Introduction
Depression is a prevalent mental condition that is
characterized by depressed mood, anhedonia, fatigue or loss of
energy, weight loss, psychomotor retardation or agitation (1,2). It
affects 15-18% of the population and is generally more prevalent in
women (3-5).
Depression is a leading cause of reduction in the quality of life.
Furthermore, the World Health Organization projected that
depression would rank as the leading contributor to the global
burden of disease by 2030 (1,2).
Emerging evidence has revealed that folate
deficiency is a risk factor for depression (6,7).
Folate refers to a group of water-soluble compounds that are also
known as vitamin B9(8). It is
considered to be associated with the function and development of
the central nervous system (8).
Previous studies have suggested that individuals with depression
exhibited lower serum folate levels compared with those in
individuals without depression (7,9,10).
Additionally, a sex-stratified analysis of the National Health and
Nutrition Examination Survey data revealed that serum folate levels
are negatively associated with depression in women but not in men
(11). However, to the best of our
knowledge, no studies have investigated the effects of folate
deficiency on depression-like behavior in the two sexes. Therefore,
it is necessary to determine the relationship between chronic
folate deficiency and depression, in addition to establishing if
there are sex-associated differences related to this condition.
The mechanism by which folate deficiency affects
female-associated depression has yet to be fully determined.
However, a previous study reported that folate deficiency can lead
to reduced estradiol (E2) levels (12). It has also been documented that
women are more susceptible to depression when estrogen
concentrations are low (13,14).
Previous studies have demonstrated that plasma E2 levels tended to
be significantly lower among women with depression, suggesting that
E2 supplementation can be beneficial for the treatment of
depression (15-17).
Another study suggested that E2 serves a role in depression
(13), where the effects mediated
by E2 on depressive behavior may involve estrogen receptor β (ERβ)
signaling. Apart from estrogen receptor α (ERα), which serves an
important role in neuroendocrine reproductive function (18), ERβ is more likely to affect mood
(18). ERβ is a nuclear receptor
that is localized to the cell membrane and is widely distributed in
the cerebral cortex whilst also being present in both neurons and
glia (18-20).
E2 can cross the blood-brain barrier and exert its actions through
ERβ to regulate the PI3K/AKT signaling pathway (18). PI3K/AKT signaling is ubiquitous in
cells and regulates the proliferation and differentiation of cells
(21-23).
Its downregulation or inhibition can increase cleaved caspase-3
expression, which promotes neuronal apoptosis (24,25).
In individuals with depression, it has been shown that neuronal
cell density is reduced in the cerebral cortex (26).
By applying animal models, the present study aimed
to investigate whether folate deficiency can result in differences
in parameters associated with depression between males and females.
In addition, another aim of the present study was to elucidate the
potential underlying mechanism by which this occurs. It was
hypothesized that a chronic folate-deficient diet may reduce E2
levels in mice, leading to neuronal apoptosis in the cerebral
cortex to induce depression-like behavior, by inhibiting the
expression of ERβ and suppressing the PI3K/AKT signaling
pathway.
Materials and methods
Folic acid diet groups
Folic acid is a derivative of folates due to the
high chemical lability of naturally occurring folates, which is
used in supplements and for food fortification (8,27).
Based on the standard AIN-93G diet (28), two folic acid diet (cat. nos.
LAD-3001G-F2 and LAD-3001G-F0; Trophic Animal Feed High-Tech Co.,
Ltd.) groups were designed: i) The control diet (consisting of 2
mg/kg folic acid); and ii) the chronic folate-deficient (CFD) diet,
consisting of 0 mg/kg folic acid. It is generally accepted that 2
mg/kg folic acid is the basic requirement for rodents (28). In addition to the difference in
folic acid content between the two diets, the CFD diet contained
succinyl sulfathiazole (1%), which inhibits folic acid production
by gut bacteria (29), ensuring
folic acid deficiency in mice in the CFD group.
Animals and treatments
A total of 40 male (weight, 31-33 g) and 40 female
(weight, 30-32 g) CD-1 mice were purchased from Beijing Vital River
Laboratory Animal Technology Co., Ltd. at 8 weeks of age. All
procedures performed on animals were in accordance with the
guidelines for humane treatment set by the Association of
Laboratory Animal Sciences and the Center for Laboratory Animal
Science at Anhui Medical University (GB/T 35892-2018) (30). The present study was approved by
the Ethics Committee of Anhui Medical University (approval no.
LLSC20150350; Hefei, China). A total of four mice were housed per
cage under a 12-h dark/light cycle in a controlled environment
(temperature, 20-24˚C; relative humidity, 50-55%) and left to
acclimatize for 2 weeks. Standard control diet and water were
available ad libitum during this time. Bahous et al
(31) previously indicated that a
folate-deficient diet administered to mice for ~30 weeks reduced
the duration spent in the center areas of an open field test due to
long-term folate deficiency (31).
Therefore, mice in the present study were administered the control
or folate-deficient diet from 10 to 38 weeks of age (totaling 28
weeks).
Mice at 10 weeks of age were randomly divided into
four groups (n=20 per group) as follows: i) Female control group
(F-Ctrl); ii) female chronic folate deficiency group (F-CFD); iii)
male control group (M-Ctrl); and iv) male chronic folate deficiency
group (M-CFD). F-Ctrl and M-Ctrl mice were fed with a controlled
diet, whilst F-CFD and M-CFD mice received a folate-deficient diet.
The body weight of each mouse was recorded every 7 days. Behavioral
assessments were performed on mice during their 38th week. All mice
were subsequently sacrificed by dislocation of cervical vertebra on
the second day following the behavioral experiments. After
sacrifice, serum samples of all mice were collected, centrifuged at
12,000 x g for 15 min at 4˚C and stored at -80˚C. Serum folate,
homocysteine, E2 and testosterone levels were then measured. In the
present study, considering that stimulation may cause changes in
the mouse brain, such as decreased protein expression in the medial
prefrontal cortex (32), cerebral
cortex samples of mice that were not subjected to behavioral assays
were extracted following sacrifice and were stored at -80˚C for
subsequent experimentation.
Behavioral assays
Mice that underwent the aforementioned diet regimens
were used for behavioral testing from during the 38th week of age
(n=10 per group). The experiments were performed in the following
sequence: i) Open field test (OFT); ii) sucrose preference test
(SPT); and iii) forced swim test (FST). All behavioral tests were
performed during the light cycle of housing in a dedicated
sound-proof behavioral facility located in Laboratory Animal Center
of Anhui Medical University. All mice were brought to the testing
room and underwent 30 min of acclimatization before the start of
each behavioral test and remained in the same room once the test
was initiated. Behaviors in OFT and FST were evaluated using SMART
software (SMART v3.0.02; Panlab, Inc.). All animals were sacrificed
on the second day following FST, to obtain the aforementioned
samples.
