Introduction
Type 1 diabetes (T1D) is characterized by the
destruction of pancreatic β-cells, which results in insulin
deficiency. The etiology and pathogenesis of the disease remain to
be fully elucidated. Insulin replacement is currently the only
treatment for T1D (1).
Blood glucose is tightly maintained within its
normal range by numerous factors that affect glucose production and
utilization. The key hormones involved in blood glucose regulation
mainly include insulin, glucagon, epinephrine, glucocorticoid and
growth hormone (2). As the primary
organ of biochemical metabolism, the liver serves an important role
in glucose homeostasis (3). Net
hepatic glucose production is the summation of gluconeogenesis,
glycogenolysis, glycogen synthesis and glycolysis, among other
metabolic pathways (4). Glycogen
is a multibranched polysaccharide of glucose, whereby liver
glycogen is involved in the regulation of blood glucose
homeostasis. Under normal physiological conditions, glycogen is
synthesized following ingestion and broken down during fasting
periods. However, dysregulated glycogen metabolism is observed in
the diabetic liver (5).
Furthermore, other metabolic pathways display abnormalities to
varying degrees as a result of T1D (6).
Mitochondria are the main organelles for substance
and energy metabolism. Hepatic mitochondrial dysfunction serves an
important role in excessive hepatic glucose production and impaired
glucose utilization (7,8). Previous studies have demonstrated
that niclosamide ethanolamine salt (NEN) and artemether (Art)
displayed significant hypoglycemic effects in diabetes (9-12).
Their pharmacological actions are closely related to the regulation
of mitochondrial function (13,14).
However, their combined therapeutic effects on the liver in T1D
have remained elusive.
In the present study, the hepatoprotective effects
of combined NEN and Art treatment in T1D mice and the potential
underlying mechanisms were investigated.
Materials and methods
Animal experiment
A total of 60 male C57BL/6 mice (body weight, 22-26
g, ~8 weeks) were purchased from Guangdong Medical Laboratory
Animal Center and were housed in the Central Animal Facility at
Shenzhen Graduate School of Peking University (Shenzhen, China).
The temperature was controlled at 20-23˚C, the relative humidity
was controlled at 50-60%, the light and dark cycle was 12/12 h. All
mice had free access to water and food. The diabetic mice were
randomly allocated to the following groups (n=8 per group): T1D
group (T1D), T1D+NEN group (NEN), T1D+Art group (Art) and
T1D+NEN+Art group (NEN+Art). T1D was established by administering
multiple intraperitoneal injections of streptozotocin (STZ; 55
mg/kg) to mice on five consecutive days. Control mice (n=8) were
intraperitoneally injected with the same volume of the vehicle.
Successful induction of T1D was confirmed according to fasting
blood glucose levels of ≥11.1 mmol/l at 9 days after the last
injection of STZ. Mice in the control and T1D groups were fed a
conventional standard diet; Mice in the NEN group were fed a
standard diet containing 10 g/kg NEN; mice in the Art group were
fed a standard diet containing 0.67 g/kg Art; and mice in the
NEN+Art group were fed a standard diet containing 10 g/kg NEN and
0.67 g/kg Art. The diet was freely available to each group mice in
their cage. The doses of NEN and Art used in the present study were
selected according to previous studies (9,12).
The treatment lasted for 8 weeks. NEN was purchased from Hubei
ShengTian HengChuang Biological Technology Co., Ltd. and Art was
purchased from Chengdu ConBon Biotech Co., Ltd. All animal
procedures were approved by the Guangzhou University of Chinese
Medicine Institutional Animal Care and Use Committee (Shenzhen,
China).
Tissue preparation
The body weight of the animals in each group was
determined at the end of the study. The mice were anesthetized with
~1% isoflurane and euthanized by cervical dislocation. Blood
samples and liver tissues were rapidly collected and the livers
were weighed. Partial liver tissues were fixed in 10% formalin and
used for histopathological examination and immunohistochemical
staining. The remaining liver tissues were immediately snap-frozen
in liquid nitrogen and stored at -80˚C for later analysis.
Physiological and metabolic
parameters
Fasting blood glucose was analyzed using a blood
glucose meter (Roche Diagnostics). Urine was collected using
metabolic cages (Tecniplast). Glycated hemoglobin (HbA1c) levels
were analyzed using an Ultra2 HbA1c Analyzer (Primus). Serum
alanine transaminase (ALT), aspartate transaminase (AST), total
protein (TP), albumin (ALB), triglyceride (TG), total cholesterol
(TC) and urinary glucose were detected using an automatic
biochemical analyzer (Roche Diagnostics). Liver glycogen was
quantified using the Anthrone method (15). Prior to the end of the experiment,
the respiratory exchange ratio (RER) of the mice was determined
using the Comprehensive Lab Animal Monitoring System (CLAMS;
Columbus Instruments). The limb grip strength was measured using a
dynamometer (ZH-YLS-13A; Anhui Zhenghua Biological Instrument
Equipment Co., Ltd.) according to the manufacturer's instructions.
Three measurements were performed and the mean value was
calculated.
Light microscopy
From each specimen, three to six liver sections were
randomly selected and stained with periodic acid-Schiff (PAS) and
diastase (D)-PAS (Beijing Solarbio Science & Technology Co.,
Ltd.) according to the manufacturer's protocols. The sections were
scanned using the Digital Slide Scanner (3DHistech Ltd.) to
evaluate the liver glycogen content.
ELISA
Serum insulin levels were determined using an ELISA
kit (EMD Millipore; cat. no. #EZRMI-13K) according to the
manufacturer's protocol.
Immunohistochemical staining
Liver sections (3-6 slices) were deparaffinized and
rehydrated. Subsequently, the sections were subjected to antigen
retrieval by boiling in citric acid buffer (pH 6) for 20 min. After
rinsing three times with PBS, the sections were incubated with
primary antibodies against glucose-6-phosphatase (G6Pase; Abcam,
ab83690, 1:100) overnight at 4˚C. After washing with PBS, the
sections were incubated with HRP-polymer conjugated
anti-mouse/rabbit IgG secondary antibody for 1 h at room
temperature. Diaminobenzidine solution was used as a chromogen. The
sections were counterstained with hematoxylin and images were
acquired using the Digital Slide Scanner (3DHistech Ltd.).
