Introduction
Dementia is a syndrome that is clinically
characterized by progressive loss of intelligence and is
accompanied by different degrees of personality changes.
Alzheimer's disease (AD) is the most common cause of dementia,
accounting for 60-80% of all types of dementia (1). AD is divided into two types based on
the age of onset: Early-onset AD (age, <65 years) and late-onset
AD (age, >65 years) (2).
β-amyloid (Aβ) deposition and tau protein hyperphosphorylation
comprise two major neuropathological features of AD (3). To date, the etiology of AD is unknown
and may be associated with the interactions of the environment,
genetics and other factors (4).
For example, results of a previous study demonstrated that
Ginkgo biloba extract exhibited neuroprotective antioxidant
effects in rat models of AD (5).
Moreover, variants of presenilin (PSEN), including PSEN1 and PSEN2,
are associated risk factors of AD (6). Therefore, studies on AD biomarkers
have become a hot topic in recent years. It has been shown that
increased levels of neurofilament and neurogranin in the
cerebrospinal fluid of patients with AD had the potential to
predict the progression of AD (7).
It has also been found that an increase or decrease in the
expression levels of FOXO3A, lysosomal proteins and flotillin in
the serum may act as novel diagnostic markers of AD (8-10).
At present, only five drugs have been approved for the treatment of
AD, and these drugs can only improve the clinical symptoms in
patients with AD and not delay the progression of the disease
(11,12). Therefore, identifying effective
biomarkers is essential for early clinical diagnosis and guidance
of drug therapy for patients with AD.
MicroRNAs (miRNA/miR) are a class of endogenous
small non-coding RNAs. The function of miRNAs is to regulate gene
expression by inhibiting the translation or promoting the
degradation of target mRNAs (13).
There are abundant and stable miRNAs in the blood, cerebrospinal
fluid and other body fluids (14).
Yang et al (15)
demonstrated that serum exosome miR-135a and miR-384 were
upregulated in patients with AD. The expression levels of miR-1291
and miR-597-5p were also increased in the cerebrospinal fluid of
patients with AD (16). Recent
studies have shown that some miRNAs in body fluids are
overexpressed or expressed at low levels in numerous
neurodegenerative diseases, such as AD, suggesting that miRNAs in
body fluids could be used as novel biomarkers for the early
diagnosis of these neurodegenerative diseases. The expression
levels of miR-29a and miR-29c were significantly decreased in the
serum of patients with Parkinson's disease (PD) and were negatively
associated with the severity of PD (17). Dobrowolny et al (18) reported that serum miR-423-3p and
miR-151a-5p were significantly downregulated in mild and terminal
stages of the amyotrophic lateral sclerosis. Zhang et al
(19) found that the expression
level of serum miR-128 was increased in patients with AD, and this
may serve as a promising diagnostic biomarker of AD. MiR-34c played
important roles in the decline of synaptic function and memory
impairment via the SYT1/ROS-JNK-p53 pathway (20). Hou et al (21) reported that miR-124 promoted tau
protein phosphorylation, inducing the occurrence of AD. It has also
been confirmed that miRNA-22 could inhibit the release of
inflammatory cytokines and improve the cognitive ability in a mouse
model of AD (22). Increased mRNA
expression levels of miR-326 in a mouse model of AD reduced the
deposition of Aβ, and the contents of Aβ1-40 and Aβ1-42(23).
Results of a previous study demonstrated that
miR-4722-5p and miR-615-3p were AD-related biological markers from
the analysis of AD samples at different stages of the disease
(24). However, to the best of our
knowledge, the mRNA expression levels and functional roles of
miR-4722-5p and miR-615-3p in AD have not been reported. In the
present study, the mRNA expression levels of miR-4722-5p and
miR-615-3p in the serum of patients with AD were analyzed, and an
Aβ25-35-induced PC12 cell model was established to investigate
their value as potential biomarkers for AD. This could provide a
new theoretical basis and research direction for the early
diagnosis, and further treatment of AD.
Materials and methods
Demographic data and clinical
characteristics
A total of 33 patients with AD were recruited from
the Department of Neurology, the Affiliated Hospital of Jiangsu
University (Jiangsu, China), between November 2019 and June 2021.
The patients with AD were diagnosed according to the 1984 National
Institute of Neurological and Communicative Disorders and Stroke,
and the AD and Related Disorders Association criteria (25). A total of 33 healthy volunteers,
who were matched for age and sex, were admitted to the Health
Examination Center of the Affiliated Hospital of Jiangsu University
(Jiangsu, China) at the same period as previously described for
patients with AD. Patient characteristics, including sex, age,
years spent in education, and dementia risk factors, such as
hypertension, diabetes, hyperhomocysteinemia, total cholesterol,
low-density lipoprotein cholesterol, vitamin B12 and the folic acid
of patients were determined using medical history and blood tests.
Power analysis and sample size software (v15; NCSS, LLC) was used
for sample size estimation to ensure the significance and
reliability of the results. The mini-mental state examination
(MMSE) scale is the most widely used cognitive function screening
scale in clinical practice, and it can be used to assess the degree
of cognitive impairment in patients with AD (26). The severity of AD, according to the
MMSE scores, was defined as follows: Mild dementia (21≤ MMSE scores
≤26), moderate dementia (15≤ MMSE scores ≤20) and severe dementia
(MMSE scores <15) (27). The
MMSE scores have been associated with literacy levels and are
divided according to literacy levels (illiterate, ≤17; primary
school, ≤20; junior high school or above, ≤24) (28). The clinical characteristics and
demographic data of the patients with AD and the volunteers are
shown in Table I. The present
study was approved by the Scientific Research Ethics Committee of
the Affiliated Hospital of Jiangsu University (Jiangsu, China), and
written informed consent was obtained from each participant.
 | Table IComparisons of clinical
characteristics and demographic data between patients with AD and
the healthy controls. |
Table I
Comparisons of clinical
characteristics and demographic data between patients with AD and
the healthy controls.
| Characteristic | Healthy controls
(n=33) | Patients with AD
(n=33) | F | Χ² | Z | P-value |
|---|
| Mean age ± SD,
years | 42±6.85 | 73.58±8.41 | 0.487 | N/A | N/A | 0.331 |
| Female, n (%) | (57.58) | 16 (48.48) | N/A | 0.547 | N/A | 0.459 |
| Male, n (%) | (42.42) | 17 (51.52) | | | | |
| Education, n
(%) | | | | | | |
|
High school
and above | (15.15) | 1 (3.03) | 2.889 | N/A | N/A | 0.089 |
|
Junior high
school and above | (84.85) | 32 (96.97) | | | | |
| Hypertension, n
(%) | (54.55) | 19 (57.58) | N/A | 0.062 | N/A | 0.804 |
| Diabetes, n
(%) | (39.40) | 15 (45.45) | N/A | 0.248 | N/A | 0.618 |
| HHcy, n (%) | (48.48) | 11 (33.33) | N/A | 0.262 | N/A | 0.211 |
| Mean ± SD total
cholesterol, mmol/l | 86±0.95 | 4.52±0.98 | 0.048 | N/A | N/A | 0.169 |
| Mean ± SD LDL-C,
mmol/l | 88±0.70 | 2.62±0.82 | 0.856 | N/A | N/A | 0.178 |
| Mean ± SD vitamin
B12, pg/ml | 36±206.93 | 401.33±161.71 | 0.878 | N/A | N/A | 0.712 |
| Mean ± SD folic
acid, ng/ml | 6.09±2.65 | 5.09±1.91 | 2.667 | N/A | N/A | 0.083 |
| Mean (IQR) MMSE
scores | 28 (27-30) | 16 (12-17) | N/A | N/A | -7.017 | <0.001 |
Serum sample collection
Venous blood samples were collected from all
subjects after fasting for at least 8 h and immediately placed at
4˚C for 1 h. The upper serum was separated by centrifugation for 15
min at 1,500 x g and 4˚C, and stored at -80˚C until further
analysis.