OFT
The OFT is used to measure the activity of mice in
an enclosed open area. Mice that travelled the least distance,
spend the shortest amount of time in the center and enter into the
center area the fewest times are considered to be more depressed
(33). In the present study, mice
were gently placed into the same corner of the open field, which
consisted of a wooden box (46x46x40 cm) with its floor marked into
25 squares (Fig. S1). Of these,
nine squares were defined as the center whereas the outer 16
squares along the walls were defined as the periphery. A 5-min
video was then recorded to observe the locomotor activity of mice.
The following parameters were assessed during the test: i) Total
distance; ii) number of squares crossed; and iii) time spent in
each square. Between tests, 75% alcohol was used to disinfect the
chamber.
SPT
The SPT was used to evaluate anhedonia in mice, a
core symptom of depression (34).
Decreased sucrose preference is considered to indicate
depression-like behavior (34).
Mice were housed separately and were habituated to two bottles
containing either tap water or 1% sucrose solution for 2 days. The
location of the two bottles was changed every 12 h to prevent the
possible effects of side preference on drinking behavior. On the
day 3, mice were deprived of water and food for 24 h. The next day,
each mouse was given a free choice between two bottles, one with
tap water and the other with 1% sucrose solution. The left/right
position of the bottles was alternated after 12 h. Sucrose
preference (%) was calculated using the following formula: Sucrose
preference rate (%)=Sucrose intake (g)/[Water intake (g) + sucrose
intake (g)] x100.
FST
The FST is used to assess despair in animals. Longer
immobility times indicate that a mouse is exhibiting depression
(35). The Mice were placed into a
vertical transparent cylinder containing temperature-controlled
water (24±1˚C). The cylinder was 20 cm deep, with a height of 30 cm
and a diameter of 12 cm, to ensure that mice could neither escape
nor touch the bottom of the container. The mice were forced to swim
for a total of 6 min, during which behavior was monitored over the
last 4 min. Immobility time was recorded and analyzed using the
aforementioned software, SMART.
Serum detection
Serum samples of all mice were collected through
centrifugation at 12,000 x g for 15 min at 4˚C and were stored at
-80˚C once behavioral experiments were completed at the end of 38
weeks of age. Serum concentrations of folate were measured using
chemiluminescence (cat. no. A98032; Beckman Coulter, Inc.)
according to the manufacturer's instructions (36). Serum concentrations of total
homocysteine (HCY) were measured using an enzymatic cycling assay
[cat. no. 1.02.4802; Shanghai Fosun Pharmaceutical (Group) Co.,
Ltd.] (37). Serum E2 (Elecsys
Estradiol III; cat. no. 6656021190; Roche Diagnostics GmbH) and
testosterone (Elecsys Testosterone II; cat. no. 5200067190; Roche
Diagnostics GmbH) were measured using electrochemiluminescence
immunoassays according to the manufacturer's instructions (38,39).
Western blot analysis
At the end of the behavioral experiments, the
cerebral cortex of mice that were not subjected to behavior assays
was collected and homogenized using 0.4 ml lysis buffer containing
50 mM Tris-HCl, 150 mM NaCl, 1 mM EDTA, 1% Triton X-100, 1% sodium
deoxycholate and 0.1% SDS, supplemented with 1% protease
inhibitors, 1% PMSF and 2% phosphatase inhibitor. After
centrifugation at 15,000 x g for 15 min at 4˚C, protein
concentrations were measured using BCA assay. The samples were then
boiled for 10 min at 100˚C, after which equal amounts of protein
(30 µg) were separated by SDS-PAGE (12.5 or 15%) and transferred
onto PVDF membranes. After blocking in blocking buffer (5% skimmed
milk powder) at room temperature for 1.5 h, blots were incubated
overnight with antibodies against ERβ (1:1,000; cat. no. 8974;
Santa Cruz Biotechnology, Inc.), phosphorylated (p)-PI3K (1:1,000;
cat. no. 4228T; Cell Signaling Technology, Inc.), PI3K (1:1,000;
cat. no. 4257S; Cell Signaling Technology, Inc.), p-AKT (1:1,000;
cat. no. 4060S; Cell Signaling Technology, Inc.), AKT (1:1,000;
cat. no. 4691S; Cell Signaling Technology, Inc.), caspase-3 (1:500;
cat. no. 14220T; Cell Signaling Technology, Inc.) and GAPDH
(1:1,000; cat. no. 365062; Sant Cruz, Inc.) at 4˚C. After washing
three times, the membrane was probed with HRP-conjugated goat
anti-rabbit IgG antibodies (1:10,000; cat. no. sc-2005; Santa Cruz
Biotechnology, Inc.) for 1 h at room temperature. The blots were
subsequently detected using an ECL detection kit (Pierce; Thermo
Fisher Scientific, Inc.) and quantified using a ChemiDoc Imaging
system (version, 2.3.0.07; cat. no. 12003154; BioRad, Inc.).
Statistical analysis
SPSS 24.0 (IBM Corp.) and GraphPad Prism 9 (GraphPad
Software, Inc.) software were used to analyze data, which were
presented as the mean ± SD. Body weight was analyzed using mixed
ANOVA followed by Tukey's test to study the effects of different
diets on the weight of mice over time. The remaining data were
analyzed using an unpaired two-tailed Student's t-test. P<0.05
was considered to indicate a statistically significant
difference.
Results
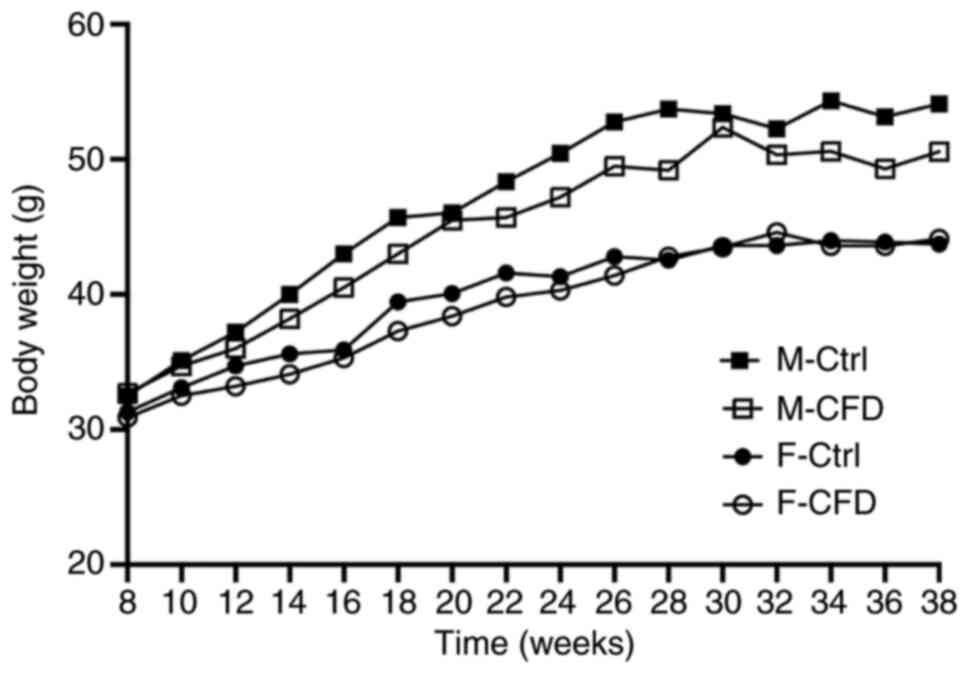
CFD diet does not change murine body
weight but increases homocysteine concentrations
Body weight was recorded every week. Fig. S2 is the result of statistical
analysis, which represents the influence of age factors
(intra-group factors) on the body weight of mice, and its weight
value is the same as Fig. 1. Mixed
ANOVA analysis revealed that the body weights of mice in the F-Ctrl
(P<0.01), F-CFD (P<0.01), M-Ctrl (P<0.01) and M-CFD
(P<0.01) groups increased with age (Fig. S2). However, the factor of diet did
not induce a statistically significant difference in the weight of
female (P=0.70) or male mice (P=0.16; Fig. 1). In conclusion, there was no
statistical difference in body weight in both female and male mice
on a folate-deficient diet.