Immunoblotting analysis
Liver tissues were homogenized and prepared in
sample loading buffer (Bio-Rad Laboratories, Inc.). Total protein
was separated using 10% SDS-PAGE and transferred onto
polyvinylidene difluoride membranes (EMD Millipore). After blocking
with 5% non-fat dry milk (Bio-Rad Laboratories, Inc.), the
membranes were incubated at 4˚C overnight with the following
primary antibodies: G6Pase (Abcam, ab83690, 1:1,000), transcription
factor A mitochondrial (TFAM; EMD Millipore, ABE483, 1:1,000),
cytochrome c oxidase IV (COX IV), translocase of the outer
mitochondrial membrane 20 (TOM20), voltage-dependent anion channel
(VDAC), pyruvate dehydrogenase (PDH), phosphoenolpyruvate
carboxykinase (PCK2), branched-chain α-keto acid dehydrogenase
complex (BCKDH) and β-actin. COX IV (cat. no. #4844, 1:1,000),
TOM20 (#13929, 1:1,000), VDAC (#4661, 1:1,000), PDH (#2784,
1:1,000), PCK2 (#6924, 1:1,000) and BCKDH (#90198, 1:1,000)
antibodies were purchased from Cell Signaling Technology, Inc. and
β-actin antibody was from MilliporeSigma (A5441, 1:2,000). The
membranes were then incubated with HRP-conjugated Goat anti-Rabbit
or Goat anti-Mouse secondary antibodies (Invitrogen; Thermo Fisher
Scientific, Inc.; Cat. nos. 65-6120 or 62-6520, respectively; all
1:2,000) and detected by the ChemiDoc™ MP Imaging System (Bio-Rad
Laboratories, Inc.).
Statistical analysis
Values are expressed as the mean ± standard
deviation. Data analysis was performed using SPSS statistics
software version 22.0 (IBM Corporation). One-way ANOVA followed by
Bonferroni's post-hoc test was used for data analysis. P<0.05
was considered to indicate a statistically significant
difference.
Results
NEN+Art improves glycometabolism and
diabetic symptoms
Compared with the control group, significantly
increased fasting blood glucose, HbA1c and urinary glucose, and
decreased serum insulin were observed in the T1D group mice
(Fig. 1A-D). Consistent with a
glycometabolic disorder, the mice in the T1D group exhibited
typical diabetic symptoms including polydipsia, polyuria,
polyphagia and increased feces production (Fig. 1E-H). These results confirmed that
T1D in mice was well established and the success rate of
establishing the model was ~85%. The combined treatment of NEN+Art
reduced hyperglycemia and improved diabetic symptoms to a greater
extent than treatment with NEN or Art alone.
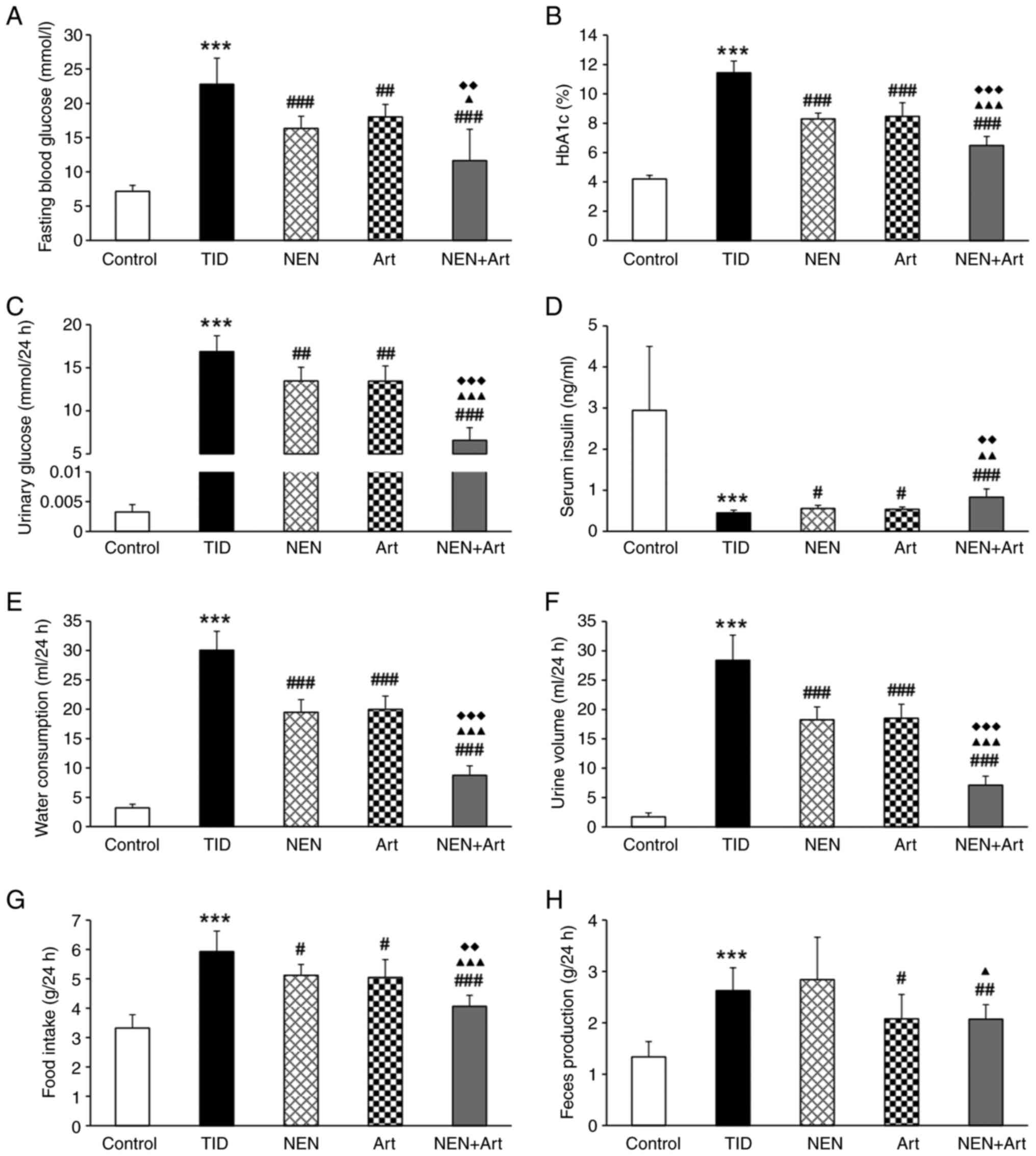 | Figure 1NEN+Art improves glycometabolism and
T1D symptoms. (A-D) Changes in glycometabolism-related indices,
including (A) fasting blood glucose, (B) HbA1c, (C) urinary
glucose, and (D) serum insulin in the different groups after 8
weeks of treatment. (E) Water consumption, (F) urine volume, (G)
food intake, and (H) feces production after 8 weeks treatment in
each group (n=8 per group). ***P<0.001 vs. the
control group; #P<0.05, ##P<0.01 and
###P<0.001 vs. the T1D group; ▲P<0.05,
▲▲P<0.01 and ▲▲▲P<0.001 vs. the NEN
group; ◆◆P<0.01 and ◆◆◆P<0.001 vs. the
Art group. NEN, niclosamide ethanolamine salt; Art, artemether;
T1D, type 1 diabetes; HbA1c, glycated hemoglobin. |
NEN+Art significantly ameliorates
liver injury and reduces serum lipid levels
The liver serves important roles in glucose and
lipid metabolism homeostasis. Compared to the control group, the
T1D group exhibited elevated serum ALT, AST, TG and TC levels and
decreased TP and ALB levels (Fig.
