Introduction
The severe acute respiratory syndrome coronavirus 2
(SARS-CoV-2) virus has infected hundreds of millions of
individuals, according to the World Health Organization coronavirus
disease 2019 (COVID-19) Dashboard, and raised worldwide caution. It
is able to lead to serious lung inflammation, pneumonia, acute lung
injury (ALI) and even acute respiratory distress syndrome (ARDS) in
vulnerable individuals (1). One of
the most common target organs attacked by bacteria and viruses is
the lung (2-4),
and lung injury is frequently associated with inflammation.
Lipopolysaccharide (LPS), a vital component of the outer cell wall
of gram-negative bacteria, is thought to be one of the major causes
of inflammation (5,6). LPS is also considered the main toxic
substance damaging the lung. It usually enters the organs as part
of the bacterial outer membrane, contributing to local inflammation
and systemic toxicity (7). LPS is
closely associated with the occurrence of lung injury, which has
multiple etiologies and may result in fulminant respiratory failure
and death (8-10).
A large number of studies have confirmed that LPS induces ALI/ARDS
in animals (11). During ALI/ARDS,
the injured cells trigger a cascade of events, including acute
inflammatory response, recruitment of immune cells and release of
cytokines and chemokines (12).
A549 cells, although a lung cancer cell line, are characterized as
type II epithelial cells and frequently used as a model system for
analyzing type II epithelial cells; they have been used in research
investigating the mechanism of lung injury (13,14).
Prior to LPS being able to enter cells, it requires to be first
recognized by and bind with LPS binding protein; it is then
accepted and binds to the LPS receptor (15) molecule CD14(16). However, CD14 lacks intracellular
domains and is unable to transport signals through the cell
membrane. Rather, CD14 presents LPS to Toll-like receptor 4 (TLR4).
The compound is then bound with TLR4, which leads to the activation
of multiple intracellular signaling components, including NF-κB
(17-19).
Following its activation, NF-κB is able to enter into the nucleus
to activate the transcription and translation of proinflammatory
factors, such as IL-6, IL-8 and tumor necrosis factor (TNF)-α
(20). Overwhelming
pro-inflammatory responses are hallmarks of inflammation, which may
lead to multiple organ failure and death. SARS-CoV-2 destroys the
type II alveolar cells that secrete pulmonary surfactants and block
TLR4 in the lungs, promoting ARDS and inflammation (21). Furthermore, the levels of soluble
CD14 and TNF receptors 1 and 2 may be predictive of the risk of
death in severe COVID-19(22).
Approximately 50% of patients with COVID-19 with critical disease
die from the infection (23).
COVID-19 morbidity and mortality are also associated with
hyperinflammation (24,25). Therefore, the modulation of
CD14/TLR4-mediated LPS signaling may be an attractive target for
defending against inflammation, including SARS-Cov-2 infection.
Anesthetic agents, including propofol, are commonly
used for general anesthesia, as well as sedation in intensive care
units (ICUs). Apart from its sedative effect, 2,6-diisopropylphenol
(propofol) has been indicated to exert protective effects in
various disease models, particularly in sepsis/endotoxemia models
(26-29).
In clinical practice, patients with inflammation that end up in the
operating room or ICU are administered different anesthetics,
including propofol. Since the start of the COVID-19 pandemic, an
increasing number of patients with preexisting lung injury and
inflammation are undergoing surgery or artificial ventilation under
sedation at the ICU (30). In
clinical situations, the onset of lung injury usually occurs prior
to the administration of anesthesia or sedation to facilitate
artificial ventilation. Whether post-treatment with propofol has an
anti-inflammatory effect on these patients requires further
exploration.
Despite the increase in the understanding of its
pathophysiological processes, there are no specific pharmacological
treatments for inflammation. The aim of the present study was to
identify potential molecules that may effectively attenuate or
inhibit the inflammatory and immune responses in ALI and ARDS, and
evaluate the effect of post-treatment with propofol on the
inflammatory and immune responses.
Materials and methods
Cell culture
A549 cells (donated by the cell bank in the Central
Lab of China Medical University) were cultured in RPMI-1640 media
(Invitrogen; Thermo Fisher Scientific, Inc.) supplemented with 10%
FBS (Invitrogen; Thermo Fisher Scientific, Inc.). Cells were grown
at 37˚C in a humidified incubator with 5% CO2 and then
seeded at a density of 1x106 cells/ml. Cells were then
treated with LPS (final concentration, 1 µg/ml for 2 h; cat. no.
055:B5; L-2880; MilliporeSigma) and propofol at clinically relavant
concentrations (10, 25 and 50 µM, dissolved using 5% glucose; 3 h;
Corden Pharma Caponago S.P.A.) following cell attachment to the
bottom of the wells for 24 h. Cell viability was determined using a
trypan blue dye exclusion assay.