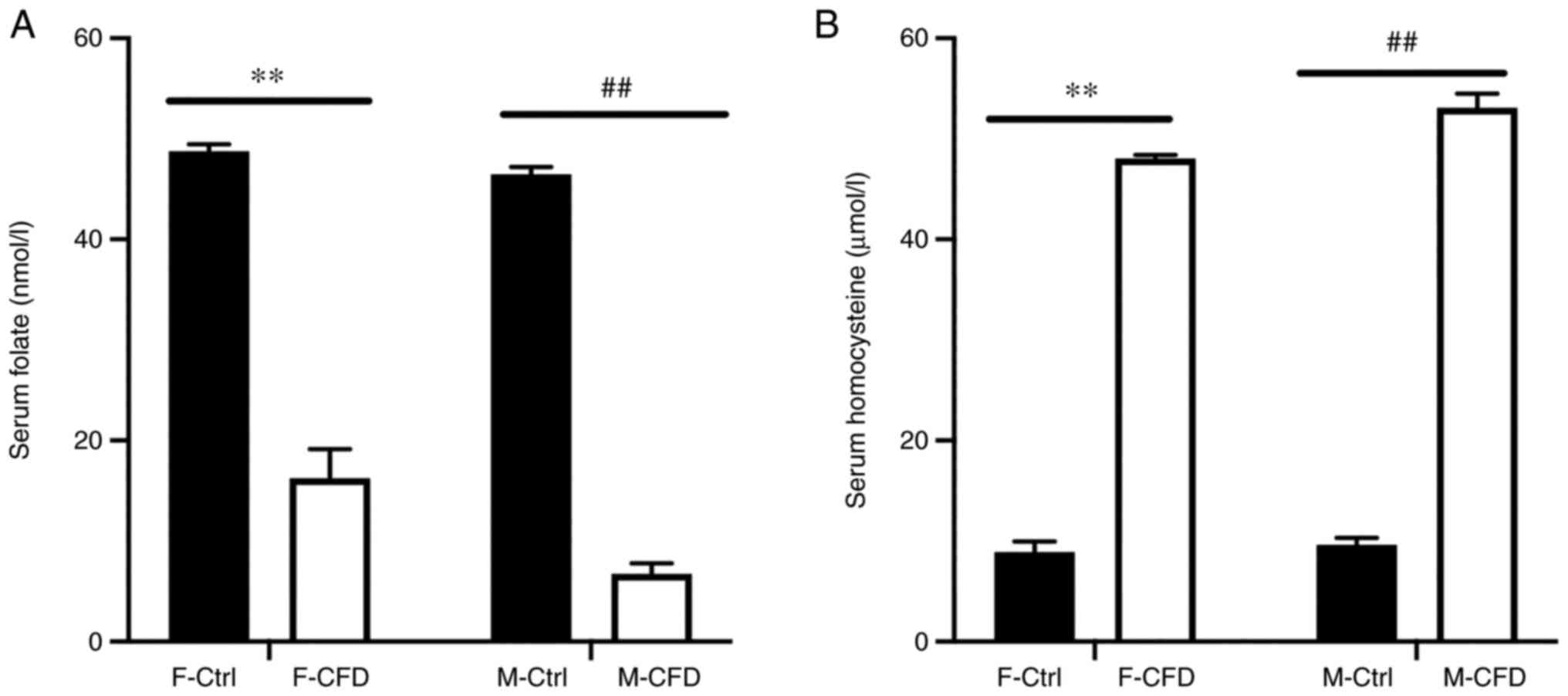
Serum folate and homocysteine were measured to
investigate the effect of chronic folate deficiency. Folate is a
water-soluble vitamin that serves a fundamental role as a methyl
donor in the re-methylation of homocysteine to produce methionine
(40). During folate deficiency,
increased levels of homocysteine are observed in humans (40). Therefore, serum homocysteine can be
used as a useful functional indicator of the folate status
(40). In the present study, after
animals received a folate-deficient diet, both F-CFD (P<0.01)
and M-CFD (P<0.01) mice exhibited significantly lower serum
folate concentrations compared with those in their respective
controls (Fig. 2A). Accordingly,
serum homocysteine levels were significantly higher in mice in the
F-CFD (P<0.01) and M-CFD (P<0.01) groups compared with those
in their respective controls (Fig.
2B). In summary, a folate-deficient diet caused reduced
concentrations of serum folate and increased concentrations of
homocysteine in F-CFD and M-CFD mice.