2). NEN treatment alone significantly reduced serum ALT and AST
levels and increased TP and ALB levels, whereas TG and TC levels
were increased to a certain extent but not to a significant degree.
Art treatment alone significantly increased TP and ALB levels and
decreased TG levels, but did not significantly affect ALT, AST and
TC levels. Overall, NEN+Art more prominently ameliorated the
T1D-associated aberrations in these liver function biomarkers.
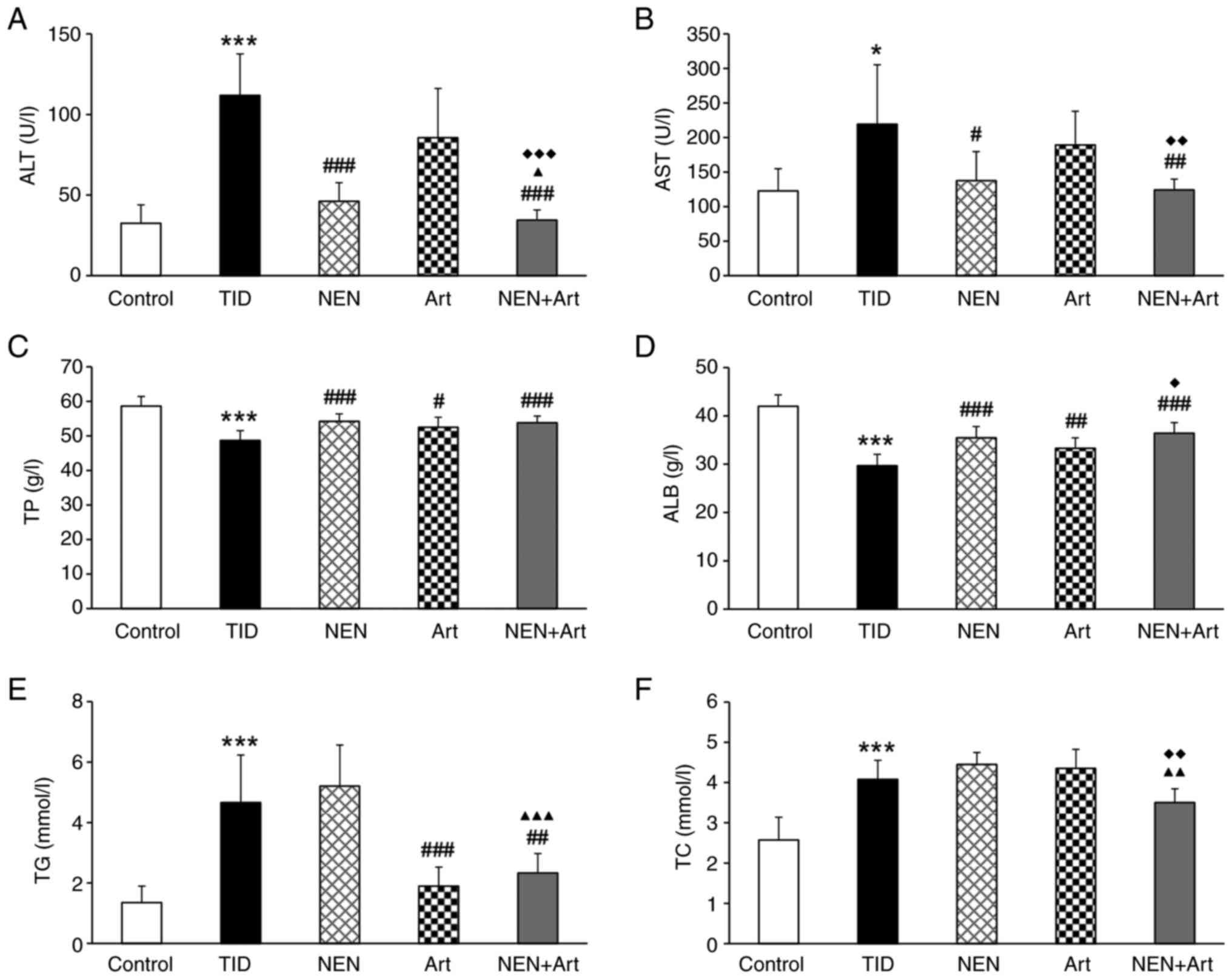 | Figure 2Effects of NEN, Art and NEN+Art on
liver function and serum lipid. (A-F) Bar graphs display the serum
levels of (A) ALT, (B) AST, (C) TP, (D) ALB, (E) TG, and (F) TC in
each group at the end of the study (n=8 per group).
