heard K: Emergency
medicine animal research: Does use of randomization and blinding
affect the results? Acad Emerg Med. 10:684–687. 2003.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Bowenkamp KE, David D, Lapchak PL, Henry
MA, Granholm AC, Hoffer BJ and Mahalik TJ: 6-hydroxydopamine
induces the loss of the dopaminergic phenotype in substantia nigra
neurons of the rat. A possible mechanism for restoration of the
nigrostriatal circuit mediated by glial cell line-derived
neurotrophic factor. Exp Brain Res. 111:1–7. 1996.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Ara J, Przedborski S, Naini AB,
Jackson-Lewis V, Trifiletti RR, Horwitz J and Ischiropoulos H:
Inactivation of tyrosine hydroxylase by nitration following
exposure to peroxynitrite and
1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP). Proc Natl Acad
Sci USA. 95:7659–7663. 1998.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Kuhn DM, Aretha CW and Geddes TJ:
Peroxynitrite inactivation of tyrosine hydroxylase: Mediation by
sulfhydryl oxidation, not tyrosine nitration. J Neurosci.
19:10289–10294. 1999.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Blanchard-Fillion B, Souza JM, Friel T,
Jiang GC, Vrana K, Sharov V, Barrón L, Schöneich C, Quijano C,
Alvarez B, et al: Nitration and inactivation of tyrosine
hydroxylase by peroxynitrite. J Biol Chem. 276:46017–46023.
2001.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Jackson-Lewis V and Przedborski S:
Protocol for the MPTP mouse model of Parkinson's disease. Nat
Protoc. 2:141–151. 2007.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Zhao Q, Yang M, Deng Y, Yu H, Wang L, Teng
F, Cho K, Ma H, Wu P, Li X, et al: The safety evaluation of
salvianolic acid B and ginsenoside Rg1 combination on mice. Int J
Mol Sci. 16:29345–29356. 2015.PubMed/NCBI View Article : Google Scholar
|
|
50
|
Zhang ZL, Fan Y and Liu ML: Ginsenoside
Rg1 inhibits autophagy in H9c2 cardiomyocytes exposed to
hypoxia/reoxygenation. Mol Cell Biochem. 365:243–250.
2012.PubMed/NCBI View Article : Google Scholar
|
|
51
|
Lu D, Zhu LH, Shu XM, Zhang CJ, Zhao JY,
Qi RB, Wang HD and Lu DX: Ginsenoside Rg1 relieves tert-Butyl
hydroperoxide-induced cell impairment in mouse microglial BV2
cells. J Asian Nat Prod Res. 17:930–945. 2015.PubMed/NCBI View Article : Google Scholar
|
|
52
|
Li SS, Ye JM, Deng ZY, Yu LX, Gu XX and
Liu QF: Ginsenoside-Rg1 inhibits endoplasmic reticulum
stress-induced apoptosis after unilateral ureteral obstruction in
rats. Ren Fail. 37:890–895. 2015.PubMed/NCBI View Article : Google Scholar
|
|
53
|
Liu Y, Yi L, Wang L, Chen L, Chen X and
Wang Y: Ginsenoside Rg1 protects human umbilical cord blood-derived
stromal cells against tert-Butyl hydroperoxide-induced apoptosis
through Akt-FoxO3a-Bim signaling pathway. Mol Cell Biochem.
421:75–87. 2016.PubMed/NCBI View Article : Google Scholar
|
|
54
|
Huo DS, Zhang M, Cai ZP, Dong CX, Wang H
and Yang ZJ: The role of nerve growth factor in ginsenoside
Rg1-induced regeneration of injured rat sciatic nerve. J Toxicol
Environ Health A. 78:1328–1337. 2015.PubMed/NCBI View Article : Google Scholar
|
|
55
|
Miao HH, Zhen Y, Ding GN, Hong FX, Xie ZC
and Tian M: Ginsenoside Rg1 attenuates isoflurane-induced caspase-3
activation via inhibiting mitochondrial dysfunction. Biomed Environ
Sci. 28:116–126. 2015.PubMed/NCBI View Article : Google Scholar
|
|
56
|
Liao B, Newmark H and Zhou R:
Neuroprotective effects of ginseng total saponin and ginsenosides
Rb1 and Rg1 on spinal cord neurons in vitro. Exp Neurol.
173:224–234. 2002.PubMed/NCBI View Article : Google Scholar
|
|
57
|
Zhang YF, Fan XJ, Li X, Peng LL, Wang GH,
Ke KF and Jiang ZL: Ginsenoside Rg1 protects neurons from
hypoxic-ischemic injury possibly by inhibiting Ca2+
influx through NMDA receptors and L-type voltage-dependent
Ca2+ channels. Eur J Pharmacol. 586:90–99.
2008.PubMed/NCBI View Article : Google Scholar
|
|
58
|
Liu Z, Qi Y, Cheng Z, Zhu X, Fan C and Yu
SY: The effects of ginsenoside Rg1 on chronic stress induced
depression-like behaviors, BDNF expression and the phosphorylation
of PKA and CREB in rats. Neuroscience. 322:358–369. 2016.PubMed/NCBI View Article : Google Scholar
|