Protein extraction and
immunoblotting
Protein was extracted from the treated cells using a
commercially available kit (cat. no. SA4378; Nanjing Sunbio
Technology Co., Ltd). The total protein concentration was
determined using a BCA protein assay kit (cat. no. PA001-1;
Signalway Antibody LCC). Total proteins (20 µg per lane) were
separated using 10% SDS-PAGE, transferred onto a PVDF membrane
(cat. no. abs931; Absin Bioscience Inc.) and blocked for 1 h at
room temperature with 5% Difco™ Skim Milk (BD Bioscience). For
primary antibody incubation, membranes were exposed to anti-CD14
(dilution, 1:400; cat. no. sc-9150; Santa Cruz Biotechnology,
Inc.), anti-TLR4 (dilution, 1:400; cat. no. sc-10741; Santa Cruz
Biotechnology, Inc.) and anti-β-actin (dilution, 1:400; cat. no.
sc-47778; Santa Cruz Biotechnology, Inc.) antibodies overnight at
4˚C. Membranes were washed in tris-buffered saline with Tween-20
and then incubated with a horseradish peroxidase-conjugated goat
anti-rabbit IgG (dilution, 1:4,000; cat. no. ZB-2301; OriGene
Technologies, Inc.) and goat anti-mouse IgG (dilution, 1:4,000;
cat. no. ZB-2305; OriGene Technologies, Inc.) for 1 h at room
temperature. Immunoreactive bands were visualized with enhanced
chemiluminescence (Pierce; Thermo Fisher Scientific, Inc.).
Densitometry was performed using ImageJ 1.37c software (National
Institutes of Health). Western blot analysis was performed in
triplicate for each experimental condition.
Reverse transcription-quantitative PCR
(RT-qPCR)
Total RNA was extracted using TRIzol®
reagent (Thermo Fisher Scientific, Inc.). Next, total RNA (500 ng)
was reverse-transcribed using 2 µl Reverse Transcription 10X
Buffer, 2 µl dNTP mixture, 4 µl MgCl2, 0.5 µl
recombinant RNasin ribonuclease inhibitor, 0.6522 µl AMV reverse
transcriptase and 1 µl Oligo primer, which were components of a
Reverse Transcription system kit (cat. no. A3500; Promega
Corporation), according to the manufacturer's protocol, incubating
at 42˚C for 15 min, 95˚C for 5 min and 5˚C for 5 min. An ABI PRISM
7500 Real-Time PCR System (Applied Biosystems; Thermo Fisher
Scientific, Inc.) was used for gene amplification and Hot-Start
Activation was performed for cDNA at 95˚C for 2 min, followed by 40
cycles of 95˚C for 3 sec, annealing/extension at 60˚C for 30 sec
and dissociation at 60˚C. The total reaction volume (25 µl)
contained 12.5 µl GoTaq qPCR Master Mix, 2 µl primer, 2 µl cDNA and
8.5 µl nuclear-free water, which were contained in GoTaq qPCR
Master Mix Kit (cat. no. A6001; Promega Corporation). GAPDH was
used as the reference gene and the relative of gene expression
level was calculated as ΔCq=Cq (gene)-Cq (reference). The fold
change of gene expression was calculated using the
2-ΔΔCq method (31).
The experiment was performed in triplicate. The primer sequences
used were as follows: CD14 forward, 5'-GAGTCAACAGGGCATTCACC-3' and
reverse, 5'-GGGACCGTAACAGGAAGGAT-3'; TLR4 forward,
5'-TAAGGTTGCCGCTTTCACTT-3' and reverse, 5'-TGACCGAGCAGTTTCTGAGG-3';
and GAPDH forward, 5'-AAACCCATCACCATCTTCCAG-3' and reverse,
5'-AGGGGCCATCCACAGTCTTCT-3'.
Immunofluorescence staining
A549 cells were cultured on Lab-Tek chamber slides
(cat. no. 155380; Nunc™; Thermo Fisher Scientific, Inc.) for 24 h.
They were then stimulated with LPS (1 µg/ml) for 2 h and then
treated with propofol (1 µg/ml) for 3 h. Cells were fixed in 4%
formaldehyde for 30 min at room temperature. For immunostaining,
cells were permeabilized in 0.2% Triton X-100 for 5 min at room
temperature and blocked with 5% BSA (cat. no. A1933;
MilliporeSigma) for 30 min at room temperature. Cells were
incubated with rabbit polyclonal CD14 antibody (dilution, 1:160;
cat. no. sc-9150; Santa Cruz Biotechnology, Inc.) and goat
polyclonal TLR4 antibody (dilution, 1:160; cat. no. sc-16240; Santa
Cruz Biotechnology, Inc.) overnight at 4˚C. Fluorescein-conjugated
anti-goat IgG (dilution, 1:50; cat. no. SA00003-2; ProteinTech
Group) and rhodamine-conjugated anti-rabbit IgG (dilution, 1:50;
cat. no. SA00007-1; ProteinTech Group) antibodies were used as
secondary antibodies with incubation at 37˚C for 45 min. Nuclei
were counterstained with DAPI (cat. no. ab228549; Abcam) and cells
were visualized using confocal microscopy (magnification, x400;
FV1000; Olympus Corporation).