*P<0.05 and ***P<0.001 vs. the control
group; #P<0.05, ##P<0.01 and
###P<0.001 vs. the T1D group; ▲P<0.05,
▲▲P<0.01 and ▲▲▲P<0.001 vs. the NEN
group; ◆P<0.05, ◆◆P<0.01 and
◆◆◆P<0.001 vs. the Art group. NEN, niclosamide
ethanolamine salt; Art, artemether; T1D, type 1 diabetes; ALT,
alanine transaminase; AST, aspartate transaminase; TP, total
protein; ALB, albumin; TG, triglycerides; TC, total
cholesterol. |
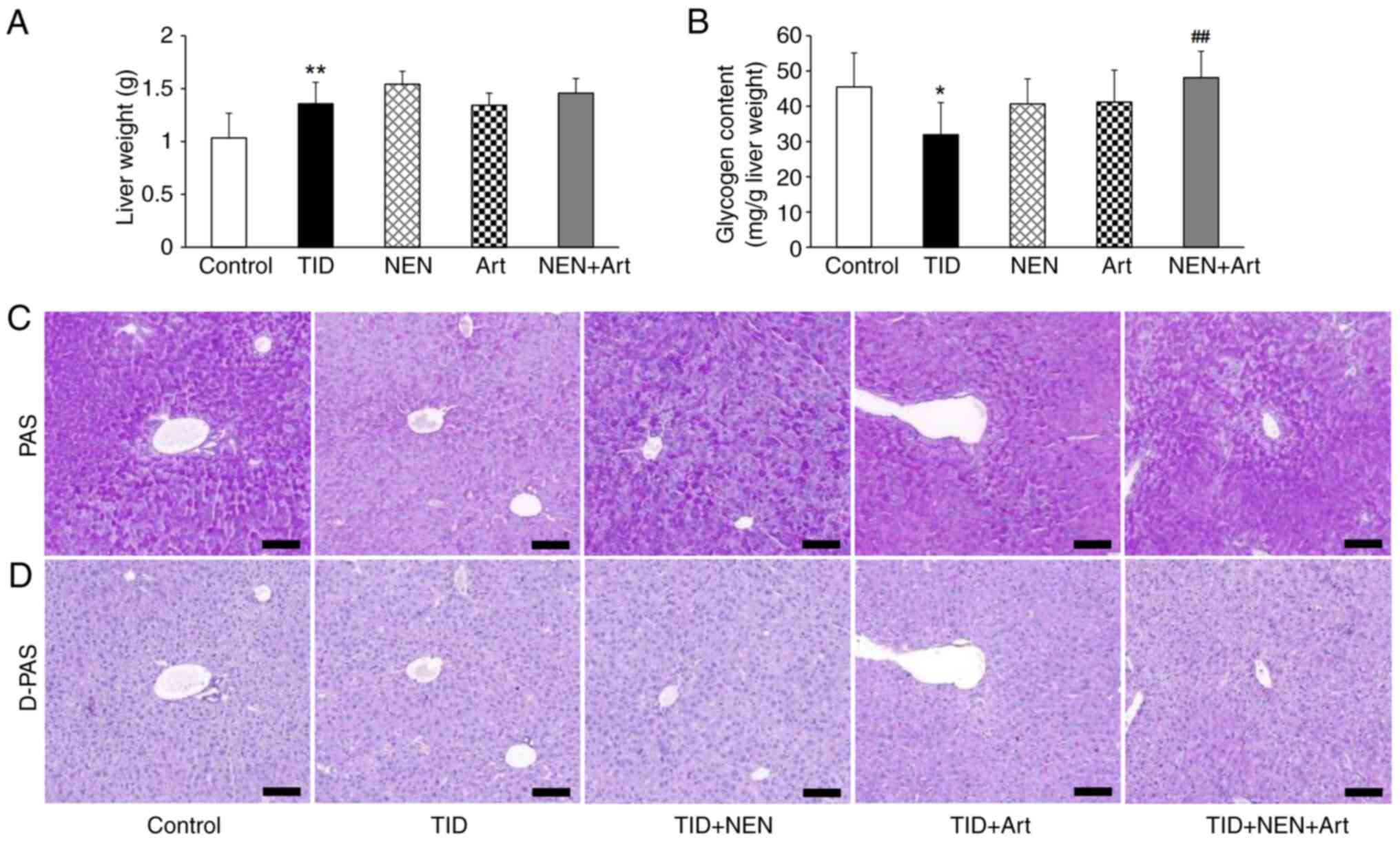
NEN+Art increases liver glycogen
storage
Significantly increased liver weight and reduced
liver glycogen were observed in the T1D group (Fig. 3A and B). NEN or Art treatment alone did not
significantly increase the liver glycogen content. However, NEN+Art
markedly increased the glycogen content (Fig. 3B). To further determine glycogen
storage in the liver, PAS and D-PAS staining on liver sections was
performed. The images demonstrated that glycogen changes in various
groups were consistent with the biochemical measurement results
(Fig. 3C and D).
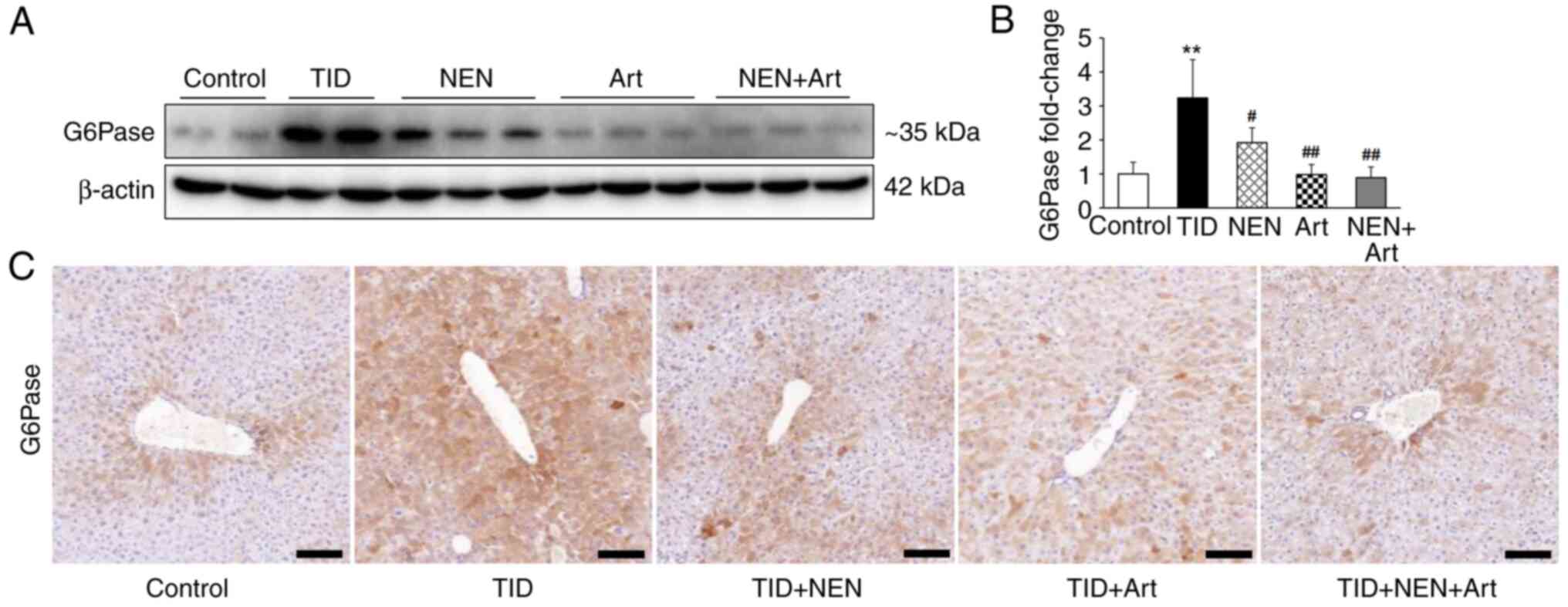
Effects of NEN and Art on hepatic
G6Pase protein expression levels
As presented in Fig.