Cell culture and treatment
The rat pheochromocytoma cell line, PC12 was
purchased from the Shanghai Institute of Cell Biology, Chinese
Academy of Sciences. The cell line was cultured at 37˚C in a
humidified incubator containing 5% CO2, and maintained
in high-glucose DMEM (Gibco; Thermo Fisher Scientific, Inc.),
supplemented with 10% FBS (Gibco; Thermo Fisher Scientific, Inc.)
and 1% penicillin-streptomycin (Invitrogen; Thermo Fisher
Scientific, Inc.). When the PC12 cells reached 90% confluence, the
cells were washed with PBS (HyClone; Cytiva) and then collected
with Trypsin-EDTA Solution (Biosharp, China). The cells were
subsequently seeded in a 6-well plate at a density of
5x105 cells each well, and treated with Aβ25-35 (30
µmol/l; 24 h; Sigma-Aldrich; Merck KGaA) to induce cell damage.
Aβ25-35 was dissolved in PBS (HyClone; Cytiva) and maintained at
37˚C for 7 days to allow for fibril formation before use.
RNA extraction and reverse
transcription-quantitative PCR (RT-qPCR)
Serum RNA was extracted using RNApure Circulating
Reagent (CoWin Biosciences), while cellular RNA was isolated using
a RNA-Quick Purification kit (ESscience Biotech), according to the
manufacturer's instructions. The purity and concentration of the
RNA was measured using a Titertek Berthold Micro Spectrophotometer
(Berthold Technologies GmbH and Co. KG), and the absorbance ratio
of A260/A280 between 1.8 and 2.2 represented high purity. The cDNA
synthesis reaction was conducted using the miRNA 1st Strand cDNA
Synthesis kit (by Stem-Loop) (Vazyme Biotech Co., Ltd) and the
reaction conditions of reverse transcription were 25˚C for 5 min,
50˚C for 15 min and 85˚C for 5 min. The ChamQ SYBR qPCR Master Mix
kit (Vazyme Biotech Co., Ltd) and a QuantStudio5 Real-Time PCR
System (Thermo Fisher Scientific, Inc.) were used to amplify the
cDNA, which was conducted in a 96-well plate. Each sample was
performed with 3 duplicate wells and U6 served as the internal
reference control. The following thermocycling conditions were
used: Initial denaturation at 95˚C for 30 sec; followed by 40
cycles at 95˚C for 10 sec, 56˚C for 30 sec and 72˚C for 60 sec; and
final extension at 95˚C for 15 sec, 60˚C for 60 sec and 95˚C for 15
sec. The relative mRNA expression levels of the miRNA were
calculated using the 2-ΔΔCq method (29). All the primers were designed and
synthesized by Sangon Biotech Co., Ltd., using stem-loop methods.
Universal reverse primers (URP) were used as the reverse primers
for miR-4722-5p and miR-615-3p. The following specific sequences
were used for RNA extracted from cells and serum: miR-4722-5p
forward, 5'-GGCAGGAGGGCTGTGCC-3'; URP, 5'-AGTGCAGGGTCCGAGGTATT-3';
U6 forward, 5'-AGAGAAGATTAGCATGGCCCCTG-3', and reverse,
5'-ATCCAGTGCAGGGTCCGAGG-3'. The forward sequences,
5'-CGTCCGAGCCTGGGTCTC-3' and 5'-GGGGGTCCCCGGTGCT-3' were used for
serum and cell samples, respectively.
miRNA target gene prediction
miRWalk version 3.0 (http://mirwalk.umm.uni-heidelberg.de/) is a
comprehensive database to predict the target genes of miRNAs, and
contains information of miRNA target genes from humans, mice, rats
and several other species. For example, the sequences of miR-211
from different species, including humans, mice, rats, fish, dogs
and cows can be found in the miRWalk database. The target genes of
miR-4722-5p and miR-615-3p were predicted using the miRWalk
database. The potential target genes were screened under the
following conditions: miRNA binding region 3'-untranslated region
scores ≥1.
Gene Ontology (GO) and Kyoto
Encyclopedia of Genes and Genomes (KEGG) enrichment analyses of the
miRNA target genes
GO and KEGG enrichment analyses of the miRNA target
genes were performed using The Database for Annotation,
Visualization and Integrated Discovery Bioinformatics Resources
database version 6.8 (https://david.ncifcrf.gov/). The R language analysis
package was used to display the top 10 GO and the top 15 KEGG
enrichment pathway information. The significant difference of
enrichment analysis was set as P<0.05.
Statistical analysis
SPSS version 22.0 software (IBM Corp.) and GraphPad
Prism version 8.0.2 software (GraphPad Software, Inc.) were used
for statistical analysis. Numerical data are presented as a number
or percentage, and either a χ2 test or Fisher's exact
test was used for comparison between patients with AD and the
healthy controls. Continuous data are expressed as the mean ± SD or
the median (interquartile range), and the difference between
patients with AD and healthy controls was analyzed using either a
t-test or the Mann-Whitney U test. The Spearman correlation
coefficient was used to analyze the correlation between the
expression of the two miRNAs and MMSE scores. A receiver operating
characteristic curve (ROC) was used to evaluate the specificity and
sensitivity of miR-4722-5p and miR-615-3p in AD. A binary logistic
regression model was used to predict the diagnostic value for AD by
combining the two miRNAs. P<0.05 was considered to indicate a
statistically significant difference.
Results
Clinical characteristics and
demographic data
A total of 33 patients with AD (17 males and 16
females) and 33 healthy individuals (14 males and 19 females) were
recruited into the present study. The average age of the patients
with AD was 73.58±8.41 years, while that in the healthy individuals
was 75.42±6.85 years. The two groups exhibited no significant
differences in sex, age or years spent in education, and exhibited
no significant differences in dementia risk factors, including
hypertension, diabetes, hyperhomocysteinemia, total cholesterol,
low-density lipoprotein cholesterol, vitamin B12 or folic acid
(P>0.05), but there were statistically significant differences
between the two groups in MMSE scores (P<0.001) (Table I).
Relative expression level of
miR-4722-5p and miR-615-3p in patients with AD
The melting curve of RT-qPCR was unimodal, which
ensured the specificity of the amplified products. The results
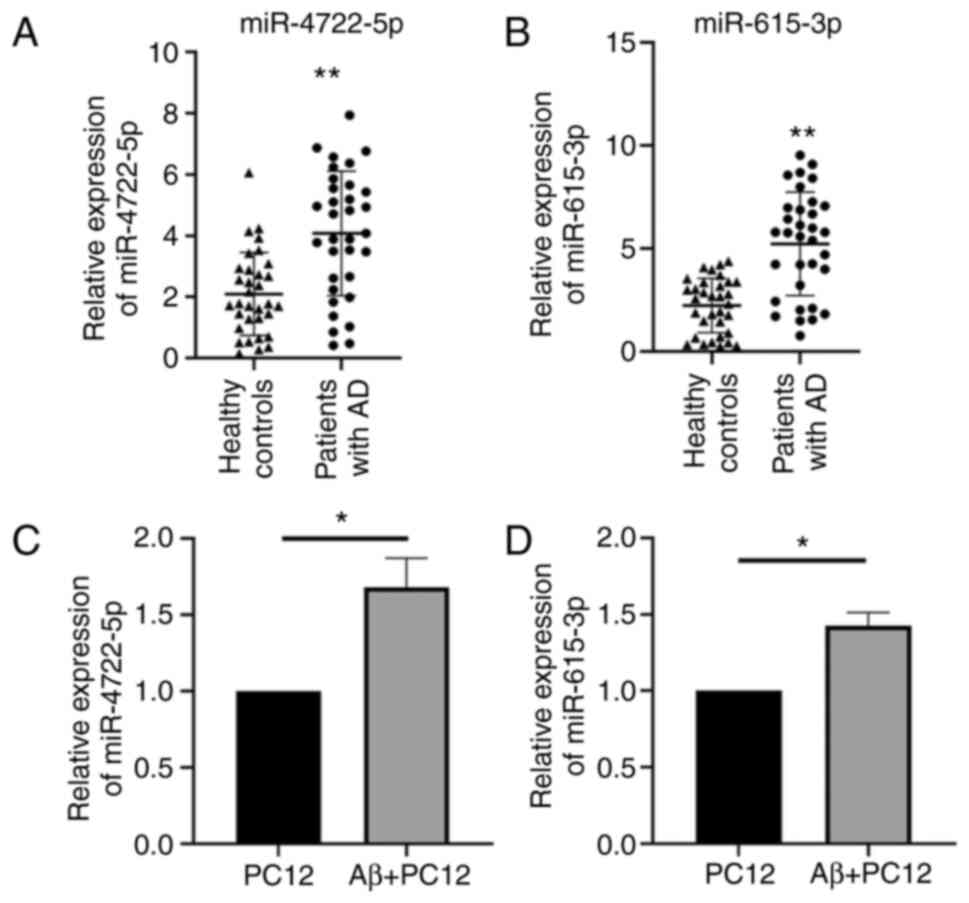
showed that the mRNA expression levels of miR-4722-5p (Z, -3.918)
and miR-615-3p (Z, -4.675) in the patients with AD were both higher
compared with that in the healthy controls (P<0.001) (Fig. 1A and B). The mRNA expression levels of
miR-4722-5p (t, 6.169) and miR-615-3p (t, 8.345) in the
Aβ25-35-treated PC12 cells were also higher compared with that in
the control group (P<0.05) (Fig.