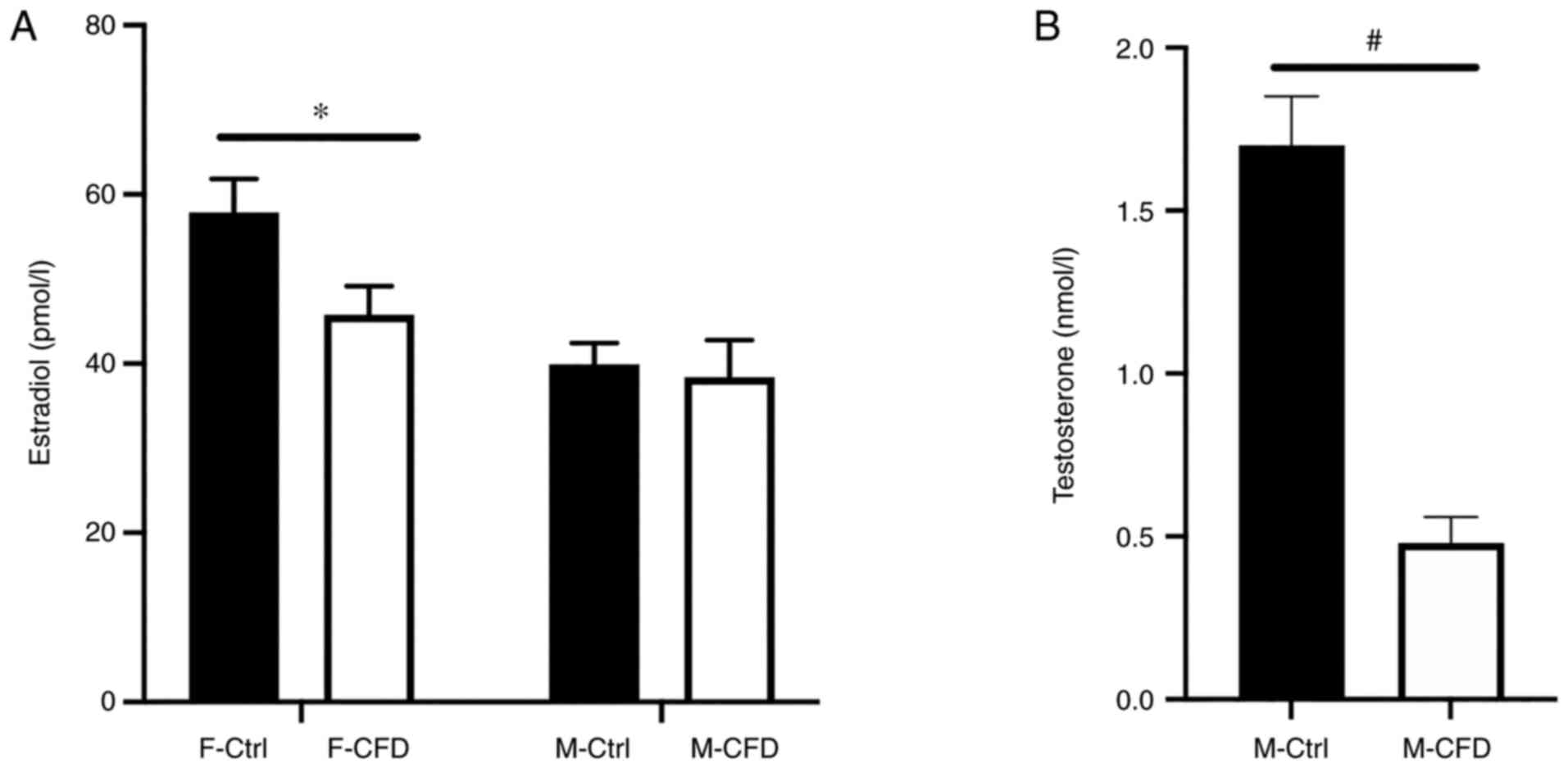
CFD diet reduces E2 concentrations in
female mice
Serum E2 levels were measured in the mice of the
present study. Following administration of the CFD diet, E2 levels
were significantly reduced in female mice (P<0.05; Fig. 3A). However, the CFD diet did not
affect the E2 levels (P=0.760) of male mice, even though
testosterone concentrations were significantly lower in the M-CFD
group compared with those in the M-Ctrl group (P<0.05; Fig. 3A and B). In conclusion, folate-deficient diet
caused lower E2 levels only in F-CFD.
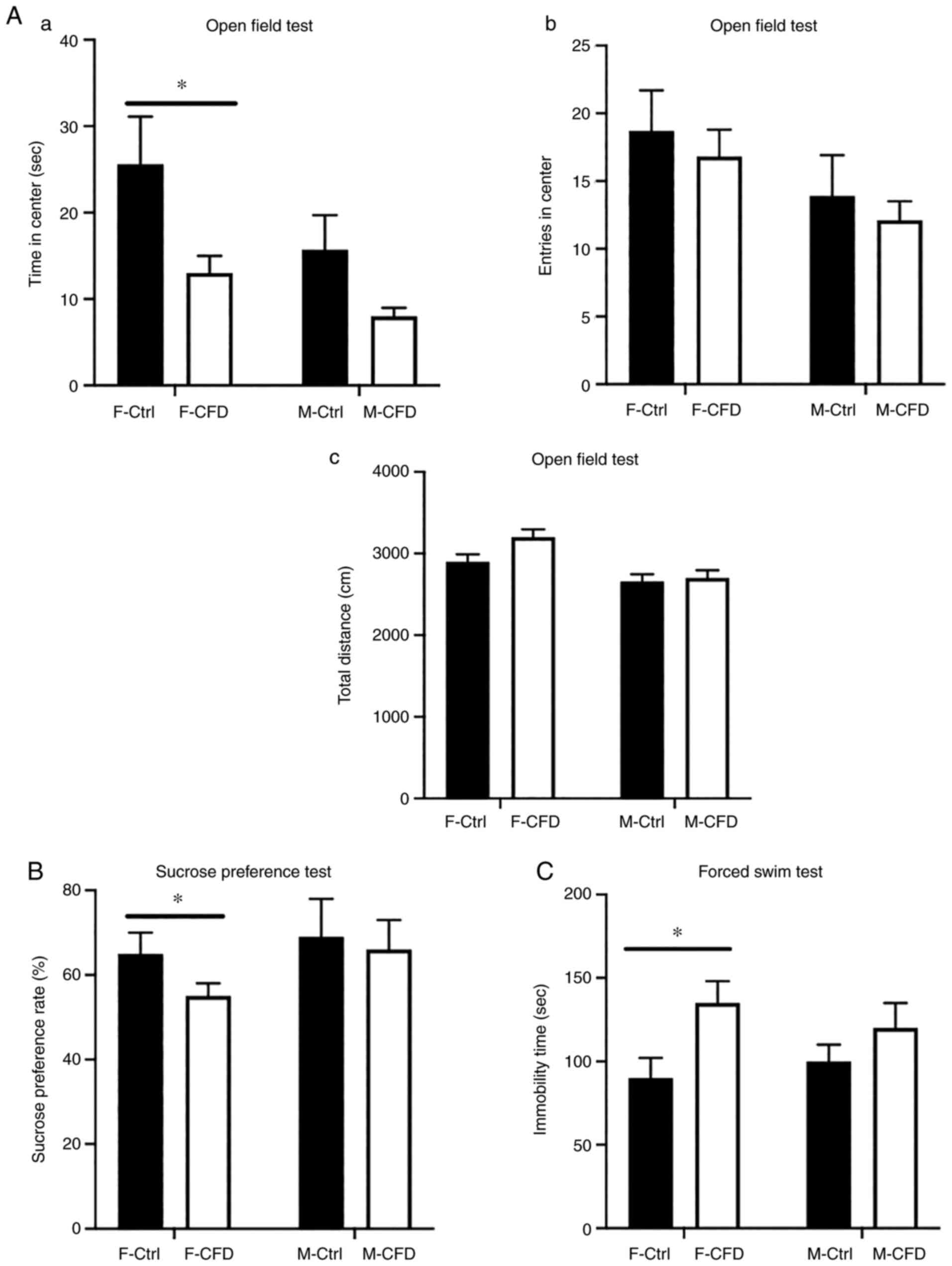
CFD diet leads to depression-like
behaviors in female mice
To assess whether folate deficiency could lead to
depression-like behavior, OFT, SPT and FST were performed to
evaluate the extent of depression-associated behaviors in mice
following the administration of the two different diets. OFT
results revealed that F-CFD mice spent significantly less time in
the central areas compared with time spent by F-Ctrl mice (Fig. 4Aa), suggesting that the CFD diet
caused a decrease in exploratory behavior. No statistically
significant differences in the total distance traveled or entries
into the center could be observed in either F-CFD or M-CFD mice
when compared with their respective controls (Fig. 4Ab and Ac). In addition, the CFD diet resulted in
significantly reduced sucrose preference to <60% in female mice,
an effect not observed in males (Fig.