4A and B, the immunoblotting
results demonstrated that hepatic G6Pase protein expression levels
significantly increased in T1D mice. NEN and Art treatment alone or
as a combined therapy significantly downregulated G6Pase protein
expression levels. Immunohistochemical staining for G6Pase also
confirmed its protein expression trends in the different groups
(Fig. 4C).
NEN and Art regulate hepatic
mitochondrial biogenesis
Compared with the control group, TFAM protein
expression levels increased significantly in the T1D group
(Fig. 5A and B). Furthermore, mitochondrial-associated
proteins, including VDAC, TOM20 and COX IV also increased to
varying degrees (Fig. 5C-E). NEN
and Art both exerted suppressive effects on mitochondrial
biogenesis and these effects were significantly enhanced when the
treatments were combined.
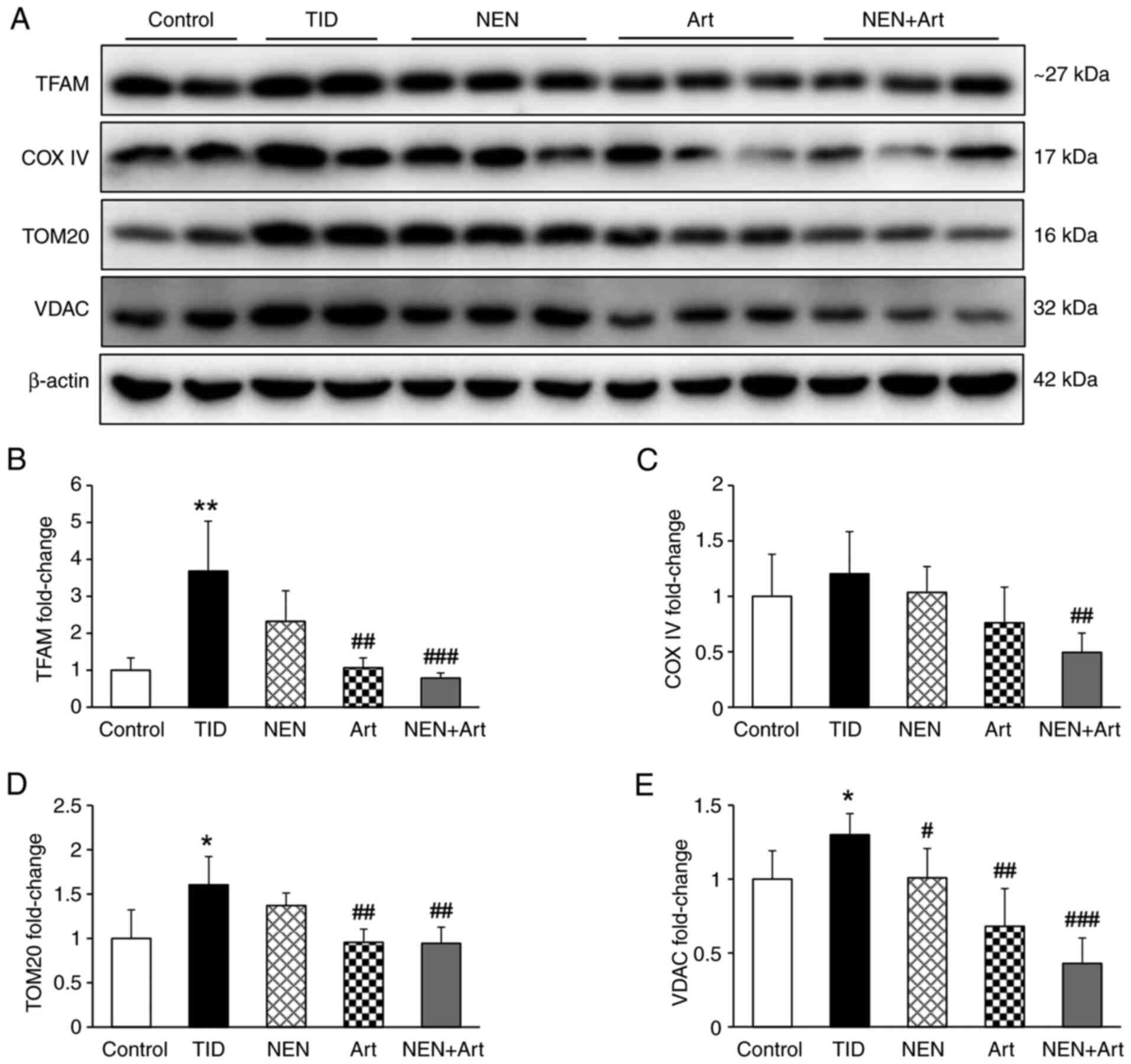 | Figure 5Effect of NEN and Art on hepatic
mitochondrial biogenesis. Western blot analysis was performed to
determine the protein expression levels of TFAM, COX IV, TOM20 and
VDAC in the hepatic tissue of the different groups. (A) Western
blot bands and (B-E) semi-quantitative analysis of (B) TFAM, (C)
COX IV, (D) TOM20 and (E) VDAC protein expression levels in each
group (n=4-6 per group). *P<0.05 and
**P<0.01 vs. the control group;
#P<0.05, ##P<0.01 and
###P<0.001 vs. the T1D group. NEN, niclosamide
ethanolamine salt; Art, artemether; T1D, type 1 diabetes; TFAM,
transcription factor A mitochondrial; COX IV, cytochrome c oxidase
IV; TOM20, translocase of the outer mitochondrial membrane 20;
VDAC, voltage-dependent anion channel. |
NEN+Art increases RER and regulates
mitochondrial metabolism
Compared with the control group, the T1D group
exhibited a significantly lower RER, which indicated that
carbohydrates were not the predominant fuel source in the diabetic
mice (Fig. 6A and B). During the dark (active) phase, either
NEN or Art were able to raise the RER and this effect was enhanced
in the group with the NEN+Art combined treatment. During the light
(sleep) phase, only NEN and the NEN+Art combination were able to
significantly raise the RER. As presented in Fig. 6C and D, there was no significant difference in
PDH protein expression levels among the groups. Compared with the
control mice, PCK2 and BCKDH protein expression levels were
significantly increased in T1D mice (Fig. 6E and F). NEN treatment alone downregulated PCK2
but did not influence BCKDH protein expression levels. Art
treatment alone significantly downregulated both PCK2 and BCKDH
protein expression levels. Similar to those in the Art group, the
protein expression levels of PCK2 and BCKDH in the NEN+Art group
were also significantly reduced.