1C and D).
Correlations between the relative mRNA
expression levels of miR-4722-5p and miR-615-3p, and MMSE
scores
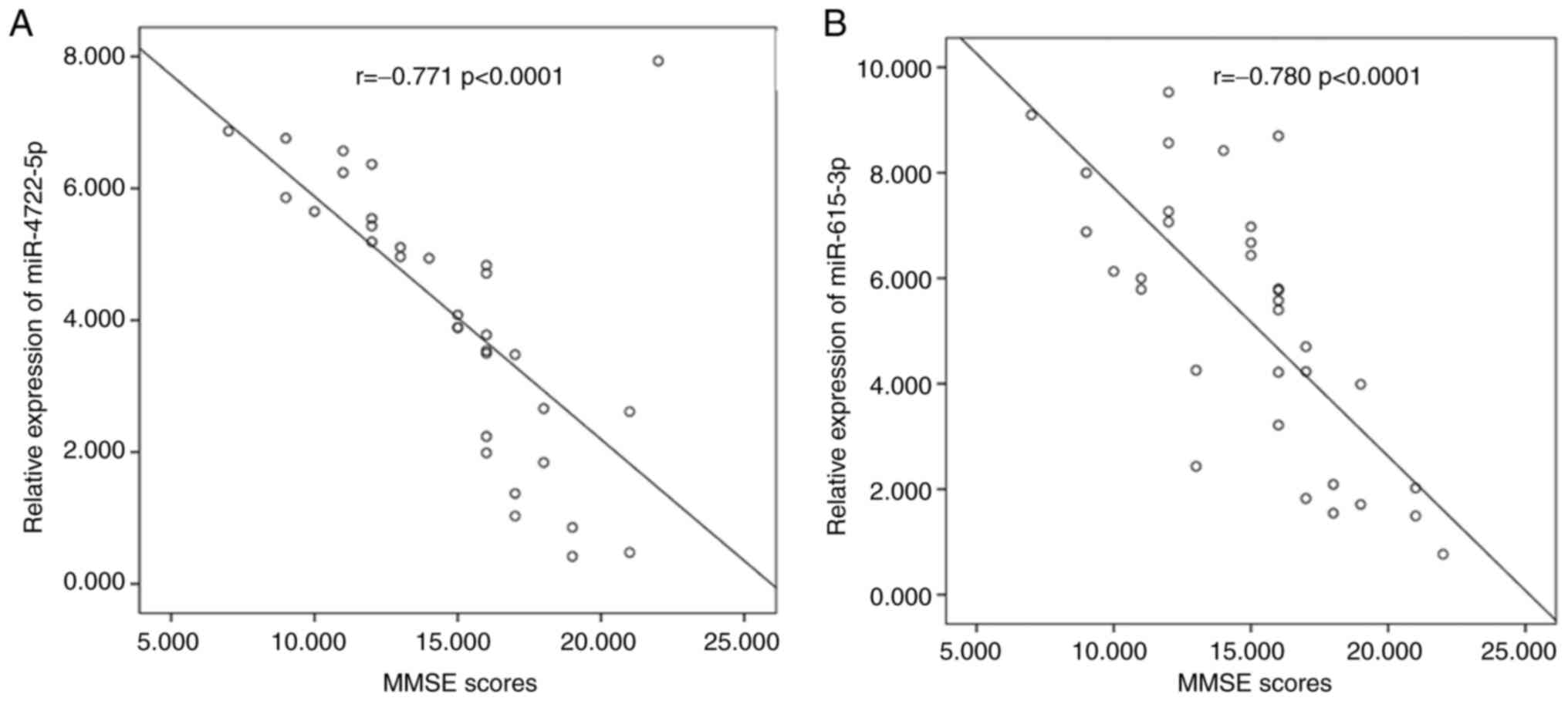
The relative mRNA expression levels of serum
miR-4722-5p and miR-615-3p were negatively correlated with MMSE
scores according to the analysis of Spearman's correlation
coefficient. The relative expression levels of serum miR-4722-5p
(r, -0.771; P<0.0001; Fig. 2A)
and miR-615-3p (r, -0.780; P<0.0001; Fig. 2B) were higher in patients with AD
and lower MMSE scores.
Diagnostic power of miR-4722-5p and
miR-615-3p in patients with AD
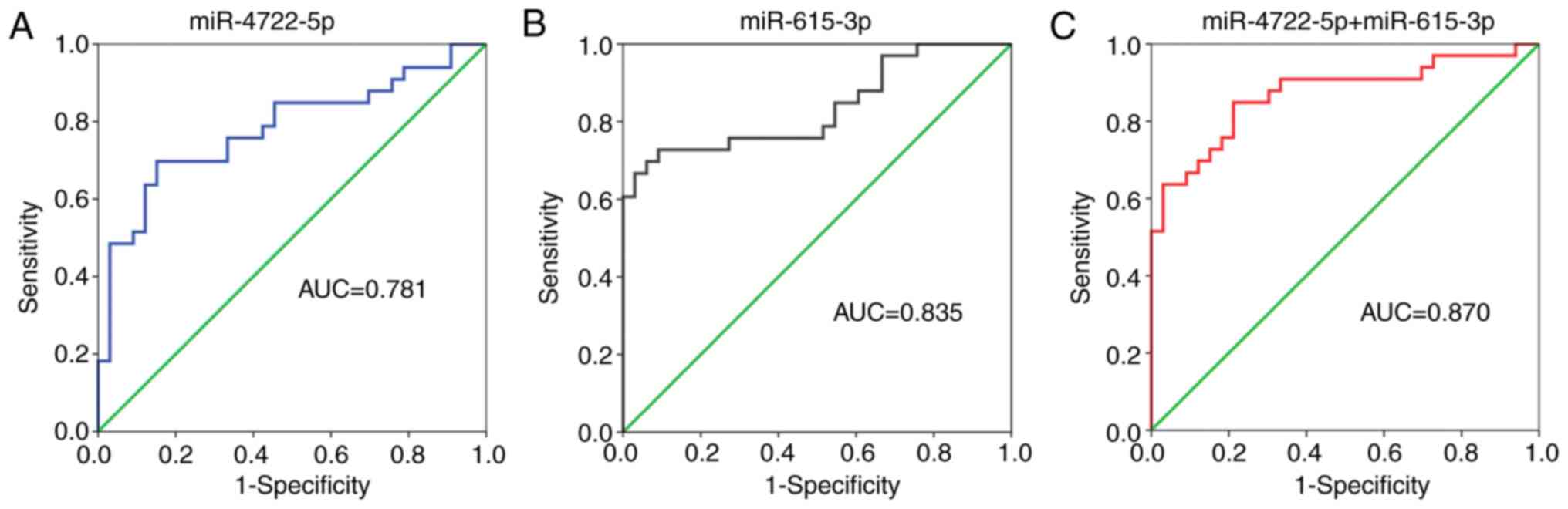
The ROC curve is mainly used to evaluate the
diagnostic value of a certain index to obtain the best index
threshold (30). The area under
the curve (AUC) of serum miR-4722-5p was 0.781, the sensitivity was
0.697 and the specificity was 0.848 (Fig. 3A). The AUC of serum miR-615-3p was
0.835, the sensitivity was 0.727 and the specificity was 0.909
(Fig. 3B). The logistic regression
model showed that the AUC of both miRNAs combined was 0.870, the
sensitivity was 0.697 and the specificity was 0.939 (Fig. 3C). The sensitivity and specificity
of miR-615-3p for the diagnosis of AD were higher compared with
that in miR-4722-5p for the diagnosis of AD. In addition, the
specificity of the two miRNAs combined for AD was higher compared
with that for each miRNA alone. The results of the ROC curve
analysis are shown in Table
II.