ELISA
Cell culture supernatants were collected and stored
at -80˚C in advance. The TNF-α levels were determined using a human
TNF-α ELISA kit (cat. no. VAL 105; R&D Systems) according to
the manufacturer's protocol. This experiment was performed three
times.
Statistical analysis
Values are expressed as the mean ± standard
deviation (n=3). SPSS version 17 (SPSS, Inc.) was used for all
analyses. One-way ANOVA followed by Tukey's post-hoc test was used
for comparisons between groups. P<0.05 was considered to
indicate a statistically significant difference.
Results
Post-treatment with propofol regulates
CD14 expression in a dose-dependent manner
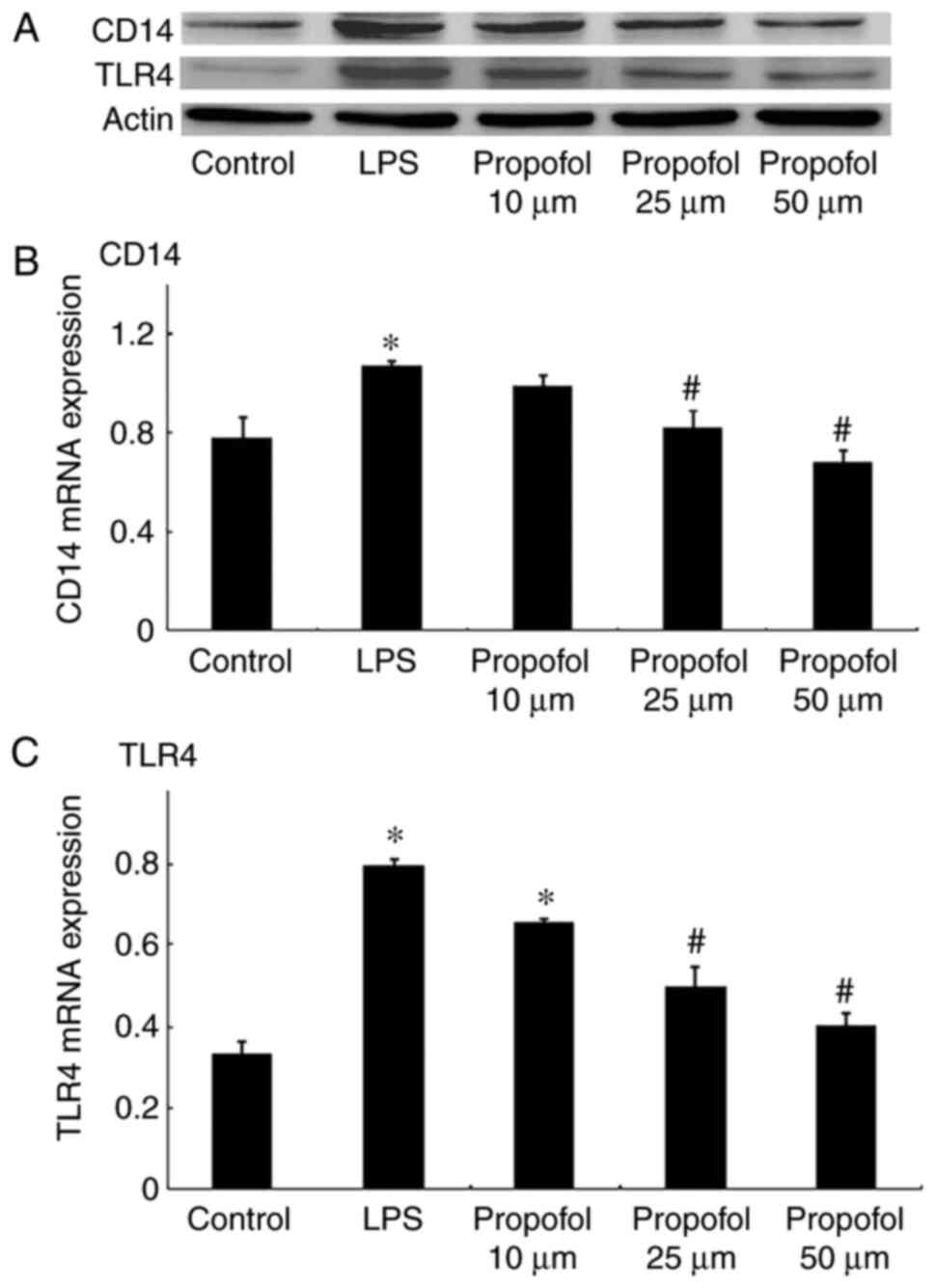
The expression of CD14 was detected by western blot
analysis and RT-qPCR. As presented in Fig. 1, the CD14 level was significantly
increased by treatment with LPS at both the protein and mRNA level
in A549 cells. CD14 expression decreased significantly following
post-treatment with propofol in a dose-dependent manner at both the
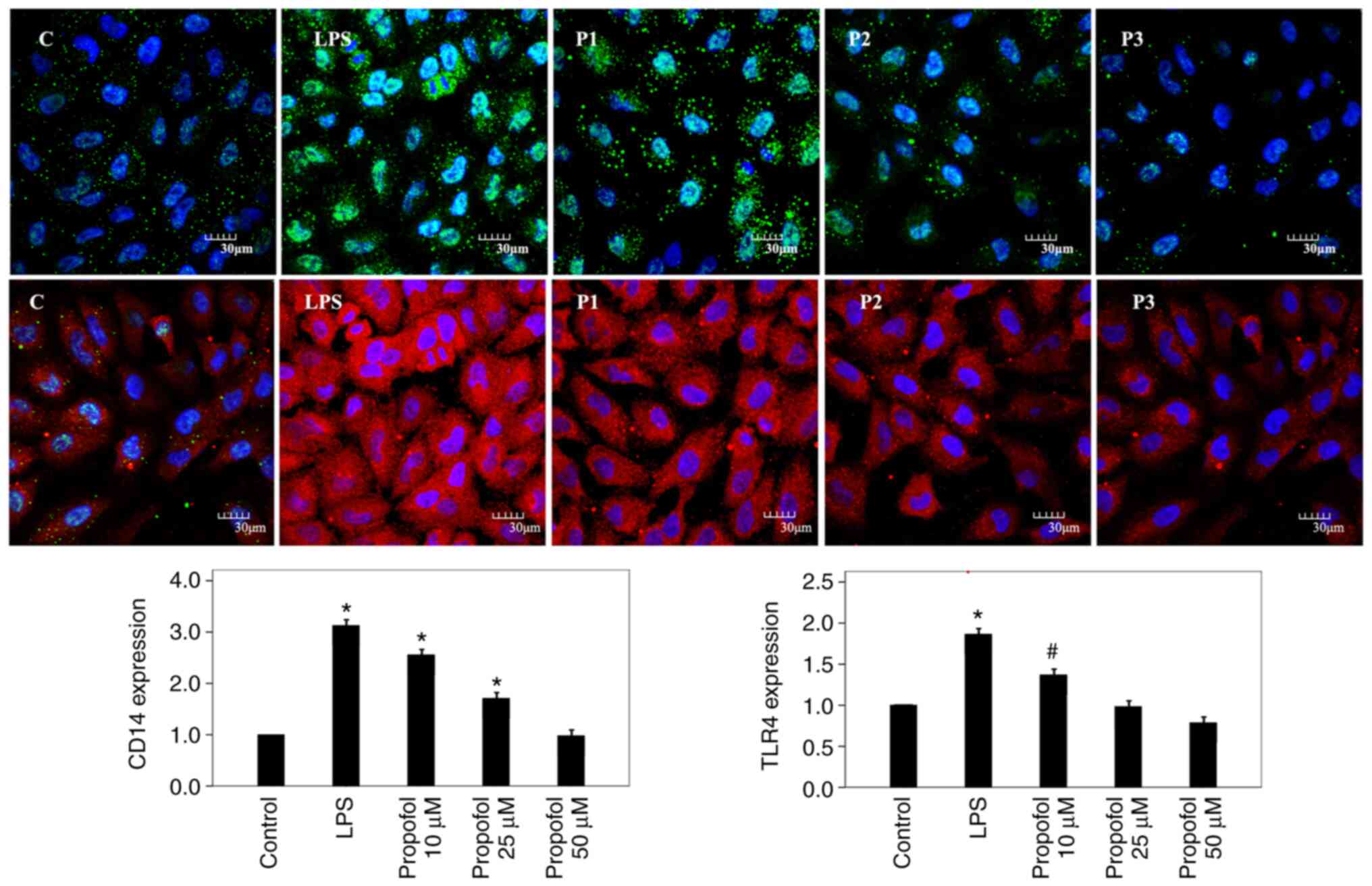
protein and mRNA levels. Furthermore, CD14 protein was visualized 3
h after post-treatment with propofol using immunofluorescence
microscopy and suppressed CD14 expression was observed (Fig. 2).
Post-treatment with propofol regulates
TLR4 expression in a dose-dependent manner
TLR4 expression was analyzed in A549 cells using
western blot analysis and RT-qPCR. The western blot results
indicated that TLR4 expression increased significantly following
treatment with LPS in A549 cells (Fig.
1C). Post-treatment with propofol decreased TLR4 expression in
the A549 cells in a concentration-dependent manner. The RT-qPCR
results exhibited a similar trend (Fig. 3), namely that LPS upregulated the
mRNA levels of TLR4 and post-treatment with propofol reversed these
increases in the mRNA levels of TLR4 in a concentration-dependent
manner in A549 cells. The TLR4 protein levels were also determined
using immunofluorescence microscopy following propofol treatment
for 3 h and similar results were observed; TLR4 expression was
increased following LPS treatment and suppressed following
post-treatment with propofol (Fig.
2).