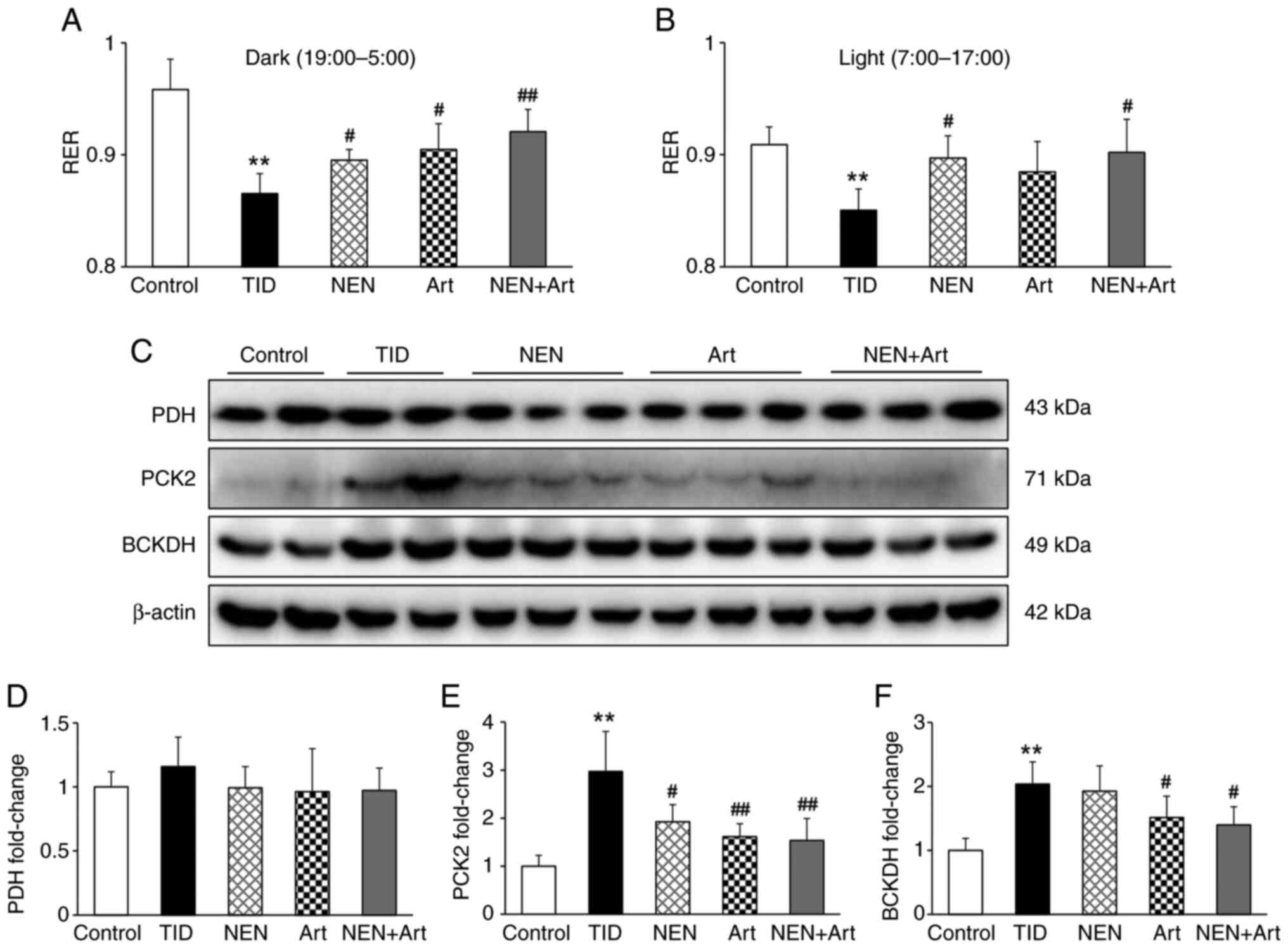 | Figure 6Effects of NEN and Art on the RER and
mitochondrial metabolism. (A and B) RER values for (A) the dark and
(B) light phase in each group (n=4 per group). (C-F) Western blot
analysis was performed to determine the protein expression levels
of PDH, PCK2, and BCKDH in the hepatic tissue of various groups.
(C) Western blot bands and semi-quantitative analysis of (D) PDH,
(E) PCK2 and (F) BCKDH in each group (n=4-6 per group).