 | Table IIResults of receiver operating
characteristic curve analysis. |
Table II
Results of receiver operating
characteristic curve analysis.
| miRNA | AUC (95% CI) | Sensitivity | Specificity | Cut-off value | P-value |
|---|
| miR-4722-5p | 0.781
(0.666-0.895) | 0.697 | 0.848 | 3.455 | <0.001 |
| miR-615-3p | 0.835
(0.734-0.935) | 0.727 | 0.909 | 3.976 | <0.001 |
| miR-4722-5p and
miR-615-3p | 0.870
(0.780-0.959) | 0.697 | 0.939 | 0.615 | <0.001 |
GO enrichment analysis of the miRNA
target genes
GO is a database established by the Association of
Gene Ontology Consortium and includes three main categories:
Biological process (BP), cellular component (CC) and molecular
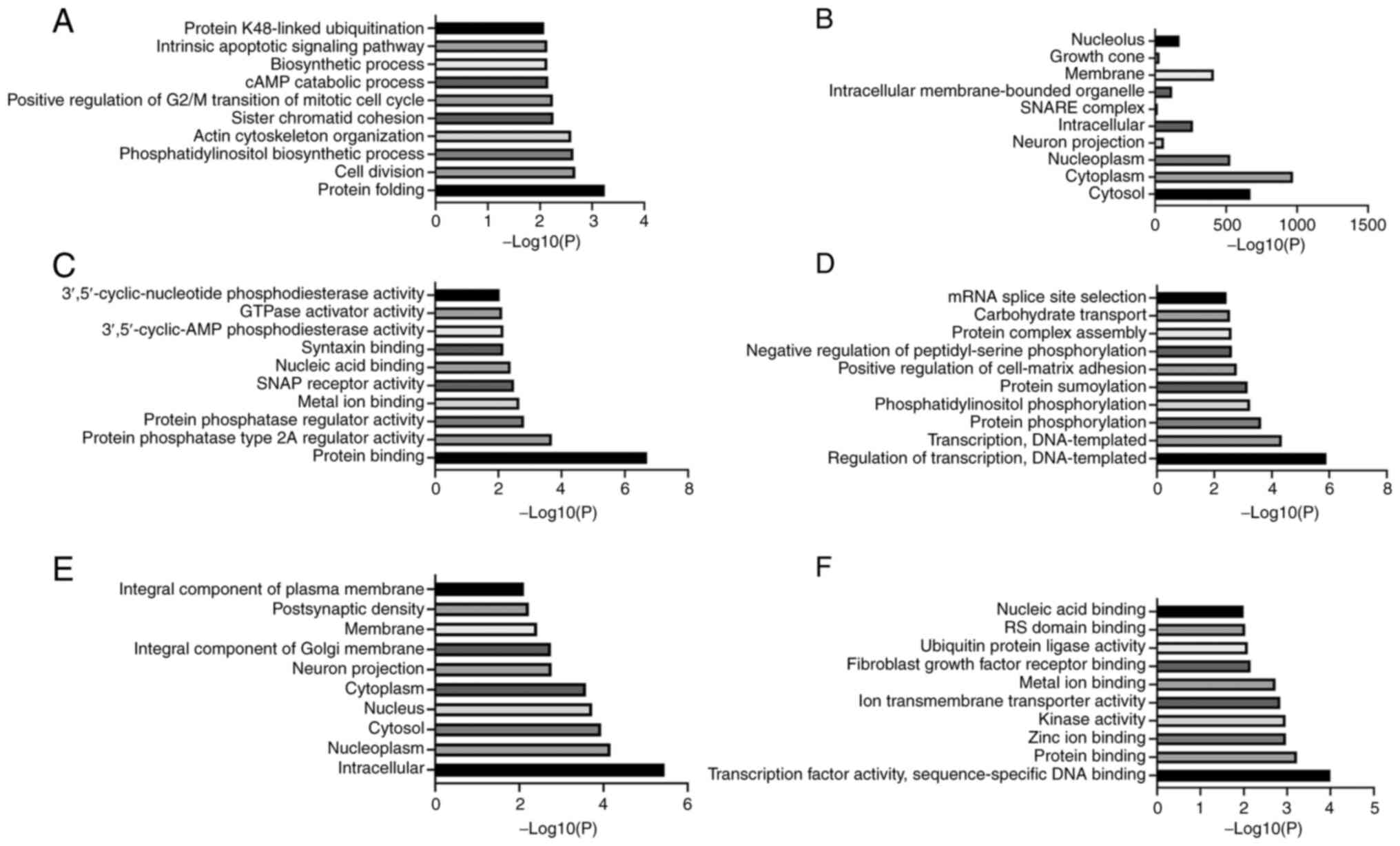
function (MF) (31). BP analysis
of the target genes of miR-4722-5p showed that these genes were
mainly involved in ‘cell division’, ‘protein folding’, ‘sister
chromatid cohesion’, ‘actin cytoskeleton organization’ and ‘cAMP
catabolic process’ (Fig. 4A). CC
analysis showed that the target genes of miR-4722-5p were mainly
located in the ‘cytoplasm’, ‘cytosol’, ‘nucleoplasm’ and ‘membrane’
(Fig. 4B). MF analysis indicated
that the target genes of miR-4722-5p performed the functions of
‘protein binding’, ‘metal ion binding’, ‘nucleic acid binding’ and
‘GTPase activator activity’ (Fig.
4C). BP analysis of the target genes of miR-615-3p indicated
that these genes mainly participated in the ‘regulation of
transcription, DNA-templated’, ‘protein phosphorylation’, ‘protein
sumoylation’ and ‘protein complex assembly’ (Fig. 4D). The target genes of miR-615-3p
were found in the ‘nucleus’, ‘cytoplasm’, ‘cytosol’ and
‘nucleoplasm’ (Fig. 4E) and played
roles in ‘protein binding’, ‘metal ion binding’, ‘zinc ion binding’
and ‘transcription factor activity, sequence-specific DNA binding’
(Fig. 4F).