Post-treatment with propofol regulates
TNF-α expression in a dose-dependent manner
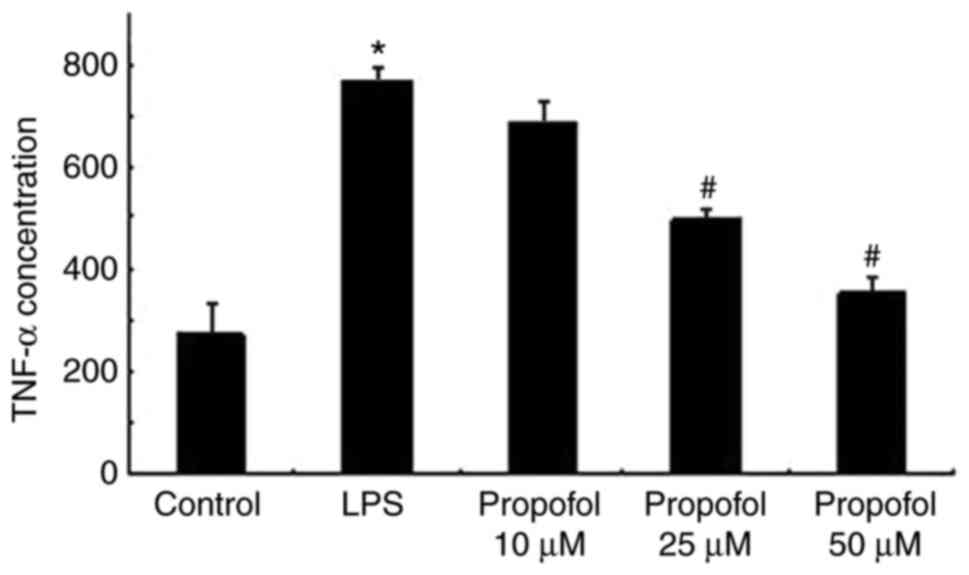
TNF-α expression in A549 cells treated with LPS and
propofol was determined using ELISA. As presented in Fig. 3, LPS significantly promoted the
expression of TNF-α in the A549 cells. Post-treatment with propofol
suppressed the expression of TNF-α in a dose-dependent manner in
A549 cells.
Discussion
The present study investigated whether
post-treatment with propofol has a positive role in protecting A549
cells against LPS-induced inflammation, and how propofol exerted
its protective function. It was indicated that post-treatment with
propofol markedly restored immune function in LPS-induced A549
cells, attenuating the stimulation of proinflammatory cytokines. It
was also observed that the mechanism of action of post-treatment
with propofol may involve the modulation of CD14 and TLR4
expression during protection against lung injury. The results of
the present study indicated the protective role of post-treatment
with propofol in LPS-induced inflammation and revealed that
post-treatment with propofol mitigated inflammation by modulating
CD14 and TLR4 expression.
With the COVID-19 pandemic, inflammation and its
alleviation have drawn ever increasing attention. Alveolar
epithelial cells are frequently the first type of cell to suffer
the damage caused by pathogenic microbial cells, which include not
only the inflammatory and target cells but also active inflammatory
and effector cells (32,33). The A549 cell line, orignally a lung
cancer cell line, is a widely used cell line in research on
alveolar epithelial cell biology and its shared characteristics
with type II alveolar epithelial cells have been demonstrated in
vitro; the response of this cell line to various interventions
is constant and repeatable, so it may be used in the study of
relevant interventions (34,35).
TLR-4 is one of the most important receptors that
recognize and initiate the inflammatory signal of LPS, as well as
that of viruses, such as SARS-CoV-2 (36,37).
TLR4-CD14 complexes may initiate the inflammatory signaling pathway
of LPS and activate downstream cellular signaling pathways
(38). TNF-α is an early
endogenous mediator and an important signaling factor, produced
mainly by the alveolar macrophages, which is released early in the
inflammatory response. TNF-α is able to initiate, amplify and
continue the systemic or local inflammatory reaction, as well as
accelerate pulmonary toxicity (39).
Propofol is considered to be one of the most
commonly used drugs for anesthesia and sedation in clinical
practice. Certain studies have proven its immunoregulatory and
anti-inflammatory effects. Propofol alleviated lung injury in
neonatal rats with LPS-induced ALI by preventing inflammation and
oxidative stress through the regulation of p38 MAPK/NF-κB signaling
pathway activity and NLR family pyrin domain containing 3 (NLRP3)
inflammasome expression (40).
Furthermore, Zhao et al (41) reported that propofol reduced
endotoxin-induced cardiomyocyte injury by inhibiting inflammation
and apoptosis through the peroxisome proliferator-activated
receptor γ/high mobility group box protein 1/NLRP3 axis. However,
the effects and mechanisms remain to be fully elucidated,
particularly when patients with pre-existing inflammation or
ALI/ARDS are placed under propofol-induced sedation or anesthesia.
In the present study, TLR4 was indicated to have a marked impact on
the molecular signaling pathways of inflammation, particularly in
the identification of pathogens associated with inflammatory
molecular patterns by combining with CD14. Certain previous reports
have indicated that propofol was able to inhibit the expression of
TLR4 and the activation of downstream molecules (42,43),
which is in line with the results of the present study.