4B). FST revealed that administration of the CFD diet
significantly increased the immobility time of female mice but not
in their male counterparts (Fig.
4C). In conclusion, folate-deficient diet caused
depression-like behaviors only in F-CFD.
CFD diet elevates cleaved caspase-3
protein expression by inhibiting the PI3K/AKT signaling pathway in
female mice
To investigate whether the PI3K/AKT signaling
pathway was associated with cell death and the effect of E2/ERβ
deficiency in mice, the protein levels of ERβ, p-PI3K, PI3K, p-AKT,
AKT and caspase-3 were assessed. E2 exerts its actions via ERβ to
further regulate the PI3K/AKT signaling pathway (18). The PI3K/AKT signaling pathway
serves a crucial role in the regulation of cell survival,
differentiation and apoptosis (21-23).
Furthermore, caspase-3 is a key enzyme that is involved in the
execution of apoptosis (24).
Western blotting results revealed that the protein expression level
of ERβ and the p-PI3K/PI3K expression ratio were significantly
decreased in the cerebral cortex of F-CFD mice compared with those
in the F-Ctrl (Fig. 5A and
B). AKT is a downstream signaling
molecule of PI3K (41,42). However, the reduced expression
ratio of p-PI3K/PI3K did not appear to affect AKT expression but
inhibited its phosphorylation in F-CFD mice compared with that in
the F-Ctrl group (Fig. 5C).
Furthermore, cleaved caspase-3 (19 kDa) expression was
significantly increased in F-CFD mice compared with that in the
F-Ctrl group, despite total caspase-3 levels exhibiting no
significant difference between F-CFD and F-Ctrl mice (Fig. 5D). No significant difference was
observed in the protein expression levels of ERβ, p-PI3K/PI3K and
p-AKT/AKT ratios, cleaved caspase-3 or caspase-3 expression between
the M-CFD and M-Ctrl groups (Fig.
5A-D). In conclusion, a folate-deficient diet reduced the
expression levels of ERβ and the PI3K/AKT pathway, and increased
the expression levels of cleaved caspase-3 in the cerebral cortex
only in F-CFD.
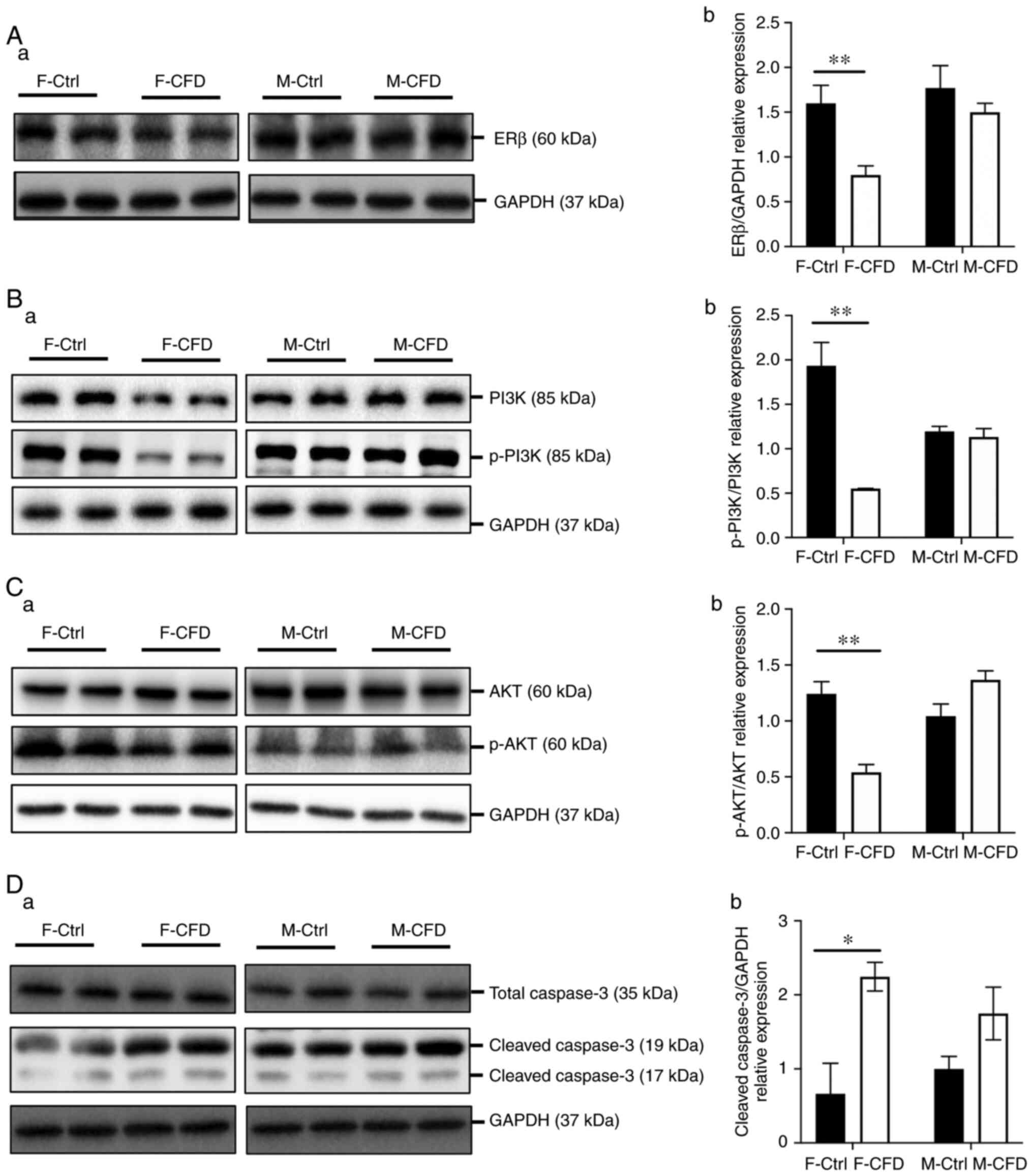 | Figure 5CFD diet inhibits PI3K/AKT signaling
and upregulates cleaved caspase-3 levels in female mice. Western
blot measurements of (Aa) ERβ, (Ba) PI3K and p-PI3K, (Ca) AKT and
p-AKT, (Da) caspase-3 and cleaved caspase-3 in brain samples.
Semi-quantification of (Ab) ERβ, (Bb) p-PI3K/PI3K, (Cb) p-AKT/AKT
and (Db) cleaved caspase-3 protein expression in brain samples
(n=20 per group). *P<0.05 and **P<0.01.