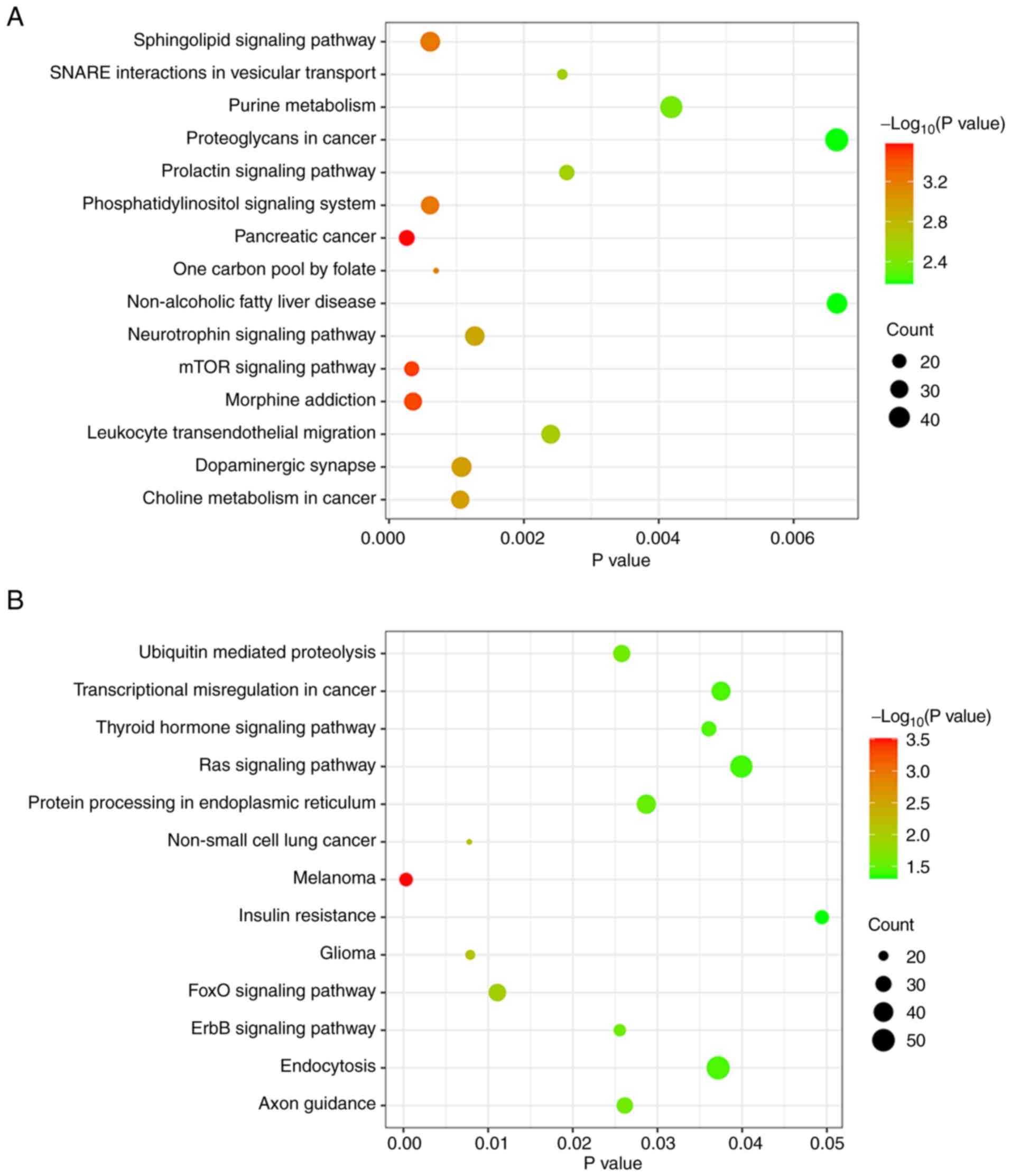
KEGG enrichment analysis of miRNA
target genes
KEGG is a database integrating genomic, chemical and
system functional information (32). KEGG enrichment analysis of the
target genes for miR-4722-5p showed that these genes were mainly
involved in ‘proteoglycans in cancer’, ‘purine metabolism’,
‘neurotrophin signaling pathway’ and ‘mTOR signaling pathway’
(Fig. 5A). The target genes for
miR-615-3p were associated with ‘endocytosis’, ‘insulin
resistance’, ‘Ras signaling pathway’ and ‘FoxO signaling pathway’
(Fig. 5B).
Discussion
AD is a chronic progressive neurodegenerative
disease that affects ~47 million people worldwide, and that number
is expected to increase by 62% by 2030(33). It has been hypothesized that the Aβ
waterfall theory (34), the tau
protein theory (35), oxidative
stress (36), inflammatory
mechanisms (37), mitochondrial
dysfunction (38) and other
theories are involved in the pathogenesis of AD. An increase in Aβ
levels in the brain may lead to Aβ aggregation into oligomers, thus
initiating a series of events leading to cell dysfunction and death
(39). Abnormal phosphorylation,
aggregation and proteolysis of the tau protein in a pre-tangle
stage of neurofibrillary degeneration has also been proved to be an
early and crucial event in the pathogenesis of AD (40). It has been found that reduction or
loss of trigger receptor expressed on myeloid cells 2 function in a
mouse model of tauopathy was neuroprotective, reducing gliosis and
neuroinflammation (41). The
clinical diagnosis of AD mainly depends on the clinical
manifestations of the patient, neuropsychological scales, genetic
testing and imaging techniques (42). Studies have shown that Aβ-positron
emission tomography (PET) has a certain value in the diagnosis of
AD (43). However, Aβ-PET has not
been widely used in the diagnosis of AD in a clinical setting as
the method for analyzing the fluorodeoxyglucose-PET data has not
reached the same degree of standardization and the cost is high. It
has also been confirmed that Aβ42, the ratio of Aβ42/Aβ40, total
tau protein and phosphorylated tau protein in the cerebrospinal
fluid are important biomarkers for the diagnosis of AD (44). However, lumbar puncture is an
invasive examination (45) and it
is difficult to obtain the cooperation of the patient or the family
members. miRNAs are non-invasive biomarkers and their specific
expressions have been associated with the pathogenesis of AD
(46). However, finding miRNAs
expressed only in the serum of patients with AD is not easy.
In the present study, the mRNA expression levels of
miR-4722-5p and miR-615-3p in the serum of patients with AD, and
the Aβ25-35-induced PC12 cell model were higher compared with that
in the control groups. GO analysis indicated that the target genes
of miR-4722-5p might be involved in the cAMP catabolic process.
cAMP produced by ATP from the adenylate cyclase family is the
second messenger required for long-term enhancement and memory
consolidation (47,48). Nassireslami et al (49) demonstrated that the upregulation of
cAMP analogues activated the cAMP/PKA signaling pathway, and
improved synaptic plasticity and memory deficits. In addition, the
cAMP/PKA signaling pathway can reduce the production of tau protein
and improve cognitive deficits (50). KEGG analysis of the target genes
for miR-4722-5p revealed that they were associated with the mTOR,
neurotrophin, prolactin, ‘SNARE interactions in vesicular
transport’, ‘proteoglycans in cancer’, ‘non-alcoholic fatty liver
disease’, ‘purine metabolism’, ‘pancreatic cancer’, ‘morphine
addiction’, phosphatidylinositol, sphingolipid, ‘one carbon pool by
folate’, ‘leukocyte transendothelial migration’, ‘choline
metabolism in cancer’ and ‘dopaminergic synapse’. mTOR is the main
regulator of autophagy and plays an important role in
neurodegenerative diseases (51,52).
mTOR inhibitors, including rapamycin, have been shown to be
effective in improving cognitive deficits in AD (53). Therefore, it was hypothesized that
miR-4722-5p might regulate the mTOR pathway, inducing the
occurrence of AD.