Post-treatment with propofol reduced the LPS-induced expression of
TLR4 and CD14 at both the protein and mRNA levels in a
concentration-dependent manner in A549 cells. Therefore, it may be
hypothesized that post-treatment with propofol is able to inhibit
the inflammatory reaction by reducing TLR4 and CD14 levels during
ALI/ARDS. To further investigate the anti-inflammatory effect of
post-treatment with propofol, the expression of TNF-α in A549 cells
treated with LPS and propofol was analyzed. The present results
indicated that post-treatment with propofol can alleviate the
LPS-induced inflammation of A549 cells.
In a previous study, patients required an average
blood propofol concentration of 4.05±1.01 µg/ml for major surgery
and 2.97±1.07 µg/ml for non-major surgery (12-29 µM) (42,44).
Blood concentrations of propofol may reach 56 µM after a bolus
injection (43,45), with a peak of 67 µM (44,46).
Therefore, 10-50 µM was considered as the range of clinically
achievable concentrations during propofol anesthesia. However, 10
µM propofol had no statistically significant anti-inflammatory
effect in LPS-induced A549 cells, suggesting it was too low to
exert an anti-inflammatory effect.
To the best of our knowledge, no studies have
investigated post-treatment with propofol for the suppression of
inflammation. The present study indicated that propofol suppressed
LPS-induced CD14, TLR4 and TNF-α expression in a
concentration-dependent manner in A549 cells, providing guidance on
choosing anesthetics. Propofol may be a better choice for patients
with pre-existing lung injury due to its anti-inflammatory effects.
For patients with pre-existing ALI and ARDS, propofol may be a
suitable choice for anesthesia or sedation.
The present study was not without its limitations.
First, it was an in vitro study; further in vivo
animal studies should be performed to verify the mechanism.
Furthermore, since propofol is widely used in the clinic, clinical
trials with actual patients will provide more reliable results on
its effect on inflammation and guidance for medical practice.
In conclusion, it was confirmed that post-treatment
with a clinically relevant concentration of propofol had important
anti-inflammatory effects on LPS-induced alveolar epithelial cells.
This beneficial effect of post-treatment with propofol on cell
viability was mediated by inhibition of CD14 and TLR4 expression.
The present study provided a pharmacological basis for the clinical
application of the anesthetic compound propofol in patients with
inflammation.
Acknowledgements
The authors deeply appreciate the kind help from Ms.
Min Shi (National Institute of Environmental Health Science,
National Institutes of Health) with the language editing of the
manuscript.
Funding
Funding: This work was supported by the National Natural Science
Foundation of China (grant no. 81302534).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
XY performed the experiments and the data analysis.
LM contributed to the design of the experiments and writing the
manuscript. XY and LM confirm the authenticity of all the raw data.
All authors have read and approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Chen N, Zhou M, Dong X, Qu J, Gong F, Han
Y, Qiu Y, Wang J, Liu Y, Wei Y, et al: Epidemiological and clinical
characteristics of 99 cases of 2019 novel coronavirus pneumonia in
Wuhan, China: A descriptive study. Lancet. 395:507–513.
2020.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Dorward DA, Russell CD, Um IH, Elshani M,
Armstrong SD, Penrice-Randal R, Millar T, Lerpiniere CEB,
Tagliavini G, Hartley CS, et al: Tissue-specific immunopathology in
fatal COVID-19. Am J Respir Crit Care Med. 203:192–201.
2021.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Pairo-Castineira E, Clohisey S, Klaric L,
Bretherick AD, Rawlik K, Pasko D, Walker S, Parkinson N, Fourman
MH, Russell CD, et al: Genetic mechanisms of critical illness in
COVID-19. Nature. 591:92–98. 2021.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Baedorf Kassis E, Schaefer MS, Maley JH,
Hoenig B, Loo Y, Hayes MM, Moskowitz A and Talmor D: Transpulmonary
pressure measurements and lung mechanics in patients with early
ARDS and SARS-CoV-2. J Crit Care. 63:106–112. 2021.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Smith S, Skerrett SJ, Chi EY, Jonas M,
Mohler K and Wilson CB: The locus of tumor necrosis factor-alpha
action in lung inflammation. Am J Respir Cell Mol Biol. 19:881–891.
1998.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Knox KW, Vesk M and Work E: Relation
between excreted lipopolysaccharide complexes and surface
structures of a lysine-limited culture of Escherichia coli. J
Bacteriol. 92:1206–1217. 1996.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Nova Z, Skovierova H and Calkovska A:
Alveolar-capillary membrane-related pulmonary cells as a target in
endotoxin-induced acute lung injury. Int J Mol Sci.