Data are presented as the mean ± SD. CFD, chronic folate-deficient;
F-Ctrl, female mice with standard control diet; F-CFD, female mice
with folate deficiency diet; M-Ctrl, male mice with standard
control diet; M-CFD, male mice with folate deficiency diet; p-,
phosphorylated; ERβ, estrogen receptor β. |
Discussion
The results of the present study revealed that CFD
led to depression-like behavior in female mice but not in male
mice, suggesting that CFD had a sex-dependent effect. OFT, SPT and
FST were performed to explore whether CFD was involved in the
depression-like behavior of mice. The results revealed that only
F-CFD mice exhibited lower sucrose preferences in the SPT, longer
immobility times in the FST and reduced exploratory behaviors in
the OFT.
Previous studies have indicated that the differences
observed in sex may be associated with E2 levels (13,43).
Differences in emotion processing is most apparent between men and
women that are exhibiting low E2 levels. In women, lower E2
concentrations occur at certain phases of the menstrual cycle and
are associated with an increasingly negative mood, increased
depressive symptoms and postpartum depression (13,43).
Mohanty and Das (44) previously
demonstrated that folate deficiency may impair the ovarian
synthesis of E2 in monkeys. It has also been reported that folate
deficiency can reduce circulating testosterone levels in micropigs
(45). Unlike women, men generally
experience consistently higher E2 activity as androgens are
aromatized into E2 (13,46). This process remains efficient even
at low androgen concentrations (46). Although the percentage of androgen
that is converted into E2 is <1%, the hormonal effects exhibited
may remain high, since E2 is 100-1,000X more active than androgens
(46). In the present study, E2
levels were measured in mouse serum using chemiluminescence. The
results revealed that F-CFD mice exhibited lower E2 levels compared
with those in F-Ctrl mice. Additionally, there was no significant
difference in E2 levels between either groups of male mice.
Hormonal differences provide the basis for proposals regarding the
effects of sexual dimorphism on disorders between men and women
(3,46). This may explain why only F-CFD mice
exhibited depression-like behavior in the present study.
Accumulating evidence has suggested that the ability
of E2 to ameliorate mood symptoms is associated with ERβ activation
(18,19). Activated ERβ modulates the PI3K/AKT
signaling pathway, which regulates both cell proliferation and cell
death (41,47). The present study demonstrated that
decreased E2/ERβ expression levels in F-CFD mice led to the
downregulation of the PI3K signaling pathway and upregulation of
cleaved caspase-3 protein expression. Caspase-3 serves a key role
in apoptosis activation (25). Liu
et al (48) reported that
the induction of apoptosis by tanshinone I in leukemia cells was
mainly associated with the inactivation of the PI3K/AKT signaling
pathway and the activation of caspase-3. Additionally, Tang et
al (49) demonstrated that
ursolic acid induced the apoptosis of human hepatocellular
carcinoma cells by downregulating PI3K/AKT and increasing
caspase-3. Zhang et al (50) also revealed that purple-colored
sweet potato significantly inhibited the activity of caspase-3 and
raised PI3K and p-AKT protein levels to exert hepatoprotective
effects. The present study found that a CFD diet increased the
expression levels of cleaved caspase-3 in female mice but not male
mice, suggesting that decreased E2 levels in CFD-fed female mice
increased the apoptosis of cells in the cerebral cortex, which was
consistent with observations previously made by Patten et al
(51) and Khan et al
(52). Increased cleaved caspase-3
activity promotes neuronal apoptosis (24,52).
A number of neural regions are involved in the processing and
regulation of emotions, such as the prefrontal cortex and cingulate
cortex (53,54). Neuronal apoptosis decreases
neurogenesis and is accompanied by the occurrence of
depression-like behavior (23-25).
A recent study documented that post-weaning folate
deficiency reduced the degree of maturation in neonatal hippocampal
neurons and subsequently induced depression-like symptoms in male
mice (55). However, the rate of
hippocampal neurogenesis declines with increasing age, meaning that
folate deficiency may adversely affect hippocampal function in
older individuals (56). By
contrast, the present study revealed that folate deficiency led to
neural apoptosis in female mice through reducing the
E2/ERβ/PI3K/AKT signaling pathway. This may be the underlying
mechanism in which decreased neurogenesis is associated with
depression-like behavior (24).
By applying animal models, the present study aimed
to investigate whether folate deficiency led to differences in
depression between males and females, in addition to elucidating
the potential underlying mechanism by which this occurred.
Unfortunately, phases of the estrous cycle were not evaluated in
the behavioral assays. In addition, E2 rescue and estrogen receptor
agonists are needed to examine the effects of E2 on depressive
behaviors. Further study is also required to examine the effects of
folate deficiency on the estrous cycle and behavior, in addition to
the importance of E2 in this process.
In conclusion, the CFD diet led to depressive
behavior only in female mice. Furthermore, the CFD diet may be
associated with decreased E2 levels, which in turn inhibited the
PI3K/AKT signaling pathway and increased the expression levels of
cleaved caspase-3, resulting in neuronal cell damage, apoptosis and
depression-like behavior.
Supplementary Material
Open field test design (n=10 per
group). (A) Apparatus of open field test. The apparatus consists of
a square arena (46x46 cm) that is 40-cm high with a white and
opaque wall. All mice were gently placed at the same corner (point
M). (B) Floor of the apparatus of the open field test. The floor
was separated into 25 squares. The 16 squares along the walls were
considered periphery whereas the other nine squares were considered
to be center.
Estimated weight trend of the mice in
the present study (n=20 per group). (A) Female and (B) male mouse
weights. The horizontal axis represents the number of time levels.
The weight of mice at week 8 corresponds to time level 1, the
weight of mice at week 10 corresponds to time level 2, until the
weight of mice at week 38 corresponds to time point 16. CFD,
chronic folate-deficient; F-Ctrl, female mice with standard control
diet; F-CFD, female mice with folate deficiency diet; M-Ctrl, male
mice with standard control diet; M-CFD, male mice with folate
deficiency diet.
Acknowledgements
Thanks to Professor Dexiang Xu, head of the Key
Laboratory of Environmental Toxicology of Anhui Higher Education
Institutes, Anhui Medical University, China, for his technical
assistance.
Funding
Funding:The present study was supported by the National Natural
Science Foundation of China (grant no. 81671471), the Key
Cultivation Program of School of Nursing, Anhui Medical University
(grant no. hlzd2020003) and the Seedling Cultivation Program of
School of Nursing, Anhui Medical University (grant no.
hlqm2021006).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
WS and QQ wrote the manuscript and performed the
experiments. XC, LZ, NY and JC analyzed the data. MZ analyzed the
data and revised the manuscript. WS and QQ confirm the authenticity
of all the raw data. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
The present protocol has received ethics approval
from the Ethics Committee of Anhui Medical University (approval no.