It has been confirmed that miR-615-3p was associated
with the occurrence and development of numerous diseases, such as
esophageal, gastric and non-small lung cancers. The expression of
miR-615-3p was upregulated in the brain of patients with
Huntington’s disease, and was associated with the pathogenesis of
HD (54). Miyamoto et al
(55) found that the enhancement
of miR-615-3p expression levels might be of therapeutic benefit for
nonalcoholic fatty liver disease by inhibiting palmitate-induced
hepatocyte lipoapoptosis. Feng et al (56) demonstrated that miR-615-3p could
inhibit the apoptosis of epileptiform hippocampal neurons via the
PI3K/Akt/mTOR pathway. GO analysis of the target genes for
miR-615-3p was associated with BP, such as transcription, ‘protein
phosphorylation’, ‘protein complex assembly’, ‘phosphatidylinositol
phosphorylation’, ‘protein sumoylation’, ‘positive regulation of
cell-matrix adhesion’, ‘negative regulation of peptidyl-serine
phosphorylation’, ‘carbohydrate transport’, ‘mRNA splice site
selection’ and other biological processes. Wang et al
(57) reported that miR-615-3p
promoted gastric cancer proliferation and migration by suppressing
the expression of CUGBP- and ETR-3-like family 2. KEGG analysis
demonstrated that its target genes were engaged in regulating FoxO,
ErbB, melanoma, ‘non-small cell lung cancer’, glioma, ‘ubiquitin
mediated proteolysis’, ‘axon guidance’, ‘protein processing in
endoplasmic reticulum’, ‘thyroid hormone’, endocytosis,
‘transcriptional misregulation in cancer’, ‘insulin resistance’ and
Ras signaling pathway. FoxO transcription factors have been
associated with nerve cell survival, and neuronal signal
transmission exists in the hippocampus, amygdala and nucleus
accumbens (58,59). FoxO transcription factors have also
been identified as potential targets for a variety of
neurodegenerative diseases, such as AD, Parkinson's disease and
Huntington's disease (60). Maiese
(61) demonstrated that FoxO
transcription factors could not only promote apoptotic cell death
in the nervous system but also offer protection against
degenerative disease through the induction of autophagy that could
lead to dementia. In some circumstances, Aβ could induce the
dephosphorylation and mitochondrial translocation of FoxO3a leading
to mitochondrial dysfunction (62). Increased activity of FoxO could
result in the apoptosis and autophagy in Aβ-induced neuron death
(63). EGFR, a transmembrane
glycoprotein, is a member of the ErbB receptor tyrosine kinase
superfamily (64). Stupack et
al (65) found that SORLA, a
transmembrane transporter associated with the risk of AD, promoted
neurite regeneration by activating EGF receptors. Mansour et
al (66) proposed that EGFR
inhibitors had neuroprotective effects on AD models. Therefore,
miR-615-3p may be crucial to the pathogenesis of AD by regulating
the FoxO and ErbB signaling pathways.
The present study is preliminary and some
limitations must be acknowledged. Firstly, the basic information of
the individuals recruited into the study were matched as best as
possible, including sex and age to minimize the influence of
confounding factors. Nevertheless, the results may be limited due
to the small sample size, different lifestyles of the patients,
genetic variation and external effects, such as regional
differences among patients with AD. Replication of the results in
further studies with larger sample sizes is required. Secondly,
Parkinson's disease is another well-known neurodegenerative
disease. Therefore, it is necessary to detect the expression levels
of miR-4722-5p and miR-615-3p in patients with Parkinson's disease
in future studies. Moreover, results of the present study
demonstrated that miR-4722-5p and miR-615-3p are highly expressed
in the AD cell model and the serum of patients with ADs. Thus,
animal models will be used in future investigations to verify the
conclusions of the present study, and to investigate the specific
mechanisms underlying miR-4722-5p and miR-615-3p in AD. In
addition, previous studies have demonstrated that the sequences of
most miRNAs are highly conserved among different species (67,68).
To date, the rat sequence of miR-4722-5p has not been published in
the database; therefore, human primers were used for RT-qPCR from
RNA extracted from the PC12 cell line. Finally, the mRNA expression
levels of miR-495-3p in the serum of patients with AD and healthy
controls showed no statistically significant difference in our
previous experiment (data not published). However, there was no
relevant reports on the mRNA expression levels of miR-495-3p in
patients with AD in current studies. Eun et al (69) demonstrated that the overexpression
of miR-495-3p could cause the death of gastric cancer cells.
Further investigation into other miRNAs in the serum of patients
with AD and healthy controls will be a new goal for future
research.
In summary, it was first confirmed that the mRNA
expression levels of miR-4722-5p and miR-615-3p were increased in
patients with AD, and the Aβ25-35-induced PC12 cell model compared
with that in the control groups in the present study. Furthermore,
MMSE scores were negatively correlated with the mRNA expression
levels of the two miRNAs. These findings suggest that miR-4722-5p
and miR-615-3p may be potential biomarkers for the diagnosis of AD;
however, the specific mechanism requires further investigation.
Acknowledgements
The authors would like to thank Professor Ming Yu
(Chief Physician at the Department of Neurology, the Affiliated
Hospital of Jiangsu University) and Yuhao Xu (Resident Physician at
the Department of Neurology, the Affiliated Hospital of Jiangsu
University) for their guidance on the design of the experimental
scheme and the revision of the paper.
Funding
Funding: This study was supported by the Zhenjiang Key Research
and Development Plan (Social Development) (grant no.
SH2019036).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
MY and YL conceived and designed the study. YL and
YX completed all the experiments and analyzed the experimental
data. MY, YL and YX wrote and revised the manuscript. MY and YL
confirmed the authenticity of all the raw data. All authors read
and approved the final manuscript.
Ethics approval and consent to
participate
The study was reviewed and approved by the
Scientific Research Ethics Committee of the Affiliated Hospital of
Jiangsu University (Jiangsu, China), and written informed consent
was provided by each participant.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Eratne D, Loi SM, Farrand S, Kelso W,
Velakoulis D and Looi JC: Alzheimer's disease: Clinical update on
epidemiology, pathophysiology and diagnosis. Australas Psychiatry.
26:347–357. 2018.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Di Resta C and Ferrari M: New molecular
approaches to Alzheimer's disease. Clin Biochem. 72:81–86.
2019.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Takeda S: Progression of Alzheimer's
disease, tau propagation, and its modifiable risk factors. Neurosci
Res. 141:36–42. 2019.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Jiang L, Dong H, Cao H, Ji X, Luan S and
Liu J: Exosomes in pathogenesis, diagnosis, and treatment of
Alzheimer's Disease. Med Sci Monit. 25:3329–3335. 2019.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Tian X, Wang J, Dai J, Yang L, Zhang L,
Shen S and Huang P: Hyperbaric oxygen and Ginkgo Biloba extract
inhibit Aβ25-35-induced toxicity and oxidative stress in vivo: A
potential role in Alzheimer's disease. Int J Neurosci. 122:563–569.
2012.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Karch CM, Cruchaga C and Goate AM:
Alzheimer's disease genetics: from the bench to the clinic. Neuron.
83:11–26. 2014.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Pereira JB, Westman E and Hansson O:
Association between cerebrospinal fluid and plasma
neurodegeneration biomarkers with brain atrophy in Alzheimer's
disease. Neurobiol Aging. 58:14–29. 2017.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Pradhan R, Yadav SK, Prem NN, Bhagel V,
Pathak M, Shekhar S, Gaikwad S, Dwivedi SN, Bal CS, Dey AB and Dey
S: Serum FOXO3A: A ray of hope for early diagnosis of Alzheimer's
disease. Mech Ageing Dev. 190(111290)2020.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Goetzl EJ, Boxer A, Schwartz JB, Abner EL,
Petersen RC, Miller BL and Kapogiannis D: Altered lysosomal
proteins in neural-derived plasma exosomes in preclinical Alzheimer
disease. Neurology. 85:40–47. 2015.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Abdullah M, Kimura N, Akatsu H, Hashizume
Y, Ferdous T, Tachita T, Iida S, Zou K, Matsubara E and Michikawa
M: Flotillin is a novel diagnostic blood marker of Alzheimer's
Disease. J Alzheimers Dis. 72:1165–1176. 2019.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Briggs R, Kennelly SP and O'Neill D: Drug
treatments in Alzheimer's disease. Clin Med (Lond). 16:247–253.
2016.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Sancesario GM and Bernardini S:
Alzheimer's disease in the omics era. Clin Biochem. 59:9–16.