20(831)2019.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Chacko B, Peter JV, Tharyan P, John G and
Jeyaseelan L: Pressure-controlled versus volume-controlled
ventilation for acute respiratory failure due to acute lung injury
(ALI) or acute respiratory distress syndrome (ARDS). Cochrane
Database Syst Rev. 1(CD008807)2015.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Zhu T, Zhang W, Feng SJ and Yu HP: Emodin
suppresses LPS-induced inflammation in RAW264.7 cells through a
PPARγ-dependent pathway. Int Immunopharmacol. 34:16–24.
2016.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Sivanantham A, Pattarayan D, Bethunaickan
R, Kar A, Mahapatra SK, Thimmulappa RK, Palanichamy R and
Rajasekaran S: Tannic acid protects against experimental acute lung
injury through downregulation of TLR4 and MAPK. J Cell Physiol.
234:6463–6476. 2019.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Li HF, Wu YL, Tseng TL, Chao SW, Lin H and
Chen HH: Inhibition of miR-155 potentially protects against
lipopolysaccharide-induced acute lung injury through the
IRF2BP2-NFAT1 pathway. Am J Physiol Cell Physiol. 319:C1070–C1081.
2020.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Wang H, Wang T, Yuan Z, Cao Y, Zhou Y, He
J, Shen Y, Zeng N, Dai L, Wen F and Chen L: Role of receptor for
advanced glycation end products in regulating lung fluid balance in
lipopolysaccharide-induced acute lung injury and infection-related
acute respiratory distress syndrome. Shock. 50:472–482.
2018.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Meyer K, Patra T, Vijayamahantesh
and Ray R: SARS-CoV-2 spike protein induces paracrine senescence
and leukocyte adhesionin endothelial cells. J Virol.
95(E0079421)2021.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Schiller HB, van Breugel M and Nawijn MC:
SARS-CoV-2-specific hotspots in virus-host interaction networks.
Nat Immunol. 22:806–808. 2021.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Nakamura M, Takeuchi T, Shirakawa K and
Furusako S: Anti-human CD14 monoclonal antibody improves survival
following sepsis induced by endotoxin, but not following
polymicrobial infection. Eur J Pharmacol. 806:18–24.
2017.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Lappin MJ, Brown V, Zaric SS, Lundy FT,
Coulter WA and Irwin CR: Interferon-γ stimulates CD14, TLR2 and
TLR4 mRNA expression in gingival fibroblasts increasing
responsiveness to bacterial challenge. Arch Oral Biol. 61:36–43.
2016.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Huynh DTN, Baek N, Sim S, Myung CS and Heo
KS: Minor ginsenoside Rg2 and Rh1 attenuates LPS-induced acute
liver and kidney damages via downregulating activation of
TLR4-STAT1 and inflammatory cytokine production in macrophages. Int
J Mol Sci. 21(6656)2020.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Iannucci A, Caneparo V, Raviola S,
Debernardi I, Colangelo D, Miggiano R, Griffante G, Landolfo S,
Gariglio M and De Andrea M: Toll-like receptor 4-mediated
inflammation triggered by extracellular IFI16 is enhanced by
lipopolysaccharide binding. PLoS Pathog.
16(e1008811)2020.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Plociennikowska A, Hromada-Judycka A,
Dembinska J, Roszczenko P, Ciesielska A and Kwiatkowska K:
Contribution of CD14 and TLR4 to changes of the PI(4,5)P2 level in
LPS-stimulated cells. J Leukoc Biol. 100:1363–1373. 2016.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Liu P, Cui L and Shen L: Knockdown of
TRIM52 alleviates LPS-induced inflammatory injury in human
periodontal ligament cells through the TLR4/NF-ĸB pathway. Biosci
Rep. 40(BSR20201223)2020.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Aboudounya MM and Heads RJ: COVID-19 and
toll-like receptor 4 (TLR4): SARS-CoV-2 may bind and activate TLR4
to Increase ACE2 expression, facilitating entry and causing
hyperinflammation. Mediators Inflamm. 2021(8874339)2021.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Bowman ER, Cameron CMA, Avery A, Gabriel
J, Kettelhut A, Hecker M, Sontich CU, Tamilselvan B, Nichols CN,
Richardson B, et al: Levels of soluble CD14 and tumor necrosis
factor receptors 1 and 2 may be predictive of death in severe
coronavirus disease 2019. J Infect Dis. 223:805–810.
2021.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Singhal S, Kumar P, Singh S, Saha S and
Dey AB: Clinical features and outcomes of COVID-19 in older adults:
A systematic review and meta-analysis. BMC Geriatr.
21(321)2021.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Fernandez-Botran R, Furmanek S,
Ambadapoodi RS, Exposito Gonzalez E, Cahill M, Carrico R, Akca O
and Ramirez JA: University of Louisville COVID-19 Study Group.
Association and predictive value of biomarkers with severe outcomes
in hospitalized patients with SARS-CoV-2 infection. Cytokine.
149(155755)2022.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Tufan A, Avanoglu Guler A and
Matucci-Cerinic M: COVID-19, immune system response,
hyperinflammation and repurposing antirheumatic drugs. Turk J Med
Sci. 50:620–632. 2020.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Zheng X, Huang H, Liu J, Li M, Liu M and
Luo T: Propofol attenuates inflammatory response in LPS-activated
microglia by regulating the miR-155/SOCS1 pathway. Inflammation.