**P<0.01 vs. the control group; #P<0.05
and ##P<0.01 vs. the T1D group. NEN, niclosamide
ethanolamine salt; Art, artemether; T1D, type 1 diabetes; PDH,
pyruvate dehydrogenase; PCK2, phosphoenolpyruvate carboxykinase;
BCKDH, branched-chain α-keto acid dehydrogenase complex; RER,
respiratory exchange ratio. |
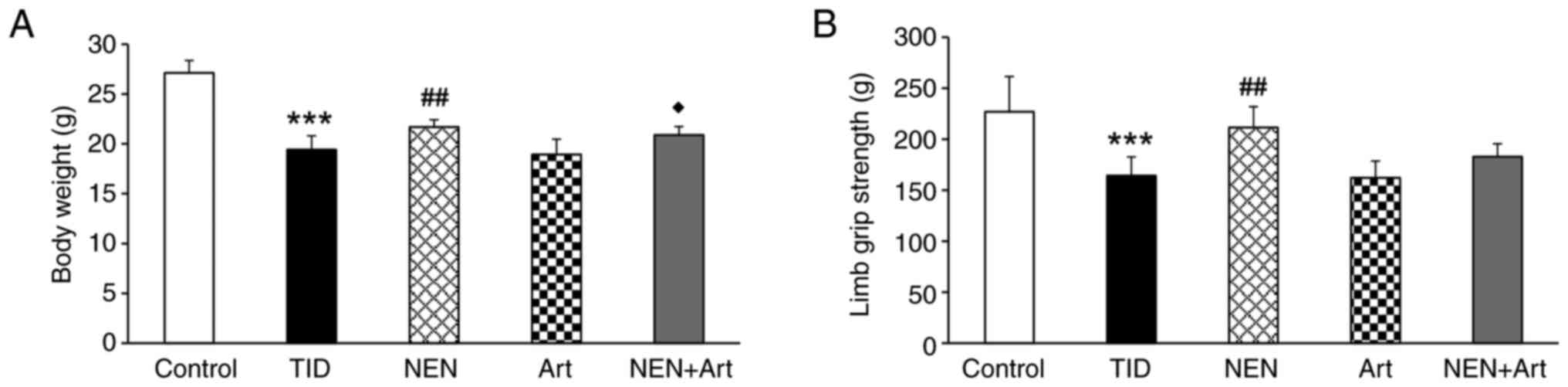
Effects of NEN and Art on body weight
and limb grip strength
In order to explore the contribution of muscle
function on the RER in T1D, the body weight and limb grip strength
in each group of mice were measured. As presented in Fig. 7, significantly reduced body weight
and limb grip strength were observed in the T1D group when compared
with those in the control group. These results may suggest that the
contribution of muscle metabolism on the RER was weakened in the
T1D group. NEN and NEN+Art treatment significantly increased the
body weight, but only NEN treatment enhanced limb grip strength.
Art treatment did not significantly affect the body weight and limb
grip strength.
Discussion
In the present study, it was demonstrated that a
combined treatment approach with NEN and Art more effectively
improved T1D symptoms and glycometabolism compared with either
treatment administered separately. The therapeutic effects may be
related to the regulation of hepatic glycogen metabolism and
mitochondrial function.
Consistent with previous studies, NEN or Art
treatment alone displayed a hypoglycemic effect; however, the two
treatments do not necessarily use the same mechanism (9-12).
The therapeutic effect was significantly enhanced in the NEN+Art
combined treatment group. Considering that NEN and Art have
different pharmacological actions (16,17),
it may be hypothesized that their effect may be synergistic.
Hepatic metabolic disorder is characteristic in patients with T1D.
Changes in various liver injury biomarkers indicated that NEN
treatment alone had a hepatoprotective effect. Although Art
treatment alone did not reduce abnormally elevated serum levels of
ALT and AST, the liver function improved with the combination of
Art and NEN. These results indicated that NEN and Art may
ameliorate liver injury via multiple signaling pathways.
Abnormal blood glucose and liver glycogen metabolism
frequently occur simultaneously and restoration of one usually
normalizes the other (18).
Consistent with previous findings (19), a lower liver glycogen content was
detected in the T1D mice. G6Pase is responsible for completing the
final step in gluconeogenesis, which hydrolyzes glucose-6-phosphate
to glucose in the endoplasmic reticulum (20,21).
In the present study, significantly increased hepatic G6Pase
protein expression levels were observed in T1D mice. Taken
together, these results suggested that augmented hepatic
gluconeogenesis may contribute to impaired blood glucose and liver
glycogen metabolism. The increased G6Pase protein expression levels
were significantly reduced by NEN and Art alone or together to
various degrees. A combination of NEN and Art also increased liver
glycogen storage.
Mitochondria are the prime metabolic platform for
various factors involved in energy metabolism (22). TFAM is a transcription factor that
activates mitochondrial biogenesis (23). VDAC, COX IV and TOM20 are the main
structural components of the mitochondria. In the present study,
all these aforementioned mitochondria-related proteins were
indicated to be significantly upregulated in T1D mice, which
demonstrated that the hepatic mitochondrial content was increased
under T1D conditions. PCK2 and BCKDH are metabolic enzymes located
in the mitochondrial matrix that are associated with
gluconeogenesis (24-26).
Their upregulated protein expression levels in the diabetic liver
in the present study suggested that the mitochondria were working
harder to promote gluconeogenesis. Furthermore, the hepatic protein
expression level of PDH (the key enzyme regulating pyruvate
oxidation) did not increase with diabetic liver mitochondria
accumulation. The RER is an important indicator to evaluate the
utilization ratio of metabolic substrates (10). Diabetic T1D mice exhibited a
reduced RER during both the dark and light phases of their daily
cycles when compared with the control mice. The declining trend was
more prominent during the dark phase. Taken together, these results
indicated that mitochondrial pyruvate aerobic oxidation is
relatively weakened and this is consistent with the reduction in
the RER. NEN and Art ameliorated the pathological alterations of
mitochondria and increased RER to various degrees. These results
demonstrated that NEN and Art were able to increase the aerobic
oxidation of glucose and recover the impaired mitochondrial glucose
metabolism. More importantly, the effects were significantly
enhanced by the combined treatment. However, the detailed
mechanisms underlying the influences of NEN and Art on the RER
require further investigation.
In conclusion, the present study demonstrated that
combined treatment with NEN and Art improved glycometabolism and
liver function more effectively than NEN or Art treatment alone in
T1D mice. The underlying mechanisms may be associated with hepatic
glycogen and mitochondrial metabolism. Combination therapy may be a
promising approach for treating T1D in the future.
Acknowledgements
Not applicable.
Funding
Funding: This study was supported by grants from the National
Natural Science Foundation of China (grant nos. 82004156 and
81673794), Shenzhen Science and Technology Project (grant no.
JCYJ20190812183603627) and Shenzhen Fund for Guangdong Provincial
High-level Clinical Key Specialties.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
HS and PH designed the research. WW and HL performed
most of the experiments and analyzed most of the data. ZS, PZ, XY
and MS performed part of the experiments. PH and HS wrote the
manuscript and confirmed the authenticity of all the raw data. All
authors read and approved the final manuscript.