2018.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Serpente M, Fenoglio C, D'Anca M, Arcaro
M, Sorrentino F, Visconte C, Arighi A, Fumagalli GG, Porretti L,
Cattaneo A, et al: MiRNA profiling in plasma neural-derived small
extracellular vesicles from patients with Alzheimer's Disease.
Cells. 9(1443)2020.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Silvestro S, Bramanti P and Mazzon E: Role
of miRNAs in Alzheimer's Disease and possible fields of
application. Int J Mol Sci. 20(3979)2019.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Yang TT, Liu CG, Gao SC, Zhang Y and Wang
PC: The serum exosome derived MicroRNA-135a, -193b, and -384 Were
potential Alzheimer's Disease Biomarkers. Biomed Environ Sci.
31:87–96. 2018.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Lusardi TA, Phillips JI, Wiedrick JT,
Harrington CA, Lind B, Lapidus JA, Quinn JF and Saugstad JA:
MicroRNAs in human cerebrospinal fluid as biomarkers for
Alzheimer's Disease. J Alzheimers Dis. 55:1223–1233.
2017.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Bai X, Tang Y, Yu M, Wu L, Liu F, Ni J,
Wang Z, Wang J, Fei J, Wang W, et al: Downregulation of blood serum
microRNA 29 family in patients with Parkinson's disease. Sci Rep.
7(5411)2017.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Dobrowolny G, Martone J, Lepore E, Casola
I, Petrucci A, Inghilleri M, Morlando M, Colantoni A, Scicchitano
BM, Calvo A, et al: A longitudinal study defined circulating
microRNAs as reliable biomarkers for disease prognosis and
progression in ALS human patients. Cell Death Discov.
7(4)2021.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Zhang M, Han W, Xu Y, Li D and Xue Q:
Serum miR-128 Serves as a potential diagnostic biomarker for
Alzheimer's Disease. Neuropsychiatr Dis Treat. 17:269–275.
2021.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Shi Z, Zhang K, Zhou H, Jiang L, Xie B,
Wang R, Xia W, Yin Y, Gao Z, Cui D, et al: Increased miR-34c
mediates synaptic deficits by targeting synaptotagmin 1 through
ROS-JNK-p53 pathway in Alzheimer's Disease. Aging Cell.
19(e13125)2020.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Hou TY, Zhou Y, Zhu LS, Wang X, Pang P,
Wang DQ, Liuyang ZY, Man H, Lu Y, Zhu LQ and Liu D: Correcting
abnormalities in miR-124/PTPN1 signaling rescues tau pathology in
Alzheimer's disease. J Neurochem. 154:441–457. 2020.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Han C, Guo L, Yang Y, Guan Q, Shen H,
Sheng Y and Jiao Q: Mechanism of microRNA-22 in regulating
neuroinflammation in Alzheimer's disease. Brain Behav.
10(e01627)2020.PubMed/NCBI View Article : Google Scholar
|
|
23
|
He B, Chen W, Zeng J, Tong W and Zheng P:
MicroRNA-326 decreases tau phosphorylation and neuron apoptosis
through inhibition of the JNK signaling pathway by targeting VAV1
in Alzheimer's disease. J Cell Physiol. 235:480–493.
2020.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Soleimani Zakeri NS, Pashazadeh S and
MotieGhader H: Gene biomarker discovery at different stages of
Alzheimer using gene co-expression network approach. Sci Rep.
10(12210)2020.PubMed/NCBI View Article : Google Scholar
|
|
25
|
McKhann G, Drachman D, Folstein M, Katzman
R, Price D and Stadlan EM: Clinical diagnosis of Alzheimer's
disease: Report of the NINCDS-ADRDA Work Group under the auspices
of department of health and human services task force on
Alzheimer's Disease. Neurology. 34:939–944. 1984.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Folstein MF, Folstein SE and McHugh PR:
‘Mini-mental state’. A practical method for grading the cognitive
state of patients for the clinician. J Psychiatr Res. 12:189–198.
1975.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Kahle-Wrobleski K, Andrews JS, Belger M,
Ye W, Gauthier S, Rentz DM and Galasko D: Dependence levels as
interim clinical milestones along the continuum of Alzheimer's
Disease: 18-Month results from the GERAS Observational study. J
Prev Alzheimers Dis. 4:72–80. 2017.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Zeng Q, Zou L, Qian L, Zhou F, Nie H, Yu
S, Jiang J, Zhuang A, Wang C and Zhang H: Expression of
microRNA-222 in serum of patients with Alzheimer's disease. Mol Med
Rep. 16:5575–5579. 2017.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) Method. Methods. 25:402–408.
2001.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Hoo ZH, Candlish J and Teare D: What is an
ROC curve? Emerg Med J. 34:357–359. 2017.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Gene Ontology Consortium. Gene ontology
consortium: Going forward. Nucleic Acids Res. 43(Database
issue):D1049–D1056. 2015.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Kanehisa M, Furumichi M, Tanabe M, Sato Y
and Morishima K: KEGG: New perspectives on genomes, pathways,
diseases and drugs. Nucleic Acids Res. 45:D353–D361.
2017.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Dos Santos Picanco LC, Ozela PF, de Fatima
de Brito Brito M, Pinheiro AA, Padilha EC, Braga FS, de Paula da
Silva CHT, Dos Santos CBR, Rosa JMC and da Silva Hage-Melim LI:
Alzheimer's Disease: A review from the pathophysiology to
diagnosis, new perspectives for pharmacological treatment. Curr Med
Chem. 25:3141–3159. 2018.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Van der Kant R, Goldstein LSB and
Ossenkoppele R: Amyloid-β-independent regulators of tau pathology
in Alzheimer disease. Nat Rev Neurosci. 21:21–35. 2020.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Wei S, Peng W, Mai Y, Li K, Wei W, Hu L,
Zhu S, Zhou H, Jie W, Wei Z, et al: Outer membrane vesicles enhance
tau phosphorylation and contribute to cognitive impairment. J Cell
Physiol. 235:4843–4855. 2020.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Bello-Medina PC, González-Franco DA,
Vargas-Rodríguez I and Díaz-Cintra S: Oxidative stress, the immune
response, synaptic plasticity, and cognition in transgenic models
of Alzheimer disease. Neurologia (Engl Ed) S0213-4853(19)30109-4,
2019 (Epub ahead of print).
|
|
37
|
Yoo SM, Park J, Kim SH and Jung YK:
Emerging perspectives on mitochondrial dysfunction and inflammation
in Alzheimer's disease. BMB Rep. 53:35–46. 2020.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Devi L, Prabhu BM, Galati DF, Avadhani NG
and Anandatheerthavarada HK: Accumulation of amyloid precursor
protein in the mitochondrial import channels of human Alzheimer's
disease brain is associated with mitochondrial dysfunction. J
Neurosci. 26:9057–9068. 2006.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Cheignon C, Tomas M, Bonnefont-Rousselot
D, Faller P, Hureau C and Collin F: Oxidative stress and the
amyloid beta peptide in Alzheimer's disease. Redox Biol.
14:450–464. 2018.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Šimić G, Babić Leko M, Wray S, Harrington
C, Delalle I, Jovanov-Milošević N, Bažadona D, Buée L, de Silva R,
Di Giovanni G, et al: Tau Protein Hyperphosphorylation and
Aggregation in Alzheimer's Disease and other Tauopathies, and
possible Neuroprotective Strategies. Biomolecules.
6(6)2016.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Leyns CEG, Ulrich JD, Finn MB, Stewart FR,
Koscal LJ, Remolina Serrano J, Robinson GO, Anderson E, Colonna M
and Holtzman DM: TREM2 deficiency attenuates neuroinflammation and
protects against neurodegeneration in a mouse model of tauopathy.