41:11–19. 2018.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Yang N, Liang Y, Yang P and Ji F: Propofol
suppresses LPS-induced nuclear accumulation of HIF-1α and tumor
aggressiveness in non-small cell lung cancer. Oncol Rep.
37:2611–2619. 2017.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Yeh CH, Cho W, So EC, Chu CC, Lin MC, Wang
JJ and Hsing CH: Propofol inhibits lipopolysaccharide-induced lung
epithelial cell injury by reducing hypoxia-inducible factor-1alpha
expression. Br J Anaesth. 106:590–599. 2011.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Huang T, Zhang Y, Wang C and Gao J:
Propofol reduces acute lung injury by up-regulating
gamma-aminobutyric acid type a receptors. Exp Mol Pathol.
110(104295)2019.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Witenko CJ, Littlefield AJ, Abedian S, An
A, Barie PS and Berger K: The safety of continuous infusion
propofol in mechanically ventilated adults with coronavirus disease
2019. Ann Pharmacother. 56:5–15. 2022.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408.
2001.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Gerard L, Lecocq M, Bouzin C, Hoton D,
Schmit G, Pereira JP, Montiel V, Plante-Bordeneuve T, Laterre PF
and Pilette C: Increased angiotensin-converting enzyme 2 and loss
of alveolar type II Cells in COVID-19 Related ARDS. Am J Respir
Crit Care Med. 204:1024–1034. 2021.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Bridges JP, Vladar EK, Huang H and Mason
RJ: Respiratory epithelial cell responses to SARS-CoV-2 in
COVID-19. Thorax. 77:203–209. 2022.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Sasaki M, Kishimoto M, Itakura Y, Tabata
K, Intaruck K, Uemura K, Toba S, Sanaki T, Sato A, Hall WW, et al:
Air-liquid interphase culture confers SARS-CoV-2 susceptibility to
A549 alveolar epithelial cells. Biochem Biophys Res Commun.
577:146–151. 2021.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Wang Y, Fan Y, Huang Y, Du T, Liu Z, Huang
D, Wang Y, Wang N and Zhang P: TRIM28 regulates SARS-CoV-2 cell
entry by targeting ACE2. Cell Signal. 85(110064)2021.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Choudhury A and Mukherjee S: In silico
studies on the comparative characterization of the interactions of
SARS-CoV-2 spike glycoprotein with ACE-2 receptor homologs and
human TLRs. J Med Virol. 92:2105–2113. 2020.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Leifer CA and Medvedev AE: Molecular
mechanisms of regulation of Toll-like receptor signaling. J Leukoc
Biol. 100:927–941. 2016.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Kim S, Kim SY, Pribis JP, Lotze M, Mollen
KP, Shapiro R, Loughran P, Scott MJ and Billiar TR: Signaling of
high mobility group box 1 (HMGB1) through toll-like receptor 4 in
macrophages requires CD14. Mol Med. 19:88–98. 2013.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Sancho Ferrando E, Hanslin K, Hultström M,
Larsson A, Frithiof R and Lipcsey M: Uppsala Intensive Care
COVID-19 Research Group. Soluble TNF receptors predict acute kidney
injury and mortality in critically ill COVID-19 patients: A
prospective observational study. Cytokine.
149(155727)2022.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Yu X and Li C: Protective effects of
propofol on experimental neonatal acute lung injury. Mol Med Rep.
19:4507–4513. 2019.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Zhao H, Gu Y and Chen H: Propofol
ameliorates endotoxin induced myocardial cell injury by inhibiting
inflammation and apoptosis via the PPARγ/HMGB1/NLRP3 axis. Mol Med
Rep. 23(176)2021.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Ma L, Yang Y, Sun X, Jiang M, Ma Y, Yang X
and Guo Z: Propofol regulates the expression of TLR4 through miR-21
in human umbilical vein endothelial cells. Mol Med Rep.
16:9074–9080. 2017.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Wang Y, Lin C, Wang J, Zhou M, Fang T,
Miao L and Wei Y: Propofol rescues LPS-induced toxicity in
HRT-8/SVneo cells via miR-216a-5p/TLR4 axis. Arch Gynecol Obstet:
Jan 4, 2022 (Epub ahead of print).
|
|
44
|
Shafer A, Doze VA, Shafer SL and White PF:
Pharmacokinetics and pharmacodynamics of propofol infusions during
general anesthesia. Anesthesiology. 69:348–356. 1988.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Gepts E, Camu F, Cockshott ID and Douglas
EJ: Disposition of propofol administered as constant rate
intravenous infusions in humans. Anesth Analg. 66:1256–1263.
1987.PubMed/NCBI
|
|
46
|
Schüttler J and Ihmsen H: Population
pharmacokinetics of propofol: A multicenter study. Anesthesiology.
92:727–738. 2000.PubMed/NCBI View Article : Google Scholar
|