LLSC20150350; Hefei, China).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
McCarron RM, Shapiro B, Rawles J and Luo
J: Depression. Ann Intern Med. 174:ITC65–ITC80. 2021.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Malhi GS and Mann JJ: Depression. Lancet.
392:2299–2312. 2018.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Jääskeläinen E, Juola T, Korpela H,
Lehtiniemi H, Nietola M, Korkeila J and Miettunen J: Epidemiology
of psychotic depression-systematic review and meta-analysis.
Psychol Med. 48:905–918. 2018.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Kessler RC and Bromet EJ: . The
epidemiology of depression across cultures. Annu Rev Public Health.
34:119–138. 2013.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Bromet E, Andrade LH, Hwang I, Sampson NA,
Alonso J, de Girolamo G, de Graaf R, Demyttenaere K, Hu C, Iwata N,
et al: Cross-national epidemiology of DSM-IV major depressive
episode. BMC Med. 9(90)2011.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Reus GZ, Maciel AL, Abelaira HM, de Moura
AB, de Souza TG, Dos Santos TR, Darabas AC, Parzianello M, Matos D,
Abatti M, et al: ω-3 and folic acid act against depressive-like
behavior and oxidative damage in the brain of rats subjected to
early- or late-life stress. Nutrition. 53:120–133. 2018.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Bender A, Hagan KE and Kingston N: The
association of folate and depression: A meta-analysis. J Psychiatr
Res. 95:9–18. 2017.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Zheng Y and Cantley LC: Toward a better
understanding of folate metabolism in health and disease. J Exp
Med. 216:253–266. 2019.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Nguyen B, Weiss P, Beydoun H and Kancherla
V: Association between blood folate concentrations and depression
in reproductive aged U.S. women, NHANES (2011-2012). J Affect
Disord. 223:209–217. 2017.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Miyaki K, Song Y, Taneichi S, Tsutsumi A,
Hashimoto H, Kawakami N, Takahashi M, Shimazu A, Inoue A, Kurioka S
and Shimbo T: Socioeconomic status is significantly associated with
the dietary intakes of folate and depression scales in Japanese
workers (J-HOPE study). Nutrients. 5:565–578. 2013.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Huang X, Fan Y, Han X, Huang Z, Yu M,
Zhang Y, Xu Q, Li X, Wang X, Lu C and Xia Y: Association between
serum vitamin levels and depression in U.S. adults 20 years or
older based on national health and nutrition examination survey
2005-2006. Int J Environ Res Public Health. 15(1215)2018.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Li Y, Gao R, Liu X, Chen X, Liao X, Geng
Y, Ding Y, Wang Y and He J: Folate deficiency could restrain
decidual angiogenesis in pregnant mice. Nutrients. 7:6425–6445.
2015.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Albert KM and Newhouse PA: Estrogen,
stress and depression: Cognitive and biological interactions. Annu
Rev Clin Psychol. 15:399–423. 2019.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Sassarini DJ: Depression in midlife women.
Maturitas. 94:149–154. 2016.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Li D, Li Y, Chen Y, Li H, She Y, Zhang X,
Chen S, Chen W, Qiu G, Huang H and Zhang S: Neuroprotection of
reduced thyroid hormone with increased estrogen and progestogen in
postpartum depression. Biosci Rep. 39(BSR20182382)2019.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Xu Y, Sheng H, Bao Q, Wang Y, Lu J and Ni
X: NLRP3 inflammasome activation mediates estrogen
deficiency-induced depression- and anxiety-like behavior and
hippocampal inflammation in mice. Brain Behav Immun. 56:175–186.
2016.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Liu T, Ma Y, Zhang R, Zhong H, Wang L,
Zhao J, Yang L and Fan X: Resveratrol ameliorates estrogen
deficiency-induced depression- and anxiety-like behaviors and
hippocampal inflammation in mice. Psychopharmacology (Berl).
236:1385–1399. 2019.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Gillies GE and McArthur S: Estrogen
actions in the brain and the basis for differential action in men
and women: A case for sex-specific medicines. Pharmacol Rev.
62:155–198. 2010.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Rettberg JR, Yao J and Brinton RD:
Estrogen: A master regulator of bioenergetic systems in the brain
and body. Front Neuroendocrino. 35:8–30. 2014.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Walf AA and Frye CA: A review and update
of mechanisms of estrogen in the hippocampus and amygdala for
anxiety and depression behavior. Neuropsychopharmacology.
31:1097–1111. 2006.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Wang X, Yi L, Zhu Y, Zou J, Hong Y and
Zheng W: AKT signaling pathway in invasive ductal carcinoma of the
breast: Correlation with ERa, ERβ and HER-2 expression. Tumori.
97:185–190. 2011.PubMed/NCBI View
Article : Google Scholar
|
|
22
|
Hernández-Silva CD, Villegas-Pineda JC and
Pereira-Suárez AL: Expression and role of the G protein-coupled
estrogen receptor (GPR30/GPER) in the development and immune
response in female reproductive cancers. Front Endocrinol
(Lausanne). 11(544)2020.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Hoxhaj G and Manning BD: The PI3K-AKT
network at the interface of oncogenic signalling and cancer
metabolism. Nat Rev Cancer. 20:74–88. 2020.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Lossi L, Castagna C and Merighi A:
Caspase-3 mediated cell death in the normal development of the
mammalian cerebellum. Int J Mol Sci. 19(3999)2018.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Sangaran PG, Ibrahim ZA, Chik Z, Mohamed Z
and Ahmadiani A: LPS preconditioning attenuates apoptosis mechanism
by inhibiting NF-κB and caspase-3 activity: TLR4 pre-activation in
the signaling pathway of LPS-induced neuroprotection. Mol
Neurobiol. 58:2407–2422. 2021.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Phillips ML, Drevets WC, Rauch SL and Lane
R: Neurobiology of emotion perception II: Implications for major
psychiatric disorders. Biol Psychiatry. 54:515–528. 2003.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Wright AJ, Dainty JR and Finglas PM: Folic
acid metabolism in human subjects revisited: Potential implications
for proposed mandatory folic acid fortification in the UK. Br J
Nutr. 98:667–675. 2007.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Reeves PG: Components of the AIN-93 diets
as improvements in the AIN-76A diet. J Nutr. 127(Suppl
5):S838–S841. 1997.PubMed/NCBI View Article : Google Scholar
|
|
29
|
O'Leary K and Sheehy PJ: Effects of
preparation and cooking of folic acid-fortified foods on the
availability of folic acid in a folate depletion/repletion rat
model. J Agric Food Chem. 49:4508–4512. 2001.PubMed/NCBI View Article : Google Scholar
|
|
30
|
MacArthur Clark JA and Sun D: Guidelines
for the ethical review of laboratory animal welfare People's
Republic of China National Standard GB/T 35892-2018 (issued 6
february 2018 effective from 1 september 2018). Animal Model Exp
Med. 3:103–113. 2020.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Bahous RH, Cosín-Tomás M, Deng L, Leclerc
D, Malysheva O, Ho MK, Pallàs M, Kaliman P, Bedell BJ, Caudill MA
and Rozen R: Early manifestations of brain aging in mice due to low
dietary folate and mild MTHFR deficiency. Mol Neurobiol.