Proc Natl Acad Sci USA. 114:11524–11529. 2017.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Langbaum JB, Fleisher AS, Chen K,
Ayutyanont N, Lopera F, Quiroz YT, Caselli RJ, Tariot PN and Reiman
EM: Ushering in the study and treatment of preclinical Alzheimer
disease. Nat Rev Neurol. 9:371–381. 2013.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Niemantsverdriet E, Ottoy J, Somers C, De
Roeck E, Struyfs H, Soetewey F, Verhaeghe J, Van den Bossche T, Van
Mossevelde S, Goeman J, et al: The cerebrospinal fluid
Aβ1-42/Aβ1-40 ratio improves concordance with Amyloid-PET for
diagnosing Alzheimer's Disease in a clinical setting. J Alzheimers
Dis. 60:561–576. 2017.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Blennow K and Zetterberg H: Biomarkers for
Alzheimer's disease: Current status and prospects for the future. J
Intern Med. 284:643–663. 2018.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Cao F, Liu Z and Sun G: Diagnostic value
of miR-193a-3p in Alzheimer's disease and miR-193a-3p attenuates
amyloid-β induced neurotoxicity by targeting PTEN. Exp Gerontol.
130(110814)2020.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Jia LH and Liu YN: Downregulated serum
miR-223 servers as biomarker in Alzheimer's disease. Cell Biochem
Funct. 34:233–237. 2016.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Ravnskjaer K, Madiraju A and Montminy M:
Role of the cAMP pathway in glucose and lipid metabolism. Handb Exp
Pharmacol. 233:29–49. 2016.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Ricciarelli R and Fedele E: cAMP, cGMP and
Amyloid β: Three ideal partners for memory formation. Trends
Neurosci. 41:255–266. 2018.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Nassireslami E, Nikbin P, Payandemehr B,
Amini E, Mohammadi M, Vakilzadeh G, Ghadiri T, Noorbakhsh F and
Sharifzadeh M: A cAMP analog reverses contextual and tone memory
deficits induced by a PKA inhibitor in Pavlovian fear conditioning.
Pharmacol Biochem Behav. 105:177–182. 2013.PubMed/NCBI View Article : Google Scholar
|
|
50
|
Myeku N, Clelland CL, Emrani S, Kukushkin
NV, Yu WH, Goldberg AL and Duff KE: Tau-driven 26S proteasome
impairment and cognitive dysfunction can be prevented early in
disease by activating cAMP-PKA signaling. Nat Med. 22:46–53.
2016.PubMed/NCBI View Article : Google Scholar
|
|
51
|
Heras-Sandoval D, Pérez-Rojas JM,
Hernández-Damián J and Pedraza-Chaverri J: The role of
PI3K/AKT/mTOR pathway in the modulation of autophagy and the
clearance of protein aggregates in neurodegeneration. Cell Signal.
26:2694–2701. 2014.PubMed/NCBI View Article : Google Scholar
|
|
52
|
Wang Y and Zhang H: Regulation of
autophagy by mTOR signaling pathway. Adv Exp Med Biol. 1206:67–83.
2019.PubMed/NCBI View Article : Google Scholar
|
|
53
|
Wang C, Yu JT, Miao D, Wu ZC, Tan MS and
Tan L: Targeting the mTOR signaling network for Alzheimer's disease
therapy. Mol Neurobiol. 49:120–135. 2014.PubMed/NCBI View Article : Google Scholar
|
|
54
|
Hoss AG, Kartha VK, Dong X, Latourelle JC,
Dumitriu A, Hadzi TC, Macdonald ME, Gusella JF, Akbarian S, Chen
JF, et al: MicroRNAs located in the Hox gene clusters are
implicated in huntington's disease pathogenesis. PLoS Genet.
10(e1004188)2014.PubMed/NCBI View Article : Google Scholar
|
|
55
|
Miyamoto Y, Mauer AS, Kumar S, Mott JL and
Malhi H: Mmu-miR-615-3p regulates lipoapoptosis by inhibiting C/EBP
homologous protein. PLoS One. 9(e109637)2014.PubMed/NCBI View Article : Google Scholar
|
|
56
|
Feng H, Gui Q, Wu G, Zhu W, Dong X, Shen
M, Fu X, Shi G, Luo H, Yang X, et al: Long noncoding RNA Nespas
inhibits apoptosis of epileptiform hippocampal neurons by
inhibiting the PI3K/Akt/mTOR pathway. Exp Cell Res.
398(112384)2021.PubMed/NCBI View Article : Google Scholar
|
|
57
|
Wang J, Liu L, Sun Y, Xue Y, Qu J, Pan S,
Li H, Qu H, Wang J and Zhang J: MiR-615-3p promotes proliferation
and migration and inhibits apoptosis through its potential target
CELF2 in gastric cancer. Biomed Pharmacother. 101:406–413.
2018.PubMed/NCBI View Article : Google Scholar
|
|
58
|
Maiese K: Forkhead transcription factors:
New considerations for alzheimer's disease and dementia. J Transl
Sci. 2:241–247. 2016.PubMed/NCBI View Article : Google Scholar
|
|
59
|
Santo EE and Paik J: FOXO in neural cells
and diseases of the nervous system. Curr Top Dev Biol. 127:105–118.
2018.PubMed/NCBI View Article : Google Scholar
|
|
60
|
Maiese K: FoxO proteins in the nervous
system. Anal Cell Pathol (Amst). 2015(569392)2015.PubMed/NCBI View Article : Google Scholar
|
|
61
|
Maiese K: Forkhead transcription factors:
Formulating a FOXO target for cognitive loss. Curr Neurovasc Res.
14:415–420. 2017.PubMed/NCBI View Article : Google Scholar
|
|
62
|
Shi C, Zhu J, Leng S, Long D and Luo X:
Mitochondrial FOXO3a is involved in amyloid β peptide-induced
mitochondrial dysfunction. J Bioenerg Biomembr. 48:189–196.
2016.PubMed/NCBI View Article : Google Scholar
|
|
63
|
Saleem S and Biswas SC: Tribbles
pseudokinase 3 induces both apoptosis and autophagy in
amyloid-β-induced neuronal death. J Biol Chem. 292:2571–2585.
2017.PubMed/NCBI View Article : Google Scholar
|
|
64
|
Seshacharyulu P, Ponnusamy MP, Haridas D,
Jain M, Ganti AK and Batra SK: Targeting the EGFR signaling pathway
in cancer therapy. Expert Opin Ther Targets. 16:15–31.
2012.PubMed/NCBI View Article : Google Scholar
|
|
65
|
Stupack J, Xiong XP, Jiang LL, Zhang T,
Zhou L, Campos A, Ranscht B, Mobley W, Pasquale EB, Xu H and Huang
TY: Soluble SORLA enhances neurite outgrowth and regeneration
through activation of the EGF Receptor/ERK signaling axis. J
Neurosci. 40:5908–5921. 2020.PubMed/NCBI View Article : Google Scholar
|
|
66
|
Mansour HM, Fawzy HM, El-Khatib AS and
Khattab MM: Potential repositioning of anti-cancer EGFR inhibitors
in Alzheimer's Disease: Current perspectives and challenging
prospects. Neuroscience. 469:191–196. 2021.PubMed/NCBI View Article : Google Scholar
|
|
67
|
Liu F, Lv Q, Du WW, Li H, Yang X, Liu D,
Deng Z, Ling W, Zhang Y and Yang BB: Specificity of miR-378a-5p
targeting rodent fibronectin. Biochim Biophys Acta. 1833:3272–3285.
2013.PubMed/NCBI View Article : Google Scholar
|
|
68
|
Kenny NJ, Sin YW, Hayward A, Paps J, Chu
KH and Hui JH: The phylogenetic utility and functional constraint
of microRNA flanking sequences. Proc Biol Sci.
282(20142983)2015.PubMed/NCBI View Article : Google Scholar
|
|
69
|
Eun JW, Kim HS, Shen Q, Yang HD, Kim SY,
Yoon JH, Park WS, Lee JY and Nam SW: MicroRNA-495-3p functions as a
tumor suppressor by regulating multiple epigenetic modifiers in
gastric carcinogenesis. J Pathol. 244:107–119. 2018.PubMed/NCBI View Article : Google Scholar
|