Ethics approval and consent to
participate
The animal experiments in this study were approved
by the Guangzhou University of Chinese Medicine Institutional
Animal Care and Use Committee (Shenzhen, China).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Warshauer JT, Bluestone JA and Anderson
MS: New frontiers in the treatment of type 1 diabetes. Cell Metab.
31:46–61. 2020.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Guemes M, Rahman SA and Hussain K: What is
a normal blood glucose? Arch Dis Child. 101:569–574.
2016.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Han HS, Kang G, Kim JS, Choi BH and Koo
SH: Regulation of glucose metabolism from a liver-centric
perspective. Exp Mol Med. 48(e218)2016.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Petersen MC, Vatner DF and Shulman GI:
Regulation of hepatic glucose metabolism in health and disease. Nat
Rev Endocrinol. 13:572–587. 2017.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Hu Z, Li E, Sullivan MA, Tan X, Deng B,
Gilbert RG and Li C: Glycogen structure in type 1 diabetic mice:
Towards understanding the origin of diabetic glycogen molecular
fragility. Int J Biol Macromol. 128:665–672. 2019.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Marinkovic T and Oresic M: Modeling
strategies to study metabolic pathways in progression to type 1
diabetes-Challenges and opportunities. Arch Biochem Biophys.
589:131–137. 2016.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Morio B, Panthu B, Bassot A and Rieusset
J: Role of mitochondria in liver metabolic health and diseases.
Cell Calcium. 94(102336)2021.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Franko A, von Kleist-Retzow JC, Neschen S,
Wu M, Schommers P, Böse M, Kunze A, Hartmann U, Sanchez-Lasheras C,
Stoehr O, et al: Liver adapts mitochondrial function to insulin
resistant and diabetic states in mice. J Hepatol. 60:816–823.
2014.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Wang Y, Han P, Wang M, Weng W, Zhan H, Yu
X, Yuan C, Shao M and Sun H: Artemether improves type 1 diabetic
kidney disease by regulating mitochondrial function. Am J Transl
Res. 11:3879–3889. 2019.PubMed/NCBI
|
|
10
|
Han P, Wang Y, Zhan H, Weng W, Yu X, Ge N,
Wang W, Song G, Yi T, Li S, et al: Artemether ameliorates type 2
diabetic kidney disease by increasing mitochondrial pyruvate
carrier content in db/db mice. Am J Transl Res. 11:1389–1402.
2019.PubMed/NCBI
|
|
11
|
Han P, Zhan H, Shao M, Wang W, Song G, Yu
X, Zhang C, Ge N, Yi T, Li S and Sun H: Niclosamide ethanolamine
improves kidney injury in db/db mice. Diabetes Res Clin Pract.
144:25–33. 2018.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Han P, Shao M, Guo L, Wang W, Song G, Yu
X, Zhang C, Ge N, Yi T, Li S, et al: Niclosamide ethanolamine
improves diabetes and diabetic kidney disease in mice. Am J Transl
Res. 10:1071–1084. 2018.PubMed/NCBI
|
|
13
|
Chen W, Mook RA Jr, Premont RT and Wang J:
Niclosamide: Beyond an antihelminthic drug. Cell Signal. 41:89–96.
2018.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Tsui KH, Wu MY, Lin LT, Wen ZH, Li YH, Chu
PY and Li CJ: Disruption of mitochondrial homeostasis with
artemisinin unravels anti-angiogenesis effects via auto-paracrine
mechanisms. Theranostics. 9:6631–6645. 2019.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Carroll NV, Longley RW and Roe JH: The
determination of glycogen in liver and muscle by use of anthrone
reagent. J Biol Chem. 220:583–593. 1956.PubMed/NCBI
|
|
16
|
Wang Y, Wang Y, You F and Xue J: Novel use
for old drugs: The emerging role of artemisinin and its derivatives
in fibrosis. Pharmacol Res. 157(104829)2020.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Park JS, Lee YS, Lee DH and Bae SH:
Repositioning of niclosamide ethanolamine (NEN), an anthelmintic
drug, for the treatment of lipotoxicity. Free Radic Biol Med.
137:143–157. 2019.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Lopez-Soldado I, Guinovart JJ and Duran J:
Increasing hepatic glycogen moderates the diabetic phenotype in
insulin-deficient Akita mice. J Biol Chem.
296(100498)2021.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Hwang JH, Perseghin G, Rothman DL, Cline
GW, Magnusson I, Petersen KF and Shulman GI: Impaired net hepatic
glycogen synthesis in insulin-dependent diabetic subjects during
mixed meal ingestion. A 13C nuclear magnetic resonance spectroscopy
study. J Clin Invest. 95:783–787. 1995.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Hutton JC and O'Brien RM:
Glucose-6-phosphatase catalytic subunit gene family. J Biol Chem.
284:29241–29245. 2009.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Lizak B, Szarka A, Kim Y, Choi KS, Németh
CE, Marcolongo P, Benedetti A, Bánhegyi G and Margittai É: Glucose
transport and transporters in the endomembranes. Int J Mol Sci.
20(5898)2019.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Chipuk JE, Mohammed JN, Gelles JD and Chen
Y: Mechanistic connections between mitochondrial biology and
regulated cell death. Dev Cell. 56:1221–1233. 2021.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Pfanner N, Warscheid B and Wiedemann N:
Mitochondrial proteins: From biogenesis to functional networks. Nat
Rev Mol Cell Biol. 20:267–284. 2019.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Yu S, Meng S, Xiang M and Ma H:
Phosphoenolpyruvate carboxykinase in cell metabolism: Roles and
mechanisms beyond gluconeogenesis. Mol Metab.
53(101257)2021.PubMed/NCBI View Article : Google Scholar
|
|
25
|
White PJ, McGarrah RW, Grimsrud PA, Tso
SC, Yang WH, Haldeman JM, Grenier-Larouche T, An J, Lapworth AL,
Astapova I, et al: The BCKDH kinase and phosphatase integrate BCAA
and lipid metabolism via regulation of ATP-Citrate lyase. Cell
Metab. 27:1281–1293.e7. 2018.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Lynch CJ and Adams SH: Branched-chain
amino acids in metabolic signalling and insulin resistance. Nat Rev
Endocrinol. 10:723–736. 2014.PubMed/NCBI View Article : Google Scholar
|