56:4175–4191. 2019.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Martorell AJ, Paulson AL, Suk HJ, Abdurrob
F, Drummond GT, Guan W, Young JZ, Kim DN, Kritskiy O, Barker SJ, et
al: Multi-sensory gamma stimulation ameliorates
Alzheimer's-associated pathology and improves cognition. Cell.
177:256–271.e22. 2019.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Zhang M, Liu Y, Zhao M, Tang W, Wang X,
Dong Z and Yu S: Depression and anxiety behaviour in a rat model of
chronic migraine. J Headache Pain. 18(27)2017.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Liu MY, Yin CY, Zhu LJ, Zhu XH, Xu C, Luo
CX, Chen X, Zhu DY and Zhou QG: Sucrose preference test for
measurement of stress-induced anhedonia in mice. Nat Protoc.
13:1686–1698. 2018.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Trunnell ER: Use of the forced swim test
to assess ‘despair’. Brain Stimul. 12:1317–1318. 2019.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Gao R, Ding Y, Liu X, Chen X, Wang Y, Long
C, Li S, Guo L and He J: Effect of folate deficiency on promoter
methylation and gene expression of Esr1, Cdh1 and Pgr, and its
influence on endometrial receptivity and embryo implantation. Hum
Reprod. 27:2756–2765. 2012.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Roberts RF and Roberts WL: Performance
characteristics of a recombinant enzymatic cycling assay for
quantification of total homocysteine in serum or plasma. Clin Chim
Acta. 344:95–99. 2004.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Kanso H, Inguimbert N, Istamboulie G,
Barthelmebs L, Calas-Blanchard C and Noguer T: Chemiluminescence
immunoassays for estradiol and ethinylestradiol based on new
biotinylated estrogen derivatives. Anal Biochem. 537:63–68.
2017.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Brandhorst G, Streit F, Kratzsch J,
Schiettecatte J, Roth HJ, Luppa PB, Körner A, Kiess W, Binder L,
Oellerich M and von Ahsen N: Multicenter evaluation of a new
automated electrochemiluminescence immunoassay for the
quantification of testosterone compared to liquid chromatography
tandem mass spectrometry. Clin Biochem. 44:264–267. 2011.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Chan YM, Bailey R and O'Connor DL: Folate.
Adv Nutr. 4:123–125. 2013.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Jafari M, Ghadami E, Dadkhah T and
Akhavan-Niaki H: PI3k/AKT signaling pathway: Erythropoiesis and
beyond. J Cell Physiol. 234:2373–2385. 2019.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Fruman DA, Chiu H, Hopkins BD, Bagrodia S,
Cantley LC and Abraham RT: The PI3K pathway in human disease. Cell.
170:605–635. 2017.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Shors TJ and Leuner B: Estrogen-mediated
effects on depression and memory formation in females. J Affect
Disord. 74:85–96. 2003.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Mohanty D and Das KC: Effect of folate
deficiency on the reproductive organs of female rhesus monkeys: A
cytomorphological and cytokinetic study. J Nutr. 112:1565–1576.
1982.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Wallock-Montelius LM, Villanueva JA,
Chapin RE, Conley AJ, Nguyen HP, Ames BN and Halsted CH: Chronic
ethanol perturbs testicular folate metabolism and dietary folate
deficiency reduces sex hormone levels in the Yucatan micropig. Biol
Reprod. 76:455–465. 2007.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Blakemore J and Naftolin F: Aromatase:
Contributions to physiology and disease in women and men.
Physiology (Bethesda). 31:258–269. 2016.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Fernandez JW, Grizzell JA and Wecker L:
The role of estrogen receptor β and nicotinic cholinergic receptors
in postpartum depression. Prog Neuropsychopharmacol Biol
Psychiatry. 40:199–206. 2013.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Liu JJ, Liu WD, Yang HZ, Zhang Y, Fang ZG,
Liu PQ, Lin DJ, Xiao RZ, Hu Y, Wang CZ, et al: Inactivation of
PI3k/Akt signaling pathway and activation of caspase-3 are involved
in tanshinone I-induced apoptosis in myeloid leukemia cells in
vitro. Ann Hematol. 89:1089–1097. 2010.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Tang C, Lu YH, Xie JH, Wang F, Zou JN,
Yang JS, Xing YY and Xi T: Downregulation of survivin and
activation of caspase-3 through the PI3K/Akt pathway in ursolic
acid-induced HepG2 cell apoptosis. Anticancer Drugs. 20:249–258.
2009.PubMed/NCBI View Article : Google Scholar
|
|
50
|
Zhang ZF, Lu J, Zheng YL, Hu B, Fan SH, Wu
DM, Zheng ZH, Shan Q and Liu CM: Purple sweet potato color protects
mouse liver against d-galactose-induced apoptosis via inhibiting
caspase-3 activation and enhancing PI3K/Akt pathway. Food Chem
Toxicol. 48:2500–2507. 2010.PubMed/NCBI View Article : Google Scholar
|
|
51
|
Patten RD, Pourati I, Aronovitz MJ, Baur
J, Celestin F, Chen X, Michael A, Haq S, Nuedling S, Grohe C, et
al: 17beta-estradiol reduces cardiomyocyte apoptosis in vivo and in
vitro via activation of phospho-inositide-3 kinase/Akt signaling.
Circ Re. 95:692–699. 2004.PubMed/NCBI View Article : Google Scholar
|
|
52
|
Khan M, Ullah R, Rehman SU, Shah SA, Saeed
K, Muhammad T, Park HY, Jo MH, Choe K, Rutten BPF and Kim MO:
17β-estradiol modulates SIRT1 and Halts oxidative stress-mediated
cognitive impairment in a male aging mouse model. Cells.
8(928)2019.PubMed/NCBI View Article : Google Scholar
|
|
53
|
Gong Q and He Y: Depression, neuroimaging
and connectomics: A selective overview. Biol Psychiatry.
77:223–235. 2015.PubMed/NCBI View Article : Google Scholar
|
|
54
|
Nestler EJ, Barrot M, DiLeone RJ, Eisch
AJ, Gold SJ and Monteggia LM: Neurobiology of depression. Neuron.
34:13–25. 2002.PubMed/NCBI View Article : Google Scholar
|
|
55
|
Nishida S, Araki R, Baba A, Asari S,
Tachibana S, Nakajima Y, Iwakumo A and Yabe T: Post-weaning folate
deficiency induces a depression-like state via neuronal immaturity
of the dentate gyrus in mice. J Pharmacol Sci. 143:97–105.
2020.PubMed/NCBI View Article : Google Scholar
|
|
56
|
Snyder JS, Soumier A, Brewer M, Pickel J
and Cameron HA: Adult hippocampal neurogenesis buffers stress
responses and depressive behaviour. Nature. 476:458–461.
2011.PubMed/NCBI View Article : Google Scholar
|